CHAPTER 5 Complications, toxicity, and safety
Complications and toxicity
Principles underlying complications and errors
Human factors can be defined as lapses in, or lack of, safe habit, or the occurrence of a vigilance decrement resulting from sleep deprivation, fatigue, recent alcohol or drug ingestion, or boredom. Inexperience on the part of the anesthesiologist is likely to contribute to poor decision-making or an error in judgment. An important component in the avoidance of complications arising from these factors is awareness of anesthesiologists as individuals and of their role in complication development. They should thus seek to establish safe working practices and individual self-discipline that may act to counterbalance these risks.1–3
Central nervous system toxicity
The signs and symptoms of local anesthetic-induced CNS toxicity are shown in Figure 5.1. An initial phase of CNS excitability, as demonstrated by light-headedness, dizziness, visual and auditory disturbance, muscle twitching, and convulsions, is followed by CNS depression, with coma, then respiratory depression and arrest. This sequence of events occurs because of an initial inhibition, at lesser concentrations, of inhibitory pathways in the amygdala. At greater concentrations, both inhibitory and excitatory pathways are inhibited, resulting in generalized CNS depression.5–8
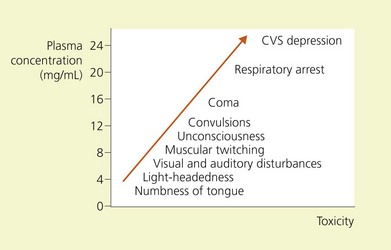
Figure 5.1 Relations of signs and symptoms of local anesthetic toxicity to plasma concentrations of lidocaine.
(From Ref. 4, Covino BG, Wildsmith JAW. Clinical pharmacology of local anesthetic agents. In: Cousins MJ, Bridenbaugh PO (eds). Neural blockade in clinical anesthesia and management of pain, 3rd edn. Philadelphia: © Lippincott-Raven; 1998.)
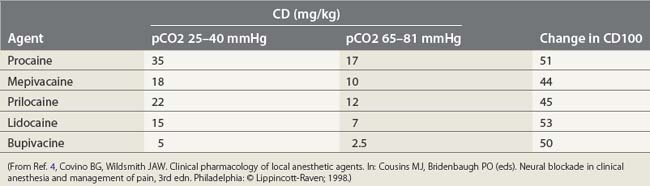
The toxic potential of each anesthetic drug is related to its potency as an anesthetic agent (Table 5.1),9 and the rate at which it is injected or absorbed (Fig. 5.2). Hypercapnia and acidosis lower the convulsive threshold for local anesthetic drugs. This occurs in a number of ways. A high pCO2 will increase cerebral blood flow, resulting in greater rates of drug delivery; decreased intracellular pH facilitates the formation of the cationic form of drug, i.e. the active form; and hypercapnia and acidosis result in diminished protein-binding of drug, thereby making available a greater proportion of free drug.10
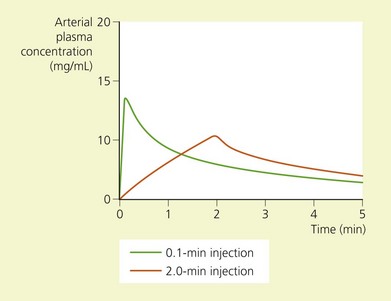
(From Ref. 4, Covino BG, Wildsmith JAW. Clinical pharmacology of local anesthetic agents. In: Cousins MJ, Bridenbaugh PO (eds). Neural blockade in clinical anesthesia and management of pain, 3rd edn. Philadelphia, © Lippincott-Raven; 1998.)
Local anesthetic-induced seizures are effectively terminated by administration of barbiturate or benzodiazepine drugs.11,12 The doses required are small and one should remain mindful that their myocardial depressant effects are additive to those of local anesthetic drugs.
