General Approach to the Pregnant Patient
Perspective
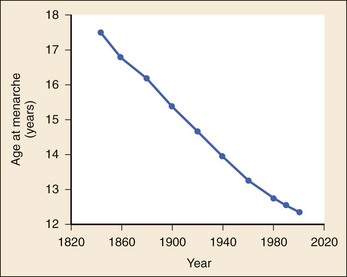
The 2008 report from the U.S. National Center for Health Statistics noted that the birth rate for women younger than 25 years, the principal childbearing age, had fallen from 43% in 1990 to 34.4% in 2009.1 The overall birth rate for teens aged 15 to 19 years dropped from 15% in 1990 to 10% in 2009,1 which resulted in a historic low of 66.7 pregnancies per 1000 women.2 Nearly half of all pregnancies in the United States occur among unmarried women, and this number is rising, increasing from 2.7 million in 1990 to 2.8 million in 2004, whereas rates among married women continue to fall, from 4.1 million in 1990 to 3.5 million in 2004 (newer data do not exist at the time of publication). Pregnancy outcomes in the United States vary across the black, Hispanic, and white populations. The rate of pregnancies resulting in live births is highest in Hispanic women (98%) compared with non-Hispanic whites (69%) and non-Hispanic blacks (50%), whereas the rate of pregnancy resulting in abortion is highest in non-Hispanic blacks (37%) compared with non-Hispanic whites (12%) and Hispanic women (19%).1 The median age at menarche for adolescent American girls fell from 15.5 years in 1900 to 12.3 years in 20003 (Fig. 177-1).
From 1980 to 2008, the number of twin births in the United States rose more than 100% (from 68,339 to 138,660), and triplet and higher order births rose just less than 400% (from 1377 to 6268 births).2 At the same time, differences in twin birth rates between non-Hispanic white and non-Hispanic black mothers disappeared. Non-Hispanic white women were more than twice as likely as non-Hispanic black or Hispanic women to have a triplet birth.2
Maternal mortality related to complications of pregnancy and childbirth in the United States has slowly been declining during the past several years from 2960 maternal deaths in 1950 to 548 deaths in 2007.4 The highest number of deaths is attributed to women older than 35 years, with 32 deaths per 100,000 live births, which is four times the rate for women 29 years of age and younger.2 The most common causes of death are pulmonary embolism, diseases of the circulatory system, and postpartum hemorrhage.5
Rates of elective cesarean deliveries in industrialized countries are continuing to rise secondary to a common perception of safety and potential benefits of elective low-risk cesarean delivery compared with vaginal delivery. In Canada, the rate of planned cesarean deliveries has increased to 26% in 2003 compared with 5% in 1969.4 A retrospective cohort study, which evaluated more than 2.3 million healthy pregnant patients, demonstrated a composite severe morbidity of 27 per 1000 deliveries for planned cesarean delivery for breech presentation versus 9 per 1000 deliveries for planned vaginal delivery for any reason, respectively.4 Furthermore, although the study showed an increased risk of maternal cardiac arrest of 2% in the planned cesarean delivery group and 0.4% in the vaginal delivery group, the difference in the rate of in-hospital maternal death was nonsignificant (P = .87). Decisions about planned cesarean sections for other than conventional indications require a discussion of the risks and benefits of this intervention.
Statistical data concerning pregnant patient demographics and frequency of use of the ED and the diagnostic profile are incomplete because most observational data sets group perinatal and pregnancy as “other medical diagnosis,” which accounts for less than 2% of visits to the ED in the United States and Canada5; however, persons between the ages of 18 and 44 years account for approximately 22% of all ED visits, so the risk of an unknown pregnancy complicating the diagnosis may be higher than anticipated.6 A study of random pregnancy tests performed on female trauma patients revealed that 2% were pregnant.7,8 Even when the chance of pregnancy was remote, female patients seeking treatment in the ED had a pregnancy rate of at least 10%.7
Maternal cardiac arrest is rare, although recent data from the Confidential Enquiries into Maternal and Child Health data set show a maternal cardiac arrest frequency that is on the rise with a current rate of 1 : 20,000 pregnancies, up from the previous rate of 1 : 30,000.9
Pregnant patients may present with symptoms and signs related to an undiagnosed, normal pregnancy (abdominal discomfort, nausea, urinary frequency, breast swelling and tenderness, fatigue, or near-syncope), symptoms and signs related to complications of pregnancy (see Chapter 178), or clinical manifestations of chronic medical illnesses that may be exacerbated by pregnancy (see Chapter 179). This chapter covers the following aspects of a normal pregnancy: common initial signs and symptoms, diagnostic tests, and considerations for treatment and referral.
Clinical Features
The reliability of the sexual and menstrual history has been notably poor. Ramoska and colleagues reported that 11.5% of women with abdominal pain or vaginal bleeding had positive pregnancy test results, in contrast to their perception that there was “no chance” they were pregnant.7 Missed diagnosis of pregnancy in adolescents was associated with failure to document a sexual or menstrual history. Adolescents rarely mentioned the possibility of pregnancy at triage (10% of those found to be pregnant), and 10% of those with a positive pregnancy test result denied being sexually active.10 Interestingly, pregnancy rates in adolescents continue to rise in the United States, with estimates of 42.5 per 1000 women of this age group, and have increased 5% from 2005-2007 data.1 Thus one should have a high index of suspicion of pregnancy in adolescents who present with symptoms of pregnancy, which include cessation of menses, anorexia, nausea, easy fatigability, urinary frequency, breast swelling and tenderness, and perception of fetal movement or “quickening” (beginning at 16 to 20 weeks).
Cessation of menses becomes suggestive of pregnancy at 10 days or longer after the time that menstruation is expected. Intermittent, spontaneous vaginal bleeding or vaginal discharge is common during the first half of pregnancy, particularly in multiparous women. Most women without cervical lesions experience bleeding on or before the 40th day of pregnancy. This bleeding has been interpreted by some investigators to be physiologic in origin and a result of implantation.11 Nevertheless, any patient presenting with vaginal bleeding during pregnancy should be considered to be at risk for a pathologic cause of the bleeding.
