55 Glaucoma
Pathophysiology
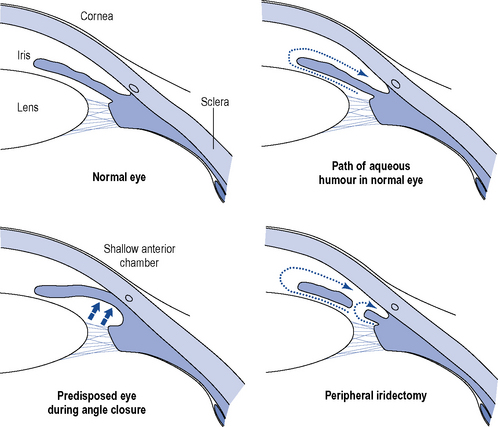
The physiological precipitating factors of PACG in predisposed eyes are not fully understood. Two theories currently exist. The dilator muscle theory suggests that contraction of the dilator muscle causes a posterior movement, which increases the apposition between the iris and anteriorly located lens and the degree of physiological pupillary block. The simultaneous dilation of the pupil renders the peripheral iris more flaccid, and causes the pressure in the posterior chamber to increase and the iris to bow anteriorly. Eventually, the peripheral iris obstructs the angle and the IOP rises (Fig. 55.1).
Investigations
IOP may be measured by tonometry, such as indentation tonometry in which a plunger is applied to the cornea and the amount of indentation on the eye reflects the pressure within it. Tonography is a technique used to measure the outflow of aqueous humour from the eye, resulting from indentation of the eye, using a tonometer. Gonioscopy is used to estimate the width of the chamber angle, with the aid of a slit lamp. Perimetry is important for both the diagnosis and management of glaucoma by detecting early scotomata and larger changes in visual field. Other investigations which should be routinely offered to patients include a central corneal thickness measurement (CCT), as this has an effect on the measured IOP and may affect the efficacy of certain drug treatments (Johnson et al., 2008), and fundus examination for optic nerve assessment with dilatation using stereoscopic slit-lamp biomicroscopy. There are also guidelines for the monitoring of these patients and at what intervals their IOP, optic nerve head and visual fields should be checked (National Institute for Health and Clinical Excellence, 2009).
Treatment
Chronic open-angle glaucomas
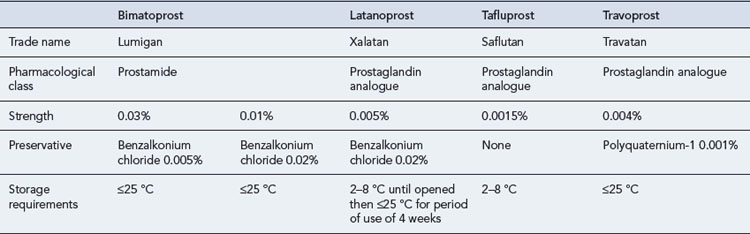
The initial treatment of COAG is usually medical. Topical administration is the preferred type of therapy, and there is a wide range of preparations available (Table 55.1). The chosen drug should be administered at its lowest concentration and as infrequently as possible to obtain the desired effect over the whole 24-h period as a high level of diurnal variation in IOP has been shown to be a more important factor in visual field development than the average IOP. A drug with few potential side effects should be chosen, with oral therapy retained as the final option. The prostaglandin analogues latanoprost and travoprost and the prostamide bimatoprost are indicated for first-line use, as are the β-blockers as these produce the greatest fall in IOP (Table 55.2). Carbonic anhydrase inhibitors and sympathomimetics, which result in a smaller fall in IOP, are reserved for patients unresponsive to first-line drugs, in patients in whom the first-line agents are contraindicated or as adjunctive therapy.
Table 55.1 Drugs used in the treatment of chronic open-angle glaucoma
| Therapeutic category | Primary mechanism |
|---|---|
| Topical prostaglandins | Increase aqueous outflow |
| Topical prostamides | Increase aqueous outflow |
| Topical β-blocking agents | Decrease aqueous formation |
| Topical miotics | Increase aqueous outflow |
| Topical adrenergic agonists | Increase aqueous outflow and decrease aqueous formation |
| Topical carbonic anhydrase inhibitors | Decrease aqueous formation |
| Oral carbonic anhydrase inhibitors | Decrease aqueous formation |
Table 55.2 Comparison of reduction in intraocular pressure (IOP) with a range of ocular hypotensive drugs (adapted from van der Valk et al., 2009)
| Drug | Relative IOP change from baseline at trough (%) (95% CI) | Relative IOP change from baseline at peak (%) (95% CI) |
|---|---|---|
| Bimatoprost | −28 (−29 to −27) | −33 (−35 to −31) |
| Travoprost | −29 (−32 to −25) | −31 (−32 to −29) |
| Latanoprost | −28 (−30 to −26) | −31 (−33 to −29) |
| Timolol | −26 (−28 to −25) | −27 (−29 to −25) |
| Betaxolol | −20 (−23 to −17) | −23 (−25 to −22) |
| Dorzolamide | −17 (−19 to −15) | −22 (−24 to −20) |
| Brinzolamide | −17 (−19 to −15) | −17 (−19 to −15) |
| Brimonidine | −18 (−21 to −14) | −25 (−28 to −22) |
| Placebo | −5 (−9 to −1) | −5 (−10 to 0) |
In most cases, the initial topical treatment is with a prostaglandin analogue or prostamide. If this is ineffective, another prostaglandin or prostamide may be substituted or a β-blocker used instead. Patients not reaching their target pressure on one first-line agent may be prescribed another concomitantly. Alternatively, a carbonic anhydrase inhibitor or a sympathomimetic may be added to one of the first-line drugs. Guidance on the treatment of people with OHT or suspected COAG has been published (National Institute for Health and Clinical Excellence, 2009). The treatment options (Table 55.3) take into account central corneal thickness and age, although age thresholds are only appropriate where vision is normal (OHT with or without suspected COAG) and the treatment is purely preventative. Pilocarpine is no longer recommended by NICE but is recommended as a second-line agent in European guidelines (European Glaucoma Society, 2008). Oral therapy with carbonic anhydrase inhibitors is usually reserved for use as the final stage of treatment in those complex glaucomas awaiting surgery (Fig. 55.2).