Cardiovascular system toxicity
The depolarization phase of the action potential in cardiac tissue differs from nerve tissue in that the fast influx of Na+ is followed by a slow influx of Ca2+. This influx of Ca2+ is responsible for the spontaneous depolarization that is characteristic of cardiac tissue (Fig. 5.3, Table 5.2). Local anesthetic drugs depress the maximal depolarization rate of the cardiac action potential, Vmax, secondary to inhibition of Na+ conductance. With increasing concentrations of local anesthetics, prolongation of conduction times occurs, producing an increase in the P–R interval and QRS duration. At greater concentrations this is followed by sinus bradycardia, sinus arrest, and atrioventricular dissociation.14,15 Local anesthetics also profoundly depress cardiac contractility, a phenomenon that may be related to the displacement of Ca2+ from the sarcolemma.16–18
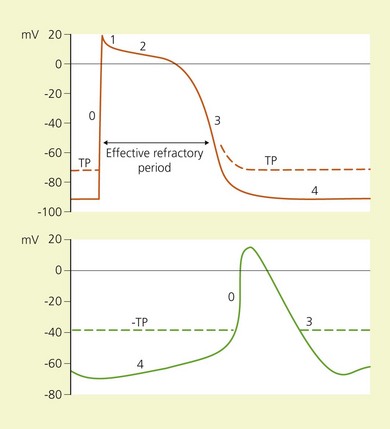
(From Ref. 13, Stoelting RK. Heart. In: Pharmacology and physiology in anesthetic practice. 2nd ed. Philadelphia, © JB Lippincott; 1991.)
Table 5.2 Ion movement during phases of the cardiac action potential
| Phase | Ion | Movement across cell membrane |
|---|---|---|
| 0 | Na+ | In |
| 1 | K+ | Out |
| Cl− | In | |
| 2 | Ca2+ | In |
| K+ | Out | |
| 3 | K+ | Out |
| 4 | Na+ | In |
(From Ref. 13, Stoelting RK. Heart. In: Pharmacology and physiology in anesthetic practice. 2nd ed. Philadelphia: © JB Lippincott; 1991.)
The CC/CNS ratio is that of the dosage required for cardiovascular collapse (CC) to the dosage required to produce convulsions. It is approximately 7.1 for lidocaine and 3.7 for bupivacaine, suggesting a greater margin of safety in the use of lidocaine.19 The high lipid-solubility of bupivacaine results in a slow rate of dissociation from the tissues, and thus a persistent effect on Vmax. Cardiovascular collapse resulting from bupivacaine is therefore resistant to treatment. The potential for cardiac toxicity is enhanced in pregnancy, for reasons not fully understood, and also in the presence of hypoxia and hypercapnia.20–22 These factors enhance the toxic potential of bupivacaine to a greater degree than they do lidocaine.
Peripheral vasculature
Local anesthetic drugs have a biphasic action on vascular smooth muscle.23 At low concentrations they produce vasoconstriction. As the concentration increases, the effect becomes one of vasodilatation. These observations have been explained as being due to stimulation of spontaneous myogenic activity at low concentrations and inhibition of the same at greater concentrations.
