Chapter 9 Genetics and dysmorphology
Long Cases
Down syndrome
Down syndrome (DS) represents a common chromosomal disorder. The incidence has changed with the increase in the proportion of pregnancies diagnosed prenatally. In addition, the maternal age at parturition has changed, with more women over 35 giving birth; in 1986, 72% of babies with DS were born to younger mothers, whereas in 2004, 46% were born to younger mothers. However, the worldwide birth incidence of DS has not increased, but decreased from what it could have been by 2–18% per year. One in 350 pregnancies are affected, but only 1 in 1150 live births. Now (2010), 95% of fetuses identified with DS are terminated. In the USA, between 1989 and 2005, there was a 49% decrease between expected and observed rates, and in the UK, between 1989 and 2006, there was a 54% decrease between expected and observed rates. These trends can be attributed to availability of prenatal testing and women preferring selective terminations.
The increased risk of developing acute leukaemias in children with DS is being investigated, as transient leukaemia (TL), and the myeloid leukaemia of DS (ML DS) offer unique models to understand better the stepwise progression of leukaemia, and of gene dosage effects mediated by aneuploidy. (Aneuploid cells have an abnormal number of chromosomes: the most common aneuploidies are trisomies; most trisomies are not compatible with life, with the exception of DS and the sex chromosomal trisomies.)
History
Past treatment
• CVS: past surgery, complications thereof, plans for further operative procedures. Past interventional catheter procedures. Medications, past and present, side effects of these, monitoring levels, treatment plans for the future. Antibiotic prophylaxis for dental procedures, maintenance of dental hygiene. Compliance with treatment. Any identification bracelet. Instructions for air travel and high altitude. Any recent investigations monitoring treatment. Any recent changes in treatment regimen, and indications for these.
• GIT: past surgery, complications thereof, plans for further operative procedures. Compliance with treatment (e.g. following diet for coeliac disease). Any recent investigations monitoring treatment. Any recent changes in treatment regimen, and indications for these.
• Hearing and vision: past ear, nose and throat surgical intervention, hearing aid placement, ophthalmological intervention, glasses.
• OSA: past adenotonsillectomy, nasal mask continuous positive airway pressure (CPAP).
• Treatments for other conditions: hypothyroidism, atlantoaxial subluxation, seizures, leukaemia, arthritis, diabetes mellitus.
Current state of health
Note any of the following symptoms:
• CVS disease (fatigue, shortness of breath, cough, sweating, poor feeding, recurrent chest infections; symptoms suggesting arrhythmias, such as syncope, alteration of consciousness, dizziness, palpitations, ‘funny feeling’ in the chest, chest pain).
• GIT disease (nausea, vomiting, change in bowel habit).
• Recurrent infection (how often, what sites [usually upper or lower respiratory tract], treatment required, any prophylactic antibiotics).
• Hearing impairment (compliance/problems with hearing aids, impacted cerumen, ventilation tubes for chronic otitis media).
• Visual impairment (development of refractive disorders, keratoconus, corneal opacities, cataracts).
• Weight concerns (obesity, non-compliance with diet, exercise, or sign of hypothyroidism).
• OSA symptoms (snoring, restless sleep, daytime somnolence).
• Skin problems (in children—seborrhoeic dermatitis, palmar/plantar hyperkeratosis, xerosis; in adolescents—folliculitis [especially back, buttocks, thighs, perigenital area], fungal infections [skin and nails], atopic dermatitis).
• Oral health (level of oral hygiene, dental caries, peridontal disease, bruxism [stereotyped orofacial movements with teeth grinding], intervention for malocclusion, non-compliance with dental recommendations).
• Respiratory problems (recurrent pneumonia due to silent aspiration).
• Orthopaedic issues (limping can be due to atlantoaxial subluxation, acetabular dysplasia with subluxing hips [not more common in DS, but may appear later], slipped femoral epiphysis, arthritis or leukaemia).
• Foot problems (hallux valgus, hammer toe deformities, plantar fasciitis, pedal arthritis).
• Joint problems (polyarticular onset juvenile arthritis-like arthropathy).
• Diabetes mellitus (increased drinking or eating, weight loss, lethargy).
• Hypothyroidism (dry skin, cold intolerance, lethargy).
• Haematological neoplasia (acute lymphoblastic leukaemia [ALL] or acute non-lymphoblastic leukaemia [ANLL] occur 10–15 times more frequently in DS, with usual symptoms of pallor, bruising, fever, hepatosplenomegaly and lymphadenopathy).
