Gastrointestinal Bleeding
Perspective
Gastrointestinal bleeding (GIB) accounts for more than 1 million hospitalizations annually in the United States, with significant morbidity, mortality, and economic burden.1 GIB is traditionally classified by the bleeding source—upper GIB (UGIB) is proximal and lower GIB (LGIB) is distal to the ligament of Treitz in the terminal duodenum.
UGIB accounts for more than 500,000 U.S. hospital admissions annually, with approximately 165 incidents per 100,000 patients.1 Mortality rates have remained consistent at 13 to 14% over the past two decades despite advances in medical therapy, intensive care unit (ICU) management, endoscopy, and surgery.2 An increasing proportion of elderly patients, who may die owing to comorbid conditions, and increases in the number of cirrhotic and variceal patients may contribute to the lack of change in mortality rates. In the United States, hospitalized patients with and without complications of nonvariceal UGIB had a mean length of stay of 4.4 and 2.7 days and hospitalization costs of $5632 and $3402 (2004 U.S. dollars), respectively.3
Diagnostic Approach
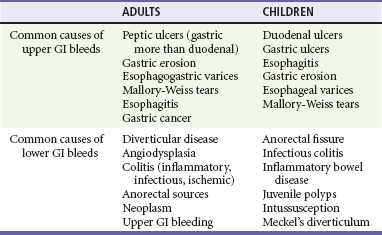
The characteristics of the bleed and the age of the patient can help determine the cause of the GIB. UGIB can routinely manifest as bloody or coffee-ground–like vomit known as hematemesis or as dark, tarry stools known as melena. In adults, peptic ulcer disease, erosive gastritis, and esophageal varices account for the majority of these cases4,5 (Table 30-1). In fact, peptic ulcers make up more than half of all acute cases of UGIB seen in the emergency department (ED). However, in inner city populations, varices and gastritis are more prevalent. In pediatric patients, gastric and duodenal ulcers, esophagitis, gastritis, esophageal varices, and Mallory-Weiss tears account for most cases of UGIB, in descending order of frequency. Contrary to this, LGIB usually produces bright red or maroon blood per rectum, known as hematochezia. Anorectal sources, such as hemorrhoids, are the most common causes of LGIB in all age groups.6 In adults, the most common sources of hematochezia are colonic diverticula and angiodysplasia.7 Other noteworthy causes include colitis caused by ischemia, infection, and inflammatory bowel disease. Major causes of LGIB in children include anorectal fissures and infectious colitis. Bleeding can also be caused by intussusception and Meckel’s diverticulum in infants and toddlers. Despite diagnostic advances for all ages, the source of GIB is not identified in 8 to 14% of patients.2
Death from exsanguination resulting from GIB is rare. However, there are two causes of GIB that may rapidly cause death if not recognized and mitigated: esophageal varices and aortoenteric fistula. The former is the single most common source of massive UGIB and has a mortality rate of 30%. The latter is caused when an abdominal aortic aneurysm or, more commonly, an aortic graft adheres to and erodes through a bowel wall. Aortoenteric fistula is a rare but rapidly fatal cause of LGIB.7 As is the case for any patient with acute massive hemorrhage, prompt consultation with a surgeon is warranted when aortoenteric fistula is a likely diagnosis.
Finally, in the differential considerations, one must determine if in fact the patient is having actual GIB. Epistaxis, dental bleeding, or red food coloring can mimic the appearance of hematemesis. Bismuth-containing medications and iron supplements can create melanotic-appearing (but guaiac-negative) stools. Vaginal bleeding, gross hematuria, and partially digested red foods (such as beets) can all be mistaken for hematochezia.8 Unless an alternative diagnosis is clearly evident, the appropriate approach is to continue with the workup for GIB.
Pivotal Findings
Medical History
1. Context: The context of the bleeding can help explain its cause. For instance, if a patient complains of bright red blood per rectum after several days of constipation and straining, that presentation suggests an anorectal source for the bleeding. Alternatively, a patient with hematemesis after several earlier episodes of retching would lead one to suspect an esophageal tear. Finally, a patient with easy bruising and recurrent gingival bleeding might suggest a long-standing, underlying coagulopathy.
