CHAPTER 4 General indications and contraindications
Peripheral nerve block: indications
Surgery
A thorough knowledge of descriptive and topographic anatomy, especially with regard to nerve distribution, is beyond discussion. It is a condition which anyone desirous of attempting the study of regional anesthesia should fulfil. The anatomy of the human body must, besides, be approached from an angle hitherto unknown to the medical student and with which the average surgeon is not at all familiar.1
Gaston Labat wrote these words at a time when deep ether anesthesia was required to provide adequate muscle relaxation, especially for abdominal surgery. The problems associated with deep ether anesthesia included nausea, vomiting, and atelectasis and subsequent pneumonia. Therefore, the benefits of regional anesthesia were readily apparent. The practice of regional anesthesia still holds attraction, possibly because of its positive effects on secondary outcomes such as postoperative nausea and vomiting, postoperative confusion, and rapid return to ‘street fitness’. Evidence of a positive influence on the ‘hard’ postoperative outcomes of morbidity and mortality is more difficult to come by, although a number of studies have shown benefit in specific circumstances.2–4 Practicing regional anesthesia is also an opportunity for anesthesiologists to employ their individual skills, and so can be an important source of professional satisfaction. Practitioners are responsible for acquainting themselves with the anatomy to which Labat refers and to which a large part of this textbook and DVD-ROM is directed. This knowledge lies at the core of successful regional anesthetic practice and the avoidance of many of its complications.
