CHAPTER 35 Glaucoma filtration surgery
Historical background
Filtration surgery creates a fistula between the anterior chamber and the subconjunctival space (Fig. 35.1). The earliest fistulizing procedures were described in the nineteenth century. MacKenzie (1830) was the first to suggest the surgical relief of raised intraocular pressure (IOP) by a sclerotomy. In 1857 Crichet attempted to make a permanent paracentesis by including the iris in a limbal wound. De Wecker devised an anterior sclerotomy with a view to increasing the drainage of the aqueous by the formation of a filtering cicatrix. The results remained unsatisfactory for the wound tended to close1.
In 1906 LaGrange performed a sclerecto-iridectomy and in 1909 Elliot described limbal trephination that provided a permanent fistula to the subconjunctival space1. To decrease the rate of flow through the filtering ostium during the early postoperative period, iris was used as a wick to act as a partial plug, or iridencleisis2. Thermal cautery of the scleral wound edges with entry into the anterior chamber (Preziosi, 1934)1, Scheie’s modification with peripheral iridectomy, a thermal sclerostomy (1958)3, and posterior lip sclerectomy1 (Iliff and Haas, 1962) were the most widely used operations until guarded filtration procedures were developed1.
In 1968 Cairns reported good results with ‘trabeculectomy’ in a series of glaucoma patients4. This procedure was supposed to remove a portion of trabecular meshwork to allow flow into the cut ends of the Schlemm’s canal, and used a partial-thickness scleral flap to cover the sclerostomy. Interestingly, it had been described in 1961 by Sugar, who should be acknowledged as the first to use the term ‘trabeculectomy’, but the flap was sutured tightly, with no subconjunctival filtration, and those cases were unsuccessful5. The ‘trabeculectomy’, more appropriately described as a guarded filtering procedure, became the most commonly used filtration procedure because of the reduction in the frequency of postoperative complications associated with over-filtration.
Mechanisms of IOP lowering
Filtration surgery lowers the IOP by creating a fistula between the anterior chamber and the subconjunctival space (see Fig. 35.1). The intraocular fluid beneath the conjunctiva and/or Tenon’s capsule form a filtering bleb and can either be absorbed through veins, conjunctival lymphatics or, in some cases where the conjunctiva is thin, pass directly into the tear film6,7.
Epidemiology
The prevalence of glaucoma in the developed world has been calculated to be between 1.1 and 2.1%, increasing with age. Even though it is usually a slowly progressive disease and the majority of people do not become blind, glaucoma represents a leading cause of blindness worldwide, and the commonest cause of preventable blindness. There are over 5 million blind from this disease, and more than 67 million people affected with glaucoma worldwide8.
Glaucoma care in the developed world has changed with the introduction of new medications and diagnostic technologies. Overall, there is a large increase in the volume of prescribing, accompanied by a large reduction in the number of guarded filtration operations. For example, in Scotland the number of guarded filtration procedures per 100 000 population was 61% less in 2004 than in 1994. This downward trend in Scotland has also been found in the UK, Australia, the USA, Canada, and France. In the USA there has been a substantial increase in laser trabeculoplasty procedures9–14.
Indications for glaucoma filtration surgery
Glaucoma treatment aims to prevent visual field loss progression and maintain patients’ quality of life. Factors that affect glaucoma patients’ quality of life include functional loss, inconvenience and side effects caused by medication(s), treatment expense, anxiety associated with the diagnosis of a chronic, and potentially blinding disease and the possibility of surgery15,16. Currently, IOP is the only major glaucoma risk factor that can be treated, and lowering IOP has been shown to reduce visual field loss progression17,18.
Preoperative assessment and anesthesia
The preoperative assessment and preparation of patients undergoing guarded filtration surgery under local anesthesia varies worldwide19. The standard preoperative assessment includes specific enquiry about bleeding disorders and drugs. There is an increased risk of hemorrhage in patients receiving anti-coagulants and a clotting profile assessment is required prior to injection techniques. Patients receiving anticoagulants are usually advised to continue medication. Clotting results should be within the recommended therapeutic range. Currently there is no recommendation (lack of data) for patient receiving anti-platelet agents. SubTenon’s block and/or topical anesthetic are favored in these patients.
There are numerous modes of anesthesia from which a surgeon can choose. Overall, there is not one type of anesthesia right for all cases, and the best choice varies from surgeon to surgeon, and patient to patient. The use of general anesthesia or regional (i.e. retrobulbar or peribulbar) block has declined with the availability of other safer and equally effective means of local anesthesia including subTenon’s, subconjunctival, and topical anesthesia associated with intracameral lidocaine20,21.
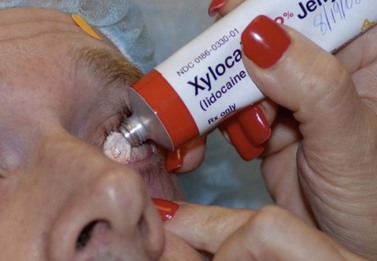
Topical ocular anesthesia has been demonstrated to be a safe and effective alternative to retro bulbar or peribulbar anesthesia for guarded filtration surgery. Local anesthetic eye drops are instilled three or four times, separated by a few minutes just prior to surgery. Another popular practice is the administration of topical anesthesia using viscous lidocaine gel instead of or in addition to drops (Fig. 35.2). Topical anesthesia does not provide ocular akinesia and may provide inadequate sensory blockade for the iris and ciliary body, thus intracameral injection of local anesthetics (preservative-free 1% lidocaine injected in doses of 1–5 ml) is also used22.
SubTenon’s anesthesia (block) is a simple, safe, effective and versatile alternative to a sharp needle block for orbital anesthesia. Access to the space by the inferonasal quadrant is the most commonly described approach because the placement of the cannula in this quadrant allows good fluid distribution superiorly while avoiding the area of surgery and reducing the risk of damage to the vortex veins. After instillation of local anesthetic eye drops the patient is asked to look upwards and outwards, to expose the inferonasal quadrant. The conjunctiva and Tenon capsule are gripped with non-toothed forceps 5–10 mm away from the limbus. A small incision is made through these layers with scissors and sclera is exposed. A blunt curved metal subTenon’s cannula, (19 G, 25 mm long, curved, with a flat profile with end hole) securely mounted on to a 5 ml syringe containing the local anesthetic solution, is inserted through the hole along the curvature of the sclera. The local anesthetic agent of choice is injected slowly and the cannula is removed. With the above technique adequate anesthesia is achieved for the majority of ocular surgeries. Akinesia is volume dependent and, if 4–5 ml of local anesthetic agent is injected, most patients develop akinesia23,24.
