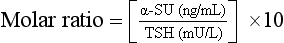Glycoprotein-secreting pituitary tumors
1. What are glycoprotein hormones?
The glycoprotein hormones, luteinizing hormone (LH), thyrotropin (TSH), follicle-stimulating hormone (FSH), and chorionic gonadotropin (CG), are composed of two noncovalently bound subunits. The alpha subunits (α-SUs) are similar in all four hormones. In contrast, the beta subunits (LHβ, FSHβ, and so on) are unique both immunologically and biologically for each hormone.
2. Name two types of glycoprotein-secreting pituitary tumors and their secretory products.
3. Do pituitary tumors secrete only a single hormone?
No. Many tumors make two or more hormones or subunits. At times, sufficient quantities of multiple hormones are secreted to produce clinical symptoms characteristic of several syndromes within the same patient.
4. Under what circumstances should a TSH-secreting tumor be considered?
5. Describe the differential diagnosis for patients with a transient increase in serum T4 and detectable or elevated TSH.
6. Describe the differential diagnosis for patients with a permanent increase in serum total T4 and detectable or elevated level of serum TSH.
7. What tests aid in the differential diagnosis of the patient with elevated serum total T4 and detectable or elevated TSH?
The history and physical examination usually rule out medications and nonthyroidal illnesses. The most important laboratory test is the free T4 measurement. A normal free T4 value with an elevated total T4 value strongly suggests one of the binding protein disorders. An elevated free T4 value, in contrast, generally narrows the differential to two disorders: a thyroid hormone resistance syndrome or a TSH-secreting pituitary tumor. Clinical thyrotoxicosis is commonly present in patients with either condition. One should confirm the abnormal test results in a second laboratory before initiating a workup for these uncommon disorders.
8. How can one distinguish between the hyperthyroid patient with thyroid hormone resistance and one with a pituitary tumor?
TSH tumors may secrete α-SU in excess of the whole TSH molecule. Therefore, the molar ratio of serum α-SU to TSH is increased in many patients with TSH tumors but is normal in those with thyroid hormone resistance. A thyrotropin-releasing hormone (TRH; protirelin) test is also helpful. Fewer than 20% of patients with a TSH tumor have a twofold increase in serum TSH after TRH administration, whereas those with resistance show a brisk response. T3 (triiodothyronine) suppression does not lower TSH in TSH-producing pituitary tumors but does do so in thyroid hormone resistance disorders. T3 suppression reduces Doppler color-flow and peak systolic velocity on thyroid ultrasound in most patients with thyroid hormone resistance, but not usually in patients with TSH-producing tumors. If a tumor is suspected, magnetic resonance imaging (MRI) of the pituitary should be obtained. Most TSH tumors (approximately 90%) are macroadenomas (i.e., ≥10 mm). Most microadenomas (<10 mm) are also visualized on MRI, but rarely, sampling of inferior petrosal sinus blood may be helpful in localizing a tumor. Dynamic MRI or somatostatin receptor scintigraphy (OctreoScan) is also useful. Long-term (2-month) administration of a long-acting somatostatin analog decreases serum free T4/T3 and TSH in patients with TSH tumors. Rarely, patients with TSH-secreting pituitary adenomas may have coexisting Graves’ hyperthyroidism or thyroid carcinoma.
9. Describe how to calculate an alpha subunit/TSH molar ratio.
TSH values are expressed as μU/mL (or mU/L). One must know the bioactivity and convert these units to ng/mL, the units of α-SU. Furthermore, the molecular weight of the subunit is only half the molecular weight of the whole TSH molecule; this fact must also be considered in calculating the molar ratio. From a practical standpoint, the following formula can be used:
10. Name the treatment of choice for TSH-secreting tumors.
Pituitary surgery is the treatment of choice and produces remission in up to 50% of patients. Results are somewhat better if surgery is followed by radiation therapy. Because more microadenomas are being identified, results of cure or control of disease are improving.
