Genital Tract Infections
1. Describe the basic anatomy of the male and female reproductive systems.
2. Define the following conditions: vaginitis, cervicitis, proctitis, bartholinitis, pelvic inflammatory disease (PID), epididymitis, prostatitis, orchitis, neoplasia, urethritis, and dysuria.
3. List microorganisms that commonly are associated with vaginitis, cervicitis, and PID.
4. Describe the normal flora of the male and female genital tracts, and differentiate normal flora from pathogenic organisms.
5. List the media used to selectively isolate and differentiate genital tract pathogens including Modified Thayer-Martin (MTM), New York City (NYC), Colistin Nalidixic Agar (CNA), and JEMBEC System and the organisms capable of growth on each.
6. Compare and contrast the recommended use of various collection swabs for genital tract specimens including the organisms inhibited by each (cotton-tipped, with or without charcoal; calcium alginate; Dacron; rayon).
7. Determine specimen acceptability based on collection, transport, and diagnostic test orders for a genital tract specimen.
8. Explain the significance of gram-negative intracellular diplococci in genital specimens from both men and women.
9. Correlate signs and systems of infections with the results of laboratory diagnostic procedures for identification of the etiologic agent associated with infections of the genital tract.
General Considerations
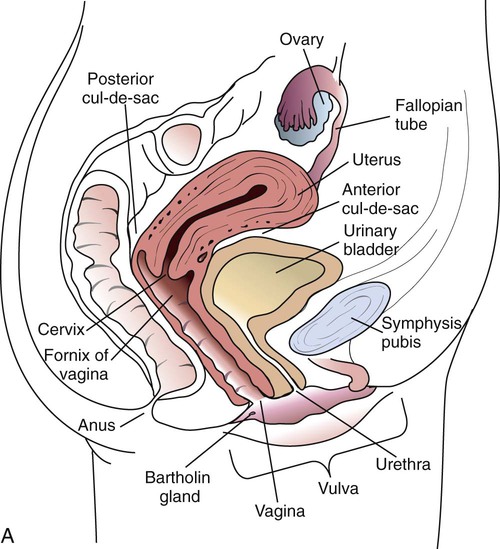
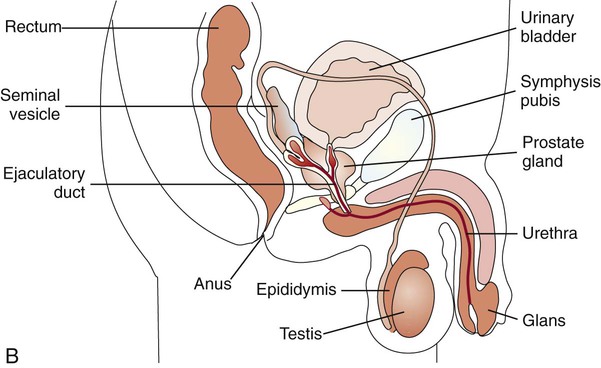
Anatomy
Familiarity with the anatomic structures is important for appropriate processing of specimens from genital tract sites and interpretation of microbiologic laboratory results. The key anatomic structures for the female and male genital tract in relation to other important structures are shown in Figure 74-1.
Genital Tract Infections
Sexually Transmitted Diseases and Other Lower Genital Tract Infections
Epidemiology/Etiologic Agents
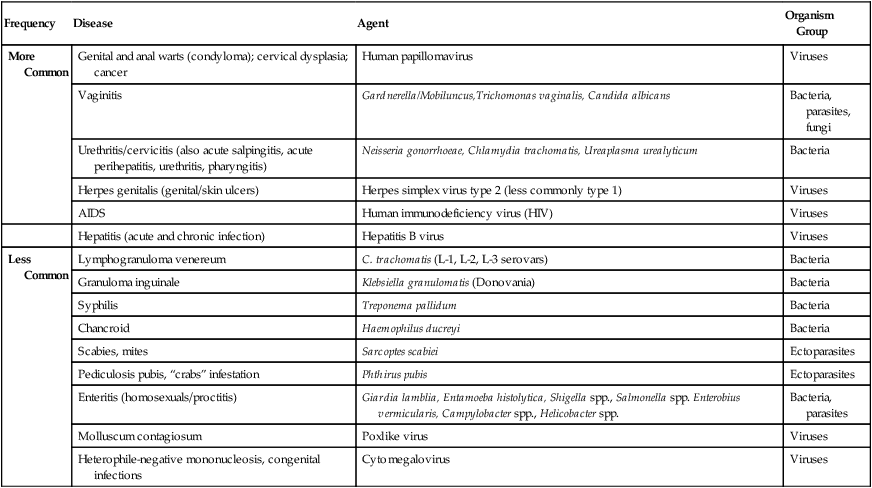
The number of microorganisms that can cause genital tract infections is large. These organisms are diverse, representing all four major groups of microorganisms (bacteria, viruses, fungi, and parasites). The major causes of genital tract infections are listed in Table 74-1.