Sight and life-threatening complications have been reported but are very rare. The rise in IOP after administration of a subTenon’s block is small or even non-significant25,26.
The current retrobulbar technique used by most ophthalmologists today was described by Atkinson in 1934, and until recently served as the most commonly used technique for intraocular surgery27.
Davis and Mandel are credited with introducing the peribulbar block in 1986 as a safer alternative to retrobulbar anesthesia28.
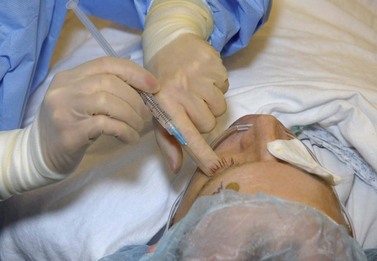
The decision between retrobulbar anesthesia and peribulbar anesthesia presents the surgeon with a choice between speed and safety. With a retrobulbar block a surgeon can ensure that adequate akinesia and anesthesia will result for glaucoma surgery; however, a blind injection into the orbit poses several potential complications including, but not limited to, retrobulbar hemorrhage, globe perforation, optic nerve damage, and brainstem anesthesia. Peribulbar anesthesia, involving the injection(s) of local anesthetic external to the muscle cone, decreases the likelihood of optic nerve and globe perforation while maintaining the desirable qualities of excellent akinesia and anesthesia (Fig. 35.3).
General anesthesia provides the most controlled environment for surgery. However, it is associated with an increased risk of systemic complications (e.g. malignant hyperthermia, hemodynamic fluctuation, myocardial infarction, postoperative nausea and vomiting), and requires more medication, equipment, and personnel. As a result, it is the most costly form of anesthesia. General anesthesia remains the technique of choice for children, intellectually handicapped individuals, and demented or psychologically unstable patients. Patients may feel that they will not be able to cooperate during surgery and insist on general anesthesia29.
Surgical technique
Choosing the surgical site
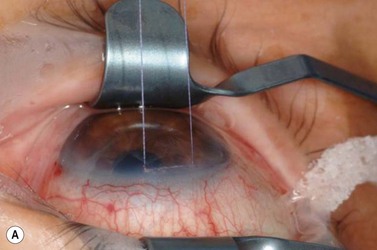
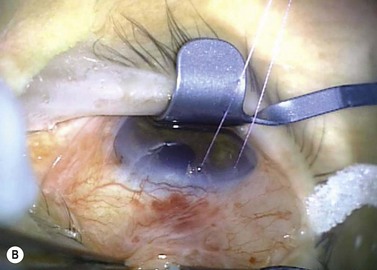
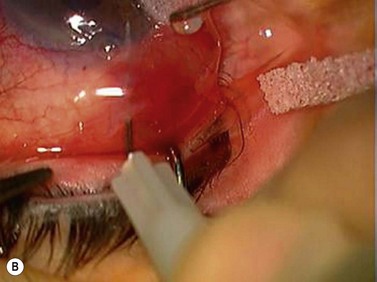
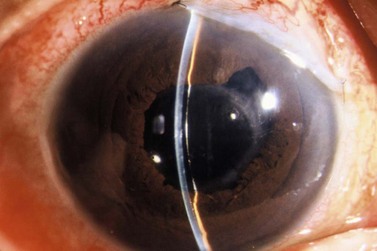
A fixation or traction suture keeps the eye in a downward position to give a good area of exposure superiorly. A corneal traction suture in the quadrant of the planned surgery (7-0 or 8-0 black silk or nylon, or 7-0 or 8-0 Vicryl on a spatula needle) is preferred by the authors (Fig. 35.4). The needle is passed through clear, mid-stromal cornea approximately 2 mm from the limbus for approximately 3–4 mm. Alternatively a superior rectus traction suture (4-0 or 5-0 black silk on a tapered needle) can be used to rotate the globe inferiorly and bring the superior bulbar conjunctival into view. Using a muscle hook to rotate the globe downwards, the conjunctiva and superior rectus are grasped with toothed forceps and the threaded needle is passed through the tissue bundle.
Conjunctival dissection
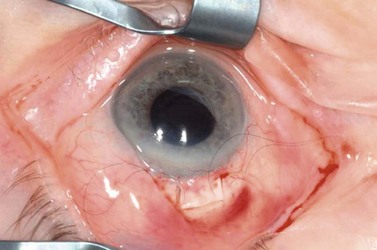
The advantages of the fornix-based conjunctival flap include improved exposure and access, reduced risk of conjunctival button-hole formation, and the formation of a more posterior and diffuse bleb, which may be associated with decreased incidence of late endophthalmitis (Fig. 35.5). However, with a fornix-based flap there is an increased risk of conjunctival wound leak in the early postoperative period.
Internal block excision and peripheral iridectomy
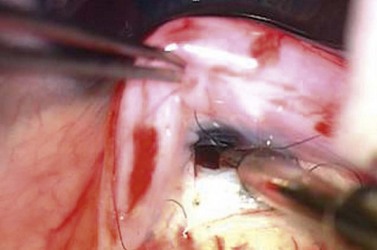
A block of tissue at the corneo-scleral junction is excised either with a sharp blade (e.g. a 15° or 30° blade) and Vannas scissors or with a punch, such as a Kelly’s punch. With the former, two radial incisions are made with the blade starting in clear cornea, at the most anterior point adjacent to the scleral flap, and extending posteriorly approximately 1–1.5 mm. The radial incisions are made approximately 2 mm apart. The blade, or the Vannas scissors, is used to connect the radial incisions, allowing the removal of a rectangular piece of tissue. Alternatively, the fistula can be created with a punch. An anterior corneal incision, parallel to the limbus, is made to enter into the anterior chamber, and a scleral punch is used to excise the limbal tissue (Fig. 35.6).
