Chapter 87 Gastrointestinal Pharmacology
Nausea and Vomiting
Introduction and Definitions
Uncontrolled nausea and vomiting in the pediatric intensive care unit may result in consequences that range from minor patient discomfort to life-threatening infection or electrolyte imbalance. To the patient this can mean wound dehiscence, gastrointestinal (GI) bleeding, malnutrition, dehydration, pulmonary aspiration, and increased anxiety with future medical interventions.1 Indeed, nausea and vomiting has been referred to as the “big little problem” with the capacity to delay discharge, monopolize nursing care, and impact financial resources.2 These costs can be limited with a focus on prophylactic treatment, employing recent drug developments and guideline-driven therapy when possible. The data that drive these guidelines are, particularly in pediatrics, largely based on studies that look at vomiting more specifically than nausea.3 Because nausea is an unpleasant, largely subjective sensation in the epigastrium that may be associated with vomiting, it is more difficult to quantify objectively.3,4 Vomiting, defined as the forceful expulsion of gastric contents from the mouth, can be more easily measured for medication assessment purposes. Although distinct, when vomiting is discussed here, it can be inferred to also pertain to managing nausea (unless otherwise noted).
Pathophysiology
The vomiting center or emetic center or “central pattern generator,” located in the medulla oblongata, is a collection of neurons that may trigger vomiting after receiving input from the chemoreceptor trigger zone, abdominal vagal afferents or cerebral cortex.1,5–7 The chemoreceptor trigger zone, located in the area postrema region near the fourth ventricle, is not fully protected by the blood-brain barrier; and is therefore vulnerable to antineoplastic agents, or other stimuli in the blood or cerebral spinal fluid.5–7 The vagus nerve provides important stimuli to both the chemoreceptor trigger zone and vomiting center, and appears to have the largest role in CINV.5,7 This occurs when cells within the GI tract are exposed to chemotherapy and release mediators, including 5-hydroxytryptamine (5-HT), substance P, and cholecystokinin, that stimulate the vagus nerve.5,7 Finally, the role of the cerebral cortex is less well defined, but is thought to play a role in anticipatory nausea and vomiting.1
The mechanism described is most dependent upon the neurotransmitter receptors for dopamine, serotonin (5-hydroxytryptamine type 3), and substance P.1,5–7 Additional receptors that are thought to play a supporting role include corticosteroid, histamine, cannabinoid, acetylcholine, opiate, neurokinin-1, and gamma-aminobutyric acid.1,6,8 The intricate cascade briefly described here and multiple neurotransmitter involvement, foreshadow treatment guidelines that will often be rooted in multiple agents that target various pathways.
Chemotherapy-Induced Nausea and Vomiting: Types of Emesis
Emesis that occurs from chemotherapy is divided into three distinct categories. Anticipatory CINV occurs before chemotherapy administration and occurs as a conditioned response in patients that have had significant past CINV. Acute CINV occurs within 24 hours of chemotherapy administration and is the most widely studied. Delayed nausea and vomiting occur after 24 hours and may persist for 7 days. Pediatric patients that have well-controlled acute nausea and vomiting are less likely to have delayed nausea and vomiting.8 This highlights the importance of “staying ahead” of symptoms with prophylactic treatment.
Treatment Guidelines
The advent of 5-HT3 receptor antagonists in the early 1990s provided a new cornerstone in how acute nausea and vomiting is managed in patients receiving highly or moderately emetogenic chemotherapy. The first-generation 5-HT3 receptor antagonists of dolasetron, granisetron, and ondansetron have been established in pediatrics to be equally effective, and equally toxic, with the most common side effects including headache, constipation, and dizziness.3,9–13 It should be noted that the approved pediatric dose of granisetron of 10 ug/kg once daily is likely ineffective, and a dose of 40 ug/kg once daily is more appropriate.3,13,14,20 The 5-HT3 receptor antagonists have been established, at the appropriate doses, to be as effective when given orally as when given intravenously, and that single daily dose schedules are as effective as multiple-dose schedules.15–20 These agents are most useful for CINV occurring within the first 24 hours after administration of chemotherapy.21
In 2003, what may be considered a “second-generation” 5-HT3 receptor antagonist, palonosetron, gained Food and Drug Administration approval for both acute and delayed CINV. It is unique from other 5-HT3 antagonists because of its long half-life (21 to 37 hours in children and 40 hours in adults) and greater affinity for 5-HT3 receptors.14,22 Three phase III trials have established palonosetron as not inferior to older 5-HT3 antagonists.23–25 A trial comparing palonosetron with ondansetron in pediatric patients found a significant reduction in emesis on the first 3 days after treatment, and in nausea intensity the first 4 days after treatment favoring the palonosetron group.26 Further prospective study is needed before palonosetron can be established as superior to other 5-HT3 receptor antagonists. To date, CINV guidelines have not stated any one 5-HT3 receptor antagonist to be superior to another.20
Corticosteroids, most commonly dexamethasone or methylprednisolone, are potent antiemetics and have a critical role in managing acute and delayed CINV in pediatrics. Their exact mechanism of action is unclear, but may involve decreasing 5-HT3 release in the periphery.27 However, corticosteroids also have the potential to decrease the effect of antineoplastic drugs on brain tumors, osteosarcomas and carcinomas.27 Dexamethasone may augment the ability of a number of carcinomas to resist both radiation and chemotherapy.3 Interferon or interleukin-2 may be less effective when used along with steroids.1 Corticosteroids may also impact the quality of brain tumor images generated by computed tomography or magnetic resonance imaging by altering the distribution of contrast media.3 In addition to these concerns, the usual side effects of steroids must be kept in mind, including: hyperglycemia, hypokalemia, anxiety, euphoria, and insomnia. It would be prudent to consult a patient’s chemotherapy protocol or an oncologist before initiating corticosteroids in a pediatric patient with a malignancy to verify the appropriateness of this approach.
The neurokinin-1 receptor antagonist is the most recent antiemetic class. These are effective for both acute and delayed nausea and vomiting in moderately and highly emetogenic chemotherapy. Such agents are approved in CINV only when used in conjunction with a 5-HT3 antagonist and a corticosteroid. Currently the only medications that represent this class on the market are oral aprepitant and intravenous fosaprepitant. They are generally well tolerated with diarrhea, fatigue, headache, and hiccups being common side effects.28–30 Both aprepitant and fosaprepitant are substrates, inhibitors and inducers of the cytochrome P450 enzymes 3A4 and 2C9.14 A thorough review of potential interactions for individual patients is merited. Most notable of these cytochrome interactions are: The effect of Coumadin (brand name form of the generic warfarin) on coagulation pathway (altered prothrombin time, International Normalized Ratio) may be reduced, the concentration of corticosteroids will be increased with a dose reduction needed when used for CINV with aprepitant, and a backup contraception method should be used for patients taking oral contraceptives.30 This class of medication does not have approval for patients younger than 18 years, nor are suggested dose modifications available.14 One study examined aprepitant dosed for young adults in patients ages 11 to 19 years weighing 43 to 105 kg.31 Aprepitant containing regimens performed comparably to non-aprepitant-containing regimens, but an increase in the number of cases of febrile neutropenia was noted in the aprepitant patients.31 Further prospective study is necessary before neutrokinin-1 receptor antagonists are prescribed in pediatric patients.
Other agents commonly seen in pediatrics for CINV include benzodiazepines, most often lorazepam, which is routinely employed for anticipatory nausea and vomiting at a dose of 0.04 to 0.08 mg/kg/dose (maximum dose, 4 mg).14 Diphenhydramine is anecdotally used in pediatric patients despite its absence from guidelines and reviews of CINV.3,20 Dopamine receptor antagonists, including phenothiazines (e.g., prochlorperazine), butyrophenones (e.g., droperidol), and benzamides (e.g., metoclopramide), cause a high incidence of dystonic reactions when used at high doses for several days and potentially oculogyric crisis.20,32 Cannabinoids (i.e., dronabinol) are limited by their lack of pediatric data, and their side effects, which include euphoria, sedation, depression, and hallucinations.14 A scopolamine patch may have some utility in patients older than 12 years that are able to wait up to 12 hours for relief.3 Generally speaking, these agents should only be considered for pediatric patients who do not tolerate first-line therapy alone.
Determining appropriate antiemetic therapy requires one to first evaluate the likelihood that a particular chemotherapy regimen will produce nausea or vomiting.20,33–35 This has been accomplished with literature and expert opinion and is summarized in Table 87-1, with subsequent dosing information provided in Table 87-2.14,20,33–40 This categorization is largely based on adult data, so it is vital to take individual patient parameters and CINV history into account. If a chemotherapy regimen is close to the next highest emetic risk category, it is better to be aggressive with antiemetic management.
Table 87–1 Antiemetic Selection for Chemotherapy Based on Emetogenicity of Frequently Encountered Single-Day Pediatric Chemotherapy
| Emetic Risk (Incidence of Emesis with no Prophylaxis) | Antineoplastic Agent∗ | Recommended Antiemetic |
|---|---|---|
| High (>90%) |
5-HT3, 5-Hydroxytryptamine-3; NK-1, neurokinin-1.
∗ Table focuses on more common pediatric chemotherapy (see references 20 and 33 through 38 for complete list). Medication is parental unless otherwise stated.
Table 87–2 Antiemetic Dosing in Chemotherapy-Induced and Postoperative Nausea and Vomiting14,36,39,40
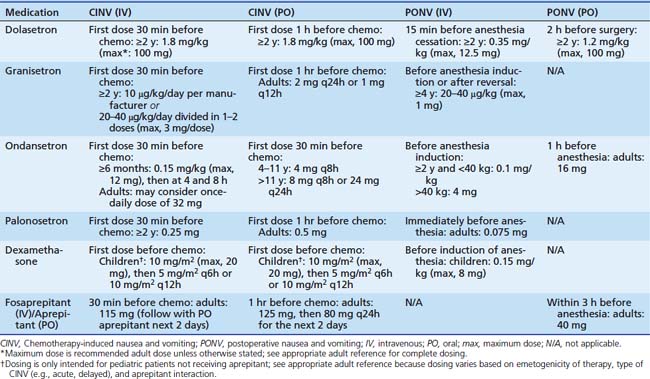
Table 87-1 includes aprepitant and dexamethasone that may be contraindicated for multiple reasons in children as discussed previously. In such patients, medications that are less-well supported for delayed CINV, such as diphenhydramine or 5HT3 receptor antagonists, may be employed based on individual practitioner preference. Other areas of future research include breakthrough emesis that occurs despite adequate prophylaxis in a single cycle, and refractory emesis that occurs despite adequate prophylaxis over multiple cycles.8 Additional areas that require further investigation in pediatric CINV include multiple-day chemotherapy, high-dose chemotherapy with stem cell rescue, and intractable symptoms in terminal patients.41–43 Finally, each institution would be well advised to conduct a pharmacoeconomic review to aid in antiemetic selection of pharmacologically equivalent products.
