CHAPTER 34 Gastrointestinal and Hepatic Complications of Solid Organ and Hematopoietic Cell Transplantation
COMPLICATIONS OF SOLID ORGAN TRANSPLANTATION
Gastrointestinal complaints after solid organ transplant (SOT) are reported in 20% to 35% of recipients, with a frequency as high as 60% reported in India.1 Most of the problems relate to graft dysfunction, adverse effects of medications, opportunistic infections, or malignancy (Table 34-1).1–3 Infectious complications remain a major source of morbidity and mortality following SOT, particularly within the first six months. During the first month following SOT, infections include those present prior to transplant (e.g., urinary tract infection), those related to technical complications of the procedure itself (e.g., biliary sepsis), or those transmitted with the allograft. Opportunistic viral, fungal, and parasitic infections are more likely to develop after the first month, with herpesvirus infections being the most common (Fig. 34-1). Several noninfectious complications can mimic infection (see Table 34-1).
Cytomegalovirus (CMV) is the predominant viral pathogen occurring within the first year after SOT, with the intestine and hepatobiliary tracts major sites of infection (see Fig. 34-1). Factors predisposing to CMV infection include the type of immunosuppression used, that is, use of antilymphocyte antibody in addition to conventional immunosuppression or maintenance mycophenolate mofetil (MMF) therapy, and the recipient’s risk of infection.4,5 CMV-negative recipients who received a CMV-positive graft are at the greatest risk of primary CMV infection.6 The peak incidence is generally four to six months after transplantation, with fever, malaise, myalgia, and occasionally cough and minor elevations of serum alanine aminotransferase (ALT).7 CMV-deoxyribonucleic acid (DNA) or antigen is generally detected in the bloodstream, but CMV can be recovered from intestinal biopsy tissue in the absence of detectable virus in the bloodstream. Either post-transplant antiviral prophylaxis or preemptive therapy with either ganciclovir or valganciclovir significantly reduces the risk of CMV disease.8–11 Valganciclovir should not be used in the setting of liver transplantation, however, because there is a higher rate of tissue-invasive disease.12 In this setting, ganciclovir is recommended.
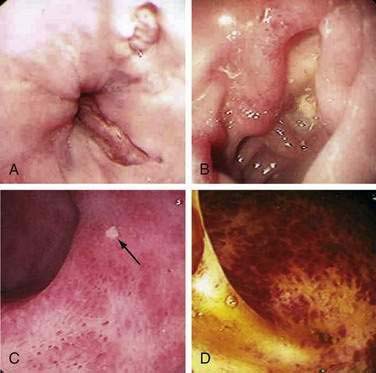
Herpes simplex virus (HSV) is the second most commonly seen viral infection and characteristically represents reactivation of latent virus within the recipient, typically two to four weeks after transplant. HSV has tropism for squamous epithelium (nose, mouth, esophagus) but can involve the intestine and liver if patients are not receiving prophylaxis (see Fig. 34-1B). Other herpesvirus infections—Epstein-Barr virus (EBV), varicella-zoster virus (VZV), and human herpesvirus 6 (HHV-6)—are less common. MMF immunotherapy may increase the risk of VZV dissemination.
Fungal infections usually develop after the first month post-transplant, particularly among patients who have discontinued fungal prophylaxis. The most common fungi are candidal species (Candida albicans, Candida tropicalis), but molds such as Aspergillus and Zygomycetes are emerging as pathogens.13 Less common infections (Nocardia, Pneumocystis, Toxoplasma; parasites such as Strongyloides) also may occur after the first month. Once beyond the first six months following SOT, opportunistic infections occur less frequently, but recipients remain at risk for community-acquired infections. Post-transplant lymphoproliferative disease continues to be a problem for SOT recipients, who require continued high-level immune suppression. B and T cell lymphomas can be seen (Fig. 34-2).
KIDNEY AND KIDNEY/PANCREAS TRANSPLANTATION
Gastrointestinal complications are among the most prevalent complications post KT, seen in up to 50% of patients, and correlate with patient long-term survival.14–16 It has been reported that KT patients who experience gastroesophageal reflux disease (GERD) or dyspepsia have an increased risk of graft loss and death, the mechanism of which is unclear.17 Graft pancreatitis and graft duodenitis generally occur early after kidney/pancreas transplant (KPT) and may lead to intra-abdominal infection.18,19 The frequency of hepatitis C virus (HCV) or hepatitis B virus (HBV) infection ranges from 5% to 66% of KT and KPT recipients, depending on country of origin. The effect of HCV on patient and graft outcomes remains controversial.20 Many have shown outcomes to be inferior in patients who are chronically infected with either HCV or HBV.21–24 HBV antiviral therapy has improved clinical outcome, but HCV antiviral therapy with interferon alpha and ribavirin cannot be used in the post-KT setting because of increased risk of allograft rejection. Cirrhotic patients who undergo KT have a significantly worse 10-year survival (about 20% to 30%).