Closure of scleral flap
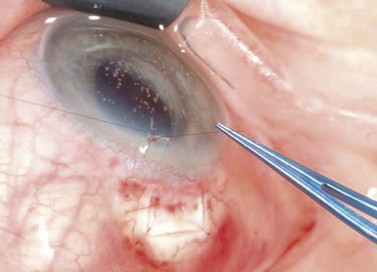
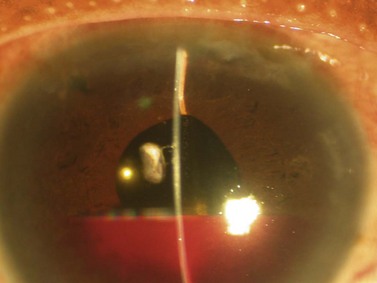
The use of releasable sutures offers another strategy to close the scleral flap so that the flow can be increased postoperatively (Figs 35.7 and 35.8). Externalized releasable sutures may be removed without lasers. They offer advantages over planned laser suture lysis when the conjunctiva is inflamed or hemorrhagic, or the Tenon’s tissue thickened so that suture lysis is difficult. Several techniques have been described by Wilson (mattress-type suture with an externalized knot on the cornea)30, Shin (removable knot passed through the conjunctival bleb)31, Cohen and Osher (loop-knot suture externalized through the cornea)32, Hsu and Yarng (an externalized hemi-bow tie in the center of the filtering bleb)33, Maberley et al. (a two-arm ‘U’ suture that leaves no exposed suture end until one arm of the suture is removed)34, and Johnstone et al. (releasable ‘tamponade suture’)35.
Indications and technique of intraoperative application of anti-metabolites
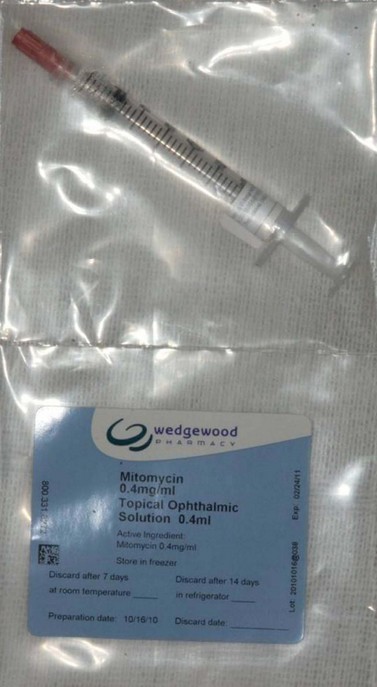
To reduce postoperative subconjunctival fibrosis and preserve bleb function postoperative topical steroids are frequently used36. The use of anti-fibrotic agents in filtering procedures is associated with a higher success but also with a higher complication rate (hypotony due to over-filtration, bleb leak, and ocular infection). For this reason an individualized consideration of the risk/benefit ratio is recommended. A survey of glaucoma specialists in the American Glaucoma Society indicated that anti-fibrotic agents are used in the majority of operations37, but in the UK they are used less frequently38. The agent 5-fluorouracil (5FU) is usually administered postoperatively but some surgeons use it intraoperatively (50 mg/ml) for 5 minutes. Mitomycin C (0.1–0.5 mg/ml solution) (Fig. 35.9) is more potent than 5FU, and increases the chances of success and the risks of complications39–42.
Postoperative care and prevention of failure of the filtering bleb
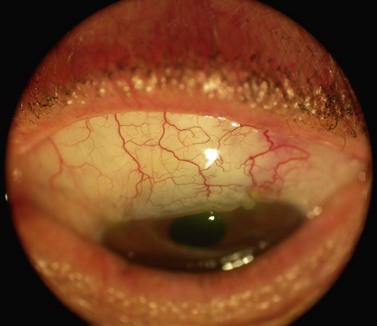
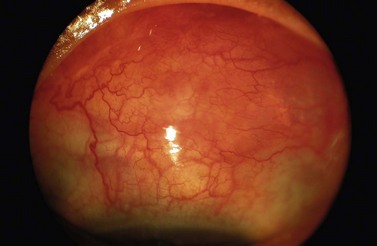
Failed blebs are those associated with inadequate IOP control and impending or established obstruction of aqueous outflow. Early failure of filtering blebs is characterized by a high IOP, deep anterior chamber, and low and hyperemic bleb. Failing blebs should be recognized promptly because if obstruction is not relieved permanent adhesions between conjunctiva and episclera can lead to closure of the fistula (see below). Failure of filtering operations is most commonly due to subconjunctival scarring (Fig. 35.10). Internal obstruction of the fistula by blood clot, vitreous, iris, or incompletely excised Descemet’s membrane is also possible.
Digital ocular compression and focal compression can be used to improve the function of a temporarily non- or poorly functioning filtering bleb43. Digital ocular compression (DOC) can be applied to the inferior sclera or cornea through the inferior eyelid, or to the sclera posterior to the scleral flap through the superior eyelid. Focal compression can be applied with a moistened cotton tip at the edge of the scleral flap. In the early postoperative period laser suture lysis or removal of an externalized releasable suture can enhance the filtration. Gonioscopy performed prior to the laser should confirm an open sclerostomy with no tissue or clot occluding its entrance (Fig. 35.11). After the suture is cut, if the bleb and IOP are unchanged, ocular massage or focal pressure can be applied. Usually only one suture is cut at a time to avoid the possible complications of over-filtration, hypotony, and flat anterior chamber. The timing of suture release is critical. Suture lysis is effective within the first 2 weeks after surgery without anti-metabolites. If anti-metabolites have been used at the time of surgery, suture lysis can be effective several weeks or even months after surgery.
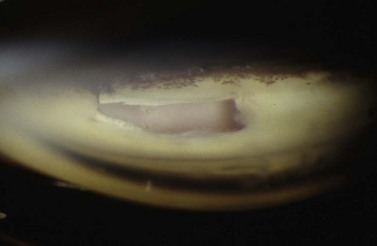
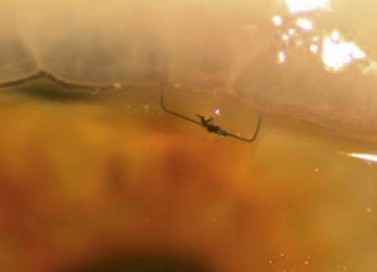
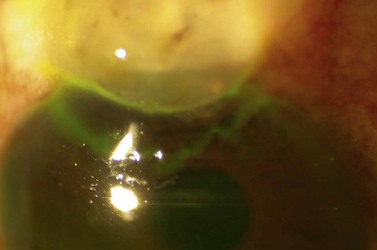
Fig. 35.11 The internal osteum is open, assuring that there is no iris blocking the filtration site.
‘Warning signs’ of failing filtration are increased bleb vascularization, bleb inflammation, and/or bleb thickening. The recognition of these signs is important because treatment with additional topical and even subconjunctival steroids and subconjunctival injections of 5-fluorouracil (5 mg in 0.1 ml of a 50 mg/ml solution) may control the healing response. In cases of established subconjunctival–episcleral fibrosis an external revision or ‘bleb needling’ can be tried44–47.