Nerve injury
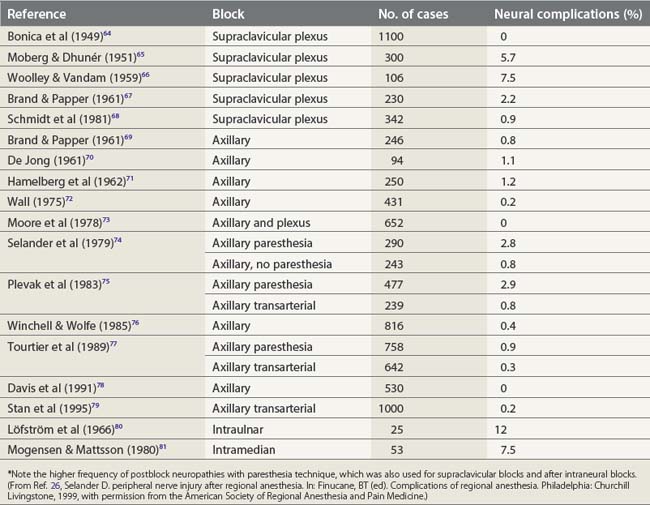
Nerve stimulation is one effective technique for locating a peripheral nerve. Prospective studies have demonstrated that a paresthesia technique can significantly increase the risk of postblock neuropathies (2.8%), while the transarterial approach to the brachial plexus is associated with paresthesia in as many as 40% of cases,24,25 producing neuropathy in 0.8% (Table 5.3). In contrast, a nerve stimulation technique aims to avoid nerve contact and has been shown to produce important block-related neuropathies in only 0–0.3% of cases.27
The risk of penetrating a nerve fascicle is reduced when a short-bevel (45°) needle is used, compared with a standard long-bevel (15°) needle, the reason being that nerve fascicles tend to roll away more readily from the advancing short-bevel needle tip.28 Although the incidence of injury is less with short-bevel needles, when injury does occur it is more severe.
Intraneural needle position is associated with painful paresthesias on injection, and intraneural injection causes nerve damage and cell death by mechanical disruption, disruption of the blood–nerve barrier, high endoneural pressure (above capillary perfusion pressure) (Fig. 5.4), and direct neurotoxicity of local anesthetic agent. This situation is further aggravated if the solution contains epinephrine.29,30 Therefore it is important to maintain verbal contact with the patient, avoid paresthesias, administer small incremental doses of drug, and reposition the needle if paresthesias are elicited.
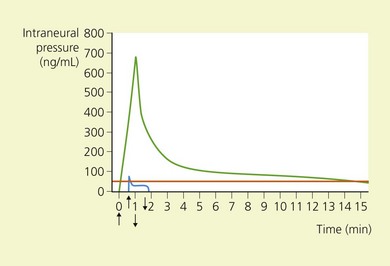
(From Ref. 26, Selander D. Peripheral nerve injury after regional anesthesia. In: Finucane, BT (ed). Complications of regional anesthesia. Philadelphia, Churchill Livingstone, 1999, with permission from the American Society of Regional Anesthesia and Pain Medicine.)
In attempting to establish the etiology of nerve lesions in the postoperative period, the differential diagnosis must initially take into account patient positioning, tourniquet use, surgical trauma, and the presence of tight casts or dressings.31–33 Follow-up of the patient in the immediate postoperative period will help to avoid inaccurate labeling of the deficit as ‘anesthesia-related’.
Allergic reactions
Allergic reactions to local anesthetics occur rarely.34 Indeed, most ‘allergic reactions’ to local anesthetics are in fact adverse reactions. Nevertheless, para-aminobenzoic acid is a product of the hydrolysis of ester local anesthetics and is a known allergen.35–37 Allergy to amide local anesthetics is still rarer. However, some preparations contain methylparaben (an allergen), because of its excellent bacteriostatic and fungistatic properties.38 After a case of allergy to a local anesthetic agent, intradermal testing of the full range of anesthetic agents is worthwhile, because allergy to one agent does not necessarily imply allergy to another.37,39
Infection
The presence of infection at the site of puncture is generally accepted as being a contraindication to regional anesthesia. The paucity of reports detailing infective complications of peripheral nerve block suggests that local and generalized infections following nerve blocks are rare. Disastrous infective complications continue to be reported following central neuraxial block, however, and the increasing use of peripheral nerve catheters suggests some elementary precautions be taken in this regard.40 Unfortunately, no recommendations exist as to aseptic technique for spinal, epidural, or peripheral nerve block.41 A review of the literature serves to highlight the following points:40
Neural toxicity of local anesthetics
All clinically used local anesthetic agents are potentially toxic at high concentrations. Under normal conditions, the drug is rapidly diluted and absorbed. However, if the nerve is ischemic or the drug is injected intraneurally, the nerve is exposed to greater than normal concentrations of local anesthetic and for a longer than expected period of time. This situation is exacerbated with epinephrine-containing solutions.55 Lidocaine was shown to have a greater neurotoxic potential in this regard than the other clinically used agents.