2. Quantity: Efforts should also be made to quantify the amount of blood lost during the bleeding event. Patients may describe the passage of large clots, blood changing the toilet bowl water red, or simply streaks of blood on the toilet paper. The patient’s recollection of the bleed and its amount is usually poorly quantified and inaccurate. Often, the degree of bleeding is better gauged by assessing symptoms associated with significant intravascular loss such as dyspnea, lightheadedness, or chest pain.9 These findings demonstrate signs of decreased oxygen-carrying capacity that often accompanies significant blood loss and should prompt a thorough and expeditious workup.
3. Appearance: Classifying the blood as hematemesis, melena, or hematochezia provides the initial clues to the source of bleeding. Vomiting of fresh blood or blood with the appearance of coffee grounds strongly suggests an upper GI (UGI) source. The passage of melena, dark digested stools, also suggests likely UGIB. In contrast, the presence of hematochezia, bright red or maroon stools, usually signifies LGIB. There are exceptions, however. In a hemodynamically unstable patient, bright red blood per rectum can represent brisk UGIB. Hematemesis rarely can arise from a source in the lower GI (LGI) tract that is proximal to an obstruction. Although the definitive cause and location of the bleed will usually be determined by the gastroenterologist, the emergency clinician uses the history to make a rough estimate of the source and help guide the initial workup.8
4. Relevant medical history: A review of the patient’s relevant medical history and risk factors for bleeding should note whether a patient has had similar bleeding before and the location of the causative lesion (Table 30-2). This is especially important with UGIB, as the majority of these presentations are caused by rebleeding of previously identified sources. Next, identification of relevant comorbid diseases helps to risk stratify these patients in the context of their bleed. Patients with GIB and a history of coronary artery disease, congestive heart failure, liver disease, or diabetes have a higher mortality and therefore may require earlier or more extensive intervention.8 A review of the patient’s medications should pay particular attention to gastrotoxic substances, anticoagulants, and antiplatelet drugs. Medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, warfarin, clopidogrel, corticosteroids, and certain chemotherapeutic agents are known to increase the risk of GIB.5,8 In addition, reviewing the patient’s social history can identify activities that increase risk for GIB. Alcohol abuse is associated with gastritis and peptic ulcer disease. It can also result in cirrhosis, portal hypertension, and ultimately esophageal variceal bleeding. Smoking cigarettes results in slower healing and greater recurrence of ulcers. These two social habits are also closely associated with gastrointestinal malignancy—another, albeit rare, risk factor for GIB.
Physical Examination
Vital Signs.: Hypotension and tachycardia can suggest moderate hypovolemia and can be the early indicators of impending shock.5,8 Orthostatic vital signs, although frequently used, are of dubious value in determining volume status in the context of acute blood loss.
General Examination.: Eye examination may show conjunctival pallor, suggesting blood loss anemia. In similar fashion, dermatologic changes can lend supporting evidence to the presence and cause of a GI bleed. Generalized pallor in a hemodynamically stable patient might indicate the anemia of a subacute or chronic GIB; in the unstable patient, pallor might reinforce the impression of massive blood loss. Cold, clammy skin on the extremities might signal significant volume loss consistent with hemorrhagic shock. Ecchymoses or petechiae suggest a coagulopathy. Finally, jaundice, palmar erythema, or spider angiomata suggests the possibility of UGIB from esophageal varices.5
The abdomen should be carefully examined for subtle findings that can help identify the source of bleeding. Hyperactive bowel sounds are a nonspecific finding, but might indicate UGIB, as intraluminal blood is a known cathartic that can stimulate peristalsis.5 Tenderness to palpation can be seen in many cases of peptic ulcer disease. Severe, diffuse tenderness on examination warrants the consideration of bowel ischemia, mechanical obstruction, ileus, or bowel perforation. Evidence of peritonitis merits a rapid surgical consultation for possible operative management. The abdominal examination may also show further signs of portal hypertension with the presence of hepatomegaly, ascites, or caput medusae.5 A rectal examination, with determination of the type of bleeding, should be performed in most patients with GIB. The examination should include evaluation of the external anus, a digital rectal examination, and anoscopy looking to identify possible bleeding sources such as hemorrhoids, polyps, or fissures.5,7,8
Emergency Department Studies
Occult Blood and Guaiac Bedside Testing.: In patients with suspected UGIB, guaiac testing can be performed at the bedside to evaluate for occult blood even when stool appears normal. The test makes use of the pseudoperoxidase activity found in hemoglobin. When hydrogen peroxide is dripped onto the guaiac paper containing the stool sample, an oxidative reaction rapidly turns the paper blue. The test can actually be positive for up to 2 weeks after an acute bleed and thus is more useful at diagnosing chronic occult bleeding.8 False positives can be triggered by ingestions of red meat, turnips, horseradish, vitamin C, methylene blue, and bromide preparations. Iron- and bismuth-containing medications can cause dark stools that will be guaiac negative. Similar testing is available for gastric contents but can be unreliable if minor trauma from the passage of a nasogastric (NG) tube or vomiting has caused occult bleeding.
