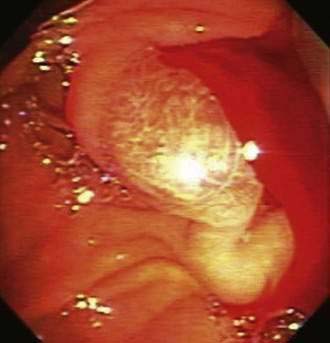
CHAPTER 19 Gastrointestinal Bleeding
The annual rate of hospitalization for any type of gastrointestinal (GI) hemorrhage in the United States is estimated to be 350 hospital admissions/100,000 population, with more than 1,000,000 hospitalizations annually.1 Approximately 50% of admissions for GI bleeding are for upper GI (UGI) bleeding (from the esophagus, stomach, duodenum), 40% are for lower GI (LGI) bleeding (from the colon and anorectum), and 10% are for obscure bleeding (from the small intestine).
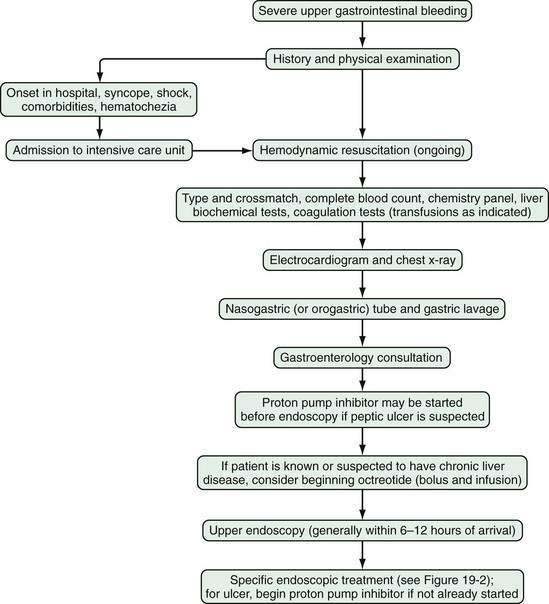
Severe gastrointestinal bleeding is defined as documented gastrointestinal bleeding (i.e., hematemesis, melena, hematochezia, or positive nasogastric lavage) accompanied by shock or orthostatic hypotension, a decrease in the hematocrit value by at least 6% (or a decrease in the hemoglobin level of at least 2 g/dL), or transfusion of at least two units of packed red blood cells. Most patients with severe gastrointestinal bleeding are admitted to the hospital for resuscitation and treatment. Overt bleeding implies visible signs of blood loss from the GI tract. Hematemesis is defined as vomiting of blood, which is indicative of bleeding from the esophagus, stomach, or duodenum. Hematemesis includes vomiting of bright red blood, which suggests recent or ongoing bleeding, and dark material (coffee-ground emesis), which suggests bleeding that stopped some time ago. Melena is defined as black tarry stool and results from degradation of blood to hematin or other hemochromes by intestinal bacteria. Melena can signify bleeding that originates from UGI, small bowel, or proximal colonic source. Melena generally occurs when 50 to 100 mL or more of blood is delivered into the GI tract (usually the upper tract), with passage of characteristic stool occurring several hours after the bleeding event.2,3 Hematochezia refers to bright red blood per rectum, and suggests active UGI or small bowel bleeding, or distal colonic or anorectal bleeding. Occult gastrointestinal bleeding refers to subacute bleeding that is not clinically visible. Obscure gastrointestinal bleeding is bleeding from a site that is not apparent after routine endoscopic evaluation with esophagogastroduodenoscopy (upper endoscopy) and colonoscopy, and possibly small bowel radiography. An algorithm for the initial management of acute, severe UGI bleeding is shown in Figure 19-1.
INITIAL ASSESSMENT AND MANAGEMENT OF ACUTE GASTROINTESTINAL BLEEDING
HISTORY
Initial assessment of the patient with acute GI bleeding includes medical history taking, obtaining vital signs, performing a physical examination, including a rectal examination, and nasogastric lavage. During history taking, patients should be questioned about risk factors and historical features that help identify diagnostic possibilities for the bleeding source (Table 19-1). Bleeding from a peptic ulcer should be suspected in patients with a history of an ulcer or those taking daily aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). Patients who have known or suspected liver disease or who are alcoholics should be suspected of bleeding related to portal hypertension. Patients with heavy alcohol intake, a feeding or chronic nasogastric tube, or a history of gastroesophageal reflux disease are at risk of erosive esophagitis. Patients who have had prior surgical repair of an abdominal aortic aneurysm should be considered to have a fistula from the graft to the duodenum until proven otherwise. Patients on an anticoagulant such as warfarin should be evaluated for the possibility of excessive anticoagulation. Prior radiation to the abdomen should raise the possibility of radiation enteritis or colitis. Weight loss suggests possible malignancy. Abdominal pain may suggest malignancy, inflammatory bowel disease, or ischemic colitis. A change in stool caliber suggests colon cancer or a colonic stricture. Chest pain or syncope suggests possible cardiovascular complications related to blood loss.
Table 19-1 Suspected Source of Gastrointestinal Bleeding as Suggested by a Patient’s History
| SUSPECTED SOURCE OF BLEEDING | PATIENT HISTORY |
|---|---|
| Nasopharynx |
IBD, inflammatory bowel disease.
PHYSICAL EXAMINATION
On initial evaluation, physical examination should focus on the patient’s vital signs, with attention to signs of hypovolemia such as hypotension, tachycardia, and orthostasis. The abdomen should be examined for surgical scars, tenderness, and masses. Signs of chronic liver disease include spider angiomata, palmar erythema, gynecomastia, ascites, splenomegaly, caput medusae, and Dupuytren’s contracture. The skin, lips, and buccal mucosa should be examined for telangiectasias, which are suggestive of hereditary hemorrhagic telangiectasia (HHT), or Osler-Weber-Rendu disease. Pigmented lip lesions may suggest Peutz-Jeghers syndrome. Purpuric skin lesions may suggest Henoch-Schönlein purpura. Acanthosis nigricans may suggest underlying malignancy, especially gastric cancer. The patient’s feces should be observed to identify melena or maroon and red stool; however, the subjective description of stool color varies greatly among patients and physicians.4
LABORATORY STUDIES
Blood from the patient with acute GI bleeding should be sent for standard hematology, chemistry, liver biochemical, and coagulation studies and for typing and crossmatching for packed red blood cells. The hematocrit value immediately after the onset of bleeding may not reflect blood loss accurately because over 24 to 72 hours there is equilibration of red blood cells in the vascular space with extravascular fluid and hemodilution resulting from intravenous administration of saline.5 The mean corpuscular volume (MCV) is an important indicator of the chronicity of blood loss; an MCV lower than 80 fL suggests chronic GI blood loss and iron deficiency, which can be confirmed by the finding of low blood iron, total iron-binding capacity (TIBC), and ferritin levels. A low MCV and negative fecal occult blood test result raise the possibility of celiac disease. A high MCV (>100 fL) suggests chronic liver disease or folate or vitamin B12 deficiency. An elevated white blood cell count may occur in more than half of patients with UGI bleeding and has been associated with greater severity of bleeding.6 A low platelet count can contribute to the severity of bleeding and suggests chronic liver disease or a hematologic disorder.
The blood urea nitrogen (BUN) and serum creatinine levels can help assess the patient for hemoconcentration (elevated levels) or chronic kidney disease, which may lead to chronic anemia because of decreased erythropoietin production. In patients with UGI bleeding, the BUN level typically increases to a greater extent than the serum creatinine level because of increased intestinal absorption of urea after the breakdown of blood proteins by intestinal bacteria.7
CLINICAL DETERMINATION OF THE BLEEDING SITE
Presentation with hematemesis, coffee-ground emesis, or nasogastric lavage with return of a large amount of blood or coffee-ground emesis indicates an UGI source of bleeding. A small amount of coffee-ground material or pink-tinged fluid that clears easily may represent mucosal trauma from the nasogastric tube rather than active bleeding from an UGI source. A clear (nonbloody) nasogastric aspirate does not necessarily indicate a more distal GI source bleeding, because 16% of patients with actively bleeding UGI lesions have a clear nasogastric aspirate.8 The presence of bile in the nasogastric aspirate makes UGI bleeding unlikely but can be seen with an intermittently bleeding UGI source.
Melena generally indicates an UGI source but can be seen with small intestinal or proximal colonic bleeding. Hematochezia generally implies a colonic or anorectal source of bleeding unless the patient is hypotensive, which could indicate a severe, brisk UGI bleed with rapid transit of blood through the GI tract.4 Maroon-colored stool can be seen with an actively bleeding UGI source or a small intestinal or proximal colonic source.
HOSPITALIZATION
On the basis of the patient’s initial history, physical examination, and laboratory test results, the location of bleeding (upper or lower), suspected bleeding lesion, and severity of bleeding can be predicted. Patients with severe GI bleeding require hospitalization, whereas those who present with only mild acute bleeding (self-limited hematochezia or infrequent melena) and who are hemodynamically stable (not suspected to be volume depleted), have normal blood test results, and can be relied on to return to the hospital if symptoms recur may be candidates for semiurgent outpatient endoscopy rather than direct admission to the hospital.9,10 On the other hand, patients should be hospitalized in an intensive care unit if they have large amounts of red blood in the nasogastric tube or per rectum, have unstable vital signs, or have had severe acute blood loss that may exacerbate other underlying medical conditions. Patients who have had an acute GI bleed but are hemodynamically stable can be admitted to a monitored bed (step-down unit) or standard hospital bed, depending on their clinical condition.
Several small studies have suggested that urgent endoscopy performed in the emergency department in patients with suspected UGI bleeds can help determine optimal hospital placement; however, widespread implementation of this practice is unlikely.11,12
INITIAL MEDICAL THERAPY
Administration of a proton pump inhibitor (PPI) is useful for reducing rebleeding rates in patients with peptic ulcer disease (see later). Starting a PPI in the emergency department or intensive care unit (ICU) before endoscopy is performed in patients with severe UGI bleeding has become a common practice but is still controversial.13 Several clinical studies and meta-analyses have shown that infusion of a high-dose PPI before endoscopy accelerates the resolution of endoscopic stigmata of bleeding in ulcers (see later) and reduces the need for endoscopic therapy but does not result in improved clinical outcomes in the transfusion requirement, rebleeding rate, need for surgery, or death rate.14–17 Patients with a strong suspicion of portal hypertension and variceal bleeding should be started empirically on intravenous octreotide (bolus followed by infusion [see later and Chapter 90]), which can reduce the risk of rebleeding to a rate similar to that associated with endoscopic therapy (Fig. 19-2; also see Fig. 19-1).18,19
ENDOSCOPY
GI endoscopy will identify the bleeding site and permit therapeutic hemostasis in most patients with GI bleeding. Endoscopy should be done only when it is safe to do so and when the information obtained from the procedure will influence patient care. Ideally, the patient should be hemodynamically stable, with a heart rate of less than 100 beats/min and a systolic blood pressure higher than 100 mm Hg. Respiratory insufficiency, altered mental status, or ongoing hematemesis indicates the need for endotracheal intubation before emergency upper endoscopy to stabilize the patient and protect the airway. Coagulopathy and thrombocytopenia should be corrected with transfusions prior to endoscopy. Proper medical resuscitation will not only allow safer endoscopy, but also ensure a better diagnostic examination for lesions, such as varices, that are volume dependent and will allow more effective hemostasis because of the correction of coagulopathy (Figs. 19-3 and 19-4; also see Figs. 19-1 and 19-2).
