Chapter 1 General notes
Radiology
The procedures are laid out under a number of sub-headings, which follow a standard sequence. The general order is outlined below, together with certain points that have been omitted from the discussion of each procedure in order to avoid repetition. Minor deviations from this sequence will be found in the text where this is felt to be more appropriate.
Contraindications
Risk due to radiation
Radiation effects on humans may be:
There are legal regulations which guide the use of diagnostic radiation (see Appendix I). These are the basic principles:
Justification is particularly important when considering the irradiation of women of reproductive age, because of the risks to the developing fetus. The mammalian embryo and fetus are highly radiosensitive. The potential effects of in-utero radiation exposure on a developing fetus include prenatal death, intrauterine growth restriction, small head size, mental retardation, organ malformation and childhood cancer. The risk of each effect depends on the gestational age at the time of the exposure and the absorbed radiation dose.2 Most diagnostic radiation procedures will lead to a fetal absorbed dose of less than 1 mGy for imaging beyond the maternal abdomen/pelvis and less than 10 mGy for direct abdominal/pelvic or nuclear medicine imaging.7 There are important exceptions which result in higher doses. Computed tomography (CT) scanning of the maternal pelvis may result in fetal doses below the level thought to induce neurologic detriment to the fetus, but, theoretically, may double the fetal risk for developing a childhood cancer.8
Almost always, if a diagnostic radiology examination is medically indicated, the risk to the mother of not doing the procedure is greater than the risk of potential harm to the fetus.9 However, whenever possible, alternative investigation techniques not involving ionizing radiation should be considered before a decision is taken to use ionizing radiation in a female of reproductive age. It is extremely important to have a robust process in place that prevents inappropriate or unnecessary ionizing radiation exposure to the fetus. Previous guidelines recommended that all non-emergency examinations that would involve irradiation of the lower abdomen or pelvis in women of child-bearing age be restricted to the first 10 days (10-day rule) or 28 days (28-day rule) following the onset of a menstrual period.10 This led to some potential practical difficulties, e.g. for women who denied recent sexual intercourse, for those with an irregular menstrual cycle or for those who had been taking the oral contraceptive pill. There are also concerns:
Joint guidance from the National Radiological Protection Board, the College of Radiographers and the Royal College of Radiologists recommends the following:11
If the examination is necessary, a technique that minimizes the number of views and the absorbed dose per examination should be utilized. However, the quality of the examination should not be reduced to the level where its diagnostic value is impaired. The risk to the patient of an incorrect diagnosis may be greater than the risk of irradiating the fetus. Radiography of areas that are remote from the pelvis and abdomen may be safely performed at any time during pregnancy with good collimation and lead protection.
Equipment
For many procedures this will also include a trolley with a sterile upper shelf and a non-sterile lower shelf. Emergency drugs and resuscitation equipment should be readily available (see Chapter 17).
See Chapter 9 for introductory notes on angiography catheters.
Patient preparation
The radiologist must assess a child’s capacity to decide whether to give consent for or refuse an investigation. At age 16 years a young person can be treated as an adult and can be presumed to have the capacity to understand the nature, purpose and possible consequences of the proposed investigation, as well as the consequences of non-investigation. Following case law in the United Kingdom (Gillick v West Norfolk and Wisbech Area Health Authority) and the introduction of The Children Act 1989, in which the capacity of children to consent has been linked with the concept of individual ability to understand the implications of medical treatment, there has come into existence a standard known as ‘Gillick competence’. Under the age of 16 years children may have the capacity to consent depending on their maturity and ability to understand what is involved. When a competent child refuses treatment, a person with parental responsibility or the court may authorize investigations or treatment which is in the child’s best interests. In Scotland the situation is different: parents cannot authorize procedures a competent child has refused. Legal advice may be helpful in dealing with these cases.14
Preliminary film
The purpose of these films is:
Technique
1 Wall B.F., Kendall G.M., Edwards A.A., et al. What are the risks from medical X-rays and other low dose radiation? Br. J. Radiol.. 2006;79(940):285-294.
2 McCollough C.H., Scheuler B.A., Atwell T.D., et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27(4):909-917.
3 Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317-323.