TABLE 74-1
Major Causes of Genital Tract Infections and Sexually Transmitted Diseases
| Frequency | Disease | Agent | Organism Group |
| More Common | Genital and anal warts (condyloma); cervical dysplasia; cancer | Human papillomavirus | Viruses |
| Vaginitis | Gardnerella/Mobiluncus,Trichomonas vaginalis, Candida albicans | Bacteria, parasites, fungi | |
| Urethritis/cervicitis (also acute salpingitis, acute perihepatitis, urethritis, pharyngitis) | Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum | Bacteria | |
| Herpes genitalis (genital/skin ulcers) | Herpes simplex virus type 2 (less commonly type 1) | Viruses | |
| AIDS | Human immunodeficiency virus (HIV) | Viruses | |
| Hepatitis (acute and chronic infection) | Hepatitis B virus | Viruses | |
| Less Common | Lymphogranuloma venereum | C. trachomatis (L-1, L-2, L-3 serovars) | Bacteria |
| Granuloma inguinale | Klebsiella granulomatis (Donovania) | Bacteria | |
| Syphilis | Treponema pallidum | Bacteria | |
| Chancroid | Haemophilus ducreyi | Bacteria | |
| Scabies, mites | Sarcoptes scabiei | Ectoparasites | |
| Pediculosis pubis, “crabs” infestation | Phthirus pubis | Ectoparasites | |
| Enteritis (homosexuals/proctitis) | Giardia lamblia, Entamoeba histolytica, Shigella spp., Salmonella spp. Enterobius vermicularis, Campylobacter spp., Helicobacter spp. | Bacteria, parasites | |
| Molluscum contagiosum | Poxlike virus | Viruses | |
| Heterophile-negative mononucleosis, congenital infections | Cytomegalovirus | Viruses |
Clinical Manifestations
Lesions of the Skin and Mucous Membranes.
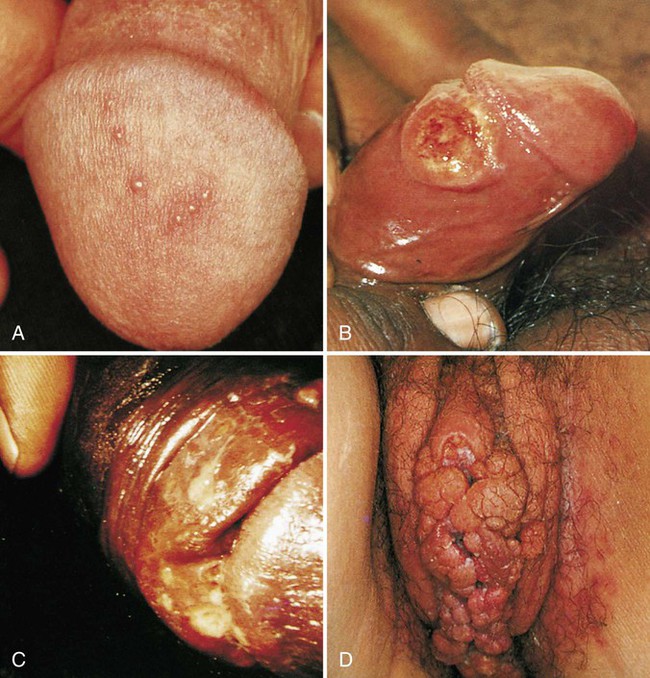
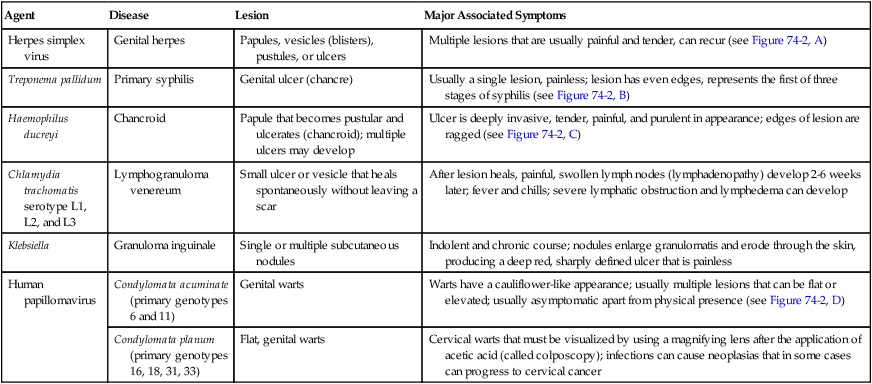
Numerous organisms can cause genital lesions that are diverse in both their appearance and their associated symptoms (Figure 74-2). The agents and their features of infection are summarized in Table 74-2. Some of these infections, such as genital herpes (caused by HSV) or genital warts (caused by HPVs and discussed in Chapter 66), are common, whereas others, such as lymphogranuloma venereum and granuloma inguinale, are uncommon in the United States. Specific HPV genotypes infect mucosal cells in the cervix and can cause a progressive spectrum of abnormalities classified as low-grade and high-grade squamous intraepithelial neoplasia (process of rapid cell growth that is faster than normal and continues to grow, i.e., a tumor) and in some cases, progress to invasive cervical cancer.
TABLE 74-2
Summary of Common Causes of Genital Lesions of the Skin and Mucous Membranes
| Agent | Disease | Lesion | Major Associated Symptoms |
| Herpes simplex virus | Genital herpes | Papules, vesicles (blisters), pustules, or ulcers | Multiple lesions that are usually painful and tender, can recur (see Figure 74-2, A) |
| Treponema pallidum | Primary syphilis | Genital ulcer (chancre) | Usually a single lesion, painless; lesion has even edges, represents the first of three stages of syphilis (see Figure 74-2, B) |