A 27- or 25-G needle is used to cut the edge of the scleral flap and restore aqueous outflow. Entry of the needle tip into the anterior chamber beneath the flap is important but should be undertaken with extreme caution in phakic eyes (Fig. 35.12). The technique can be repeated as needed. The outcome may be more favorable if there was a previously well-established filtration bleb before the fistula became occluded. Repeated subconjunctival injections of 5FU after revision may increase the probability of success. Mitomycin C before or after needling has also been proposed. The most common complications are associated with postoperative hypotony (see above).
Appearance of filtering blebs
The morphologic characteristics of filtering blebs are highly variable. In the early postoperative period the conjunctiva and subconjunctival tissues are edematous and hyperemic. After several weeks the conjunctiva overlying the scleral flap usually shows the greatest elevation and the least hyperemia. A late functioning bleb may be diffuse or localized, thin-walled or spongy, and differ in height, pallor, and extent of conjunctival microcystic edema. Late successful filtering blebs change in appearance and size over time. After an average follow-up of 7 years of 210 eyes that underwent successful filtering procedure, Sugar reported that the filtering bleb increased in size in 71.8%, decreased in size in 8.7%, and remained unchanged in 19.4% of eyes48,49.
Factors that affect the gross morphology and function of the bleb
In general, previous scarring and causes of an increased wound healing response influence the function and morphology of the filtering bleb. There are also several surgical and postoperative factors that can affect the bleb appearance50,51. The likelihood of development of a cystic bleb may be greater with a limbus-based conjunctival flap than with a fornix-based incision52. The use of corticosteroids and anti-fibrotic agents influences the appearance of the bleb by inhibiting inflammatory and healing processes. The postoperative use of corticosteroids can cause thinner and more cystic filtering blebs51. Thin, cystic, avascular blebs are common after trabeculectomy with anti-fibrosis therapy (see above).
Bleb histology
In functioning blebs the subepithelial connective tissue is typically thin, loosely arranged, and contains clear spaces. Electron microscopy reveals channel-like spaces (of 50 to 200 µ in diameter) throughout the stroma. The epithelium appears normal53–55.
Anti-fibrosis agents are likely to induce a hypocellular or acellular bleb with less fibrovascular proliferation56–61. Mitomycin produces filtering blebs that have a thinner and irregular epithelium, with fewer goblet cells, and a more atrophic and avascular stroma57–59. Scattered inflammatory cells and viable fibroblasts can be found. In spite of these abnormalities, Taniguchi et al. reported that the epithelial barrier function of the filtering bleb and cornea was not affected by the use of mitomycin61.
Topical anti-glaucoma medications induce several changes in the conjunctiva, Tenon’s capsule, and episclera, such as increasing the number of inflammatory cells, epithelial metaplasia, and decreased goblet cell numbers62–64. Topical anti-glaucoma medications adversely affect the outcome of filtration surgery65,66.
Complications of guarded filtration surgery
Overall, severe visual loss is uncommon but the incidence of transient complications is high. For example, in the Collaborative Initial Glaucoma Treatment Study (CIGTS) early complications occurred in 50% of 465 trabeculectomies. The most frequently complications were shallow or flat anterior chamber (13%), encapsulated bleb (12%), ptosis (12%), serous choroidal detachment (11%), and hyphema (10%). Suprachoroidal hemorrhage occurred in 0.7% of cases; there were no cases of endophthalmitis67. In the UK, a national survey of trabeculectomy was conducted and of 1240 reported cases, early complications were reported in 46%, and late complications in 42% of cases68. The most common early complications were hyphema (24%), shallow anterior chamber (23%), hypotony (24%), wound leak (17%), and choroidal detachment (14%) (Fig. 35.13). The most frequent late complications were cataract (20%), visual loss (18%) and encapsulated bleb (3%). Recently, the Tube Versus Trabeculectomy (TVT) study in 212 patients found intraoperative complications in 10% and 7% of cases during trabeculectomy and tube surgery respectively, and postoperative complications in 57% and 34% of patients after trabeculectomy and tube surgery respectively. Most complications were self-limited69.
Intraoperative complications
Suprachoroidal hemorrhage (Fig. 35.14) is a serious complication that can be seen during or after any intraocular surgery70–74. If it occurs intraoperatively and cannot be controlled (i.e., expulsive hemorrhage), it can lead to loss of vision. The incidence of suprachoroidal hemorrhage in glaucoma patients undergoing various types of intraocular surgery has been reported to be 0.73%. Ocular risk factors for suprachoroidal hemorrhage include glaucoma, aphakia, pseudophakia, previous vitrectomy, vitrectomy at the time of glaucoma surgery, myopia, and postoperative hypotony. Systemic risk factors are arteriosclerosis, high blood pressure, tachycardia, and bleeding disorders. The source of the hemorrhage is usually one of the posterior ciliary arteries, particularly at the point of entrance of the short posterior ciliary vessels into the suprachoroidal space.
Postoperative complications
Hypotony
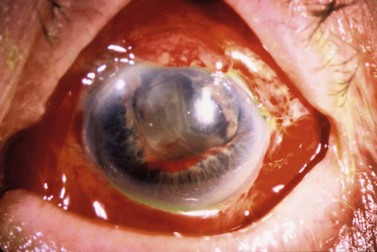
Hypotony can be caused by excessive aqueous outflow, most frequently due to excessive filtration, wound leak, or cyclodialysis cleft. Reduced aqueous production should also be considered (ciliochoroidal detachment, inflammation, inadvertent use of aqueous suppressants). Transient hypotony is very common after glaucoma surgery, but it may lead to other possible complications including flat anterior chamber, Descemet’s membrane folds, choroidal effusions, suprachoroidal hemorrhage, cataract, macular and optic disc edema, and chorio-retinal folds (predominantly in young myopic patients)75–89 (Fig. 35.15).
The initial management of early postoperative hypotony with a formed anterior chamber is conservative with topical steroids and cycloplegics. Intervention is indicated in cases when hypotony is associated with other complications such as persistent low IOP with loss of visual acuity and hypotony maculopathy (see below). Treatment should be aimed at correcting the specific cause of hypotony. When there is a flat anterior chamber with lens–corneal touch, immediate surgical intervention is necessary to prevent endothelial damage and cataract formation. Reformation of the anterior chamber with viscoelastic can be done at the slit lamp or under the operating microscope through the paracentesis made intraoperatively. When there are large appositional choroidal effusions, drainage of the fluid is recommended (Fig. 35.16).