Gastrointestinal CMV infection is seen in about 7% of KT and KPT recipients, with pancreas recipients at greater risk due to higher levels of immunosuppression.25 About 4% develop intestinal fungal infections, most often with candidal species. HSV infection post KT is generally asymptomatic and self-limited, presenting as stomatitis, mononucleosis, hepatitis, or pneumonia.26 Cholecystitis is seen in KT recipients, and the incidence is higher among diabetic patients.27
Traditionally gastrointestinal hemorrhage occurred in up to 20% of KT recipients and had a high mortality.28 Many KT recipients with gastroduodenal ulcers have no history of gastroduodenal disease. Approximately 50%, however, have complaints of dyspepsia, and about 30% are colonized with Helicobacter pylori.29 With decreased use of glucocorticoids and use of proton pump inhibitors, ulcer formation and hemorrhage are rare.3 Renal recipients are at particular risk for the development of intestinal ischemia compared with other SOT recipients. However, the incidence is low (<5%) and the etiology is multifactorial.30 Recipients with polycystic kidney disease more often develop intestinal ischemia and obstruction.31 Intestinal ischemia in this setting carries a high mortality. Ischemia should be considered in KT recipients with abdominal pain, particularly older patients (>40 years of age) who have received a cadaveric kidney.30
LIVER TRANSPLANTATION
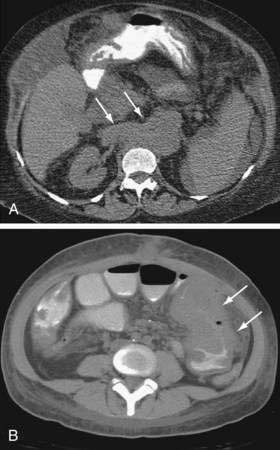
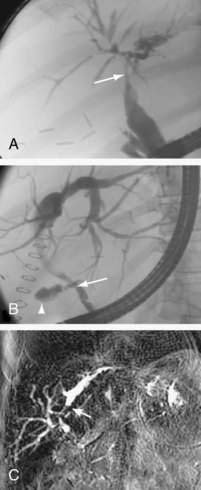
Gastrointestinal complications unique to orthotopic liver transplant (OLT) are generally related to the surgery itself, that is, hemorrhage, hepatic arterial stenosis or thrombosis, biliary tract dysfunction, bowel perforation, bowel obstruction, and gastrointestinal bleeding.32 Hepatic artery thrombosis presents with a spectrum of consequences, ranging from mildly elevated liver enzymes to fulminant hepatic failure. Post-OLT, the biliary tree receives its entire blood supply from the hepatic artery, thus loss of flow results in bile duct necrosis and leakage with development of bilomas and abscesses (Fig. 34-3A and B). Gradual loss of hepatic arterial flow can result in ductopenia, which is indistinguishable from ductopenic rejection. Portal vein thrombosis can lead to hepatic ischemia and severe hepatic dysfunction if it occurs early in the post-transplant course; later, signs of portal hypertension develop. Rarely, hepatic vein thrombosis and inferior vena cava thrombosis/stenosis can create a Budd-Chiari–like syndrome.
Biliary leakage and stricture formation, generally at the anastomotic site, are the most common biliary abnormalities seen following OLT (see Fig. 34-3A and B).33 Anastomotic strictures generally occur within two to six months post OLT, but can occur in the newly transplanted patient as well. Strictures and leaks in patients with duct-to-duct anastomoses are often amenable to endoscopic therapy, whereas those with choledochojejunostomies may require percutaneous or surgical correction. The incidence of biliary cast syndrome has decreased to 5% to 20%, and generally occurs within the first year post OLT.34 Clinical factors associated with development of biliary casts include hepatic ischemia and biliary strictures. Endoscopic and percutaneous therapy is successful in up to 70%, but surgical intervention may be required, and mortality is reported at 10% to 30%.35
CMV hepatitis is more severe in OLT recipients than in recipients of other organs.6,36 Patients often have elevations in serum aminotransferases, which can be confused with rejection, and therefore liver biopsy is essential for differentiation. The diagnosis can usually be confirmed by detection of CMV in the bloodstream. Asymptomatic low-level CMV viremia does not require antiviral therapy.37 Liver transplant recipients more often develop invasive fungal infections than other SOT recipients, with a high mortality. In the absence of prophylaxis, intestinal colonization with Candida is nearly universal post OLT, and Candida accounts for the majority of all invasive fungal infections following OLT.38 A serum galactomannan assay is useful for detecting mold infections.39,40
There is a risk for recurrence of the underlying liver disease following OLT, including HCV, HBV, autoimmune hepatitis, nonalcoholic steatohepatitis (NASH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC) (see Fig. 34-3C).41–43 Recurrence of HCV in the liver allograft is nearly universal, with 75% developing signs of liver damage and 25% progressing to cirrhosis within 5 years, which leads to increased graft loss.44–46 HBV recurrence may be prevented with the use of hepatitis B immunoglobulin (HBIG) and antiviral medications. PBC recurs in about 26% of patients post-liver transplant.41
HEART, LUNG, AND HEART/LUNG TRANSPLANTATION
Up to half of heart (HT), lung (LT), and heart-lung transplantation (HLT) recipients experience gastrointestinal complications, with up to 20% requiring surgery.47,48 The most common complications include diarrhea, GERD, dyspepsia, nausea and vomiting, abdominal pain, pancreatitis, herpesvirus infections (especially CMV), cholelithiasis, ulcers, and hepatobiliary disease.47–49 GERD and gastroparesis are particularly problematic after LT or HLT and may be related to medications and vagal nerve injury during the operation.48,50,51 Symptomatic gastroparesis has been described in 25% of LT recipients and up to 80% in HLT recipients.52,53 The course is often waxing and waning, suggesting a neuropathic, infectious (CMV), or medication-induced etiology, but ultimately there is partial or complete remission.52,54 Recipients with GERD and/or gastroparesis are at particular risk for the development of obliterative bronchiolitis, which significantly threatens the longevity of LT recipients.51,52 Proton pump inhibitors can be used to help control reflux; however, if reflux disease is unremitting, laparoscopic fundoplication may be successful.55–57
LT recipients may develop giant gastric ulcers (>3 cm in diameter) that occur despite routine use of acid suppression. These ulcers carry significant morbidity and mortality, and are more often associated with bilateral LT, high-dose nonsteroidal anti-inflammatory drugs (NSAIDs) after transplant, acute rejection requiring high-dose glucocorticoids, and cyclosporine immunosuppression. For this reason, some authors believe NSAIDs should not be used in the post-transplant setting. Recipients of LT and HT more often develop CMV infection (15% to 25%) than other SOT recipients. Generally CMV infection presents as pneumonitis, but gastrointestinal CMV infection remains a major cause of morbidity (see Fig. 34-1). LT and HLT recipients have the highest incidence of fungal infection in the SOT setting in which Aspergillus, not Candida species, predominates.