Postoperative Nausea and Vomiting
Assessing the risk of the pediatric surgical candidate is the first step in determining PONV prophylaxis. Strabismus repair, tonsillectomy, hernia repair, orchiopexy, penile surgery, or any procedure where anesthesia time is 30 minutes or longer have an increased likelihood of PONV.4,40,44 Children aged 3 years and older continue to increase their risk of PONV until they become pubescent.40,44 Patients who have a history of PONV, motion sickness, or patients with first-degree relatives with such histories are more prone to have PONV.40,44 Prophylaxis is recommended only for children thought to be at moderate or high risk of PONV.4 Regional anesthesia is optimal in such patients.4 However, if general anesthesia is necessary, the following may reduce the risk of PONV: using intraoperative supplemental oxygen, adequate hydration, minimizing opioid or neostigmine exposure, and avoiding nitrous oxide or volatile anesthetics when possible.40
Individual institutions should determine which patients are at moderate or high risk. These patients should receive a combination of two or three agents with distinct mechanisms of action for PONV prophylaxis.4,40 A 5HT3 receptor antagonist and dexamethasone combination is a reasonable first choice.4 Other options include dimenhydrinate, perphenazine, or droperidol.4,40,44 Because of potential for QT prolongation and torsades des pointes, droperidol should be reserved for patients who have failed first-line therapy and are hospital inpatients.4 Droperidol should be dosed once for prophylaxis at 0.05 mg/kg (maximum, 1.25 mg), or at 0.01 to 0.03 mg/kg/dose for PONV treatment.14,40 Finally, NK-1 receptor antagonists have been established as potent agents for PONV in adult patients, but even if approved in pediatrics it seems likely a multiple agent approach to PONV as described above will remain the standard of care.45–48
Diarrhea
Introduction and Definition
It is estimated that in the United States, diarrhea-based illnesses result in approximately 200,000 hospital admissions and 400 deaths per year.49 The majority of acute diarrhea episodes stem from infections to the GI tract and it is estimated that 20% to 40% of children treated with broad-spectrum antibiotics will develop diarrhea.50,51 Infectious causes include viruses (e.g., Rotavirus), bacteria (e.g., Escherichia coli, Shigella, Clostridium difficile), and parasites (e.g., Cryptosporidium parvum, Giardia lamblia). Noninfectious causes include appendicitis, intussusception, food allergies, irritable bowel syndrome, and ulcerative colitis. Diarrhea may be defined for practical purposes as a decrease in stool consistency, along with an increase in frequency to more than three bowel movements in 24 hours.49 After diarrhea is identified, rehydration is paramount. Identifying the underlying cause will guide specific treatment.
Treatment
If C. difficile is identified, any potentially contributing broad-spectrum antibiotics should be discontinued, or streamlined to more a specific agent if possible. Oral metronidazole for 10 days is first line therapy in mild to moderate disease.51,52 Cases that require protracted courses of metronidazole should include monitoring for peripheral neuropathy. Oral vancomycin should be considered for patients with severe symptoms, intolerance to metronidazole, or metronidazole failure.51–53 Indeed, there is growing evidence in the adult population of superior outcomes when vancomycin is used first line for severe disease.52,53 Probiotics, bile-acid sequestrants, intravenous immunoglobulin, or tapering doses of vancomycin may be considered for recurrent cases. The impact of C. difficile can be minimized with prevention that includes good hand hygiene, infection control, and appropriate antibiotic usage. It would be prudent to limit acid suppressing agents (e.g., proton pump inhibitors, histamine H2 antagonists) to legitimate indications, as a positive association has been identified in adult populations between acid suppression and C. difficile disease.54,55
Probiotics are widely defined as live microorganisms that offer health benefits. These agents have been studied in pediatric patients in terms of potential reduction in antibiotic-associated diarrhea. One meta-analysis found that for every seven pediatric patients administered probiotics, one fewer would develop antibiotic-associated diarrhea. Positive risk reduction was identified, in decreasing strength, for those treated with Lactobacillus GG, Saccharomyces boulardii, and the combination of Bifidobacterium lactis and Streptococcus thermophilus.56 Significant reductions were not found with L. acidophilus/Bifidobacterium infantis or L. acidophilus/L. bulgaricus.56 A second meta-analysis reviewing Lactobacillus GG, L. sporogenes, and Saccharomyces boulardii found a significant decrease in antibiotic-associated diarrhea but nonsignificant overall results in intent-to-treat analysis.57 Such agents require further study from an efficacy and pharmacoeconomic standpoint before they become the standard of care. If these agents are used, sufficient live organism content should be verified. These agents should be used cautiously, if at all, in premature infants and in patients with any of the following: short bowel syndrome, central catheters, cardiac valve disease, and immune compromise.58,59
Other important treatments for diarrhea include the antimotility agents loperamide and diphenoxylate. Both inhibit excessive GI peristalsis and increase GI transit time.14 Neither medication should be used in diarrhea resulting from pseudomembranous enterocolitis or any infectious cause, because it is critical that toxins produced are cleared from the GI tract.14,49 Underlying electrolyte imbalances and dehydration should be addressed before these antimotility agents are initiated, because the potential exists that intestinal fluid retention may exacerbate such underlying conditions. Side effects to be mindful of include: sedation, confusion, ileus, and respiratory depression (with younger children at increased risk).14
Constipation
Introduction and Definition
Constipation is defined as a reduction in the number of bowel movements. This number varies significantly based on the infant or child’s age and breastfeeding status with exclusively breast-fed infants having an increased frequency of bowel movements.60,61 The differential diagnosis underlying constipation in a pediatric intensive care unit is extensive and includes: anatomical (e.g., anal stricture, rectal abscess), metabolic (e.g., hypothyroidism, cystic fibrosis), neurologic (e.g., Hirschsprung disease, cerebral palsy), and connective tissue disorder (e.g., systemic lupus erythematosus).61 Medications such as opiates, phenobarbital, antacids, antihypertensives, anticholinergics, and antidepressants, and lead may all contribute to a child’s constipation.61
Treatment
Any underlying disease state or anatomical anomaly should be managed individually. If medication related, the offending agent should be discontinued if possible; or the minimal effective dose should be targeted. Constipation itself is addressed as a two-step process of disimpaction lasting 3 to 5 days, followed by maintenance. The duration of the latter stage is determined by symptom recurrence when treatment is withdrawn.60,62
Disimpaction may be accomplished with oral or rectal medication administration. The oral route is less invasive and may give a sense of empowerment to the child, but the rectal route is quicker and may be necessary if the child has abdominal pain. Oral options include mineral oil, oral electrolyte solutions, lactulose, sorbitol, magnesium hydroxide, magnesium citrate, senna, and bisacodyl. Mineral oil may result in lipoid pneumonia if aspirated and is not recommended in children younger than 1 year or any patient on aspiration precautions. Options for rectal disimpaction include phosphate soda, saline, or mineral oil enemas followed by a phosphate enema. Also effective are glycerin suppositories in infants, and bisacodyl suppositories in older children. Soapsuds, tap water, and magnesium enemas are discouraged because of their potential toxicity.60 Enemas are to be avoided in children younger than 3 years and in young children with severe neurologic deficiencies as they are more likely to be retained with subsequent resulting toxicities.62
Maintenance therapy with polyethylene glycol 3350 without electrolytes (MiraLax) has demonstrated superior palatability in children, and although not yet recommended by guidelines in infants, there is growing evidence of its safe use in this population.60,62 Other maintenance therapies include mineral oil, magnesium hydroxide, lactulose, and sorbitol. Bisacodyl or senna may be necessary as a stimulant laxative as rescue therapy, but should be avoided for extended use.60
Gastroesophageal Reflux Disease
Treatment of gastroesophageal reflux disease (GERD) should alleviate symptoms and prevent or heal esophageal damage. Although researchers conducting multiple pediatric studies have used varying doses, multiple drug therapies, and non-pharmacologic therapies, guidelines do exist for the treatment of GERD in infants and children.63 Many patients who may be admitted to the intensive care unit for other conditions are likely to have preexisting gastroesophageal reflux conditions and corresponding surgical and pharmacologic therapies. These populations include patients with reactive airway disease, recurrent pneumonia, neurologic impairment, premature infants, and genetic syndromes including Down syndrome.
Histamine-2 Receptor Antagonists
Histamine-2 (H2) receptor antagonists inhibit gastric acid secretion by means of competitive inhibition of H2 receptors of the gastric parietal cells. H2 receptor antagonists are generally well tolerated. Common adverse effects include nausea and headache. Although thrombocytopenia may occur, it is often difficult to determine the sole cause in critically ill patients. Ranitidine should be avoided in patients with acute porphyria. Tachyphylaxis is well documented for intravenous and oral H2 receptor blockers in adult and pediatric populations. Infants may suffer from irritability, headache, and somnolence, which when mistaken for GERD symptoms, may lead to an unwarranted increase in dose.63 All of the currently available H2 receptor blockers require dosage adjustment for renal impairment. Although fewer pediatric studies exist for ranitidine and famotidine, these agents are frequently used and may be preferred over cimetidine because of fewer drug interactions and central nervous system adverse effects. Availability of both oral and intravenous formulations allow for easy continuation of therapy when patients are admitted to the intensive care unit.