• Reproductive issues in adolescents (difficulties with menstrual hygiene, use of oral contraceptives, Depo-provera, presentation of premenstrual syndrome [PMS] with temper tantrums, autistic behaviour episodes, seizures, sex education, desire to reproduce).
• Neurological issues (seizures [more frequent than general population, but less than other causes of intellectual impairment], strokes [due to cyanotic CHD, or moyamoya disease]).
Current state of behaviour
Ask about possible co-morbid psychiatric/behavioural issues:
• Symptoms of ADHD (inattention, hyperactivity, impulsivity); any treatment for these.
• Symptoms of ASD (impaired social interaction, impaired communication, behaviour patterns including preferring own company, tendency to be loner, ‘in their own world’), most problematic behaviours at present (e.g. rituals, anxiety, aggression, self-injury); any treatment for these.
• Other behavioural concerns: depression, conduct disorder, oppositional defiant disorder, aggressive behaviour; any treatment for these.
• Impact of these co-morbid issues (on family, educational facility [e.g. special school], therapists, carers).
Social history
1. DS impact on child: level of functioning in activities of daily living (ADLs), schooling (type of school, level of support from education department, therapists, academic performance, teachers’ attitudes, peer attitudes, teasing, amount of school missed and whether schooling is appropriate).
2. DS impact on parents: for example, financial situation, financial burden of disease so far, government allowances being received, marriage/partnership stability, restrictions on social life, plans for further children, genetic counselling, availability of prenatal diagnosis, contingency plans for child’s future, guardianship and power of attorney issues.
3. DS impact on siblings: for example, effect of family’s financial burden, whether siblings feel comfortable to bring friends home, whether siblings miss out on parental time, plans for siblings to act as guardians in future.
4. Social supports: for example, social worker, contact with DS parent support groups, any available respite.
5. Coping: contingency plans (e.g. plan if child develops severe febrile illness); parents’ degree of understanding regarding health supervision issues in DS.
6. Access to local doctor, paediatrician, neurodevelopmental clinic, various subspecialty clinics attended (where, how often), other clinics attended, alternative practitioner (e.g. homeopathy) involvement.
Examination
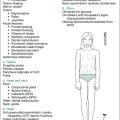
The examination of the child with DS in a long-case setting includes a full cardiological appraisal if the child has any CVS involvement, a documentation of the dysmorphic features of DS occurring in that child, plus an assessment for the development of any of numerous associated problems that may have arisen (e.g. thyroid disease, leukaemia). In addition, an assessment of function with respect to ADLs, a developmental assessment and an impression of behaviour based on direct interaction will give a complete picture, but time constraints may preclude these being assessed adequately. The approach given in Table 9.1 deals with the physical aspects of the child with DS that are able to be assessed objectively and within the time requirements. This approach can also be used in a short-case setting.
| A. Measurements |
| Height |
| Head circumference |
| Request/plot weight |
| Assess percentile charts specific for Down syndrome |
| Calculate height velocity |
| Request/plot birth parameters |
| Request/plot parents’ percentiles and ages at puberty |
| B. Systematic examination |
| The following is a selected listing of possible physical findings in children with Down syndrome—it does not include behavioural aspects |
| General inspection |
| Diagnostic facies |
| Tanner staging |
| Nutritional status |
• Scars: repairs of gastrointestinal tract anomalies (e.g. duodenal atresia, pyloric stenosis, Hirschsprung disease, omphalocoele, imperforate anus), repairs of urinary tract anomalies (e.g. vesicoureteric reflux, posterior urethral valves, other obstructive uropathy), renal transplantation (e.g. dysplastic kidneys, glomerulosclerosis), operative interventions for other associated conditions (e.g. Crohn’s disease)
• Prune-like appearance (prune belly syndrome)
• Tanner staging pubic hair (pubic hair tends to be straight)
• Quality of gait: often ‘Chaplinesque’ (externally rotated hips, flexed knees (in valgus), externally rotated tibiae)
• Limp (hip dysplasia, dislocation, avascular necrosis, slipped femoral capital epiphysis)
• Hemiplegic/circumducting gait (cerebrovascular accident [CVA] or cerebral abscess complicating cyanotic CHD)
Management issues
The following directs you to most areas of management relevant in the long case.