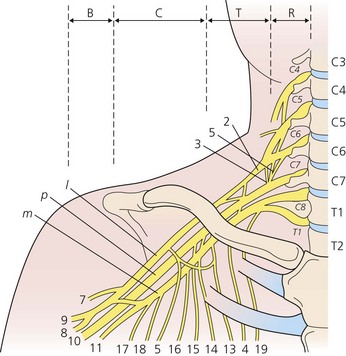
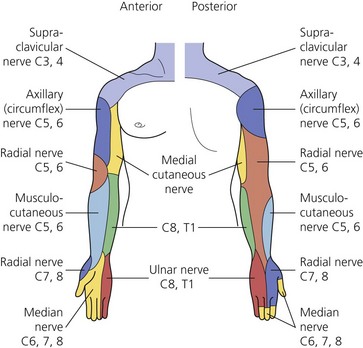
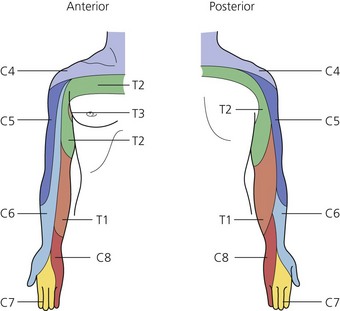
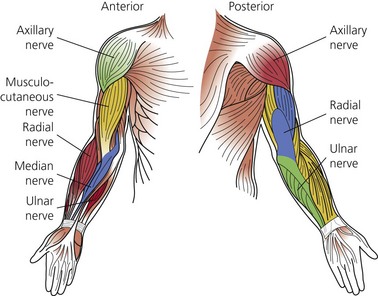
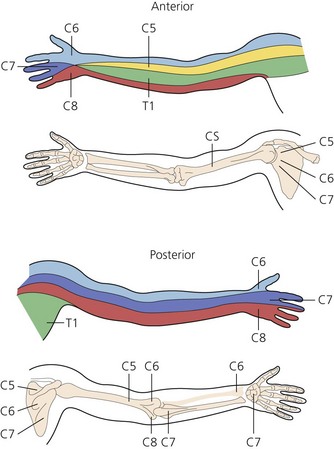
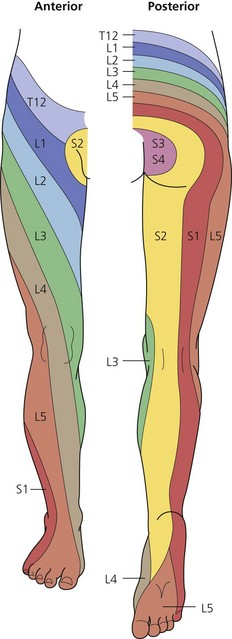
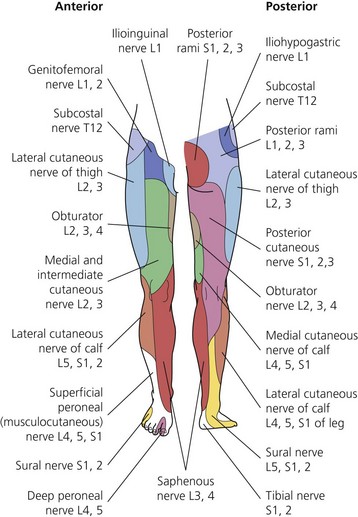
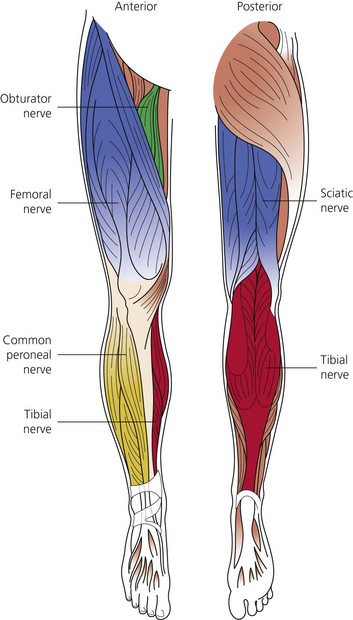
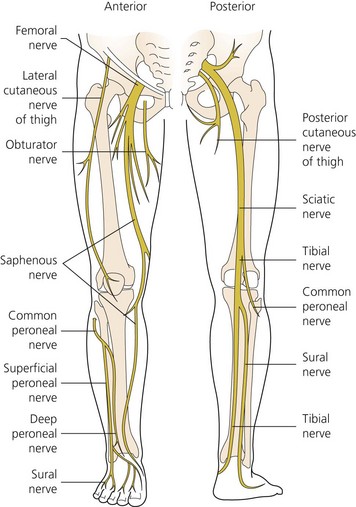
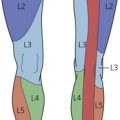
The dermatomes and myotomes of the body and limbs are shown in Figures 4.1–4.9.5 The selection of a regional anesthetic technique appropriate to a particular surgical intervention becomes more straightforward when one can answer the following questions:
Management of acute pain
Pain arises from the direct activation of primary afferent neurons. It is often associated with tissue damage and an inflammatory response, especially in the clinical setting. The inflammatory response has both cellular and neurogenic components. Activation of lymphocytes, macrophages, and mast cells, and the release of neuropeptides such as substance P and neurokinin A result in the further release of inflammatory mediators such as histamine, bradykinin, and the products of arachidonic acid metabolism.6–9 These chemicals can sensitize high-threshold nociceptors to produce the phenomenon of peripheral sensitization. The resultant area of primary hyperalgesia is characterized by an increased responsiveness to thermal and low-threshold mechanical stimuli at the site of injury.