4 Cardis E., Vrijheid M., Blettner M., et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat. Res.. 2007;167(4):396-416.
5 Berrington de González A., Darby S. Risk of cancer from diagnostic X-rays: estimates from the UK and 14 other countries. Lancet. 2004;363(9406):345-351.
6 International Commission on Radiological Protection. The optimization of radiological protection: broadening the process. ICRP publication 101. Ann. ICRP. 36(3), 2006.
7 Lowe S.A. Diagnostic radiography in pregnancy: risks and reality. Aust. N. Z. J. Obstet. Gynaecol.. 2004;44(3):191-196.
8 Hurwitz L.M., Yoshizumi T., Reiman R.E., et al. Radiation dose to the fetus from body MDCT during early gestation. Am. J. Roentgenol.. 2006;186(3):871-876.
9 International Commission on Radiological Protection. Pregnancy and medical radiation. Ann. ICRP. 2000;30(1):1-43.
10 Bury B., Hufton A., Adams J. Editorial. Radiation and women of childbearing potential. BMJ. 1995;310:1022-1023.
11 Sharp C., Shrimpton J.A., Bury R.F. Diagnostic Medical Exposures: Advice on Exposure to Ionising Radiation during Pregnancy. London: National Radiological Protection Board, 1998.
12 The Royal College of Radiologists. Standards for Patient Consent Particular to Radiology. London: The Royal College of Radiologists, 2005.
13 General Medical Council. Consent: Patients and Doctors Making Decisions Together. London: General Medical Council, 2008.
14 General Medical Council. 0–18 years: Guidance for all Doctors. London: General Medical Council, 2007.
Radionuclide Imaging
RADIOPHARMACEUTICALS
Radioactive injections
In the UK, the Administration of Radioactive Substances Advisory Committee (ARSAC) advises the health ministers on the Medicines (Administration of Radioactive Substances) Regulations 1978 (MARS). These require that radioactive materials may only be administered to humans by a doctor or dentist holding a current ARSAC certificate or by a person acting under their direction. Administration of radioactive substances can only be carried out by an individual who has received appropriate theoretical and practical training, as specified in the Ionising Radiation (Medical Exposure) Regulations 20001 (see Appendix III). These will place responsibilities on the referrer to provide medical data to justify the exposure, the practitioner (ARSAC licence holder) to justify individual exposure, and operators (persons who carry out practical aspects relating to the exposure).
Activity administered
The maximum activity values quoted in the text are those currently recommended as diagnostic reference levels in the ARSAC Guidance Notes.2 The unit used is the SI unit, the megabecquerel (MBq). Millicuries (mCi) are still used in some countries, notably the US; 1 mCi = 37 MBq.
The regulations require that doses to patients are kept as low as reasonably practicable (the ALARP principle) and that exposure follows accepted practice. Centres will frequently be able to administer activities below the maximum, depending upon the capabilities of their equipment and local protocols. Typical figures are given in the text where they differ from the diagnostic reference levels. In certain circumstances, the person clinically directing (ARSAC licence holder) may use activity higher than the recommended maximum for a named patient, for example for an obese patient where attenuation would otherwise degrade image quality.
Technique
Patient positioning
The resolution of gamma camera images is critically dependent upon the distance of the collimator surface from the patient, falling off approximately linearly with distance. Every effort should, therefore, be made to position the camera as close to the patient as possible. For example, in posterior imaging with the patient supine, the thickness of the bed separates the patient from the camera, as well as interposing an attenuating medium. In this case, imaging with the patient sitting or standing directly against the camera is preferable.
‘Oldendorf’ bolus injection3
For some investigations the Oldendorf technique is recommended to provide an abrupt bolus:
Aftercare
Radiation safety
Special instructions should be given to patients who are breast feeding regarding expression of milk and interruption of feeding.2 Precautions may have to be taken with patients leaving hospital or returning to wards, depending upon the radionuclide and activity administered. These precautions were reviewed following the introduction of the Ionising Radiation Regulations 1999 and the adoption of lower dose limits to members of the public, and appropriate guidance has been published.4
1 The Ionising Radiation (Medical Exposure) Regulations. London: HMSO, 2000.
2 Administration of Radioactive Substances Advisory Committee. Notes for Guidance on the Clinical Administration of Radiopharmaceuticals and use of Sealed Radioactive Sources. Didcot: NRPB, 1998.