Proton Pump Inhibitors
By covalently bonding to the hydrogen-potassium-adenosine triphosphatase (ATP) of parietal cells, proton pump inhibitors (PPIs) suppress gastric acid secretion for the life of the parietal cell. Ideally, therapy should be given 30 minutes before feeding so that peak plasma concentrations coincide with parietal cell stimulation. Administration with food reduces bioavailability by fifty percent. Although their serum half-lives are relatively short; the pharmacological effect persists until new parietal cells are generated.64 Omeprazole, esomeprazole, and lansoprazole have been approved for use in pediatrics. Extemporaneously compounded suspensions of omeprazole and lansoprazole are frequently used, even in patients with various types of feeding tubes. Intravenous lansoprazole is no longer available. Neither intravenous pantoprazole nor esomeprazole is Food and Drug Administration (FDA)–approved for use in pediatric patients. Until sufficient pediatric dosing information becomes available, intravenous PPIs are likely to be reserved for patients with active GI bleeding or contraindication to alternative therapies. Although PPIs are generally thought of as well tolerated, both long- and short-term adverse effects are gaining more attention. Reactions include idiosyncratic reactions, drug-drug interactions, drug-induced hypergastrinemia, and drug-induced hypochlorhydria.63 The most common side effects are headache, diarrhea, constipation, and nausea.14 Case reports of biopsy proven interstitial nephritis associated with PPI use have emerged. Thus far no pediatric cases have been reported.63 The FDA and Health Canada are currently investigating potential links between PPIs and decreased effectiveness of clopidogrel. Although the data are primarily from adult reports, the potential interaction may be relevant in pediatrics as well. PPIs should not be discontinued abruptly because a hypersecretory phase results.63,65 Acid suppression, whether from H2 receptor antagonists or PPIs, may increase the rates of community-acquired pneumonia, gastroenteritis, candidemia, and necrotizing enterocolitis.63,66–68
Antacids
Both aluminum hydroxide and magnesium hydroxide are effective treatments of GERD. In infants, however, aluminum hydroxide formulations can increase plasma levels of aluminum to those reported to cause osteopenia, microcytic anemia, and neurotoxicity. Aluminum-based antacids should be avoided in patients with renal impairment. Efficacy of calcium carbonate antacids has not been demonstrated in pediatric patients. Depending on the antacid formulation chosen, adverse effects may include electrolyte disturbances, nausea, flatulence, diarrhea, or constipation. Because alternative therapies are available and generally well tolerated, antacids should be reserved for intermittent symptom management rather than chronic treatment of GERD.63
Surface Agents
Sucralfate, an aluminum salt of sulfated sucrose, forms a protective barrier by binding to damaged mucosa in an acidic environment. Although it has demonstrated effectiveness in the treatment of esophagitis, it is not recommended for long-term treatment of GERD in children because of a lack of efficacy data and safety concerns associated with aluminum toxicity.63
Prokinetic Therapy
Erythromycin is a macrolide antibiotic and motilin agonist. It increases motility in the proximal GI tract. The most frequent adverse effects include abdominal cramping, nausea, vomiting, diarrhea, cardiac dysrhythmias with interacting medications, and risk of bacterial overgrowth. Metoclopramide promotes gastric emptying by acting as an antagonist to the inhibitory actions of dopamine in the gut. It also sensitizes the gut to acetylcholine and increases lower esophageal sphincter tone.69 Metoclopramide blocks dopamine receptors in the chemoreceptor trigger zone and accelerates gastric emptying and intestinal transit time without stimulating gastric, biliary, or pancreatic secretions.14 Adverse effects are common; are dose dependent; and can include extrapyramidal effects, seizures, and Parkinsonian reactions, which may be irreversible.14,63 The FDA required the addition of a boxed warning to metoclopramide drug labels regarding the risks of long-term or high-dose use. Chronic use of metoclopramide has been linked to tardive dyskinesia, which may include involuntary and repetitive movements of the body, even after drug discontinuation. It is noted that these adverse effects are more prevalent in elderly populations.70 Extrapyramidal reactions in children and younger adults are more likely after intravenous administration of high doses, particularly within 24 to 48 hours after starting therapy.14 Prescribers are urged to carefully consider legitimate indications for initiating drug therapy. Current GERD guidelines recommend against use of prokinetic agents.
Gastroesophageal Reflux Disease and Acute Life-Threatening Event
An acute life-threatening event (ALTE) is an episode of combined apnea, color change (cyanosis, pallor, plethora), abnormal muscle tone (limpness and stiffness), choking, and gagging that requires intervention. Some patients have succumbed to sudden infant death syndrome who have had a history of ALTE with documented GER. This combination is rare and assigning the timing and causality of the event is extremely difficult. Neither pharmacotherapy nor surgical intervention has been adequately studied in secondary prevention of ALTE. Those patients who may benefit from pharmacologic intervention may include those whose ALTEs were obviously associated with vomiting, regurgitation, or obstructive apnea.63
Stress-Induced Mucosal Damage: Ulcer Prophylaxis
Prospective studies reveal similar incidence of GI hemorrhage between adult and pediatric intensive care units. Of 1006 patients admitted to a pediatric intensive care unit, 10.2% had upper GI hemorrhage; however, only 1.6% of the admissions were viewed as clinically significant. Respiratory failure, coagulopathy, and a Pediatric Risk of Mortality score greater than or equal to 10 were identified as independent risk factors for GI hemorrhage.71,72 Other studies also identified pneumonia, multitrauma, a surgical operation longer than 3 hours, and circulatory shock in patients as high-risk factors.73,74 One subsequent pediatric study demonstrated mechanical ventilation as the only statistically significant risk factor for clinically significant bleeds after multivariate analysis.75 Children ventilated for greater than 48 hours were at additional risk of hemorrhage if thrombocytopenia, coagulopathy, organ failure, high pressure ventilator settings (>25 cm H2O), or Pediatric Risk of Mortality score greater than 10 were present.76 Mechanical ventilation and coagulopathy are recognized as independent risk factors in adult patients.72 Because of the low incidence of clinically significant hemorrhage, prophylaxis may only be warranted in those patients with risk factors.77
Sucralfate, H2 receptor antagonists, antacids, and PPIs have been used for prophylaxis of stress-induced mucosal damage in pediatric critical care patients. Individual trials including comparative studies and case series have shown each agent to be effective. With the low incidence of clinically significant bleeds, studies intending to demonstrate superiority of one drug class or agent over another would require an extremely large sample size. Therefore pediatric studies use a surrogate endpoint of gastric pH 4 or greater to evaluate efficacy of various dosing regimens.78–80
One study included 165 patients treated with ranitidine, antacid, sucralfate, or placebo. There were no differences among the treatment groups and all treatment groups had a lower incidence of bleeding than the placebo group.81 Another study compared ranitidine, omeprazole, sucralfate, or placebo for stress ulcer prophylaxis and also compared adverse events. No differences were found among groups with regard to macroscopic stress ulcer bleeding, mortality, or ventilator-associated pneumonia.82 Because multiple agents appear to be effective for stress ulcer prophylaxis, selecting an appropriate agent may be patient specific. However, when prescribing prophylaxis to adult patients, the inadequacy of sucralfate it is widely recognized after the landmark trial by Cook et al.83 published in 1998 that demonstrated significantly lower rates of clinically relevant bleeding in the ranitidine treatment group. In addition there was no significant difference in pneumonia rates in this 1200-patient trial. Critical care patients do present challenges with regard to drug administration for antacids and sucralfate. Both agents require multiple doses per day, lack intravenous formulations, and may chelate or inhibit absorption of other drugs. In addition, aluminum toxicity may be of greater concern in critical care patients because of the potential for renal impairment. H2 receptor antagonists are commonly used because of the availability of intravenous formulations. Adverse effects of the central nervous system, particularly in patients with renal impairment who have not been dose adjusted; thrombocytopenia; and drug interactions remain a concern. Most of these adverse events, however, can be prevented or at least monitored for early detection. PPIs may also be used orally, intravenously, or off-label through feeding tubes.
Stress ulcer prophylaxis is recommended, along with other supportive therapies, for acute lung injury and acute respiratory distress syndrome in children.84 Stress ulcer prophylaxis is also recommended as supportive care for patients with sepsis in the 2008 Surviving Sepsis Campaign. H2 receptor antagonists use was recommended with a stronger level of evidence than PPIs.85 Some experts believe that although greater acid suppression is achieved with PPIs over H2 receptor antagonists, PPIs should be used as second-line agents for stress ulcer prophylaxis because of the greater association with adverse effects such as community-acquired pneumonia, ventilator acquired pneumonia, and C. difficile infection.86
Gastrointestinal Hemorrhage
Clinical guidelines for treatment of nonvariceal upper GI bleeding in adults include recommendations for pharmacotherapy.87 Unfortunately, this body of evidence does not exist in the pediatric population. H2 receptor antagonist use is discouraged as primary treatment for patients with acute bleeding. Although octreotide and somatostatin are more effective than H2 receptor antagonists, these agents are also not recommended for routine management. PPIs are cited as the drugs of choice for treatment of acute nonvariceal upper GI bleeding.87 High-dose intravenous proton pump inhibitor therapy (80 mg pantoprazole bolus followed by 8 mg/hr for 72 hours after endoscopic therapy39) is preferred over H2 receptor antagonists alone, or in combination with octreotide, to prevent rebleeding in adult patients. As more data become available regarding the use of intravenous proton pump inhibitors in pediatric patients, parallels may be seen between the treatments of adult and pediatric nonvariceal upper GI bleeding.