Most commonly hypotony results from over-filtration of a filtering bleb. Treatment options are available. Several methods have been reported to induce an inflammatory or healing reaction in the filtering bleb, which modifies the morphology of the filtering bleb and increases IOP75–89. Surgical revision is the most efficacious option. Resuturing the scleral flap (occasionally with scleral patch grafting when resuturing is not possible) is our favored option. Resuturing the scleral flap can be done through the conjunctiva.
‘Malignant’ glaucoma
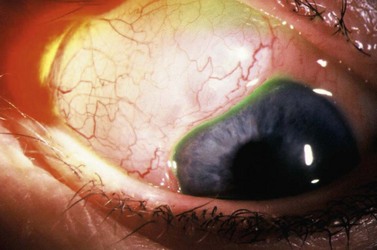
Malignant glaucoma (also called aqueous misdirection or ciliary block glaucoma) is characterized by increased IOP, and shallowing or flattening of the anterior chamber without pupillary block (i.e., in presence of a patent iridectomy) or chorio-retinal pathology such as suprachoroidal hemorrhage. It is more common after surgery for angle closure, in phakic, hyperopic (small) eyes. In this condition increased pressure within the vitreous cavity displaces the lens forward, and shallows the anterior chamber90 (Fig. 35.17).
In pseudophakic eyes a peripheral hyaloidotomy with the Nd:YAG laser may be efficient and can often be accomplished through an existing peripheral iridectomy91,92. If not successful, zonulo-hyaloido-vitrectomy via the anterior segment has been used successfully in a series of pseudophakic patients93. Pars plana vitrectomy should be considered when other therapies fail94,95, removing the anterior vitreous and part of the anterior hyaloid. Pars plana tube-shunt insertion with vitrectomy has been recommended to treat patients with aqueous misdirection, especially in cases with angle closure glaucoma95.
Wipe-out
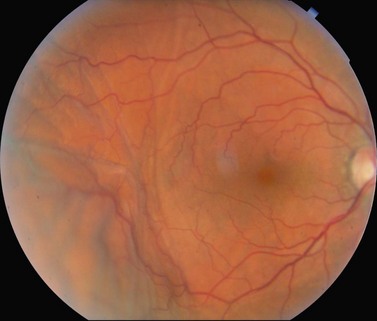
The phenomenon of severe visual loss after surgery, with no obvious cause, is known as ‘wipe-out’ or ‘snuff syndrome’ (Fig. 35.18). Wipe-out may affect patients who have very severe glaucomatous damage; while it is uncommon, it remains an important concern among glaucoma surgeons96–99.
Possible mechanisms for this wipe-out include: direct damage to the optic nerve from the anesthetic technique (local pressure from a hematoma or simply from the volume of anesthetic injected), an IOP spike, decreased blood flow to the optic nerve, and postoperative hypotony100–103.
Early bleb leak
An inadvertent buttonhole in the conjunctiva during a filtering procedure or a wound leak, through the conjunctival incision, can lead to an early leaking bleb69–71,77. Bleb leaks are more likely with extensive conjunctival scarring. Early bleb leaks usually heal spontaneously, but if there are associated complications such as shallow or flat anterior chamber, then active intervention is recommended. Our preferred strategies to treat leaking blebs include bandage contact lens, fibrin tissue glue, and surgical revision (see above).
Late bleb leak
Spontaneous late bleb leaks are more frequent in avascular, thin blebs, which occur most often when anti-metabolites are used in the filtering procedure. Leakage of the filtering bleb can be associated with hypotony, and may increase the chances for bleb infection and subsequent endophthalmitis77 (Fig. 35.19).
Bleb leaks can heal spontaneously and conservative management with prophylactic antibiotics is recommended if there are no associated problems. Multiple techniques have been proposed to resolve a non-healing bleb leak104–111. We recommend surgical revision, attempting to save the established initial filtration site. The ischemic and thin-walled bleb tissue is excised, or denuded of conjunctival epithelium by blade debridement or cautery to allow long-term adherence of grafted conjunctiva, or fresh conjunctiva adjacent to the bleb is mobilized and sutured to cover the de-epithelialized bleb by rotational, sliding, or free conjunctival grafts.
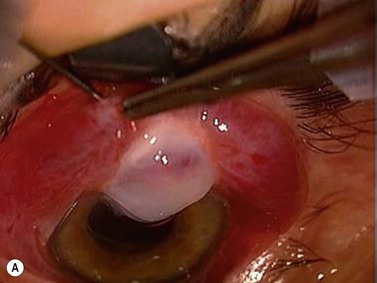
Encapsulated blebs
Encapsulated blebs are localized, elevated, and tense, with vascular engorgement of the overlying conjunctiva and thick connective tissue. Commonly appearing within 2–6 weeks after surgery, bleb encapsulation leads to a rise of IOP after a period of control112–116. Initial management of encapsulated blebs includes anti-glaucoma medications and topical steroids. When surgical revision is needed the simplest technique is to cut the fibrotic wall with a 27-gauge needle, a Ziegler knife, or an MVR blade and apply anti-fibrotic agents.
Symptomatic blebs
Filtering blebs are usually asymptomatic. Some patients have discomfort, which is most common with nasal, large blebs extending onto the cornea (Fig. 35.20). Tear film abnormalities with Dellen formation and superficial punctate keratopathy may occur. Artificial tears and ocular lubricants can be helpful, especially in cases with an abnormal tear film. Partial surgical excision, or trans-conjunctival compression sutures, is usually helpful although bleb failure is possible. Large blebs that extend onto the cornea can be freed by blunt dissection. The corneal extension can be excised with a cut parallel to the limbus, usually with excellent results, and without leakage.
Bleb-related ocular infection
Ocular infections related to filtration procedures can occur months to years after the initial surgery116–126. The incidence of bleb-related ocular infections after filtration procedures ranges from 0.2 to 1.5% after mid- to long-term follow-up. Thin bleb wall and most importantly bleb leaks are associated with a higher risk of infection. A surprising risk factor is long-term use of topical antibiotics. Bleb-related ocular infections can affect the subconjunctival space, the anterior segment, and the vitreous cavity.
1 Duke-Elder S. Simple glaucoma. Treatment. In: Duke-Elder S, editor. System of Ophthalmology, vol. XI. London: Henry Kimpton; 1969:528-533.
2 Holth S. Iridenclesis cum iridotomia meridionali. Arch Ophthalmol. 1930;4:803-816.
3 Scheie H. Retraction of scleral wound edges as a fistulizing procedure for glaucoma. Am J Ophthalmol. 1958;45:20-28.
4 Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66:673-679.
