Objectives
• Identify the clinical manifestations of GI disorders.
• Explain the treatment of GI disorders.
• Discuss the nursing priorities for managing a patient with GI dysfunction.
![]()
Be sure to check out the bonus material, including free self-assessment exercises, on the Evolve web site at http://evolve.elsevier.com/Urden/priorities.
Understanding the pathology of a disease, the areas of assessment on which to focus, and the usual medical management allows the critical care nurse to accurately anticipate and plan nursing interventions. This chapter focuses on gastrointestinal disorders commonly seen in the critical care environment.
Acute Gastrointestinal Hemorrhage
Gastrointestinal hemorrhage is a potentially life-threatening emergency that remains a common complication of critical illness1 and results in 400,000 hospital admissions yearly.2 Despite advances in medical knowledge and nursing care, the mortality rate for patients with acute gastrointestinal bleeding remains at 10%.1,3
Etiology
Gastrointestinal hemorrhage occurs from bleeding in the upper or lower gastrointestinal tract. The ligament of Treitz is the anatomic division used to differentiate between the two areas. Bleeding proximal to the ligament is considered to be upper gastrointestinal in origin, and bleeding distal to the ligament is considered to be lower gastrointestinal in origin.1,4 The various causes of acute gastrointestinal hemorrhage are listed in Box 22-1.4,5 Only the three main causes of gastrointestinal hemorrhage commonly seen in the intensive care unit (ICU) are discussed further.
Peptic Ulcer Disease
Peptic ulcer disease (gastric and duodenal ulcers), which results from the breakdown of the gastromucosal lining, is the leading cause of upper gastrointestinal hemorrhage, accounting for approximately 21% of cases.4 Normally, protection of the gastric mucosa from the digestive effects of gastric secretions is accomplished in several ways. First, the gastroduodenal mucosa is coated by a glycoprotein mucous barrier that protects the surface of the epithelium from hydrogen ions and other noxious substances present in the gut lumen.6,7 Adequate gastric mucosal blood flow is necessary to maintain this mucosal barrier function. Second, gastroduodenal epithelial cells are protected structurally against damage from acid and pepsin because they are connected by tight junctions that help prevent acid penetration. Third, prostaglandins and nitric oxide protect the mucosal barrier by stimulating mucus and bicarbonate secretion and inhibiting the secretion of acid.7
Peptic ulceration occurs when these protective mechanisms cease to function, allowing gastroduodenal mucosal breakdown. After the mucosal lining is penetrated, gastric secretions autodigest the layers of the stomach or duodenum, leading to injury of the mucosal and submucosal layers. This results in damaged blood vessels and subsequent hemorrhage. The two main causes of disruption of gastroduodenal mucosal resistance are nonsteroidal antiinflammatory drugs and the bacterial action of Helicobacter pylori.4,8,9
Stress-Related Mucosal Disease
Stress-related mucosal disease (SRMD) is an acute erosive gastritis that covers both types of mucosal lesions that are often found in the critically ill patient: stress-related injury and discrete stress ulcers.10,11 These abnormalities develop within hours of admission.10 They range from superficial mucosal erosions to deep focal lesions and usually affect the upper gastrointestinal tract.10 SRMD occurs by means of the same pathophysiological mechanisms as peptic ulcer disease, but the main cause of disruption of gastric mucosal resistance is increased acid production and decreased mucosal blood flow, resulting in ischemia and degeneration of the mucosal lining.10,11 Patients at risk include those in situations of high physiological stress, such as occur with mechanical ventilation, extensive burns, severe trauma, major surgery, shock, sepsis, coagulopathy, or acute neurological disease.10,11 Gastroduodenal lesions are estimated to occur in 75% to 100% of ICU patients within 24 hours of admission.10 SRMD is a leading cause of upper gastrointestinal hemorrhage, accounting for approximately 15% of cases.11
Esophagogastric Varices
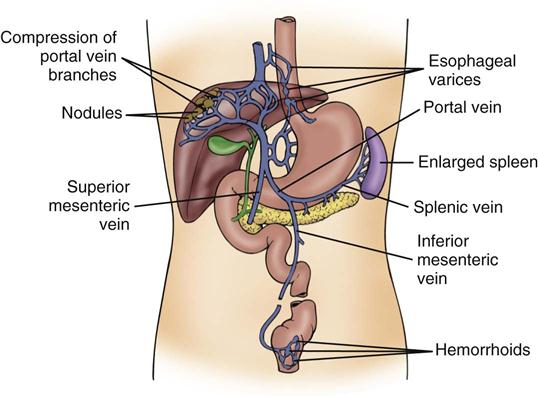
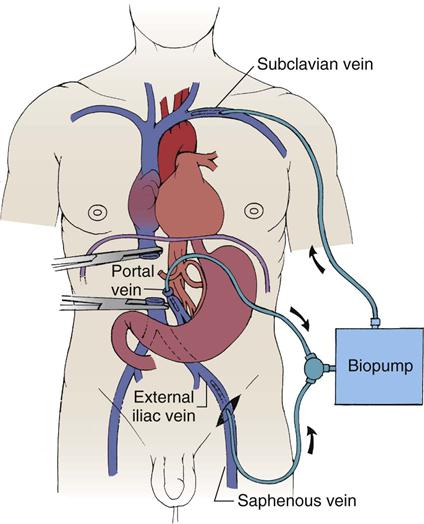
Esophagogastric varices are engorged and distended blood vessels of the esophagus and proximal stomach that develop as a result of portal hypertension caused by hepatic cirrhosis, a chronic disease of the liver that results in damage to the liver sinusoids (Figure 22-1). Without adequate sinusoid function, resistance to portal blood flow is increased, and pressures within the liver are elevated. This leads to increased portal venous pressure (portal hypertension), causing collateral circulation to divert portal blood from areas of high pressure within the liver to adjacent areas of low pressure outside the liver, such as into the veins of the esophagus, the spleen, the intestines, and the stomach. The tiny, thin-walled vessels of the esophagus and proximal stomach that receive this diverted blood lack sturdy mucosal protection. The vessels become engorged and dilated, forming esophagogastric varices that are vulnerable to damage from gastric secretions and that may result in subsequent rupture and massive hemorrhage.12 The risk of variceal bleeding increases with disease severity and variceal size, but overall, bleeding occurs in up to 30% of patients with medium or large varices, and only 50% of patients stop bleeding spontaneously.12,13
Pathophysiology
Gastrointestinal hemorrhage is a life-threatening disorder that is characterized by acute, massive bleeding. Regardless of the cause, acute gastrointestinal hemorrhage results in hypovolemic shock, initiation of the shock response, and development of multiple organ dysfunction syndrome if left untreated (Concept Map on Acute Gastrointestinal Hemorrhage).7 However, the most common cause of death in cases of gastrointestinal hemorrhage is exacerbation of the underlying disease, not intractable hypovolemic shock.
Assessment and Diagnosis
The initial clinical presentation of the patient with acute gastrointestinal hemorrhage is that of a patient in hypovolemic shock, and the clinical presentation depends on the amount of blood lost (Table 22-1).7 Hematemesis (bright red or brown, coffee grounds emesis), hematochezia (bright red stools), and melena (black, tarry, or dark red stools) are the hallmarks of gastrointestinal hemorrhage.1,4,5
TABLE 22-1
CLINICAL CLASSIFICATION OF HEMORRHAGE
| CLASS | BLOOD LOSS (%) | CLINICAL SIGNS AND SYMPTOMS |
| 1 | ≤15 | Pulse rate: normal or <100 beats/min (supine) |
| Capillary refill <3 sec | ||
| Urine output: adequate (30-35 mL/hr) | ||
| Orthostatic hypotension | ||
| Apprehensive | ||
| 2 | 15-30 | Pulse rate: increased (>100 beats/min) |
| Capillary refill: sluggish | ||
| Pulse pressure: decreased | ||
| Blood pressure: normal (supine) | ||
| Tachypnea | ||
| Urine output: low (25-30 mL/hr) | ||
| 3 | 30-40 | Pulse rate: 120+ beats/min (supine) |
| Hypotension | ||
| Skin: cool, pale | ||
| Confused | ||
| Hyperventilating | ||
| Urine output: low (5-15 mL/hr) | ||
| 4 | ≥40 | Profoundly hypotensive |
| Pulse rate: 140+ beats/min | ||
| Confused, lethargic | ||
| Urine output minimal |
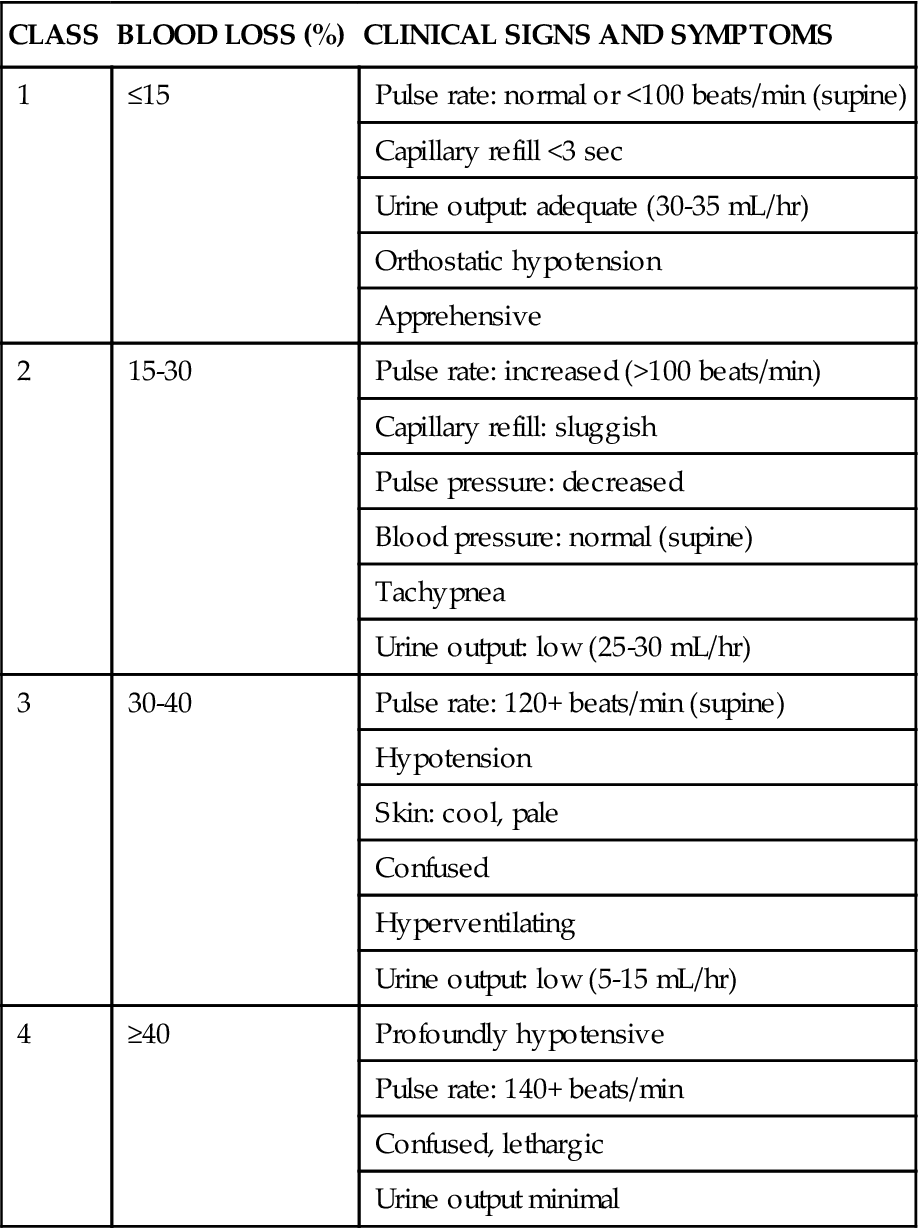
From Klein DG: Physiologic response to traumatic shock, AACN Clin Issues Crit Care Nurs 1(3):505, 1990.