3 Oldendorf W.H., Kitano M., Shimizu S. Evaluation of a simple technique for abrupt intravenous injection of radioisotope. J. Nucl. Med.. 1965;6:205-209.
4 Working Party of the Radiation Protection Committee of the British Institute of Radiology. Patients leaving hospital after administration of radioactive substances. Br. J. Radiol.. 1999;72:121-125.
Computed Tomography
Patient preparation
Many CT examinations require little physical preparation. An explanation of the procedure, the time it is likely to take, the necessity for immobility and the necessity for breath-holding whilst scanning chest and abdomen should be given. Waiting times should be kept to a minimum, as a long wait may increase anxiety. The patient should be as pain-free as is practical but too heavy sedation or analgesia may be counter-productive – patient cooperation is often required. Children under the age of 4 years will usually need sedation; please see Chapter 18. Children should also have an intravenous (i.v.) cannula inserted at the time sedation is administered or local anaesthetic cream applied to two sites if i.v. contrast medium is needed. If these simple steps are taken, the number of aborted scans will be reduced and the resultant image quality improved.
Intravenous contrast medium
Many CT examinations will require i.v. contrast medium. Essential information should be obtained from the patient and appropriate guidelines followed (see Chapter 2). An explanation of the need for contrast enhancement should be given to the patient.
Oral contrast medium
For examinations of the abdomen, opacifying the bowel satisfactorily can be problematic. Water-soluble contrast medium (e.g. 20 ml Urografin 150 diluted in 1 l of orange squash to disguise the taste, preflavoured contrast such as 20 ml Gastromiro diluted in 1 l of water) or low-density barium suspensions (2% w/v) can be used. Timing of administration is given in Table 1.1. Doses of contrast media in children depend upon age.
| Volume (ml) | Time before scan (min) | |
|---|---|---|
| Adult | ||
| Full abdomen and pelvis | 1000 | Gradually over 1 h before scanning |
| Upper abdomen, e.g. pancreas | 500 | Gradually over 0.5 h before scanning |
| Child | ||
| Newborn | 60–90 |  |
| 1 month–1 year | 120–240 | |
| 1–5 years | 240–360 | |
| 5–10 years | 360–480 | |
| Over 10 years | As for adult | |
| If the large bowel needs to be opacified then give the contrast medium the night before or 3–4 h before scanning | ||
MAGNETIC RESONANCE IMAGING
Patient preparation
As for CT scanning, a full description of the purpose and nature of the examination should be given to the patient and waiting times kept to a minimum. Some patients find the interior of the scanner a very disconcerting environment, and report claustrophobic and even acute anxiety symptoms. This may occur in as many as 10% of patients. Most of these patients are able to complete their examination, but approximately 1% of investigations may have to be curtailed as a result. To decrease the number of scans aborted, the counselling, explanation to and reassurance of patients by well-trained staff should be routine. A small number of adult patients may require sedation before an MRI scan is undertaken. Sedation or general anaesthesia are often required for MRI scans in young children; details of suggested protocols are given in Chapter 18.
SAFETY IN MAGNETIC RESONANCE IMAGING
MRI has generally been a very safe process because of the care taken by equipment manufacturers and MRI staff. However, there are significant potential hazards1 to patients and staff due to:
Effects due to magnetic fields
Static field
The strength of the static magnetic field used in MRI is measured in units of gauss or tesla (10000 gauss = 1 tesla (T)). The earth’s magnetic field is approximately 0.6 gauss. Current guidelines on MRI field strength are given in Table 1.2.