5 Sugar HS. Experimental trabeculectomy in glaucoma. Am J Ophthalmol. 1961;51:623-627.
6 Benedikt OP. Drainage mechanism after filtration. Glaucoma. 1979;1:71-77.
7 Kronfeld PC. Chemical demonstrations of transconjunctival passage after antiglaucomatous operations. Am J Ophthalmol. 1952;35:38-45.
8 Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-267.
9 Macleod SM, Clark R, Forrest J, et al. A review of glaucoma treatment in Scotland 1994–2004. Eye. 2008;22:251-255.
10 Schmier JK, Covert DW, Lau EC, et al. Trends in annual Medicare expenditures for glaucoma surgical procedures from 1997 to 2006. Arch Ophthalmol. 2009;127:900-905.
11 Keenan TD, Salmon JF, Yeates D, et al. Trends in rates of trabeculectomy in England. Eye. 2009;23:1141-1149.
12 Kenigsberg PA. Changes in medical and surgical treatments of glaucoma between 1997 and 2003 in France. Eur J Ophthalmol. 2007;17(4):521-527. Erratum in: Eur J Ophthalmol 2007;17:1010
13 Rachmiel R, Trope GE, Chipman ML, et al. Effect of medical therapy on glaucoma filtration surgery rates in Ontario. Arch Ophthalmol. 2006;124:1472-1477.
14 Gaskin BJ, Carroll SC, Gamble G, et al. Glaucoma management trends in Australia and New Zealand. Clin Exp Ophthalmol. 2006;34:208-212.
15 Gutierrez P, Wilson MR, Johnson C, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997;115:777-784.
16 Mills RP, Janz NK, Wren PA, et al. Correlation of visual field with quality-of-life measures at diagnosis in the Collaborative Initial Glaucoma Treatment Study (CIGTS). J Glaucoma. 2001;10:192-198.
17 Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression. Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268-1279.
18 AGIS Investigators. The advanced glaucoma intervention study (AGIS). 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429-440.
19 Sweitzer BJ. Preoperative medical testing and preparation for ophthalmic surgery. Ophthalmol Clin North Am. 2006;19:163-177.
20 Eke T. Anesthesia for glaucoma surgery. Ophthalmol Clin North Am. 2006;19:245-255.
21 Azuara-Blanco A, Moster MR, Marr BP. Subconjunctival versus peribulbar anesthesia in trabeculectomy: a prospective, randomized study. Ophthalmic Surg Lasers. 1997;28(11):896-899.
22 Karp CL, Cox TA, Wagoner MD, et al. Intracameral anesthesia: a report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:1704-1710.
23 Kumar CM, Dodds C. Sub-Tenon’s anesthesia. Ophthalmol Clin North Am. 2006;19:209-219.
24 Kumar CM, Dodds C. Evaluation of the Greenbaum subTenon’s block. Br J Anaesth. 2001;87:631-633.
25 Alwitry A, Koshy Z, Browning AC, et al. The effect of sub-Tenon’s anaesthesia on intraocular pressure. Eye. 2001;15:733-735.
26 Pianka P, Weintraub-Padova H, Lazar M, et al. Effect of sub-Tenon’s and peribulbar anesthesia on intraocular pressure and ocular pulse amplitude. J Cataract Refract Surg. 2001;27:1221-1226.
27 Atkinson WS. Retrobulbar injection of anesthetic within the muscular cone. Arch Ophthalmol. 1936;16:494-503.
28 Davis DB2nd, Mandel MR. Posterior peribulbar anesthesia: an alternative to retrobulbar anesthesia. J Cataract Refract Surg. 1986;12:182-184.
29 McGoldrick KE, Foldes PJ. General anesthesia for ophthalmic surgery. Ophthalmol Clin North Am. 2006;19:179-191.
30 Wilson RP. Technical advances in filtration surgery. In: McAllister JA, Wilson RP, editors. Glaucoma. Boston: Butterworths; 1986:243-350.
31 Shin DH. Removable-suture closure of the lamellar scleral flap in trabeculectomy. Ann Ophthalmol. 1987;19:51-53.
32 Cohen JS, Osher RH. Releasable scleral flap suture. Ophthalmol Clin North Am. 1988;1:187-197.
33 Hsu CT, Yarng SS. A modified removable suture in trabeculectomy. Ophthal Surg. 1993;24:579-584.
34 Maberley D, Apel A, Rootman DS. Releasable ‘U’ suture for trabeculectomy surgery. Ophthal Surg. 1994;25:251-255.
35 Johnstone MA, Wellington DP, Ziel CJ. A releasable scleral-flap tamponade suture for guarded filtration surgery. Arch Ophthalmol. 1993;111:398-403.
36 Starita RJ, Fellman RL, Spaeth GL, et al. Short- and long-term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology. 1985;92:938-946.
37 Joshi AB, Parrish RK2nd, Feuer WF. 2002 Survey of the American Glaucoma Society: Practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma. 2005;14:172-174.
38 Siriwardena D, Edmunds B, Wormald RP, et al. National survey of antimetabolite use in glaucoma surgery in the United Kingdom. Br J Ophthalmol. 2004;88:873-876.
39 Goldenfeld M, Krupin T, Ruderman JM, et al. 5-fluorouracil in initial trabeculectomy. A prospective, randomized, multicenter study. Ophthalmology. 1994;101:1024-1029.
40 The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol. 1996;121:349-366.
41 Kitazawa Y, Suemori-Matsushita H, Yamamoto T, et al. Low-dose and high-dose mitomycin trabeculectomy as an initial surgery in primary open-angle glaucoma. Ophthalmology. 1993;100:1624-1628.
42 Wu-Dunn D, Cantor LB, Palanca-Capistrano AM, et al. A prospective randomized trial comparing intraoperative 5-fluorouracil versus mitomycin C in primary trabeculectomy. Am J Ophthalmol. 2002;134:521-528.
43 Traverso CE, Greenidge KC, Spaeth GL, et al. Focal pressure: a new method o encourage filtration after trabeculectomy. Ophthalmic Surg. 1984;15:62-65.
44 Ewing RH, Stamper RL. Needle revision with and without 5-fluorouracil for the treatment of failed filtering blebs. Am J Ophthalmol. 1990;110:254-259.
45 Greenfield DS, Miller MP, Suner IJ, et al. Needle revision of failed filtering blebs using mitomycin-C. Ophthalmology. 1996;122:195-204.
46 Mardelli PG, Lederer CM, Murrary PL, et al. Slit-lamp needle revision of filtering blebs using mitomycin C. Ophthalmology. 1996;103:1946-1955.