| Haemophilus ducreyi | Chancroid | Papule that becomes pustular and ulcerates (chancroid); multiple ulcers may develop | Ulcer is deeply invasive, tender, painful, and purulent in appearance; edges of lesion are ragged (see Figure 74-2, C) |
| Chlamydia trachomatis serotype L1, L2, and L3 | Lymphogranuloma venereum | Small ulcer or vesicle that heals spontaneously without leaving a scar | After lesion heals, painful, swollen lymph nodes (lymphadenopathy) develop 2-6 weeks later; fever and chills; severe lymphatic obstruction and lymphedema can develop |
| Klebsiella | Granuloma inguinale | Single or multiple subcutaneous nodules | Indolent and chronic course; nodules enlarge granulomatis and erode through the skin, producing a deep red, sharply defined ulcer that is painless |
| Human papillomavirus | Condylomata acuminate (primary genotypes 6 and 11) | Genital warts | Warts have a cauliflower-like appearance; usually multiple lesions that can be flat or elevated; usually asymptomatic apart from physical presence (see Figure 74-2, D) |
| Condylomata planum (primary genotypes 16, 18, 31, 33) | Flat, genital warts | Cervical warts that must be visualized by using a magnifying lens after the application of acetic acid (called colposcopy); infections can cause neoplasias that in some cases can progress to cervical cancer |
Vaginitis.
Although uncommon, there are other infectious causes of vaginitis. Three are briefly mentioned here because Gram stain of vaginal secretions may be helpful. First, Sobel described a number of premenopausal patients with a diffuse, exudative vaginitis with massive vaginal cell exfoliation, purulent vaginal discharge, and an occasional vaginal and cervical spotted rash. Laboratory findings included elevated pH of vaginal secretions. Also, numerous polymorphonuclear cells, an increased number of parabasal cells, the absence of gram-positive bacilli, and their replacement by occasional gram-positive cocci are observed on direct Gram stain (Figure 74-3). Basal cells appear as a result of the extensive exfoliation of epithelial cells. This clinical syndrome is referred to as desquamate inflammatory vaginitis. Symptoms associated with another disorder, lactobacillosis, resemble those of candidiasis and often follows antifungal therapy. Gram stain or wet mount typically reveals a large number of very long lactobacilli. These predominately anaerobic lactobacilli are 40 to 75 µ in length and are significantly longer than the average normal flora lactobacillus (5 to 15 µ). Finally, preexisting lesions due to other diseases may become secondarily infected with a mixed anaerobic flora of fusobacteria and spirochetes. This is referred to as fusiform-spirochete disease; this infection can progress rapidly. Gram stain examination reveals inflammatory cells in conjunction with gram-negative, fusiform bacterial morphotypes and spirochetes.