Table 55.3 Treatment of people with ocular hypertension or suspected COAG (National Institute for Health and Clinical Excellence, 2009)
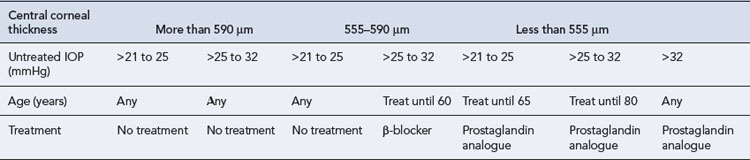
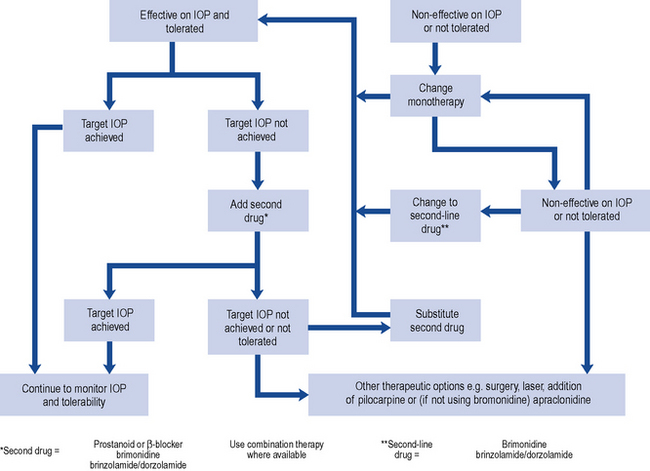
Fig. 55.2 Topical therapy for chronic open-angle glaucoma (COAG) based on recommendations of European Glaucoma Society (2008).
It has been proposed that the improved control of IOP with newer therapies has led to a reduction in surgery for glaucoma (Kenigsberg, 2007). However, some patients still require surgical intervention to reach their target pressure.
The most frequently performed surgical procedures create a fistula to act as a new route for aqueous outflow and the most frequently performed surgery of this type, trabeculectomy, appears more effective in controlling IOP than laser trabeculoplasty, in which a series of laser burns is applied to the trabecular meshwork to improve the outflow of aqueous humour. (Rolim de Moura et al., 2007).
Primary angle-closure glaucoma
The medical management of acute PACG is essentially to prepare the eye for surgical treatment. The aim of treatment is to decrease the IOP and associated inflammation. Analgesics and antiemetics are sometimes needed, dependent on symptom severity, to make the patient comfortable. It is usual to treat the unaffected eye prophylactically with miotics (Fig. 55.3).
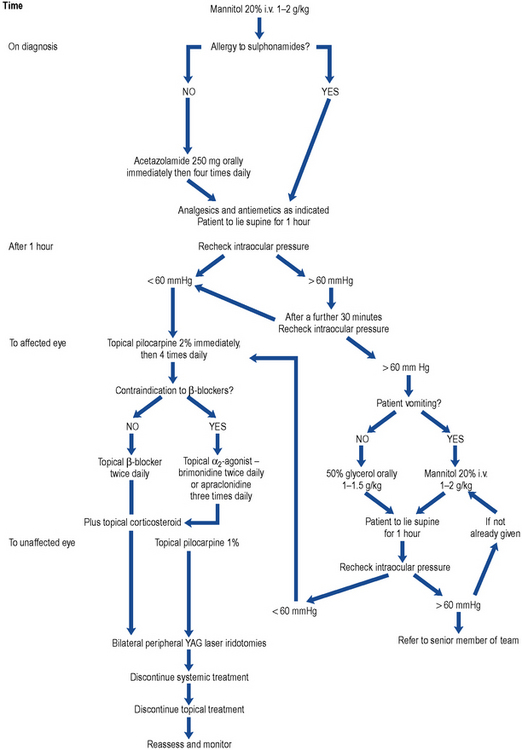
Fig. 55.3 Algorithm for the treatment of primary angle-closure glaucoma (PACG). YAG, yttrium aluminium garnet.
Once the IOP has been reduced medically, the condition is treated surgically, by either surgical peripheral iridectomy or laser iridotomy, to remove an area of the peripheral iris to allow flow of aqueous humour through an alternative pathway (see Fig. 55.1). Filtration surgery is indicated if a large proportion of the angle has been permanently closed by adhesions between the iris and the cornea.
Drug treatment
Ocular prostanoids: prostaglandin analogues and prostamides
The National Institute for Health and Clinical Excellence (2009) guideline does not differentiate between the prostaglandin analogues and the prostamide bimatoprost, but recommends one of this class for the treatment of COAG, certain patients with OHT and certain COAG suspects (see Table 55.4)
The prostaglandin analogues, latanoprost, travoprost and tafluprost, are ester compounds thought to achieve a fall in IOP primarily by increasing the uveoscleral outflow with no significant effect on other parameters of aqueous humour dynamics, while the prostamide bimatoprost is thought to increase outflow through both trabecular and uveoscleral outflow pathways. However, there is still debate about the precise mechanisms of action of this group (Lim et al., 2008; Toris et al., 2008).
In a meta-analysis, these drugs have been shown to have a greater effect on both peak and trough readings of IOP than timolol, betaxolol, dorzolamide, brinzolamide or brimonidine, producing falls in IOP of 28–29% at trough and 31–33% at peak as shown in Table 55.2 (van der Valk et al., 2009).
Randomised head-to-head evaluations of prostaglandin therapy demonstrate similar efficacy, but differing hyperemia effects with bimatoprost and travoprost causing more hyperaemia than latanoprost (Eyawo et al., 2009; Honrubia et al., 2009). However, these studies were conducted before the introduction of the new formulations of bimatoprost 0.01% and the benzalkonium chloride–free travoprost.