It is possible to calculate the estimated date of delivery in a regularly menstruating woman who has 28-day cycles and can give a reliable history. Franz K. Nägele, a German obstetrician in the 18th century, developed Nägele’s rule, which is still the current standard: add 7 days to the date of the first day of the last menstrual period and subtract 3 months. Currently, with the increasing use of smart phones, hand-held devices, and the Internet, literally hundreds of calculators are available to clinicians to aid in the estimation of delivery date. In the absence of reliable recall of the first day of the last menstrual period, first-trimester ultrasonography may estimate the date of delivery. Ultrasonography dating is most accurate if it is done between 10 and 12 weeks.12
Physical Examination
Vital signs in a pregnant patient are essentially the same as those in a nonpregnant patient. The only changes are seen in the heart rate and blood pressure. The resting heart rate increases approximately 10 to 15 beats/minute, and blood pressure demonstrates a mean arterial decrease of 6 to 10 mm Hg, which reaches a nadir in mid–second trimester and slowly increases to the patient’s nonpregnant value by term. Blood pressure in the brachial artery is highest in the sitting position, lowest when lying in the left lateral position, and intermediate in the supine position, except in pregnant women who have supine hypotension syndrome. Pathologic hypertension of pregnancy is defined as a sustained rise of 30 mm Hg systolic or 15 mm Hg diastolic above baseline values on at least two occasions, 6 hours or more apart. A study demonstrated a steady increase in prevalence of hypertension in pregnant women from 67 per 1000 pregnant patients in 1998 to 83 per 1000 patients in 2006. The highest risk cohort is women older than 35 years, with a reported rate of 89 per 1000 patients.13 The authors concluded that hypertension and related illnesses are major risk factors for severe obstetric morbidity. Furthermore, as more women have children later in life, in addition to the increasing prevalence of obesity, the number of patients with hypertension-related illness due to pregnancy will most likely continue to rise.13
Fetal heart activity should be included as the fifth vital sign in the evaluation of a pregnant patient. It can be auscultated with a stethoscope at 17 weeks of gestation on average and by 19 weeks in nearly all pregnancies in nonobese women. With the use of Doppler ultrasonography, fetal cardiac activity can be detected as early as 8 weeks; however, it is more commonly heard by 10 weeks. With improved transvaginal ultrasonography technology, it has been possible to detect fetal cardiac activity in embryos as small as 6 mm.14
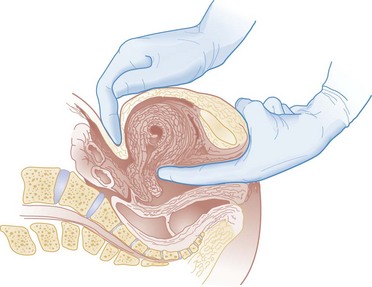
Hegar’s sign, which is softening of the lower uterine segment caused by hyperemia, can be appreciated during an internal pelvic examination at approximately 6 to 8 weeks of pregnancy. In some cases the softening is sufficient to allow the examiner to easily distinguish the cervix from the fundus on palpation. It can be found in other conditions, but it is not as easily detected as it is in pregnancy (Fig. 177-2).
The vaginal mucosa becomes hyperemic during pregnancy and with any other condition that causes congestion of the pelvic organs. In response, the color of the mucosa of the vaginal walls changes from pink to blue to violet and can be visualized at the vaginal introitus (Chadwick’s sign).15
At midpregnancy the fetus can be detected by ballottement or palpated through the maternal abdominal wall in a nonobese patient. The fetus floats in a large volume of amniotic fluid. Thus, with ballottement, pressure exerted on the uterus causes the fetus to sink and then to rebound to its original position. Palpation of the outline of the fetal body becomes easier near the end of pregnancy; however, subserosal myomas can simulate the fetal head and small parts, or both.10
Diagnostic Strategies
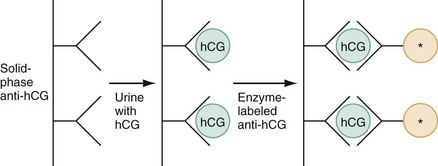
The fundamental process of ELISA involves an antibody adherent to a solid-faced support (usually plastic) that binds to a region of the hCG molecule. A second antibody chemically linked to an enzyme (e.g., alkaline phosphatase) binds to another side of the trapped hCG molecule. The hCG molecule is sandwiched between the two antibodies. Excess enzyme-linked antibody is washed away, and a color developer is added and becomes blue when it comes in contact with the enzyme (Fig. 177-3). The antibodies can be polyclonal or monoclonal. Many variations exist with respect to the solid-faced support and the method of developing the color reaction.
In 1988 the first one-step pregnancy test became available. It was developed and patented by Unipath and sold as Clearblue One Step. This test uses monoclonal antibodies specific for the beta subunit of hCG. It includes a unique built-in control that shows a blue line when the test is complete and performed correctly.16 This test appeals to consumers because it is rapid (requiring less than 5 minutes), requires no manipulation by the user, offers hygienic sample handling, provides clear and easy-to-read results, and has a sensitivity level of hCG between 25 and 50 mIU/mL. This level of sensitivity means that more than 95% of pregnancies within 3 days after missed menses will be detected by a home pregnancy device.17 For detection to occur on the day of missed menses, a lower sensitivity level of 12.5 mIU/mL is required. A study in 2001 compared routine urinary hCG point-of-care qualitative tests performed by nurses in the ED with laboratory tests and found the former to be equally accurate and much faster, shaving 35 minutes off wait times for results.18
A well-designed ELISA applied to urine is not affected by drugs or a concurrent physiologic state. The only exception is patients taking exogenous hCG for induction of ovulation.19
Normal hCG levels in men and premenopausal women range from 0.02 to 0.8 mIU/mL. Postmenopausal women may have higher levels.20 The blastocyst begins to secrete hCG 7 days after fertilization.21 Thus the hCG released can be detected as early as 6 to 8 days after conception.22
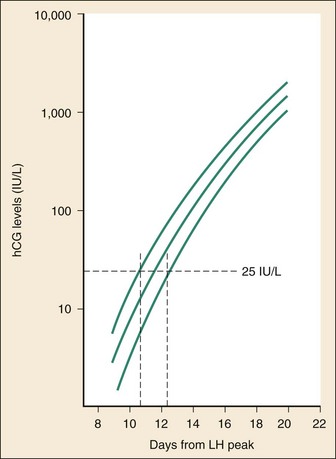
The initial doubling time is fast and has been attributed to the actual process of implantation. This is followed by a slower rate of doubling as trophoblast hCG and maternal circulatory hCG levels equilibrate22 (Fig. 177-4). Levels of hCG peak at 7 to 10 weeks of pregnancy, with a mean value of 50,000 mIU/mL and a range of 20,000 to 200,000 mIU/mL.21
The test results for hCG become positive in 98% of patients 7 days after implantation, which coincides with the time of the expected period. When a test with a sensitivity of 25 to 50 mIU/mL is used, a negative result 1 week from the expected time of the missed period essentially guarantees that a woman is not pregnant.19
False-positive results can be attributed to postmenopausal status, abortion in the first trimester, exogenous hCG for induction of ovulation, or hCG-secreting tumor. Levels of hCG may take as long as 60 days to return to zero after an abortion.23 Failure to decline during 60 days and persistent elevation of hCG beyond 60 days after abortion may indicate an incomplete abortion, a twin pregnancy with only one fetus removed, or an ectopic pregnancy.1
The hCG assay continues to be the standard for the diagnosis of pregnancy. Routine clinical detection of pregnancy by ultrasonography is not possible until at least 5 weeks’ gestation, when the hCG level is 1000 mIU/mL or more.24–26
Radiology
Ultrasonography is reliable for the diagnosis of intrauterine pregnancy during the first trimester. Systematic reviews demonstrate that ultrasonography scans performed in the ED have a sensitivity of 90% and specificity of 98% in the detection of an intrauterine pregnancy.27 In addition to diagnosis, it also locates the embryo and assesses gestational age and viability. Transabdominal sonography is more commonly used; however, transvaginal sonography may be necessary during the first trimester if transabdominal sonography is not diagnostic.