Infections of the Reproductive Organs and Other Upper Tract Infections
Females.
Infections Associated with Pregnancy.
Prenatal infections (those that occur any time before birth) may be acquired hematogenously (circulation) or ascending genital tract routes from mother to infant. If the mother has a bloodstream infection, organisms can reach and cross the placenta, with possible spread of infection to the developing fetus. Organisms that can cross the placenta are listed in Table 74-3. Alternatively, organisms can also infect the fetus by the ascending route from the vagina through torn or ruptured fetal membranes. Chorioamnionitis is an infection of the uterus and its contents during pregnancy. This infection is commonly acquired when organisms spread from the vagina or cervix after premature or prolonged rupture of the membranes or during labor. Organisms that are commonly isolated from amniotic fluid are listed in Box 74-1. Other maternal infections associated with adverse pregnancy outcomes that are not generally sexually transmitted include parvovirus B19, rubella, and Listeria monocytogenes.
TABLE 74-3
Common Etiologic Agents of Prenatal and Neonatal Infections
| Time of Infection* | Route of Infection | Common Agents |
| Prenatal | Transplacental | Bacteria: Listeria monocytogenes, Treponema pallidum, Borrelia burgdorferi Viruses: Cytomegalovirus (CMV), rubella, HIV, parvovirus B19, enteroviruses Parasites: Toxoplasma gondii, Plasmodium spp. |
| Ascending | Bacteria: Group B streptococci, Escherichia coli, L. monocytogenes, Chlamydia trachomatis, genital mycoplasmas Viruses: CMV, herpes simplex virus (HSV) |
|
| Natal | Passing through the birth canal | Bacteria: Group B streptococci, E. coli, L. monocytogenes, N. gonorrhoeae, C. trachomatis Viruses: CMV, HSV, enteroviruses, hepatitis B virus, HIV |
| Postnatal | All of the above routes, from the nursery environment, or from maternal contact (e.g., breastfeeding) | All agents listed above and various organisms from the nursery environment, including gram-negative bacteria and viruses such as respiratory syncytial virus |
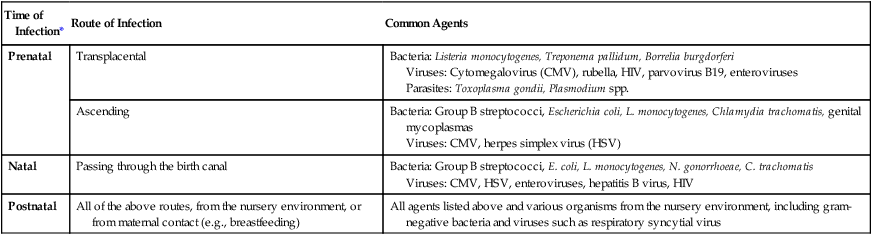
*Some newborns develop infections during the first 4 weeks of postnatal life. Infections may be delayed manifestations of earlier prenatal (before birth), natal, or postnatal (after birth) acquisition of pathogens.
Males.
Syphilis.
Many individuals can be infected and remain asymptomatic for years, making the control of this disease difficult. The disease is characterized by three stages: primary, secondary, and tertiary (also referred to as late or latent syphilis). Direct diagnosis may be accomplished by dark-field microscopy of material from an infectious lesion. However, serology provides a more accurate and reliable method for diagnosis. See Chapter 46 for a more detailed description of the disease and laboratory diagnosis.
Laboratory Diagnosis of Genital Tract Infections
Lower Genital Tract Infections
Urethritis, Cervicitis, and Vaginitis
Specimen Collection.
This discussion focuses on those specimens submitted for culture or direct examination. Procedures for the collection and transport of specimens for detection of agents by other noncultural methods (e.g., detection of Chlamydia trachomatis by amplification) should be followed according to the respective manufacturer’s instructions. Refer to Table 5-1 for a review of collection, transport, and processing of genital tract specimens.
Urethral.