Myotoxicity of local anesthetics
Injection of local anesthetic into muscle results in focal necrosis; the more potent the agent, the greater the degree of injury that results. This effect is localized and regeneration has been shown to be complete within 2 weeks. The changes are of a subclinical nature and do not appear to contribute to the peri-operative morbidity of regional anesthesia.56 Some concern has been raised, however, regarding the role of local anesthetic drugs in the development of diplopia following cataract surgery performed under regional anesthesia. A 0.25% incidence of diplopia related to anesthetic factors has been reported.57 It appears to be more common following peribulbar block than retrobulbar block and does not occur following topical or general anesthesia. The inferior rectus muscle is typically involved following infraorbital injection. Possible mechanisms underlying this complication are direct muscle injury from the block needle, vascular compromise secondary to elevated local pressures, and myotoxicity of the local anesthetic.
Safe conduct of regional anesthesia
Patient selection
When general anesthesia poses serious risks – for example in the patient with a full stomach, difficult airway, or poor general medical condition – regional anesthesia is quite often the anesthetic technique of choice. However, there are various medical conditions where the choice of anesthetic technique remains controversial. These include degenerative neurologic disease, diabetes, and severe cardiovascular and respiratory disease. These conditions are dealt with in more detail in Chapter 4. As for all patients, a thorough pre-anesthetic medical and laboratory evaluation is indicated in order to perform an appropriate risk–benefit analysis.
Morbid obesity, physical deformities, arthritis, fractures, local infection, or locally enlarged lymph glands may serve to hinder the administration of an adequate block and so should be noted prior to the formulation of an anesthetic plan. The disoriented or psychologically deranged patient may not only make intra-operative management difficult, but render the safe performance of a block impossible. A history of concurrent medication, such as anticoagulants (Box 5.1) or vasoactive drugs, should also be taken into account because of the implications for the performance and administration of regional anesthesia (see Ch. 4).
Box 5.1
Precautions for combined use of anticoagulants and neuraxial block
(From Ref. 58, Vandermeulen EP, VanAken H, Vermylen J: Anticoagulants and spinal-epidural anesthesia. Anesth Analg 1994; 79: 1165.)
The patient interview allows one to obtain the relevant information outlined above; it is also an opportunity to prepare the patient psychologically for the procedure and the peri-operative experience in general and to obtain informed consent. Informed consent implies that the material risks and benefits of the proposed procedure have been explained, as well as those of the available alternatives. When one explains the reasons for choosing one technique over another, and when appropriate assurances as to the standard of care are given, most patients will accept regional anesthesia. Assurances as to the availability of block supplementation and sedation up to the level of general anesthesia should be given, as well as a detailed description of the performance of the block(s). Patients may perceive sensations of an unusual or bizarre nature under regional anesthesia; therefore, explanation, reassurance, and appropriate sedation should be provided. Most patients who have benefited from a well-executed regional anesthetic will choose the same technique for future interventions when possible.59
Pre-operative fasting
Traditionally, adult patients scheduled for a surgical intervention were required to abstain from both oral solids and fluids for a minimum of 6h, and, more often, from midnight the night before. However, unrestricted clear fluids (water and apple juice) up to 2 h before surgery have been shown to result in no significant difference in mean residual gastric volume or pH.60 As a consequence, a number of organizations have amended their fasting guidelines (Table 5.4).61
Table 5.4 Example of fasting protocol for sedation and analgesia for elective procedures*
| Patient group | Solids and non-clear liquids† | Clear liquids |
|---|---|---|
| Adults | 6–8 h or none after 12 midnight‡ | 2–3 h |
| Children >36 months old | 6–8 h | 2–3 h |
| Children 6–36 months old | 6 h | 2–3 h |
| Children <6 months old | 4–6 h | 2 h |
* Gastric emptying may be influenced by many factors including anxiety, pain, abnormal autonomic function (e.g. diabetes), pregnancy, and mechanical obstruction. Therefore the suggestions above do not guarantee that complete gastric emptying has occurred. Unless contraindicated, children should be offered clear liquids until 2–3 h before sedation to minimize the risk of dehydration.