In addition to the area of primary hyperalgesia, a zone of secondary hyperalgesia develops in the uninjured tissues surrounding the site of injury. No changes occur in the threshold to stimuli of the nerves in this area. Changes in the dorsal horn of the spinal cord and elsewhere account for this central sensitization.10 Changes that occur in the dorsal horn in association with central sensitization include an expansion in receptive field size, increased response to stimuli, and a reduction in threshold. These changes are important in the development of both acute and chronic pain.11,12
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their action by blocking the cyclo-oxygenase (COX) enzyme pathway. With traditional agents, this has involved the inhibition of both the COX1 and COX2 isoforms. Reductions in pain scores and opioid requirements have been reported with their use. The COX2 isoform is predominantly induced by the inflammatory process, and the recent development and introduction into clinical practice of specific COX2 inhibitors, holds promise for a reduction in side-effects of these drugs.13 Evidence also exists to support a central mechanism of action of NSAIDs in the modification of pain mechanisms.14
The role of opioid drugs in the modification of central pain mechanisms has been long recognized. They act presynaptically to inhibit the release of neurotransmitters from the nociceptive primary afferent neuron. Peripheral nerves are known to manufacture opioid receptors in the cell body and transport them to both the periphery and the dorsal horn. Following tissue injury, the peripheral receptors become active.15,16 Initial interest in exploiting these features has waned somewhat as equivocal results following the intra-articular administration of morphine to treat arthroscopic procedure-related pain have been published.17
Damage to peripheral nerves results in pathophysiologic changes in the nerves themselves.18 Such damage manifests as spontaneous firing, increased sensitivity to non-noxious stimuli, demyelination, and the sprouting of nerve fibers. These changes form the basis for the development of peripheral chronic pain states. Low concentrations of local anesthetic can reduce ectopic activity in damaged nerves, a feature utilized during their systemic administration for the treatment of neuropathic pain.19 Local anesthetic field block combined with wound infiltration has been shown to significantly reduce pain scores and opioid requirements for up to a week following hernia repair.20 Wound infiltration is an integral part of this technique; however, definitive evidence showing prevention in the development of the above changes remains lacking.
The concept and effectiveness of pre-emptive analgesia remain controversial.21 At its heart, however, is the hypothesis that the prevention of noxious inputs occurring during and after surgery will prevent the development of central sensitization. Although it has been demonstrated that early postoperative pain is a predictor of long-term pain, it is not known what degree of noxious input is required, or for how long it must be present to produce long-term changes in the nervous system.22 The logic of combining NSAIDs, opioids, and a regional anesthetic technique (with or without perineural catheter) appears self-evident, yet definitive evidence of benefit in the clinical setting is lacking, and the standardization of study methods is required to allow firm conclusions to be drawn.23–25
Chronic pain
The indications for somatic peripheral nerve block in the management of chronic pain are limited, and the results require careful interpretation. A common indication has been to determine the likelihood of success following surgical decompression or neurolysis of a peripheral nerve. Small volumes of local anesthetic need to be used in this setting to prevent spread to other nerves, and long-acting agents allow one to differentiate the results from the placebo effect, which in itself tends to be short-lived.26
Continuous nerve block
Continuous catheter techniques are gaining widespread use in a number of clinical settings. These include acute pain relief in the inpatient and ambulatory settings, early postoperative rehabilitation, continuous sympathectomy following re-implantation procedures, and the diagnosis and treatment of chronic pain syndromes.27–31 Indeed, there have been published reports of improved surgical outcomes with these techniques, in addition to improved secondary outcomes.32
The concerns regarding continuous techniques have related to infection, catheter migration, high plasma levels of local anesthetic, local myelotoxicity, and neurologic complications. Infection has been reported, yet despite a colonization rate of up to 27%, overt problems appear to be rare.33 Catheter migration can be detected early with regular and routine examination of catheter site and assessment of the nerve block.
Plasma levels of local anesthetic may rise progressively during an infusion. Although the peri-operative rise in α1-acid glycoprotein (GP) has been shown to ameliorate the effect, a seizure rate of 1.2 per 1000 procedures has been reported.34,35 Postoperative protocols, education of carers, and patient cooperation are necessary to detect the early signs of local anesthetic toxicity and ensure the optimal use of this technology.