The prostanoids have some interesting local side effects (Table 55.5). Pigmentation of the iris occurs in patients with mixed colour (green-brown or blue-brown) irides after 3–6 months of use and is a result of increased deposition of melanin in the melanocytes. An increase in the length and thickness of the eyelashes and pigmentation of the palpebral skin are also known side effects. Use of these drugs may lead to disruption of the blood–aqueous barrier in patients with aphakia and pseudophakia (no or false lens, respectively) and increase the risk of developing cystoid macular oedema; they should be used with caution in such patients. Reactivation of herpes simplex infection has been reported with bimatoprost, travoprost and latanoprost.
| Ocular | Systemic |
|---|---|
| Asthenopia | Abdominal cramp |
| Allergic conjunctivitis | Asthenia |
| Blepharitis | Asthma |
| Blepharospasm | Bradycardia |
| Browache | Dizziness |
| Cataract | Dyspnoea |
| Conjunctival follicles, papillae | Elevated liver function |
| Conjunctival hyperaemia | Headache |
| Cystoid macular oedema | Hirsutism |
| Corneal erosion | Hypertension |
| Conjunctival oedema | Hypotension |
| Deepening of lid sulcus | Infection (primarily URTIs) |
| Distichiasis | Peripheral oedema |
| Eye discharge | Skin rash |
| Eye pain | |
| Eyelash changes – increased number, length, thickness, pigmentation, misdirection, poliosis | |
| Eyelid oedema, eyelid retraction | |
| Eyelid and periocular skin darkening | |
| Foreign body sensation | |
| Increase in vellus hair on eyelids | |
| Increased iris pigmentation | |
| Iritis | |
| Lid margin crusting | |
| Localised skin reactions on the eyelids | |
| Ocular burning, dryness, irritation pruritus, fatigue | |
| Photophobia | |
| Punctate epithelial erosions | |
| Retinal haemorrhage | |
| Tearing | |
| Uveitis | |
| Visual disturbance |
URTIs, upper respiratory tract infection
Latanoprost
Latanoprost, which is converted to its active free acid on entering the eye, was the first of the prostanoids to be launched and is the market leader. Like timolol amongst the β-blockers, latanoprost is the drug in this class against which new drugs or combinations are assessed. It must be administered in the evening for maximum effect (Stewart et al., 2008). Latanoprost is generally very well tolerated. Serious adverse drug reactions were reported in only 17/3936 (0.43%) patients using latanoprost over a 5-year period (Goldberg et al., 2008), and patient persistence with latanoprost therapy is better than that with all other frequently used monotherapies (Rahman et al., 2009). Latanoprost is not heat stable and requires refrigeration; however, it is stable enough to be stored at room temperature for the 4-week in-use period applied in the UK. The concentration of the preservative benzalkonium chloride in latanoprost eye drops and bimatoprost 0.01% may prevent their use in certain patients. The benzalkonium chloride concentration in these formulations is 0.02%, which is four times that in bimatoprost 0.03% eye drops. The current formulation of travoprost, launched in January 2011, contains polyquaternium-1 rather than benzalkonium chloride.
Travoprost
Patients treated with travoprost show good diurnal fluctuation control, and an ocular hypotensive effect was shown to last for over 3 days. Patients previously treated with β-blockers, α2-agonists, carbonic anhydrase inhibitors, latanoprost and the dorzolamide-timolol fixed combination respond to travoprost. Administration time is not as important with travoprost as with latanoprost as no statistical difference was noted in the percentage fall in IOP whether travoprost was administered in the morning or in the evening (Stewart et al., 2008). Travoprost is a stable compound throughout a range of temperatures, and the commercially available product does not require refrigeration.
Tafluprost
Tafluprost is the first of the prostanoids available in a preservative-free form. In terms of efficacy and safety, it has been shown to be equivalent to a preserved preparation of tafluprost (Hamacher et al., 2008) and useful in patients allergic to, or intolerant of, benzalkonium chloride.
Tafluprost as adjunctive therapy to timolol results in consistently greater reductions in IOP compared with vehicle in patients with glaucoma or OHT that is inadequately controlled with timolol monotherapy (Egorov and Ropo, 2009).
Bimatoprost
Bimatoprost lowers the IOP to a greater extent than any other topical ocular hypotensive (van der Valk et al., 2009), an effect (see Table 55.2) which is sustained for at least 4 years (Williams et al., 2008). It is superior to latanoprost in terms of response rate, fall in IOP and the percentage of patients reaching their target IOP. In some studies, bimatoprost is reported to be as effective as the fixed combination of latanoprost and timolol. Many patients are non-responsive to latanoprost as they do not achieve a fall in IOP greater than 10%, although a large proportion of patients show a 20% or more fall in IOP with bimatoprost. Patients not reaching their target on latanoprost achieved lower IOPs when switched to bimatoprost (Sonty et al., 2008). Such patients achieve better diurnal control with bimatoprost than with a fixed combination of dorzolamide and timolol (Sharpe et al., 2008).
Generally, bimatoprost causes similar ocular side effects to latanoprost and travoprost, but while all the ocular prostanoids cause subclinical ocular inflammation, bimatoprost and travoprost cause this to a lesser degree than latanoprost. However, bimatoprost causes hyperaemia more frequently than latanoprost, although this is described as mild. This may contribute to the higher discontinuation rate seen with bimatoprost therapy (Rahman et al., 2009). To address this issue, the manufacturers of bimatoprost have introduced a lower strength (0.01%) version containing a higher concentration of benzalkonium chloride to aid penetration of the drug. In a 12-month study, the bimatoprost 0.01% was equivalent to bimatoprost 0.03% in lowering IOP and demonstrated improved tolerability, including less frequent and severe conjunctival hyperemia (Katz et al., 2010).
β-Adrenoceptor antagonists
The exact mechanism of action of β-adrenoceptor antagonists (β-blockers) in lowering IOP has not been fully established but is thought to result from the blockade of ciliary β-receptors, preventing the cyclic AMP-induced rise in aqueous secretion, as they have been shown to reduce aqueous humour formation rather than increase outflow. Although there are both β1– and β2-receptors in the eye, the latter predominate and even cardioselective β-blockers are thought to work by blockade of β2-receptors. Genetic factors have been shown to be important in patients’ response to this group of drugs (Nieminen et al., 2007, Sidjanin et al., 2008).
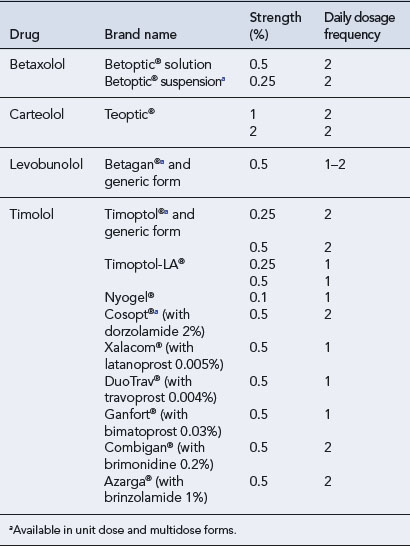
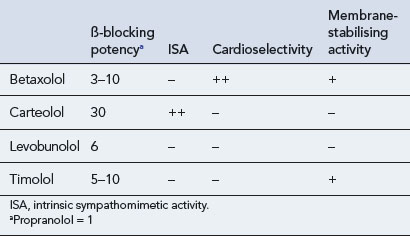
Four drugs are available in the UK for topical administration: betaxolol, carteolol, levobunolol and timolol (Table 55.6). β-Blockers have a number of important properties in addition to β-adrenoceptor blockade. These include intrinsic sympathomimetic activity (ISA), cardioselectivity and membrane-stabilising activity, which are all of importance when considering the side effects seen with these agents (Table 55.7). The property of membrane stabilisation is relevant to the incidence of ocular side effects. The absence of anaesthetic properties reduces the number and severity of foreign body and dryness sensations, anaesthesia of the cornea and dry eye syndrome.