47 Shetty RK, Wartluft L, Moster MR. Slit-lamp needle revision of failed filtering blebs using high-dose mitomycin C. J Glaucoma. 2005;14:52-56.
48 Sugar HS. Course of successfully filtering blebs. Ann Ophthalmol. 1971;3:485-487.
49 Sugar HS. The course of change in size of successful filtering cicatrices. Am J Ophthalmol. 1960;49:795-800.
50 Sanders R, MacEwen C, Haining WM. Trabeculectomy: effect of varying surgical site. Eye. 1993;7:440-443.
51 Starita RJ, Fellman RL, Spaeth GL, et al. Short- and long-term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology. 1985;92:938-946.
52 Agbeja AM, Dutton GN. Conjunctival incisions for trabeculectomy and their relationship to the type of bleb formation. A preliminary study. Eye. 1987;1:738-743.
53 Addicks CA, Quigley HA, Green R, et al. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983;101:795-798.
54 Cioffi GA, Van Buskirk M. Corneal trabeculectomy without conjunctival incision. Extended follow-up and histologic findings. Ophthalmology. 1993;100:1077-1082.
55 Mietz H, Brunner R, Addicks K, et al. Histopathology of an avascular filtering bleb after trabeculectomy with mitomycin C. J Glaucoma. 1993;2:266-270.
56 Hutchinson AK, Grossniklaus HA, Brown RH, et al. Clinicopathologic features of excised mitomycin filtering blebs. Arch Ophthalmol. 1994;112:74-79.
57 Khaw PT, Doyle JW, Sherwood MB, et al. Effects of intraoperative 5-fluorouracil or mitomycin C on glaucoma filtering surgery in the rabbit. Ophthalmology. 1993;100:367-372.
58 Mietz H, Arnold G, Kirchhof B, et al. Histopathology of episcleral fibrosis after trabeculectomy with and without mitomycin C. Graefe’s Arch Clin Exp Ophthalmol. 1996;234:364-368.
59 Nuyts RM, Felten PC, Pels E, et al. Histopathologic effects of mitomycin C after trabeculectomy in human glaucomatous eyes with persistent hypotony. Am J Ophthalmol. 1994;118:251-253.
60 Shields MB, Scroggs MW, Sloop CM, et al. Clinical and histopathologic observations concerning hypotony after trabeculectomy with adjunctive mitomycin C. Am J Ophthalmol. 1993;116:673-683.
61 Taniguchi T, Yamamoto T, Mochizuki K, et al. Epithelial barrier function of the filtering bleb and the cornea after trabeculectomy with mitomycin C. J Glaucoma. 1996;5:233-236.
62 Broadway DC, Grierson I, O’Brien C, et al. Adverse effects of topical antiglaucoma medications. I. The conjunctival cell profile. Arch Ophthalmol. 1994;112:1437-1445.
63 Herreras JM, Pastor JC, Calonge M, et al. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99:1082-1088.
64 Sherwood MB, Grierson I, Millar L, et al. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96:327-335.
65 Broadway DC, Grierson I, O’Brien C, et al. Adverse effects of topical antiglaucoma medications. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112:1446-1454.
66 Lavin MJ, Wormald RPL, Migdal CS, et al. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol. 1990;108:1543-1548.
67 Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2005;140:16-22.
68 Edmunds B, Thompson JR, Salmon JF, et al. The national survey of trabeculectomy. III. Early and late complications. Eye. 2002;16:297-303.
69 Gedde SJ, Herndon LW, Brandt JD, et al. Surgical complications in the tube versus trabeculectomy study during the first year of follow up. Am J Opthalmol. 2007;143:23-31.
70 Cantor LB, Katz LJ, Spaeth GL. Complications of surgery in glaucoma: suprachoroidal expulsive hemorrhage in glaucoma patients undergoing intraocular surgery. Ophthalmology. 1985;92:1266-1270.
71 Ruderman JM, Harbin TS, Campbell DG. Postoperative suprachoroidal hemorrhage following filtering procedures. Arch Ophthalmol. 1986;104:201-205.
72 Givens K, Shields MB. Suprachoroidal hemorrhage after glaucoma filtering surgery. Am J Ophthalmol. 1987;103:689-694.
73 Frenkel REP, Shin DH. Prevention and management of delayed suprachoroidal hemorrhage after filtration surgery. Arch Ophthalmol. 1986;104:1459-1463.
74 Spaeth GL, Baez KA. Long-term prognosis of eyes having had operative suprachoroidal expulsive hemorrhage. Ger J Ophthalmol. 1994;3:159-163.
75 Eha J, Hoffmann EM, Wahl J, et al. Flap suture – a simple technique for the revision of hypotony maculopathy following trabeculectomy with mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2008;246:869-874.
76 Costa VP, Arcieri ES. Hypotony maculopathy. Acta Ophthalmol Scand. 2007;85:586-597.
77 Azuara-Blanco A, Katz LJ. Dysfunctional filtering blebs. Surv Ophthalmol. 1998;43:93-126.
78 Blok MDW, Kok JHC, van Mil C, et al. Use of the megasoft bandage lens for treatment of complications after trabeculectomy. Am J Ophthalmol. 1990;110:264-268.
79 Simmons RJ, Kimbrough RL. Shell tamponade in filtering surgery for glaucoma. Ophthalmic Surg. 1984;10:17-34.
80 Fink AJ, Boys-Smith JW, Brear R. Management of large filtering blebs with the argon laser. Am J Ophthalmol. 1986;101:695-699.
81 Lynch MG, Roesch M, Brown RH. Remodeling filtering blebs with the Neodynium:YAG laser. Ophthalmology. 1986;103:1700-1705.
82 Leen MM, Moster MR, Katz LJ, et al. Management of overfiltering and leaking blebs with autologous blood injection. Arch Ophthalmol. 1995;113:1050-1055.
83 Wise JB. Treatment of chronic postfiltration hypotony by intrableb injection of autologous blood. Arch Ophthalmol. 1993;111:827-830.
84 Lu DW, Azuara-Blanco A, Katz LJ. Severe visual loss after autologous blood injection for mitomycin-associated hypotony maculopathy. Ophthalmic Surg Lasers. 1997;28:244-245.
85 Schwartz GF, Robin AL, Wilson RP, et al. Resuturing the scleral flap leads to resolution of hypotony maculopathy. J Glaucoma. 1996;5:246-251.
86 Haynes WL, Alward WLM. Rapid visual recovery and long-term intraocular pressure control after donor scleral-patch grafting for trabeculectomy-induced hypotony maculopathy. J Glaucoma. 1994;4:200-201.