The incidence of neurologic complications is less than 1% with the use of perineural catheters. This is similar to the rates recorded following multiple single-dose techniques.36 Whether the incidence of complications can be reduced further using ultrasound to guide catheter placement remains to be seen. It should be noted that catheter techniques are often used in major joint surgery such as distraction interposition arthroplasty, which carries an inherent high risk of nerve injury.37
Peripheral nerve block: contraindications
Anticoagulant medication
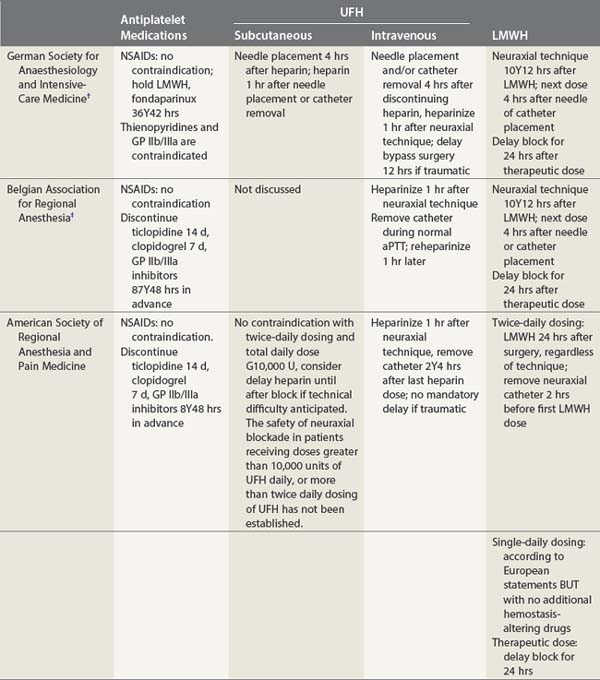
Hematoma formation following peripheral nerve block is considered to be uncommon and usually of little importance. However, it can produce significant patient discomfort, persistent paresthesias, and occasionally be severe and extensive. The administration of anticoagulant medication is a risk factor for the development of prolonged bleeding following venous or arterial puncture. Precautions to be observed for the peri-operative use of anticoagulants have been outlined by the American Society of Regional Anesthesia and Pain Medicine (see Table 4.1, Guidelines) as well as equivalent organizations outside the USA.38 These guidelines have been informed by the contrasting US and European experiences in neuraxial block, specifically in relation to the occurrence of spinal and epidural hematoma. Until and unless further studies suggest otherwise, it appears prudent to observe the same recommendations when performing peripheral nerve block, particularly if the nerves are deep or lie in proximity to non-compressible vessels.
Heparin
Intravenous heparin has a half-life of 1.5–2 h and is cleared within 4–6 h of administration. It stimulates the formation of antithrombin III, which forms a complex with activated thrombin, thus neutralizing thrombin activity and preventing the conversion of fibrinogen to fibrin. Its effects can be reversed with protamine; 1 mg per 100 U of heparin. Protamine forms an inactive complex with heparin. The activity of heparin can be measured with the activated partial thromboplastin time (APTT) and the activated clotting time, both being sensitive tests of heparin function. There is a wide variation in dose responses between individuals; this variation is further affected by diet, liver function, renal function, and cardiac status.40 Laboratory testing should be performed on patients who have received heparin prior to nerve block.
Respiratory disease
The phrenic nerve (C3, 4, 5) is a branch of the cervical plexus, its three roots usually joining at the lateral border of the scalenus anterior muscle. The nerve passes across the anterior aspect of the muscle and descends to enter the thorax, having passed between the subclavian artery and vein. The incidence of ipsilateral phrenic nerve paresis following supraclavicular block ranges from 36%, regardless of technique used, to 100% with the interscalene approach.41,42 With ultrasonographic assessment, this 100% incidence remains, despite a reduction in the mass of local anesthetic used. A 25% reduction in forced vital capacity (FVC) and forced expiratory volume in 1s (FEV1), as well as a reduction in peak expiratory flow rate (PEFR), can be expected following interscalene block. This persists for the duration of action of the anesthetic agent.43,44 The patient with normal pulmonary function can tolerate this embarrassment easily. However, those with poor respiratory reserve are at risk of developing acute respiratory failure. The wisdom of performing these blocks in such patients must be questioned, and bilateral blocks are absolutely contraindicated. An FEV1 < 1 L, FVC < 15–20 mL/kg, FEV/FVC < 35%, PEFR < 100 L/min, and pCO2 > 50 mmHg are predictors of serious respiratory compromise following supraclavicular block.45 Further absolute contraindications to interscalene brachial plexus block include a history of pre-existing contralateral hemidiaphragmatic paralysis or contralateral pneumonectomy.