Patients undergoing LT for cystic fibrosis experience a unique set of gastrointestinal complications.58 Pancreatic insufficiency, a marker for severe cystic fibrosis, is common. Cystic fibrosis–induced secondary biliary cirrhosis can complicate absorption of immunosuppressive medications such as cyclosporine. If severe liver disease is detected prior to LT, lung-liver transplant should be considered. Distal intestinal obstruction syndrome occurs in about 20% and is similar to the incidence in the nontransplant setting. Cystic fibrosis patients also may experience cholecystitis, peptic ulcer disease, and GERD.
Primary HCV infection following HT leads to significantly decreased one- and three-year survival. However, acquisition of HBV following HT does not appear to affect survival, at least up to five years.59,60
INTESTINAL TRANSPLANTATION
Most complications are related to underlying diseases, graft rejection, intestinal ischemia, and anastomotic leaks. Bacterial and fungal infections are common, often associated with mucosal disruption following surgery, but a source may not be identifiable. Two types of malignancy related to intense immunosuppression have been reported, EBV lymphoproliferative disease (EBV-LPD) and de novo cancers of nonlymphomatous origin.61,62 Surveillance for EBV DNA and preemptive treatment reduces the frequency of lymphoproliferative disease (see following). Altered intestinal motility and anorexia have been reported.
A PROBLEM-ORIENTED APPROACH TO DIAGNOSIS IN SOLID ORGAN TRANSPLANT RECIPIENTS
Upper Gastrointestinal Symptoms and Signs
The approach to SOT patients with esophageal or gastric symptoms is influenced by a high frequency of nonspecific symptoms as harbingers of serious infection (for example, CMV infection presenting as nausea and vomiting) and by the rapidity with which disease can progress. GERD is the most common cause of heartburn and midchest pain, particularly following lung transplantation (see lung transplant earlier), but viral and fungal esophagitis may underlie these symptoms, particularly after chemoprophylaxis has been discontinued. Candidal esophagitis is seen with particular frequency in those with diabetes; other risk factors include use of broad-spectrum antibiotics, high-dose immunosuppression, and the presence of a Roux-en-Y anastomosis in liver transplant recipients. Severe necrotizing fungal esophagitis can lead to perforation, which can have a fatal outcome in up to one third of patients. Odynophagia, dysphagia, or hematemesis should lead to consideration of an esophageal infection; herpesviruses (CMV, HSV) and fungal species (Candida) are responsible for the largest proportion, but unusual organisms can be seen.63 Dysphagia secondary to pill esophagitis may develop in SOT recipients, caused by antibiotics, antivirals, potassium chloride, bisphosphonates, and NSAIDs. Esophageal strictures following severe esophageal infection have been reported and may present a long time after eradication of the organism.