Hematemesis
The patient who is vomiting blood is usually bleeding from a source above the duodenojejunal junction; reverse peristalsis is seldom sufficient to cause hematemesis if the bleeding point is below this area. The hematemesis may be bright red or look like coffee grounds, depending on the amount of gastric contents at the time of bleeding and the length of time the blood has been in contact with gastric secretions. Gastric acid converts bright red hemoglobin to brown hematin, accounting for the coffee grounds appearance of the emesis. Bright red emesis results from profuse bleeding with little contact with gastric secretions.1
Hematochezia and Melena
The presence of blood in the gastrointestinal tract results in increased peristalsis and diarrhea. Hematochezia (bright red stool) occurs from massive lower gastrointestinal hemorrhage and, if rapid enough, upper gastrointestinal hemorrhage. Melena (black, tarry, or dark red stool) occurs from digestion of blood from an upper gastrointestinal hemorrhage and may take several days to clear after the bleeding has stopped.2,4
Laboratory Studies
Laboratory tests can help to determine the extent of bleeding, although the patient’s hemoglobin level and hematocrit are poor indicators of the severity of blood loss if the bleeding is acute. As whole blood is lost, plasma and red blood cells are lost in the same proportion; if the patient’s hematocrit is 45% before a bleeding episode, it will be 45% several hours later.7 It may take as long as 72 hours for the redistribution of plasma from the extravascular space to the intravascular space to occur and cause the patient’s hemoglobin level and hematocrit value to decrease.14
Diagnostic Procedures
To isolate and treat the source of bleeding, an urgent fiberoptic endoscopy is usually undertaken. If performed within 12 hours of the bleeding event, endoscopy therapy has a 90% effectiveness rate in achieving hemostasis and reducing mortality.1,2 Before endoscopy, the patient must be hemodynamically stabilized.15 Tagged red blood cell scanning or angiography, or both, may be done to assist with localizing and treating a bleeding lesion in the gastrointestinal tract when it is impossible to clearly view the gastrointestinal tract because of continued active bleeding.1,5
Medical Management
To reduce mortality related to gastrointestinal hemorrhage, patients at risk should be identified early, and interventions should be implemented to reduce gastric acidity and support the gastric mucosal defense mechanisms. Management of the patient at risk for gastrointestinal hemorrhage should include prophylactic administration of pharmacological agents for gastric acid neutralization. These agents include antacids, histamine-2 (H2) antagonists, cytoprotective agents, and proton-pump inhibitors (PPIs).10,11
Priorities in the medical management of the patient with gastrointestinal hemorrhage include airway protection, fluid resuscitation to achieve hemodynamic stability, correction of comorbid conditions (e.g., coagulopathy), therapeutic procedures to control or stop bleeding, and diagnostic procedures to determine the exact cause of the bleeding.1,2,15
Stabilization
The initial treatment priority is the restoration of adequate circulating blood volume to treat or prevent shock. This is accomplished with the administration of intravenous infusions of crystalloids, blood, and blood products.13,15,16 Hemodynamic monitoring can help to guide fluid replacement therapy,8 particularly in patients at risk for cardiac failure. Supplemental oxygen therapy is initiated to increase oxygen delivery and improve tissue perfusion.2,5 Intubation may be necessary in the patient at risk for aspiration or to facilitate gastric lavage.15 A large nasogastric tube may be inserted to confirm the diagnosis of active bleeding and to prepare the esophagus, stomach, and proximal duodenum for endoscopic evaluation.1,2 A urinary drainage catheter should be inserted to monitor urine output.14
Controlling the Bleeding
Interventions to control bleeding are the second priority for the patient with gastrointestinal hemorrhage.
Peptic Ulcer Disease
In the patient with gastrointestinal hemorrhage related to peptic ulcer disease, bleeding hemostasis may be accomplished by endoscopic injection therapy in conjunction with thermal or hemostatic clips.2,17 Endoscopic thermal therapy uses heat to cauterize the bleeding vessel, and endoscopic injection therapy uses a variety of agents such as hypertonic saline, epinephrine, ethanol, and sclerosants to induce localized vasoconstriction of the bleeding vessel.1,2,17 Intraarterial infusion of vasopressin into the gastric artery or intraarterial injection of an embolizing agent (e.g., Gelfoam pledgets, polyvinyl alcohol particles, coils) can be performed during arteriography to control bleeding after the site has been identified.2
Stress-Related Mucosal Disease
In the patient with gastrointestinal hemorrhage caused by SRMD, bleeding hemostasis may be accomplished by intraarterial infusion of vasopressin and intraarterial embolization. Endoscopic therapies provide minimal benefit because of the diffuse nature of the disease.15
Esophagogastric Varices
In acute variceal hemorrhage, control of bleeding may be initially accomplished through the use of pharmacological agents and endoscopic variceal ligation.13 Intravenous vasopressin, somatostatin, and octreotide can reduce portal venous pressure and slow variceal hemorrhaging by constricting the splanchnic arteriolar bed.18 Endoscopic variceal ligation is the preferred endoscopic therapy for controlling acute gastrointestinal bleeding related to varices. Bands are placed around the varices to create an obstruction to stop the bleeding.13
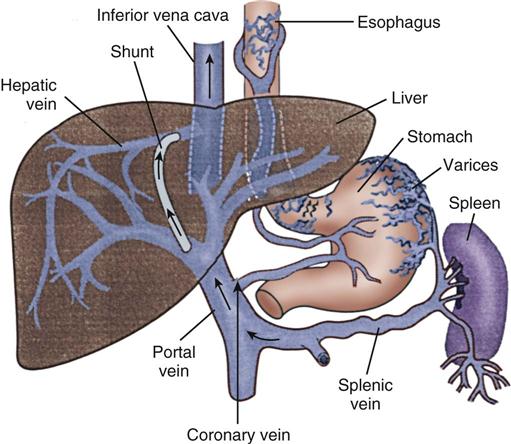
If these initial therapies fail, transjugular intrahepatic portosystemic shunting (TIPS) or esophagogastric balloon tamponade may be necessary. In a TIPS procedure, a channel between the systemic and portal venous systems is created to redirect portal blood, thereby reducing portal hypertension and decompressing the varices to control bleeding (Figure 22-2).1,13 Balloon tamponade tubes (Sengstaken-Blakemore, Linton, and Minnesota tubes) stop hemorrhaging by applying direct pressure against bleeding vessels while decompressing the stomach.19 This therapy is rarely needed anymore given the success rate of the other therapies.
Surgical Intervention
The patient who remains hemodynamically unstable despite volume replacement may need urgent surgery.
Peptic Ulcer Disease
The operative procedure of choice to control bleeding from peptic ulcer disease is a vagotomy and pyloroplasty. During this procedure, the vagus nerve to the stomach is severed, eliminating the autonomic stimulus to the gastric cells and reducing hydrochloric acid production. Because the vagus nerve also stimulates motility, a pyloroplasty is performed to provide for gastric emptying.9
Stress-Related Mucosal Disease
Several operative procedures can be used to control bleeding from SRMD. A total gastrectomy is performed when bleeding is generalized. The ulcers are oversewn when bleeding is localized.20 Total gastrectomy involves the complete removal of the stomach with anastomosis of the esophagus to the jejunum. During an oversew of the ulcers, the bleeding vessel is ligated, and the ulcer crater is closed.16,20
Esophagogastric Varices
Operative procedures to control bleeding gastroesophageal varices include portacaval shunt, mesocaval shunt, and splenorenal shunt (Figure 22-3).20 These shunt procedures are also referred to as decompression procedures, because they result in the diversion of portal blood flow away from the liver and decompression of the portal system. The portacaval shunt procedure has two variations. An end-to-side portacaval shunt procedure involves the ligation of the hepatic end of the portal vein with subsequent anastomosis to the vena cava. During a side-to-side portacaval shunt procedure, the side of the portal vein is anastomosed to the side of the vena cava. A mesocaval shunt procedure involves the insertion of a graft between the superior mesenteric artery and the vena cava. During a distal splenorenal shunt procedure, the splenic vein is detached from the portal vein and anastomosed to the left renal vein.20
Nursing Management
All critically ill patients should be considered at risk for stress ulcers and therefore gastrointestinal hemorrhage. Patients at risk also should be assessed for the presence of bright red or coffee grounds emesis; bloody nasogastric aspirate; and bright red, black, or dark red stools. Any signs of bleeding should be promptly reported to the physician.
Nursing management of a patient experiencing acute gastrointestinal hemorrhage incorporates a variety of nursing diagnoses (Nursing Diagnosis Priorities Box on Acute Gastrointestinal Hemorrhage). Nursing priorities are directed toward (1) administering volume replacement, (2) controlling the bleeding, (3) providing comfort and emotional support, (4) maintaining surveillance for complications, and (5) educating the patient and family.
Administering Volume Replacement
Measures to facilitate volume replacement include obtaining intravenous access and administering prescribed fluids and blood products. Two large-diameter peripheral intravenous catheters should be inserted to facilitate the rapid administration of prescribed fluids.13
Controlling the Bleeding
One measure to control active bleeding is gastric lavage. It is used to decrease gastric mucosal blood flow and evacuate blood from the stomach. Gastric lavage is performed by inserting a large-bore nasogastric tube into the stomach and irrigating it with normal saline or water until the returned solution is clear. It is important to keep accurate records of the amount of fluid instilled and aspirated to ascertain the true amount of bleeding.1 Historically, iced saline was favored as a lavage irrigant. Research has shown, however, that low-temperature fluids shift the oxyhemoglobin dissociation curve to the left, decrease oxygen delivery to vital organs, and prolong bleeding time and prothrombin time. Iced saline also may further aggravate bleeding; therefore room-temperature water or saline is the preferred irrigant for use in gastric lavage.21
Maintaining Surveillance for Complications
The patient should be continuously observed for signs of gastric perforation. Although a rare complication, gastric perforation constitutes a surgical emergency. Signs and symptoms include sudden, severe, generalized abdominal pain with significant rebound tenderness and rigidity. Perforation should be suspected when fever, leukocytosis, and tachycardia persist despite adequate volume replacement.9
Educating the Patient and Family
Early in the hospital stay, the patient and family should be taught about acute gastrointestinal hemorrhage and its causes and treatments. As the patient moves toward discharge, teaching should focus on the interventions necessary for preventing the recurrence of the precipitating disorder. If an alcohol abuser, the patient should be encouraged to stop drinking and be referred to an alcohol cessation program (Patient Education Box on Acute Gastrointestinal Hemorrhage). Collaborative management of the patient is outlined in the Box on Collaborative Management of Acute Gastrointestinal Hemorrhage.
Acute Pancreatitis
Acute pancreatitis is an inflammation of the pancreas that produces exocrine and endocrine dysfunction that may also involve surrounding tissues and/or remote organ systems. The clinical course can range from a mild, self-limiting disease to a systemic process characterized by organ failure, sepsis, and death. In approximately 80% of patients, it takes the milder form of edematous interstitial pancreatitis, whereas the other 20% develop severe acute necrotizing pancreatitis.22 Reported mortality rates for acute pancreatitis range from 2% to 15% overall and from 20% to 50% for patients with severe disease.22,23 Several prognostic scoring systems have been developed to predict the severity of acute pancreatitis. One of the most commonly used is Ranson’s criteria (Box 22-2). If the patient has 0 to 2 factors present, the predicted mortality rate is 2%; with 3 to 4 factors, the rate is 15%; with 5 to 6 factors, the rate is 40%; and with 7 to 8 factors, predicted mortality rate is 100%.22,24
Etiology
The two most common causes of acute pancreatitis are gallstones and alcoholism. Together, they account for approximately 80% of cases. Less common causes are quite diverse and include surgical trauma, hypercalcemia, various toxins, ischemia, infections, and the use of certain drugs (Box 22-3). In 10% to 20% of patients with acute pancreatitis, no etiologic factor can be determined.23
Pathophysiology
In acute pancreatitis, the normally inactive digestive enzymes become prematurely activated within the pancreas itself, leading to autodigestion of pancreatic tissue. The enzymes become activated through various mechanisms, including obstruction of or damage to the pancreatic duct system, alterations in the secretory processes of the acinar cells, infection, ischemia, and other unknown factors.8,22
Trypsin is the enzyme that becomes activated first. It initiates the autodigestion process by triggering the secretion of proteolytic enzymes such as kallikrein, chymotrypsin, elastase, phospholipase A, and lipase. Release of kallikrein and chymotrypsin results in increased capillary membrane permeability, leading to leakage of fluid into the interstitium and the development of edema and relative hypovolemia. Elastase is the most harmful enzyme in terms of direct cell damage. It dissolves the elastic fibers of blood vessels and ducts, leading to hemorrhage. Phospholipase A, in the presence of bile, destroys the phospholipids of cell membranes, causing severe pancreatic and adipose tissue necrosis. Lipase flows into the damaged tissue and is absorbed into the systemic circulation, resulting in fat necrosis of the pancreas and surrounding tissues.8,22
The extent of injury to the pancreatic cells determines the type of acute pancreatitis that develops. If injury to the pancreatic cells is mild and without necrosis, edematous pancreatitis develops. The acinar cells appear structurally intact, and blood flow is maintained through small capillaries and venules. This form of acute pancreatitis is self-limiting. If injury to the pancreatic cells is severe, acute necrotizing pancreatitis develops.22,23 Cellular destruction in pancreatic injury results in the release of toxic enzymes and inflammatory mediators into the systemic circulation and causes injury to vessels and other organs distant from the pancreas; this may result in systemic inflammatory response syndrome (SIRS), multiorgan failure, and death.22,23 Local tissue injury results in infection, abscess and pseudocyst formation, disruption of the pancreatic duct, and severe hemorrhage with shock.22
Assessment and Diagnosis
The clinical manifestations of acute pancreatitis range from mild to severe and often mimic those of other disorders (Box 22-4). Acute onset of abdominal pain is a hallmark symptom.23 Epigastric to periumbilical pain may vary from mild and tolerable to severe and incapacitating. Many patients report a twisting or knifelike sensation that radiates to the low dorsal region of the back. The patient may obtain some comfort by leaning forward or assuming a semifetal position. Nausea and vomiting are common.23 Other clinical findings include fever, diaphoresis, weakness, tachypnea, hypotension, and tachycardia. Depending on the extent of fluid loss and hemorrhage, the patient may exhibit signs of hypovolemic shock.22,23,25
Physical Examination
The results of physical assessment usually reveal hypoactive bowel sounds and abdominal tenderness, guarding, distention, and tympany. Findings that may indicate pancreatic hemorrhage include Grey Turner’s sign (gray-blue discoloration of the flanks) and Cullen’s sign (discoloration of the umbilical region); however, they are rare and usually seen several days into the illness.22 A palpable abdominal mass indicates the presence of a pseudocyst or abscess.26
Laboratory Studies
Assessment of laboratory data usually demonstrates elevated levels of serum amylase and lipase. Serum lipase is more pancreas-specific than amylase and a more accurate marker for acute pancreatitis. Amylase is present in other body tissues, and other disorders (e.g., intraabdominal emergencies, renal insufficiency, salivary gland trauma, liver disease) may contribute to an elevated level. Unlike other serum enzymes, however, amylase is excreted in urine, and this clearance increases with acute pancreatitis. Measurement of urinary versus serum amylase should be considered in light of the patient’s creatinine clearance. The serum amylase level may be elevated for only 3 to 5 days; if the patient delays seeking treatment, a normal level (false-negative result) may be detected. Leukocytosis, hypocalcemia, hyperglycemia, hyperbilirubinemia, and hypoalbuminemia may also be present (Table 22-2).8,22,23
TABLE 22-2
LABORATORY TESTS AND DIAGNOSTIC PROCEDURES FOR ACUTE PANCREATITIS
| STUDY | FINDING IN PANCREATITIS |
| Laboratory Studies | |
| Serum amylase | Elevated |
| Serum isoamylase | Elevated |
| Urine amylase | Elevated |
| Serum lipase (if available) | Elevated |
| Serum triglycerides | Elevated |
| Glucose | Elevated |
| Calcium | Decreased |
| Magnesium | Decreased |
| Potassium | Decreased |
| Albumin | Decreased or increased |
| White blood cell count | Elevated |
| Bilirubin | May be elevated |
| Liver enzymes | May be elevated |
| Prothrombin time | Prolonged |
| Arterial blood gases | Hypoxemia, metabolic acidosis |
| Diagnostic Procedures | |
| Abdominal ultrasonography | |
| Computed tomography scan | |
| Magnetic resonance imaging | |
| Endoscopic retrograde cholangiopancreatography | |
| Abdominal radiographs (flat plate and upright or decubitus) | |
| Chest radiographs (posteroanterior and lateral) |
Modified from Krumberger JM: Acute pancreatitis, Crit Care Nurs Clin North Am 5(1):185, 1993.