Table 1.2 Guidelines on whole-body exposure to static magnetic fields4
| Level | Magnetic field (T) |
|---|---|
| Normal operating mode | <2 |
| First level operating mode (one or more outputs reach a value that may cause physiological stress and which requires medical supervision) | 2–4 |
| Second level operating mode (one or more outputs reach a value that may produce significant risk for patients, for which explicit ethical approval is required) | >4 |
Biological effects
Despite extensive research, no significant deleterious physiological effects have been proven. There have been reports of minor changes, such as alteration in electrocardiogram (T-wave elevation)2 presumed to be due to electrodynamic forces on moving ions in blood vessels which might result in a reduction of blood-flow velocity. This change is purely temporary and disappears on removal from the field. Studies of volunteers exposed to 8 T static magnetic fields have shown no clinically significant effect on heart rate, respiratory rate, systolic and diastolic blood pressure, finger pulse oxygenation levels and core body temperature.3 However, it was noted that movement within the 8 T field could cause vertigo. Teratogenesis in humans is thought unlikely at the field strengths used in clinical MRI.
Non-biological effects
There are two main areas of concern:
Gradient field
Biological effects
The rapidly switched magnetic gradients used in MRI can induce electric fields in a patient which may result in nerve or muscle stimulation, including cardiac muscle stimulation. The strength of these is dependent on the rate of change of the field and the size of the subject.5 Studies have shown that the threshold for peripheral nerve stimulation is lower than that for cardiac or brain stimulation.6 Although possible cardiac fibrillation or brain stimulation are major safety issues, peripheral nerve stimulation is a practical concern because, if sufficiently intense, it can be intolerable and result in termination of the examination. Recommendations for safety limits on gradient fields state that the system must not have a gradient output that exceeds the limit for peripheral nerve stimulation.5 This will protect against cardiac fibrillation.
Non-biological effects
Rapidly varying fields can induce currents in conductors. Metal objects may heat up rapidly and cause tissue damage. Instances of partial- and full-thickness burns, arising when conducting loops (e.g. ECG electrodes or surface imaging coils) have come into contact with skin, are well recorded.
Radiofrequency field
For whole-body exposures, no adverse health effects are expected if the increase in body core temperature does not exceed 1 °C. In the case of infants and those with circulatory impairment, the temperature increase should not exceed 0.5 °C. With regard to localized heating temperatures, measured temperature in focal regions of the head should be less than 38 °C, of the trunk less than 39 °C, and in the limbs less than 40 °C.5
Recommendations for safety
Detailed MRI safety recommendations have been published by the American College of Radiology.7 It is essential that all MRI units have clear MRI safety policies and protocols, including a detailed screening questionnaire for all patients and staff (Table 1.3).
Table 1.3 Questions which should be included in a screening questionnaire for magnetic resonance imaging patients and staff
| Question to patient or staff member | Action |
|---|---|
| Do you have a pacemaker or have you had a heart operation? | Pacemaker – if present patient must not enter controlled area. |
| Heart operation – establish if any metal valve prosthesis, intravascular device or recent metallic surgical clip insertion and check MR compatibility. | |
| Could you possibly be pregnant? | See text. |
| Have you ever had any penetrating injury, especially to the eyes, even if it was years ago? | Establish details. If necessary arrange X-ray of orbits or relevant area to determine if there is any metallic foreign body. |
| Have you ever had any operations to your head, chest or neck? | Find out details. If any metallic aneurysm or haemostatic clips or metallic prosthesis/implant then check MR compatibility. |
| Do you have any joint replacements or any other body implants? | Check details of surgery and MR compatibility of joint replacement or implant. |
| Have you removed all metal objects and credit cards from your clothing and possessions? | This must be done before entering the controlled area. |
Controlled and restricted access
Access to the scanning suite should be limited. Areas should be designated as restricted (5 gauss line) and, closer to the scanner, controlled (10 gauss line). No patient with a pacemaker should be allowed to enter the restricted area. Any person who enters this area should be made aware of the hazards; in particular the ‘missile effect’. Any person entering the controlled area should remove all loose ferromagnetic materials, such as paperclips and pens, and it is advisable that wristwatches and any magnetic tape or credit cards do not come near the magnet. Other considerations include the use of specially adapted cleaning equipment, wheelchairs and trolleys. Fire extinguishers and all anaesthetic or monitoring equipment must be constructed from non-ferromagnetic materials.