Clinical Laboratory Testing
BUN levels can be elevated in the setting of UGIB owing to the absorption of digested blood into the circulatory system during intestinal transit. The BUN can also be elevated from prerenal azotemia in the setting of hypovolemia. A BUN-to-creatinine ratio greater than 36 in the setting of no renal failure can be highly suggestive of UGIB.5,8
Electrocardiogram
Because GIB and its subsequent anemia can reduce the oxygen-carrying capacity of blood, patients should be screened for signs of myocardial ischemia. An electrocardiogram (ECG) is recommended and is especially relevant in elders and those with known coronary artery disease who are at higher risk for ischemic events.5,8–10 ECG findings consistent with myocardial ischemia should prompt immediate treatment according to current American College of Cardiology (ACC) guidelines; transfusion with packed RBCs will optimize oxygen delivery to the ischemic tissue.
Radiographic Imaging
Emergent imaging of the chest and abdomen in the ED setting is rarely indicated in the patient with acute GIB.11 Although an upright chest radiograph may show free air if hollow viscus perforation has occurred, it is insensitive and nonspecific, and abdominal computed tomography (CT) scan is the preferred imaging modality. Identification of subdiaphragmatic air and peritoneal findings on abdominal examination suggest the possibility of bowel perforation and the need for immediate surgical evaluation. Abdominal plain radiographs are of no value for patients with GIB, unless bowel obstruction is strongly suspected. Unless perforation or significant gut ischemia is suspected, CT imaging of the solid abdominal organs is not indicated and does not alter the acute management and disposition of the patient with a GIB. Multidetector CT may have a role in identifying angiodysplasia and other localized bleeding sites throughout the GI tract.4 Scintigraphy and angiography can also be used to localize the source of bleeding, and angiography may allow for therapeutic options via embolization.4,12 These imaging modalities are rarely used in the acute ED setting, and their use is often based on hospital availability and consultant preference.
Empirical Management
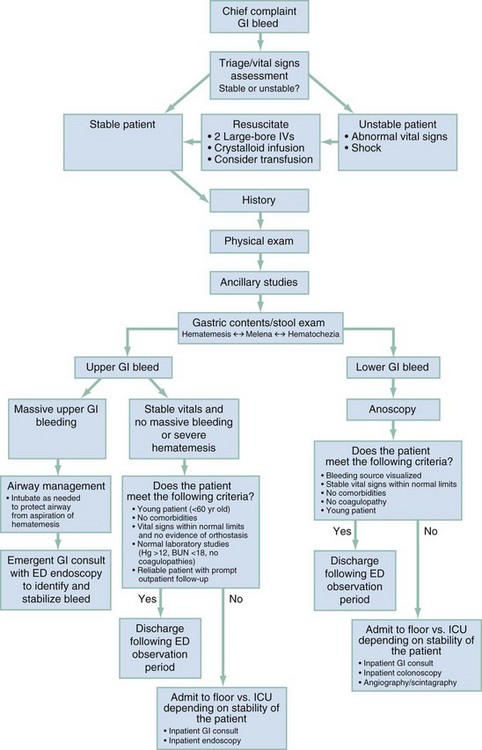
Rapid identification of the bleeding source (i.e., UGI versus LGI tract), risk stratification, resuscitation, consultation, and disposition are the integral elements of this process. Initial management includes an assessment of airway, breathing, and circulation. Massive bleeding, active hematemesis, hypoxia, severe tachypnea, or altered mental status may necessitate tracheal intubation for protection and to supplement tissue oxygenation. Figure 30-1 outlines both diagnostic and management guidelines.