87 Suner IJ, Greenfield DS, Miller MP, et al. Hypotony maculopathy after filtering surgery with mitomycin-C: incidence and treatment. Ophthalmology. 1997;104:207-215.
88 Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with mitomycin C. Am J Ophthalmol. 1993;116:314-326.
89 Costa VP, Wilson RP, Moster MR, et al. Hypotony maculopathy following the use of topical mitomycin C in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:389-394.
90 Quigley HA, Frieadman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12:167-180.
91 Ruben S, Tsai J, Hitchings RA. Malignant glaucoma and its management. Br J Ophthalmol. 1997;81:163-167.
92 Epstein DL, Steinert RF, Puliafito CA. Neodynium:YAG laser therapy to the anterior hyaloid in aphakic malignant (ciliary block) glaucoma. Ophthalmology. 1980;87:1155-1159.
93 Lois N, Wong D, Groenwald C. New surgical approach in the management of pseudophakic malignant glaucoma. Ophthalmology. 2001;108:780-783.
94 Byrnes GA, Leen MM, Wong TP, et al. Vitrectomy for ciliary block (malignant) glaucoma. Ophthalmology. 1995;102:1308-1311.
95 Azuara-Blanco A, Katz LJ, Gandham S, et al. Pars plana tube insertion of aqueous shunt with vitrectomy in malignant glaucoma. Arch Ophthalmol. 1988;116:808-810.
96 Lichter PR, Ravin JG. Risk of sudden visual loss after glaucoma surgery. Am J Ophthalmol. 1974;78:1009-1013.
97 Costa VP, Smith M, Spaeth GL, et al. Loss of visual acuity after trabeculectomy. Ophthalmology. 1993;100:599-612.
98 Law SK, Nguyen AM, Coleman AL, et al. Severe loss of central vision in patients with advanced glaucoma undergoing trabeculectomy. Arch Ophthalmol. 2007;125:1044-1050.
99 Moster MR, Moster ML. Wipe-out: a complication of glaucoma surgery or a just a blast from the past. Am J Ophthalmol. 2005;140:705-706.
100 Eke T. Anesthesia for glaucoma surgery. Ophthalmol Clin North Am. 2006;19:245-255.
101 Kumar CM, Dowd TC, Dodds C, et al. Orbital swelling following peribulbar and subTenon’s anaesthesia. Eye. 2004;18:418-420.
102 Hulbert MF, Yang YC, Pennefather PM, et al. Pulsatile ocular blood flow and intraocular pressure during retrobulbar injection of lignocaine: influence of additives. J Glaucoma. 1998;7:413-416.
103 Huber KK, Remky A. Effect of retrobulbar versus subconjunctival anaesthesia on retrobulbar haemodynamics. Br J Ophthalmol. 2005;89:719-723.
104 Zalta AH, Wieder RH. Closure of leaking filtering blebs with cyanoacrylate tissue adhesive. Br J Ophthalmol. 1991;75:170-173.
105 Asrani SG, Wilenski JT. Management of bleb leaks after glaucoma filtering surgery. Use of autologous fibrin tissue glue as an alternative. Ophthalmology. 1996;103:294-298.
106 Kajiwara K. Repair of a leaking bleb with fibrin glue. Am J Ophthalmol. 1990;109:599-601.
107 Wilensky JT. Management of late bleb leaks following glaucoma filtering surgery. Trans Am Ophthalmol Soc. 1992;93:161-168.
108 Wadhwani RA, Resham A, Bellows AR, et al. Surgical repair of leaking filtering blebs. Ophthalmology. 2000;107:1681-1687.
109 Burnstein AL, WuDunn D, Knotts L, et al. Conjunctival advancement versus nonincisional treatment for late-onset glaucoma filtering bleb leaks. Ophthalmology. 2002;109:71-75.
110 Catoria Y, Wudunn D, Cantor LB. Revision of dysfunctional filtering blebs by conjunctival advancement with bleb preservation. Am J Ophthalmol. 2000;130:574-579.
111 Harris L, Yang G, Feldman RN, et al. Autologous conjunctival resurfacing of leaking filtering blebs. Ophthalmology. 2000;107:1675-1680.
112 Feldman RM, Gross RL, Spaeth GL, et al. Risk factors for the development of Tenon’s capsule cysts after trabeculectomy. Ophthalmology. 1989;96:336-341.
113 Scott DR, Quigley HA. Medical management of a high bleb phase after trabaculectomies. Ophthalmology. 1988;95:1169-1173.
114 Azuara-Blanco A, Bond BJ, Wilson RP, et al. Encapsulated filtering blebs after trabeculectomy with mitomycin-C. Ophthalmic Surg Lasers. 1997;28:805-809.
115 Yarangümeli A, Köz OG, Kural G. Encapsulated blebs following primary standard trabeculectomy: course and treatment. J Glaucoma. 2004;13:251-255.
116 Katz LJ, Cantor LB, Spaeth GL. Complications of surgery in glaucoma. Early and late bacterial endophthalmitis following glaucoma filtering surgery. Ophthalmology. 1985;92:959-963.
117 Caronia RM, Liebmann JM, Friedman R, et al. Trabeculectomy at the inferior limbus. Arch Ophthalmol. 1996;114:387-391.
118 Greenfield DS, Suner IJ, Miller MP, et al. Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996;114:943-949.
119 Mandelbaum S, Forster RK, Gelender H, et al. Late onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985;92:964-972.
120 DeBry PW, Perkins TW, Heatley G, et al. Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol. 2002;120:297-300.
121 Soltau JB, Rothman RF, Budenz DL, et al. Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol. 2000;118:338-342.
122 Jampel HD, Quigley HA, Kerrigan-Baumrid LA, et al. The Glaucoma Surgical Outcomes Study Group. Risk factors for late-onset infection following glaucoma filtration surgery. Arch Ophthalmol. 2001;119:1001-1008.
123 Song A, Scott IU, Flynn HWJr, et al. Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. Ophthalmology. 2002;109:985-991.
124 Kangas TA, Greenfield DS, Flynn HWJr, et al. Delayed-onset endophthalmitis associated with conjunctival filtering blebs. Ophthalmology. 1997;104:746-752.
125 Reynolds AC, Skuta GL, Monlux R, et al. Management of blebitis by members of the American Glaucoma Society: a survey. J Glaucoma. 2001;10:340-347.
126 Wand M, Quintiliani R, Robinson A. Antibiotic prophylaxis in eyes with filtration blebs: survey of glaucoma specialists, microbiological study, and recommendations. J Glaucoma. 1995;4:103-109.