Diagnostic Procedures
An abdominal ultrasound scan is obtained as part of the diagnostic evaluation to determine the presence of biliary stones. A contrast-enhanced computed tomography (CT) scan is considered the gold standard for diagnosing pancreatitis and for ascertaining the overall degree of pancreatic inflammation and necrosis.22,23
Medical Management
Initial management of the patient with severe acute pancreatitis includes ensuring adequate fluid and electrolyte replacement, providing nutritional support, and correcting metabolic alterations.23 Careful monitoring for systemic and local complications is critical.22,24,25
Fluid Management
Because pancreatitis if often associated with massive fluid shifts, intravenous crystalloids and colloids are administered immediately to prevent hypovolemic shock and maintain hemodynamic stability. In severe forms of acute pancreatitis, a pulmonary artery catheter may be used to guide ongoing fluid management.25 Electrolytes are monitored closely, and abnormalities such as hypocalcemia, hypokalemia, and hypomagnesemia are corrected.22,25 If hyperglycemia develops, exogenous insulin may be required.25
Nutritional Support
Over the past 3 decades, nutritional support has shifted. Previously, conventional nutritional management was to place the patient on a no oral intake (NPO) regimen and institute intravenous hydration. The rationale was to rest the inflamed pancreas and prevent enzyme release. Oral feeding was initiated only when the attack had subsided and enzymes had normalized. Total parenteral nutrition (TPN) was started for patients anticipated to have oral feedings held for more than 5 days. Randomized clinical trials have demonstrated that enteral feeding (gastric or jejunal) is safe and cost-effective and that it is associated with fewer septic and metabolic complications than other methods.27,28 Enteral feeding enhances immune modulation and maintenance of the intestinal barrier, and it avoids complications associated with parental nutrition.27–29 Early initiation of enteral feeding is preferred over TPN.27–29 However, TPN still has a role for the critically ill patient with acute pancreatitis who does not tolerate enteral feeding or when nutritional goals are not reached within 2 days.23,27 In the past, nasogastric suction was also recommended, but this intervention has not been shown to be of benefit and should be instituted only if the patient has persistent vomiting, obstruction, or gastric distention.22
Systemic Complications
Acute pancreatitis can affect every organ system, and recognition and treatment of systemic complications are crucial to management of the patient (Box 22-5). The most serious complications are hypovolemic shock, acute lung injury (ALI), acute renal failure (ARF), and gastrointestinal hemorrhage. Hypovolemic shock is the result of relative hypovolemia resulting from third spacing of intravascular volume and vasodilation caused by the release of inflammatory immune mediators. These mediators also contribute to the development of ALI and ARF. Other possible pulmonary complications include pleural effusions, atelectasis, and pneumonia.
Local Complications
Local complications include the development of infected pancreatic necrosis and pancreatic pseudocyst.23,27 The necrotic areas of the pancreas can lead to development of a widespread pancreatic infection (infected pancreatic necrosis), which significantly increases the risk of death.27
Prophylactic antibiotics reduce sepsis and mortality and are initiated in patients suspected of having necrotizing pancreatitis.27 After the patient develops infected necrosis, however, surgical débridement is necessary.23,27 The procedure of choice is a minimally invasive necrosectomy, which entails careful débridement of the necrotic tissue in and around the pancreas. A pancreatic pseudocyst is a collection of pancreatic fluid enclosed by a nonepithelialized wall.26 Cyst formation may result from liquefaction of a pancreatic fluid collection or from direct obstruction in the main pancreatic duct.24 A pancreatic pseudocyst may (1) resolve spontaneously; (2) rupture, resulting in peritonitis; (3) erode a major blood vessel, resulting in hemorrhage; (4) become infected, resulting in abscess; or (5) invade surrounding structures, resulting in obstruction.24 Treatment involves drainage of the pseudocyst surgically,30 endoscopically, or percutaneously.23,27
Nursing Management
Nursing management of the patient with pancreatitis incorporates a variety of nursing diagnoses (Nursing Diagnosis Priorities Box on Acute Pancreatitis). Nursing priorities are directed toward (1) providing pain relief and emotional support, (2) maintaining surveillance for complications, and (3) educating the patient and family.
Providing Comfort and Emotional Support
Pain management is a major priority in acute pancreatitis. Administration of analgesics to achieve pain relief is essential. For years, meperidine (Demerol) was considered to be the preferred agent in the patient with acute pancreatitis because morphine produced spasms at the sphincter of Oddi. However, studies have demonstrated that all opioids have a spasmogenic effect on the sphincter of Oddi. There is no evidence to indicate that morphine is contraindicated for use in acute pancreatitis, and it may provide more effective analgesia with fewer side effects than meperidine.22,27 Relaxation techniques and positioning the patient in the knee-chest position can also assist in pain control.
Maintaining Surveillance for Complications
The patient must be routinely monitored for signs of local or systemic complications (see Box 22-5). Intensive monitoring of each of the organ systems is imperative, because organ failure is a major indicator of the severity of the disease.31 The patient must be closely monitored for signs and symptoms of pancreatic infection, which include increased abdominal pain and tenderness, fever, and increased white blood cell count (Box 22-6).22
Educating the Patient and Family
Early in the patient’s hospital stay, the patient and family should be taught about acute pancreatitis and its causes and treatment. As the patient moves toward discharge, teaching should focus on the interventions necessary for preventing the recurrence of the precipitating disorder. If there is sustained, permanent damage to the pancreas, the patient will require teaching specific to diet modification and supplemental pancreatic enzymes. Diabetes education may also be necessary. If an alcohol abuser, the patient should be encouraged to stop drinking and be referred to an alcohol cessation program (Patient Education Box on Acute Pancreatitis).25 Collaborative management of the patient is outlined in the Box on Collaborative Management of Acute Pancreatitis.
Acute Liver Failure
Description
Acute liver failure (ALF) is a life-threatening condition characterized by severe and sudden liver cell dysfunction, coagulopathy, and hepatic encephalopathy.32,33 Although uncommon, ALF is associated with a mortality rate as high as 33%, and it usually occurs in patients without preexisting liver disease.33,34 Because liver transplantation is one of the few definitive treatments, the patient with ALF should be transferred to a critical care unit and strongly considered for referral to a major medical center where transplantation services are available.32–34
Etiology
The causes of ALF include infections, drugs, toxins, hypoperfusion, metabolic disorders, and surgery (Box 22-7); however, viral hepatitis and drug-induced liver damage are the predominant causes in North America. Patients are usually healthy before the onset of symptoms because ALF tends to occur in patients with no known liver history. A thorough medication and health history is imperative to determine a possible cause. The patient should be questioned about exposure to environmental toxins, hepatitis, intravenous drug use, and sexual history. Viral hepatitis, drug toxicity, poisoning, and vascular and metabolic disorders such as Reye’s syndrome and Wilson’s disease should be considered.32
Pathophysiology
ALF is a syndrome characterized by the development of acute liver failure over 1 to 3 weeks, followed by the development of hepatic encephalopathy within 8 weeks, in a patient with a previously healthy liver. The interval between the failure of the liver and the onset of hepatic encephalopathy usually is less than 2 weeks. The underlying cause is massive necrosis of the hepatocytes.32,33
Acute liver failure results in a number of derangements, including impaired bilirubin conjugation, decreased production of clotting factors, depressed glucose synthesis, and decreased lactate clearance. This results in jaundice, coagulopathies, hypoglycemia, and metabolic acidosis. Other effects of acute liver failure include increased risk of infection and altered carbohydrate, protein, and glucose metabolism. Hypoalbuminemia, fluid and electrolyte imbalances, and acute portal hypertension contribute to the development of ascites.33,34 Hepatic encephalopathy is thought to result from failure of the liver to detoxify various substances in the bloodstream, and it may be worsened by metabolic and electrolyte imbalances.33
The patient may experience a variety of other complications, including cerebral edema, cardiac dysrhythmias, acute respiratory failure, sepsis, and acute renal failure. Cerebral edema and increased intracranial pressure (ICP) develop as a result of breakdown of the blood-brain barrier and astrocyte swelling. Circulatory failure that mimics sepsis is common in ALF and may exacerbate low cerebral perfusion pressure (CPP).34 Hypoxemia, acidosis, electrolyte imbalances, and cerebral edema can precipitate the development of cardiac dysrhythmias. Acute respiratory failure, progressing to ALI, can result from pulmonary edema, aspiration pneumonia, and atelectasis. ARF may be caused by acute tubular necrosis, hypotension, or hemorrhage.33,34
Assessment and Diagnosis
Early recognition of ALF is essential. The diagnosis should include potentially reversible conditions (e.g., autoimmune hepatitis) and should differentiate ALF from decompensating chronic liver disease. Prognostic indicators such as coma grade, serum bilirubin, prothrombin time, coagulation factors, and pH should be assessed and potential causes investigated.33,34
Signs and symptoms of ALF include headache, hyperventilation, jaundice, mental status changes, palmar erythema, spider nevi, bruises, and edema. The patient should be evaluated for the presence of asterixis or “liver flap,” best described as the inability to voluntarily sustain a fixed position of the extremities. Asterixis is best demonstrated by having the patient extend the arms and dorsiflex the wrists, resulting in downward flapping of the hands. Hepatic encephalopathy is assessed using a grading system that stages the encephalopathy according to the patient’s clinical manifestations (Box 22-8).33,34 Diagnostic findings include elevated levels of serum bilirubin, aspartate aminotransferase (AST), alkaline phosphatase, and serum ammonia and decreased levels of serum albumin. Arterial blood gases (ABGs) reveal respiratory alkalosis or metabolic acidosis, or both. Hypoglycemia, hypokalemia, and hyponatremia also may be present.33,34
Factors I (fibrinogen), II (prothrombin), V, VII, IX, and X are produced exclusively by the liver. Prothrombin time may be the most useful of tests of these in the evaluation of ALF because levels may be 40 to 80 seconds above control values. Test results show decreased levels of plasmin and plasminogen and increased levels of fibrin and fibrin-split products. Platelet counts may be less than 100,000/mm3.33
Medical Management
Medical interventions are directed toward management of the multiple system impact of ALF.