Cardiac disease
Around 30–50% of children with DS have CVS disease, the most common being septal defects (particularly atrioventricular (AV) canal, then VSD, then ASD), patent ductus arteriosus and tetralogy of Fallot; left-side defects are uncommon. These abnormalities are now more easily corrected, and are largely responsible for the increased life span of patients with DS. Cardiac disease may be entirely asymptomatic, so all neonates diagnosed with DS should have an echocardiogram after birth, and again at 6 weeks. The success rate for repair of septal defects is improving steadily: if not corrected, these conditions can lead to pulmonary hypertension, particularly if there is OSA, and shortened life span. Eisenmenger’s syndrome (reversal of shunting, to cause right-to-left shunt), more common in DS than in the general population, is now rare. Children with DS under 2 years of age with cyanotic CHD have an increased risk of cerebrovascular accident (CVA); they may present with seizures or acute onset hemiplegia. Children with DS over 2 years of age with cyanotic congenital heart disease (CHD) have an increased incidence of brain abscess, the severity of which relates to the degree of hypoxia. They may present with fever, headache, seizures or focal neurological signs. Around 50% of adolescents with DS syndrome and no previously known cardiac diagnosis develop mitral valve prolapse; aortic incompetence also can develop in adolescence. These are indications for continued monitoring. Despite obesity and unfavourable lipid levels in DS, the incidence of hypertension and atheroma is low. See further discussion of cardiac disease issues in the long case on cardiology (Chapter 6).
Developmental issues
Nearly all children with DS have a mild (IQ 50–70) to moderate (IQ 35–50) intellectual disability (ID). A small number have a severe ID, with an IQ in the 20–35 range. The IQ alone gives little clue as to function. Language milestones are slower to develop; aspects affected include articulation, expression, comprehension, phonological awareness, syntax and semantics, with reading being a relative strength. In around two thirds of DS patients, language comprehension is equal to the mental age, but expressive language is more delayed. Gross and fine motor skills are delayed. Academic attainment is higher in mainstream schools rather than specialist schools, and is influenced also by IQ, gender (females tend to do better) and the father’s role in the child’s life. Self-sufficiency is determined by IQ, excitability, extent of behaviour problems (see below) and whether the mother uses practical means to cope with things, showing the child what, when and why, with more showing and less talking; the level of social activity in which the child is engaged also affects self-sufficiency.
Behavioural and psychiatric issues
The most common behavioural issues include problems associated with ADHD-like behaviour, autism (appears in around 7% of children with DS), conduct/oppositional defiant disorder and aggressive behaviour. See the discussion in the long cases in Chapter 5 (Behavioural paediatrics) on each of these topics for relevant clinical aspects and their management. The diagnosis of autism is usually much later than in non-DS children, and parents often are reassured by various people that it is due to DS; there appear to be two groups, one where atypical behaviours appear in infancy, and the second when children have autistic regression at 3–7 years (this is a loss, or plateauing, of social and language skills). Depression is the other common psychiatric issue in DS, but tends to present beyond the paediatric age group. In those who appear depressed, thyroid function should be checked, as hypothyroidism can present in this way. Both the intellectual impairment and speech problems in DS complicate the prompt recognition of mental illness. Excluding sensory impairment in either vision or hearing, or both, should always occur before attributing new unusual behaviours to mental illness or Alzheimer’s syndrome. In the adult population, 25% of those with DS have major depression or aggressive behaviour.
Dental problems
A high level of dental hygiene should be maintained. The immunological deficiency of DS results in periodontal disease in almost all children with DS, probably due to aberrations of mouth flora. Any carious teeth should be dealt with promptly, with appropriate antibiotic cover. All DS children with congenital heart disease should be given a letter or card to show to any dentist or doctor, explaining the need for antibiotic prophylaxis for any dental or similar procedure (e.g. tonsillectomy), including the recommended doses of antibiotics. Regular visits to the dentist and routine brushing can prevent periodontal disease. Orthodontic problems occur in almost all patients with DS, but compliance with orthodontic procedures and braces can be problematic.
Thyroid disease
The rate of congenital hypothyroidism in DS is around 1 in 140, versus 1 in 4000 for the general population. The recommendation is thyroid screening (for thyroxine and thyroid-stimulating hormone [TSH]) at birth, 6 months and then every year thereafter. The frequency of thyroid disease increases with age, such that it eventually affects 15% of people with DS. The symptoms and signs of hypothyroidism can be difficult to differentiate from those of DS itself. Children with DS who have a normal thyroxine level, but a TSH concentration above 10 mU/L, are considered to have compensated hypothyroidism and should be treated. Treatment with thyroxine in this group often results in increased growth velocity. For further discussion of the symptoms of hypothyroidism, see the discussion in the short-case section on thyroid disease (Chapter 7).