Anorexia, nausea, and/or vomiting are common following SOT, particularly early in the post-transplant course.15,47,48 These symptoms are often related to herpesvirus infections or to medications (including immunosuppressive drugs), and thus, endoscopic evaluation is necessary for diagnosis in most patients. Tacrolimus (Prograf) is a macrolide lactone that can cause nausea, abdominal pain, and diarrhea, often leading to anorexia, food aversion, and weight loss. These side effects are dose dependent and can be managed with dose reduction or, more rarely, drug discontinuation. Sirolimus (Rapamune), a newer macrolide immunosuppressant, has a GI side effect profile similar to tacrolimus. MMF (CellCept) is an inhibitor of nucleic acid synthesis with gastrointestinal side effects of nausea, vomiting, and diarrhea, often requiring dosing modifications. A formulation of mycophenolic acid delayed-release tablets (Myfortic) appears to have significantly fewer gastrointestinal side effects with similar therapeutic efficacy.64 Less common causes of anorexia and nausea include pancreatitis, cholecystitis, or cystitis. Rarely following SOT, graft-versus-host disease (GVHD) presents with fever, skin rash, and gastrointestinal symptoms, particularly nausea, vomiting, and diarrhea.65,66 Endoscopic evaluation with biopsy is essential if GVHD is suspected and skin lesions are absent, recognizing that other conditions such as viral infections and drug reactions can have a GHVD-like histologic pattern.67 Symptomatic gastroparesis is frequently seen in the setting of lung transplant but is less often reported in the setting of other solid organ transplant.52 CMV and VZV may rarely involve intestinal neural plexuses, leading to intestinal dilation or gastroparesis. H. pylori infection may be associated with symptomatic dyspepsia, gastritis, and gastroduodenal ulceration, but there is no relationship between the use or degree of immunosuppression and H. pylori colonization; its incidence is similar to that seen in the nontransplant setting.2
Diarrhea and Constipation
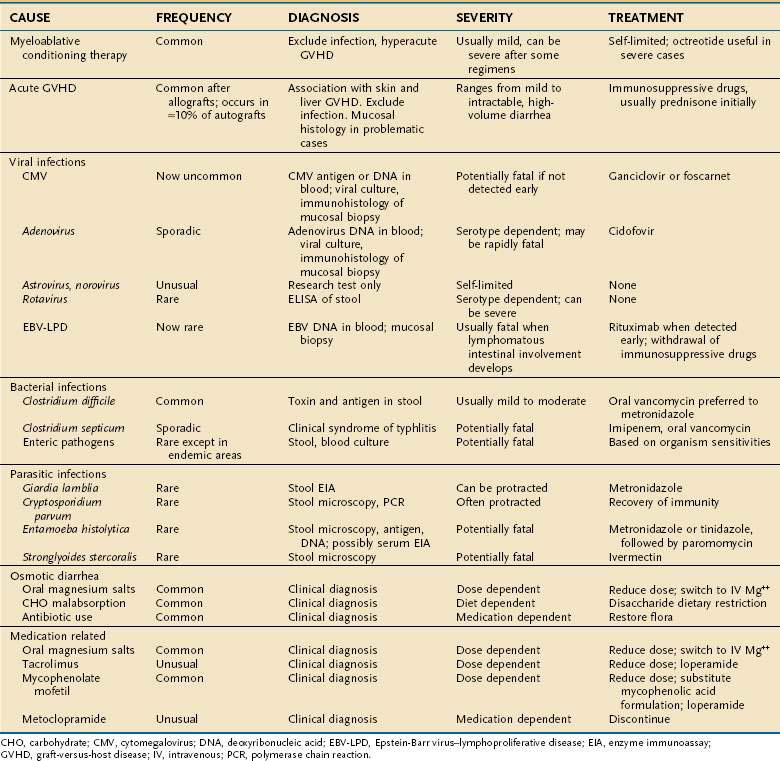
Colonic and small bowel complications (diverticulitis, ischemic colitis, malignancy, and infections) have been reported to occur following all types of SOT. Early in the post-transplant setting, infections predominate. Diarrhea is commonly infectious and may be accompanied by fever,68 abdominal pain (46%), nausea (32%) and vomiting (22%).69,70 The microbes usually responsible are CMV and Clostridium difficile, but the literature describes a wide range of organisms in SOT recipients, particularly when they are cared for in infection-endemic areas (for example, adenovirus, rotavirus, coxsackievirus, bacterial enteric pathogens, enterohemorrhagic Escherichia coli, Yersinia enterocolitica, Giardia lamblia, Candida species, cryptosporidium, Enterocytozoon bieneusi, Isospora belli, and Strongyloides stercoralis).70 Diagnosis can be made by examination of stool specimens in nearly all cases; the exceptions are CMV, certain parasites, and EBV-LPD. Small intestinal involvement with CMV often causes profuse watery diarrhea with protein-losing enteropathy, particularly if the diagnosis is delayed.71 Colonic involvement may appear as an inflammatory colitis resulting in bloody diarrhea and is often associated with fever, abdominal distention, and pain.72 Diagnosis of CMV may require mucosal biopsy, particularly if blood specimens are negative for CMV DNA or antigen. C. difficile infection may present with a more severe course post SOT, and patients with fulminant colitis and toxic megacolon require prompt surgical intervention to prevent perforation and peritonitis.70 Signs of colitis may be subtle due to concomitant immunosuppression. Only about 70% of patients respond to treatment with metronidazole; persistent and more severe cases require oral vancomycin. Recurrence may develop in up to 20% of cases.73 The use of probiotics (e.g., Saccharomyces boulardii) in SOT recipients remains controversial because there have been reports of yeast dissemination and infection in the immunocompromised host.74 Intestinal fungal infections can be seen in up to 25% of SOT recipients. In the absence of prophylaxis, intestinal fungal overgrowth and diarrhea can result from antibiotic use or intestinal dysmotility. Common parasitic infections also must be considered in an immunocompromised host, particularly in areas of high endemicity. The protozoa are a much less frequent cause of acute diarrhea post SOT. Microsporidia (E. bieneusi) is a more rarely reported cause of chronic diarrhea, perhaps reflecting the fact that it is often not sought out in the post-SOT setting. Clinically, patients with this infection experience fatigue, intermittent diarrhea, and weight loss. There are no clearly effective therapies for E. bieneusi. Symptoms of colitis or toxic megacolon are most often associated with infection, but in up to 20% of cases, no clear etiology can be found.70,75 Early recognition, diagnosis, and treatment of colitis can decrease disease-associated mortality. Eosinophilic colitis with diarrhea has been reported with the use of tacrolimus and cyclosporine. Histologically this is characterized by eosinophilic colonic infiltrates and peripheral eosinophilia. Elevated serum immunoglobulin E may be present in some patients.