11. How effective is radiation as the sole therapy?
12. List the medical therapies used for TSH-secreting tumors.
Octreotide or lanreotide (somatostatin analog) decreases TSH in more than 90% of cases and normalizes free T4 in 75% of cases. Tumor size decreases, and vision improves. Bromocriptine has limited success. Dexamethasone reduces TSH, but its side effects exclude long-term use. Iopanoic acid is effective preoperatively.
13. Summarize the role of thyroid gland ablation in the treatment of TSH-secreting tumors.
Thyroidectomy and radioactive iodine (I 131) should be avoided. They do not control TSH secretion and may enhance pituitary activity and growth, although two reported patients monitored for 8 and 12 years had no tumor growth.
14. Do all patients with an enlarged pituitary gland and an elevated serum TSH value have thyrotropinomas?
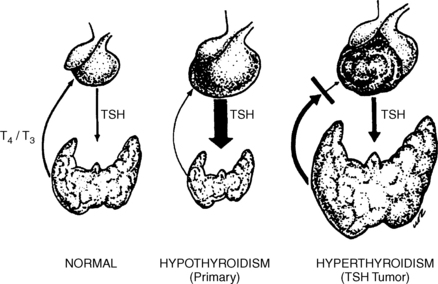
No. In patients with long-standing hypothyroidism, pituitary hyperplasia and a pseudotumor may develop (Fig. 22-1). The mass can extend into the suprasellar region, causing visual field defects. The serum T4 value is always low. Shrinkage of the enlarged gland usually occurs with l-T4 therapy. Hyperplasia of lactotrophs may also occur, causing elevated prolactin levels. No patient should undergo pituitary gland surgery without preoperative measurement of serum T4 and TSH.
15. What clinical features raise suspicion of a TSH-secreting pseudotumor?
Almost all patients have symptoms of hypothyroidism and the serum T4 level is always low. The underlying abnormality is usually autoimmune thyroiditis. About 80% of cases of pituitary enlargement with hypothyroidism have occurred in women, whereas only 55% of true TSH tumors are in women. In children, precocious puberty may occur. Thyroid antibodies are present in more than 75% of patients with pseudotumor, compared with about 10% of patients with TSH tumors that produce hyperthyroidism.
16. Does the presence of abnormal visual fields help distinguish between pituitary hyperplasia due to primary hypothyroidism and TSH-secreting tumors?
No. Abnormal visual fields have been reported in 28% of patients with hyperplasia versus 42% of those with tumors. In contrast, patients with thyroid hormone resistance have normal vision.
17. Does family history provide any clues for distinguishing these disorders?
In pseudotumor from thyrotroph hyperplasia, the family history may be positive for autoimmune diseases (e.g., thyroiditis, Graves’ disease, type 1 diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, vitiligo, Addison disease, pernicious anemia). In TSH tumors, family history is usually absent. Most cases of generalized thyroid hormone resistance are familial with autosomal dominant inheritance (i.e., 50% of the family members have the abnormality).
18. Which hormones are elevated in the serum of patients with gonadotroph adenomas?
Serum FSH is increased much more often than LH. An increase in alpha subunit level is not specific for gonadotrophs because it may also derive from thyrotrophs. Furthermore, determination of alpha subunit/LH (or alpha subunit/FSH) molar ratio has not been clinically useful.
19. List the presenting symptoms of patients with gonadotropinomas.
| Mass effect (common) | Large tumors with extrasellar growth |
| Visual impairment/diplopia | |
| Headaches | |
| Apoplexy | |
| Hypopituitarism | |
| Endocrine excesses (uncommon) | Ovarian hyperstimulation |
| Testicular enlargement | |
| Precocious puberty |
20. In a patient in whom gonadotropin values are elevated, how can one distinguish a gonadotroph adenoma from primary hypogonadism?