Blood Product Transfusion
Continued hemodynamic instability and/or ongoing hemorrhage dictates the need for blood transfusion. Factors such as age, comorbidities (ischemic heart disease, peripheral vascular disease, or heart failure), baseline hemoglobin and hematocrit levels, and evidence of cardiac, renal, or cerebral hypoperfusion should be considered in determining transfusion quantity. Blood transfusion side effects should be weighed against adverse outcomes of anemia. A recent meta-analysis of trauma, surgical, and intensive care observational studies found that massive transfusion was associated with a higher risk of death, nosocomial infection, multiorgan dysfunction, and acute respiratory distress.13
Coagulopathy, especially in patients with underlying liver disease or those requiring massive transfusions, should be corrected promptly. Correction of coagulopathy with fresh frozen plasma (FFP) or platelets is recommended but should not delay endoscopy.14 Consensus guidelines call for the transfusion of one unit of FFP for every four units of packed RBCs transfused to replace coagulation factors.14 Patients with fewer than 50,000 platelets/µL and active bleeding may need platelet transfusion.
Nasogastric Aspiration and Lavage
Once considered a standard emergent intervention, NG aspiration with gastric lavage is generally not indicated for evaluation of GIB. The sensitivity of NG aspiration and lavage for predicting UGIB is low, and the negative likelihood ratio in patients with melena or hematochezia without hematemesis is poor.15 Fifteen percent of patients without bloody or coffee-ground material in NG aspirates are found to have high-risk lesions on endoscopy.16 NG tube placement has been associated with severe complications including aspiration, pneumothorax, perforation, and gastric lesions and is a painful procedure. On some occasions a consulting gastroenterologist may wish to place an NG tube in hopes of improving endoscopic visibility (and accuracy) by evacuating gastric blood, but absent such an indication, placement of an NG tube in patients with suspected UGIB is not recommended.
Medications
Several medications improve GIB outcomes. Infusion of high-dose proton pump inhibitors (PPIs) before endoscopy accelerates the resolution of signs of bleeding in ulcers and reduces the need for endoscopic therapy.17 The recommended dosage is an 80-mg bolus of omeprazole intravenously, followed by 8 mg/hr for 3 days. High-dose oral PPIs have been shown in Asian populations to reduce the risk of rebleeding, the need for surgery, and the risk of death, but additional data are needed to determine whether those findings are generalizable to Western patient populations.16
Somatostatin and octreotide, synthetic analogues, are splanchnic vasoconstrictors that reduce portal hypertension and the risk of persistent bleeding, rebleeding, and transfusion requirements in patients with variceal bleeding.18 Octreotide therapy should be empirically administered in patients with GIB and significant liver disease, a history of variceal bleeding, a history of alcoholism, or highly abnormal liver function tests. The recommended dose of octreotide is a 50-µg bolus followed by 50 µg/hr intravenously.