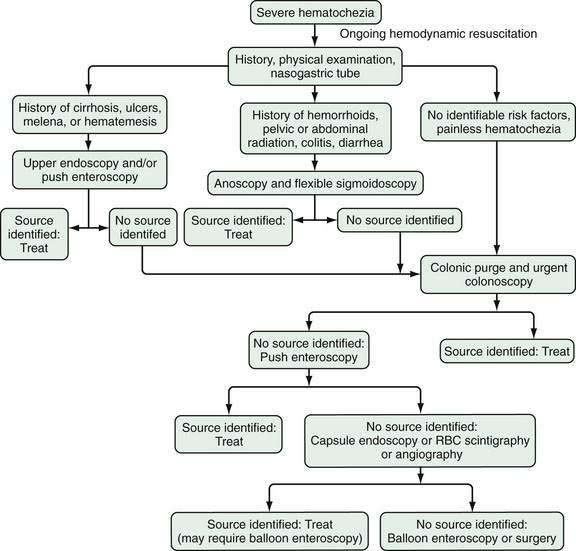
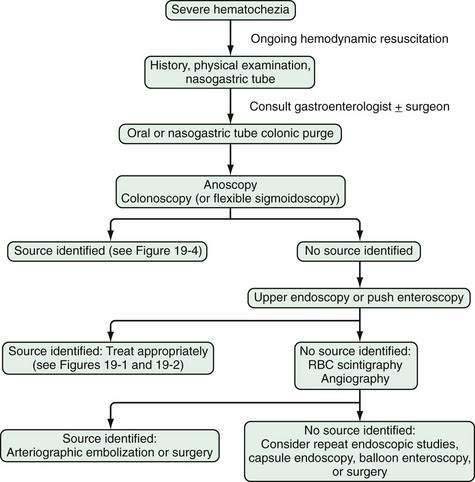
Figure 19-4. Algorithm for the management of severe hematochezia modified according to the patient’s history. RBC, red blood cell.
In patients with severe UGI bleeding, gastric lavage with a large (34-Fr) orogastric tube should be performed to evacuate blood and clots from the stomach to prevent aspiration and allow good endoscopic visualization. Special lavage systems can help remove blood rapidly. The intravenous administration of erythromycin (a gastric prokinetic agent) 30 to 90 minutes before upper endoscopy to induce gastric contraction and push blood from the stomach into the small intestine helps endoscopic visualization.20,21 Therapeutic single- or double-channel endoscopes with large-diameter suction channels should be used to allow quick removal of fresh blood from the GI tract during endoscopy. Additionally, a water pump can be used to irrigate target lesions through an accessory channel and dilute blood for suctioning, both of which facilitate visualization. Using iced saline lavage to prevent or decrease UGI bleeding is of no particular value and may impair coagulation and cause hypothermia. Gastric lavage with lukewarm tap water is as safe as lavage with sterile saline and much less expensive.
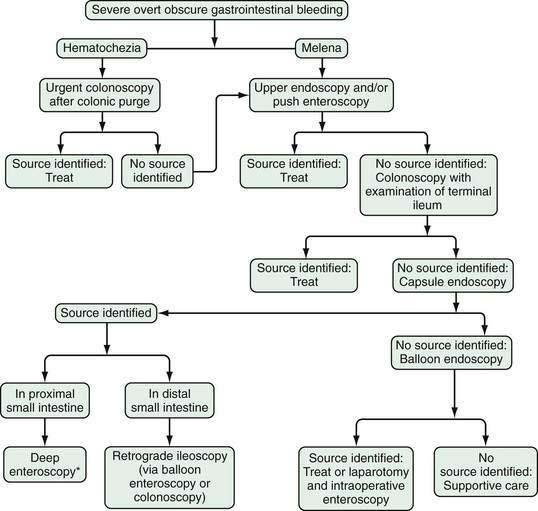
In patients with severe hematochezia and suspected active colonic bleeding, urgent colonoscopy can be undertaken after a rapid purge (see Figs. 19-3 and 19-4).22,23 Patients should receive 4 to 8 L of polyethylene glycol purge orally or via a nasogastric tube over four to six hours until the rectal effluent is clear of stool, blood, and clots. Additional polyethylene glycol bowel purge may be required in some patients, particularly those with active bleeding, severe constipation, or the onset of hematochezia in the hospital. Metoclopramide, 10 mg, may be given intravenously before the purge and repeated every four to six hours to facilitate gastric emptying and reduce nausea. In patients with severe or ongoing active hematochezia, urgent colonoscopy should be performed within 12 hours, but only after thorough cleansing of the colon. Patients with mild or moderate self-limited hematochezia should undergo colonoscopy within 24 hours of admission, and a colonic purge is also recommended in this situation to cleanse the colon thoroughly.
Patients with maroon stool in whom there is pretest uncertainty about the bleeding source should be considered for an urgent polyethylene bowel preparation as well. Colonoscopy immediately after push enteroscopy (see later), while the patient is still sedated, will expedite a patient’s care if push enteroscopy does not provide a diagnosis (and is also indicated for colon cancer screening in patients older than 50 years; Fig. 19-5).
Wireless small bowel capsule endoscopy (or capsule endoscopy; see later) can be useful in patients with overt GI bleeding who have normal push enteroscopy and colonoscopy results and in whom a small bowel source of bleeding is suspected.24 Capsule endoscopy has the advantages of directly visualizing the small intestine to identify potential sources or active bleeding. Disadvantages are that the procedure takes eight hours to complete and additional time to download and review the images, does not permit therapeutic hemostasis, and is difficult to perform in inpatients because of limited availability of staff trained to place the capsule. A follow-up endoscopic procedure, such as single- or double-balloon enteroscopy or retrograde ileoscopy, may be indicated for definitive diagnosis and treatment if a focal bleeding site is found on capsule endoscopy.
Complications related to emergency endoscopy and endoscopic hemostasis may occur in up to 1% of patients, depending on the type of endoscopy and treatment performed.25,26 The most common complications include GI tract perforation, aspiration pneumonia, induced hemorrhage, an adverse medication reaction, hypotension, and hypoxia (see Chapter 40).
ENDOSCOPIC HEMOSTASIS
Thermal contact probes have been the mainstay of endoscopic hemostasis since the 1970s. These probes come in diameters of 7 and 10 Fr and in lengths that can fit through panendoscopes, enteroscopes, or colonoscopes. Contact probes can physically tamponade a blood vessel to stop bleeding and interrupt underlying blood flow, and thermal energy is then applied to seal the underlying vessel (coaptive coagulation). The most commonly used probe is a multipolar electrocoagulation (MPEC) probe, also referred to as a bipolar electrocoagulation probe, with which heat is created by current flowing between intertwined electrodes on the tip of the probe. Animal studies in which MPEC probes were used to stop bleeding in mesenteric vessels have shown that optimal coagulation occurs with low-power settings (12 to 16 W) applied for a moderate amount of time (8 to 10 seconds), with moderate pressure on the bleeding site.27 Heater probes can provide a predetermined amount of joules of energy, which does not vary with tissue resistance. Animal studies have shown that heater probes can effectively coagulate arteries up to 2 mm in diameter, a diameter considerably larger than most secondary or tertiary branches of arteries (usually 1 mm) found in resected bleeding human peptic ulcers.28,29 The main risk of using a thermal probe is perforation with excessive application of coagulation or pressure, especially in acute or nonfibrotic lesions. Thermal probes can also cause a coagulation injury that can make lesions larger and deeper and may induce delayed bleeding in patients with a coagulopathy.
Injection therapy is performed most commonly with the use of a sclerotherapy needle to inject epinephrine, diluted to a concentration of 1 : 10,000 or 1 : 20,000, submucosally into or around the bleeding site or stigma of hemorrhage (see later). The advantages of this technique are its wide availability, relatively low cost, and safety in patients with a coagulopathy. Additionally, it is associated with a lower risk of perforation and thermal burn damage than the thermal techniques. The disadvantage of epinephrine injection is that it is not as effective for definitive hemostasis as thermal coagulation, hemoclipping (see later), or combination therapy.30,31 Injection therapy can also be performed with a sclerosant, such as ethanolamine or alcohol, but these agents are associated with increased tissue damage and other risks.
Endoscopic hemoclips (or clips) have been available since 1974, and have become popular as technical improvements have been introduced.32 Hemoclips serve to apply mechanical pressure to a bleeding site, as is done with surgical clips or sutures. Endoscopic hemoclips differ from surgical clips, however, in that they do not have as much compressive strength, and the currently available clips do not close completely but leave a small space between the prongs. Animal studies have shown that the first-generation hemoclips could not stop bleeding in vessels larger than a diameter of 1 mm.33 Subsequent hemoclips have been larger and stronger and have had a grasp and release mechanism that improves endoscopic deployment and hemostasis. By not causing significant thermal damage, hemoclips are especially useful for patients with malnutrition or coagulopathy.34 Nevertheless, hemoclips can also be difficult to deploy depending on the location of the bleeding site, the degree of fibrosis of the underlying lesion, and limitations to endoscopic access.
Band ligation is a technique in which mucosal (with or without submucosal) tissue is suctioned into a cap placed on the end of the endoscope, and a rubber band is rolled off the cap and over the lesion to compress its base. This technique is widely used for the treatment of esophageal varices (see Chapter 90) and occasionally can be used for other bleeding lesions. An advantage of band ligation is that it is relatively easy to perform; however, a disadvantage is that sufficient mucosa must be suctioned into the cap for ligation to be successful. Depending on the manufacturer, some band ligation devices can only fit on diagnostic endoscopes, and switching from a larger therapeutic endoscope to a smaller diagnostic endoscope during a case is time-consuming and inefficient.
RADIOLOGIC IMAGING
Angiography may be used to diagnose and treat severe bleeding, especially when the cause cannot be determined by upper and lower endoscopy. Angiography generally is diagnostic of extravasation into the intestinal lumen only when the arterial bleeding rate is at least 0.5 mL/min.35 The sensitivity of mesenteric angiography is 30% to 50% (with higher sensitivity rates for active GI bleeding than for recurrent acute or chronic occult bleeding), and the specificity is 100%.36 An advantage of angiography is that it permits therapeutic intra-arterial infusion of vasopressin or transcatheter embolization for hemostasis if active bleeding is detected, without the need for bowel cleansing. Nevertheless, the rate of major complications, including hematoma formation, femoral artery thrombosis, contrast dye reactions, acute kidney injury, intestinal ischemia, and transient ischemic attacks, is 3%.37 Another disadvantage of angiography is that it usually does not identify the specific cause of bleeding, only its location.