This distinction can be difficult, especially in women, because their levels of LH and FSH increase after menopause. This fact may be why most gonadotroph adenomas are recognized in men. Historically, men with such tumors experienced normal puberty and may have fathered children.
On examination, testicular size may be normal. In contrast, men with hypogonadism may have had abnormal pubertal development or a history of testicular injury; the testes are small.
21. What laboratory tests are helpful?
In primary hypogonadism, both FSH and LH are increased, whereas FSH is elevated, but LH is usually normal in patients with gonadotropinomas. When LH is high in men with gonadotropinomas, testosterone also is high rather than low, as in hypogonadism. For unclear reasons, about one third of patients with such a tumor have an anomalous rise in serum FSH or LHβ when given a TRH injection. MRI of the pituitary reveals a large tumor. Occasionally, a patient with long-standing hypogonadism may have some pituitary enlargement.
22. How are gonadotropinomas treated?
Pituitary surgery is the treatment of choice. Although cure is often impossible, substantial reductions in tumor size and hormone secretion are common. Reduced hormone secretion provides a convenient marker for monitoring tumor recurrence; an abrupt increase in FSH or alpha subunit should prompt repeat imaging. Radiation therapy is often given after surgery to prevent tumor recurrence.
23. Is medical therapy effective?
Agonist analogs of gonadotropin-releasing hormone (GnRH) reduce secretion from normal gonadotrophs. Unfortunately, they often have the opposite effect on gonadotropinomas. An antagonist analog (Nal-Glu-GnRH) reduced serum FSH in a small group of men with gonadotropinomas but did not reduce tumor size. Bromocriptine has reduced hormone levels in an occasional patient, whereas octreotide has lowered alpha subunit and improved visual fields in certain patients. Cabergoline can reduce estradiol levels and ovarian size in women with ovarian hyperstimulation.
Al-Gahtany, M, Horvath, E, Kovacs, K. Pituitary hyperplasia. Hormones. 2003;2:149–158.
Beck-Peccoz, P, Brucker-Davis, F, Persani, L, et al. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610–638.
Beck-Peccoz, P, Persani, L, Mannavola, D, et al. TSH-secreting adenomas. Best Practice and Research Clinical Endocrinology and Metabolism. 2009;23:597–606.
Bogazzi, F, Manetti, L, Tomisti, L, et al, Thyroid color flow Doppler sonography. an adjunctive tool for differentiating patients with inappropriate thyrotropin (TSH) secretion due to TSH-secreting pituitary adenoma or resistance to thyroid hormone. Thyroid. 2006;16(10):989–995.
Brown, RL, Muzzafar, T, Wollman, R, et al, A pituitary carcinoma secreting TSH and prolactin. a non-secreting adenoma gone awry. Eur J Endocrinol 2006;154:639–643.
Buchfelder, M. Thyrotroph pituitary adenomas. Endocrinologist. 2002;12:117–125.
Caron, P, Arlot, S, Bauters, C, et al. Efficacy of the long-acting octreotide formulation (octreotide-LAR) in patients with thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 2001;86:2849–2853.
Chaidarun, SS, Klibanski, A. Gonadotropinomas. Semin Reprod Med. 2002;20:339–348.
Clarke, M, Erickson, D, Castro, R, et al. Thyroid-stimulating hormone pituitary adenoma. J Neurosurg. 2008;109:17–22.
Cooper, O, Geller, J, Melmed, S. Ovarian hyperstimulation syndrome caused by an FSH-secreting pituitary adenoma. Nat Clin Pract Endocrinol Metab. 2008;4(4):234–238.
Daousi, C, Foy, PM, MacFarlane, IA. Ablative thyroid treatment for thyrotoxicosis due to thyrotropin-producing pituitary tumours. J Neurol Neurosurg Psychiatry. 2007;78:93–95.
Dhillon, KS, Cohan, P, Kelly, DF, et al. Treatment of hyperthyroidism associated with thyrotropin-secreting pituitary adenomas with iopanoic acid. J Clin Endocrinol Metab. 2004;89:708–711.