Coeliac disease
The average time between the first symptoms of coeliac disease and definitive diagnosis approaches 4 years in patients with DS. The frequency of coeliac disease in DS is around 5–7%. It is sensible to screen all children with DS for coeliac disease: the Down Syndrome Medical Interest Group recommends this be done at 24 months of age, but there is yet to be consensus as to whether screening should be repeated later in life. See the short case on malabsorption for more details on screening tests (Chapter 8).
Obstructive sleep apnoea (OSA)
OSA is increasingly recognised in DS, and in children previously incorrectly labelled as having ADHD. OSA has also been a major contributor to pulmonary hypertension and the development of Eisenmenger’s syndrome in children with DS and cyanotic CHD historically. As soon as OSA is identified, referral to an ear, nose and throat surgeon for tonsillectomy and adenoidectomy is appropriate in most cases. See the further discussion of clinical and management aspects in the long case on OSA in Chapter 15.
Haematological disorders (including leukaemia)
Chemotherapy in children with DS is associated with a higher morbidity and mortality. Anthracycline dosages are reduced in children with DS because of the increased long-term risk of cardiotoxicity in a population that may have compromised cardiac function due to congenital heart disease. For further discussion of leukaemia, see the long-case section on oncology (Chapter 14). The other haematological aberrations seen in DS are neonatal polycythaemia (occurs in two thirds of babies with DS) and macrocytosis (also in two thirds).
Seizures
Seizures occur in around 8% of patients with DS. There is a bimodal distribution of age of onset: 40% of seizures commence by 12 months of age, and 40% commence just outside of the paediatric age group, in the third decade of life. Children with DS tend to develop infantile spasms and generalised tonic clonic seizures with myoclonus; about half of those with infantile spasms will go into remission without relapse, and there can be some restoration of development. For further discussion on seizures, see the long-case section on seizures and epilepsy in Chapter 13 (Neurology).
Orthopaedic problems
Apart from AAI, discussed above, there are a number of musculoskeletal problems that are more common in DS, including developmental dysplasia of the hip, acquired hip dislocation (risk related to ligamentous laxity), chronic patellar dislocation, pes planus, ankle pronation, scoliosis and degenerative joint disease (the latter two most likely related to obesity, hypotonia and premature ageing).
Turner syndrome
The physical signs of TS may be very subtle in childhood and adolescence. In the newborn, there may be characteristic features, such as lymphoedema of the hands and feet, nuchal folds, left-sided heart lesions, webbed neck and a low hairline. In childhood, TS should be considered in any girl with declining growth velocity (falling below the 5th centile), even if there are no obvious dysmorphic features. In adolescence, TS may cause short stature, absence of breast development by 13 years of age, or amenorrhoea with elevated follicle-stimulating hormone (FSH) levels. TS can also present with complete phenotypic expression of X-linked recessive conditions in a female, such as haemophilia A, Duchenne muscular dystrophy, or red–green colour-blindness, which suggests X monosomy.
Definitions
Haplodeficiency is presence in the cell of one set of genes instead of the usual two sets.
History
Initial treatment
1. Surgical (e.g. CVS—correction of coarctation, hypoplastic left-heart syndrome [HLHS]; urinary tract—correction of duplex system).
2. Medical (e.g. antihypertensives, growth hormone (GH), oestrogen, progesterone, calcium supplementation, vitamin D, thyroxine for hypothyroidism, gluten-free diet for coexistent coeliac disease).
Past treatment
• CVS. Past surgery, complications thereof, plans for further operative procedures. Past interventional catheter procedures. Medications, past and present, side effects of these, monitoring levels, treatment plans for the future. Antibiotic prophylaxis for dental procedures; maintenance of dental hygiene. Compliance with treatment. Any identification bracelet. Instructions for air travel and high altitude. Any recent investigations monitoring treatment. Any recent changes in treatment regimen, and indications for these. Frequency of echocardiography or cardiac MRI to screen for aortic root dilatation.