Radionuclide imaging is occasionally helpful for patients with unexplained GI bleeding, although it is used less frequently now than in the past because of the widespread use of endoscopy and lack of availability of nuclear medicine services for emergencies, particularly at night and on weekends. Radionuclide imaging can be performed relatively quickly and may help localize the general area of bleeding and thereby guide subsequent endoscopy, angiography, or surgery. The technique involves injecting a radiolabeled substance intravenously into the patient’s bloodstream and then performing serial scintigraphy to detect focal collections of radiolabeled material. Radionuclide imaging has been reported to detect bleeding at a rate of 0.04 mL/min.38 The two tracers used for radionuclide imaging for bleeding (bleeding scans) are technetium sulfur colloid and technetium pertechnetate–labeled autologous red blood cells. Technetium sulfur colloid is cleared rapidly from the bloodstream and is therefore useful for identifying acute, active bleeding. Technetium pertechnetate–labeled red blood cells remain in the circulation for up to 24 hours and therefore can be used for repeated scanning in patients with intermittent bleeding. A comparative study of patients with suspected GI bleeding has found technetium pertechnetate–labeled red blood cell scans to be more sensitive, specific, and accurate than technetium sulfur colloid scans.39
The overall rate of a diagnostic radionuclide scan is approximately 45%, with a 78% accuracy rate in the localization of the true bleeding site.40,41 Up to 25% of bleeding scans suggest a site of bleeding that proves to be incorrect.41–43 The rate of true-positive scans is higher for active bleeding with hemodynamic instability than for less severe bleeding.44 The most common reason for a false-positive result is rapid transit of luminal blood, so that labeled blood is detected in the colon even though it originated from a more proximal site in the GI tract. Caution is recommended in using the results of delayed scans to localize and target lesions for resective surgery.45
Technetium pertechnetate scintigraphy can identify ectopic gastric mucosa in a Meckel’s diverticulum. This diagnosis should be considered in a pediatric or young adult patient with unexplained GI bleeding. The positive predictive value, negative predictive value, and overall accuracy of a so-called Meckel’s scan has been reported to be higher than 90% in young patients.46,47 In patients older than 25 years, however, Meckel’s scans are much less sensitive (<50%). Caution is recommended in interpreting a negative Meckel’s scan result in an adult patient with GI bleeding.48
Conventional radiographic imaging is usually not needed in a patient with GI bleeding but occasionally may provide some important information. In patients with a prior abdominal aortic aneurysm repair and graft, computed tomography (CT) with intravenous contrast can identify inflammation between the graft and duodenum and thus suggest graft fistulization into the duodenum.49 In selected patients, an abdominal CT scan can also identify a mass lesion, such as an intra-abdominal tumor, or small bowel abnormalities that may suggest a cause of bleeding. Advances in CT scan technology have permitted CT enterography and CT angiography to be performed, with promising results.50,51
UPPER GASTROINTESTINAL BLEEDING
EPIDEMIOLOGY
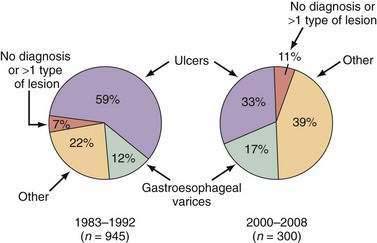
UGI bleeding is a common medical emergency that accounts for more than 500,000 hospital admissions each year, or approximately 170 patients/100,000 population/year.1 Of the potential causes of severe UGI bleeding, peptic ulcer is the most common, accounting for approximately 40% of cases (Table 19-2).52,53 Despite advances in medical therapy, ICU care, endoscopy, and surgery, the mortality rate of 5% to 10% for severe UGI bleeding has not changed since the 1970s.1,52–56 The lack of decline in the mortality rate may be explained by an increase in the proportion of older patients with GI bleeding, who may die as a result of worsening of other medical conditions rather than from exsanguination, and an increase in the number of patients with cirrhosis and variceal bleeding.
Table 19-2 Causes of Severe Upper Gastrointestinal Bleeding in the UCLA CURE Database
| CAUSE | FREQUENCY (%) |
|---|---|
| Peptic ulcer | 38 |
| Gastric or esophageal varix | 16 |
| Esophagitis | 13 |
| No cause found | 8 |
| Upper gastrointestinal tract tumor | 7 |
| Angioma* | 6 |
| Mallory-Weiss tear | 4 |
| Erosions | 4 |
| Dieulafoy’s lesion | 2 |
| Other | 2 |
CURE, Center for Ulcer Research and Education; UCLA, University of California, Los Angeles.
* Angioectasia and telangiectasia.
Bleeding is self-limited in 80% of patients with UGI hemorrhage, even without specific therapy.54,57 Of the remaining 20% who continue to bleed or rebleed, the mortality rate is 30% to 40%.8 Patients at high risk for continuous bleeding or for rebleeding potentially can benefit the most from acute medical, endoscopic, and surgical therapy.
RISK FACTORS AND RISK STRATIFICATION
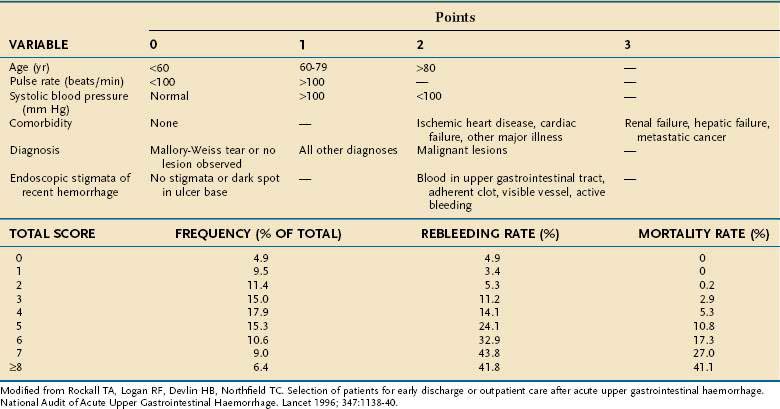
Pre-endoscopy scoring systems for nonvariceal bleeding include the Blatchford Score, the Clinical Rockall Score, and an artificial neural network score. The Blatchford Score uses pre-endoscopy variables such as blood pressure, BUN level, hemoglobin level, heart rate, syncope, melena, liver disease, and heart failure to assess a patient’s risk for needing clinical interventions to control bleeding (e.g., blood transfusions, endoscopic therapy, or surgery).58 The clinical Rockall Score uses the patient’s age, shock, and coexisting illnesses.59 The artificial neural network instrument uses 21 clinical variables to help predict the presence of stigmata of recent hemorrhage at endoscopy (see later) and the need for endoscopic therapy.60
The most commonly used postendoscopy scoring system is the Complete Rockall Score (Table 19-3).59 The Rockall Score was developed after an audit of hospitalizations for UGI bleeding at 74 hospitals in England and validated in another audit of 45 hospitals in 1994, to identify risk factors for death or rebleeding. The Complete Rockall Score includes the Clinical Rockall Score (pre-endoscopy variables—the patient’s age, shock, and coexisting illnesses) and endoscopic findings, including endoscopic stigmata of recent bleeding. The Rockall score after endoscopic therapy correlates well with mortality but does not seem to correlate as well with the risk of rebleeding.61–63
The Rockall risk stratification schemes can be used not only to identify patients at highest risk for poor outcomes, but also to identify patients at low risk for poor outcomes (i.e., Rockall scores of 0 to 2) who should be considered for early discharge from the hospital.64
Other scoring systems to predict outcomes from UGI bleeding after endoscopy include the Baylor Scoring System and the Cedars-Sinai Bleeding Index.65–68 In general, all these scoring systems are better at determining mortality than rebleeding.69
PEPTIC ULCER
In the past, peptic ulcer, most commonly gastric or duodenal ulcer, was identified as the leading cause of severe UGI bleeding in the United States, accounting for 50% of UGI bleeds and approximately 100,000 hospitalizations/year.70,71 Some data have suggested that the incidence of bleeding peptic ulcer decreased between 1993 and 2002, whereas the proportion of ulcers caused by NSAIDs increased.72 Other data related to peptic ulcer bleeding between 1990 and 2000, however, found no change in overall bleeding rates but an increase in the rate of bleeding in the subgroup of older patients taking NSAIDs (Fig. 19-6).73 The mortality rate associated with peptic ulcer bleeding is 5% to 10%.52,53 The estimated costs of hospitalization for peptic ulcer bleeding is estimated to be more than $2 billion/year in the United States (see Chapter 52).74
Clinical and endoscopic factors in patients with peptic ulcer bleeding associated with increased morbidity and mortality are shown in Table 19-4. Knowledge of these risk factors can be used to identify patients at high risk for rebleeding and assist in planning the timing of endoscopy.
Table 19-4 Factors Predictive of a Poor Prognosis after Hemorrhage from Peptic Ulcer
| Age > 60 yr |
| Bleeding onset in hospital |
| Comorbid medical illness |
| Shock or orthostatic hypotension |
| Fresh blood in nasogastric tube |
| Coagulopathy |
| Multiple transfusions required |
| Higher lesser curve gastric ulcer (adjacent to left gastric artery) |
| Posterior duodenal bulb ulcer (adjacent to gastroduodenal artery) |
| Endoscopic finding of arterial bleeding or visible vessel |
Pathogenesis
Peptic ulcers are most commonly caused by a decrease in mucosal defense mechanisms attributable to aspirin or other NSAIDs, Helicobacter pylori infection, or both.75,76 In one large multicenter study of patients with severe peptic ulcer bleeding, 57% of those with bleeding from a gastric ulcer (n = 2057) took aspirin or other NSAID, and 45% were infected with H. pylori, whereas 53% of those with a bleeding duodenal ulcer (n = 2033) took aspirin or other NSAID, or both, and 50% were infected with H. pylori.77 Of the patients with a bleeding peptic ulcer in this study, 10% had no obvious cause for the ulcer (H. pylori–negative, no aspirin or other NSAID use, no cancer, no gastrinoma).
H. pylori infection is common, with a prevalence of over 80% of the population in many developing countries and 20% to 50% in industrialized countries.78 H. pylori gastritis most commonly involves the antrum and predisposes patients to duodenal ulcers, whereas gastric body–predominant gastritis is associated with gastric ulcers. The lifetime risk of peptic ulcer disease from H. pylori infection ranges from 3% in the United States to 25% in Japan (see Chapter 50).
NSAIDs are the most widely used medication in the United States, with 11% of the adult population using NSAIDs on a daily basis.79 NSAIDs, including aspirin, predominantly cause ulceration by inhibiting cyclooxygenase-mediated prostaglandin synthesis and thereby impairing mucosal protection, rather than causing direct topical injury.76 Gastroduodenal ulcers are found at endoscopy in 15% to 45% of patients who take NSAIDs regularly.80,81 Gastric ulcers are approximately four times as common as duodenal ulcers in patients who take NSAIDs.82 In a large study of patients with UGI hemorrhage and NSAID-associated ulcers, however, gastric and duodenal ulcers occurred with equal frequencies.77
Histopathology
In a landmark study by Swain and colleagues, the pathologic examination of 27 surgically resected bleeding gastric ulcers with endoscopically visible vessels revealed an underlying artery in 96% of specimens.28 Approximately 50% of the vessels protruded above the surface of the ulcer, whereas the other 50% had clot in continuity with a breach in the vessel wall. The bleeding arteries had a mean diameter of 0.7 mm, with a range of 0.1 to 1.18 mm.