† This includes milk, formula, and breast milk. (High fat content may delay gastric emptying.)
‡ There are no data to establish whether a 6–8-h fast is equivalent to an overnight fast prior to sedation or analgesia.
(From the American Society of Anesthesiologists,61 with permission of ASA.)
Equipment
In 1986, the Department of Anesthesia of Harvard Medical School published detailed, mandatory standards for minimal patient monitoring during anesthesia.62 For the safe conduct of regional anesthesia, in addition to the presence of an anesthesiologist or nurse anesthetist, the following equipment should be available and used:
The equipment included in Box 5.2 should also be available for immediate use, and a checklist performed prior to anesthesia. In addition, the nerve stimulator to be used should be checked according to the manufacturer’s instructions prior to each use.
Box 5.2
Pre-anesthetic checklist
(From Ref. 63, McIntyre JWR. Regional Anesthesia Safety. In: Finucane BT (ed). Complications of Regional Anesthesia. Philadelphia, Churchill Livingstone, 1999, with permission from the American Society of Regional Anesthesia and Pain Medicine.)
Injection and safe practice
Once the needle has been correctly located, slow, deliberate injection should be made of no more than 5 mL at a time. The injection should be discontinued and the needle repositioned immediately if the patient complains of pain or paresthesia. Low pressure local anesthetic injections can be made by using the compressed air injection technique.82
1 Cooper JB, Gaba DM. A strategy for preventing accidents. Int Anesthesiol Clin. 1989;27:148-152.
2 Weinger MB, Englund CE. Ergonomic and human factors affecting anesthetic vigilance and monitoring performance in the operating room environment. Anesthesiology. 1990;73:995-1021.
3 Gaba DM. Human error in anesthetic mishaps. Int Anesthesiol Clin. 1989;27:137-147.
4 Covino BG, Wildsmith JAW. Clinical pharmacology of local anesthetic agents. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 3rd edn. Philadelphia: Lippincott-Raven; 1998:97-128.
5 DeJong RH, Robles R, Corbin RW. Central actions of lidocaine – synaptic transmission. Anesthesiology. 1969;30:19-23.
6 Huffman RD, Yim GKW. Effects of diphenylaminoethanol and lidocaine on central inhibition. Int J Neuropharmacol. 1969;8:217-225.
7 Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidocaine. Nature. 1966;209:207-208.
8 Wagman IH, DeJong RH, Prince DA. Effects of lidocaine on the central nervous system. Anesthesiology. 1967;28:155-172.
9 Liu PL, Feldman HS, Giasi R, et al. Comparative CNS toxicity of lidocaine, etidocaine, bupivacaine and tetracaine in awake dogs following rapid IV administration. Anesth Analg. 1983;62:375-379.
10 Englesson S. The influence of acid–base changes on central nervous system toxicity of local anesthetic agents. Acta Anaesthesiol Scand. 1974;18:79-87.
11 Covino BG. Toxicity and systemic effects of local anesthetic agents. In: Strichartz G, editor. Local anesthetics, handbook of experimental pharmacology, Vol. 81. New York: Springer-Verlag; 1987:187-209.
12 Davis NL, DeJong RH. Successful resuscitation following massive bupivacaine overdose. Anesth Analg. 1982;61:62-64.
13 Stoelting RK. Heart. In: Pharmacology and physiology in anesthetic practice, 2nd edn. Philadelphia: JB Lippincott; 1991.