• Growth. When GH started (fell below 5th centile), supervised by whom (paediatric endocrinologist), dosage given (usual commencing dosage 0.05 mg/kg/day [0.1 IU/kg/day]), any combination therapy (e.g. with oxandrolone in girls aged 9–12); when oestrogen replacement therapy commenced (after age 12), when cyclic menstruation achieved.
• Hearing. When impairment detected, level of loss, whether hearing aids prescribed, compliance with hearing aids.
Current state of health
Note any symptoms of the following:
• CVS disease (symptoms suggesting dilation of root of ascending aorta: chest or epigastric pains, or ‘funny feeling’ in chest or epigastrium (preceding aortic dissection and rupture); symptoms of hypertension (headache); other symptoms of CVS involvement (fatigue, shortness of breath, sweating, poor feeding [infants], syncope, alteration of consciousness, dizziness, palpitations).
• Loss of weight (Graves’ disease, inflammatory bowel disease [IBD], coeliac disease, renal disease, malignancy [colon, germ cell tumours]).
• Gain in weight (obesity, non-compliance with diet, exercise, Hashimoto’s thyroiditis causing hypothyroidism).
• Hypothyroidism (dry skin, cold intolerance, lethargy).
• Orthopaedic problems: limp (associated developmental dysplasia of hips, juvenile arthritis, IBD-associated arthritis), back pain (scoliosis, kyphosis, lordosis, juvenile arthritis, IBD-associated arthritis).
• GIT disease: diarrhoea (coeliac, IBD), nausea, abdominal pain, rectal bleeding (IBD).
• Urinary tract problems (infection, haematuria, proteinuria).
• Hearing impairment (recurrent otitis media, recent hearing testing).
• Visual problems (development of strabismus).
• Weight concerns (obesity, non-compliance with diet, exercise, Hashimoto’s thyroiditis causing hypothyroidism).
• Reproductive issues in adolescents (education about advisability or otherwise of pregnancy [increased risk of maternal complications, including risk of dilatation and dissection of aorta], education about donor oocyte pregnancies, sex education, discussion of desire to reproduce).
• Psychosocial/behavioural issues: being bullied, unsatisfactory peer interactions, ADHD-like symptoms (inattention, hyperactivity, impulsivity), immature behaviour, anxiety, depression; any treatment for these.
Social history
• TS impact on child: schooling (type of school, level of support from teachers, therapists, academic performance, teachers’ attitudes, peer attitudes, teasing, amount of school missed and whether it is appropriate).
• TS impact on parents: for example, financial situation, financial burden of disease so far, government allowances received, marriage/partnership stability, restrictions on social life, plans for further children, genetic counselling, availability of prenatal diagnosis, contingency plans for child’s future, guardianship and power of attorney issues.
• TS impact on siblings: for example, effect of family’s financial burden, siblings missing out on parental time.
• Social supports: for example, contact with TS parent support groups.
• Coping: contingency plans (e.g. parents’ degree of understanding regarding health supervision issues in TS).
• Access to local doctor, paediatrician, paediatric endocrinologist, neurodevelopmental clinic, various subspecialty clinics attended (where, how often), other clinics attended.
Examination
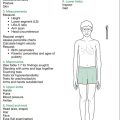
Growth parameters are assessed first: measurements and manoeuvres are given below.
Manoeuvres
With each manoeuvre, stand opposite the child and demonstrate, so that she will mirror your movements. The skin should be evaluated concurrently with inspecting from front, back and side. Note any pigmented naevi, any scars (keloid common).
1. Inspect from in front
• Screen for carrying angle: have the child hold her arms by her side, with the palms forward. This angle can be increased in TS.
• Screen for short limbs: have the child touch the tips of the thumbs to the tips of the shoulders. If the thumbs do not reach the shoulders, there may be middle-segment (mesomelic) limb-shortening, found in TS and the associated Leri–Weill dyschondrostenosis (caused by abnormalities of the same SHOX gene—the haplodeficiency [loss of one allele] which contributes to the TS stature).
• Screen the hands. Have the child hold the palms up: look for short mid-phalanx of fifth finger, Madelung deformity (congenital subluxation or dislocation of head of ulna with radial deviation of hand). Turn the hands over (palms down) and check the nails (e.g. hyperconvex).
• Screen for short metacarpals by having the child make a fist and look for shortened third, fourth or fifth metacarpal.