Ammonia Levels
Neomycin or lactulose is administered to remove or decrease production of nitrogenous wastes in the large intestine. Neomycin, given orally or rectally, reduces bacterial flora of the colon. This aids in decreasing ammonia formation by decreasing bacterial action on protein in the feces. Side effects include renal toxicity and hearing impairment. Lactulose, a synthetic ketoanalogue of lactose split into lactic acid and acetic acid in the intestine, is given orally through a nasogastric tube or as a retention enema. The result is the creation of an acidic environment that decreases bacterial growth. Lactulose also traps ammonia and has a laxative effect that promotes expulsion.34
Complications
Bleeding is best controlled through prevention. Because these patients are at risk for acute gastrointestinal hemorrhage, stress ulcer prophylaxis is essential.35 If an invasive procedure (e.g., central line placement, ICP monitor) will be performed or the patient develops active bleeding, vitamin K, fresh-frozen plasma (to maintain a reasonable prothrombin time), and platelet transfusions are necessary.34 Metabolic disturbances such as hypoglycemia, metabolic acidosis, hypokalemia, and hyponatremia should be monitored and treated appropriately. Prophylactic antibiotic administration may be initiated because the patient is at high risk for an infection.33,34
The development of cerebral edema necessitates ICP monitoring. Mannitol is the only treatment shown to be of benefit in managing increased ICP in the patient with ALF, but it must be used with caution in patients with renal failure.35 Other interventions to control intracranial hypertension include elevating the head of the bed (HOB) to 30 degrees, treating fever and hypertension, minimizing noxious stimulation, and correcting hypercapnia and hypoxemia.35 Various experimental therapies to prevent or treat cerebral edema, such as induction of hypothermia, prophylactic phenytoin, and induction of hypernatremia, have been studied but have not improved survival.35 If renal failure develops, continuous renal replacement therapy (CRRT) should be initiated.35 Intubation and mechanical ventilation may be necessary as the inability to protect the airway and hypoxemia develops.34 Hemodynamic instability is a common complication necessitating fluid administration and vasoactive medications to prevent prolonged episodes of hypotension. A pulmonary artery catheter may be used to guide clinical management.34
If ALF continues and the patient shows no immediate signs of improvement or reversal, the patient should be considered for a liver transplant. Prompt referral to a transplantation center should be a high priority for patients experiencing ALF.33–35
Nursing Management
Nursing management of the patient with ALF incorporates a variety of nursing diagnoses (Nursing Diagnosis Priorities Box on Acute Liver Failure). Nursing priorities are directed toward (1) protecting the patient from injury, (2) providing comfort and emotional support, (3) maintaining surveillance for complications, and (4) educating the patient and family.
Protecting the Patient from Injury
Use of benzodiazepines and other sedatives is discouraged in the ALF patient because pertinent neurological changes may be masked and hepatic encephalopathy may be exacerbated.34 These patients are often very difficult to manage because they may be extremely agitated and combative. Physical restraint may be necessary to prevent patient injury.
Maintaining Surveillance for Complications
As the neurological condition worsens, respiratory depression and arrest can occur quickly. Continuous pulse oximetry monitoring and ABG analysis are helpful in assessing adequacy of respiratory efforts. A thorough neurological assessment should be performed at least every hour.
Educating the Patient and Family
Early in the patient’s hospital stay, the patient and family should be taught about ALF and its causes and treatment. As the patient moves toward discharge, teaching should focus on the interventions necessary for preventing the recurrence of the precipitating cause. If the patient is considered a candidate for liver transplantation, the patient and family will need specific information regarding the procedure and care. Liver transplant evaluation may include screening for medical contraindications, human immunodeficiency virus (HIV) serology, anticipated compliance, and assessment of the social support system. Psychiatric and other specialty team consultations are necessary for a thorough evaluation of the patient’s suitability for a liver transplant (Patient Education Box on Acute Liver Failure). Collaborative management of the patient with ALF is outlined in the Collaborative Management Box on Acute Liver Failure.
Therapeutic Management
Gastrointestinal Intubation
Because gastrointestinal intubation is used so often in critical care units, it is important for nurses to know the clinical indications and responsibilities inherent in tube use. The four categories of gastrointestinal tubes are based on function: nasogastric suction tubes, long intestinal tubes, feeding tubes, and esophagogastric balloon tamponade tubes (Patient Safety Priorities Box on Tubing Misconnections).
Nasogastric Suction Tubes
Nasogastric tubes remove fluid regurgitated into the stomach, prevent accumulation of swallowed air, may partially decompress the bowel, and reduce the patient’s risk for aspiration. Nasogastric tubes also can be used for collecting specimens, assessing the presence of blood, and administering tube feedings. The most common nasogastric tubes are the single-lumen Levin tube and the double-lumen Salem sump. The Salem sump has one lumen that is used for suction and drainage and another that allows air to enter the patient’s stomach and prevents the tube from adhering to the gastric wall and damaging the mucosa. The tube is passed through the nose into the nasopharynx and then down through the pharynx into the esophagus and stomach. The length of time the nasogastric tube remains in place depends on its use. The tube is then placed to gravity, or low continuous suction, and in rare instances, it is clamped.36,37
Nursing management focuses on preventing complications common to this therapy, such as ulceration and necrosis of the nares, esophageal reflux, esophagitis, esophageal erosion and stricture, gastric erosion, and dry mouth and parotitis from mouth breathing. Interference with ventilation and coughing, aspiration, and loss of fluid and electrolytes can be critical problems. Interventions include irrigating the tube every 4 hours with normal saline, ensuring the blue air vent of the Salem sump is patent and maintained above the level of the patient’s stomach, and providing frequent mouth and nares care.36
Long Intestinal Tubes
Miller-Abbott, Cantor, and Andersen tubes are examples of long, weighted-tip intestinal tubes that are placed preoperatively or intraoperatively. The long length allows removal of contents from the intestine to treat an obstruction that cannot be managed by a nasogastric tube. These tubes can decompress the small bowel and can splint the small bowel intraoperatively or postoperatively. Because progression of the tubes depends on bowel peristalsis, their use is contraindicated in patients with paralytic ileus and severe mechanical bowel obstructions. Older devices such as the Cantor and Miller-Abbott tubes are rarely used today because the balloon and the distal end is filled with mercury; the newer Andersen tube has a preweighted tungsten tip and is a safer option.36
Interventions used in the care of the patient with a long intestinal tube are similar to those with a nasogastric tube. The patient should be observed for (1) gaseous distention of the balloon section, which makes removal difficult; (2) rupture of the balloon or spillage of mercury into the intestine; (3) overinflation of the balloon, which can lead to intestinal rupture; and (4) reverse intussusception if the tube is removed rapidly. Intestinal tubes should be removed slowly; usually 6 inches of the tube is withdrawn every hour.36
Feeding Tubes
Small-diameter (8- to 12-Fr) flexible feeding tubes, such as Dobhoff tubes, are commonly placed at the bedside for patients who cannot take nourishment orally. The feeding tube may be inserted orally or nasally so that the tip ends up in the stomach or duodenum. To facilitate passage into the gastrointestinal tract, these tubes have a weighted tungsten tip, and a guidewire is needed to prevent them from curling up in the back of the patient’s throat. An x-ray film must be obtained to verify correct placement of the tube before initiating feeding.36–39 The tube should also be marked with indelible ink where it exits the mouth or nares so that the nurse can later verify that the tube has not been dislodged.39
Nursing management of the patient with a feeding tube includes prevention of complications and monitoring the tolerance of feeding. Tracheobronchial aspiration of gastric contents is a serious potential complication.38 Before administering medications or feedings, it is important to ensure that the tube is in the patient’s stomach or duodenum. Assessing the exit point marked on the tube helps to determine whether the tube has maintained the same position. Looking for coiling in the mouth or throat can help detect upward displacement that may have occurred as a result of vomiting. The traditional practice of confirming placement by auscultating air inserted through the tube over the epigastrium is not reliable and is not recommended.37–39 If there is any doubt about the tube’s position, a repeat radiograph should be obtained. During feedings, the head of bed should be elevated at least 30 degrees to minimize the risk of aspiration, and gastric residuals should be checked at least every 4 to 6 hours.38 Large gastric residuals, cramping, and abdominal distention may indicate intolerance of feeding, and the physician should be notified.38 Other interventions include nares and oral care and flushing the tube with normal saline or water to maintain patency.36,37
Endoscopic Injection Therapy
Endoscopic injection therapy is used to control bleeding of varices and ulcers. It may be performed emergently, electively, or prophylactically. An endoscope is introduced through the patient’s mouth, and endoscopy of the esophagus and stomach is performed to identify the bleeding varices or ulcers. An injector with a retractable 23- to 25-gauge needle is introduced through the biopsy channel of the endoscope. The needle then is inserted in or around the varices or into the area around the ulcer, and a liquid agent is injected. The most commonly used agent is epinephrine, which results in localized vasoconstriction and enhanced platelet aggregation. Sclerosing agents such as ethanolamine, alcohol, and polidocanol also may be used. These agents cause an inflammatory reaction in the vessel that results in thrombosis and eventually produces a fibrous band. Repeated sclerotherapy results in the development of supportive scar tissue around the varices. Other agents used include fibrinogen and thrombin, which when injected together react to form an active fibrin clot, and cyanoacrylate glue, which is used as a sealant to stop the bleeding.17,18
Endoscopic injection therapy controls acute variceal bleeding in as many as 80% of patients.40 Complications can vary from mild to severe and include esophageal perforation, extravasation of the injection agent, and strictures of the esophagus. This procedure is contraindicated in patients with severe coagulopathies.3
Endoscopic Variceal Ligation
Endoscopic variceal ligation involves applying bands or metal clips around the circumference of the bleeding varices to induce venous obstruction and control bleeding. Between 1 and 2 days after the procedure, necrosis and scar formation promote band and tissue sloughing. Fibrinous deposits within the healing ulcer potentiate vessel obliteration. Band ligation is accomplished through endoscopy, with 5 to 8 bands placed per session.40 The procedure may be repeated on an inpatient or outpatient basis over 2 to 3 weeks until all the varices are obliterated.13
Endoscopic variceal ligation controls bleeding approximately 80% to 90% of the time.13 This procedure is reported to require fewer endoscopic treatment sessions and has a lower rebleeding rate and fewer complications than endoscopic sclerotherapy. The most common complication of endoscopic variceal ligation is the development of superficial mucosal ulcers. Systemic complications are rare.40
Transjugular Intrahepatic Portosystemic Shunt
Transjugular intrahepatic portosystemic shunting (TIPS) is an angiographic interventional procedure for decreasing portal hypertension. TIPS is advocated for (1) patients with portal hypertension who are also experiencing active bleeding or have poor liver reserve, (2) transplant recipients, and (3) patients with other operative risks. The TIPS procedure is usually performed by a gastroenterologist, vascular surgeon, or interventional radiologist.
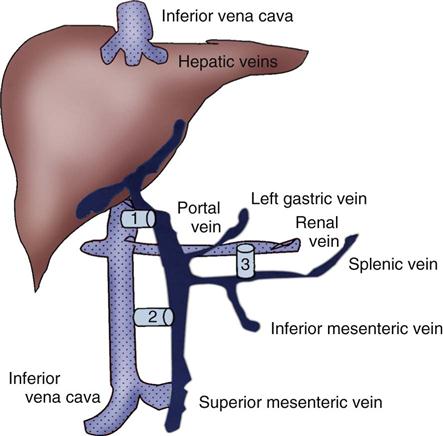
Portal hypertension is confirmed by direct measurement of the pressure in the portal vein (gradient greater than 10 mm Hg). Cannulation is achieved through the internal jugular vein, and an angiographic catheter is advanced into the middle or right hepatic vein. The mid-hepatic vein is then catheterized, and a new route is created connecting the portal and hepatic veins using a needle and guidewire with a dilating balloon. An expandable stainless steel stent is then placed in the liver parenchyma to maintain that connection (Figure 22-4). The increased resistance in the liver is bypassed.41
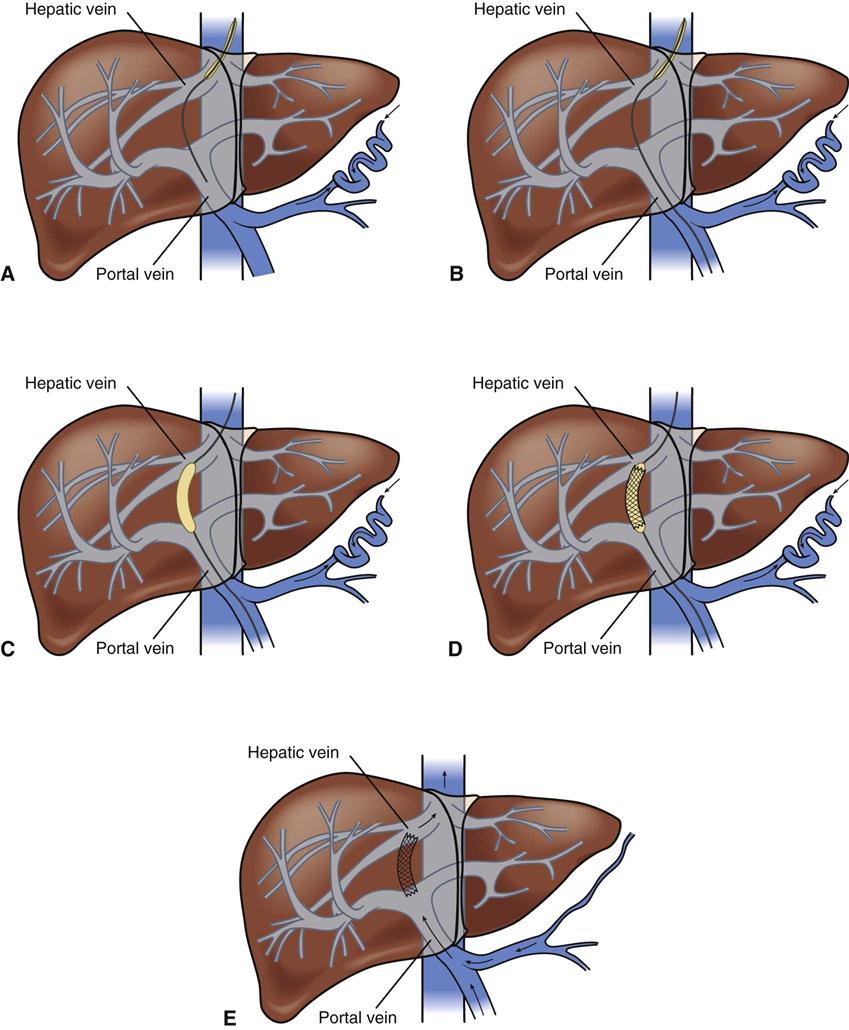
A, Needle directed though liver parenchyma to portal vein. B, Needle and guidewire passed down to midportal vein. C, Balloon dilation. D, Deployment of stent. E, Intrahepatic shunt from portal to hepatic vein.