Ocular side effects of topically administered β-blockers are shown in Table 55.8.
Table 55.8 Ocular and systemic side effects of topical β-blockers
| Ocular | Systemic |
|---|---|
| Allergic blepharoconjunctivitis | Central nervous system |
| Burning and itching | Anxiety |
| Blurred vision | Depression |
| Conjunctival hyperaemia | Fatigue |
| Corneal anaesthesia | Hallucinations |
| Dryness | Irritability |
| Foreign body sensation | Sleep disturbances |
| Macular oedema | Endocrine |
| Nasolacrimal duct obstruction | Hypoglycaemia (insulin induced) |
| Pain | Gastro-intestinal |
| Punctate keratitis | Nausea |
| Uveitis | Diarrhoea |
| Vascular | |
| Arrhythmias | |
| Bradycardia | |
| Hypotension | |
| Peripheral vasoconstriction | |
| Reduced stroke volume | |
| Respiratory | |
| Bronchoconstriction | |
| Dyspnoea |
A degree of bradycardia is commonly seen with all β-blockers, and β-blocker eye drops have been identified as the cause of episodes of orthostatic hypotension and syncope in elderly patients (Müller et al., 2006). The use of topical β-blockers in patients on verapamil, diltiazem, disopyramide, quinidine or amiodarone could potentiate bradycardia and should be avoided. Topical β-blockers are generally avoided in patients with normal-tension glaucoma because the nocturnal fall in arterial pressure causes a potentially dangerous reduction in ocular perfusion.
Systemic side effects of topically administered β-blockers are shown in Table 55.8.
Betaxolol
In theory, because of its cardioselectivity, betaxolol should have fewer adverse effects on the pulmonary system. It should also have fewer adverse cardiovascular effects because of comparatively lower systemic β-receptor occupancy after ocular administration. Maximum occupancies for β1 and β2 receptors after ophthalmic administration were 52% and 88% for carteolol, 62% and 82% for timolol, and 44% and 3% for betaxolol, respectively. The lack of systemic effects of betaxolol compared with timolol has been highlighted by Easton who reported the case of an 85-year-old lady who developed atrial fibrillation on having her β-blocker eye drops changed from timolol to betaxolol (Easton, 2007)
However, betaxolol is less effective than other β-blockers as an ocular hypotensive agent (van der Valk, 2009). On initiation of treatment, the fall in IOP is slower than with other topical β-blockers. The 0.25% suspension is as effective as the 0.5% solution and is better tolerated by the patient (Yalvac et al., 2007). Experimental studies showed the drug reaches the retina after topical administration and displays a voltage-dependent L-type calcium channel-blocking activity, which probably leads to improved retinal perfusion. This effect may explain the significant improvement in visual field performance seen with betaxolol in a comparison study with timolol in open-angle glaucoma. The significant improvement with betaxolol occurred despite the more effective reductions in IOP with timolol.
Levobunolol
Levobunolol is the potent l-isomer of bunolol. It is metabolised to dihydrolevobunolol in the eye, prolonging the drug’s half-life and making it one of only two topical β-blockers licensed for once-daily use. It is as effective with once-daily dosing as the usual twice-daily regimen. It is not cardioselective, showing greater affinity for the β2-receptor, and does not possess ISA. It is reported to be as effective as timolol 0.5% and metipranolol 0.6% (now discontinued) in lowering IOP and more effective than betaxolol 0.5%. The incidence of allergic contact dermatitis is greater with levobunolol than timolol (Jappe et al., 2006), and a greater percentage of patients on levobunolol than timolol or betaxolol discontinued the drug due to adverse effects (Rahman et al., 2009). Its effect on tear volume and corneal epithelial barrier function is similar to that produced by timolol, and it has less effect on non-invasive break-up time of the precorneal tear film. This may be attributable to its formulation which includes polyvinyl alcohol. Levobunolol appears to have no effect on the retinal and choroidal vasculature.
Timolol
This non-cardioselective β-blocker without ISA was the first to be introduced, and as such is the agent against which all newer β-blockers were compared. It is effective in the long-term treatment of glaucoma, often in conjunction with other antiglaucoma therapy in terms of IOP lowering. Many patients can be placed on once-a-day therapy provided the IOP is maintained at satisfactory levels. However, the presentation of timolol in a prolonged-release formulation (a polysaccharide-based, gel-forming solution) leads to a prolonged corneal contact time and increased penetration of timolol into the eye. This is the preferred form for once-daily administration. Both timolol eye gels, 0.1% and 0.5%, have been shown to be as effective as the 0.5% solution administered twice daily. The 0.1% gel has the advantage of a much lower drug load, giving rise to plasma levels of timolol 10 times lower than achieved after twice-daily dosage of timolol 0.5% eye drops. The concentration of timolol achieved in the aqueous humour following administration of the 0.1% gel formulation, despite being approximately 40% of that following administration of the 0.5% solution is sufficient to occupy 100% of β1– and β2-receptors (Volotinen et al., 2009). Timolol is available in combination products with the carbonic anhydrase inhibitors brinzolamide and dorzolamide, the prostaglandin analogues latanoprost and travaprost, the prostamide bimatoprost and the sympathomimetic brimonidine (see Table 55.6).
Sympathomimetic agents
Apraclonidine
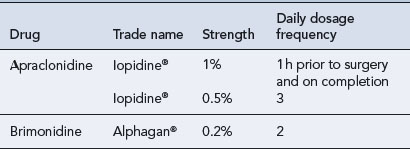
Eye drops containing a 0.5% solution are licensed for the short-term adjunctive treatment of patients on maximally tolerated medical therapy who require additional IOP reduction to delay laser treatment or glaucoma surgery, but the benefit for most patients is less than 1 month. Apraclonidine 0.5% is as effective in lowering IOP as brimonidine 0.2%, but has less effect on blood pressure and heart rate. Although an off-licence use, apraclonidine is sometimes used in children in whom brimonidine is strictly contraindicated (Wright and Freedman, 2009).