Sonography during the second trimester is used to survey fetal anatomy. The anatomic survey includes the cerebral ventricles, four-chamber view of the heart, spine, stomach, urinary bladder, umbilical cord insertion on the abdominal wall, and kidneys.28
Much debate exists about whether ultrasonography should be performed as part of routine prenatal screening in all pregnancies. The American College of Obstetricians and Gynecologists (ACOG) recommends that all physicians discuss the benefits and limitations of ultrasonography with patients and come to a decision together about the screening examination.28 Although prenatal screening is still an object of contention, the ACOG states that there is good evidence for the safety and accuracy of ultrasonography examination to determine gestational age, major fetal anomalies, fetal number, viability, and placental location and recommends the test if these areas are in question.28 First-trimester ultrasonography, in conjunction with biochemical parameters, is an integral part of first-trimester genetic screening for aneuploidy and is usually performed at 11 to 13 weeks of age.
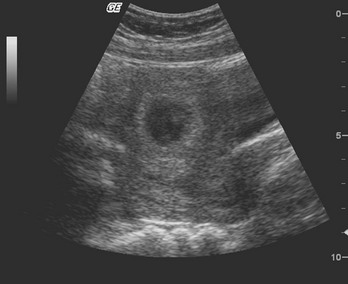
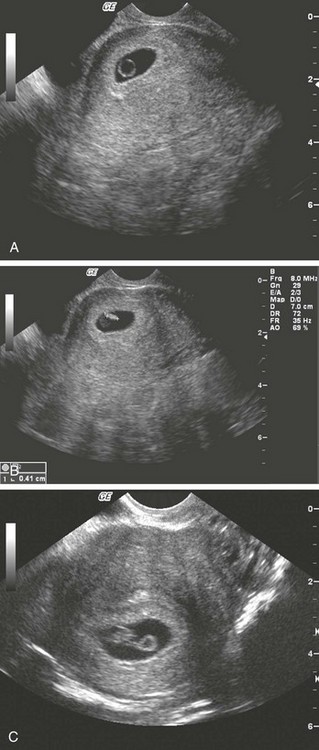
Transabdominal Sonography.: At 6 weeks the gestational sac can be visualized by transabdominal sonography (Fig. 177-5). This sonographic finding disappears after the 11th week of gestation. Presence of the gestational sac is confirmed when two central uterine echoes are visualized within the hypertrophied endometrium. This diagnostic echo is called the double ring sign. The gestational sac is eccentrically positioned within an asymmetrically thickened decidua. A healthy pregnancy can be determined by correlating the size of the sac with gestational age and quantitative hCG values. At 8 weeks the fetal pole and fetal heart activity can be visualized reliably with transabdominal ultrasonography in a normal pregnancy. Diagnosis of an intrauterine pregnancy with ultrasonography can be made with the following findings: yolk sac or fetal pole visualized inside myometrium, bladder-uterine juxtaposition confirmed, and myometrial mantle of 5 mm29 (Fig. 177-6). Diagnosis of a live intrauterine pregnancy also requires visualization of intrauterine fetal heart activity.
Transvaginal Sonography.: Transvaginal sonography affords greater resolution than transabdominal sonography and does not require a full bladder. Transvaginal ultrasonography examination can identify an intrauterine gestational yolk sac at 4 to 5 weeks’ gestation. Fetal heart motion can be detected with transvaginal ultrasonography evaluation at 6 weeks’ gestation.
An intrauterine pregnancy can be diagnosed by sonographic findings in the following order of appearance: the double ring sign, the double gestational sac, the intrauterine fetal pole, and intrauterine fetal heart activity (see Fig. 177-6).
Magnetic Resonance Imaging
No significant side effects have been documented to date with the magnetic field and radio waves used in magnetic resonance imaging (MRI). Gadolinium is the most common intravenous contrast agent, and allergic reactions with this agent are rare. Currently, the ACOG recommends that nonionizing imaging procedures like MRI and ultrasonography be used preferentially, when appropriate, and they are not known to be associated with adverse fetal effects.30
Diagnostic Imaging Considerations in Pregnant Patients
The decision to perform imaging investigations in a pregnant patient must take into consideration the risk to the developing fetus from radiation exposure and the inherent risks with such investigations for the mother, balanced with the risk to the mother and fetus associated with missing the diagnosis because of reluctance to use available diagnostic imaging techniques. The potential for adverse outcomes with intrauterine fetal exposure to the radiation used in radiography is minimal.31 Case-control studies report a slight but statistically significant increase in the relative risk for childhood cancer.32 Radiation is a dose-dependent teratogen.33 The fetal central nervous system is most vulnerable to the teratogenic effects of radiation at 8 to 15 weeks after conception. The fetal dose of radiation is estimated from the ovarian or uterine dose. Pregnant women exposed to less than 50 mGy have pregnancy outcomes similar to those of control subjects.34 A study of childhood cancers in the offspring of female radiologists revealed a cancer frequency of 0.16% in children exposed to 10 mGy of radiation in utero. In comparison, the frequency of cancer is 0.07% among children who were not exposed to radiation in utero.
Table 177-1 summarizes the estimated fetal radiation exposure associated with the more common diagnostic imaging modalities. Radiation exposure depends on the equipment and technique, and estimates listed may not be generalizable to every radiology department.
Table 177-1
Fetal Radiation Exposure with Various Imaging Modalities
| PROCEDURE | ESTIMATED FETAL RADIATION EXPOSURE (µGy) |
| Chest plain film with an abdominal shield | 10* |
| Chest CT with an abdominal shield | 100* |
| Abdomen plain film | 2400* |
| Abdomen CT: with and without contrast agent | 20,000 and 10,000* |
| Cardiac catheterization: with and without pelvic fluoroscopy | 13,000 and 1000* |
| Head CT | 100* |
| Unilateral venography without an abdominal shield | 3050 |
| Limited venography with an abdominal shield | 500 |
| Pulmonary angiography via femoral access | 4050 |
| Pulmonary angiography via brachial access with an abdominal shield | 500 |
| Perfusion lung scan using 99mTc-MAA | |
| 3 mCi | 180 |
| 1-2 mCi | 60-120 |
| Ventilation lung scan using | |
| 133Xe | 30-200 |
| 99mTc-DTPA | 70-350 |
| 99mTc-SC | 10-50 |
| Ventilation-perfusion scan | 500 |
*Data from Duke University and Duke Medicine Radiation Safety Division: Fetal radiation dose estimates. Available at www.safety.duke.edu/radsafety/fdose/fdxray.asp. Accessed March 12, 2011.