Completing the examination
Formal physical examination flows well if commenced at the hands, working up to the head and then downward—essentially a head-to-toe pattern. Table 9.2 gives a selected listing of possible physical findings in children with Turner syndrome. It does not include behavioural aspects (see below).
Table 9.2 The child with Turner syndrome: physical examination
| A. Measurements |
| Height |
| Lower segment (LS) |
| Calculate upper segment (US) by subtracting LS from height |
| Calculate US:LS ratio |
| Arm span |
| Head circumference |
| Record weight |
| Assess percentile charts |
| Calculate height velocity |
| Record birth parameters |
| Record parents’ percentiles and ages at puberty |
| B. Manoeuvres |
|
• Arms by side with palms forward (to detect cubitus valgus over 15 degrees between extended supinated forearm to upper arm) • Thumbs on shoulders, to detect short limbs: can detect middle segment shortening • Palms up: to detect short mid-phalanx of fifth finger; Madelung deformity (congenital subluxation or dislocation of head of ulna with radial deviation of hand) • Make a fist (to detect short third, fourth, fifth metacarpal) |
Specific complications and associations
The mnemonic TURNER ULLRICH’S helps recall most of these:
T. Thyroiditis and other autoimmune disease (JIA, coeliac disease)
R. Root of aorta dilatation risks (bicuspid aortic valve, aortic stenosis, aortic coarctation, hypertension)
 Neck short, webbed/Nails hyperconvex/Normal intelligence (but impairments in memory, maths, spatial perception, goal-setting, goal-attainment, attention span)
Neck short, webbed/Nails hyperconvex/Normal intelligence (but impairments in memory, maths, spatial perception, goal-setting, goal-attainment, attention span)
E. Endocrine deficiency (pubertal failure, short stature)/Eye findings (epicanthic folds, strabismus, ptosis, congenital glaucoma)/Ears (unusual shape, rotation)
R. Reproductive technology assistance (egg donor programs)
Ulcerative colitis/Crohn’s disease
• Left-sided heart problems/Linear growth deficiency
• Lymphoedema/Lymphatic malformations (e.g. cystic hygroma)
R. Renal anomalies (hydronephrosis)
C. Cancer risks (gonadoblastoma, neuroblastoma, colonic carcinoma)
H. Hearing loss/Hypertension/Hepatic cirrhosis
S. Skeletal (DDH, Madelung)/Spine (scoliosis, kyphosis, lordosis)
Management issues
The following directs you to most areas of management relevant in the long case.
Cardiovascular disease
Hypertension occurs in around 25% of girls, and a higher percentage of adults, with TS; hypertension is a risk factor for aortic dilation and dissection. It has been noted that some babies with TS have an unusual resting tachycardia, that starts in utero, and the occurrence of impaired sympathovagal tone infers that there could be a problem with autonomic regulation of the cardiovascular system in TS. Many patients with TS have nocturnal hypertension, such that 24-hour monitoring may be helpful.
Lymphatic abnormalities
In TS fetuses that do not survive, there are often cardiovascular and lymphatic abnormalities found; fetuses with cardiac failure almost always have obstructed jugular lymphatics with nuchal cystic hygromas. In TS girls who survive, there is often peripheral oedema and webbed neck, which are the residua of fetal lymphoedema and cystic hygromas, respectively. The newborn lymphoedema usually resolves by 2 years, although it may reoccur at any age, particularly when growth hormone or oestrogen are commenced, as these both can cause salt retention. Support stockings and elevation may be required. ‘Decongestive physiotherapy’ has been recommended, comprising skin and nail care, massage for manual lymph drainage, compression bandaging and exercise. These patients should avoid any vascular surgery or diuretics. There is a National Lymphoedema Network in the USA, which provides useful information for families, at www.lymphnet.org.
Growth
GH increases the rate of growth without increasing bone age. Increases in final height of 15 cm have been described if there were at least 6 years of GH therapy, and oestrogen was delayed. The advantages and disadvantages must be covered fully in discussion with the parents. When GH is commenced, it can lead to enlargement of naevi, and recurrence of lymphoedema. The approved doses are 4.7–9.3 mg/m2/week (0.16–0.32 mg/kg/week) in Australia.
Ophthalmological disorders
Approximately 16% of children with TS have ptosis. Other ocular findings include hypertelorism, epicanthal folds, upward-slanting palpebral fissures, strabismus (25–35%), hyperopia (farsightedness) (25–35%), amblyopia (due to the preceding two), blue sclerae and cataracts. Red–green colour-blindness occurs in around 8%. In addition to standard visual screening, all children with TS should be assessed by a paediatric ophthalmologist yearly.