Ocular and systemic side effects of α2-agonists are shown in Table 55.9.
Table 55.9 Ocular and systemic side effects of topical α2-agonists
| Ocular | Systemic |
|---|---|
| Ocular pruritus | Dry mouth/nose |
| Discomfort | Headache |
| Tearing | Asthenia |
| Hyperaemia | Bradycardia |
| Conjunctival and lid oedema | Depression |
| Lid retraction, conjunctival blanching and mydriasis (reported after perioperative use of apraclonidine) | |
| Miosis (reported with brimonidine) | |
| Uveitis |
Brimonidine
A database containing details of drug use in 956 glaucoma patients over 18 years shows that brimonidine had the highest proportion of discontinuations due to adverse effects (Rahman et al., 2009). It has high allergenicity and may increase the likelihood of allergy to preparations subsequently used.
A formulation of brimonidine, brimonidine-Purite 0.15%, given twice daily, has been shown to be as effective as brimonidine 0.2% twice a day. It has a more favourable safety and tolerability profile, a reduced incidence of allergic conjunctivitis and better patient satisfaction and comfort rating. In a 4-month study, co-administration of latanoprost and brimonidine 0.15% results in a greater fall in IOP than co-administration of latanoprost and brinzolamide or dorzolamide (Bournias and Lai, 2009). This product is not available in the UK.
Brimonidine is contraindicated in patients receiving monoamine oxidase inhibitors or antidepressants which affect noradrenergic transmission, and there is the possibility of brimonidine potentiating or causing an additive effect with CNS depressants. Its use is contraindicated in children in whom it causes drowsiness, ataxia, pallor, irritability, hypotension, bradycardia, miosis and respiratory depression (Lai Becker et al., 2009).
Experimental evidence in animals has demonstrated that brimonidine is a potential neuroprotective agent. However, to date, clinical trials have failed to translate into similar efficacy in humans (Saylor et al., 2009).
Commercially available preparations of sympathomimetic agents are shown in Table 55.10.
Miotics
Ocular side effects of pilocarpine are shown in Box 55.1. In eyes with narrow angles, PACG may be precipitated by an aggravation of pupillary block. Systemic side effects are due to parasympathetic stimulation and include anxiety, bradycardia, diarrhoea, nausea, vomiting and sweating (Box 55.2).
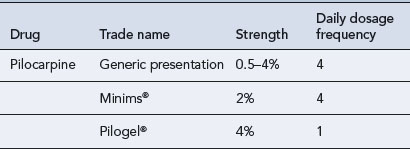
The onset of action of pilocarpine is 20 min, but its short duration of action necessitates four times daily dosing. The frequency of instillation of pilocarpine eye drops is a major disadvantage, and advances have been made to reduce the inconvenience of a four-times-daily dosage regimen. A slow-release gel preparation, Pilogel (Table 55.11), has been introduced. A single daily application, administered at bedtime, allows low IOP to be maintained for 24 h, with the patient sleeping through the more troublesome ocular side effects.
Carbonic anhydrase inhibitors
Acetazolamide
This is the only systemic carbonic anhydrase inhibitor available in the UK. Although it is amongst the most potent ocular hypotensive agents available, it has limited use in the long-term management of glaucoma due to poor patient adherence following occurrence of side effects. The systemic side effects are shown in Box 55.3.
Topical carbonic anhydrase inhibitors
The topical carbonic anhydrase inhibitors dorzolamide and brinzolamide are useful alternatives to acetazolamide in glaucoma management. They are licensed as monotherapy for patients with OHT or open-angle glaucoma resistant to β-blockers or those in whom use of β-blockers is contra-indicated. Dorzolamide is also licensed for the treatment of pseudo-exfoliative glaucoma, and limited clinical data in paediatric patients are available. Both drugs are licensed as adjunctive therapy to β-blockers and brinzolamide to prostaglandin analogues, although there is evidence to suggest that dorzolamide is as efficacious as brinzolamide when added to latanoprost (Nakamura et al., 2009).
Side effects similar to those of systemic sulphonamides may occur and should be watched for. The most common side effects are shown in Table 55.12. Of the two topical carbonic anhydrase inhibitors, brinzolamide appears to cause less burning and stinging on instillation due to the neutral pH (7.5) of the formulation. This is reflected in a much greater discontinuation rate with dorzolamide (31%) than with brinzolamide (14%) (Rahman et al., 2009).
Table 55.12 Side effects of topical carbonic anhydrase inhibitors
| Ocular | Systemic |
|---|---|
| Blurred vision | Dry mouth |
| Burning/stinging | Dyspnoea |
| Conjunctivitis | Headache |
| Eyelid pain/discomfort | Nausea |
| Fatigue | Taste perversion |
| Itching | |
| Nasolacrimal duct obstruction | |
| Ocular discharge | |
| Tearing |
Combination products
Fixed combination of timolol and dorzolamide
A combination of timolol 0.5% and dorzolamide 2% (Cosopt®) was the first topical ocular hypotensive combination to be marketed. It is indicated in the treatment of elevated IOP in patients with open-angle glaucoma or pseudoexfoliative glaucoma when topical β-blocker monotherapy is not sufficient. The fixed combination of dorzolamide and timolol is more effective than either timolol or dorzolamide alone, and as effective as its two components administered separately. In a review of its efficacy compared with other ocular hypotensives, an 80% response rate was reported for the fixed combination of dorzolamide and timolol (Yeh et al., 2008). It has also been reported to be a useful agent when used adjunctively with latanoprost, resulting in a further fall in IOP. Although generally well tolerated, the main problem with Cosopt® is burning and stinging on instillation. Although discontinuation of the combination in trials is low, Cosopt ® has been found to be the third most frequently discontinued eye product in practice (Rahman et al., 2009).
Fixed combination of timolol and latanoprost
A combination of timolol 0.5% and latanoprost 0.005%, marketed as Xalacom®, was first launched in the UK in 2001, shortly before the launch of travoprost and bimatoprost. Xalacom® is indicated for the reduction of IOP in patients with open-angle glaucoma and OHT who are insufficiently responsive to topical beta-blockers or prostaglandin analogues. It is administered once a day and has been shown to be more effective when administered in the evening than in the morning (Takmaz et al., 2008). It is unclear whether fixed combinations of prostaglandin analogues with timolol are superior to prostaglandin analogues alone.