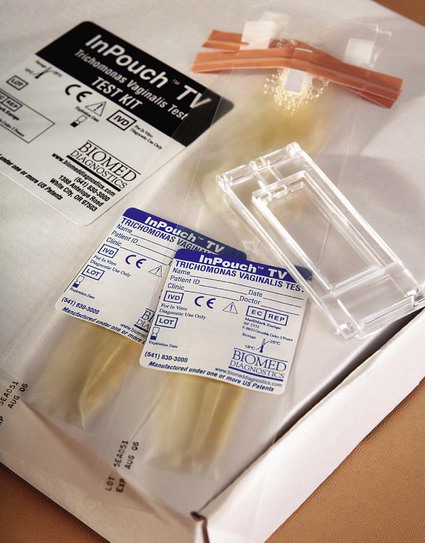
Because T. vaginalis may be present in urethral discharge, material for culture should be collected by swab as described and another specimen collected on a swab and placed into a tube containing 0.5 mL of sterile physiologic saline. This specimen should be delivered to the laboratory immediately. Direct wet mounts and cultures for T. vaginalis can be performed from this second specimen. Commercial media for culture of Trichomonas are available. The first few drops of voided urine is a suitable specimen for recovery of Trichomonas from infected males, if it is inoculated into culture media immediately. Alternatively, material may be smeared onto a slide for a fluorescent antibody stain. Plastic envelopes for direct examination and subsequent culture are also available (InPouch TV, BIOMED, White City, Oregon); sensitivity of this system is superior to other available methods, and organism viability is maintained up to 48 hours (Figure 74-4). In addition, several other techniques are available, including enzyme immunoassay, latex agglutination tests, and the Affirm VPIII probe (Becton Dickinson, Cockeysville, Maryland); polymerase chain reaction (PCR) has also been used to detect T. vaginalis directly in clinical specimens.
Direct Microscopic Examination.
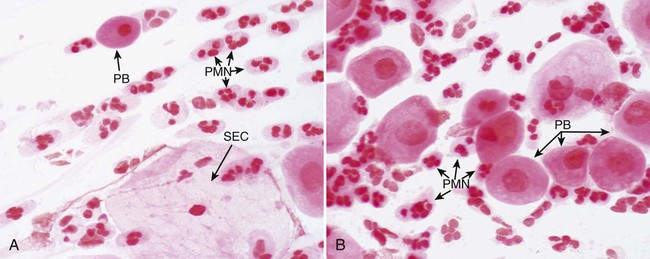
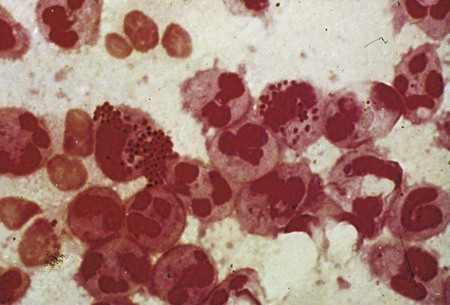
In addition to culture, urethral discharge may be examined by Gram stain for the presence of gram-negative intracellular diplococci (Figure 74-5), usually indicative of gonorrhea in males. After inoculation to culture media, the swab is rolled over the surface of a glass slide, covering an area of at least 1 cm2. If the Gram stain is characteristic, cultures of urethral discharge need not be performed. Urethral smears from females may also be examined. If extracellular organisms resembling N. gonorrhoeae are seen, the microbiologist should continue to examine the smear for intracellular diplococci. Presumptive diagnosis can be useful when decisions are to be made regarding immediate therapy, but confirmatory cultures or an alternative nonculture method should always be performed on specimens from females. Some strains of N. gonorrhoeae are sensitive to the amount of vancomycin present in selective media. If suspicious organisms seen on smear fail to grow in culture, re-culture on chocolate agar without antibiotics may be warranted.
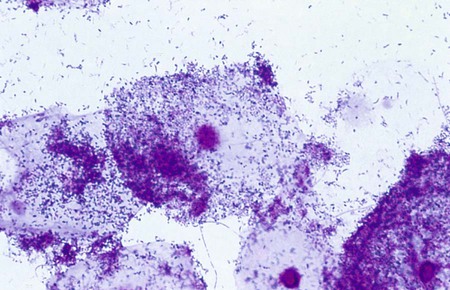
BV, characterized by a foul-smelling discharge, can be diagnosed microscopically or clinically. The discharge is primarily sloughed epithelial cells, many of which are completely covered by tiny, gram-variable rods, and coccobacilli. These cells are called clue cells (Figure 74-6). The absence of inflammatory cells in the vaginal discharge is another sign of BV. Although Gardnerella vaginalis has been historically associated with the syndrome and can be cultured on a human blood bilayer plate, culture is not recommended for diagnosis of BV. A clinical diagnosis of BV is dependent on the presence of three or more of the following criteria: homogeneous, gray discharge; clue cells seen on wet mount or Gram stain; a pH greater than 4.5; and an amine or fishy odor elicited by the addition of a drop of 10% KOH to the discharge on a slide or on the speculum.