Endoscopic Risk Stratification
Endoscopy not only detects a peptic ulcer, but also can be used to evaluate the ulcer for stigmata associated with an increased risk of rebleeding. The Forrest classification is used to categorize findings during endoscopic evaluation of bleeding peptic ulcers, as follows: active spurting bleeding (Forrest IA); oozing bleeding (Forrest IB); pigmented protuberance or nonbleeding visible vessel (NBVV; Forrest IIA); adherent clot (Forrest IIB); flat pigmented spot (Forrest IIC); and clean-based ulcer (Forrest III).83 Overall interobserver agreement among experts for classifying these stigmata of recent bleeding is only fair to moderate, with poor agreement for NBVVs.84,85
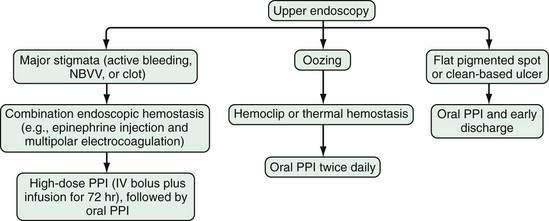
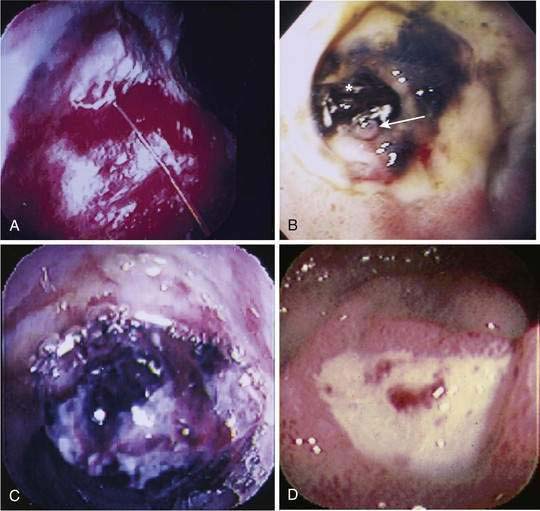
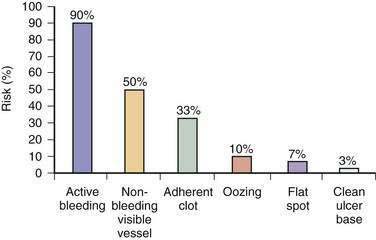
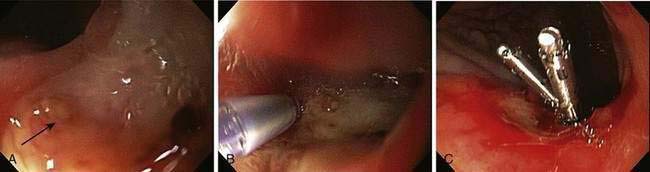
Endoscopic stigmata of recent hemorrhage from an ulcer are shown in Figure 19-7, and the risk of rebleeding associated with each stigma is shown in Figure 19-8. Patients at high risk of rebleeding without treatment are those with active arterial bleeding (90%), an NBVV (50%), or an adherent clot (33%).86,87 These patients benefit from endoscopic hemostasis (see later). An endoscopically identified NBVV that has a translucent (pearl or whitish) color has a higher risk of rebleeding than a darkly colored pigmented protuberance (clot), because the translucent stigma likely represents the arterial wall.88,89 A multivariate analysis of predictors of persistent or recurrent bleeding in patients with nonvariceal UGI bleeding is shown in Table 19-5. Patients with major stigmata of ulcer hemorrhage (spurting, NBVV, or adherent clot) benefit most from endoscopic hemostasis, whereas those with a flat spot or clean ulcer base do not. Patients with oozing bleeding and no other stigma (e.g., a clot or NBVV) have an intermediate risk of rebleeding and may benefit from endoscopic hemostasis but not from high-dose PPI infusion (see later).
Table 19-5 Independent Risk Factors for Persistent or Recurrent Gastrointestinal Tract Bleeding
| RISK FACTOR | RANGE OF ODDS RATIOS FOR INCREASED RISK |
|---|---|
| Clinical Factors | |
| Health status (ASA class 1 vs. 2-5) | 1.94-7.63 |
| Comorbid illness | 1.6-7.63 |
| Shock (systolic blood pressure < 100 mm Hg) | 1.2-3.65 |
| Erratic mental status | 3.21 |
| Ongoing bleeding | 3.14 |
| Age ≥ 70 yr | 2.23 |
| Age > 65 yr | 1.3 |
| Transfusion requirement | NA |
| Presentation of Bleeding | |
| Hematemesis | 1.2-5.7 |
| Red blood on rectal examination | 3.76 |
| Melena | 1.6 |
| Laboratory Factors | |
| Coagulopathy | 1.96 |
| Initial hemoglobin ≤ 10 g/dL | 0.8-2.99 |
| Endoscopic Factors | |
| Ulcer location on superior wall of duodenum | 13.9 |
| Ulcer location on posterior wall of duodenum | 9.2 |
| Active bleeding | 2.5-6.48 |
| High-risk stigmata | 1.91-4.81 |
| Ulcer size ≥ 2 cm | 2.29-3.54 |
| Ulcer location high on lesser curve | 2.79 |
| Diagnosis of gastric or duodenal ulcer | 2.7 |
| Clot over ulcer | 1.72-1.9 |
ASA, American Society of Anesthesiologists; NA, not applicable.
Data from Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843-57.
The risk of rebleeding from a peptic ulcer decreases significantly 72 hours after the initial episode of bleeding. This conclusion is based on studies in which serial endoscopies were performed and only active bleeding was treated endoscopically, with all other stigmata observed. All patients were treated with an intravenous histamine H2 antagonist and cessation of aspirin and other NSAIDs.88,90,91 Natural history studies of untreated NBVVs have found that these lesions resolve over four days and adherent clots tend to resolve over two days.92
Doppler Probe Ultrasound
Portable Doppler ultrasound probes can be passed through the working channel of an endoscope and applied to an ulcer to determine if blood flow is present beneath a stigma in the ulcer base.93,94 The presence of a blood flow signal correlates with the risk of rebleeding before and after endoscopic therapy. Conflicting results have been reported, however, as to whether use of Doppler ultrasound improves the outcome of endoscopic hemostasis in patients with acute peptic ulcer bleeding.95,96 A decision-analysis study has found that Doppler ultrasound is the preferred cost-minimizing strategy over conventional endoscopic therapy alone in patients with acute peptic ulcer bleeding,97 but the area remains one of active investigation.
Endoscopic Hemostasis
Active Bleeding and Nonbleeding Visible Vessels
Many well-conducted randomized controlled trials, meta-analyses, and consensus conferences have concluded that endoscopic hemostasis with epinephrine injection or coaptive thermal probe therapy significantly decreases the rates of ulcer rebleeding, urgent surgery, and mortality in patients with high-risk stigmata, such as active bleeding and NBVVs.98–101 The rebleeding rates for peptic ulcers with various endoscopic stigmata are shown in Figure 19-8. These rebleeding rates are based on studies performed before the widespread use of high-dose infusions of PPIs and predominantly used injection therapy, MPEC therapy, or a combination of injection and thermal probe therapy. In general, for the lesions at highest risk, including those with active bleeding (90% risk of ongoing bleeding) or NBVVs (50% risk of ongoing bleeding), endoscopic hemostasis alone decreases the rebleeding rate to approximately 15% to 30% (Table 19-6). The adjunctive administration of a high-dose intravenous PPI (e.g., pantoprazole, 80-mg bolus and 8 mg/hr for 72 hours) decreases this rate even further, as discussed in the next section. Intravenous formulations of pantoprazole, lansoprazole, and esomeprazole are available in the United States.
The most commonly used treatment for ulcer bleeding worldwide is epinephrine injection therapy, because it is widely available, easy to perform, safe, and inexpensive. Therapy with epinephrine alone seems to be more effective when used in high doses (13 to 20 mL) than in low doses (5 to 10 mL).102 Injection of epinephrine results in a fivefold increase in circulating plasma epinephrine levels but rarely is thought to cause clinically significant cardiovascular events.103
Although epinephrine injection alone is effective compared with placebo, numerous studies and meta-analyses have shown that the addition of a thermal or mechanical hemostatic modality further decreases the rates of rebleeding, surgery, and mortality significantly.30,104,105 Several studies have suggested that the only benefit to adding epinephrine injection to thermal probe therapy is in patients with active bleeding and that no benefit is seen in patients with NBVVs.106,107
Mechanical endoscopic clips have not been studied as well as injection and thermal probe techniques but seem to be more effective than epinephrine injection alone and have shown mixed results when compared with thermal probe therapy.108–111 In a meta-analysis of outcomes for ulcer hemorrhage, application of hemoclips was shown to be superior to epinephrine injection alone but comparable to thermocoagulation.31 Hemoclips have the advantage of being able to be used in patients with severe coagulopathy without the risk of inducing bleeding. The disadvantage of hemoclips is that they can be difficult to deploy, depending on the position of the endoscope in approaching an ulcer.
Adherent Clots
An adherent clot is generally defined as a blood clot over an ulcer that is resistant to several minutes of vigorous target jet water irrigation. The rebleeding rate for ulcers with an adherent clot with medical therapy alone is 8% to 35%, with most large studies reporting rebleeding rates of 30% to 35%.112–115 Randomized controlled studies have shown that endoscopic treatment of adherent clots can decrease the rebleeding rate to less than 5% (see Table 19-6). A meta-analysis has found that endoscopic therapy is superior to medical therapy for preventing recurrent bleeding from peptic ulcers with an adherent clot, but no differences in the need for surgery, duration of hospitalization, number of transfusions, or mortality rate are observed.116 These studies were performed prior to the widespread use of PPIs, which also decrease rates of rebleeding.
Clean-Based Ulcers
Patients with clean-based ulcers at endoscopy after target irrigation have a rebleeding rate of less than 5%. Laine and colleagues have found no difference in outcomes between patients who immediately resumed eating and those who waited several days before they resumed eating after an UGI bleed.117 Longstreth and Feitelberg showed that selected low-risk patients with clinically mild UGI bleeds and clean-based ulcers can be discharged safely to home with a significant savings in cost.9,10
Techniques for Endoscopic Hemostasis
Active Bleeding
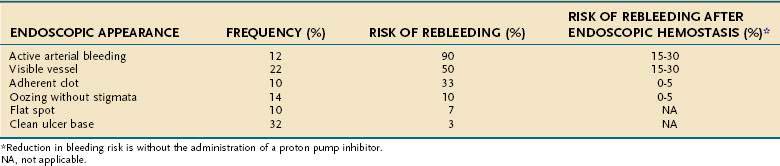
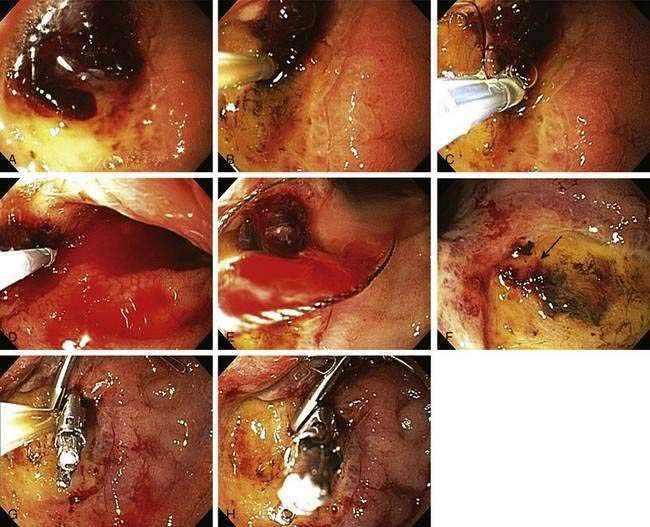
The technique used at the University of California, Los Angeles (UCLA) Center for Ulcer Research and Education (CURE) for actively spurting ulcer bleeding is to inject 0.5- to 1.0-mL aliquots of epinephrine (1 : 20,000) via a sclerotherapy needle into four quadrants of the ulcer within 1 to 2 mm of the bleeding site (Table 19-7). When combination therapy is performed, coagulation is performed with a large 10-Fr multipolar probe. After epinephrine injection, the thermal probe is placed directly on the bleeding site to tamponade the site and stop the bleeding, and coagulation is applied with long (10-second) pulses and firm pressure at a low (12- to 15-W) power setting (Fig. 19-9). The probe is then removed slowly from the ulcer (sometimes with gentle irrigation to prevent pulling coagulated tissue), and thermal coagulation is repeated as needed to stop bleeding and flatten any underlying visible vessel. Epinephrine injection can be repeated if rebleeding persists. With successful endoscopic hemostasis, the rebleeding rate can be decreased to 30% with monotherapy and 15% with combination therapy (see Table 19-6). Alternatively, injection of epinephrine followed by hemoclip placement directly across the actively bleeding site is also effective, although some investigators recommend that clips be placed prior to injection of epinephrine to allow placement of the clip directly on the vessel rather than on a submucosal epinephrine-filled cushion.