Modified from Ginsberg JS, et al: Thrombotic complications in the obstetric patient. In Coleman RW, et al (eds): Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 3rd ed. Philadelphia, JB Lippincott, 1994.
A thoughtful, common-sense approach to the differential diagnosis will provide a sound basis for imaging investigations that will undoubtedly be in the best interests of the mother and the fetus. It is important not to defer investigations that are medically necessary, so long as the anticipated fetal doses are less than 50 mGy.35
Specific Disorders
In general, a pregnant patient should be treated and assessed in the same way as all other patients are. However, the normal physiologic changes of pregnancy may mask or unmask underlying medical conditions. Thus it is important to ascertain whether the presenting complaint is due to the pregnancy, an unrelated problem that is affected by the pregnancy, or a problem that affects the pregnancy.36 In addition, consideration should be given to the increased medical risks of pregnancy (e.g., deep venous thrombosis) and risk to the fetus associated with diagnostic imaging or surgical interventions.
Headache
The most common headache during pregnancy is a muscle contraction headache.37 The patient usually has had a history of headaches before the pregnancy. The headaches begin on waking and increase in intensity during the day. The headache is described as a broad band, squeezing or constricting in quality, and localized to the vertex. The paracervical muscles are taut, and palpation usually reproduces or exacerbates the pain. During pregnancy these headaches are worsened by postural changes and psychosocial stress. Commonly used abortive medications for migraines in pregnancy include acetaminophen (e.g., Tylenol), aspirin, opioids, triptans, antiemetics, and caffeine.37 From 18 to 86% of classic migraine sufferers experience remission from attacks during pregnancy, particularly women with menstrual migraines.37,38 Therefore, any headache in a pregnant patient requires investigation, except a typical headache in a patient with a known primary headache disorder.37,39
Brain tumors enlarge during pregnancy and shrink temporarily postpartum.40 Most brain tumors become symptomatic during the second half of pregnancy, and they account for 10% of maternal deaths during pregnancy. Both pituitary gland and prolactin-secreting adenomas swell during pregnancy. Headache usually develops during the first trimester and precedes visual changes by approximately 1 month.40 On occasion, central venous thrombosis may occur during pregnancy and mimic eclampsia. The lack of proteinuria and hypertension points to central venous thrombosis.37 Central venous thrombosis is an unusual cause of postpartum headache that is associated with a mortality rate of 40%.37
Pseudotumor cerebri is benign intracranial hypertension in the absence of a mass lesion and hydrocephalus. Symptoms and signs often include headache and papilledema. Other associated findings include decreased visual acuity, loss of color vision, defects in visual fields, and horizontal diplopia caused by abducens nerve palsy. Pseudotumor cerebri associated with pregnancy usually occurs in obese women and begins during the third to fifth months of pregnancy. Symptoms resolve spontaneously after 1 to 3 months.41
Spontaneous subarachnoid hemorrhage accounts for 10% of maternal deaths. Reviews of the risk for subarachnoid hemorrhage in pregnant versus nonpregnant patients have been conflicting. The absolute risk has been estimated to be 1 in 2000 to 10,000. In a majority of cases, the intracranial bleeding is attributable to rupture of an arteriovenous malformation or a ruptured intracranial aneurysm. The overall risk for rupture of an arteriovenous malformation is identical in pregnant and nonpregnant patients without previous bleeding.42 Arteriovenous malformations usually bleed during the second trimester and during labor. Berry aneurysms typically bleed during the third trimester, and the incidence of bleeding increases as the pregnancy advances.
Chest Pain
The pregnant patient is predisposed to thromboembolic disease as a result of increased venous pressure, decreased distal venous flow, and significant venous stasis from compression of the pelvic veins and inferior vena cava by the enlarging uterus. Other predisposing factors may include the use of oral contraceptives before conception and the propensity for stasis secondary to lack of activity in the workplace and at home as the pregnancy progresses. The annual incidence of thromboembolism is five times higher in postpartum women than in pregnant women.43 The incidence of deep venous thrombosis is three times higher than that of pulmonary embolism; however, the incidence of deep venous thrombosis during pregnancy has remained constant during the last 30 years, whereas that of pulmonary embolism during pregnancy doubled.43 This increased risk for pulmonary embolism in pregnancy should prompt thorough evaluation of pregnant patients who present with pleuritic chest pain, syncope, acute right-sided heart failure, or more subtle signs, such as unexplained fever, dyspnea, or tachycardia.
Abdominal Pain
Obstetric causes of abdominal pain early in pregnancy include vascular congestion of the pelvic tissues, round ligament tension, ectopic pregnancy, and threatened abortion. With increasing rates of infertility and use of fertility-enhancing drugs, there has been a corresponding increased incidence of women with ovarian hyperstimulation syndrome (OHSS). OHSS is an iatrogenic syndrome characterized by ovarian enlargement (from multiple ovarian cysts) and fluid shifts to the extravascular space, causing ascites, hypovolemia, and electrolyte abnormalities. Studies have suggested that in vitro fertilization treatments have been associated with up to 33% of mild forms of OHSS and 3 to 8% of severe cases.44 Severe forms of OHSS require urgent diagnosis and treatment to mitigate the high risk of associated thromboembolic events, respiratory failure, and renal failure.
Appendicitis is the most common surgical emergency in pregnancy.45,46 The incidence ranges from 0.06 to 0.1%, or 1 in 1500 deliveries. Although the incidence is relatively close to that of nonpregnant patients, the perforation rate is higher during pregnancy.46–48 The state of pregnancy results in delays in diagnosis of appendicitis that contribute significantly to maternal and fetal morbidity and mortality.48 The perinatal mortality rate for women with nonperforated appendicitis is 4.8%. It rises, however, to 27.8% in those experiencing perforation at the time of surgery. The perforation rate is as high as 30% during the third trimester.48
The diagnosis of appendicitis is more difficult during the latter half of pregnancy, but it is challenging even in early pregnancy. Anorexia, nausea, and vomiting are symptomatic of appendicitis and may be misinterpreted as hyperemesis of pregnancy. As the uterus enlarges, the appendix moves upward and outward toward the right. As the appendix moves higher, the omentum is unable to surround the appendix and to contain the infection if rupture occurs. Thus appendiceal rupture in pregnant women results in generalized peritonitis. However, even though there is a change in the relative position of the appendix during pregnancy, the most common symptom of appendicitis is right lower quadrant pain that occurs close to McBurney’s point in the majority of women regardless of the stage of pregnancy.44,49,50 A retrospective review comparing MRI findings with surgical confirmation of disease suggested that MRI is useful for triage of pregnant patients with acute abdominal and pelvic pain.50 When it is available, MRI should be considered in this setting of nonconclusive findings on ultrasonography, particularly during the first trimester of pregnancy, because of the higher levels of radiation exposure associated with computed tomography of the abdomen.