TIPS may be performed on patients with bleeding varices, with refractory bleeding varices, or as a bridge to liver transplantation if the candidate becomes hemodynamically unstable. Postprocedural care should include observation for overt (cannulation site) or covert (intrahepatic site) bleeding, hepatic or portal vein laceration (resulting in rapid loss of blood volume), and inadvertent puncture of surrounding organs. Other complications include bile duct trauma, stent migration, and stent thrombosis.41 Portal hypertension recurs almost universally after TIPS, whereas shunt dysfunction occurs in 50% to 60% of patients within 6 months.42
Gastrointestinal Surgery
Types of Surgery
Gastrointestinal surgery refers to a wide variety of surgical procedures that involve the esophagus, the stomach, the intestine, the liver, the pancreas, or the biliary tract. Indications for gastrointestinal surgery are numerous and include bleeding or perforation from peptic ulcer disease, obstruction, trauma, inflammatory bowel disease, and malignancy. Patients may be admitted to the critical care unit for monitoring after gastrointestinal surgery as a result of their underlying medical condition; however, this portion of the chapter focuses only on several surgical procedures that commonly require postoperative critical care.
Esophagectomy
Esophagectomy is usually performed for cancer of the distal esophagus and gastroesophageal junction. The technically difficult procedure involves the removal of part or the entire esophagus, part of the stomach, and lymph nodes in the surrounding area. The stomach is then pulled up into the chest and connected to the remaining part of the esophagus. If the entire esophagus and stomach must be removed, part of the bowel may be used to form the esophageal replacement (Figures 22-5 and 22-6).43,44
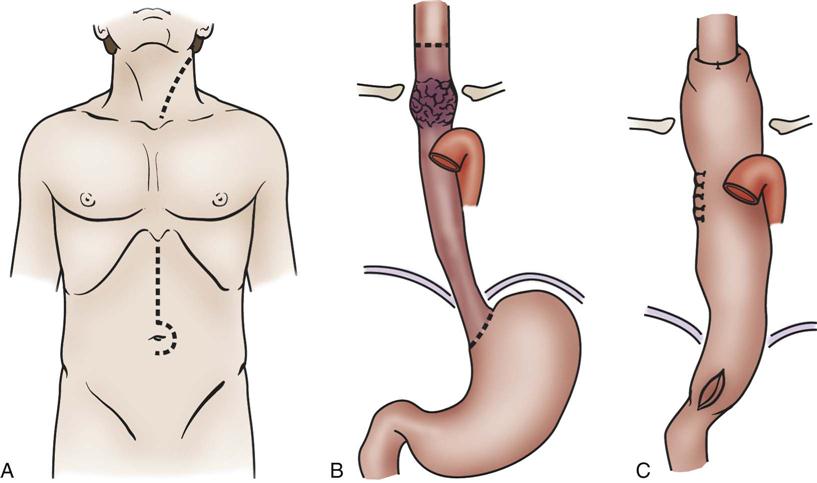
A, With gastric mobilization B, and gastric pull-up C, for cervical-esophagogastric anastomosis. (A to C, Modified from Ellis F: Esophagogastrectomy for carcinoma: technical considerations based on anatomic location of lesion, Surg Clin North Am 60[2]:265, 1980.)
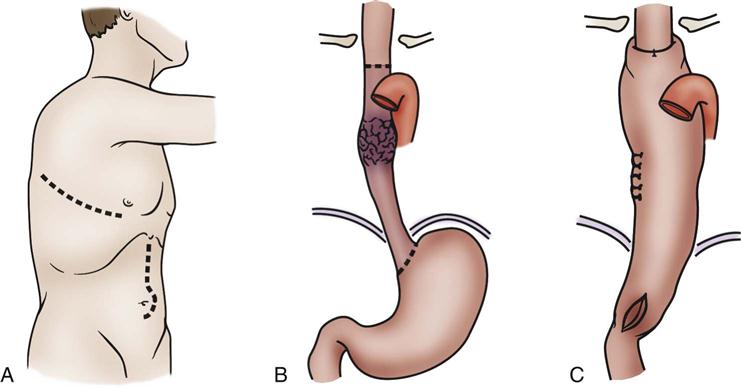
A, With esophageal resection, gastric mobilization B, and intrathoracic anastomosis C, for a midesophageal tumor. (A to C, Modified from Ellis FH: Esophagogastrectomy for carcinoma: technical considerations based on anatomic location of lesion, Surg Clin North Am 60[2]:265, 1980.)
Pancreaticoduodenectomy
The standard operation for pancreatic cancer is a pancreaticoduodenectomy, also called a Whipple procedure. In the Whipple procedure, the pancreatic head, the duodenum, part of the jejunum, the common bile duct, the gallbladder, and part of the stomach are removed. The continuity of the gastrointestinal tract is restored by anastomosing the remaining portion of the pancreas, the bile duct, and the stomach to the jejunum (Figure 22-7).44,45
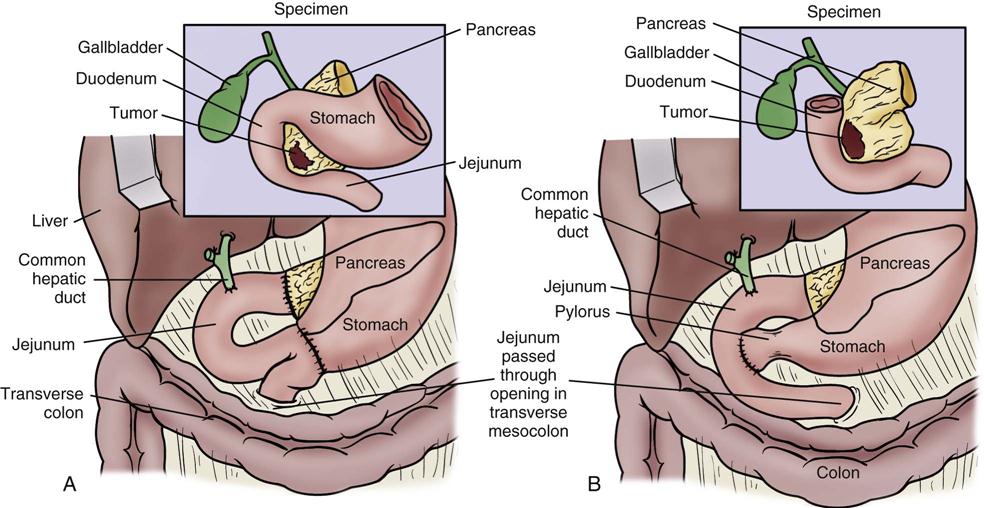
A, The standard Whipple procedure involves resection of the gastric antrum, head of pancreas, distal bile duct, and entire duodenum with reconstruction as shown. B, The pylorus-preserving Whipple procedure does not include resection of the distal stomach, pylorus, or proximal duodenum. (From Cameron JL: Current status of the Whipple operation for periampullary carcinoma, Surg Rounds 77, 1988.)
Bariatric Surgery
Bariatric surgery refers to surgical procedures of the gastrointestinal tract that are performed to induce weight loss. Bariatric procedures are divided into three broad types: restrictive, malabsorptive, and combined restrictive and malabsorptive.46 Restrictive procedures such as vertical banded gastroplasty (VBG) (Figure 22-8, A) and gastric banding (Figure 22-8, B) reduce the capacity of the stomach and limit the amount of food that can be consumed. Malabsorptive procedures such as the biliopancreatic diversion (BPD) (Figure 22-8, C) alter the gastrointestinal tract to limit the digestion and absorption of food. The Roux-en-Y gastric bypass (RYGBP) (Figure 22-8, D) combines both strategies by creating a small gastric pouch and anastomosing the jejunum to the pouch. Food then bypasses the lower stomach and duodenum, resulting in decreased absorption of digestive materials.46,47
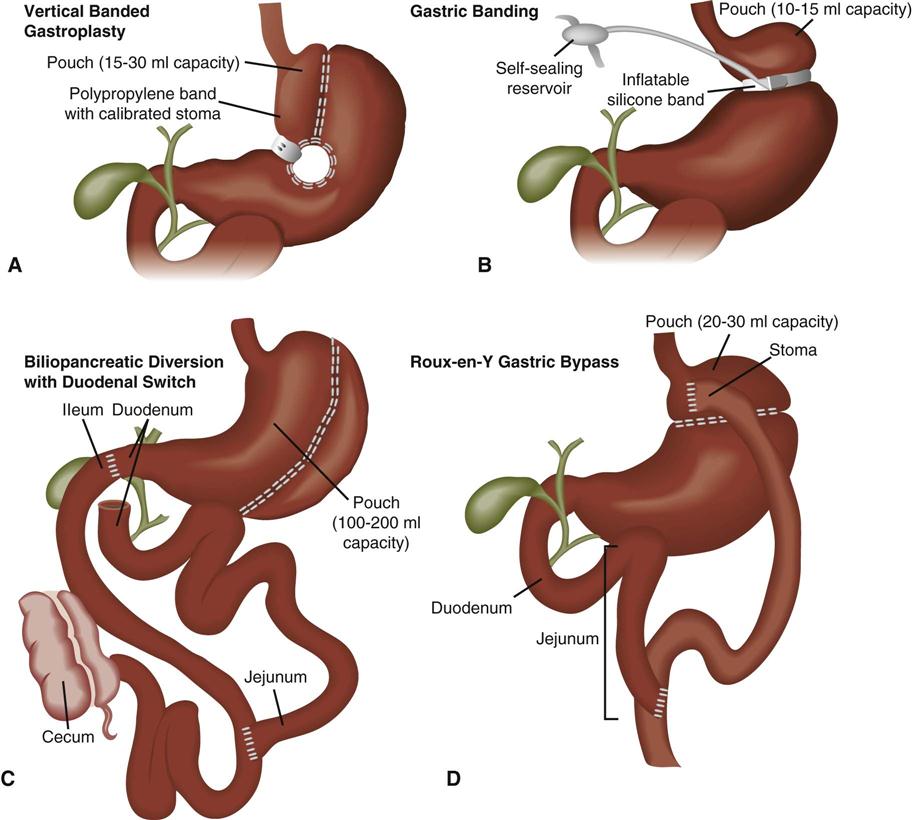
A, Vertical banded gastroplasty creates a tubular stomach that is restrictive. B, Gastric banding systems are adjustable and reversible, and they can be placed laparoscopically. C, Biliopancreatic diversion with duodenal switch and vertical gastroplasty/sleeve gastrectomy. D, Roux-en-Y proximal gastric bypass. (From Lewis et al: Medical surgical nursing: Assessment and management of clinical problems, ed 7, St Louis, 2007, Mosby. Redrawn from Price SA, Wilson LM: Pathophysiology: Clinical concepts of disease processes, ed 6, St Louis, 2003, Mosby.)
Preoperative Care
A thorough preoperative evaluation should be conducted to evaluate the patient’s physical status and identify risk factors that may affect the postoperative course. Because obesity is associated with a higher incidence of comorbidities such as cardiovascular disease, hypertension, diabetes, gastroesophageal reflux, obstructive sleep apnea, and heart failure, an extensive workup may be required for the bariatric patient.47 Before esophagectomy or pancreaticoduodenectomy, the patient may undergo multiple diagnostic tests, such as CT, positron emission tomography (PET), and endoscopic ultrasound (EUS), to determine the invasiveness of the tumor.43
Surgical Considerations
Two approaches may be used for esophageal resection: transhiatal or transthoracic (see Figures 22-6 and 22-7). In both approaches, the stomach is mobilized through an abdominal incision and then transposed into the chest. The anastomosis of the stomach to the esophagus is performed in the chest (transthoracic) or in the neck (transhiatal). The approach selected depends on the location of the tumor, the patient’s overall health and pulmonary function, and the experience of the surgeon. After surgery, the patient has a nasogastric tube in place that should not be manipulated because of the potential to damage the anastomosis. Those who undergo transthoracic esophagectomy have one or more chest tubes.43,44
Most bariatric procedures can be performed using an open or laparoscopic surgical technique. Although laparoscopic approaches are more technically difficult to perform, they have largely replaced open procedures because they are associated with decreased pulmonary complications, less postoperative pain, reduced length of hospital stay, fewer wound complications (e.g., infections, incisional hernia), and an earlier return to full activity.46,48 Open procedures are performed on patients who have had prior upper abdominal surgery, are morbidly obese, or who may not be able to tolerate the increased abdominal pressure associated with laparoscopic procedures.44
Complications and Medical Management
Several complications are associated with gastrointestinal surgery, including respiratory failure, atelectasis, pneumonia, anastomotic leak, deep vein thrombosis, pulmonary embolus, and bleeding. The morbidly obese patient is at even greater risk for many postoperative complications.46,47
Pulmonary Complications
The risk for pulmonary complications is substantial after gastrointestinal surgery, and adverse respiratory events such as atelectasis and pneumonia are twice as likely to occur in the obese patient.46 Aggressive pulmonary exercise should be initiated in the immediate postoperative period. Early ambulation and adequate pain control assist in reducing the risk of atelectasis development. Suctioning, chest physiotherapy, or bronchodilators may be needed to optimize pulmonary function. Patients should be closely monitored for the development of oxygenation problems. Treatment should be aimed at supporting adequate ventilation and gas exchange. Mechanical ventilation may be required in the event of respiratory failure.