14 Lieberman NA, Harris RS, Katz RI, et al. The effects of lidocaine on the electrical and mechanical activity of the heart. Am J Cardiol. 1968;22:375-380.
15 Sugimoto T, Schaal FS, Dunn NM, et al. Electrophysiological effects of lidocaine in awake dogs. J Pharmacol Exp Ther. 1969;166:146-150.
16 Block A, Covino BG. Effect of local anesthetic agents on cardiac conduction and contractility. Reg Anesth. 1981;6:55-61.
17 Feldman HS, Covino BG, Sage DJ. Direct chronotropic and inotropic effects of local anesthetic agents in isolated guinea pig atria. Reg Anesth. 1982;7:149-156.
18 Josephson I, Sperelakis N. Local anesthetic blockade of Ca2+-mediated action potentials in cardiac muscle. Eur J Pharmacol. 1976;40:201-208.
19 Morishima HO, Pederson H, Finster M, et al. Is bupivacaine more cardiotoxic than lidocaine? Anesthesiology. 1983;59:A409.
20 Morishima HO, Pederson H, Finster M, et al. Bupivacaine toxicity in pregnant and nonpregnant ewes. Anesthesiology. 1985;63:134-139.
21 Sage DJ, Feldman HS, Arthur GR, et al. Influence of lidocaine and bupivacaine on isolated guinea pig atria in the presence of acidosis and hypoxia. Anesth Analg. 1984;63:1-7.
22 Thigpen JW, Kotelko DM, Shnider SM, et al. Bupivacaine cardiotoxicity in hypoxic-acidotic sheep. Anesthesiology. 1983;59:A204.
23 Blair MR. Cardiovascular pharmacology of local anesthetics. Br J Anaesth. 1975;47:247-252.
24 Selander D, Edshage S, Wolff T. Paresthesiae or no paresthesiae? Nerve lesions after axillary blocks. Acta Anaesth Scand. 1979;23:27-33.
25 Plevak DJ, Linstromberg JW, Danielsson DR. Paresthesia vs non-paresthesia – the axillary block. Anesthesiology. 1983;59:A216.
26 Selander D. Peripheral nerve injury after regional anesthesia. In: Finucane BT, editor. Complications of regional anesthesia. Philadelphia: Churchill Livingstone; 1999:105-115.
27 Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France. The SOS regional anesthesia hotline service. Anesthesiology. 2002;97:1274-1280.
28 Selander D, Dhuner KG, Lundborg G. Peripheral nerve injury due to injection needles used for regional anesthesia. Acta Anaesthesiol Scand. 1977;21:182-188.
29 Selander D, Sjöstrand J. Longitudinal spread of intraneurally injected local anesthetics: an experimental study in the initial distribution following intraneural injections. Acta Anaesthesiol Scand. 1978;22:622-634.
30 Selander D, Brattsand R, Lundborg G. Local anesthetics: importance of mode of application, concentration and adrenaline for the appearance of nerve lesions: an experimental study of axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anaesthesiol Scand. 1979;23:127-136.
31 Nicholson MJ, McAlpine FS. Neural injuries: association with surgical positions and operations. In: Martin JT, editor. Positioning in anesthesia and surgery. Philadelphia: WB Saunders; 1978:193.
32 Winchell SW, Wolfe R. The incidence of neuropathy following upper extremity nerve blocks. Reg Anesth. 1985;10:12-15.
33 Kroll DA, Caplan RA, Posner K, et al. Nerve injury associated with anesthesia. Anesthesiology. 1990;73:202-207.
34 Adriani J. Reactions to local anesthetics. JAMA. 1966;196:405-408.
35 Aldrete JA, Johnson DA. Evaluation of intracutaneous testing for investigation of allergy to local anesthetic agents. Anesth Analg. 1970;49:173-183.