Musculoskeletal Pain
Hand symptoms are common during pregnancy. The median nerve is at risk for compression distally within the carpal tunnel. Approximately 25% of pregnant women complain of hand symptoms compatible with median nerve compression, but only 2.3% are actually found to have carpal tunnel syndrome.51 Symptoms of carpal tunnel syndrome include the classic triad of burning pain, numbness, and tingling in the thenar area or median nerve distribution. The patient often is awakened from sleep with the onset of symptoms. In 80% of cases, the symptoms are bilateral. Tapping over the median nerve at the wrist (Tinel’s sign) or hyperflexing the wrist (Phalen’s test) reproduces the symptoms and is diagnostic. Because the symptoms usually resolve during the postpartum period, only supportive treatment is required. A sleep splint applied with the hand in slight flexion resolves the symptoms in 80% of patients.51
Postural changes in the cervical spine, the shoulder girdle, and the lumbar spine result in pregnancy-related posterior pelvic pain and low back pain. Lordosis is exaggerated and becomes progressively more symptomatic during pregnancy. Low back pain usually is localized to the lumbar region just above the sacroiliac joint, worsened by forward flexion at the waist, and results in decreased range of motion and tenderness to palpation of the erector spinae muscles. The pain may radiate to one or both of the legs. The nature of the referred pain lacks the radicular quality of diskogenic pain and does not extend beyond the knee. Patients should avoid wearing high-heeled shoes because this will increase the lumbar lordosis with exacerbation of the pain. Swimming and conditioning exercises are recommended. Pregnancy-related posterior pelvic pain is hypothesized to occur because of asymmetrical sacroiliac joint laxity. It is important to distinguish this from low back pain because the treatments are different. Pregnancy-related pelvic pain is a stabbing pain in the buttocks, distal and lateral to the L5-S1 area, which may or may not radiate to the posterior thigh or knee, with a normal range of motion at hips and spine. The posterior pelvic pain provocation test has a positive predictive value of 0.91 for pregnancy-related pelvic pain. The test is performed with the patient in the supine position with the hips and knees bent at 90 degrees. The examiner applies pressure with one hand at the knee along the long axis of the femur from anterior to posterior while using the other hand to stabilize the pelvis at the contralateral anterior iliac spine. A positive test result is gluteal pain on the side of femoral pressure.52
Trauma
The ACOG reports that 1 in every 12 pregnancies is complicated by physical trauma.53 Trauma is the leading cause of maternal death secondary to nonobstetric etiologic conditions or disorders.54 One study suggested that fetal mortality after trauma is significantly associated with higher injury severity score (of 28 or higher), lower final hemoglobin level, higher number of transfusions, longer hospital stay, and higher incidence of disseminated intravascular coagulation.55 A retrospective institutional review during 5 years demonstrated that older maternal age, first-trimester presentation, elevated serum lactate level, and high injury severity score were associated with poor fetal outcome.56 Other studies have not demonstrated a correlation between injury severity scores and maternal and fetal morbidity, although the authors advocated enhanced clinical vigilance for the unexpected in caring for obstetric trauma victims (see Chapter 37).57
Maternal Cardiac Arrest
Maternal cardiac arrest affects 1 in every 20,000 pregnancies, with an estimated survival rate of 6.9%.58 A number of anatomic and physiologic changes in pregnancy contribute to cardiac arrest; the most common causes are trauma, hemorrhage, embolism, eclampsia, cardiac disease, and acute coronary syndrome.59 When a health care provider first approaches a maternal cardiac arrest, important initial measures include activation of the maternal cardiac arrest and neonatal resuscitation teams, placement of the patient supine, and initiation of chest compressions per normal basic life support algorithms.9,59 When the maternal arrest team arrives, a member of the group should perform a manual displacement of the uterus to the left. Performance of this maneuver will help remove the gravid uterus from the inferior vena cava, thereby relieving some aortocaval compression.9,59 Other modifications to the nonpregnant cardiac arrest are starting of intravenous lines above the diaphragm, early assessment of hypovolemia and appropriate fluid resuscitation, and termination of any magnesium intravenous therapy. While active resuscitative measures are in progress, the obstetric and neonatal teams should prepare for an emergent cesarean section if there is no return of spontaneous circulation within 4 minutes of the onset of the maternal cardiac arrest; the goal is to perform the delivery of the child at 5 minutes if maternal circulation is not restored.9,59
Domestic Violence
Up to 35% of all ED visits by women are due to injuries or illnesses related to domestic violence. Domestic violence is conservatively estimated to occur in 10 to 20% of North American spousal relationships. Estimates of abuse during pregnancy vary widely, ranging from 1% up to 37%. The wide variation in estimates is attributable to different study designs, definitions of abuse, and sample population demographics.60–66 Moreover, it generally is accepted that violence in the home is under-reported and the prevalence is higher than studies estimate.
A survey taken between 2004 and 2007 of 134,955 pregnant women in the United States showed that the prevalence of violence during pregnancy is approximately 3.6%.66 Risk factors for violence during pregnancy include a recent divorce or separation (15.6%), a partner who expresses that he did not want the pregnancy (14.6%), and being close with someone who has a substance abuse or drinking problem (12.7%).66 Other significant demographic variables associated with abuse and pregnancy can be classified under the major headings of social instability, unhealthy lifestyle, and physical health problems.67 Abused pregnant women are more commonly of lower educational levels, unmarried, unemployed, eating an unhealthy diet, abusing drugs and alcohol, and experiencing an unplanned pregnancy; they may also delay seeking of prenatal care.67–69 Evidence of physical abuse is more common in the facial area in a nonpregnant patient, whereas blunt abdominal trauma is the most common injury type in a pregnant patient.67,70 Blunt abdominal trauma can result in miscarriage, abruptio placentae, fetal loss, premature labor, fetal fractures, low birth weight, and premature delivery. Other common injury sites in abused pregnant patients are the breasts and genitals.