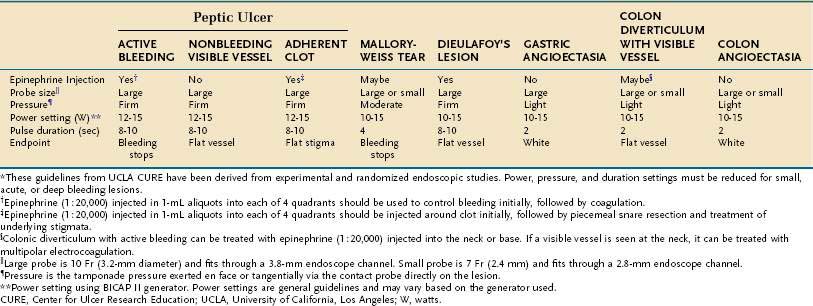
Table 19-7 Endoscopic Technical Parameters for Using Multipolar Electrocoagulation in the Treatment of Bleeding Lesions*
Nonbleeding Visible Vessel
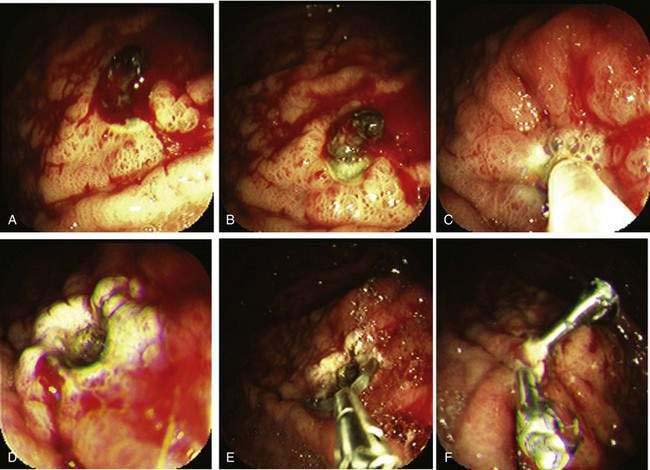
In contrast to active arterial bleeding, no significant difference in results between thermal therapy alone and combination thermal and epinephrine injection therapy is seen with NBVVs. We use the same technique as that used to stop active bleeding; visible vessels are flattened using a large probe, firm pressure, and a low power setting (Fig. 19-10). Hemoclipping can also be effective for preventing rebleeding from an NBVV if the clip is placed across the NBVV and a high-dose PPI is administered intravenously for 72 hours (Fig. 19-11).77,118 With successful endoscopic hemostasis, the rebleeding rate can be reduced to 30% with injection alone and 10% to 15% with thermal coagulation, hemoclipping, or combination therapy (see Table 19-6).
Adherent Clot
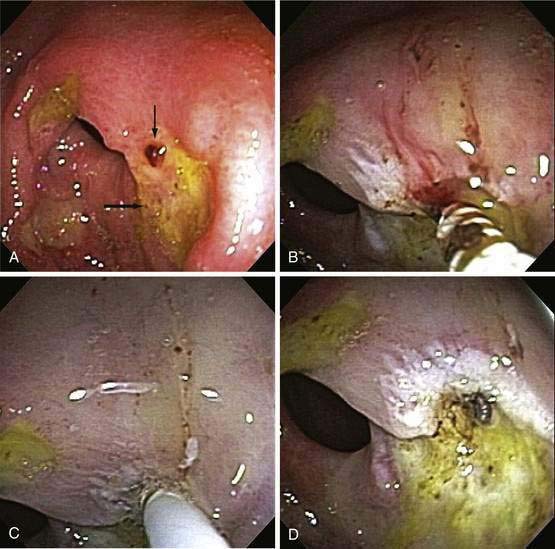
Our current recommendations for treating an adherent clot on an ulcer are first to inject epinephrine (1 : 20,000) in 1-mL increments in four quadrants around the pedicle of the clot and then use a rotatable cold snare to guillotine the clot piecemeal, without pulling it off the base, until an underlying stigma of hemorrhage is identified in the ulcer base or a 3-mm or smaller clot pedicle is left. Coagulation or hemoclipping is performed if active bleeding, a visible vessel, or residual pedicle is seen. Figure 19-12 shows an example of combination treatment for an adherent clot. The combination technique decreases the rebleeding rate from up to 35% (with medical therapy alone) to 5%. Adherent clots are considered a high-risk stigma, and administration of a high-dose PPI is recommended after endoscopic hemostasis.115,116
Oozing of Blood from an Ulcer without Other Stigmata
Minor bleeding from the edge or base of an ulcer (without other stigmata) that continues despite water irrigation and observation suggests the need for endoscopic treatment. The rebleeding rate for ulcers with persistent oozing treated medically varies from 10% (UCLA CURE) to 27% (Hong Kong). Monotherapy with probes or epinephrine injection reduces the rebleeding rate to less than 5%. In patients with oozing, the bleeding arteries may be small and the outcomes better than those in patients with active arterial bleeding.119 Patients with oozing and no other stigmata of hemorrhage (e.g., a clot or NBVV) can be treated effectively with epinephrine injection alone and have an added benefit from combination therapy. After successful endoscopic hemostasis, patients with oozing and no other stigmata do not benefit from administration of a high-dose PPI.
Clean-Based Ulcers
Patients with clean-based ulcers at endoscopy have a rebleeding rate of less than 5% and therefore do not require endoscopic therapy. If the patient has a clean-based gastric ulcer, biopsies from the ulcer edge should be considered to exclude underlying malignancy as well as to assess for H. pylori infection (see Chapter 54). These patients can be fed after the endoscopy and treated with oral acid suppression medication; they do not require continued hospitalization unless indicated for other medical problems.
Assessing for Helicobacter pylori Infection
In a patient with a bleeding gastric or duodenal ulcer, endoscopic mucosal biopsies of the normal appearing antrum and midbody greater curvature should be obtained to assess for the presence of H. pylori infection. Biopsies can be obtained safely after successful endoscopic hemostasis; however, bleeding reduces the sensitivity of rapid urease testing (see Chapter 50).
Pharmacologic Therapy
Acid Suppression Medication
In vitro studies have shown that a luminal gastric pH higher than 6.8 is required for normal clotting function (platelet aggregation and fibrin formation) and that a pH less than 5.4 almost abolishes platelet aggregation and plasma coagulation.120 Platelet aggregates lyse at an acidic pH, an effect that is enhanced by the presence of pepsin. Therefore, reducing the risk of acute bleeding and rebleeding from a peptic ulcer is theoretically possible by maintaining a gastric pH higher than 6. Intravenous H2 receptor antagonists can raise the intragastric pH acutely, but tolerance to these agents develops rapidly and the pH usually returns to 3 to 5 within 24 hours. Several studies have shown that in normal subjects, administration of an intravenous PPI can consistently keep gastric pH higher than 4 (and often 6) over a 72-hour infusion in contrast to an intravenous H2 receptor antagonist.121,122 Trials of intravenous H2 receptor antagonists for the prevention of recurrent ulcer bleeding have shown no definite benefit.123,124
Several studies of PPIs have shown that these agents are effective in reducing rebleeding rates from peptic ulcer. In a study from India, patients with endoscopic high-risk stigmata of peptic ulcer bleeding (active bleeding, NBVV, clot, oozing) who did not undergo endoscopic hemostasis were randomized to omeprazole, 40 mg orally twice daily, or placebo. The rebleeding rate in the omeprazole-treated group was 11% compared with 36% in the placebo-treated group (P < 0.001).125 Another study from the same investigators showed that omeprazole, 40 mg orally twice daily for five days, decreased the rebleeding rate after endoscopic hemostasis with injection therapy for ulcers with active bleeding, an NBVV, or a clot from 21% in the placebo-treated group to 7% in the oral omeprazole-treated group (P = 0.02).126 In a study from Hong Kong, patients who had undergone successful endoscopic hemostasis for active bleeding or an NBVV were randomized to high-dose intravenous omeprazole, 80-mg bolus, followed by 8 mg/hour or placebo. The 30-day rebleeding rate was 6.7% in the omeprazole-treated group compared with 22.5% in the placebo-treated group (P < 0.05).127 The same investigators from Hong Kong found that the 30-day rebleeding rate in patients with an adherent clot or NBVV who received intravenous omeprazole alone was 12% compared with 1% in those who received intravenous omeprazole and underwent endoscopic hemostasis (P < 0.05).128 Another study from Hong Kong found that starting intravenous omeprazole before upper endoscopy in patients with UGI bleeding resulted in a decrease in the number of high-risk stigmata found and the need for endoscopic therapy, but no difference in clinical outcomes such as the number of units transfused, frequency of recurrent bleeding, or rates of surgery and death.129
Systematic and Cochrane reviews of the clinical effectiveness and cost-effectiveness of PPIs in acute UGI bleeding by Leontiadis and colleagues have found that PPI treatment initiated after endoscopic diagnosis of peptic ulcer bleeding significantly reduces the rates of rebleeding and surgery compared with placebo or H2 receptor blockers and that the benefit is more pronounced in Asian than in non-Asian studies.130–132 PPI treatment was associated with decreased mortality in the Asian studies as well as in patients with high-risk endoscopic stigmata. The initiation of PPI treatment prior to endoscopy significantly reduced the proportion of patients with stigmata of recent hemorrhage at index endoscopy compared with placebo or H2 receptor blockers but did not reduce the rate of mortality, rebleeding, or surgery.
Some caution is advised in generalizing the results of PPI trials in Asian patients with peptic ulcer hemorrhage to heterogeneous non-Asian populations. The Asian patients are generally more responsive than heterogeneous populations or whites to PPIs.133 Asian patients have a smaller average parietal cell mass, are slower metabolizers of PPIs, and often have H. pylori infection, all of which increase the effectiveness of PPIs. These factors may explain the lower mortality rates in Asians compared with non-Asians in meta-analyses of PPI trials for peptic ulcer hemorrhage.