Any procedure in which a needle is directed toward the lung carries a risk of pneumothorax. The incidence of pneumothorax with supraclavicular blocks has variously been reported as being 6–25%.46,47 A sudden cough or inspiratory effort should alert the operator to the possibility of pneumothorax, because the symptoms and signs may not develop for hours or until the pneumothorax reaches 20% of lung volume. Radiographic evidence may take 24 h to develop. Interest in the supraclavicular block has been resurrected in more recent times with the widespread adoption of ultrasound-guided techniques. The ability to identify vital structures in addition to relevant nerves holds the promise of this becoming a safe block in the hands of appropriately trained and experienced practitioners.
The performance of intercostal or paravertebral nerve block for analgesia is preferable to no analgesia or high-dose narcotics, especially in the elderly. Dilute solutions sufficient to provide analgesia without significant motor blockade should be advocated, because case reports of respiratory failure secondary to intercostal motor block and without pneumothorax following intercostal block have appeared.48,49
Multiple sclerosis
Multiple sclerosis is a demyelinating disease of the brain and spinal cord characterized by a series of remissions and exacerbations occurring over many years. Multiple sclerosis does not affect the peripheral nervous system. Epidural and, more especially, spinal anesthesia have been implicated in exacerbations of multiple sclerosis.50 Theories to explain this suggest that demyelinated nerves may be more susceptible to the neurotoxic effects of local anesthetic agents.51 While peripheral nerve block is performed at a ‘safe’ distance from the disease process of multiple sclerosis, there always exists the potential for exacerbations secondary to stress or infection in the peri-operative period. Patients should be informed of this and their neurologic status documented before and after any intervention.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is a degenerative disease of upper and lower motor neurons and the motor nuclei of the brainstem. Its cause is unknown. Amyotrophic lateral sclerosis is associated with bulbar muscle weakness, the risk of aspiration, autonomic system dysfunction, and poor ventilatory reserve. Little information exists on the safety of performing peripheral nerve blocks in patients with amyotrophic lateral sclerosis. Epidural block has been successfully employed, suggesting that it may be safe to use local anesthetic agents in this group.52
Myasthenia gravis
The following factors identify patients at high risk of respiratory compromise and the need for postoperative ventilation:53
Guillain–Barré syndrome
Guillain–Barré syndrome is an acute demyelinating disease of the peripheral nervous system. An autoimmune mechanism following a recent viral illness is thought to be responsible. It is characterized by the cephalad progression of flaccid paralysis, respiratory weakness, and bulbar and autonomic dysfunction; 20% of patients have residual neurologic deficits. Epidural anesthesia has been employed successfully in this population, although the hemodynamic changes that may occur and an exaggerated response to indirect vasopressors may render this a high-risk intervention.54
Diabetes mellitus
Diabetes is a disease that produces multi-organ dysfunction. In many respects it may be preferable to proceed with a regional anesthesia technique in these patients. The risk of peri-operative myocardial ischemia, hypoglycemia, autonomic dysfunction, and possible difficult intubation would make this so. Unfortunately, the peripheral neuropathy common to diabetes may involve the area to be blocked. Careful mapping of any neurologic deficit is therefore necessary. Motor responses may be difficult to elicit at normal nerve stimulator settings, and sensation may not be fully intact, heightening the risk of nerve injury. In addition, coma, secondary to a central conduction block of the normal physiologic response to hypoglycemia, has been reported.55 This further highlights the dangers faced by patients with diabetes.
Summary
The contraindications to peripheral nerve block are broadly summarized in Table 4.2. The reader is advised to consult the text relating to specific blocks for further detail of individual contraindications.
| Absolute | Relative |
|---|---|
1 Labat G. Regional anesthesia: techniques and clinical applications. Philadelphia: WB Saunders; 1922. 2
2 Sorensen RM, Pace NL. Anesthetic techniques during surgical repair of femoral neck fractures: a meta-analysis. Anesthesiology. 1992;77:1095-1104.
3 Urwin SC, Parker MJ, Griffiths R. General versus regional anesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84:450-455.
4 Sharrock NE, Cazan MG, Hargett MJL, et al. Changes in mortality after total hip and knee arthroplasty over a ten year period. Anesth Analg. 1995;80:242-248.