Anastomotic Leak
An anastomotic leak is a severe complication of gastrointestinal surgery. It occurs when there is a breakdown of the suture line in a surgical anastomosis and results in leakage of gastric or intestinal contents into the abdomen or mediastinum (transthoracic esophagectomy).43,46,47 The clinical signs and symptoms of a leak can be subtle and often go unrecognized. They include tachycardia, tachypnea, fever, abdominal pain, anxiety, and restlessness.46,47 In the patient with an esophagectomy, a leak of the esophageal anastomosis may manifest as subcutaneous emphysema in the chest and neck.43 If undetected, a leak can result in sepsis, multiorgan failure, and death. Patients with progressive tachycardia and tachypnea should have a radiological study (upper gastrointestinal study with Gastrografin or CT scan with contrast) to rule out an anastomotic leak.47 The type of treatment depends on the severity of the leak. If the leak is small and well contained, it may be managed conservatively by maintaining the NPO status, administering antibiotics, and draining the fluid percutaneously. If the patient is deteriorating rapidly, an urgent laparotomy is indicated to repair the defect.49
Deep Vein Thrombosis and Pulmonary Embolism
Pulmonary embolism (PE) is a very serious complication of any surgical procedure. Deep vein thrombosis (DVT) prophylaxis should be initiated before surgery and continue until the patient is fully ambulatory to reduce the risk of clot development. Typically, a combination of sequential compression devices and subcutaneous unfractionated heparin or low-molecular-weight heparin is used. Patients determined to be at high risk for PE may benefit from prophylactic inferior vena cava filter placement.43,46,48
Bleeding
Upper gastrointestinal bleeding is an uncommon but life-threatening complication of gastrointestinal surgery. Early bleeding typically occurs at the site of the anastomosis and can usually be treated through endoscopic intervention. Surgical revision may be needed for persistent, uncontrolled bleeding. Late bleeding is usually a result of ulcer development. Medical therapy is aimed at the prevention of this complication through administration of H2-antagonists or PPIs.43
Postoperative Nursing Management
Nursing care of the patient who has had gastrointestinal surgery incorporates a number of nursing diagnoses (Nursing Diagnosis Priorities Box on Gastrointestinal Surgery). Nursing priorities are directed toward (1) optimizing oxygenation and ventilation, (2) providing comfort and emotional support, and (3) maintaining surveillance for complications.
Optimizing Oxygenation and Ventilation
Nursing interventions in the postoperative period are focused on promoting ventilation and adequate oxygenation and preventing complications such as atelectasis and pneumonia. After the patient is extubated, deep-breathing exercises and incentive spirometry should be initiated and then performed regularly. Early ambulation is encouraged to promote maximal lung inflation and thereby reduce the risk of pulmonary complications and to reduce the potential for pulmonary embolus.
Providing Comfort and Emotional Support
It is imperative to appropriately manage the patient’s pain after gastrointestinal surgery. Adequate analgesia is necessary to promote mobility of the patient and decrease pulmonary complications. Initial pain management may be accomplished by intravenous opioid (morphine, hydromorphone) administration by means of a patient-controlled analgesia (PCA) pump, or through continuous epidural infusion of an opioid and local anesthetic (bupivacaine).44,46,47 Oral pain medications can be started after an anastomosis leak is ruled out. Nonpharmacological interventions such as positioning, application of heat or cold, and distraction may also be used. If the patient’s pain is not being sufficiently relieved, the pain management service should be consulted.43,47
Liver Transplantation
Indications and Selection
Liver transplantation must be considered for any patient who suffers from irreversible acute or chronic liver disease that is progressive and for which there is no therapy of established efficacy. Diseases of the liver may be categorized as chronic, vascular, acute liver failure, and include inborn errors of metabolism and hepatic malignancies. Box 22-9 lists the most common diseases seen in patients who undergo liver transplantation. In the United States, the single most common indication for liver transplantation in adults is chronic viral hepatitis C.50
Candidate selection is an important aspect of transplantation. Given the shortage of available organs, the transplantation team must have reasonable assurance of a successful outcome. The timing of transplantation is of utmost importance. The patient must not be so ill as to be unable to survive the surgery but yet be experiencing deterioration in the quality of life. Body mass index (BMI) plays a role in posttransplantation survival. Patients who are underweight (BMI < 20) or morbidly obese (BMI > 40) are at greater risk for death after transplantation.51 In general, liver transplantation is not to be offered to persons in the following groups:
• Those who would not be likely to survive major surgery
• Those who would not survive the effects of long-term immunosuppression
• Those who have a disease that is likely to recur quickly and fatally after transplantation
The absolute contraindications listed in Box 22-10 fall under these four specific categories.
Having one relative contraindication may not rule out transplantation, but having several predicts poor outcome. Chronological age is less important than physiological age. Reports of transplantation in older patients describe favorable results.52 Certain diseases can recur after transplantation, including viral hepatitis,53 sclerosing cholangitis,54 and biliary malignancies.55 In the case of viral hepatitis, serologic indicators of viral replication are monitored closely. In the presence of aggressively replicating virus and in certain malignancies, it is in the patient’s best interest not to proceed to transplantation, because it would actually hasten death. Multicenter protocols are important in evaluating the outcomes and efficacies of transplantations in patients with diseases that recur. The decision to offer liver transplantation to any patient must be based on evaluation criteria, which vary among institutions and are modified as advances in technical ability, immunosuppression, and perioperative management continue. In the absence of complications, the average hospital stay after liver transplantation is 7 to 14 days.
Recipient Evaluation
The candidate for liver transplantation undergoes a thorough evaluation to determine the cause and severity of the liver disease, to establish the need for transplantation rather than other interventions, and to identify objective indications and contraindications. Evaluation begins with a carefully elicited patient history (Box 22-11). A comprehensive approach includes laboratory, radiographic, and diagnostic testing and multidisciplinary consultations (Box 22-12). Not every patient undergoes every test and consultation. Careful history taking and a good physical examination direct the initial diagnostic testing. For instance, a patient with a past history of malignancy would undergo extensive testing to rule out metastases, whereas a patient with acute liver failure may have a more abbreviated workup that is focused on determining the cause and potential for hepatic recovery.56
During the workup, the candidate’s support systems are evaluated by the entire transplantation team, which includes the surgeon, the hepatologist, the clinical transplantation nurse coordinator, the social worker, the dietitian, and the financial counselor. Other services, such as cardiology, nephrology, psychiatry, gynecology, anesthesia, infectious disease, endocrinology, hematology, rheumatology, and oral surgery or dentistry, may also be included in the evaluation. Ideally, all immunizations are brought up to date in an attempt to minimize postoperative infections. The patient and family receive education regarding the evaluation, waiting list, surgery, postoperative management including immunosuppression, and long-term follow-up. At the conclusion of the evaluation, one of several outcomes is possible: (1) the patient is deemed a transplant candidate, (2) the patient is deemed not a candidate, or (3) the patient may be a candidate sometime in the future if certain criteria are met. These criteria may be of a physical nature (e.g., it is too early in the disease process to list now, in which case the patient will be re-evaluated at set intervals), or they may be of a psychosocial nature (e.g., the patient must attend a formal alcohol or drug rehabilitation program or undergo treatment of depression).56
After candidacy has been determined and the patient is ready for transplantation, his or her social security number is entered into the national computer system operated by United Network for Organ Sharing (UNOS). Objective criteria are used to place a patient on the waiting list. These data are used in a formula to determine the patient’s score, which is directly associated with the patient’s risk of death within 3 months (the higher the score, the higher the risk).
Model for End-Stage Liver Disease
The Model for End-Stage Liver Disease (MELD) formula is used in all U.S. transplant centers to calculate risk of mortality in patients 12 years old or older. The MELD objective criteria include serum total bilirubin, serum creatinine, prothrombin time, and international normalized ratio.57
Placement on the waiting list is determined by blood type, weight, and patient urgency. Patients with acute liver failure are considered to be in most urgent need and are placed at the top of the list. Patients with chronic end-stage liver disease are prioritized by their MELD score. Those with higher scores are placed higher on the list. The duration of waiting time is used only as a tie-breaker for patients with equal scores. Each UNOS region has special exception cases that must be voted on by the regional review board (comprising one member from each transplant center in the region). In these cases, the board may be asked to assign higher-than-calculated scores for patients with special problems that are not addressed by the use of only objective criteria, such as children with intractable pruritus, ascites, hemorrhage, or infectious complications and patients with hepatocellular carcinoma.58
The frequency of recalculation of the MELD score is determined by the score itself. MELD scores greater than 25, between 19 and 24, and between 11 and 18 are evaluated every 7, 30, and 90 days, respectively.59 A score of less than 10 is recalculated yearly barring any exacerbation of the liver disease or patient condition.59
Next, one of the most difficult phases begins: the waiting period. It is not possible to anticipate when an appropriate organ will become available. The patient may feel that his or her life is being put “on hold.” Because of the shortage of donors, it is not uncommon for patients in critical care units to die while awaiting transplantation. And knowing that another person must die so that he or she may live can cause feelings of guilt as the patient hopes for a liver to become available. Patients with end-stage liver disease know that the only alternative to transplantation is death. By understanding the basic social processes that patients experience while awaiting transplantation, nurses can facilitate health-promotion activities. It is important for the patient and family to receive ongoing psychosocial assessment and to attend pretransplantation support groups, which are available at most transplant centers.60
Pretransplantation Phase
The patient with end-stage liver disease who is awaiting a transplant may pose one of the greatest care challenges in the critical care unit. Hepatic encephalopathy, coagulopathies, portal hypertension, severe fluid and electrolyte imbalances, cardiac compromise, and renal deterioration are not uncommon. Frequent mental status assessments are important in determining the patient’s continued candidacy for transplantation. Hepatic encephalopathy may improve with the administration of antibiotics and laxatives, or the patient may proceed to stage IV coma. Protection of the airway is especially important in an encephalopathic patient who is not intubated. In these circumstances, if hematemesis or vomiting occurs, intubation and use of paralytic agents may be necessary to protect the patient’s airway. Diagnostic studies may be needed to evaluate the possibility of an intracranial bleed. The head of the patient’s bed is maintained at 30 to 45 degrees to avoid even slight increases in intracranial pressure. Patients who have chronic liver disease also have nutritional deficits. They require supplements of the fat-soluble vitamins (A, D, E, and K), may be on protein restriction to reduce serum ammonia levels, and may experience severe muscle wasting.33
Consequences of portal hypertension must be corrected. Gastrointestinal hemorrhage from varices may respond to administration of propranolol or to procedures such as banding and sclerotherapy. Portal hypertension may be reduced by transjugular intrahepatic portosystemic shunting (TIPS) in interventional radiology. Rarely, the patient may need to undergo surgical intervention with a vascular shunt created between the portacaval system and the mesangial, splenic, or renal vascular system. Patients with massive ascites usually have total body fluid overload but are intravascularly contracted and require sodium restriction and administration of colloidal fluids (e.g., albumin) along with diuretics.
Careful documentation of fluid intake and output, daily weight determinations, and frequent measurement of vital signs are needed to monitor fluid status. Ascites can interfere with lung expansion and can compromise oxygenation. Patients with large, distended abdomens also find adequate oral nutrition difficult. Use of diuretics to control ascites is common but can compromise kidney function or even worsen hepatorenal syndrome. Paracentesis (removal of ascites) may be required for intractable ascites. However, frequent large-volume paracentesis procedures can contribute to renal demise and cardiovascular compromise related to fluid volume shifts.
Spontaneous bacterial peritonitis can be manifested in the patient with end-stage liver disease by an acute decline in the hepatic and renal function accompanied by fever, abdominal pain, and hepatic encephalopathy. The paracentesis fluid shows increased white blood cells with or without a positive culture. Patients are treated aggressively with antibiotics and are temporarily deferred from transplantation during treatment for and recovery from bacterial peritonitis.
Determining Donor Suitability
The two criteria necessary for matching a donor liver to a recipient are blood type and body size. Human leukocyte antigen (HLA) tissue typing is not used in the matching of donor livers, because it has not been shown to significantly affect patient outcomes. Donors are carefully screened for infectious diseases and metastatic carcinomas, because these can be transmitted to the recipient. The transplant center is notified by the regional organ procurement organization that a liver is available. If the organ is accepted, a member of the transplantation team contacts the patient.