Definitive Management
Endoscopy
Upper endoscopy is the most effective diagnostic and therapeutic intervention for UGIB, achieving hemostasis in more than 90% of cases. Endoscopic hemostasis has been shown to decrease rates of rebleeding, mortality, and urgent surgery.19 Endoscopic treatments include injection therapy (e.g., saline, vasoconstrictors, sclerosing agents, tissue adhesives, or a combination thereof), thermal therapy (with the use of contact methods, such as multipolar electrocoagulation and heater probe, or noncontact methods, such as argon plasma coagulation), and mechanical therapy (principally endoscopic clips). Endoscopy within 13 hours of bleeding reduces mortality in high-risk patients.20
Disposition
A consensus panel of 34 multidisciplinary experts from 34 countries identified the following clinical predictors as high risk: age older than 65 years; shock; poor overall health status; comorbid illness; low initial hemoglobin levels; melena; transfusion requirement; fresh red blood on rectal examination, in the emesis, or in the NG aspirate; sepsis; and elevated urea, creatinine, or serum aminotransferase levels.14
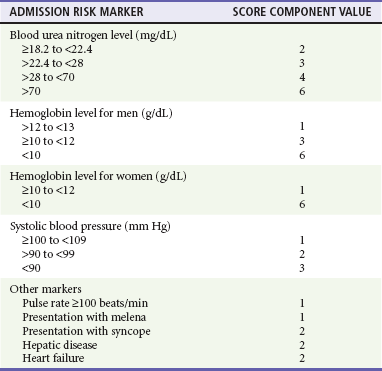
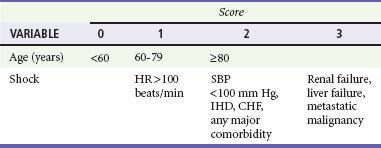
The Blatchford score (Table 30-3) and clinical (or pre-endoscopic) Rockall score (Table 30-4) use only clinical and laboratory data to risk stratify patients. In a recent retrospective review of 354 patients, the Blatchford score was 99.6% sensitive and the clinical Rockall was 90.2% sensitive at identifying patients as high risk (high-risk patients were defined as those who required a blood transfusion or endoscopic or surgical intervention during their admission).21
References
1. Lewis, JD, Bilker, WB, Brensinger, C, Farrar, JT, Strom, BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: Relationship to sales of nonsteroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol. 2002;97:2540–2549.
2. van Leerdam, ME, et al. Acute upper GI bleeding: Did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494–1499.
3. Viviane, A, Alan, BN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11:1–3.
4. Farrell, JJ, Friedman, LS. Review article: The management of lower gastrointestinal bleeding. Aliment Pharmacol Ther. 2005;21:1281–1298.
5. Cappell, MS, Friedel, D. Initial management of acute upper gastrointestinal bleeding: From initial evaluation up to gastrointestinal endoscopy. Med Clin North Am. 2008;92:491–509.
6. Manning-Dimmitt, LL, Dimmitt, SG, Wilson, GR. Diagnosis of gastrointestinal bleeding in adults. Am Fam Physician. 2005;71:1339–1346.
7. Barnert, J, Messmann, H. Management of lower gastrointestinal tract bleeding. Best Pract Res Clin Gastroenterol. 2008;22:295–312.
8. Westhoff, J. Gastrointestinal bleeding: An evidence based ED approach to risk stratification. Emerg Med Pract. 2004;6:1–20.
9. Bellotto, F, et al. Anemia and ischemia: Myocardial injury in patients with gastrointestinal bleeding. Am J Med. 2005;118:548–551.
10. Prendergast, HM, Sloan, EP, Cumpston, K, Schlichting, AB. Myocardial infarction and cardiac complications in emergency department patients admitted to the intensive care unit with gastrointestinal hemorrhage. J Emerg Med. 2005;28:19–25.
11. Andrews, AH, Lake, JM, Shorr, AF. Ineffectiveness of routine abdominal radiography in patients with gastrointestinal hemorrhage admitted to an intensive care unit. J Clin Gastroenterol. 2005;39:228–231.
12. Mellinger, JD, Bittner, JG, Edwards, MA, Bates, W, Williams, HT. Imaging of gastrointestinal bleeding. Surg Clin North Am. 2011;91:93–108.
13. Marik, PE, Corwin, HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008;36:2667–2674.
14. Barkun, AN, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113.
15. Palamidessi, N, Sinert, R, Falzon, L, Zehtabchi, S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17:126–132.
16. Gralnek, IM, Barkun, AN, Bardou, M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928–937.
17. Lau, JY, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631–1640.
18. Thabut, D, Bernard-Chabert, B. Management of acute bleeding from portal hypertension. Best Pract Res Clin Gastroenterol. 2007;21:19–29.
19. Barkun, A, Bardou, M, Marshall, JK, Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857.
20. Lim, LG, et al. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy. 2011;43:300–306.
21. Chen, IC, Hung, MS, Chiu, TF, Chen, JC, Hsiao, CT. Risk scoring systems to predict need for clinical intervention for patients with nonvariceal upper gastrointestinal tract bleeding. Am J Emerg Med. 2007;25:774–779.