Abused pregnant women often suffer from low self-esteem, despair, anxiety, fear, withdrawal, post-traumatic stress disorder, passivity, learned helplessness, and depression and have high rates of attempted suicide.67,71 Abused pregnant women may have vague physical symptoms, including headache, fatigue, insomnia, choking sensations, gastrointestinal complaints, pelvic pain, and backache. These symptoms may be manifestations of a deep depression or of a conversion reaction related to the adversity of the home environment.
Abused pregnant women do not believe that they have control over the health of their fetus. They hold a strong belief that chance is the major determinant in fetal outcome. This perception and the associated behavior (e.g., cigarette smoking, alcohol and drug abuse) are unlikely to change unless abused women leave their abusive situation. Only then will they be able to improve their sense of power and self-esteem and to make changes in their behavior that are in the best interest of the fetus.67 Identification and appropriate referral are paramount in the management of an abused pregnant patient. Most abused patients will not volunteer information about domestic violence unless they are specifically asked. Cross-sectional survey data obtained from interviews with 261 pregnant women in 1991 suggest that 33.3% of women will report abuse when asked. They are more likely to disclose such information to another woman or medical personnel of either sex who are sympathetic and offer protection, support, and access to help.71 Fear that an abused woman will be offended by direct questioning is unfounded.67 It is important to include direct queries about abuse or risk of abuse in taking a history from a patient in the absence of her partner. Direct questioning may take the form of the following:
Management
Health Promotion and Disease and Injury Prevention in Pregnancy
After complications of pregnancy and comorbid conditions have been excluded, pregnant patients should initially be counseled, appropriately referred, and encouraged to participate in regular prenatal care. The proportion of American women beginning prenatal care during the first trimester has consistently increased since the early 1990s, with a rate of 84% reported in 2004.1 Although the time for prenatal counseling is limited in the ED, such counseling can have a positive impact on both fetal and maternal health. The following discussion is not inclusive of all prenatal issues; however, it does outline some of the most commonly identified risks and patient concerns along with the appropriate advice that should be given.
Use of Seat Belts, Helmets, and Air Bags
The use of three-point seat belt restraints should be emphasized. There is no evidence that these restraints will increase the chance of fetal injury. The proper use of seat belts is a good predictor of a favorable maternal and fetal outcome in the case of vehicular trauma.72 The lap belt should be snugly and comfortably placed low across the maternal pelvis, below the uterine corpus and fundus and across the thighs, with the shoulder belt positioned between the breasts and no excessive slack allowed anywhere in the belt.73 The National Highway Traffic Safety Administration does not consider pregnancy to be an indication for the deactivation of air bags and recommends the combined use of seat belts and air bags.74 All patients should be encouraged to wear helmets when riding a bicycle or a motorcycle, whether voluntarily or in compliance with the law; these modes of transportation are ill-advised during pregnancy and certainly inappropriate during the third trimester.
Travel Restrictions during Pregnancy
Current ACOG guidelines suggest that occasional travel is generally safe for pregnant patients in the absence of maternal or obstetric complications (i.e., preeclampsia, intrauterine growth restriction).75 Most airlines allow women with normal pregnancies to travel up to 35 or 37 weeks. It should be emphasized to the pregnant traveler that she should walk about at least every 2 hours to minimize the risk for thromboembolism. Other concerns, such as noise, vibration, and cosmic radiation, are a negligible risk for the occasional pregnant air traveler.75 However, pregnant airline employees or frequent flyers should consult a physician during pregnancy because of their increased exposure to these potential risks.
Immunization during Pregnancy
The Advisory Committee on Immunization Practices posts guidelines for immunization during pregnancy on the website of the Centers for Disease Control and Prevention (CDC). Immunization of pregnant women should be guided by risk-versus-benefit logic. Relative indications for vaccination follow three guiding principles: (1) the risk of exposure is high, (2) the infection could cause harm to the mother or infant, and (3) the vaccine is unlikely to cause harm. Live virus vaccines generally are contraindicated during pregnancy because of the theoretic risk of transmission of the virus to the fetus. If a pregnant patient has received a live virus vaccine in the 3 months before the pregnancy, she should be referred for counseling on the potential risks to the fetus.76 The annual influenza vaccination is recommended by the CDC for pregnant patients as there is increased risk of acquiring the illness while pregnant and higher morbidity and mortality rates due to influenza.77 Comprehensive and specific guidelines for each vaccine are updated and posted on the CDC website (www.cdc.gov/vaccines/pubs/ACIP-list.htm).
Importance of Regular Appropriate Exercise
Patients should be encouraged to strengthen the abdominal muscles; to stretch the lower part of the back; to balance pectoralis muscle strength with that of the trapezius, rhomboids, and latissimus dorsi; to strengthen the cervical spine flexors; and to perform regular pelvic tilt exercises in all positions to correct the postural imbalance of pregnancy, to relieve discomfort, and to reduce the frequency of stress incontinence. Women with previous exercise regimens should be encouraged to continue these throughout their pregnancy and may even include high-impact activities such as jogging in the absence of medical or obstetric complications. Moderate exercise should be performed on most if not all days of the week, with special attention paid to adequate hydration and nutrition. Women starting an exercise regimen during pregnancy should generally wait until the second trimester and subsequently follow a graduated prescription for the amount and intensity of exercise throughout the remainder of their pregnancy.78 Aggressive abdominal exercises are discouraged after 12 weeks of gestation because they may exacerbate the development of diastasis recti. Moderate regular abdominal strengthening exercises will minimize the lordosis of pregnancy.
Diet, Vitamin Supplementation, and Weight Gain Recommendations
Obesity is an increasingly common concern that is complicating pregnancies worldwide. Estimates of obesity in pregnant women in the United States range from 18 to 38%.79 Obesity has been shown to complicate pregnancy by increasing rates of gestational diabetes, pregnancy-induced hypertension, preeclampsia, and venous thromboembolism.79
The ACOG recommends an average weight gain for pregnant women of between 10 and 12 kg.80 The normal physiologic changes of pregnancy account for approximately 9 kg. The remainder is maternal fat storage.81 The Institute of Medicine makes weight gain recommendations according to prepregnancy weight: underweight women (13-18 kg), normal-weight women (11-16 kg), overweight women (7-11 kg), and obese women (5-9 kg).82
Appropriate Management of Pregnant Patients with Substance Abuse Problems
The risks of smoking, illicit drug use, and excessive alcohol consumption during pregnancy are widely known to the lay public; however, despite warnings, some pregnant women continue to smoke and imbibe excessively. Cigarette smoking during pregnancy has declined steadily since 1989, to 18% in 2002-2003 and 16% in 2007-2008.83 Nonetheless, tobacco use by pregnant mothers aged 18 to 25 years continues to be high, with a reported rate of 22% in 2008. The U.S. Department of Health and Human Services Substance Abuse and Mental Health Services Administration concluded from its National Household Survey on Drug Abuse (2007-2008 database) that among women 15 to 44 years of age who were currently pregnant, 5% used one or more illicit drugs in the past month, 4.5% engaged in “binge” alcohol use, and slightly less than 20% were past-month cigarette smokers. Binge drinking was defined as drinking five or more drinks on the same day at least once during the previous 30 days. In comparison with a nonpregnant cohort, women who abuse substances reported curtailing their use during pregnancy and resuming use postpartum. Substance abuse during pregnancy was more frequent in unmarried women without a high-school education. The prevalence decreased as the amount of adult education increased. The prevalence of drug abuse during the last month of pregnancy showed no difference between Hispanic and white pregnant women. Drug abuse during pregnancy was significantly more common among black than among Hispanic patients.83
References
1. Ventura, SJ, Abma, JC, Mosher, WD, Henshaw, SK. Estimated pregnancy rates by outcome for the United States, 1990-2004. Natl Vital Stat Rep. 2008;56:1–25.