5 Gaertner E, Navez M-L, Aknin P, et al. Anesthésie régionale: anesthésie tronculaire et plexique de l’adulte. Paris: Arnette Groupe Liaisons; 2001.
6 Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273-2286.
7 Dray A, Urban L, Dickenson A. Pharmacology of chronic pain. Trends Pharmacol Sci. 1994;15:190-197.
8 Forster RW, Ramage AG. The action of some chemical irritants on somatosensory receptors of the cat. Neuropharmacology. 1981;20:191-198.
9 Perl ER. Sensitization of nociceptors and its relation to sensation. In: Bonica JJ, AlbeFessard D, editors. Advances in pain research and therapy. New York: Raven Press; 1976:17-28.
10 Bennett GJ, Kajander KC, Sahara Y, et al. Neurochemical and anatomical changes in the dorsal horn of rats with an experimental painful peripheral neuropathy. In: Cervero F, Bennett GJ, Headley PM, editors. Processing of sensory information in the superficial dorsal horn of the spinal cord. Amsterdam: Plenum Press; 1989:463-471.
11 Dubner R, Ren K. Central mechanisms of thermal and mechanical hyperalgesia following tissue inflammation. In: Boivi J, Hansson P, Lindblom U, editors. Touch, temperature and pain in health and disease: mechanisms and assessments, Vol. 3. Seattle: IASP Press; 1994:267.
12 Wilcox GL. Excitatory neurotransmitters and pain. In: Bond MR, Charlton JE, Woolf CJ, editors. Proceedings on the 6th World Congress on Pain. Pain research and clinical management series, Vol. 4. Amsterdam: Elsevier; 1991:97-117.
13 Siebert K, Zhang Y, Leahy K, et al. Pharmacological and biological demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994;91:12013-12017.
14 Walker JS. NSAID: an update on their analgesic effects. Clin Exp Pharmacol Physiol. 1995;22:855-860.
15 Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993;76:182-191.
16 Stein C, Millan MJ, Shippenberg TS, et al. Peripheral opioid receptors mediating antinociception in inflammation: evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269-1275.
17 Aasbo V, Raeder JC, Grogaard B, et al. No additional analgesic effect of intraarticular morphine or bupivacaine compared with placebo after elective knee arthroscopy. Acta Anaesthesiol Scand. 1996;40:585-588.
18 Devor M. The pathophysiology of damaged peripheral nerves. In: Wall PD, Melzack R, editors. Textbook of pain. 3rd edn. London: Churchill Livingstone; 1994:79-100.
19 Backonja MM. Local anesthetics as adjuvant analgesics. J Pain Symptom Manage. 1994;9:491-499.
20 Dahl JB, Moiniche S, Kehlet H. Wound infiltration with local anesthetics for postoperative pain relief [editorial]. Acta Anaesthiol Scand. 1994;38:7-14.
21 Kissin I. Preemptive analgesia: why its effect is not always obvious. Anesthesiology. 1996;84:1015-1019.
22 Katz J, Jackson M, Kavanagh BP, et al. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50-55.
23 Breivik H. Pre-emptive analgesia. Curr Opin Anesth. 1994;7:458-461.
24 Bridenbaugh PO. Pre-emptive analgesia – is it clinically relevant? Anesth Analg. 1994;78:203-204.
25 Pedersen JL, Crawford ME, Dahl JB, et al. Effect of pre-emptive nerve block on inflammation and hyperalgesia after human thermal injury. Anesthesiology. 1996;84:1020-1026.
26 Hogan QH, Abram SE. Diagnostic and prognostic neural blockade. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 3rd edn. Philadelphia: Lippincott-Raven; 1998:837-877.
27 Tuominen M, Haasio J, Hekali R, et al. Continuous interscalene brachial plexus block: clinical efficacy, technical problems, and bupivacaine plasma concentrations. Acta Anaesthesiol Scand. 1989;33:84-88.
28 Grant SA, Nielsen KC, Greengrass RA, et al. Continuous peripheral nerve block for ambulatory surgery. Reg Anesth Pain Med. 2001;26:209-214.