In very urgent situations, the donor blood type (e.g., type A) may not be compatible with that of the recipient (e.g., type O). Despite this incompatibility, such liver transplantations can be successful. There may be some early postoperative complications, such as mild hemolysis, higher incidence of acute cellular rejection, and increased postoperative hepatic vascular and biliary complications, but innovative use of immunosuppressive regimens and plasmapheresis have improved graft survival of patients with recipient-donor ABO incompatibility.61 Extended-criteria donor livers that are otherwise unremarkable—such as those with a cold ischemia time longer than 12 hours or those from donors older than 60 years—are expanding the donor pool and shortening waiting times.62
Living Donor Liver Transplantation
Living Donor Liver Transplantation (LDLT) began in 1989 with adult-to-child donations, commonly from a parent to his or her infant. The left lateral hepatic lobe is resected, leaving the donor with the larger mass of liver remaining.63 In such cases, the risks to the healthy donor are thought to be outweighed by the benefits of having a healthy child.64 Adult-to-adult LDLT donation began in the 1990s. The whole left hepatic lobe or right hepatic lobe is resected, taking 30% to 60% of the liver mass from the live donor.63 Complications for the donor after partial hepatectomy are usually of low severity. The most common ones are bile leak, bacterial infection, incisional hernia, pleural effusion, neuropraxia (temporary nerve dysfunction), wound infection, and abdominal abcess.59,64 More serious potential complications for the liver donor include portal vein thrombosis, inferior vena cava thrombosis, and death.59,64 Critical care nurses play an important role in caring for these donors and must be vigilant in assessing for complications and initiating early interventions.
Liver Transplantation Surgical Procedure
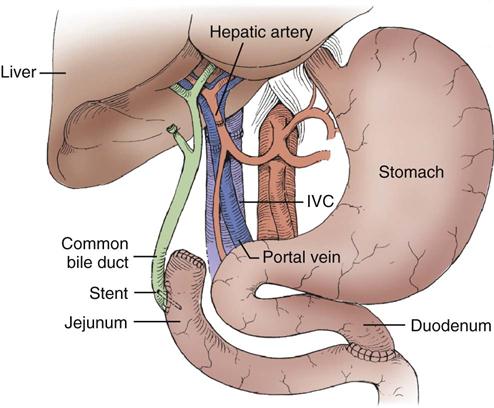
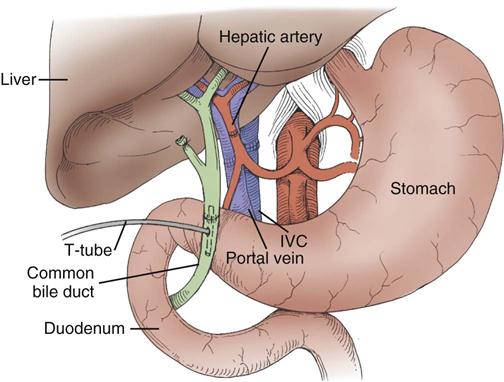
Liver transplantation surgery is lengthy and technically difficult, often lasting 4 to 12 hours. The procedure involves the combined efforts of surgeons, anesthesiologists, nurse anesthetists, operating room nurses and technicians, perfusionists, and personnel from the blood bank and laboratory and radiology departments, among others. The patient is taken to the operating room for anesthesia induction, insertion of large-bore intravenous catheters that allow high-volume fluid infusion, and insertion of a pulmonary artery catheter for hemodynamic monitoring. Continuous renal replacement may be continued or initiated in the operating room by a filter-trained critical care or dialysis nurse. Other devices, such as an arterial line, a nasogastric tube, and a urinary drainage catheter, are also inserted. The patient is positioned on the operating room table in such a way as to minimize pressure that could cause ischemia and chronic injury to tissue and peripheral nerves. Liver transplantation surgery can be divided into three stages: (1) recipient hepatectomy, (2) vascular anastomoses with donor liver, and (3) biliary anastomosis.65
Recipient Hepatectomy
Stage 1 is the longest and most difficult part of the surgery, because it involves removal of the native liver. It is complicated even more by coagulopathies, adhesions, portal hypertension, and venous collaterals. Before completion of this stage, the patient may be placed on venovenous bypass (Figure 22-9), although not all patients require this procedure. A centrifugal pump cycles the blood out through iliac and portal vein cannulas and returns it to the central circulation through the axillary or subclavian vein. Advances in surgical techniques, anesthesia, and fluid management have shortened the length of surgery enough to warrant elimination of venovenous bypass in some cases.66
Vascular Anastomoses with a Donor Liver
Stage 2 comprises the four vascular anastomoses: suprahepatic inferior vena cava, infrahepatic vena cava, hepatic artery, and portal vein. Many variations and adaptations, such as vascular patches, may be used, depending on the anatomy of the donor and recipient. If venovenous bypass is used, it is removed after the infrahepatic vena cava anastomosis and before the hepatic artery anastomosis.66
Biliary Anastomosis
Stage 3 can be achieved by choledochojejunostomy (bile duct to jejunum) or by choledochocholedochostomy (bile duct to bile duct). Choledochojejunostomy is performed in patients who have diseased bile ducts, such as those with biliary atresia or sclerosing cholangitis. It is also known as a Roux-en-Y procedure and is shown in Figure 22-10. The choledochocholedochostomy is performed in patients who have a healthy and intact common bile duct and is shown in Figure 22-11. The patient returns from surgery with or without an external stent or T-tube that is connected to a bag into which bile drains. Patients who do not have external biliary tubes may have an internal stent inserted in the bile duct across the biliary anastomosis. Eventually, the internal stent moves and is passed with the stool.66
Postoperative Medical and Nursing Management
The common nursing diagnoses associated with liver transplantation are listed in the Nursing Diagnosis Priorities Box on Liver Transplantation. After surgery, some patients are extubated before they arrive in the critical care unit, but most arrive unreversed from anesthesia and remain intubated for 12 to 24 hours. Immediate priorities include (1) reestablishment of normal body temperature, (2) hemodynamic stabilization, and (3) maintenance of an effective airway. Postoperative hypothermia is common after orthotopic liver transplantation (OLT). The critical care nurse must achieve rewarming safely by the use of methods such as warming blankets and head covers.
Hemodynamics
Hemodynamic stabilization is a particular challenge, because the patient may arrive hypervolemic, euvolemic, or hypovolemic and may be hypertensive or hypotensive. Assessment of total body fluids compared with intravascular fluid status is important. Because of inherent presurgical problems with decreased serum albumin, some centers tend to keep the patient “dry.” Hypervolemia often results in third spacing, with resultant ascites and a leaking wound. Accurate measurements of hemodynamic function, such as arterial blood pressure, peripheral blood pressure, central venous pressure, pulmonary artery pressure, pulmonary artery occlusion pressure, urinary output, patency of drains, and bile totals are assessed frequently to evaluate true volume status. Choice of replacement fluids and pharmacological agents for correction of volume and blood pressure abnormalities is specific to the transplant center. These protocols vary in their use of albumin or fresh-frozen plasma, and in the use of intravenous renal-dose dopamine or prostaglandin, as well as other agents and solutions. Still, the goals are the same: optimize tissue perfusion and deliver oxygen to all tissues, especially the newly transplanted graft.
Electrolytes
Electrolyte abnormalities can occur after OLT. Disturbances in potassium and magnesium levels are common. High serum levels of electrolytes are usually associated with renal impairment; low levels can be the result of drug side effects (e.g., diuretic therapy). The presence of hypernatremia or hyponatremia complicates the correction of volume status and fluid replacement.
Pulmonary Management
Ventilatory support of the patient is maintained until the anesthetic agent has been metabolized and cleared by the new liver and the patient awakens. Frequent measurement of arterial blood gas levels, continuous pulse oximetry, and assessment of breath sounds are needed. The patient may require changes in ventilatory settings, suctioning to remove secretions, or administration of pharmacological agents to correct acid-base imbalances. Pulmonary complications are common, as listed in Box 22-13. While the patient is on ventilatory support, pneumonia can be avoided by maintaining the head of bed elevated at 30 degrees, turning the patient frequently, providing good oral care, and brushing the patient’s teeth. After extubation, patients must be encouraged to perform incentive spirometry exercises and to turn and deep-breathe frequently to help prevent atelectasis and pneumonia.
Coagulopathy Risk
Management of coagulopathies is important in the early postoperative phase. Close monitoring of drainage tubes and incisions is required along with other nursing assessments of blood loss, such as identifying signs of hypovolemia, tachypnea, tachycardia, or poor peripheral oxygenation. Sanguineous nasogastric output, and black, tarry stools are hallmarks of bleeding problems and must be reported immediately. Laboratory monitoring during the first 24 hours after surgery is necessary to assess blood loss and coagulopathies and includes hematocrit, hemoglobin, platelet count, prothrombin time, partial thromboplastin time, fibrinogen, and fibrin split products. Reversal of coagulopathies is done judiciously, with consideration for the potential to thrombose newly anastomosed blood vessels in the liver. Blood products such as platelets, fresh-frozen plasma, and specific factors can be given along with pharmacological agents such as vitamin K.
Neurological Status
Neurological assessment of the patient is important in the early postoperative phase to determine mental status and graft function. Patients who were encephalopathic preoperatively are usually slower to clear mentally. Nevertheless, with good liver function, the patient should be alert and oriented within 1 to 2 days. The improved mental status is a reflection of a functional new liver. Certain pharmacological agents, including immunosuppressants, can cause peripheral and central neurological side effects that may alter neurological status. Induction therapy may be used to delay the initiation of immunosuppressant medications associated with neurological and nephrogenic side effects.66,67
Pain Management
The critical care nurse must always be aware of the potential for intracranial bleeds in a patient who has coagulopathies, serum sodium imbalances, and hemodynamic instability. All of these conditions can interfere with pain management, because the pharmacological agents used for pain can mask deterioration in mental status. Medications to relieve pain are administered, but other nonpharmacological nursing interventions also must be used.
Glucose Control
Intensive blood glucose control (<150 mg/dL) during liver transplantation surgery has been associated with a significant decrease in the infection rate at 30 days and in the mortality rate at 1 year.68 In the intensive care setting, insulin therapy is used to support the newly transplanted liver during the fluctuations in blood glucose related to the patient’s immunosuppressant regimen.
Kidney Function
Liver transplantation can alter kidney function by several mechanisms, including cyclosporine or tacrolimus administration, acute tubular necrosis, intrinsic kidney disease, and poor liver function. Some studies estimate that 48% to 94% of OLT patients develop renal failure.69 Patients are managed with attention to fluid and electrolyte imbalances, by avoidance of nephrotoxic drugs, and occasionally with ultrafiltration, continuous renal replacement therapy, or intermittent hemodialysis. With good liver function, kidney function usually improves. However, certain immunosuppressive agents and antimicrobials can deleteriously affect kidney function. Adjustments in dose or avoidance of use must be balanced with assessment of kidney and liver function. Daily monitoring of cyclosporine or tacrolimus serum levels is vital to determining adequate immunosuppression, and daily serum creatinine levels are necessary to watch for renal impairment. As the patient’s condition continues to improve, the frequency of laboratory testing may decrease.69
Infection Risk
Immunosuppressive therapy places the transplant recipient at an increased risk for infection. Infectious complications are common and continue to be the leading cause of death among OLT patients. The potential for infection is greatest when patients receive high doses of immunosuppressants.66 All persons who come into contact with the transplant recipient throughout the hospitalization must practice good hand hygiene techniques and standard precautions to prevent the transmission of infection.60 Infections are treated with appropriate antimicrobials specific to the invading organism. Prophylactic therapies are commonly used as well.59
Bile Drains
Careful attention to any external biliary drain line is important. If the patient has an external biliary drain, the critical care nurse documents color, character, and amount of drainage and reports any changes. Biliary complications can occur after OLT. Posttransplantation complications, including biliary ones, are listed in Box 22-13.
Nutrition
The nasogastric tube is removed when its output is minimal, bowel sounds have returned, and the patient is extubated. If the patient will be intubated longer than several days, total parenteral nutrition may be started. Consultation with a nutritionist (dietitian) should be sought after the patient is stable. Prealbumin levels may be measured to assess nutritional status. Otherwise, nutrition may begin orally or through a feeding tube as soon as bowel function returns. The diet is slowly advanced as tolerated.
Liver Function Tests
The standard laboratory biomarkers used to monitor graft function are serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and γ-glutamyltransferase (GGT); serum bilirubin; and prothrombin time. The serum levels of these markers are measured frequently during the first few postoperative days and may continue to rise before peaking and subsequently falling. As liver function improves, the frequency of laboratory testing decreases, but the critical care nurse can anticipate performing these liver function tests daily after the initial postoperative period.
Liver Graft Nonfunction
The patient with suspected primary nonfunction of a liver graft demonstrates (1) hemodynamic instability, (2) progressive deterioration of kidney function, (3) coagulopathies and abnormal serum liver function laboratory tests, (4) hypoglycemia, (5) continued ventilatory dependence, and (6) an inability to awaken from anesthesia. Continued nonfunction of the graft necessitates relisting the patient for another donor liver. Early signs of optimal graft function include improving kidney function, mental alertness, a high to normal serum glucose concentration, and early extubation. The serum ALT, AST, GGT, and alkaline phosphatase levels may peak on the third or fourth day but later decrease. The serum bilirubin concentration may take a week before beginning to fall, and there may be a mild elevation when the external biliary drainage tube is clamped or after a blood transfusion. Early mobilization and physical therapy are encouraged.