Octreotide and Somatostatin
Octreotide is a somatostatin analogue. Its pharmacologic actions include inhibition of gastric acid secretion and decreased splanchnic blood flow. Although its 1.7-hour half-life is approximately 30 times longer than that of somatostatin, octreotide is usually administered through continuous infusion for GI hemorrhage. Adverse effects include abdominal pain, sinus bradycardia, nausea, pain at the site of injection, elevation of liver enzymes, chest pain, emesis, headache, dizziness, fatigue, flushing, and diarrhea. Long-term use (longer than 1 month) has been associated with changes in thyroid function, gallstone formation, and cholecystitis.88
In adults, octreotide has been shown to decrease the duration of hemorrhage and prevent recurrence of peptic disease more so than cimetidine or ranitidine. A Cochrane Review cited improved initial hemostasis and decreased transfusion requirements; however, no change was evident in rebleeding or secondary outcomes when octreotide was used to treat variceal bleeding.88 Few researchers have evaluated octreotide for GI hemorrhage in children. In one study, continuous infusion of octreotide in seven patients was evaluated. Bleeding ceased in six of the seven patients. The duration of infusion ranged from 24 to 234 hours. Although bleeding stopped within 24 hours in nearly half of the patients, on average, bleeding stopped after 40 hours. The most notable adverse effect was hyperglycemia.89 Subcutaneous octreotide has also been used for severe chronic GI hemorrhage for periods of 24 to 50 months.90
Vasopressin
Generally vasopressin has been used for treatment of variceal bleeding more frequently than for nonvariceal upper GI bleeding. In fact, adult guidelines do not address the use of vasopressin for non-variceal bleeding.87 Vasopressin acts by mechanisms similar to octreotide; however, its effects on blood pressure make it a less desirable option in many patients.91 From a medication safety perspective, vasopressin prescribing can be confusing given the wide range of accepted dosing conventions and variety of indications in pediatric patients (e.g., diabetes insipidus, GI hemorrhage, shock). Dosing recommendations that require units per kg per unit time require careful decimal place checks. Intensive care units should decide on the accepted dosing convention and dose range for vasopressin for each indication and use infusion pumps with safety software that can accommodate the predetermined limits to prevent 10-fold dosing errors.
Helicobacter Pylori Infection
Patients who have GI bleeding of unknown etiology should be tested for Helicobacter pylori. At minimum, therapy requires three different medications administered twice daily for 1 to 2 weeks. Three different regimens are recommended for first-line treatment in pediatrics. Each consists of a proton pump inhibitor in combination with amoxicillin and clarithromycin, amoxicillin and metronidazole, or metronidazole and clarithromycin. Second-line options incorporate bismuth subsalicylate or ranitidine, and tetracycline as one of the antibiotic choices.92 Tetracycline is contraindicated in patients younger than 8 years because of permanent discoloration of teeth, enamel hypoplasia, and retardation of skeletal development and bone growth.14 Noncompliance with H. pylori regimens is the most commonly cited reason for treatment failure.92 Pediatric H. pylori treatment guidelines are currently under revision.93
Drug-Induced Liver Injury
Cause
Liver injury in children may result from autoimmune disease, metabolic disease, infectious causes, acute hepatitis, ischemia, irradiation damage, and exposure to toxins or medications.94,95 Drug-induced liver injury (DILI) has been noted to account for more than 50% of cases of acute liver failure in adults and approximately 20% in children, with acetaminophen as the agent most cited in the United States.96,97 Most cases of DILI in children are mild, and many correct after the offending medication is discontinued.97 However, some still progress to chronic liver injury.98,99 Diagnosis of DILI is largely a diagnosis of exclusion. DILI patients typically present within 12 months of starting the suspected medication.98 The Pediatric Acute Liver Failure (PALF) Study Group, a multinational, multisite consortium, was formed to prospectively study acute liver failure in children from birth through 18 years of age.94,97 The 24 sites in the PALF study group hope to improve understanding of the pathogenesis, treatment, and outcomes of acute liver failure in children.95 The PALF study group found that of the first 348 children enrolled through 2005, 14% had acute liver failure caused by acetaminophen, 5% caused by a drug or toxin other than acetaminophen, 10% caused by metabolic disease, 6% caused by autoimmune liver disease, 5% was idiosyncratic DILI, and 49% of unknown cause.95,97,98 Younger children were more likely to develop ascites, require ventilator support, and require red blood cell and plasma infusions than older children.95
Various clinical scoring systems, including the Roussel Uclaf Causality Assessment Method (RUCAM) and the Naranjo Probability Scale, have been developed as a means to increase the specificity of diagnosis of drug hepatotoxicity. These scales consider the postexposure interval, biochemical pattern, exclusion of alternative causes, extrahepatic manifestations, rechallenge attempts, and previously reported cases. Their routine use has been limited in pediatrics and is not routinely recommended.97 Table 87-3 summarizes the pharmacologic agents most cited as causing DILI in adult and pediatric patients. The corresponding references provide a more thorough discussion of the individual agents and/or case reports.
Table 87–3 Common Agents Cited in Drug-Induced Liver Injury94,96–99,103,117,145–147
| Drug Class or Group | Specific Agents and Reaction | Comments |
|---|---|---|
| Analgesics | Acetaminophen: necrosis | |
| Aspirin: Reye syndrome, necrosis | ||
| Bromfenac∗: acute liver failure | ||
| Celecoxib: hepatocellular | ||
| Diclofenac: hepatitis, cholestasis | ||
| Etodolac: fulminant hepatic failure | ||
| Ibuprofen (rare): hepatitis, vanishing bile duct syndrome | ||
| Indomethacin: cholestasis, necrosis | ||
| Meloxicam: hepatitis | ||
| Naproxen: cholestasis | ||
| Oxaprozin: hepatocellular, hepatitis | ||
| Piroxicam: hepatocellular, cholestasis, necrosis | ||
| Sulindac: hepatitis, cholestasis | ||
| Cardiovascular agents | Amiodarone: steatohepatitis, acute liver failure | |
| Angiotensin-converting enzyme inhibitors (captopril, enalapril, fosinopril): hepatitis, cholestasis | ||
| Angiotensin II receptor inhibitors (irbesartan, candesartan, losartan): cholestasis, hepatocellular | ||
| β-Adrenergic receptor blockers (propranolol, metoprolol, acebutolol, atenolol, labetalol): hepatocellular, cholestasis | ||
| Calcium channel antagonists (diltiazem, nifedipine, verapamil): hepatitis, cholestasis | ||
| Hydralazine: hepatitis, necrosis, granulomas, cholestasis | ||
| Labetalol: necrosis, hepatitis | Labetalol: most hepatotoxic β-blocker | |
| Methyldopa: hepatitis, cholestasis, steatosis, cirrhosis, granulomas | ||
| Thiazide diuretics (hydrochlorothiazide, chlorothiazide, chlorthalidone): cholestasis | ||
| Drugs used to treat diabetes mellitus | Glucosidase inhibitors (acarbose): hepatitis, cholestasis | Thiazolidinediones: monitor baseline liver tests and every 2 months after during first year of therapy |
| Human insulin: hepatitis | ||
| Metformin: hepatitis, cholestasis | ||
| Sulfonylureas (chlorpropamide, gliclazide, glipizide, glimepiride, tolazamide, tolbutamide): hepatitis, cholestasis, vanishing bile duct syndrome | ||
| Thiazolidinediones (troglitazone,∗ rosiglitazone, pioglitazone): liver failure, hepatotoxicity, fibrosis | ||
| Lipid-lowering agents | Ezetimibe: elevated serum transaminases | |
| Fibrates (fenofibrate, gemfibrozil, nicotinic acid): hepatitis, acute liver failure, cholestasis, necrosis | ||
| HMG-CoA reductase inhibitors (statins) (atorvastatin, cerivastatin,∗ fluvastatin, lovastatin, pravastatin, simvastatin): cholestasis, hepatitis, acute liver failure | Statins: monitor liver function at baseline, within 12 weeks, and then every 6 months | |
| Anticonvulsants | Carbamazepine, oxcarbazepine: cholestasis, hepatitis, vanishing bile duct syndrome, acute liver failure | |
| Felbamate: hepatitis, acute liver failure (reserve use to drug-refractory epilepsy and Lennox-Gastaut syndrome) | Felbamate: discontinue immediately if aminotransferase is elevated | |
| Lamotrigine: hepatitis | ||
| Phenobarbital: acute hepatitis, cholestasis | ||
| Phenytoin: hepatitis (rare), cholestasis, granulomas, focal necrosis | ||
| Topiramate: acute liver failure, hepatocellular | ||
| Valproic acid: steatosis, necrosis, increase in aminotransferases; greatest risk in first 2 years of life; progression to liver failure most common in patients with underlying mitochondrial disorders | ||
| Psychotropic and antidepressant medications | Atomoxetine: hepatobiliary | |
| Chlorpromazine: cholestasis | ||
| Clozapine: hepatocellular, fulminant hepatic failure | ||
| Haloperidol: cholestasis | ||
| Nefazodone: liver failure | ||
| Olanzapine: hepatitis | ||
| Paroxetine: hepatitis | ||
| Pemoline: hepatitis, acute liver failure | ||
| Prochlorperazine: cholestasis | ||
| Risperidone: hepatocellular | ||
| Thiazide diuretics (hydrochlorothiazide, chlorothiazide, chlorthalidone): cholestasis | ||
| Trazodone: hepatocellular, cholestasis | ||
| Tricyclic antidepressants (amitriptyline, imipramine): cholestasis | ||
| Chemotherapy and immunosuppressants | Azathioprine: hepatitis, hepatic veno-occlusive disease | |
| Busulfan: hepatic veno-occlusive disease | ||
| Chlorambucil: cirrhosis, fibrosis, (rare) | ||
| Cyclosporine: cholestasis | ||
| Dacarbazine: acute hepatic failure | ||
| Dactinomycin (in combination with radiotherapy): hepatitis | ||
| Erlotinib: hepatitis, hepatorenal syndrome | ||
| Fluorouracil (when given intra-arterially): hepatitis, sclerosing cholangitis | ||
| Flutamide: cholestasis | ||
| Gemcitabine: hepatitis | ||
| Interleukin-2: hepatitis | ||
| L-asparaginase, pegaspargase: liver steatosis, hepatitis, necrosis, fibrosis | ||
| Megestrol acetate: cholestasis | ||
| Mercaptopurine, azathioprine: hepatitis, cholangitis | ||
| Methotrexate: cirrhosis, fibrosis, steatosis | ||
| Natalizumab: hyperbili | ||
| Nitrosoureas (carmustine, lomustine, streptozotocin): hepatitis, hepatic veno-occlusive disease | ||
| Paclitaxel, docetaxel: hepatitis | ||
| Plicamycin: hepatitis, necrosis | Plicamycin: most hepatotoxic chemotherapy agent commercially available | |
| Recombinant α-interferon: hepatitis | ||
| Tamoxifen: cholestasis | ||
| Thioguanine: hepatic veno-occlusive disease | ||
| Anesthetics | Halothane: hepatitis, necrosis | |
| Anti-infectives | ANTIBIOTICS | |
| Amoxicillin and amoxicillin/clavulanic acid: cholestasis, hepatitis | ||
| Oxacillin, cloxacillin, dicloxacillin, flucloxacillin: cholestasis | ||
| Erythromycin: cholestasis, necrosis, hepatitis | ||
| Tetracycline, minocycline: microvesicular steatosis, hepatitis | ||
| FLUOROQUINOLONES | ||
| Ciprofloxacin, ofloxacin, levofloxacin, norfloxacin: hepatitis | ||
| Rifampin: hepatitis | ||
| SULFONAMIDES | ||
| Sulfamethoxazole and trimethoprim, sulfamethoxazole: hepatitis, cholestasis, granulomas | ||
| Nitrofurantoin: cholestasis, granulomas, chronic hepatitis | ||
| ANTIFUNGALS | ||
| Fluconazole, itraconazole, ketoconazole, flucytosine, terbinafine: cholestasis, hepatitis, necrosis | ||
| ANTIRETROVIRALS | ||
| Nucleoside analogue reverse transcriptase inhibitors (didanosine, stavudine): hepatic steatosis and lactic acidosis | ||
| Nevirapine: hepatitis | Nevirapine: monitor liver enzymes for first 18 weeks of therapy | |