Drug-related diarrhea is seen in up to a third of SOT patients, most commonly with tacrolimus or sirolimus.70,76 MMF causes watery diarrhea in up to 30% of patients, and may require dose reduction or discontinuation. The mechanism of MMF-induced diarrhea is unclear. There have also been reports of altered tacrolimus metabolism and absorption in patients suffering from MMF-induced or other causes of chronic diarrhea. Antithymocyte globulin (ATG) and anti–T cell antibody (OKT3) therapies are associated with diarrhea, which predictably lasts for three to four days and resolves spontaneously. Most cases of immunosuppressant-induced diarrhea can be managed with dose manipulation, but some are so severe that discontinuation of the immunosuppressant is required. Diarrhea also can be caused by magnesium-containing preparations prescribed to correct renal magnesium wasting and by antibiotics prescribed either prophylactically or therapeutically. Noninfectious diarrhea has been reported to increase the risk of graft loss and mortality.76
Constipation is seen in less mobile recipients who are receiving certain medications (e.g., narcotics, calcium- and aluminum-containing antacids, anticholinergics). This is generally responsive to increased patient mobility, decreased use of narcotics, use of methylnaltrexone,77 and therapy with laxatives and senna.
Abdominal Pain
Abdominal complications are common, affecting up to 30% of patients following SOT.78 Symptoms may be mild despite the presence of life-threatening complications. All patients with abdominal pain should be aggressively evaluated, with particular attention to whether the patient requires urgent surgery or a specific medical treatment. Most recipients with abdominal pain will not need surgery.
The intra-abdominal conditions presenting with pain that require urgent surgery are abscess, perforation, severe colitis, appendicitis, intestinal obstruction, intestinal ischemia, and acute cholecystitis. These disorders may appear in the early post-transplant period. Immunosuppression may mask symptoms and suppress the host response, leading to a delay in diagnosis and increase in mortality. Most transplant patients with acute appendicitis have right lower quadrant pain. Overall, intestinal perforation occurs in less than 5% of SOT recipients, although the incidence may be slightly higher in the setting of lung transplant.14,79 Perforation may occur spontaneously without clear etiology, but it is associated with colon diverticula in up to two thirds of cases (particularly renal transplant recipients) and ischemia in 15%. Perforation, especially of a diverticulum, carries a mortality of up to 55%.30,72 Risk factors for the development of colonic perforation include diverticular disease, immunosuppression (particularly glucocorticoids), CMV infection, fungal infections (e.g., mucormycosis), unrecognized lymphoma (EBV-LPD), colon cancer, and ischemia.2,72 Abdominal radiographs and helical computed tomography (CT) scans can confirm the presence of perforation, but may not reveal its source before surgery. Diverticular perforation is especially common after renal transplant, often leading to abscess formation and fistulization, sometimes without causing severe pain or findings of peritonitis. Pretransplant colonic screening for diverticulosis in patients less than 50 years of age has not been shown to predict post-transplant colonic perforations. SOT recipients also are at increased risk for the development of cholelithiasis.80 Factors related to gallstones include cyclosporine, obesity, and cystic fibrosis as an underlying disorder. Abdominal pain is frequently associated with tissue-invasive CMV disease. Although generally producing a diffuse pattern of mucosal edema, CMV may also cause focal ulceration, perforation, high-grade stricture, and intestinal obstruction (see Fig. 34-1). The first manifestation of disseminated VZV infection is often severe abdominal pain related to intestinal pseudo-obstruction and visceral neuropathy. Early treatment of both CMV and VZV infection results in improved survival.
Abdominal pain may also be a manifestation of transplant-related complications that do not usually have a dire outcome. Pain has been reported with oral tacrolimus, sirolimus, and MMF. Abdominal pain is seen in up to 19% of patients taking MMF and can significantly limit its use.81 The etiology of MMF-induced pain been postulated to involve local irritant and inflammatory effects as well as interference with rapidly dividing intestinal cells, a hypothesis supported by a study showing fewer gastrointestinal complications with delayed-release mycophenolic acid (Myfortic), than with MMF.64 Narcotic-induced ileus is common after surgery. Care must be taken to rule out an infectious etiology such as CMV or VZV, both of which can involve the intestinal nerve plexuses.2 Noninfectious pseudo-obstruction often can be managed conservatively with nasogastric decompression, vigorous correction of electrolyte imbalance, and withdrawal of opiates. Opioid-related gut symptoms can also be blocked with the use of methylnaltrexone while not interfering with central pain relief.77 Neostigmine can be safely used for treatment of intestinal pseudo-obstruction in the transplant setting.72 Surgical intervention may be required in the setting of massive colon dilation. Acute pancreatitis has been reported in 1% to 2% of renal transplant recipients, up to 6% of liver transplant recipients, and up to 18% of heart transplant recipients; it may have a fatal outcome.82 Acute pancreatitis is associated with CMV infection, hypercalcemia, cholelithiasis, biliary manipulation, malignancy, recent alcohol ingestion, and medications such as azathioprine, cyclosporine, tacrolimus, and glucocorticoids. Treatment of pancreatitis in the post-transplant setting is identical to that in the non-transplant setting, except for the need to exclude viral infection and some immune suppressive medications.