Rejection Surveillance
Acute rejection in OLT is a cellular-mediated event and should be suspected if the serum liver function laboratory tests, especially AST and ALT, become elevated compared with previous levels. Such elevations usually precede any other sign of acute rejection of the liver allograft. Sometimes, the patient also exhibits fever, a drop in bile output (if a T-tube is still connected to a drainage bag), and a change in the color and viscosity of the bile. At first the patient may not have any other physical symptoms, but eventually late signs of rejection may occur, including malaise, dark urine, and clay-colored stools. Certain infections, such as CMV, can also cause liver function test values to increase.
Rejection is suspected when liver function test values increase, but other reasons for these elevations need to be ruled out. Mechanical and vascular complications are ruled out by Doppler ultrasonography and angiography. Endoscopic retrograde cholangiopancreatography, hepatobiliary iminodiacetic acid (HIDA) scanning, or transhepatic cholangiography may reveal biliary obstruction or leakage. A liver biopsy may be indicated to determine the cause of liver dysfunction if the other tests are inconclusive. Acute rejection can occur at any time after transplantation, but most commonly it occurs during the first few months and even as early as the first week after surgery. Most liver transplant recipients experience at least one acute rejection episode. Treatment of acute rejection requires increasing immunosuppression (i.e., an increase in tacrolimus or steroid dose and possibly an addition of monoclonal or polyclonal antilymphocyte antibodies or other newer pharmacological agents). Immunosuppressant protocols vary from center to center and are usually successful at reversing acute rejection.
Chronic rejection is a humoral event and is progressive and nonreversible. Chronic rejection in a liver transplant recipient usually requires retransplantation if the patient is still a candidate.
Transfer Out of Critical Care
After the patient is stable and the transplanted liver is functional, catheters and drains are removed before the patient is transferred out of the critical care unit. Central venous catheters and arterial lines are removed. The urinary catheter is removed as soon as the patient is awake enough to be continent. Drain lines are removed as drainage outputs become minimal. As the patient begins to participate in self-care, plans are made for transfer out of the critical care unit to the transplantation nursing unit.
On the transplantation unit, laboratory data and vital signs continue to be monitored on a routine basis. Self-care is promoted. Increasing levels of physical therapy are encouraged, diet is advanced, and much of the nurse’s effort is directed toward teaching the patient and family.
Patient Education
Considerable attention is focused on patient education and discharge planning. Discharge booklets are helpful in the education process. It is important for the patient to learn how to self-administer medications, monitor vital signs, care for the incision and the T-tube (if present), prevent infections, and identify problems that must promptly receive medical attention. Because it is not uncommon for patients to be discharged within 2 weeks after OLT, it is important for discharge instructions to begin as soon as the patient is mentally alert. Patients discharged early may require home health nurse referrals to assist with follow-up of incision care, intravenous therapies, and other procedures. Education must be provided about rejection surveillance, signs and symptoms of infection, lifestyle changes as needed, long-term medication considerations, and the follow-up visit schedule.
Long-Term Follow-Up
OLT patients who do not live in the same city in which their surgery was performed usually remain in the immediate area of the transplant center after discharge before returning home. During this period, they may be monitored by a home health nurse and be seen in the clinic several times a week by the transplantation team. Continued serologic testing is done to monitor graft function, to determine blood levels of certain immunosuppressive agents, and to identify postoperative complications. Although many of these complications can be managed successfully in the outpatient setting, readmissions do occur. Because rejections, readmissions, grieving for the donor, and pharmacological side effects can create anxiety for the family and the patient, they are encouraged to attend transplantation support groups if offered by the center. After patients return home, they are encouraged to resume a close relationship with their local primary care physician and gastroenterologist.
OLT patients need long-term follow-up surveillance for hypertension, kidney failure, obesity, dyslipidemias, biliary and infectious complications, and malignancies.59 Early intervention affects the quality and length of life. Behavior modifications and therapeutic lifestyle changes should be frequently reinforced to positively affect long-term health. Financial concerns are a major source of stress in this patient population. Many transplant recipients suffered from chronic liver disease before their surgery. They often were disabled for some time and already have experienced financial stressors related to illness. As these patients live longer with liver transplants, issues of insurability, continued disability, and even the ability to obtain work will have to be addressed.
Pharmacological Agents
Many pharmacological agents are used in the care of patients with gastrointestinal disorders. Table 22-3 reviews the various agents and any special considerations necessary for administering them.
TABLE 22-3
PHARMACOLOGICAL MANAGEMENT: GASTROINTESTINAL DISORDERS
| MEDICATION | DOSAGE | ACTIONS | SPECIAL CONSIDERATIONS |
| Antacids | 30-90 mL q1-2h PO or NG; possibly titrated to NG pH | Used to buffer stomach acid and raise gastric pH | Can cause diarrhea or constipation and electrolyte disturbances. Irrigate NG tube with water after administration because antacids can clog tube. |
| Histamine2-(H2) Antagonists | |||
| Cimetidine (Tagamet) | 300 mg q6h IV or PO | Used to reduce volume and concentration of gastric secretions | Side effects include CNS toxicity (confusion or delirium) and thrombocytopenia. Separate administration of antacids and histamine blocking agents by 1 hour. |
| Ranitidine (Zantac) | 150 mg q12h PO or 50 mg q8h IV | ||
| Famotidine (Pepcid) | 40 mg daily PO or 20 mg q12h IV | ||
| Nizatidine (Axid) | 150 mg q12h PO or 300 mg q24h | ||
| Gastric Mucosal Agents | |||
| Sucralfate (Carafate) | 1 g q6h NG or PO, given 1 hour before meals and at bedtime | Forms an ulcer-adherent complex with proteinaceous exudates Covers the ulcer and protects against acid, pepsin, and bile salts |
Requires an acid medium for activation; do not administer within 30 minutes of an antacid. May cause severe constipation. May cause decreased absorption of certain drugs. |
| Gastric Proton-Pump Inhibitors | |||
| Omeprazole (Prilosec) | 20-40 mg q12h PO | Inactivates acid, or hydrogen, acid pump, blocking secretion of hydrochloric acid by gastric parietal cells | Capsules should be swallowed intact. May increase levels of phenytoin, diazepam, warfarin. May administer concomitantly with antacids. |
| Lansoprazole (Prevacid) | 15-30 mg q24h PO | ||
| 30 mg over 30 min q24h IV | |||
| Rabeprazole (AcipHex) | 20-40 mg q24h PO | ||
| Esomeprazole (Nexium) | 40 mg q12-24h PO | ||
| 20-40 mg q24h IV | |||
| Pantoprazole (Protonix) | 20-40 mg q24h PO | ||
| 80 mg q8-12h IV | |||
| Vasopressin | |||
| (Pitressin Synthetic) | Loading dose of 20 units over 20 min IV, followed by 0.2-0.4 unit/min IV infusion Doses can be increased to 0.9 unit/min if necessary |
Decreases splanchnic blood flow, reducing portal pressure | Side effects include coronary, mesenteric, and peripheral vasoconstriction. May be administered concurrently with nitroglycerin to minimize side effects. |
| Octreotide | |||
| (Sandostatin) | Bolus dose of 25-50 mcg followed by IV infusion of 25-50 mcg/hr for 48 hours | Decreases splanchnic blood flow, reducing portal pressure | May cause hyperglycemia or hypoglycemia when initiating the drip and changing dosages. |
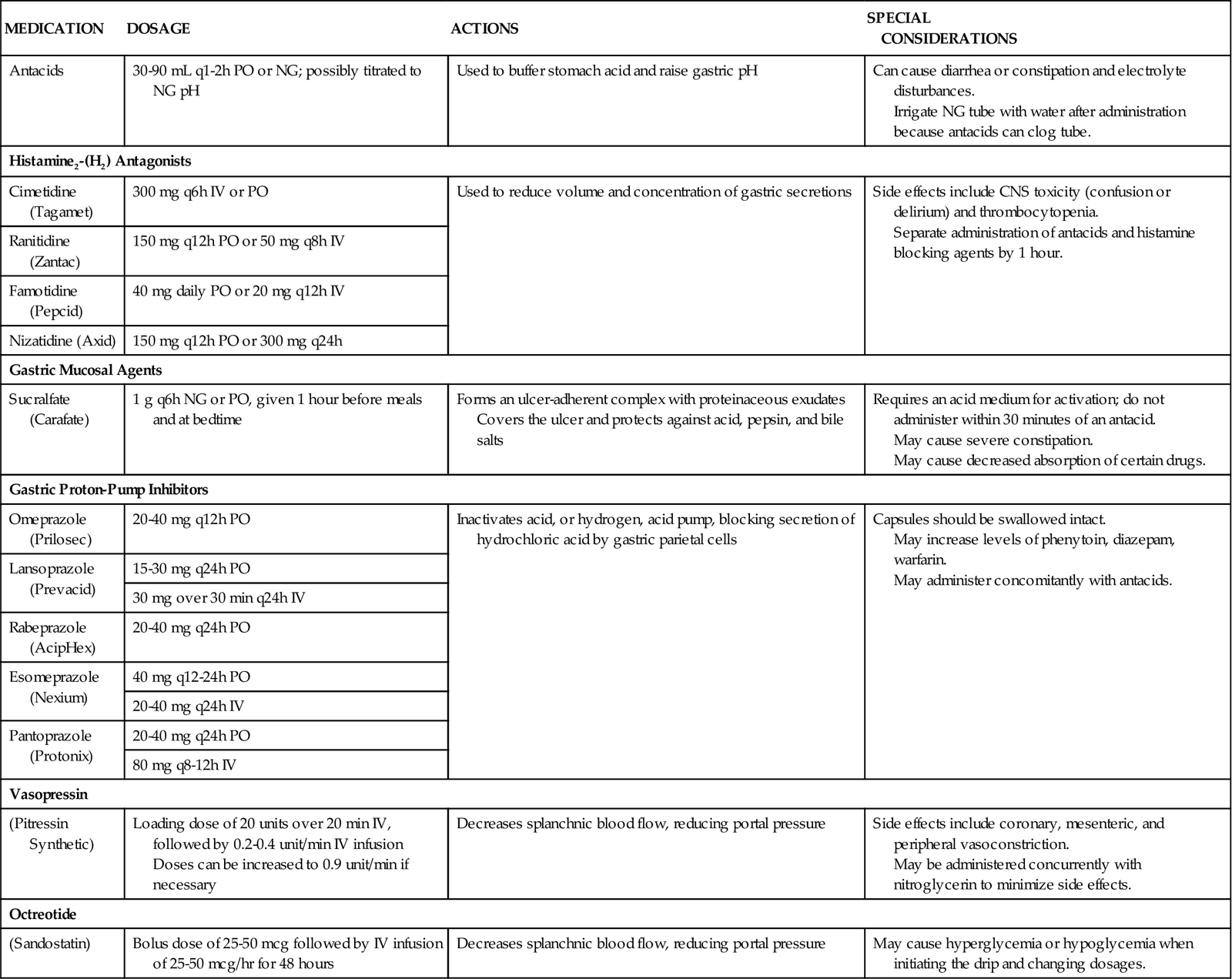
CNS, central nervous system; IV, intravenous; NG, nasogastric; PO, by mouth.
Antiulcer Agents
A number of different antiulcer agents are commonly used in the critical care setting, including H2-antagonists, gastric PPIs, and gastric mucosal agents.70 H2-antagonists are used to decrease the volume and concentration of gastric secretions and control gastric pH, decreasing the incidence of stress-related upper gastrointestinal bleeding. These agents work by blocking histamine stimulation of the H2-receptors on the gastric parietal cells, reducing acid production. Although these drugs may be administered orally, intramuscularly, or intravenously, they usually are given intravenously in the critical care setting. Proton-pump inhibitors decrease gastric acid secretion by binding to the proton pump, blocking the release of acid from the gastric parietal cells. PPIs are potent acid inhibitors and have greater suppressive ability than the H2-agonists.71,72
Unlike H2-antagonists or PPIs, sucralfate does not affect gastric acid concentration but rather exerts its action locally. Sucralfate reacts with hydrochloric acid to form a sticky, paste-like substance that adheres to the surface of the ulcer and shields it from pepsin, acid, and bile. Sucralfate predominantly binds to damaged gastrointestinal mucosa, with minimal adherence to normal tissue.72 It is administered orally or through a gastric tube. Sucralfate should not be crushed but may be dissolved in 10 mL of water to form a slurry. It is also available as a suspension.
Vasopressin
Vasopressin is used to control gastric ulcer and variceal bleeding. It is administered intraarterially, through a catheter inserted into the right or left gastric artery (through the femoral artery, aorta, and celiac trunk) or intravenously. It causes splanchnic and systemic vasoconstriction, subsequently reducing portal blood flow and pressure.71 A major side effect of the drug is systemic vasoconstriction, which can result in cardiac ischemia, chest pain, hypertension, acute heart failure, dysrhythmias, phlebitis, bowel ischemia, and cerebrovascular accident. These side effects can be offset with concurrent administration of nitroglycerin. Other complications include bradycardia and fluid retention. Nursing responsibilities associated with the use of this therapy include maintenance of a patent infusion line and continuous monitoring for vasoconstrictive complications of therapy.18
Somatostatin and Octreotide
Somatostatin is a peptide that is administered parenterally in the acutely bleeding, cirrhotic patient. It reduces splanchnic vasodilation and portal pressure through the inhibition of secretion of various vasodilator hormones, and it is as effective as vasopressin in treating variceal bleeding with minimal side effects. Octreotide is a commonly used, long-acting synthetic analogue of somatostatin.18,71