A number of issues related to PPIs and UGI bleeding are still unresolved. Whether a PPI should be given before or after endoscopy is uncertain. Although some small randomized studies have not shown pre-endoscopy administration of a PPI to improve clinical outcomes (although the number of high-risk stigmata that require treatment is reduced), most modeling studies have suggested that pre-endoscopy administration of a PPI is cost-effective.14,16,17,129,132 Intravenous administration of a PPI by high-dose continuous drip or intermittent bolus infusion is also a matter of controversy, but most data favor continuous drip, with small comparative studies suggesting that continuous infusion decreases the rate of rebleeding and need for surgery compared with intermittent dosing.134 Whether administration of an oral PPI is as effective as intravenous administration is unclear, although studies have shown that high-dose oral PPI administration (e.g., omeprazole, 40 mg twice daily) reduces rebleeding to rates that would be expected from endoscopic hemostasis. In addition, studies have shown that the increase in intragastric pH with high-dose oral PPI administration is almost identical (although delayed by one hour) to that with intravenous PPI administration.125,135 Whether intravenous administration of a PPI alone is sufficient therapy (without endoscopic hemostasis) in patients with recent UGI bleeding and some stigmata of hemorrhage, such as an NBVV, oozing, or clot, is controversial. In an Asian study, Sung and colleagues reported that the 30-day rebleeding rate with intravenous PPI administration alone (12%) is similar to that in previous studies of endoscopic hemostasis, although they also found that the rebleeding rate with a combination of endoscopic therapy and an intravenous PPI is even lower (1%).136 Finally, because almost all the major studies of PPIs in acute peptic ulcer bleeding have been conducted in Asian populations, who have a greater pharmacodynamic response to PPIs than non-Asian populations, studies in non-Asian populations are needed to confirm the Asian data. One large international study has confirmed the benefit of high-dose intravenous PPI administration in high-risk patients with active arterial bleeding, a NBVV, or an adherent clot in a study of a predominantly white population.137
Somatostatin and Octreotide
A meta-analysis has suggested that intravenous administration of somatostatin or its long-acting form octreotide decreases the risk of rebleeding from peptic ulcers when compared with placebo or an H2 receptor blocker.138 The proposed mechanisms of action include reductions in splanchnic and gastroduodenal mucosal blood flow, decreases in gastrointestinal motility, inhibition of gastric acid secretion, inhibition of pepsin secretion, and gastric mucosal cytoprotective effects. These drugs have not been studied, however, in the era of endoscopic therapy or PPI use and therefore cannot be considered for routine use.139 Somatostatin or octreotide can be considered in patients with severe ongoing bleeding who are not responsive to endoscopic therapy, an intravenous PPI, or both, and are not surgical candidates, although their effectiveness in these patients is uncertain. Intravenous octreotide may also be useful in patients with portal hypertension and peptic ulcer hemorrhage as an adjunct to endoscopic hemostasis and a PPI (see Chapter 90).
Second-Look Endoscopy
Routine repeat, or second-look, endoscopy 24 hours after initial endoscopic hemostasis, with additional endoscopic hemostasis if persistent high-risk endoscopic stigmata are found, has been proposed as a way to improve patient outcomes. The results of four prospective randomized trials have yielded conflicting results, with no benefit in the majority of studies.140–143 Therefore, routine second-look endoscopy is not recommended for most patients with peptic ulcer bleeding.100 The exception to this recommendation is for patients who had an incomplete initial endoscopic examination because of excessive blood that obscured the view or technical problems with hemostasis. Patients with clinically significant rebleeding also should undergo a second endoscopy. For patients who are treated with epinephrine injection alone, second-look endoscopy and repeat treatment are considered a routine part of the management.119 Endoscopic retreatment with administration of more epinephrine for major stigmata of hemorrhage or oozing is required in 20% to 25% of patients with peptic ulcer hemorrhage.
Rebleeding after Endoscopic Treatment
The difference between ulcer hemorrhage that starts in the outpatient setting and hemorrhage that starts in the inpatient setting is substantial (Table 19-8). Because the time to rebleeding can be much longer for inpatient (than outpatient) ulcer hemorrhage and the risk of rebleeding is high, combination endoscopic hemostasis and high-dose intravenous PPI administration for at least 72 hours ought to be considered. Further studies are warranted in this high-risk group to define the optimal management.
Table 19-8 Comparison of Outpatient and Inpatient Onset of Peptic Ulcer Bleeding*
| PARAMETER | Onset | |
|---|---|---|
| OUTPATIENT | INPATIENT | |
| Frequency (%) | 80-90 | 10-20 |
| American Society of Anesthesiologists comorbidity score† | ≤3 | >3 |
| Time to rebleeding (%) | ||
| ≤72 hr | 70-80 | 40-50 |
| 4-7 days | 10-15 | 15-20 |
| 8-30 days | 1-5 | 15-20 |
| >30 days | 0 | 5-10 |
CURE, Center for Ulcer Research Education; UCLA, University of California, Los Angeles
* Data from the UCLA CURE database.
† 1 point signifies a healthy person; 5 points signifies high likelihood of mortality within 24 hr.
If rebleeding from a peptic ulcer is severe, an urgent repeat endoscopy (rather than surgery) should be performed. A large, well-designed, randomized trial from Hong Kong has found that when endoscopic hemostasis is repeated in patients with hemodynamically significant rebleeding after initial endoscopic hemostasis, 73% of patients achieve sustained hemostasis and do not require surgery.144 The overall mortality rate was the same in both groups, but the rate of complications was significantly higher in the surgical group. Factors that predicted failure of endoscopic retreatment included an ulcer size of at least 2 cm and hypotension on initial presentation.
Angiography and Surgery
Patients with recurrent bleeding despite two sessions of endoscopic hemostasis should be considered for angiographic embolization or surgical therapy. Interventional angiography with embolization has become widely available. Several retrospective series have reported no significant difference between angiography with embolization and surgery in rates of rebleeding and mortality, despite the older age of and more serious medical problems in patients treated by angiography than in those treated by surgery.145,146 These studies suggest that angiography can be considered after failure of endoscopic therapy. If embolization therapy does not control the bleeding, surgery remains an option.
Immediate Postendoscopic Management
Low-Risk Endoscopic Stigmata
Patients with a clean-based ulcer or flat spot in the ulcer base can generally resume a normal diet immediately, begin an oral PPI once daily, and be discharged from the emergency department or hospital when stable.117 Several studies have shown that patients at low risk for rebleeding on the basis of these endoscopic findings and a stable clinical status can avoid hospitalization entirely or be discharged early.9,10,64,147 Generally these patients are young and hemodynamically stable, have no severe coexisting medical illnesses, a hemoglobin level higher than 10 mg/dL, and normal coagulation parameters, had the onset of ulcer bleeding outside the hospital, have good social support systems at home in case rebleeding occurs, and have a clean-based ulcer or ulcer with a flat spot on initial endoscopy.
Prevention of Recurrent Ulcer Bleeding
Helicobacter pylori Infection
All patients with peptic ulcer bleeding should be tested for H. pylori infection and, if the result is positive, should receive antibiotic therapy in standard fashion (see Chapter 50).78 One caveat is that bleeding can lead to a false-negative rapid urease test result, and the patient may need to undergo an alternative method of testing for H. pylori in this setting. Antibiotic therapy does not need to be started urgently and can be initiated on an outpatient basis when the patient has resumed a normal diet. Patients who are H. pylori–positive and who will need long-term PPI treatment because of the concomitant need for aspirin or other NSAID do not necessarily need to be treated for H. pylori infection, because recurrent ulceration will be prevented by the PPI. In patients who are found to have an H. pylori–induced ulcer, confirmation of the eradication of H. pylori after treatment is recommended.
Aspirin, Other Nonsteroidal Anti-inflammatory Drugs, and Clopidogrel
Ideally, patients with ulcer bleeding caused by aspirin or another NSAID should stop the drug. If the patient is also positive for H. pylori, the organism should be eradicated with antibiotics (see Chapter 50).148
In patients with a history of ulcer bleeding who are H. pylori–positive and need to continue taking low-dose aspirin (81 mg daily), eradication of H. pylori alone results in ulcer rebleeding rates similar to those associated with daily PPI therapy (if H. pylori is not eradicated).149 By contrast, in patients with a history of ulcer bleeding who are H. pylori–positive and need to continue full-dose NSAID therapy, eradication of H. pylori alone leads to a significantly higher rebleeding rate than use of a daily PPI in conjunction with the NSAID. In patients with ulcer bleeding who do not have H. pylori infection but who need to continue daily aspirin, co-therapy with a daily PPI significantly reduces the rebleeding rate compared with placebo in combination with aspirin.150
Patients who require an antiplatelet medication and have a history of ulcer bleeding will have less chance of recurrent bleeding if they take aspirin 81 mg and a PPI daily compared with clopidogrel alone.151
Patients who require an NSAID after an ulcer bleed may be considered for a selective cyclooxygenase-2 (COX-2) inhibitor. Selective COX-2 inhibitors cause fewer ulcers than nonselective NSAIDs but are associated with a greater rate of cardiovascular complications. Because selective COX-2 inhibitors result in rebleeding rates similar to those associated with NSAID and PPI cotherapy, their use may not be worth the cardiovascular risk.152
Repeat Endoscopy to Confirm Gastric Ulcer Healing
Repeat upper endoscopy should be considered in patients with a gastric ulcer after 6 to 10 weeks of acid suppressive therapy to confirm healing of the ulcer and absence of malignancy (see Chapter 54). In areas of the world in which the population is at intermediate risk for gastric cancer, 2% to 4% of repeat upper endoscopies to confirm ulcer healing have been reported to disclose gastric cancer.153–155 Some experts have suggested that when the index endoscopy with biopsies is negative for malignancy and the ulcer appears benign endoscopically, a follow-up endoscopy is unnecessary.156 A small retrospective study has found that when gastric cancer is detected on repeat endoscopy to evaluate gastric ulcer healing, survival is no better than that for patients who did not undergo the recommended follow-up endoscopy.153
OTHER CAUSES
Esophagitis
Patients with severe erosive esophagitis can present with hematemesis or melena. A multivariate analysis from a center in France, in which 8% of all UGI bleeding was caused by erosive esophagitis, found that independent risk factors for bleeding esophagitis were grade 3 or 4 (moderate to severe) esophagitis by the Savary-Miller grading system (see Chapter 43), cirrhosis, a poor performance status, and anticoagulant therapy.157 A history of heartburn was obtained in only 38% of patients. Severe bleeding from gastroesophageal reflux–induced esophagitis is treated medically with a PPI (see Chapter 43). Upper endoscopy is critical to diagnosing severe erosive esophagitis, but endoscopic therapy generally has no role unless a focal ulcer with a stigma of recent hemorrhage is found. These patients should be treated with a daily PPI for 8 to 12 weeks and undergo repeat endoscopy to exclude underlying Barrett’s esophagus (see Chapter 44).