2. Martin, JA, et al. Births: Final data for 2009. Natl Vital Stat Rep. 2011;60:1–70.
3. Chumlea, WC, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111:110.
4. Xu, JQ, et al. Deaths: Final report for 2007. National Vital Statistics Reports. 2010;58:19.
5. Clark, SL, et al. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199(36):e1–36.
6. Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, Md: National Center for Health Statistics, 2011.
7. Ramoska, EA, Sacchetti, AD, Nepp, M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18:48–50.
8. Lippman, S, et al. Detection of unknown early pregnancy: A matter of safety. Postgrad Med. 1988;83:129.
9. Vanden Hoek, TL, et al. Part 12: Cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3):S829–S861.
10. Causey, AL, Seago, K, Wahl, NG, Voelker, CL. Pregnant adolescents in the emergency department: Diagnosed and not diagnosed. Am J Emerg Med. 1997;15:125–129.
11. Speert, H, Guttmacher, AF. Frequency and significance of bleeding in early pregnancy. JAMA. 1954;155:712–715.
12. Verburg, BO, et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: Longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31:388–396.
13. Kuklina, EV, Ayala, C, Callaghan, WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–1306.
14. Ramsey, E, Shilitto, J. How early can fetal heart pulsations be detected reliably using modern ultrasound equipment? Ultrasound. 2008;16:193–195.
15. Chadwick, JR. Value of the bluish coloration of the vaginal entrance as a sign of pregnancy. Trans Am Gynecol Soc. 1986;11:399.
16. Storring, PL, Gaines-Das, RE, Bangham, DR. International Reference Preparation of Human Chorionic Gonadotrophin for Immunoassay: Potency estimates in various bioassay and protein binding assay systems; and International Reference Preparations of the alpha and beta subunits of human chorionic gonadotrophin for immunoassay. J Endocrinol. 1980;84:295–310.
17. Cole, LA, Khanlian, SA, Sutton, JM, Davies, S, Rayburn, WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol. 2004;190:100–105.
18. Lazarenko, GC, Dobson, C, Enokson, R, Brant, R. Accuracy and speed of urine pregnancy tests done in the emergency department: A prospective study. CJEM. 2001;3:292–295.
19. Chard, T. Pregnancy tests: A review. Hum Reprod. 1992;7:701–710.
20. Lee, CL, Iles, RK, Shepherd, JH, Hudson, CN, Chard, T. The purification and development of a radioimmunoassay for beta-core fragment of human chorionic gonadotrophin in urine: Application as a marker of gynaecological cancer in premenopausal and postmenopausal women. J Endocrinol. 1991;130:481–489.
21. Hay, DL, Lopata, A. Chorionic gonadotropin secretion by human embryos in vitro. J Clin Endocrinol Metab. 1988;67:1322–1324.
22. Lenton, EA, et al. Normal and abnormal implantation in spontaneous in-vivo and in-vitro human pregnancies. J Reprod Fertil. 1991;92:555–565.
23. Steier, JA, Bergsjo, P, Myking, OL. Human chorionic gonadotropin in maternal plasma after induced abortion, spontaneous abortion, and removed ectopic pregnancy. Obstet Gynecol. 1984;64:391–394.
24. Daya, S, Woods, S, Ward, S, Lappalainen, R, Caco, C. Early pregnancy assessment with transvaginal ultrasound scanning. CMAJ. 1991;144:441–446.
25. Daya, S, Woods, S, Ward, S, Lappalainen, R, Caco, C. Transvaginal ultrasound scanning in early pregnancy and correlation with human chorionic gonadotropin levels. J Clin Ultrasound. 1991;19:139–142.
26. Fossum, GT, Davajan, V, Kletzky, OA. Early detection of pregnancy with transvaginal ultrasound. Fertil Steril. 1988;49:788–791.
27. McRae, A, Murray, H, Edmonds, M. Diagnostic accuracy and clinical utility of emergency department targeted ultrasonography in the evaluation of first-trimester pelvic pain and bleeding: A systematic review. CJEM. 2009;11:355–364.
28. ACOG Practice Bulletin No. 101. Ultrasonography in pregnancy. Obstet Gynecol. 2009;113(Pt 1):451–461.
29. Canadian Emergency Ultrasound Society. Recommended Standards. www.ceus.ca/002-standards/002-00.standards.htm.
30. ACOG Committee on Obstetric Practice. ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647–651.
31. McCollough, CH, et al. Radiation exposure and pregnancy: When should we be concerned? Radiographics. 2007;27:909–917.
32. Ginsberg, JS, Hirsh, J, Rainbow, AJ, Coates, G. Risks to the fetus of radiologic procedures used in the diagnosis of maternal venous thromboembolic disease. Thromb Haemost. 1989;61:189–196.
33. Ratnapalan, S, Bona, N, Koren, G. Ionizing radiation during pregnancy. Can Fam Physician. 2003;49:873–874.
34. Bentur, Y. Ionizing and nonionizing radiation in pregnancy. In: Koren G, ed. Maternal-Fetal Toxicology. A Clinician’s Guide. New York: Marcel Dekker, 2001.
35. Duke University and Duke Medicine Radiation Safety Division. Fetal Radiation Dose Estimates. www.safety.duke.edu/radsafety/fdose/fdxray.asp.
36. Patient Safety Authority, Commonwealth of Pennsylvania. Triage of the obstetrics patient in the emergency department: Is there only one patient? Pa Patient Saf Adv. 2008;5:96.