| PROTEASE INHIBITORS | ||
| Ritonavir, tipranavir: acute liver failure, hepatitis, cholestasis | ||
| ANTITUBERCULOUS AGENTS | ||
| Isoniazid: hepatocellular necrosis | ||
| Pyrazinamide + rifampin or isoniazid | Rifampin + pyrazinamide combination use is discouraged by the CDC and ISDA because of severe liver injury | |
| Miscellaneous | Allopurinol: hepatitis, fulminant hepatic failure | |
| Amatoxin, found in wild mushrooms: acute liver failure | ||
| Cocaine: ischemic liver necrosis | ||
| Designer drugs of amphetamine (ecstasy): resemble acute viral hepatitis, acute liver failure requiring transplant | ||
| Estrogens: cholestasis | ||
| Marijuana and hashish: alterations in liver enzymes | ||
| Ondansetron: hepatitis | ||
| Propylthiouracil: hepatitis | ||
| Tolcapone: acute liver failure | ||
| Vitamin A (dose dependent): fibrosis, portal hypertension | ||
| Zafirlukast: hepatitis, necrosis | ||
| Herbals | Camphor: necrolytic hepatitis | |
| Cascara sagrada: cholestasis | ||
| Chaparral leaf, germander: acute and chronic hepatitis, cholestasis, cirrhosis, fulminant liver failure | ||
| Greater celandine: chronic hepatitis, cholestasis, fibrosis | ||
| Kava kava: acute and chronic hepatitis, fulminant liver failure | ||
| Ma huang: acute hepatitis, autoimmune hepatitis | ||
| Mistletoe: chronic hepatitis | ||
| Pyrrolizidine alkaloids (found in herbal and bush teas): veno-occlusive disease, dose-dependent hepatotoxicity | ||
| Sassafras: hepatocarcinogen | ||
| Saw palmetto: mild hepatitis | ||
| Usnic acid (lichen alkaloid): fulminant liver failure | ||
| Valerian: mild hepatitis |
HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; CDC, Centers for Disease Control and Prevention; ISDA, Infectious Disease Society of America.
Treatment
Cerebral Edema and Hepatic Encephalopathy
Protein restriction to 2 to 3 g/kg/day may help lessen excessive ammonia production,100 whereas fluid restriction to 75% of maintenance may prevent cerebral edema and reduce encephalopathy.101 Intravenous mannitol, continuous furosemide infusions, and tight glucose control have also been used for treatment of cerebral edema.101 Oral antibiotics, in particular neomycin, have been effective in reducing ammonia production in adults with hepatic encephalopathy by suppressing ammonia-forming bacteria in the gut.94,100 However, neomycin should be used cautiously in children because of potential renal toxicity and deafness. Synthetic disaccharides such as lactulose are metabolized by bacteria in the colon to form organic acids that lower the colonic stool pH and trap diffusible ammonia, thus decreasing serum ammonia levels. Lactulose forms the basis of therapy in children with hepatic encephalopathy and elevated serum ammonia levels, although the bulk of research surrounding its use is in adults.100 Levocarnitine may offer another option for reduction of ammonia levels in the brain, whereas albumin infusions have been used to reduce plasma ammonia levels.101 Sodium benzoate has been used in adults with cirrhosis, and may provide an alternative option in children.94,101,103
Ascites
Ascites is the accumulation of fluid in the peritoneal cavity. Although liver transplantation is the only definitive treatment for refractory ascites, many pharmacologic and nonpharmacologic options exist for acute management of ascites. Diagnostic abdominal paracentesis can help in directing therapy. Traditionally, bed rest has been recommended, however a lack of scientific evidence and difficulty enforcing in children may limit its role.104 Dietary restriction of sodium, such as restriction to 1 to 2 mEq/kg/day for infants and children and 1 to 2 g/day in adolescents, may help increase diuresis.104,105 Diuretics are used to increase urinary sodium excretion, leading to a negative fluid balance and weight loss. The target negative fluid balance in children of approximately 10 ml/kg/day is often used.104 Conflicting approaches to initiation of diuretics exist. Spironolactone typically is added first, with thiazide and loop diuretics (mainly furosemide in children) added if maximal doses of spironolactone are insufficient. Spironolactone acts to inhibit aldosterone at the distal renal tubule, thus inhibiting reabsorption of sodium, whereas furosemide inhibits sodium reabsorption in the ascending limb of the loop of Henle. Initial doses can be given once or twice daily, and titrated upwards as necessary.94,101,104 Another approach is to initiate both spironolactone and furosemide upfront in a ratio of 5:2, with subsequent change to spironolactone monotherapy once a desired diuresis occurs.105 Monitoring of serum electrolytes, acid base status, urine output and serum creatinine is necessary with all diuretic use, especially when using spironolactone and furosemide in combination. Potassium supplementation may be necessary. In patients with hypoalbuminemia, albumin infusion followed by furosemide may offer a brief, brisk diuresis.101, 104 Fluid restriction is typically not necessary in children. In refractory ascites, other options may include large volume paracentesis, transjugular intrahepatic shunts, although unlike with adults have limited use in children, peritoneovenous shunts, and ultimately liver transplantation.104 The most significant complication of ascites is spontaneous bacterial peritonitis.105 E. coli and Klebsiella pneumoniae are the most common causes, hence an intravenous, third-generation cephalosporin such as cefotaxime is often used for empiric therapy as it covers 95% of the associated flora.100,105 A β-lactam and β-lactamase combination agent, such as ampicillin/sulbactam, may offer an alternative,104 as well as once-daily norfloxacin in cirrhotic patients with ascites for prevention of spontaneous bacterial peritonitis.105 Trimethoprim-sulfamethoxazole has been considered a therapeutic alternative to fluoroquinolones given safety concerns in pediatrics.100
Coagulopathy and Hemorrhagic Complications
As both procoagulant and anticoagulant proteins are reduced in acute liver failure, clinically relevant bleeding rarely occurs. Intravenous vitamin K can be used to control coagulopathy, whereas severe cases may be treated with fresh frozen plasma and cryoprecipitate.101 Recombinant factor VII offers another alternative for correction of prothrombin time/INR abnormalities when active bleeding is present, although its high cost may preclude its use.94 Prophylaxis with H2 receptor blockers, PPIs, or sucralfate is started to prevent any GI bleeding.94,101 Infection may precipitate bleeding in this population; thus initiation of antibiotics should be considered if bleeding develops.94 The use of nonselective β-blockers in children, while successfully used in both primary and secondary prevention of variceal hemorrhage in adults with portal hypertension, is controversial. Insufficient data exist to demonstrate its use in this population; however, use may be considered as related to a risk reduction in bleeding. Both endoscopic injection sclerotherapy and endoscopic variceal ligation for variceal bleeding have been shown to be effective in treating varices in children and preventing future bleeding, thus these options are considered first-line, with beta blocker use adjunctive therapy.100 For emergent treatment of variceal bleeding in children, intravenous octreotide or continuous vasopressin is often initiated in an attempt to lower splanchnic vascular tone, thus decreasing portal venous pressure.100,101 However, approximately 30% of children treated with octreotide will still have a persistent hemorrhage despite conservative management and octreotide.100
Pruritus
Pruritus may be a result of prolonged cholestasis or noncholestatic liver disease. Treatment of underlying cholestatic disease should improve pruritus. Cholestyramine, an anion exchange resin, is widely used.106 Other agents that have been used and may offer benefit include ursodiol, opioid antagonists such as naloxone and naltrexone, rifampin, antihistamines, dexamethasone, and ondansetron.98,106 Trials have also evaluated prednisolone, cyclosporine, methotrexate, exposure to ultraviolet light, s-adenosyl methionine, antioxidants, macrolide antibiotics, and plasmapheresis with varying results.106
Cholangitis
Primary care consists of fasting, adequate intravenous hydration and broad spectrum antibiotics such as a third-generation cephalosporin and/or aminoglycoside to cover gram-negative bacilli, mainly Enterobacter spp.107
Miscellaneous
Acetaminophen is a widely used over the counter medication and the most common cause of DILI in children in the United States. DILI can occur after ingestion of therapeutic doses of acetaminophen over several days to treat clinical symptoms or a single overdose. Interventions for toxic acetaminophen ingestion include inhibiting drug absorption (i.e., gastric lavage), dialysis,108 prevention of the conversion to the toxic intermediate metabolite N-acetyl-p-benoquin imine (i.e., through use of cimetidine), replenishment of glutathione stores to prevent hepatotoxicity, and liver transplant. Methionine, cysteamine and N-acetylcysteine (NAC) are available agents to detoxify N-acetyl-p-benoquin imine.109 NAC is available either oral or intravenously in the United States and is the antidote of choice with fewer GI and central nervous system adverse effects compared with these other agents.109,110
It has been proposed that NAC may be useful therapy in other types of liver failure because of its ability to enhance hepatosplanchnic perfusion, to improve oxygen supply and demand, and its presumed cytoprotective and antioxidant effects. NAC may offer benefit in this patient population; however, many unanswered questions regarding dose, duration, and timing still remain.111,112
Dose Adjustments for Hepatic Dysfunction
Drugs absorbed via the GI tract undergo hepatic metabolism via a Phase I reaction (i.e., oxidation, reduction or hydrolysis) mediated be the CYP450 family of enzymes. These enzymes are found primarily in the liver.96,105 These reactions cause the biotransformation of the parent compound to a more polar compound, that is more readily excreted.99,113 Many adverse drug reactions and therapeutic failures can be attributed to genetically based differences in drug metabolism and elimination. Individuals can be divided into three groups: poor metabolizers, intermediate metabolizers, and extensive metabolizers. Average doses of medications given to poor metabolizers leads to higher drug exposure and greater risks of adverse drug reactions and drug toxicity.99 The CYP3A subfamily makes up the largest group of CYP enzymes in the liver, with CYP3A4 the most important for post natal metabolism.103,114–116 Consideration of the activity of the CYP450 isoenzymes, with specific focus on age differences, may aide in accurate drug dosing. For example, children require doses 50% to 100% higher than adults of drugs metabolized primarily by CYP2C9, whereas CYP3A4 activity is low at birth and reaches 72% of adult values at 1 year of age.115 However, age-related changes in the pharmacokinetics of various drugs may be difficult to separate from altered enzyme activity due to altered liver mass, liver blood flow, and changes in plasma drug binding.113
There are no specific diagnostic tests or pathological findings to diagnose liver dysfunction. Routine laboratory tests to assess liver function are helpful in identifying a hepatic insult; however, these findings are not specific to viral, autoimmune, metabolic disorders or drug-related causes, nor do they identify the extent that hepatic metabolism may be impaired.117 When hepatotoxicity is attributed to a dose-dependent hepatotoxin such as acetaminophen, blood levels of the suspected agent should be drawn.