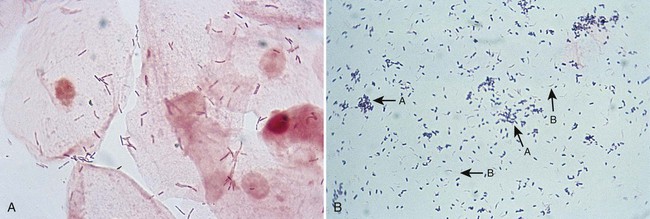
Bacterial vaginosis may be differentiated from other vaginal infection by Gram stain (Figure 74-7). Nugent and colleagues have developed a grading system for Gram stains of vaginal discharge (see Procedure 74-1 on the Evolve site). This system is based on the presence or absence of certain bacterial morphologies. Typically, in patients with BV, lactobacilli are either absent or few in number, whereas curved, gram-variable rods (Mobiluncus spp.) or G. vaginalis and Bacteroides morphotypes predominate. The Gram stain is more sensitive and specific than either the wet mount for detection of clue cells or culture for G. vaginalis, and the smear can be saved and reexamined later.
Culture.
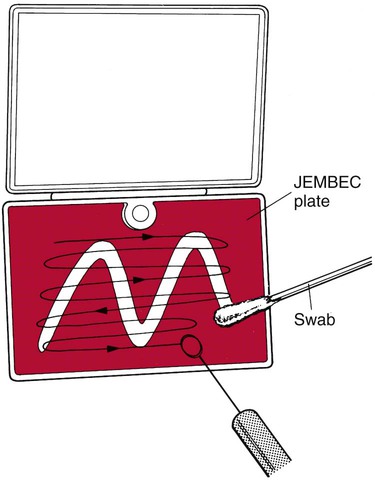
Samples for isolation of gonococci may be inoculated directly to culture media, obviating the need for transport medium. Commercially produced systems have been developed for this purpose, and many clinicians inoculate standard plates directly if convenient access to an incubator is available. Modified Thayer-Martin medium is most often used, although New York City (NYC) medium has the added advantage of supporting the growth of mycoplasmas and gonococci. Excellent recovery of gonococci results from direct inoculation to any of these media in self-contained incubation systems such as JEMBEC plates (Figure 74-8). The specimen swab is rolled across the agar with constant turning to expose all surfaces to the medium. The JEMBEC plate, which generates its own increased carbon dioxide atmosphere by means of a sodium bicarbonate tablet, is inoculated in a W pattern. The plate may be cross-streaked with a sterile loop in the laboratory (Figure 74-9).
A specimen from the lower vagina followed by the rectum using the same swab at 35 to 37 weeks’ gestation reliably predicts the presence of group B streptococci at delivery. The swab should be transported to the laboratory in a nonnutritive transport medium such as Amies’ or Stuart’s without charcoal and then inoculated into a recommended selective broth medium such as Todd-Hewitt broth supplemented either with gentamicin and nalidixic acid or with colistin and nalidixic acid. Selective enrichment broths are subcultured to agar the next day to isolate and identify group B streptococci. In addition, the presence of group B streptococci in urine in any concentration from a pregnant woman is a marker for heavy genital tract colonization. Any quantity of group B streptococci in urine from pregnant women should be worked up in the laboratory (see Chapter 73).
Nonculture Methods.