36 Incaudo G, Schatz M, Patterson R, et al. Administration of local anesthetics to patients with a history of prior adverse reaction. J Allergy Clin Immunol. 1978;61:339-345.
37 Brown DT, Beamish D, Wildsmith JAW. Allergic reaction to an amide local anaesthetic. Br J Anaesth. 1981;53:435-437.
38 Nagel JE, Fuscaldo JT, Fireman P. Paraben allergy. JAMA. 1977;237:1594-1595.
39 Fisher M, Pennington JC. Allergy to local anaesthesia. Br J Anaesth. 1982;54:893-894.
40 Videira RLR, Ruiz-Neto PP, Brandao Neto M. Post spinal meningitis and asepsis. Acta Anaesthesiol Scand. 2002;46:639-646.
41 Sellors JE, Cyna AM, Simmons SW. Aseptic precautions for inserting an epidural catheter: a survey of obstetric anaesthetists. Anaesthesia. 2002;57:593-596.
42 Lurie S, Feinstein M, Heifetz C, et al. Iatrogenic bacterial meningitis after spinal anesthesia for pain relief during labour [letter]. J Clin Anesth. 1999;11:438-439.
43 Dolinski SY, Gerancher JC. Unmasked mischief [letter]. Anesth Analg. 2001;92:279-281.
44 McLure HA, Mannam M, Talboys CA, et al. The effect of facial hair and sex on the dispersal of bacteria below a masked subject. Anaesthesia. 2000;55:173-176.
45 Hubble MJ, Weale AE, Perez JV, et al. Clothing in laminar-flow operating theatres. J Hosp Infect. 1996;32:1-7.
46 Rogers KB. An investigation into the efficiency of disposable face masks. J Clin Pathol. 1980;33:1086-1091.
47 Panikkar KK, Yentis SM. Wearing of masks for obstetric regional anesthesia: a postal survey. Anaesthesia. 1996;51:398-400.
48 Sleth JC. Evaluation des mesures d’asepsie lors de la realisation d’un catheterisme epidural et perception de son risque infectieux. Resultats d’une enquête en Languedoc-Roussillon. Ann Fr Anesth Reanim. 1998;17:408-414.
49 Handwashing Liaison Group. Hand washing: a modest measure – with big effects. Br Med J. 1999;318:686.
50 Mimoz O, Karim A, Mercat A, et al. Chlorhexidine compared with povidone iodine as skin prep before blood culture. Ann Int Med. 1999;131:834-837.
51 Rotter ML. Hand washing and hand disinfection. In: Mayhall CG, editor. Hospital epidemiology and infection control. 2nd edn. Philadelphia: Lippincott Williams & Wilkins; 1999:1339-1355.
52 American Society of Anesthesiologists. Recommendations for infection control for the practice of anesthesiology, 2nd edn. Park Ridge, IL: ASA; 1998. 10
53 Kinirons B, Mimoz O, Lafendi L. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children. A randomized controlled trial. Anesthesiology. 2001;94:239-244.
54 Tunstall ME, MacLennan FM. Contamination of opioids during preparation for regional anaesthesia [letter]. Anaesthesia. 1997;52:290.
55 Kroin JS, Penn RD, Levy FE, et al. Effect of repetitive lidocaine infusion on peripheral nerve. Exp Neurol. 1986;94:166-173.
56 Libelius R Sonesson B, Stamenovic BA, et al. Denervation-like changes in skeletal muscle after treatment with a local anesthetic (Marcain). J Anat. 1970;106:297-309.
57 Gómez-Arnau JI, Yangüela J, Gonzáles A, et al. Anaesthesia-related diplopia after cataract surgery. Br J Anaesth. 2003;90:189-193.
58 Vandermeulen EP, VanAken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165-1177.
59 Klein SM, Nielsen KC, Greengrass RA, et al. Ambulatory discharge after long-acting peripheral nerve blockade: 2382 blocks with ropivacaine. Anesth Analg. 2002;94:65-70.