Gastrointestinal Bleeding
When gastrointestinal bleeding occurs, it is often secondary to infectious ulcers. Noninfectious causes of hemorrhage include NSAID gastroduodenal ulcers, diverticular bleeding, anastomotic bleeding, and ischemic colitis. The current incidence of gastroduodenal ulcer disease in the transplant population is about 5%, with perforation rates of less than 1%.3 Prophylaxis with histamine (H2)-receptor antagonists or proton pump inhibitors decreases the occurrence of ulcer disease in this population; both therapies are equally effective.3 Patients infected with H. pylori prior to transplantation are more likely to develop peptic ulcer disease following transplant.83 In the absence of effective antiviral prophylaxis, viral ulcerations are the most common cause of intestinal bleeding. HSV-associated esophageal ulcers may present with severe bleeding even in the absence of esophageal symptoms. CMV can lead to ulceration throughout the entire intestinal tract. Although CMV esophageal ulcers are usually shallow (see Fig. 34-1A), ulcers elsewhere can be deep, erode into vessels, and lead to severe bleeding. CMV can also cause diffuse inflammation similar to that seen in idiopathic inflammatory bowel disease (see Fig. 34-1C and D). VZV and EBV are much less often associated with gastrointestinal (GI) bleeding. Although EBV itself does not cause mucosal ulceration, EBV-LPD can form mucosal tumors that can ulcerate and bleed (see Fig. 34-2). Massive bleeding has been reported in the setting of invasive fungal infection.
Gastrointestinal Malignancy
Post-transplant lymphoproliferative disorders (PTLDs), lymphoid proliferations, or lymphomas associated with EBV infection (EBV-LPD) occur in 1% to 20% of transplant recipients.84 Although most PTLDs are of B cell origin, T cell lymphoma has been reported. EBV reactivation generally presents in the early post-transplant setting as a mononucleosis-like syndrome with diffuse adenopathy and fever; detection of EBV DNA in the bloodstream may allow preemptive therapy, with lower doses of immune suppression or treatment with rituximab.85 PTLD manifesting later than a year after transplant is more insidious, often presenting with extranodal disease or visceral involvement. Gastrointestinal PTLD can present with diarrhea, intestinal obstruction (see Fig. 34-2B), bleeding, or perforation. Mucosa-associated lymphoid tissue-type (MALT) lymphomas have also been reported in the post-transplant setting.86 Fortunately, they often respond to reduction in immunosuppression, antibiotics (if associated with H. pylori), surgery, or chemotherapy.
The risk of cancer in long-lived transplant recipients is higher than in the general population, particularly for lymphomas, skin cancers, colorectal and anal cancers, and Kaposi’s sarcoma.87 Patients who underwent liver transplant for cirrhosis secondary to primary sclerosing cholangitis are at high risk for the development of colonic dysplasia and diffuse colon cancer related to underlying ulcerative colitis.88 If severe colonic dysplasia is discovered, colectomy can be performed safely as early as 10 to 12 weeks following transplant.
Hepatobiliary Complications
Bacterial sepsis can have profound effects on liver function, with severe cholestasis the most common finding (a syndrome called cholangitis lenta).89 CMV infection may lead to elevations in hepatic enzymes with either a cholestatic or hepatocellular picture. CMV hepatitis is more frequent and severe in liver transplant recipients, compared with recipients of other organs.6 VZV and HSV infection can lead to hepatitis and fulminant liver failure.26 EBV hepatitis is seen in 2% to 3% of patients after SOT but is generally mild. Primary or recurrent disease with either HCV or HBV can lead to liver disease in the post-transplant setting. These viruses may be transmitted to the recipient by any solid organ from the donor. Immunosuppression leads to a marked increase in HCV titers and in some cases, to aggressive hepatic disease post-transplant, with progression to cirrhosis within 3 to 10 years.90 The results of treatment of hepatitis C in the post-transplant setting with interferon-α (INF-α)–based regimens are disappointing. Sustained virologic clearance can be achieved in 10% to 30% of post-LT recipients, but use of interferon-based therapies is limited because of side effects.91 HCV can be successfully treated in renal transplant recipients, but because the rate of renal graft failure related to INF-α is unacceptable, treatment should not be attempted. It is unknown whether interferon alpha therapy for HCV in HT or LT recipients carries an increased risk of graft failure and its use is not recommended.92 Chronic HBV carriers (hepatitis B surface antigen-positive recipients) may develop a hepatitis flare following transplant, but disease often responds to antiviral agents.
Organ transplant recipients, particularly following LT, are at high risk for biliary tract disease. Presentation includes acalculous cholecystitis, gallbladder sludge, thickened gallbladder wall, dilated bile ducts, or cholelithiasis.80 Gallbladder and biliary disease necessitating cholecystectomy occurs in about 1% to 6% of transplant recipients. Emergent cholecystectomy in the post-transplant setting carries a high mortality (29%).80 However, pretransplant screening for gallstones and prophylactic cholecystectomy remain controversial. The etiology of biliary tract disease is multifactorial, including obesity, use of total parenteral nutrition, fasting, biliary strictures, and medications. Cyclosporine is excreted in the bile, where it may precipitate and has been implicated in an increased incidence of cholelithiasis and cholangitis.27,93 Some centers recommend that biliary calculi be removed prior to transplantation or immediately on discovery after transplantation, but this recommendation is not universal.