Fixed combination of timolol and brimonidine
The fixed combination has been shown to be superior in reducing IOP than either brimonidine or timolol alone and is as safe and effective as concomitant treatment with the individual components. Use of the combination product has been shown to result in a 24% fall in IOP (Papaconstantinou et al., 2009). Use of the brimonidine/timolol combination results in a greater number of side effects than timolol alone has fewer side effects than brimonidine alone (Sherwood et al., 2006) and fewer cases of allergy than brimonidine used alone (Motolko, 2008).
Fixed combination of timolol and travoprost
A fixed combination of travoprost 0.004% and timolol 0.5% (DuoTrav®) has been shown to be more effective than either of its components and is as efficacious as the components administered concomitantly while fewer side effects are reported with the combination product (Gross et al., 2007). The fixed combination of travoprost and timolol is indicated to decrease IOP in patients with open-angle glaucoma or OHT who are insufficiently responsive to topical β-blockers or prostaglandin analogues. Although the Summary of Product Characteristics states that the dose is one drop in the affected eye(s) once daily, in the morning or evening, it has been shown that an evening dose demonstrates better 24-h pressure control (Konstas et al., 2009).
Fixed combination of timolol and bimatoprost
A fixed combination of bimatoprost 0.03% and timolol 0.5% (Ganfort®) is available for the reduction of IOP in patients with open-angle glaucoma or OHT who are insufficiently responsive to topical β-blockers or prostaglandin analogues. The fixed combination has been shown to be more effective than either of its components used alone and as effective as the components used in their usual dosing regimen, that is bimatoprost once daily in the evening and timolol twice daily, used concomitantly. Conjunctival hyperaemia has been reported more frequently by patients on bimatoprost (39%) than the bimatoprost/timolol fixed combination (23%) with the lowest incidence in those receiving timolol (7%) (Brandt et al., 2008).
Fixed combination of timolol and brinzolamide
A fixed combination of brinzolamide 1% and timolol 0.5% (Azarga®) is a recently launched combination topical ocular hypotensive presentation. The combination is indicated to reduce IOP in adult patients with open-angle glaucoma or OHT for whom monotherapy has produced insufficient IOP reduction. It has greater IOP-lowering efficacy than brinzolamide or timolol alone (Kaback et al, 2008). Brinzolamide/timolol administration leads to a 30–33% fall in IOP at trough and 34–35% at peak and is better tolerated than, and preferred to, the dorzolamide/timolol combination, probably because of the neutral pH (7.2) of the suspension (Manni et al., 2009; Mundorf et al., 2008).
Hyperosmotic agents
Glycerol
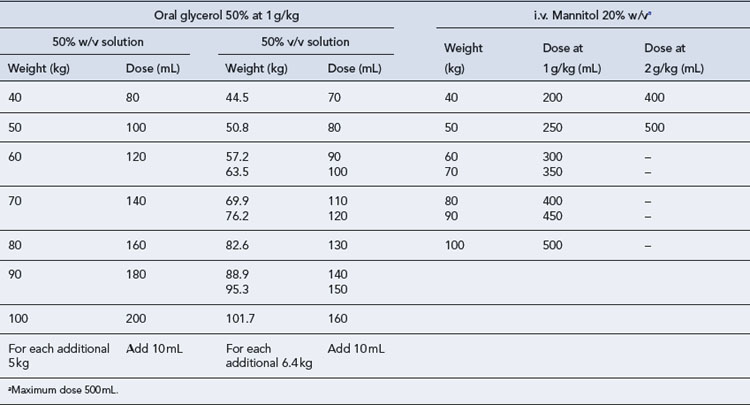
Glycerol is given orally, usually as a 50% solution in water, the dose being 1–1.5 g/kg body weight given as a single dose. It is not a strong diuretic but may cause headaches, nausea and vomiting. Although it is metabolised to glucose in the body, it may be given to diabetics who are well controlled. All practitioners should be aware of the difference in dose in mL required for a 50% solution of glycerol formulated as a 50% w/v solution and one formulated as a 50% v/v solution (Table 55.13).
Patient care
Chronic open-angle glaucoma
The existence of a patient self-help group, the International Glaucoma Association (http://www.glaucoma-association.com), should be brought to the patient’s attention. They will be able to put the patient in contact with their nearest support group.
Primary angle-closure glaucoma
Patients found to have shallow anterior chambers and narrow angles are normally promptly listed for peripheral iridectomies. However, if the procedure is delayed, they should be advised of the symptoms of an attack of acute PACG and details of the factors that are likely to precipitate an attack so that they can be avoided. They should be advised that there are a number of prescription and non-prescription drugs that they should not take (Lachkar and Bouassida, 2007). When visiting the doctor and purchasing medicines from a pharmacy, the patient should always remember to mention their condition, and the prescriber should ensure that the drug is appropriate for a patient prone to angle closure. Examples of drugs contraindicated in this condition are listed in Table 55.14. Note that the absence of a drug from this list does not imply safety.
| Therapeutic class | Examples | |
|---|---|---|
| Antimuscarinics | Topical | Atropine, cyclopentolate, homatropine, tropicamide |
| Antispasmodic | Atropine, dicycloverine, hyoscine, propantheline | |
| Motion sickness | Hyoscine, promethazine, cyclizine | |
| Bronchodilator | Ipratropium, tiotropium | |
| Urinary retention | Darifenacin, fesoterodine, flavoxate, oxybutynin, propiverine, solifenacin, tolterodine, trospium | |
| Drugs used in anaesthesia | Glycopyrronium, ketamine, suxamethonium | |
| Antidepressants | Amitriptyline, amoxapine, citalopram, clomipramine, dosulepin, doxepin,duloxetine,fluvoxamine, imipramine, lofepramine, maprotiline, mirtazapine, nortriptyline, paroxetine, phernelzine, reboxetine, sertraline, trazodone, trimipramine, venlafaxine | |
| Antipsychotics | Chlorpromazine, clozapine, flupentixol, fluphenazine, olanzapine, pericyazine, perphenazine, pipotiazine, promazine, risperidone, sulpiride, thioridazine, trifluoperazine, zuclopentixol | |
| Antihistamines | Alimemazine, antazoline, cetirizine, chlorphenamine, clemastine, cyproheptadine, diphenhydramine, hydroxyzine, loratidine, pizotifen | |
| Antiarrhythmics | Disopyramide | |
| Antiepileptics | Carbamazepine, topiramate | |
| Sympathomimetics | Adrenaline, cocaine, dipivefrine, ephedrine, isometheptene, naphazoline, phenylephrine, pseudoephedrine, salbutamol, xylometazoline | |
| Drugs used in the treatment of ADHD | Atomoxetine, dexamfetamine, methylphenidate, ritodrine | |
| Drugs used in the treatment of parkinsonism | Benserazide, carbidopa, entacapone, levodopa, orphenadrine, procyclidine, selegiline, trihexyphenidyl, | |
| Non-steroidal anti-inflammatory drugs | Mefenamic acid | |
| Sulphonamide derived drugs | Acetazolamide, co-trimoxazole, hydrochlorothiazide, sulphasalazine | |
| Miscellaneous | Botulinum toxin, carboprost, paracalcitol, pilocarpine |
Patient adherence
Patients with poor visual acuity can be helped by colour coding of eye drop labels and supplying bottles labelled with large print. Some manufacturers have endeavoured to enhance adherence by including dose-reminder caps and facilitating instillation by supplying aids to open or position the bottle. Other manufacturers have made their eye drop containers easier to squeeze or supply aids to squeeze the bottle. A list of such devices, some of which are available on prescription, others free of charge from manufacturers of drugs used in the treatment of glaucoma is available on the International Glaucoma Association’s website (http://www.glaucoma-association.com/).