Elhadd, T, Ghosh, S, Leng Teoh, W, et al, A patient with thyrotropinoma cosecreting growth hormone and follicle-stimulating hormone with low alpha-glycoprotein. a new subentity. Thyroid. 2009;19(8):899–903.
Knoepfelmacher, M, Danilovic, DLS, Rosa Nasser, RHR, et al. Effectiveness of treating ovarian hyperstimulation syndrome with cabergoline in two patients with gonadotropin-producing pituitary adenomas. Fertil Steril. 2006;86(719):715–718.
Lee, M, Wang, C. Concomitant Graves hyperthyroidism with thyrotrophin-secreting pituitary adenoma. South Med J. 2010;103:347–349.
Mannavola, D, Persani, L, Vannucchi, G, et al. Different responses to chronic somatostatin analogues in patients with central hyperthyroidism. Clin Endocrinol. 2005;62:176–181.
McGrath, GA, Goncalves, RJ, Udupa, JK, et al, New technique for quantitation of pituitary adenoma size. use in evaluating treatment of gonadotroph adenomas with a gonadotropin-releasing hormone antagonist. J Clin Endocrinol Metab 1993;76:1363–1368.
Nguyen, H, Galitz, M, Mai, V, et al, Management of coexisting thyrotropin/growth-hormone-secreting pituitary adenoma and papillary thyroid carcinoma. a therapeutic challenge. Thyroid. 2009;20(1):99–103.
Page, K, Roehmholdt, B, Jablonski, M, et al. Development of thyroid storm after surgical resection of a thyrotropin-secreting pituitary adenoma. Endocr Pract. 2008;14:732–737.
Refetoff, S, Weiss, RE, Usala, SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399.
Reubi, JC, Waser, B, Vale, W, et al. Expression of CRF1 and CRF2 receptors in human cancers. J Clin Endocrinol Metab. 2003;88:3312–3320.
Scheithauer, BW, Kovacs, K, Nose, V, et al, Multiple endocrine neoplasia type 1-associated thyrotropin-producing pituitary carcinoma. report of a probable de novo example. Human Pathology 2009;40:270–278.
Shorts-Cary, L, Xu, M, Ertel, J, et al. Bone morphogenetic protein and retinoic acid-inducible neural specific protein-3 is expressed in gonadotrope cell pituitary adenomas and induces proliferation, migration, and invasion. Endocrinology. 2007;148:967–975.
Simard, MF. Pituitary tumor endocrinopathies and their endocrine evaluation. Neurosurg Clin N Am. 2003;14:41–54.
Smallridge, RC, Thyrotropin- and gonadotropin-producing tumors. Korenman SG, Molitch ME, eds. Atlas of clinical endocrinology:. neuroendocrinology and pituitary disease, Philadelphia: Blackwell Science; 2000;95–113.
Smallridge, RC, Thyrotropin-secreting pituitary tumors. clinical presentation, investigation, and management. Curr Opin Endocrinol Diabetes 2001;8:253–258.
Smallridge, RC, Czervionke, LF, Fellows, DW, et al, Corticotropin- and thyrotropin-secreting pituitary microadenomas. detection by dynamic magnetic resonance imaging. Mayo Clin Proc 2000;75:521–528.
Snyder, PJ, Extensive personal experience. gonadotroph adenomas. J Clin Endocrinol Metab 1995;80:1059–1061.
Socin, HV, Chanson, P, Delemer, B, et al, The changing spectrum of TSH-secreting pituitary adenomas. diagnosis and management in 43 patients. Eur J Endocrinol 2003;148:433–442.
Young, WF, Jr., Scheithauer, BW, Kovacs, KT, et al, Gonadotroph adenoma of the pituitary gland. a clinicopathologic analysis of 100 cases. Mayo Clin Proc 1996;71:649–656.