The Child-Pugh score, adapted from the original Child score proposed in 1964, has been extensively used in adults as a user-friendly classification system for liver disease. This score is used as a prognosis of liver disease and as an indicator of a patient’s ability to metabolize drugs eliminated by the liver.118 However, this tool does not have the ability to predict the liver’s ability to metabolize individual drugs.116 Although various recent tools exist to classify liver disease, such as the Model for End-Stage Liver Disease (i.e., MELD) score widely used in adult liver transplantation ranking, the Child-Pugh score is the classic standard.116,119 Patients are divided into three risk groups, based on their degree of liver dysfunction. Class A is low risk/mild disease, class B is intermediate risk/moderate disease, and class C is high risk hepatic dysfunction/severe disease (Table 87-4). Even with the wide acceptance and use of the Child-Pugh score, there are a limited number of medications containing specific recommendations for dosage adjustment based on hepatic function as categorized by the Child-Pugh score.116 In 2005, a review of all Food and Drug Administration-approved medications found that only 23 included guidance for dosage adjustment based on Child-Pugh. Dose adjustments were typically recommended starting with Class B liver dysfunction, with many agents suggesting nonuse in patients with Class C rankings.120
Rectal Administration of Medication
Many drugs otherwise approved for oral and/or intravenous use have been administered rectally, in both adults and children. Rationale for using the rectal route varies. Rectal administration allows for avoidance of the orogastric route when necessary or avoidance of the intravenous route when access is an issue or when parenteral dosage forms are not available. The rectal route allows for a higher local concentration of drug in situations where limited systemic absorption is desired. Administration via this route may afford a less-costly alternative to a considerably more expensive parenteral product, and may offer an alternative when a parenteral product is unavailable. Although generally well accepted amongst patients and their families121,122 clinicians may need to overcome resistance to rectal administration from patients and families because of personal perceptions.
Interpatient variability surrounding rectal administration is a key factor for clinicians to consider. Drug absorption may be accelerated or delayed. The rate of rectal transmucosal absorption is affected by various factors. First, the formulation of the product itself varies, causing differences in the time to liquefaction with suppositories, the volume of liquid administered and the concentration of the drug. The length of the rectal catheter helps determine the site of absorption. Drugs administered high in the rectum, which is drained by the superior rectal veins, typically are carried to the liver via the portal vein, thus subject to first-pass hepatic metabolism. Drugs administered low in the rectum are delivered into the venous circulation by the inferior and middle rectal veins before passing through the liver. Presence of stool in the rectum and rectal pH can alter drug absorption. Rectal pH affects absorption by ionizing varying amounts of drug. The rectal mucosa in children typically has a more alkaline pH.123 Nonionized drugs have greater lipid solubility, causing enhanced absorption across biological membranes. The rectal retention of the drug administered also affects absorption.124
The rectal route has been commonly studied with the use of rectal diazepam gel (Diastat). This commercially available product is used for prolonged or repetitive seizures in children and has approval by the FDA for at-home administration by a trained nonprofessional caregiver. Clinical trials and postmarketing data have reported a low rate of serious respiratory adverse events,125 a major concern of many clinicians surrounding at-home use; however, cases have been reported in chronic, high-dose users of fluctuating effects of rectal diazepam, including reappearance of seizure activity.126 This product may offer an alternative to other oral and intravenous benzodiazepine options available in a critical care setting, especially in emergent situations if access cannot be readily achieved. The controlled environment of a critical care unit can easily recognize adverse effects as opposed to home administration.
Clinically, successful rectal administration of medication in children has dealt mainly with anticonvulsants such as diazepam, lorazepam, and oxcarbazepine for status epilepticus127; sedatives such as diazepam, midazolam, and ketamine in varying uses124,128; localized treatment for irritable bowel disease using mesalamine129–131; vancomycin for pseudomembranous colitis132; antibacterial agents such as erythromycin133; antiemetics134; cardiovascular agents such as nifedipine and metoprolol135–138; and analgesics, specifically acetaminophen, nonsteroidal antiinflammatory drugs, and tramadol, for temperature reduction and postoperative pain control.121,122,139,140 Although the American Academy of Pediatrics dissuades the rectal use of acetaminophen without consultation with a health care provider,141 it has been widely used and studied.139,140,142,143 In addition, rectally administered nonsteroidal anti-inflammatory medications, opioid analgesics, antiseizure medications, antiemetics, anticholinergic medications, antidepressants, psychostimulants, and antibiotics have been used with success in the hospice and palliative care settings.144
References are available online at http://www.expertconsult.com.
1. Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85-93.
2. Kapur P.A. The big “little problem,”. Anesth Analg. 1991;73(3):243-245.
3. Dupuis L.L., Nathan P.C. Options for the prevention and management of acute chemotherapy-induced nausea and vomiting in children. Paediatr Drugs. 2003;5(9):597-613.
4. Kovac A.L. Management of postoperative nausea and vomiting in children. Paediatr Drugs. 2007;9(1):47-69.
5. Baker P.D., Morzorati S.L., Ellett M.L. The pathophysiology of chemotherapy-induced nausea and vomiting. Gastroenterol Nurs. 2005;28(6):469-480.
6. Lin T.L., Ettinger D.S. Nausea and vomiting. In: Ettinger D.S., editor. Supportive care in cancer therapy. Totowa: Humana Press; 2009:179-192.
7. Hesketh P.J. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482-2494.
8. Navari R.M. Pharmacological management of chemotherapy-induced nausea and vomiting: focus on recent developments. Drugs. 2009;69(5):515-533.
9. Matera M.G., Di Tullio M., Lucarelli C., et al. Ondansetron, an antagonist of 5-HT3 receptors, in the treatment of antineoplastic drug-induced nausea and vomiting in children. J Med. 1993;24(2-3):161-170.
10. Miyajima Y., Numata S., Katayama I., et al. Prevention of chemotherapy-induced emesis with granisetron in children with malignant diseases. Am J Pediatr Hematol Oncol. 1994;16(3):236-241.
11. Craft A.W., Price L., Eden O.B., et al. Granisetron as antiemetic therapy in children with cancer. Med Pediatr Oncol. 1995;25(1):28-32.
12. Dick G.S., Meller S.T., Pinkerton G.R. Randomized comparison of ondansetron and metoclopramide plus dexamethasone for chemotherapy induced emesis. Arch Dis Child. 1995;73(3):243-245.
13. Tsuchida Y., Hayashi Y., Asami K., et al. Effects of granisetron in children undergoing high-dose chemotherapy: a multi-institutional, cross-over study. Int J Oncol. 1999;14(4):673-679.
14. Taketomo C.K., Hodding J.H., Kraus D.M. Lexi-Comp’s pediatric dosing handbook, ed 16. Lexi Comp: Hudson; 2009.
15. Seynaeve J., Schuller K., Buser H., et al. Comparison of the anti-emetic efficacy of different doses of ondansetron, given as either a continuous infusion or a single intravenous dose, in acute cisplatin-induced emesis. A multicentre, double-blind, randomised, parallel group study. Ondansetron Study Group. Br J Cancer. 1992;66(1):192-197.
16. Harman G.S., Omura G.A., Ryan K., et al. A randomized, double-blind comparison of single-dose and divided multiple-dose dolasetron for cisplatin-induced emesis. Cancer Chemother Pharmacol. 1996;38(4):323-328.
17. Ettinger S.D., Eisenberg P.D., Fitts D., et al. A double-blind comparison of the efficacy of two dose regimens of oral granisetron in preventing acute emesis in patients receiving moderately emetogenic chemotherapy. Cancer. 1996;78(1):144-151.
18. Gralla R.J., Navari R.M., Hesketh P.J., et al. Single-dose oral granisetron has equivalent antiemetic efficacy to intravenous ondansetron for highly emetogenic cisplatin-based chemotherapy. J Clin Oncol. 1998;16(4):1568-1573.