37. Menon, R, Bushnell, CD. Headache and pregnancy. Neurologist. 2008;14:108–119.
38. Marcus, DA. Managing headache during pregnancy and lactation. Expert Rev Neurother. 2008;8:385–395.
39. Von Wald, T, Walling, AD. Headache during pregnancy. Obstet Gynecol Surv. 2002;57:179–185.
40. Bickerstaff, ER, Small, JM, Guest, IA. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurochem. 1958;21:89–91.
41. Digre, KB, Varner, MW, Corbett, JJ. Pseudotumor cerebri and pregnancy. Neurology. 1984;34:721–729.
42. Grosset, DG, Ebrahim, S, Bone, I, Warlow, C. Stroke in pregnancy and the puerperium: What magnitude of risk? J Neurol Neurosurg Psychiatry. 1995;58:129–131.
43. Heit, JA, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann Intern Med. 2005;143:697–706.
44. Delvigne, A, Rozenberg, S. Systematic review of data concerning etiopathology of ovarian hyperstimulation syndrome. Int J Fertil Womens Med. 2002;47:211–226.
45. Andersson, RE, Lambe, M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281–1285.
46. Andersen, B, Nielsen, TF. Appendicitis in pregnancy: Diagnosis, management and complications. Acta Obstet Gynecol Scand. 1999;78:758–762.
47. Bickell, NA, Aufses, AH, Jr., Rojas, M, Bodian, C. How time affects the risk of rupture in appendicitis. J Am Coll Surg. 2006;202:401–406.
48. Tamir, IL, Bongard, FS, Klein, SR. Acute appendicitis in the pregnant patient. Am J Surg. 1990;160:571–575.
49. Mourad, J, Elliott, JP, Erickson, L, Lisboa, L. Appendicitis in pregnancy: New information that contradicts long-held clinical beliefs. Am J Obstet Gynecol. 2000;182:1027–1029.
50. Oto, A, et al. MR imaging in the triage of pregnant patients with acute abdominal and pelvic pain. Abdom Imaging. 2009;34:243–250.
51. Ekman-Ordeberg, G, Salgeback, S, Ordeberg, G. Carpal tunnel syndrome in pregnancy. A prospective study. Acta Obstet Gynecol Scand. 1987;66:233–235.
52. Ostgaard, HC, Zetherstrom, G, Roos-Hansson, E. The posterior pelvic pain provocation test in pregnant women. Eur Spine J. 1994;3:258–260.
53. American College of Obstetricians and Gynecologists. Trauma during pregnancy, ACOG Technical Bulletin 161—November 1991. Int J Gynaecol Obstet. 1993;40:165–170.
54. Moise, KJ, Jr., Belfort, MA. Damage control for the obstetric patient. Surg Clin North Am. 1997;77:835–852.
55. Ali, J, Yeo, A, Gana, TJ, McLellan, BA. Predictors of fetal mortality in pregnant trauma patients. J Trauma. 1997;42:782–785.
56. Aboutanos, SZ, et al. Predictors of fetal outcome in pregnant trauma patients: A five-year institutional review. Am Surg. 2007;73:824–827.
57. Schiff, MA, Holt, VL, Daling, JR. Maternal and infant outcomes after injury during pregnancy in Washington State from 1989 to 1997. J Trauma. 2002;53:939–945.
58. Lewis G, ed. Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer—2003-2005. London: CEMACH, 2007.
59. Jeejeebhoy, FM, et al. Management of cardiac arrest in pregnancy: A systematic review. Resuscitation. 2011;82:801–809.
60. Committee on Wife Assault. Reports on Wife Assault. Toronto: Ontario Medical Association; 1991.
61. Centers for Disease Control (CDC). Family and other intimate assaults—Atlanta, 1984. MMWR Morb Mortal Wkly Rep. 1990;39:525–529.
62. Bland, R, Orn, H. Family violence and psychiatric disorder. Can J Psychiatry. 1986;31:129–137.
63. Bain, JE. Wife assault: Physicians cannot ignore it. CMAJ. 1991;144:283–284.
64. Flitcraft, AH. Violence, values, and gender. JAMA. 1992;267:3194–3195.
65. Lent, B. Wife abuse in pregnancy: The role of the physician. Perinatal Outreach Program of Southwestern Ontario Newsletter. 1991;9:1.
66. Shah, PS, Shah, J. Maternal exposure to domestic violence and pregnancy and birth outcomes: A systematic review and meta-analyses. J Womens Health (Larchmt). 2010;19:2017–2031.
67. Stewart, DE, Cecutti, A. Physical abuse in pregnancy. CMAJ. 1993;149:1257–1263.
68. Gee, V. Perinatal Statistics in Western Australia. Ninth Annual Report of the Western Australian Midwives’ Notification System. Perth: Health Services Statistics and Epidemiology Branch, Health Department of Western Australia; 1991.
69. Sampselle, CM, Petersen, BA, Murtland, TL, Oakley, DJ. Prevalence of abuse among pregnant women choosing certified nurse-midwife or physician providers. J Nurse Midwifery. 1992;37:269–273.
70. Webster, J, Sweett, S, Stolz, TA. Domestic violence in pregnancy. A prevalence study. Med J Aust. 1994;161:466–470.
71. Jaffe, P, et al. Emotional and physical health problems of battered women. Can J Psych. 1986;31:625.
72. Pearlman, MD, et al. A comprehensive program to improve safety for pregnant women and fetuses in motor vehicle crashes: A preliminary report. Am J Obstet Gynecol. 2000;182:1554–1564.
73. Van Hook, JW. Trauma in pregnancy. Clin Obstet Gynecol. 2002;45:414–424.
74. Final Report: National Conference on Medical Indications for Air Bag Disconnection. George Washington University Medical Center, 1997. www.nhtsa.gov/airbags/air%20bag%20conference%20final%20report.doc.
75. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 443: Air travel during pregnancy. Obstet Gynecol. 2009;114:954–955.
76. Atkinson, WL, et al. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR Recomm Rep. 2002;51:1–35.
77. Fiore, AE, et al. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62.
78. Davies, GA, Wolfe, LA, Mottola, MF, MacKinnon, C. Joint SOGC/CSEP Clinical Practice Guideline: Exercise in pregnancy and the postpartum period. J Obstet Gynaecol Can. 2003;25:516–522.
79. Guelinckx, I, Devlieger, R, Beckers, K, Vansant, G. Maternal obesity: Pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9:140–150.
80. American College of Obstetricians and Gynecologists, Committee on Professional Standards. Standards for Obstetric-Gynecologic Services, 7th ed. Washington, DC: American College of Obstetricians and Gynecologists; 1989.
81. Hytten, F. Weight gain in pregnancy. In: Hytten F, Chamberlain G, eds. Clinical Physiology in Obstetrics. Oxford: Blackwell Scientific, 1991.
82. Institute of Medicine and National Research Council. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington DC: The National Academies Press; 2009.
83. Substance Abuse and Mental Health Services Administration, Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, Md:Office of Applied Studies; 2009. http://oas.samhsa.gov/nsduh/2k8nsduh/2k8Results.cfm.