COMPLICATIONS OF HEMATOPOIETIC CELL TRANSPLANTATION
Hematopoietic cell transplantation (HCT) uses one of three sources of hematopoietic and immune cells: bone marrow, peripheral blood stem cell, or cord blood.94 Transplanted cells can be one’s own (autologous transplant), from an identical twin (syngeneic transplant), or from another person (allogeneic transplant). Allogeneic cells can come from a sibling who is human-leukocyte-antigen (HLA) matched with the recipient, or from another family member, or from an HLA-matched unrelated donor, or from an HLA-mismatched unrelated donor (as with cord blood donors). HCT differs from solid organ transplant in three important ways: (1) the indication for HCT often involves a potentially fatal malignancy or inborn error of metabolism; (2) preparation for HCT requires either high-dose myeloablative therapy or intense immune suppression, resulting in extreme susceptibility to infection and, with some preparative regimens, organ damage; and (3) recipients of allogeneic donor cells commonly develop acute and chronic GVHD. HCT patients face combined morbidity from the toxicity of chemotherapy drugs, infections, acute and chronic GVHD, and recurrent malignancy.94
EVALUATION OF GASTROINTESTINAL AND LIVER PROBLEMS BEFORE TRANSPLANTATION
Ulcers and Tumors in the Gastrointestinal Tract
Mucosal ulcerations may bleed profusely when platelet counts drop after HCT, and in immunocompromised patients, ulcers may have an infectious etiology (e.g., CMV, HSV or fungal infection) that requires specific antimicrobial treatment.95 Intestinal ulcerations should be healed before the start of conditioning therapy. CMV, Entamoeba histolytica and C. difficile are causes of colonic ulceration that may mimic idiopathic inflammatory bowel disease. Selected patients with ulcerative colitis and Crohn’s disease have undergone allogeneic and autologous HCT without complications of bleeding, perforation, or dissemination of microorganisms.96,97 The presence of fecal occult blood should prompt colonoscopy and upper endoscopy before HCT, especially in patients older than 50 years. Endoscopic biopsy may be required for staging some forms of lymphoma with a predilection for gut involvement, such as mantle cell lymphoma.
Diarrhea
Patients with diarrhea should be investigated for organisms that may cause morbidity during the period of immunosuppression after HCT (e.g., E. histolytica, strongyloides, G. lamblia, cryptosporidia, clostridial infections, CMV, rotavirus, adenovirus).98 Cryptosporidiosis may be resistant to therapy in an immunosuppressed patient,99 but restoration of normal immunity after allogeneic HCT can result in clearance of Cryptosporidia.100 Similarly, protracted diarrhea related to immune dysregulation can be treated with allogeneic HCT, for example, by restoration of T regulatory cells in children with immundysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome.101 Typhlitis is a syndrome of cecal edema, mucosal friability, and ulceration in neutropenic patients, often associated with polymicrobial sepsis; its cause is usually an intestinal clostridial infection, particularly with Clostridium septicum.102 After treatment, the risk of post-HCT typhlitis is no different than that of other patients.
Fungal Liver Infections
Diagnosis depends on liver imaging (using high-resolution CT or magnetic resonance imaging [MRI]) in conjunction with circulating fungal biomarkers (galactomannan and glucan assays),103,104 polymerase chain reaction (PCR), or culture of liver biopsy material. Therapy with newer antifungal drugs (caspofungin or azole drugs) should be continued through HCT until engraftment is established, which can effect resolution of intractable fungal liver abscesses.105–107
Viral Hepatitis in Allogeneic Hematopoietic Cell Transplant Donors
Donors who are viremic with HBV or HCV will transmit virus to their recipients.108 When two equally HLA-matched donors are available, the uninfected donor is preferred. If the more suitable donor has chronic hepatitis B, it may be possible to prevent passage of virus by treating that donor.109,110 HBV persisting in donor peripheral blood stem cells may have to be eliminated to prevent passage.110,111 HBsAg negative/anti-HBc–positive donors can be used if their serum and peripheral blood stem cells are HBV DNA negative. A donor who is naturally anti-HBs positive may be the preferred donor if the recipient is HBsAg positive or anti-HBc positive because adoptive transfer of immunity can lead to clearance of virus.112 If a donor is infected by HCV and if time permits, treatment of the donor prior to harvest of donor cells may render them nonviremic, and much less likely to transmit infection.113 If HCV is transmitted, the acute phase of HCV infection may cause elevated liver enzymes at two to three months post HCT, after recovery of T cell function; after 10 years the outcome is no different than in transplant recipients without hepatitis C infection.114 In the long term, HCV-infected transplant recipients are at risk for development of cirrhosis and hepatocellular carcinoma.115
Chronic Liver Disease in Candidates for Hematopoietic Cell Transplantation
The risks faced by patients with fibroinflammatory liver disease include fatal sinusoidal obstruction syndrome following some myeloablative regimens and fulminant hepatitis B. In the absence of antiviral prophylaxis, fatal fulminant hepatitis B develops in approximately 15% of hepatitis B-infected HCT recipients.108 There is a 35% risk of post-HCT reactivation of HBV in patients with isolated anti-HBc antibodies, usually during treatment for acute GVHD.116 Severe hepatitis B has been seen in anti–HBc/anti–HBs-positive patients and in a patient with occult hepatitis B.117 Prior to HCT, liver biopsy should be considered if there is a clinical suspicion of cirrhosis or extensive fibrosis, as these are relative contraindications to transplant even with reduced intensity conditioning regimens.118 A reduced intensity conditioning regimen may allow congenitally immunodeficient children with chronic liver disease to be transplanted successfully, with resolution of liver disease.119
Recent Liver Dysfunction in Candidates for Hematopoietic Cell Transplantation
Patients who come to HCT following recent chemotherapy or radiation therapy that damaged the liver may be at significant risk from additional liver insults after HCT.120 These patients must be carefully evaluated with regard to the risk posed by liver-toxic conditioning regimens, particularly those containing cyclophosphamide (CY) or total body irradiation.121–123 Imatinib (Gleevec) and gemtuzumab ozogamicin (Mylotarg) deserve special mention in this context. Iminatib mesylate (and similar drugs) may cause acute hepatocellular necrosis and multiacinar collapse, with eventual healing by focal fibrosis124,125; we have successfully given CY-based myeloablative conditioning to patients who recovered from the acute injury but who had patchy fibrosis on biopsy. Gemtuzumab ozogamicin causes sinusoidal liver injury in 3% to 15% of patients126 and is a risk factor for fatal sinusoidal obstruction syndrome (SOS) if given in proximity to a liver-toxic myeloablative regimen.126,127
Gallbladder and Bile Duct Stones
HCT candidates with asymptomatic gallstones (incidentally discovered during CT or ultrasonography) do not require operative intervention.128 Patients with symptomatic cholelithiasis or stones in the common duct are at risk for biliary sepsis after HCT.