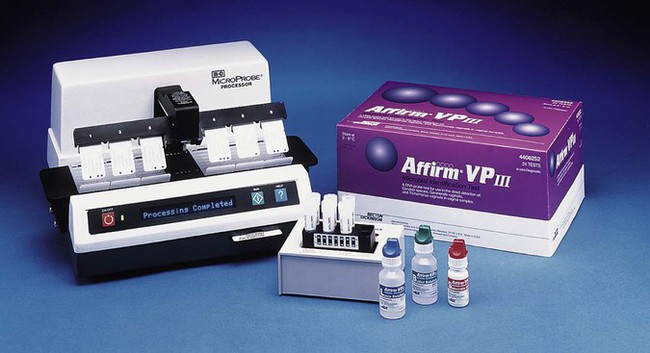
As previously discussed, BV involves several organisms. Besides the Gram stain, BV can be diagnosed by using the Amsel criteria, which include pH measurement, performance of an amine test, and wet mount microscopy of vaginal secretions. However, this approach has been considered unreliable because of the lack of microscopy-related skills and availability of pH paper in most doctors’ offices. Although the Gram stain offers high sensitivity and specificity, it is not immediately available. Currently, commercial laboratory tests are available to aid in the diagnosis of BV, but not all are available in the United States; a test for sialidase (OSOM BVBLUE, Sekisui Diagnostics, Farmingham, Massachusetts) in conjunction with measuring pH has been reported to be a rapid, highly sensitive, and specific means to diagnose BV. (Sialidases are secreted from anaerobic gram-negative rods such as Bacteroides and Prevotella as well as Gardnerella and play a role in bacterial nutrition, cellular interactions, and immune response evasion, which in turn improves the ability of bacteria to adhere, invade, and destroy mucosal tissue.) A hybridization assay (Affirm VP III Microbial Identification Test; Becton Dickinson Microbiology Systems, Sparks, Maryland) is commercially available to diagnose BV, as well as genital tract infections caused by Candida spp. and Trichomonas vaginalis. Once the appropriate reagents and specimen are added to special trays, the entire hybridization assays are then performed by instrumentation (Figure 74-10). Evaluations indicate this system is sensitive and specific.
Genital Skin and Mucous Membrane Lesions
Vesicles in the genital area are almost always attributable to viruses, and herpes simplex is the most common cause. Epithelial cells from the base of a vesicle may be spread onto the surface of a slide and examined for the typical multinucleated giant cells of HSV or stained by immunofluorescent antibody stains for viral antigens. Additionally or alternatively, the material may be transported for culture of the virus, as outlined in Procedure 74-2, which can be found on the Evolve site.
Material from lesions suggestive of syphilis should be examined by dark-field or fluorescent microscopy. These procedures are described in Chapter 46.
Infections of the Reproductive Organs
Pelvic Inflammatory Disease
Infections of Neonates and Human Products of Conception
Suspected infections acquired by the fetus as a result of a maternal infection that crosses the placenta (congenital infection) can be diagnosed culturally or serologically in the newborn. Because maternal IgG crosses the placenta, serologic tests are often difficult to interpret (see Chapter 10). For culturable agents, the most definitive diagnoses involve recovery of the pathogen in culture. HSV, varicella-zoster virus (VZV), enteroviruses, and cytomegalovirus (CMV) can be cultured easily, as can most bacterial agents. Rubella and parvovirus B19 are more difficult to culture. Nasal and urine specimens offer the greatest yield for viral isolation, although blood, cerebrospinal fluid, and material from a lesion can also be productive. Systemic neonatal herpes without lesions may be difficult to diagnose unless tissue biopsy material is examined, because the viruses may not be present in cerebrospinal fluid or blood. Bacteria and fungi can be isolated from lesions, blood, and other normally sterile sites.
Determining the presence of fetal immunoglobulin M (IgM) directed against the agent in question establishes the serologic diagnosis of congenital infection. Until recently, ultracentrifugation was required for separation of IgM from IgG, the only definitive means of preventing false-positive results caused by maternal IgG or fetal rheumatoid factor. Ion-exchange chromatography columns, antihuman IgG, and bacterial proteins that bind to IgG are commercially available for removing cross-reactive IgG and rheumatoid factor to obtain more homogeneous IgM for differentiation of fetal antibody. Indirect fluorescent antibody and enzyme-linked immunosorbent assay (ELISA) test systems are commercially available to detect IgM against T. gondii, rubella, CMV, HSV, and VZV. Interference by rheumatoid factor is still a consideration in most commercial IgM test systems (see Chapter 9). Our ability to detect viral inclusions in tissue, conjunctiva scrapings, and vesicular lesions, traditionally performed with Giemsa stain, has been improved as a result of monoclonal and polyclonal fluorescent antibody reagents, which are described in the chapters that discuss individual agents.