Implants
As mentioned above, persons with pacemakers must not enter the restricted area. All other persons must be screened to ensure there is no danger from implanted ferromagnetic objects, such as aneurysm clips, prosthetic heart valves, intravascular devices or orthopaedic implants. Where an object is not known to be ‘magnet safe’, then the person should not be scanned. Lists of safe and unsafe implants are available8 and should be consulted for each individual object.
Specific questioning is also advisable to assess the risk of shrapnel and intraocular foreign body (Table 1.3).
MAGNETIC RESONANCE IMAGING SEQUENCES
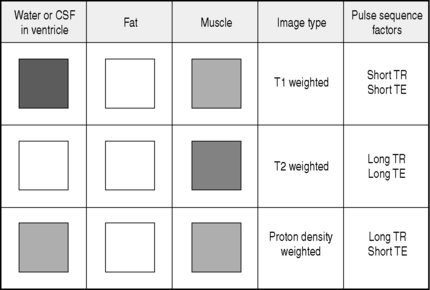
During each MRI examination a series of radiofrequency events (pulses) and magnetic field gradient events (pulses) are applied to the patient that generate nuclear magnetic resonance signals. These signals are processed to produce the image. By varying the duration and timing of the radiofrequency and magnetic field gradient events, a huge range of possible pulse sequences is available which alters the form, speed and information content of the image. The appearance of tissues varies on each pulse sequence. By changing the technical factors, including pulse sequence repetition time (TR) and echo time (TE), the following are some of the basic image types generated in MRI imaging:
The appearance of water, fat and muscle on each image type is illustrated in Figure 1.1.
1 ECRI Hazard Report. Patient death illustrates the importance of adhering to safety precautions in magnetic resonance enviroments. Health Devices. 2001;30(8):311-314.
2 Jehenson P., Duboc D., Lavergne T., et al. Change in human cardiac rhythm induced by a 2T static magnetic field. Radiology. 1988;166:227-230.
3 Chakeres D.W., Kangarlu A., Boudoulas H., et al. Effect of static magnetic field exposure of up to 8T on sequential human vital sign measurements. J. Mag. Res. Imaging. 2003;18(3):346-352.
4 International Electrotechnical Commission. Medical electrical equipment – particular requirements for the safety of magnetic resonance equipment for medical diagnosis. IEC. 1995. revised 2002, 60601–2–33
5 The International Commission on Non-Ionizing Radiation Protection. Medical magnetic resonance (MR) procedures: protection of patients. Health Physics. 2004;87(2):197-216.
6 Nyenhuis J.A., Bourland J.D., Kildishev A.V., et al. Health effects and safety of intense gradient fields. In: Shellock F.G., editor. Magnetic Resonance Procedures: Health Effects and Safety. New York: CRC Press; 2001:31-53.
7 Kanal E., Barkovich A.J., Bell C., et al. ACR guidance document for safe MR practices. Am. J. Roentgenol.. 2007;188:1447-1474.
8 Shellock F.G. Reference Manual for Magnetic Resonance Safety, Implants, and devices. Los Angeles: Biomedical Research Publishing Company, 2007. edn
ULTRASONOGRAPHY
Patient preparation
Endoscopic
Examination of the oesophagus or transoesophageal echocardiography requires preparation similar to upper gastrointestinal endoscopy. The patient should be starved for 4 h prior to the procedure to minimize the risk of vomiting, reflux and aspiration. Anaesthesia and sedation are partly a matter of personal preference of the operator. Local anaesthesia of the pharynx can be obtained using 10% lidocaine spray. Care to avoid overdose is essential as lidocaine is rapidly absorbed via this route. The maximum dose of lidocaine should not exceed 200 mg; the Xylocaine spray metered dose applicator delivers 10 mg per dose. Sedation using i.v. benzodiazepines such as Diazemuls 10 mg or midazolam 2–5 mg may also be necessary. If local anaesthetic has been used then the patient must be instructed to avoid hot food and drink until the effect has worn off (1–2 h).
Children
US examination in children can, in most cases, be performed with no preparation apart from explanation and reassurance to both child and parent. In some cases where the child is excessively frightened or where immobility is required then sedation may be necessary. With echocardiography in infants, sedation is essential to obtain optimal recordings. The sedation regimes described in Chapter 18 may be used.