19. Perez E.A., Hesketh P., Sandbach J., et al. Comparison of single-dose oral granisetron versus intravenous ondansetron in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy: a multicenter, double-blind, randomized parallel study. J Clin Oncol. 1998;16(2):754-760.
20. Kris M.G., Hesketh P.J., Somerfield M.R., et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24(18):2932-2947.
21. Dupuis L.L., Lau R., Greenberg M.L. Delayed nausea and vomiting in children receiving antineoplastics. Med Pediatr Oncol. 2001;37(2):115-121.
22. Tonini G., Vincenzi B., Santini D. New drugs for chemotherapy-induced nausea and vomiting: focus on palonosetron. Expert Opin Drug Metab Toxicol. 2005;1(1):143-149.
23. Eisenberg P., Figueroa-Vadillo J., Zamora R., et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98(11):2473-2482.
24. Gralla R., Lichinitser M., Van Der Vegt S., et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14(10):1570-1577.
25. Aapro M.S., Grunberg S.M., Manikhas G.M., et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17(9):1441-1449.
26. Sepulveda-Vildosola A.C., Betanzos-Cabrera Y., Lastiri G.G., et al. Palonosetron hydrochloride is an effective and safe option to prevent chemotherapy-induced nausea and vomiting in children. Arch Med Res. 2008;39(6):601-606.
27. Grunberg S.M. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol. 2007;18(2):233-240.
28. Hesketh P.J., Grunberg S.M., Gralla R.J., et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112-4119.
29. Poli-Bigelli S., Rodrigues-Pereira J., Carides A.D., et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97(12):3090-3098.
30. Curran M.P., Robinson D.M. Aprepitant: A Review of its use in the prevention of nausea and vomiting. Drugs. 2009;69(13):1853-1878.
31. Gore L., Chawla S., Petrilli A., et al. Aprepitant in adolescent patients for prevention of chemotherapy-induced nausea and vomiting: a randomized, double-blind, placebo-controlled study of efficacy and tolerability. Pediatr Blood Cancer. 2009;52(2):242-247.
32. Lifshitz M., Gavrilov V. Adverse reactions to metoclopramide in young children: a 6-year retrospective study and review of the literature. J Pharm Technol. 2002;18(3):125-127.
33. Hesketh P.J., Kris M.G., Grunberg S.M., et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15(1):103-109.
34. Koeller J.M., Aapro M.S., Gralla R.J., et al. Antiemetic guidelines: creating a more practical treatment approach. Support Care Cancer. 2002;10(7):519-522.
35. Grunberg S.M., Osoba D., Hesketh P.J., et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity–an update. Support Care Cancer. 2005;13(2):80-84.
36. Holdsworth M.T., Raisch D.W., Frost J. Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer. 2006;106(4):931-940.
37. The Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol. 2006;17(1):20-28.
38. Chemotherapy-induced nausea and vomiting. ESMO clinical recommendations for prophylaxis. Ann Oncol. 2007;18(Suppl 2):ii83-ii85.
39. Lacy C.F., Armstrong L.L., Goldman M.P., et al. Lexi-Comp’s drug information handbook, ed 14. Lexi Comp: Hudson; 2006.
40. Gan T.J., Meyer T., Apfel C.C., et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97(1):62-71.
41. Ellebæk E., Herrstedt J. Optimizing antiemetic therapy in multiple-day and multiple cycles of chemotherapy. Curr Opin Support Palliat Care. 2008;2(1):28-34.
42. Trigg M.E., Inverso D.M. Nausea and vomiting with high-dose chemotherapy and stem cell rescue therapy: a review of antiemetic regimens. Bone Marrow Transplant. 2008;42(8):501-506.
43. Wood G.J., Shega J.W., Lynch B., et al. Management of intractable nausea and vomiting in patients at the end of life: “I was feeling nauseous all of the time… nothing was working.”. JAMA. 2007;298(10):1196-1207.
44. Reduce the occurrence of postoperative vomiting (POV) in children with appropriate anti-emetic prophylaxis. Drugs Ther Perspect. 2008;24(2):19-22.
45. Gan T.J., Apfel C.C., Kovac A., et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104(5):1082-1089.
46. Diemunsch P., Gan T.J., Philip B.K., et al. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: a randomized, double-blind phase III trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007;99(2):202-211.
47. Diemunsch P., Joshi G.P., Brichant J.F. Neurokinin-1 receptor antagonists in the prevention of postoperative nausea and vomiting. Br J Anaesth. 2009;103(1):7-13.
48. Rowbotham D.J. Neurokinin-1 antagonists: a step change in prevention of postoperative nausea and vomiting? Br J Anaesth. 2009;103(1):5-6.
49. Guandalini S. Acute diarrhea. In: Guandalini S., editor. Essential pediatric gastroenterology, hepatology, and nutrition, New York. McGraw Hill,; 2005:15-23.
50. Elstner C.L., Lindsay A.N., Book L.S., et al. Lack of relationship of Clostridium difficile to antibiotic-associated diarrhea in children. Pediatr Infect Dis. 1983;2(5):364-366.
51. Alam S., Mushtaq M. Antibiotic associated diarrhea in children. Indian Pediatr. 2009;46(6):491-496.
52. Pepin J. Vancomycin for the treatment of Clostridium difficile infection: for whom is this expensive bullet really magic? Clin Infect Dis. 2008;46(10):1493-1498.
53. Gerding D.N., Muto C.A., Owens R.C.Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S32-S42.
54. Choudhry M.N., Soran H., Ziglam H.M. Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. Q J Med. 2008;101(6):445-448.
55. Dial S., Delaney J.A., Barkun A.N., et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989-2995.
56. Szajewska H., Ruszczynski M., Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2008;149(3):367-372.
57. Johnston B.C., Supina A.L., Vohra S. Probiotics for pediatric antibiotic-associated diarrhea: a meta-analysis of randomized placebo-controlled trials. CMAJ. 2006;175(4):377-383.
58. Snydman D.R. The safety of probiotics. CID. 2008;46(Suppl 2):S104-S111.
59. Thompson J.L., Duffy J.D. Nutrition support challenges in hematopoietic stem cell transplant patients. Nutr Clin Pract. 2008;23(5):533-546.
60. Baker S.S., Liptak G.S., Colletti R.B., et al. Clinical practice guideline: evaluation and treatment of constipation in infants and children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2006;43(3):e1-e13.
61. Bulloch B., Tenenbein M. Constipation: diagnosis and management in the pediatric emergency department. Pediatr Emerg Care. 2002;18(4):254-258.
62. Loening-Baucke V. Prevalence, symptoms and outcomes of constipation in infants and toddlers. J Pediatr. 2005;146(3):359-363.
63. Vandenplas Y., Rudolph C.D., DiLorenzo C., et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498-547.
64. Rudolph C.D., Mazur L.J., Liptak G.S., et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32(suppl 2):S1-S31.
65. Reimer C., Sondergaard B., Hilsted L., Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 2009;137(1):80-87.
66. Taira B.R., Fenton K.E., Lee T.K. Ventilator-associated pneumonia in pediatric trauma patients. Pediatr Cirt Care Med. 2009;10(4):491-494.
67. Principi N. Esposito S: Ventilator-associated pneumonia in pediatric intensive care units. Pediatr Infect Dis J. 2007;26:841-844.
68. Laheij R.J.F., Sturkenboom M.C.J.M., Jan-Hassing R. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955-1960.
69. Booth C.M., Heyland D.K., Paterson W.G. Gastrointestinal promotility drugs in the critical care setting: a systematic review of the evidence. Crit Care Med. 2002;30:1429-1435.
70. U.S. Food and Drug Administration: Safety alerts for human medical products. Available at http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm106942.htm. Accessed November 9, 2009.
71. Chaibou M., Tucci M., Dugas M.A., et al. Clinically significant upper gastrointestinal bleeding acquired in a pediatric intensive care unit: a prospective study. Pediatrics. 1998;102(4 Pt 1):933-938.
72. ASHP therapeutic guidelines on stress ulcer prophylaxis. ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347-379.
73. Cochran E.B., Phelps S.J., Tolley E.A., et al. Prevalence of, and risk factors for, upper gastrointestinal tract bleeding in critically ill pediatric patients. Crit Care Med. 1992;20:1519-1523.
74. Lacroix J., Nadeau D., Laberge S., et al. Frequency of upper gastrointestinal bleeding in a pediatric intensive care unit. Crit Care Med. 1992;20:35-42.
75. Nithiwathanapong C., Reungrongrat S., Ukarapol N. Prevalence Risk factors: of stress-induced gastrointestinal bleeding in critically ill children. World J Gastroenterol. 2005;11(43):6839-6842.
76. Deerojanawong J., Peongsujarit D., Vivatvakin B., Prapphal N. Incidence risk factor of upper gastrointestinal bleeding in mechanically ventilated children. Pediatr Crit Care Med. 2009;10(1):91-95.
77. Lacroix J., Gauthier M., Farrell C.A., et al. Prophylaxis of gastroduodenal hemorrhage caused by stress during pediatric intensive care. Pediatrics. 1991;46:393-403.
78. Haizlipp Ja Lugo R.A., Cash J.J. Vernon DD: Failure of nasogastric omeprazole suspension in pediatric intensive care patients, Pediatr Crit Care Med. 2005;6(2):182-187.
79. Lugo R.A., Marrison A.M., Cash J. et al: Pharmacokinetics and pharmacodynamics of ranitidine in critically ill children. Crit Care Med. 2001;29(4):759-764.
80. Rofil N.M., Brenner K.W., Fuller M.P. Winkler MK: Histamine 2 receptor antagonists vs. intravenous proton pump inhibitors in a pediatric intensive care unit: a comparison of gastric pH, J Crit Care. 2008;23(3):416-421.
81. Lopez-Herce J., Dorao P., Elola P., et al. Frequency and prophylaxis of upper gastrointestinal hemorrhage in critically ill children: a prospective study comparing the efficacy of almagate, ranitidine, and sucralfate. The Gastrointestinal Hemorrhage Study Group. Crit Care Med. 1992;20:1082-1089.