Common therapeutic problems in glaucoma are listed in Table 55.15.
| Problem | Comments |
|---|---|
| Lack of adherence | Treatment perceived to be worse than disease |
| Complex multiple drug regimens | |
| Frequency of dosing | |
| Inability to differentiate between different types of medication | |
| Inability to instil medication | |
| Contraindication to therapy | Pilocarpine in uveitis |
| β-Blockers in asthma, bradycardia, heart block, uncontrolled heart failure | |
| Prostaglandin analogues and prostamides in aphakia | |
| Wide range of topical and systemic drugs in shallow anterior chamber | |
| α2-agonists in depression | |
| Carbonic anhydrase inhibitors in renal failure | |
| Intolerance to drug | Miosis and ciliary spasm with pilocarpine |
| Red eye with prostaglandin analogues, bimatoprost | |
| Bronchospasm with β-blockers | |
| Paraesthesia with acetazolamide | |
| Use outside licensed indications | Paediatric patients |
| Pregnant women | |
| Nursing mothers | |
| Hypersensitivity | To active drug |
| To preservative in multidose formulations |
Case 55.1
The ophthalmologist confirms the optometrist’s findings and measures this lady’s central corneal thickness at 570 μm. In accordance with National Institute for Health and Clinical Excellence (2009) guidelines, she decides to initiate ocular hypotensive therapy in the left eye.
Answer
It is recommended that patients under 60 years of age with OHT or suspected COAG and a central corneal thickness of between 555 and 590 μm are treated with an ocular hypotensive agent (National Institute for Health and Clinical Excellence, 2009). The recommended therapy is a topical β-blocker which typically reduces IOP by approximately 25%. However, β-blockers are contraindicated in patients with asthma and the ophthalmologist needs to choose an alternative agent with equivalent or better efficacy.
If β-blockers are contraindicated, a prostaglandin analogue could be prescribed.
Bournias T.E., Lai J. Brimonidine tartrate 0.15%, dorzolamide hydrochloride 2%, and brinzolamide 1% compared as adjunctive therapy to prostaglandin analogs. Ophthalmology. 2009;116:1719-1724.
Brandt J.D., Cantor L.B., Katz L.J., et al. Ganfort Investigators Group II 2008 Bimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J. Glaucoma.. 2008;17:211-216.
Easton P.J. A cardiovascular benefit of ophthalmic beta-blockade. Age and Ageing.. 2007;36:351.
Egorov E., Ropo A. Adjunctive use of tafluprost with timolol provides additive effects for reduction of intraocular pressure in patients with glaucoma. Eur. J. Ophthalmol.. 2009;19:214-222.
European Glaucoma Society. Terminology and Guidelines for Glaucoma, third ed. Savona: Editrice Dogma; 2008. Available at http://www.eugs.org/eng/EGS_guidelines.asp
Eyawo O., Nachega J., Lefebvre P., et al. Efficacy and safety of prostaglandin analogues in patients with predominantly primary open-angle glaucoma or ocular hypertension: a meta-analysis. Clin. Ophthalmol.. 2009;3:447-456.
Goldberg I., Li X.Y., Selaru P., et al. A 5-year, randomized, open-label safety study of latanoprost and usual care in patients with open-angle glaucoma or ocular hypertension. Eur. J. Ophthalmol.. 2008;18:408-416.
Gross R.L., Sullivan E.K., Wells D.T., et al. Pooled results of two randomized clinical trials comparing the efficacy and safety of travoprost 0.004%/timolol 0.5% in fixed combination versus concomitant travoprost 0.004% and timolol 0.5%. Clin. Ophthalmol.. 2007;1:317-322.
Hamacher T., Airaksinen J., Saarela V., et al. Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: results from a pharmacodynamics analysis. Acta Ophthalmol. (Copenh). 2008;86(S242):14-19.
Honrubia F., García-Sánchez J., Polo V., et al. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: a meta-analysis of randomised clinical trials. Br. J. Ophthalmol.. 2009;93:316-321.
Jappe U., Uter W., Menezes de Pádua C.A., et al. Allergic contact dermatitis due to beta-blockers in eye drops: a retrospective analysis of multicentre surveillance data 1993–2004. Acta Derm. Venereol.. 2006;86:509-514.
Johnson T.V., Toris C.B., Fan S. Effects of central corneal thickness on the efficacy of topical ocular hypotensive medications. J. Glaucoma.. 2008;17:89-99.
Kaback M., Scoper S.V., Arzeno G., et al. Brinzolamide 1%/timolol 0.5% Study Group. Intraocular pressure-lowering efficacy of brinzolamide 1%/timolol 0.5% fixed combination compared with brinzolamide 1% and timolol 0.5%. Ophthalmology. 2008;115:1728-1734.
Katz L.J., Cohen J.S., Batoosingh A.L., et al. Twelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertension. Am. J. Ophthalmol.. 2010;149:661-671.
Kenigsberg P.A. Changes in medical and surgical treatments of glaucoma between 1997 and 2003 in France. Eur. J. Ophthalmol.. 2007;17:521-527.
Konstas A.G., Tsironi S., Vakalis A.N., et al. Intraocular pressure control over 24 hours using travoprost and timolol fixed combination administered in the morning or evening in primary open-angle and exfoliative glaucoma. Acta Ophthalmol. (Copenh). 2009;87:71-76.