Thyroid disease and autoimmunity
The incidence of Hashimoto’s thyroiditis (leading to hypothyroidism) is increased in TS after the age of 10 years; the incidence of hypothyroidism is around 10–30% in later childhood, rising to 50% in adults. Patients with structural defects of the X chromosome have increased susceptibility to autoimmune disorders, including Hashimoto’s thyroiditis and Graves’ disease, IBD, myasthenia gravis and MPGN. The recommendation is thyroid screening every year from the age of 10 years (for thyroxine and thyroid-stimulating hormone [TSH], and for anti-thyroid antibodies). The symptoms and signs of hypothyroidism can be subtle. Once identified, hypothyroidism should be treated promptly. For further information about the symptoms of hypothyroidism, see the discussion in the short-case section on thyroid disease in Chapter 7 (Endocrinology).
Gastrointestinal disease: coeliac disease, IBD and hepatic effects
The frequency of coeliac disease in TS is around 5%. It is sensible to screen all children with TS for coeliac disease, from mid-childhood, regardless of presence or absence of symptoms, repeating the screening every 2 years. See the short case on malabsorption in Chapter 8 (Gastroenterology) for more details on screening tests.
Psychosocial aspects
Adolescents with TS are at increased risk for a number of behavioural abnormalities, including anxiety, depression, significant shyness, social isolation and poor self-esteem. Many of these girls demonstrate social immaturity for their age, and they often need support in becoming independent and in interacting with others socially (particularly with males). Girls with TS may be the subjects of teasing and bullying at school: once identified, this situation must be dealt with promptly and decisively by teachers and parents. Meeting with other girls with TS and their families is very beneficial, and offers valued support for these girls and their parents. Patients with TS are not at any increased risk of significant mental health problems.
Short Case
Definitions
1. A sequence is a pattern of structural defects caused by a single problem in morphogenesis (e.g. early amnion rupture sequence, Pierre Robin sequence due to hypoplasia of the mandible before 9 weeks’ gestation)
2. A syndrome is a pattern of multiple structural defects, caused by multiple defects in one or more tissues, due to a known cause (e.g. Down syndrome).
3. An association is a pattern of structural defects that are statistically related; that is, a non-random occurrence in at least two people of multiple anomalies not due to a sequence or syndrome. The classic example is the VACTERL association:
Examination
If the child is short, then proceed as per the short-stature short-case approach (see Chapter 7, Endocrinology). If the child is tall, then proceed as per the tall-stature short-case approach (again, see Chapter 7, Endocrinology). If the child has obvious disproportion, then proceed with manoeuvres as below. If the child seems to have normal proportions, then proceed with a head-to-toe systematic examination, starting with a careful examination of the face.
Further measurements
Measure the head circumference (HC). HC can be increased in syndromes with hydrocephalus (e.g. X-linked hydrocephalus) or macrocephaly (e.g. Sotos). HC is decreased in the many syndromes with microcephaly (e.g. Seckel). The course adopted by the tape measure being placed around the skull can accentuate the abnormal skull shapes in cases of craniosynostosis (e.g. Apert, Crouzon).
Manoeuvres
Inspect from in front
Screen for short metacarpals: have the child make a fist, and look for shortened third, fourth or fifth metacarpal (e.g. Gorlin syndrome), first metacarpal (proximally placed thumb; e.g. in diastrophic dysplasia) or all metacarpals (e.g. in Poland anomaly).
Completing the examination
Formal physical examination flows well if commenced at the hands, working up to the head and then downward—essentially a head-to-toe pattern. Table 9.3 lists a small number of sample findings sought at each point.
Table 9.3 Dysmorphic child: measurements, manoeuvres and systematic examination
• Scars: repair of duodenal atresia (e.g. Down), repair of Hirschsprung (e.g. Down, Waardenburg), removal of tumour (e.g. WAGR, adrenal tumour in B–W), repair of inguinal herniae (e.g. Marfan), repair of omphalocoele (e.g. B–W)
• Prune-like appearance (prune belly syndrome)
• Tanner staging pubic hair, for precocity (e.g. McCune–Albright) or delay (Klinefelter, Noonan)
Finally, summarise your findings succinctly, and give a brief differential diagnosis, placing the most likely diagnosis first.