Patients can sometimes present with mild UGI bleeding from esophagitis not related to gastroesophageal reflux disease, such as infections (e.g., Candida, herpes simplex virus, cytomegalovirus) or pill-induced esophagitis. Endoscopy with biopsies and brushings is critical for making these diagnoses and determining the appropriate pharmacologic therapy (see Chapter 45).
Ulcer Hemorrhage in Hospitalized Patients
Bleeding from SRMI is now uncommon, with a frequency of approximately 1.5% patients in an ICU. The two main risk factors are severe coagulopathy and mechanical ventilation for longer than 48 hours.158 The frequency of clinically significant GI bleeding with either or both of these risk factors is 3.7% compared with 0.1% when neither risk factor is present. Other proposed risk factors include a history of UGI bleeding, sepsis, an ICU admission longer than seven days, occult GI bleeding for more than five days, and treatment with high-dose glucocorticoids.
ICU patients with risk factors for bleeding are the main target groups for pharmacologic prevention of bleeding SRMI. Therapy with an H2 receptor antagonist has been shown to decrease the rate of clinically significant bleeding in ICU patients at high risk of SRMI.159 Well-designed and adequately powered studies that compare H2 receptor blockers and PPIs are few in number, but one large multicenter study found that prophylactic treatment with oral omeprazole or intravenous cimetidine results in similar bleeding rates but that omeprazole is more effective than cimetidine in maintaining the luminal gastric pH above 4.160 A potential harmful effect of gastric acid suppression to prevent stress ulcers is that a decrease in gastric pH may allow proliferation of bacteria in the stomach and the potential for aspiration and ventilator-associated pneumonia; however, randomized trials in which acid suppression (with an H2 receptor blocker or antacids) and sucralfate (which does not lower gastric pH) were compared have not shown convincingly that lowering gastric pH increases the risk of pneumonia.161,162
Generally, if a patient with SRMI or an inpatient ulcer is supported hemodynamically and medically, the lesion will heal as the patient’s overall medical status improves. Because SRMI is diffuse, endoscopic therapy is generally not feasible. By contrast, focal inpatient ulcer hemorrhage often requires endoscopic hemostasis for severe hemorrhage; however, rebleeding rates are higher and healing rates are slower than those in patients in whom bleeding starts before hospitalization (see Table 19-8).163,164 A study in which epinephrine injection plus hemoclip placement was compared with epinephrine injection plus MPEC in a cohort of patients who had a high frequency of in-hospital ulcers found a significantly lower rebleeding rate in the group that underwent injection and hemoclip placement.118 Figure 19-9 illustrates combination treatment using injection therapy, bipolar probe coagulation, and endoscopic hemoclipping for an inpatient ulcer hemorrhage.
Dieulafoy’s Lesion
Dieulafoy’s lesion can be difficult to identify at endoscopy because of the intermittent nature of the bleeding; the overlying mucosa may appear normal if the lesion is not bleeding. An NBVV or adherent clot without an ulcer may be seen on endoscopy. If a massive UGI bleed seems to be emanating from the stomach, a careful inspection of the proximal stomach should be carried out to look for a protuberance that might be a Dieulafoy’s lesion. Endoscopic Doppler ultrasound has been used to help identify a Dieulafoy’s lesion that is not visualized on endoscopy.165 Because of the difficulty of identifying the bleeding site, we recommend that if a Dieulafoy’s lesion is found and treated, the site be marked with submucosal injection of ink to tattoo the area in case of rebleeding and the need for retreatment.
Endoscopic hemostasis of a Dieulafoy’s lesion can be performed with injection therapy, a thermal probe, or clip device or by band ligation.165–169,170 Large case series have reported an initial hemostasis rate of approximately 90%, with the need for surgery in 4% to 16% of cases.171 Rebleeding after successful hemostasis appears to be rare. Although all the endoscopic hemostasis techniques seem to be effective, perforation and delayed rebleeding have been reported after band ligation (see Chapter 36).
Mallory-Weiss Tears
Mallory-Weiss tears are mucosal or submucosal lacerations that occur at the gastroesophageal junction and usually extend distally into a hiatal hernia (Fig. 19-13). Patients generally present with hematemesis or coffee-ground emesis and typically have a history of recent nonbloody vomiting followed by hematemesis, although some patients do not recall any vomiting. The tear is thought to result from increased intra-abdominal pressure, possibly in combination with a shearing effect caused by negative intrathoracic pressure above the diaphragm, which is often related to vomiting in patients with a history of alcohol abuse. Mallory-Weiss tears have been reported in patients who vomit while taking a bowel purge before colonoscopy.171 Endoscopy usually reveals a single tear that begins at the gastroesophageal junction and extends several millimeters distally into a hiatal hernia sac. Occasionally, more than one tear is seen. A retroflexed view in the stomach may provide better visualization than the forward viewing position. The bleeding stigmata of Mallory-Weiss tears can include a clean base, oozing, or active spurting. Usually, the bleeding is self-limited and mild, but occasionally it can be severe. Superficial (mucosal) Mallory-Weiss tears can start healing within hours and can heal completely within 48 hours.
Although approximately 50% of patients hospitalized with UGI bleeding from a Mallory-Weiss tear receive blood transfusions, the tear manifests as mild, self-limited hematemesis in most patients, who do not seek medical care.172 The rebleeding rate among patients hospitalized for a Mallory-Weiss tear is approximately 10%; risk factors for rebleeding include shock at presentation and active bleeding at endoscopy.173 Because of the risk of continued and recurrent bleeding, patients with active bleeding from a Mallory-Weiss tear should undergo endoscopic therapy, which can be performed successfully with epinephrine injection, MPEC, hemoclip placement, or band ligation. Randomized trials that compared MPEC and medical therapy with an H2 receptor antagonist have found that endoscopic therapy reduces the rates of rebleeding, blood transfusions, and emergency surgery.174
Our current endoscopic technique for treating actively bleeding Mallory-Weiss tears in patients without portal hypertension or esophageal varices is to apply endoscopic hemoclips to stop the bleeding and close the tear. If hemoclips are not available, MPEC at a low power setting and with light pressure for one to two seconds is recommended. The management of patients with esophageal varices caused by portal hypertension who also have a Mallory-Weiss tear should be targeted toward the esophageal varices, with esophageal band ligation or variceal sclerotherapy (see later and Chapter 90).
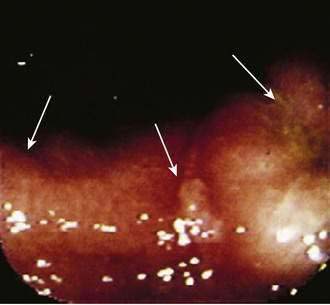
Cameron’s Lesions
Cameron’s lesions are linear erosions or ulcerations in the proximal stomach at the end of a large hiatal hernia, near the diaphragmatic pinch (Fig. 19-14).175 Cameron’s lesions are thought to be caused by mechanical trauma and local ischemia as the hernia moves against the diaphragm and only secondarily by acid and pepsin. They can be a source of acute UGI bleeding but more commonly may present as slow GI bleeding and iron deficiency anemia. Cameron’s lesions are a common cause of obscure GI bleeding (see later) and not uncommonly are missed by an unsuspecting endoscopist. Endoscopic management has been reported.176 The long-term medical management is usually with iron supplements and an oral PPI (see Chapter 36).177,178 Occasionally surgical repair of the hiatal hernia may be needed.
Upper Gastrointestinal Malignancy
Malignancy accounts for 1% of severe UGI bleeds. The tumors are usually large, ulcerated masses in the esophagus, stomach, or duodenum. Endoscopic hemostasis with MPEC, laser, injection therapy, or hemoclips can temporarily control acute bleeding in most patients and allow time to determine the appropriate long-term management.179,180 Patients with an ulcerated subepithelial mass (which usually is a gastrointestinal stromal tumor or leiomyoma) should undergo surgical resection of the mass to prevent rebleeding and to prevent the risk of metastasis. Angiography with embolization should be considered for patients with severe UGI bleeding caused by malignancy who do not respond to endoscopic therapy. External beam radiation can provide palliative hemostasis for patients with bleeding from advanced gastric or duodenal cancer (see Chapter 54).
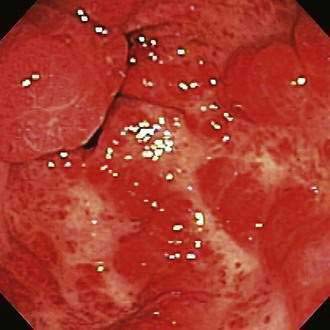
Gastric Antral Vascular Ectasia
Gastric antral vascular ectasia (GAVE), also described as watermelon stomach, is characterized by rows or stripes of ectatic mucosal blood vessels that emanate from the pylorus and extend proximally into the antrum (Fig. 19-15). The cause is uncertain, and the lesion may represent a response to mucosal trauma from contraction waves in the antrum. GAVE has been associated with cirrhosis and scleroderma (see Chapters 35 and 90). Patients with GAVE who do not have portal hypertension demonstrate linear arrays of angiomas (classic GAVE), whereas those with portal hypertension have more diffuse antral angiomas.181 The diffuse type of antral angiomas and occasionally classic GAVE are sometimes mistaken for gastritis by an unsuspecting endoscopist. Such cases are a common cause of obscure GI bleeding in referral centers (see later).48
Patients usually present with iron deficiency anemia or melena, with a mildly decreased hematocrit value suggestive of a slow UGI bleed. GAVE is most commonly reported in older women181 and also seems to be more common in patients with end-stage renal disease.
Endoscopic hemostasis with thermal heat modalities such as laser, MPEC, or argon plasma coagulation has been used successfully. Endoscopic hemostasis and ablation with thermal modalities can result in good palliation with an increase in the hematocrit value and a decrease in the need for blood transfusions and hospitalization.181,182 Usually, several sessions, approximately four to eight weeks apart, are needed to achieve eradication of the lesions and a reduction in bleeding from the antral ectasias. Endoscopic therapy with argon plasma coagulation has been shown to be equally (80%) effective in cirrhotic and noncirrhotic patients with GAVE.183 Pilot studies have demonstrated that mucosal band ligation, radiofrequency ablation, and cryotherapy can also lead to eradication of GAVE in selected patients.184–186
Placement of a transjugular intrahepatic portosystemic shunt (TIPS) in patients with portal hypertension and cirrhosis does not decrease bleeding from GAVE or diffuse antral angiomas. Patients who have ongoing severe chronic bleeding from GAVE rarely may require surgical antrectomy to control symptoms (see Chapters 36 and 90).187
Portal Hypertensive Gastropathy
Portal hypertensive gastropathy (PHG) is caused by increased portal venous pressure and severe mucosal hyperemia that results in ectatic blood vessels in the proximal gastric body and cardia and oozing of blood. Less severe grades of PHG appear as a mosaic or snakeskin pattern and are not associated with bleeding.188 Usually, patients with severe PHG present with chronic blood loss, but they occasionally can present with acute bleeding.
Severe PHG with diffuse bleeding is treated by measures that decrease portal pressure, usually with β-adrenergic receptor blockers or possibly with placement of a TIPS or surgical portacaval shunt. Endoscopic management has no role unless an obvious focal bleeding site is identified. The best treatment is liver transplantation (see Chapters 36 and 90).