82. Yildizdas D., Yapicioglu H., Yilmaz H.L. Occurrence of ventilator-associated pneumonia in mechanically ventilated pediatric intensive care patients during stress ulcer prophylaxis with sucralfate, ranitidine, and omeprazole. J Crit Care. 2002;17:240-245.
83. Cook D., Guyatt G., Marshall J. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group, N Engl J Med. 1998;338(12):791-797.
84. Randolph A.G. Management of acute lung injury and acute respiratory distress syndrome in children, Crit Care Med. 2009;37(8):2248-2254.
85. Dellinger R.P., Levy M.M., Carlet J.M., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36(1):296-327.
86. MacLauren R, Cook DJ, Rebuck JA: Stress ulcer prophylaxis: to protect or not to protect. Available at http://www.learnicu.org/Clinical_Practice/Systems/Nutrition/Pages/default.aspx. Accessed November 9, 2009.
87. Barkun A., Bardou M., Marshall J.K. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843-857.
88. Heikenen J.B., Pohl J.F., Werlin S.L., et al. Octreotide in pediatric patients. J Pediatr Gastroenterol Nutr. 2002;35:600-609.
89. Siafakas C., Fox V.L., Nurko S. Use of octreotide for the treatment of severe gastrointestinal bleeding in children. J Pediatr Gastroenterol Nutr. 1998;26:356-359.
90. Zellos A., Schwarz K.B. Efficacy of octreotide in children with chronic gastrointestinal bleeding. J Pediatr Gastroenterol Nutr. 2000;30:442-446.
91. Molleston J.P. Variceal bleeding in children. J Pediatr Gastroenterol Nutr. 2003;37:538-545.
92. Gold B.D., Colletti R.B., Abbott M., et al. Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000;31:49097.
93. North American Society for Pediatric Gastroenterology and Nutrition. http://www.naspghan.org/wmspage.cfm?parm1=295. Accessed November 9, 2009.
94. Squires R.H. Acute liver failure in children. Semin Liver Dis. 2008;28:153-166.
95. Squires R.H.Jr., Shneider B.L., Bucuvalas J., et al. Acute liver failure in children: the first 348 patients in the Pediatric Acute Liver Failure Study Group. J Pediatr. 2006;148:652-658.
96. Pugh A.J., Barve A.J., Falkner K. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13:277-294.
97. Murray K.F., Hadzic N., Wirth S. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47:395-405.
98. Fontana R.J. Acute liver failure due to drugs. Semin Liver Dis. 2008;28:175-187.
99. Kóbori L., Koñhalmy K., Porrogi P., et al. Drug-induced liver graft toxicity caused by cytochrome P450 poor metabolism. Br J Clin Pharmacol. 2007;65(3):428-436.
100. Leonis M.A., Balistreri W.F. Evaluation and management of end-stage liver disease in children. Gastroenterology. 2008;134:1741-1751.
101. Debray D., Yousef N., Durand P. New management options for end-stage chronic liver disease and acute liver failure: potential for pediatric patients. Pedriatr Drugs. 2006;8(1):1-13.
102. Reference deleted in proofs
103. Buratti S., Lavine J.E. Drugs and the liver: advances in metabolism, toxicity, and therapeutics. Curr Opin Ped. 2002;14:601-607.
104. Sabri M., Saps M., Peters J.M. Pathophysiology and management of pediatric ascites. Curr Gastroenterol Rep. 2003;5:240-246.
105. Yachha S.K., Khanna V. Ascites in childhood liver disease. Indian J Pediatr. 2006;73(9):819-824.
106. Mela M., Mancuso A., Burroughs A.K. Review article: pruritus in cholestatic and other liver diseases. Aliment Pharmacol Ther. 2003;17:857-870.
107. Yasuda H., Takada T., Kawarada Y., et al. Unusual cases of acute cholecystitis and cholangitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:98-113.
108. 2009 dialysis of drugs. Available at http://www.ckdinsights.com/downloads/DialysisDrugs2009.pdf. Accessed Nov 9, 2009.
109. Chun L.J., Tong M.J., Busuttil R.W., et al. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342-349.
110. Yarema M.C., Johnson D.W., Berlin R.J., et al. Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2009;54:606-614.
111. Lee W.M., Hynan L.S., Rossaro L., et al. Intraveous N-acetylcysteine improves transplant-free survival in early stages of non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
112. Sklar G.E., Subramaniam M. Acetylcysteine treatment for non-acetaminophen-induced acute liver failure. Ann Pharmacother. 2004;38:498-501.
113. Benedetti M.S., Whomsley R., Canning M. Drug metabolism in the paediatric population and in the elderly. Drug Discovery Today. 2007;12(15/16):599-610.
114. Björkman S. Prediction of cytochrome p450-mediated hepatic drug clearance in neonates, infants and children. How accurate are available scaling methods? Clin Pharmacokinet. 2006;45(1):1-11.
115. Anderson G.D., Lynn A.M. Optimizing pediatric dosing: a developmental pharmacologic approach. Pharmacotherapy. 2009;29(6):680-690.
116. Verbeeck R.K. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147-1161.
117. Pinñeiro-Carrero V.M., Pinñeiro E.O. Liver. Pediatrics. 2004;113:1097-1106.
118. Pugh R.N., Murray-Lyon I.M., Dawson J.L. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649.
119. Durand F., Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42:S100-S107.
120. Spray J.W., Willett K., Chase D. Dosage adjustment for hepatic dysfunction based on Child-Pugh scores. Am J Health Syst Pharm. 2007;64(7):692-693. 690
121. Owczarzak V., Haddad J. Comparison of oral versus rectal administration of acetaminophen with codeine in postoperative pediatric adenotonsillectomy patients. Laryngoscope. 2006;116:1485-1488.
122. Al-Husein M.O. Postoperative analgesia and rectal drug administration. Acta Anaesthesiol Scand. 2000;44:633-636.
123. Zwaveling J., Bubbers S., van Meurs A.H. Pharmacokinetics of rectal tramadol in postoperative paediatric patients. Br J Anaesth. 2004;93:224-227.
124. Committee on Drugs. Alternative routes of drug administration- advantages and disadvantages. Pediatrics. 1997;100(1):143-152.
125. O’Dell C., Shinnar S., Ballaban-Gil K.R. Rectal diazepam gel in the home management of seizures in children. Pediatr Neurol. 2005;33(3):166-172.
126. Brodtkorb E., Aamo T., Henriksen O., Lossius R. Rectal diazepam: pitfalls of excessive use in refractory epilepsy. Epilepsy Res. 1999;35:123-133.
127. Clemens P.L., Cloyd J.C., Kriel R.L., Remmel R.P. Relative bioavailability, metabolism and tolerability of rectally administered oxcarbazepine suspension. Clin Drug Invest. 2007;27(4):243-250.
128. Aydintug Y.S., Okcu K.M., Guner Y. Evaluation of oral or rectal midazolam as conscious sedation for pediatric patients in oral surgery. Mil Med. 2004;169(4):270-273.
129. Cortot A., Degoutte E., Delette O., et al. Mesalamine foam enema versus mesalamine liquid enema in active left-sided ulcerative colitis. Am J Gastroenterol. 2008;103(12):3106-3114.
130. Eliakim R., Tulassay Z., Kupcinskas L., et al. Clinical trial: randomized-controlled clinical study comparing the efficacy and safety of a low-volume vs. a high-volume mesalazine foam in active distal ulcerative colitis. Aliment Pharmacol Ther. 2007;26(9):1237-1249.
131. Moody G.A., Eaden J.A., Helyes Z., Mayberry J.F. Oral or rectal administration of drugs in IBD? Aliment Pharmacol Ther. 1997;11(5):999-1000.
132. Bublin J.G., Barton T.L. Rectal use of vancomycin. Ann Pharmacother. 1994;28(12):1357-1358.
133. Stratchunsky L.S., Nazarov A.D., Firsov A.A., Petrachenkova N.A.Age dependence of erythromycin rectal bioavailability in children Eur J Drug Metab Pharmacokinet Spec No 3 1991;321-323
134. Ní Chróinín M., Fallon M., Kenny D. Rectal hydrocortisone during vomiting in children with adrenal insufficiency. J Pediatr Endocrinol Metab. 2003;16(8):1101-1104.
135. de Stoppelaar F.M., Stolk L.M., Beysens A.J. The relative bioavailability if metoprolol following oral and rectal administration to volunteers and patients. Pharm World Sci. 1999;21(5):233-238.
136. Gaur A., Pande R.K., Kaushik S. Rectal nifedipine for the management of intraoperative hypertension. J Neurosurg Anesthesiol. 1993;5(4):237-240.
137. Uchiyama M., Sakai K. Rectal administration of perforated nifedipine capsules in acute severe hypertension in children. Br J Clin Pract. 1992;46(2):100-101.
138. Uchiyama M., Ogawa I. Rectal nifedipine in acute severe hypertension in young children. Arch Dis Child. 1989;64(4):632-633.
139. Goldstein L.H., Berlin M., Berkovitch M., Kozer E. Effectiveness of oral vs. rectal acetaminophen: a meta-analysis. Arch Pediatr Adolesc Med. 2008;162(11):1042-1046.
140. Cormack C.R., Sudan S., Addison R. The pharmacokinetics of a single rectal dose of paracetamol (40 mg/kg) in children with liver disease. Paediatr Anaesth. 2006;16:417-423.
141. American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108(4):1020-1024.
142. Terry D., Paolicchi J., Karn M. Acceptance of the use of diazepam rectal gel in school and day care settings. J Child Neurol. 2007;22(9):1135-1138.
143. Osterhoudt K.C., Henretig F.M. Still wary of rectal acetaminophen. Arch Pediatr Adolesc Med. 2009;163(5):491-492.
144. Davis M.P., Walsh D., LeGrand S.B., Naughton M. Symptom control in cancer patients: the clinical pharmacology and therapeutic role of suppositories and rectal suspensions. Support Care Cancer. 2002;10:117-138.
145. King P.D., Perry M.C. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162-176.
146. Liss G., Lewis J.H. Drug-induced liver injury: what was new in 2008? Expert Opin Drug Metab Toxicol. 2009;5(8):843-860.
147. Chitturi S., George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.