60 Phillips S, Hutchinson S, Davidson T. Preoperative drinking does not affect gastric contents. Br J Anaesth. 1993;70:6-9.
61 American Society of Anesthesiologists. Guidelines for sedation and analgesia by non-anesthesiologists. Park Ridge, IL: ASA; 1999. 3–11
62 Eichhorn JH, Cooper JB, Cullen DJ. Standards for patient monitoring during anesthesia at Harvard Medical School. JAMA. 1986;256:1017-1020.
63 McIntyre JWR. Regional anesthesia safety. In: Finucane BT, editor. Complications of regional anesthesia. Philadelphia: Churchill Livingstone; 1999:1-30.
64 Bonica JJ, Moore DC, Orlov M. Brachial plexus block anesthesia. Am J Surg. 1949;78:65.
65 Moberg E, Dhunér K-G. Brachial plexus block analgesia with xylocaine. J Bone Joint Surg. 1951;33A:884.
66 Wooley EJ, Vandam LD. Neurological sequelae of brachial plexus nerve block. Ann Surg. 1959;149:53-60.
67 Brand L, Papper EM. A comparison of supraclavicular and axillary techniques for brachial plexus blocks. Anesthesiology. 1961;22:226-229.
68 Schmidt E, Racenberg E, Hilderbrand G, et al. Komplikationen und gefahren der plexus-brachialis-anästhesie unter besonderer Berücksichtiging von Langzeitschaden. Anästh Intensivther Notfallmed. 1981;16:346-349.
69 Brand L, Papper EM. A comparison of supraclavicular and axillary techniques for brachial plexus blocks. Anesthesiolog. 1961;22:226-229.
70 De Jong RH. Axillary block of the brachial plexus. Anesthesiology. 1961;22:215-225.
71 Hamelberg W, Dysart R, Bosomworth P. Perivascular axillary versus supraclavicular brachial plexus block and general anesthesia. Anesth Anal. 1962;41:85-90.
72 Wall JJ. Axillary nerve blocks. Ann Sur. 1959;149:53.
73 Moore DC, Bridenbaugh LD, Thompson GE, et al. Bupivacaine: a review of 11,080 cases. Anesth Anal. 1978;57:42-53.
74 Selander D, Edshage S, Wolff T. Parasthesiae or no parasthesiae? Nerve lesions after axillary blocks. Acta Anaesth Scan. 1979;23:27-33.
75 Plevak DJ, Linstromberg JW, Danielsson DR. Paresthesiae vs non-paresthesiae – the axillary block. Anesthesiology. 1983;59:A216.
76 Winchell SW, Wolfe R. The incidence of neuropathy following upper extremity nerve blocks. Reg Anesth. 1985;10:12-15.
77 Tourtier Y, Rébillion M, Delort J, et al. Complications of axillary block using two techniques: experience with 1400 cases. Anesthesiology. 1989;71:A726.
78 Davis WJ, Lennon RL, Wedel DJ. Brachial plexus anesthesia for outpatient surgical procedures on an upper extremity. Mayo Clinic Proc. 1991;66:544-547.
79 Stan TC, Krantz MA, Solomon DL, et al. The incidence of neurovascular complications following axillary brachial plexus block using a transarterial approach. Reg Anesth. 1995;20:486-492.
80 Löfström B, Wennberg A, Widén L. Late disturbances in nerve function after block with local anesthetic agents. Acta Anesth Scand. 1966;10:111-122.
81 Mogensen BA, Mattsson HS. Posttraumatic instability of the metacarpophalangeal joint of the thumb. Hand. 1980;12:85-90.
82 Tsui BC, Knezevich MP, Pillay J. Reduced injections pressures using a compressed air injection technique (CAIT): an in vitro study. Reg Anesth Pain Med. 2008;33:168-173.