Lachkar Y., Bouassida W. Drug-induced acute angle closure glaucoma. Curr. Opin. Ophthalmol.. 2007;18:129-133.
Lai Becker M., Huntington N., Woolf A.D. Brimonidine tartrate poisoning in children: frequency, trends, and use of naloxone as an antidote. Pediatrics. 2009;123:e305-e311. Available at http://pediatrics.aappublications.org/cgi/reprint/123/2/e305.pdf
Lim K.S., Nau C.B., O’Byrne M.M., et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008;115:790-795.
Manni G., Denis P., Chew P., et al. The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. J. Glaucoma.. 2009;18:293-300.
Motolko M.A. Comparison of allergy rates in glaucoma patients receiving brimonidine 0.2% monotherapy versus fixed-combination brimonidine 0.2%-timolol 0.5% therapy. Curr. Med. Res. Opin.. 2008;24:2663-2667.
Müller M.E., van der Velde N., Krulder J.W., et al. Syncope and falls due to timolol eye drops. Br. Med. J.. 2006;332:960-961.
Mundorf T.K., Rauchman S.H., Williams R.D., et al. Brinzolamide/Timolol Preference Study Group. A patient preference comparison of Azarga (brinzolamide/timolol fixed combination) vs Cosopt (dorzolamide/timolol fixed combination) in patients with open-angle glaucoma or ocular hypertension. Clin. Ophthalmol.. 2008;2:623-628.
Nakamura Y., Ishikawa S., Nakamura Y., et al. 24-hour intraocular pressure in glaucoma patients randomized to receive dorzolamide or brinzolamide in combination with latanoprost. Clin. Ophthalmol.. 2009;3:395-400.
National Institute for Health and Clinical Excellence. Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. London: NICE; 2009. Available at http://guidance.nice.org.uk/CG85
Nieminen T., Lehtimäki T., Mäenpää J., et al. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand. J. Clin. Lab. Invest.. 2007;67:237-245.
Papaconstantinou D., Georgalas I., Kourtis N., et al. Preliminary results following the use of a fixed combination of timolol-brimonidine in patients with ocular hypertension and primary open-angle glaucoma. Clin. Ophthalmol.. 2009;3:227-230.
Rahman M.Q., Montgomery D.M., Lazaridou M.N. Surveillance of glaucoma medical therapy in a Glasgow teaching hospital: 26 years’ experience. Br. J. Ophthalmol.. 2009;93:1572-1575.
Rolim de Moura C., Paranhos JrA, Wormald R. Laser trabeculoplasty for open angle glaucoma. Cochrane Database Syst. Rev.. (4.):2007. Art. No.: CD003919. DOI: 10.1002/14651858.CD003919.pub2
Saylor M., McLoon L.K., Harrison A.R., et al. Experimental and clinical evidence for Brimonidine as an optic nerve and retinal neuroprotective agent: an evidence-based review. Arch. Ophthal.. 2009;127:402-406.
Sharpe E.D., Williams R.D., Stewart R.D., et al. A comparison of dorzolamide/timolol-fixed combination versus bimatoprost in patients with open-angle glaucoma who are poorly controlled on latanoprost. J. Ocul. Pharmacol. Therapeut.. 2008;24:408-413.
Sherwood M.B., Craven E.R., Chou C., et al. Twice-daily 0.2% brimonidine-0.5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomized trial. Arch. Ophthal.. 2006;124:1230-1238.
Sidjanin D.J., McCarty C.A., Patchett R., et al. Pharmacogenetics of ophthalmic topical beta-blockers. Personal. Med.. 2008;5:377-385.
Sonty S., Donthamsetti V., Vangipuram G., et al. Long-term IOP lowering with bimatoprost in open-angle glaucoma patients poorly responsive to latanoprost. J. Ocul. Pharmacol. Therapeut.. 2008;24:517-520.
Stewart W.C., Konstas A.G., Nelson L.A., et al. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115:1117-1122.
Takmaz T., Aşik S., Kürkçüolu P., et al. Comparison of intraocular pressure lowering effect of once daily morning vs evening dosing of latanoprost/timolol maleate combination. Eur. J. Ophthalmol.. 2008;18:60-65.
Toris C.B., Gabelt B.T., Kaufman P.L. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv. Ophthalmol.. 2008;53(Suppl. 1):S107-S120.
van der Valk R., Webers C.A., Lumley T., et al. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J. Clin. Epidemiol.. 2009;62:1279-1283.
Volotinen M., Mäenpää J., Kautiainen H., et al. Ophthalmic timolol in a hydrogel vehicle leads to minor inter-individual variation in timolol concentration in aqueous humor. Eur. J. Pharmaceuti. Sci.. 2009;36:292-296.
Williams R.D., Cohen J.S., Gross R.L., et al. Bimatoprost Study Group. Long-term efficacy and safety of bimatoprost for intraocular pressure lowering in glaucoma and ocular hypertension: year 4. Br. J. Ophthalmol.. 2008;92:1387-1392.
Wright T.M., Freedman S.F. Exposure to topical apraclonidine in children with glaucoma. J. Glaucoma.. 2009;18:395-398.
Yalvac I.S., Basci N.E., Dulger B., et al. Penetration of betaxolol HCL ionic suspension 0.25% and betaxolol HCL solution 0.50% into the aqueous humor. Eur. J. Ophthalmol.. 2007;17:368-371.
Yeh J., Kravitz D., Francis B. Rational use of the fixed combination of dorzolamide – timolol in the management of raised intraocular pressure and glaucoma. Clin. Ophthalmol.. 2008;2:389-399.
Costagliola C., dell’Omo R., Romano M.R., et al. Pharmacotherapy of intraocular pressure: part I. Parasympathomimetic, sympathomimetic and sympatholytics. Exp. Opin. Pharmacother.. 2009;10:2663-2677.
Costagliola C., dell’Omo R., Romano M.R., et al. Pharmacotherapy of intraocular pressure – part II. Carbonic anhydrase inhibitors, prostaglandin analogues and prostamides. Exp. Opin. Pharmacother.. 2009;10:2859-2870.
Harvey R. In the management of acute angle closure glaucoma is intravenous acetazolamide now an outdated treatment? Eye News. 2009;15:44-50.
Martinez A., Sanchez M. Bimatoprost/timolol fixed combination vs latanoprost/timolol fixed combination in open-angle glaucoma patients. Eye (Lond). 2009;23:810-818.