Hemobilia
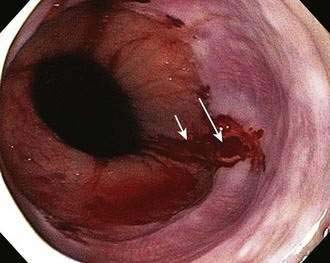
Hemobilia may occur in patients who have experienced liver trauma, undergone a liver biopsy, manipulation of the hepatobiliary system, as occurs with endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography, or TIPS, or have hepatocellular carcinoma or a biliary parasitic infection.189 Patients may present with a combination of GI bleeding and elevated liver biochemical test levels. The diagnosis can be confirmed by using a side-viewing duodenoscope to identify bleeding from the ampulla (Fig. 19-16). Ongoing or recurrent bleeding is treated with arterial embolization via arteriography.
Hemosuccus Pancreaticus
Hemosuccus pancreaticus is a rare form of UGI bleeding that occurs most commonly in patients with acute pancreatitis, chronic pancreatitis, pancreatic pseudocyst, or pancreatic cancer or after ERCP with pancreatic duct manipulation. It can also result from rupture of a splenic artery aneurysm into the pancreatic duct.190 CT can demonstrate pancreatic pathology if previously unsuspected. Endoscopy with a side-viewing duodenoscope reveals blood coming out of the ampulla. Management of severe hemorrhage is usually with angiographic embolization or surgery.
Postsphincterotomy Bleeding
Bleeding following endoscopic sphincterotomy occurs in approximately 2% of patients (see Chapter 40).191 Potential risk factors include coagulopathy, use of anticoagulants, portal hypertension, renal failure, and the type and length of sphincterotomy. Successful hemostasis of postsphincterotomy bleeding is usually achieved with endoscopic methods such as injection of epinephrine, hemoclips, or MPEC (see Chapter 40).
Aortoenteric Fistula
Bleeding from an aortoenteric fistula is usually acute and massive, with a high mortality rate.192 A primary aortoenteric fistula is a communication between the native abdominal aorta (usually an atherosclerotic abdominal aortic aneurysm) and, most commonly, the third portion of the duodenum.193 Often, a self-limited herald bleed occurs hours to months before a more severe, exsanguinating bleed. Occasionally, the diagnosis of an aortoenteric fistula is suspected by a history of an abdominal aortic aneurysm or by palpation of a pulsatile abdominal mass. The diagnosis can be difficult to make on endoscopy in the absence of active bleeding. Demonstration of an aortic aneurysm on abdominal CT scan (with intravenous contrast) suggests the diagnosis of a fistula.49
Secondary aortoenteric fistulas usually occur between the small intestine and an infected abdominal aortic surgical graft. The fistula typically occurs between the third portion of the duodenum and the proximal end of the graft but may occur elsewhere in the GI tract. The fistula usually forms between three and five years after graft placement. Patients often experience a herald bleed that is mild and self-limited, and occasionally intermittent, before massive bleeding occurs.194 A secondary fistula can also occur between the third part of the duodenum and an endovascular stent, in which case the fistula occurs as a result of pressure from the stent against the duodenum, infection of the stent, or possibly expansion of the native aneurysm.195
Patients with an acute UGI bleed and a history of an aortic aneurysm repair should undergo urgent CT with intravenous contrast, push enteroscopy to evaluate the third portion of the duodenum for compression or blood, as well as to exclude other bleeding sources, and a vascular surgery consultation. CT may show inflammation around the graft and may demonstrate the fistula. Surgical treatment is required to remove the infected graft. Therapeutic endoscopy plays no role in the management of bleeding from an aortoenteric fistula (see Chapter 36).
VARICES
Variceal hemorrhage is an important cause of UGI bleeding and is discussed in more detail in Chapter 90. Esophageal variceal bleeding related to portal hypertension is the second most common cause of severe UGI bleeding (after peptic ulcer disease). The acute mortality rate with each bleed is approximately 30%, and the long-term survival rate is less than 40% after one year with medical management alone.196 Despite advances in medical therapy, endoscopic hemostasis, and portosystemic shunt procedures, overall long-term survival rates have not improved for patients with variceal bleeding. Liver transplantation, however, can improve survival in selected patients. Survival in nontransplanted patients with variceal bleeding is heavily influenced by the severity of underlying liver disease, with poorer survival rates for patients with Child (or Child-Pugh) class C cirrhosis than for those with Child class A or B cirrhosis (see Chapter 90).
Medical Management of Acute Variceal Bleeding
Somatostatin and its long-acting analog octreotide cause selective splanchnic vasoconstriction and lower portal pressure, without causing the cardiac complications seen with vasopressin (even in combination with nitroglycerin). Studies have shown mixed results as to whether somatostatin is more effective than placebo in managing variceal bleeding, but it seems to be at least as effective as vasopressin and much safer. A meta-analysis has shown that vasoactive drugs (e.g., octreotide, somatostatin, terlipressin [a long-acting vasopressin analog]) are as effective as sclerotherapy for controlling variceal bleeding and cause fewer adverse events.19 No studies have shown a survival benefit to vasopressin or somatostatin in patients with variceal bleeding. Given the potential ability of octreotide to control acute variceal hemorrhage, its low toxicity, and its availability in the United States (unlike somatostatin and terlipressin), octreotide appears to be the pharmacologic drug of choice as an adjunct to endoscopic therapy for the treatment of variceal hemorrhage. The dose of octreotide for acute variceal hemorrhage is a 50-µg bolus followed by a continuous infusion of 50 µg/hour for up to five days.
Patients with a prolonged prothrombin time that does not correct with fresh frozen plasma may benefit from infusion of human recombinant factor VIIa. In one uncontrolled trial, a single 80-µg/kg dose of recombinant factor VIIa normalized the prothrombin time in all 10 patients within 30 minutes, with immediate control of bleeding in all patients.197 In a large, randomized, placebo-controlled study, administration of recombinant factor VII in addition to endoscopic hemostasis decreased rebleeding rates in patients with Child class B and C cirrhosis who had bled from varices.198 Because recombinant factor VIIa is expensive, its use should be reserved for patients with severe ongoing bleeding and irreversible coagulopathy, pending the results of additional clinical and cost-effectiveness studies.
Up to 20% of cirrhotic patients who are hospitalized with GI bleeding have a bacterial infection at the time of admission to the hospital, and infection develops during the hospitalization in up to 50%. Meta-analyses have suggested that administration of an antibiotic to cirrhotic patients with variceal bleeding is associated with a decrease in the rates of mortality and bacterial infections.199,200 The optimal type and duration of antibiotic is unknown. The most commonly prescribed antibiotics are fluoroquinolones, including oral norfloxacin, 400 mg twice daily, intravenous ciprofloxacin, 400 mg every 12 hours, intravenous levofloxacin, 500 mg every 24 hours, and intravenous ceftriaxone, 1 g every 24 hours, administered for seven days.
Balloon Tamponade
Balloon tamponade of varices is seldom used now to control variceal bleeding; it may be used to stabilize a patient with massive bleeding prior to definitive therapy. Varices lie in the esophageal and gastric submucosa and are amenable to physical tamponade. Three types of tamponade balloons are available. The Sengstaken-Blakemore tube has gastric and esophageal balloons, with a single aspirating port in the stomach. The Minnesota tube also has gastric and esophageal balloons and has aspiration ports in the esophagus and stomach. The Linton-Nicholas tube has a single large gastric balloon and aspiration ports in the stomach and esophagus. Most reports suggest that balloon tamponade provides initial control of bleeding in 85% to 98% of cases, but variceal rebleeding recurs soon after the balloon is deflated in 21% to 60% of patients.201 The major problem with tamponade balloons is a 30% rate of serious complications, such as aspiration pneumonia, esophageal rupture, and airway obstruction. Patients should be intubated before placement of a tamponade balloon to minimize the risk of pulmonary complications. Clinical studies have not shown a significant difference in efficacy between vasopressin administration and balloon tamponade.
Endoscopic Sclerotherapy
Prospective randomized trials have shown mixed results but suggest improved immediate hemostasis and a reduction in acute rebleeding with sclerotherapy compared with medical therapy alone for bleeding esophageal varices.202–205 Hemostasis can be achieved in 85% to 95% of cases, with a rebleeding rate of 25% to 30%.206 Complications of endoscopic variceal sclerotherapy include esophageal ulcers, which can bleed or perforate, esophageal strictures, mediastinitis, pleural effusions, aspiration pneumonia, acute respiratory distress syndrome, chest pain, fever, and bacteremia and account in part for the use of esophageal variceal band ligation as the preferred endoscopic therapy for variceal bleeding.
Endoscopic Band Ligation
The technique of endoscopic band ligation is similar to that used for band ligation of internal hemorrhoids. A rubber band is placed over a varix, which subsequently undergoes thrombosis, sloughing, and fibrosis. Prospective, randomized, controlled trials have shown that endoscopic band ligation is as effective as sclerotherapy in achieving initial hemostasis and reducing the rate of rebleeding from esophageal varices. Acute hemostasis generally can be achieved in 80% to 85% of cases, with a rebleeding rate of 25% to 30%. Subsequent studies have shown that band ligation is associated with fewer local complications, especially esophageal strictures, and requires fewer endoscopic treatment sessions than sclerotherapy.206 A meta-analysis has reported that variceal band ligation reduces the rates of rebleeding, overall mortality, and death from bleeding compared with sclerotherapy.207 Band ligation, however, may be more technically difficult to perform than sclerotherapy during active variceal bleeding. Devices used for band ligation allow up to 10 bands to be placed, without the need to remove the endoscope to reload the banding device. The strategy is to control active bleeding and place two bands on each esophageal variceal column, one distally near the gastroesophageal junction and another 4 to 6 cm proximally.
Transjugular Intrahepatic Portosystemic Shunt
Placement of a transjugular intrahepatic portosystemic shunt (TIPS) is an interventional radiologic procedure in which an expandable metal stent is placed via percutaneous insertion between the hepatic and portal veins, thereby creating an intrahepatic portosystemic shunt. TIPS is effective for the short-term control of bleeding gastroesophageal varices.208,209 Initially envisioned as a bridge to liver transplantation, it has been used with increased frequency in nontransplantation situations. Randomized trials that have compared TIPS with endoscopic sclerotherapy suggest that TIPS is more effective for the long-term prevention of rebleeding.210 The main problems with TIPS are a rate of shunt occlusion of up to 80% (less with polytetrafluoro-ethylene-coated stents) within one year and the development of new or worsening hepatic encephalopathy in approximately 20% of patients.211 TIPS does not prolong the survival of patients with variceal bleeding compared with endoscopic treatment. In the management of acute variceal bleeding, TIPS is reserved for patients who fail endoscopic treatment.
Portosystemic Shunt Surgery
A variety of portosystemic shunt operations can be performed to reduce portal venous pressure. When compared with sclerotherapy, surgical shunts decrease the rebleeding rate significantly but do not improve survival.206,212–215 Some groups have suggested that survival can be improved by using a combination of endoscopic sclerotherapy and surgical portosystemic shunt rescue for those who rebleed despite sclerotherapy. Surgical shunts may be associated with hepatic encephalopathy and can make future liver transplantation technically more difficult, but they have an advantage over endoscopic variceal therapy in reducing portal hypertension and treating gastric variceal bleeding. Surgical shunts are considered for selected patients who have failed endoscopic therapy and who are not expected to become candidates for liver transplantation (see Chapters 90 and 95).