29 O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37:179-188.
30 Taras JS, Behrman MJ. Continuous peripheral nerve block in replantation and revascularization. J Reconstr Microsurg. 1998;14:17-21.
31 Sarma VJ. Long-term continuous axillary plexus blockade using 0.25% bupivacaine: a study of 3 cases. Acta Anaesthesiol Scand. 1990;34:511-513.
32 Capdevila X, Barthelet Y, Biboulet PH, et al. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8-15.
33 Gaumann DM, Lennon RL, Wedel DJ. Continuous axillary block for postoperative pain management. Reg Anesth. 1988;13:77-82.
34 Bergman BD, Hebl JR, Kent J, et al. Neurologic complications of 405 consecutive continuous axillary catheters. Anesth Analg. 2003;96:247-252.
35 Brown DL, Ransom DM, Hall JA, et al. Regional anesthesia and local anesthetic–induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesth Analg. 1995;81:321-328.
36 Horlocker TT, Kufner RP, Bishop AT, et al. The risk of persistent paresthesia is not increased with repeated axillary block. Anesth Analg. 1999;88:382-387.
37 Cheng SL, Morrey BF. Treatment of the mobile, painful arthritic elbow by distraction interposition arthroplasty. J Bone Joint Surg Br. 2000;82:233-238.
38 Horlocker TT. Regional anesthesia in the anticoagulated patient: defining the risks. The Second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation. Reg Anesth Pain Med. 2003;28:172-197.
39 Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. (The third ASRA and PMEB guidelines neuraxial anesthesia and anticoagulation). Reg Anesthesia Pain Med. 2010;35:92-94.
40 Cooke ED. Monitoring during low-dose heparin prophylaxis. N Engl J Med. 1976;294:1066-1067.
41 Farrar MD, Scheybani M, Nolte H. Upper extremity block effectiveness and complications. Reg Anesth. 1981;6:133-134.
42 Urmey WF, Talts KH, Sharrock ME. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia diagnosed by ultrasonography. Anesth Analg. 1991;73:498-503.
43 Pere P, Pitkanen M, Rosenberg P. Effect of continuous interscalene brachial plexus block on diaphragm motion and on ventilatory function. Acta Anaesthesiol Scand. 1992;36:53-57.
44 Urmey WF, McDonald M. Hemidiaphragmatic paresis during interscalene brachial plexus block: effect on pulmonary function and chest wall mechanics. Anesth Analg. 1992;74:352-357.
45 McIntyre JWR. Regional anesthesia safety. In: Finucane BT, editor. Complications of regional anesthesia. Philadelphia: Churchill Livingstone; 1999:1-30.
46 Brand L, Papper EM. A comparison of supraclavicular and axillary techniques for brachial plexus blocks. Anesthesiology. 1961;22:226-229.
47 De Jong RH. Local anesthetics adverse effects. In: Chambers C, editor. Local anesthetics. Springfield, IL: Charles C Thomas; 1977:254.
48 Casey WF. Respiratory failure following intercostal nerve blockade. Anaesthesia. 1984;39:351-354.
49 Cory PC, Mulroy MF. Postoperative respiratory failure following intercostal block. Anesthesiology. 1981;54:418-419.
50 Bamford C, Sibley W, Laguna J. Anesthesia in multiple sclerosis. Can J Neurol Sci. 1978;5:41-44.
51 Schapira K. Is lumbar puncture harmful in multiple sclerosis? J Neurol Neurosurg Psychiatr. 1959;22:238.
52 Kochi T, Oka T, Mizuguchi T. Epidural anesthesia for patients with amyotrophic lateral sclerosis. Anesth Analg. 1989;68:410-412.
53 Leventhal SR, Orkin FK, Hirsch RA. Prediction of the need for postoperative mechanical ventilation in myasthenia gravis. Anesthesiology. 1980;53:26-30.
54 McGrady EM. Management of labour and delivery in a patient with Guillain–Barré syndrome. Anaesthesia. 1987;42:899.
55 Romano E, Gullo A. Hypoglycemic coma following epidural analgesia. Anaesthesia. 1980;35:1084-1086.