Iron Overload
HCT candidates with diseases such as thalassemia, aplastic anemia, and chronic leukemia or lymphoma may come to HCT with marked hepatic siderosis. The amount of liver iron can be accurately determined by iron-specific MRI (FerriScan or T2*).129 In patients with extreme iron overload, effective pre-HCT iron chelation therapy improves post-HCT survival.130 Although some studies suggest an association between excess tissue iron stores and regimen-related toxicity, others have failed to demonstrate this. In most patients the quantitation of tissue iron stores and a decision about iron mobilization can be deferred until after recovery from HCT.
PROBLEMS FROM TRANSPLANT THROUGH DAY 200
Anorexia, Nausea, and Vomiting
Myeloablative conditioning therapy makes most patients nauseated and anorexic,131 findings associated with delayed gastric emptying.132 Serotonin-antagonist drugs are very effective in relieving symptoms during chemotherapy. Mucositis caused by myeloablative conditioning therapy may lead to oral mucosal swelling, pain, and in severe cases sloughing of pharyngeal and esophageal epithelium, intense gagging, an inability to swallow, vomiting, retrosternal pain, and airway obstruction. Opioid therapy is effective in relieving pain but can lead to gastric stasis and intestinal ileus with worse anorexia and vomiting. Methylnaltrexone, a peripheral mu-opioid receptor antagonist, can block gut opioid symptoms while allowing pain relief.77 Appetite and food intake may remain poor for up to three weeks after myeloablative therapy, an effect that is mediated by cytokines that affect appetite (interleukin [IL]-2, IL-6, tumor necrosis factor-α [TNF-α]).131 Some regimens, notably those that contain very high-dose melphalan or multiple alkylating agents, may cause unusually severe intestinal mucosal necrosis and anorexia.
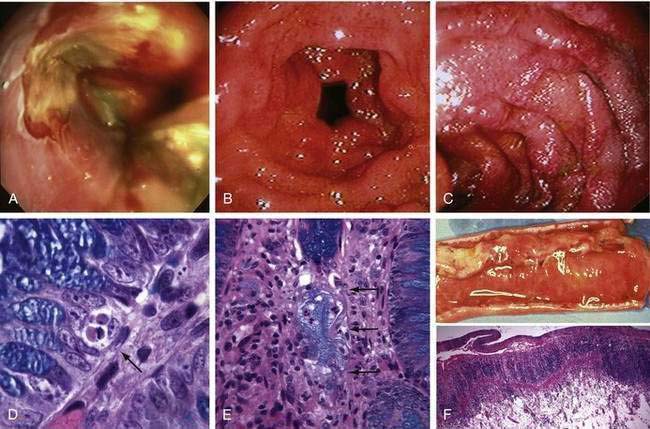
An early sign of acute GVHD is loss of appetite, often followed by nausea and vomiting. Before day 20, these symptoms overlap with effects of conditioning therapy. After day 20, more than 80% of patients with intractable anorexia, nausea, or vomiting will have gastric and duodenal GVHD as the sole explanation.133 Endoscopy shows edema of the gastric antral and duodenal mucosa, patchy erythema, and bilious gastric fluid, and histology demonstrates epithelial cell apoptosis and drop-out, often with localized lymphocytic infiltrates (Fig. 34-4).134 However, there is a false-negative rate with histology because of the patchiness of GVHD lesions. Immunosuppressive therapy using a 10-day course of prednisone 1 mg/kg/day plus oral beclomethasone dipropionate 8 mg/day is an effective therapy that avoids prolonged systemic immunosuppression and results in better outcomes.135,136 Recipients of autologous grafts may also develop a syndrome of anorexia, nausea, and vomiting that is associated with diffuse gastric edema and erythema.137 Gastric histology shows typical GVHD. Symptoms respond to a 10-day course of prednisone 1 mg/kg/day. Endoscopy also serves to rule out intestinal infection with herpesviruses, bacteria, and fungi. CMV infection of the esophagus and upper intestine accounted for a third of patients with unexplained nausea and vomiting during the pre-ganciclovir era. These infections are usually diagnosed between days 50 and 150,138 but can present earlier if patients have activated CMV before transplant.95 Gut CMV infections can be seen in the absence of evidence of CMV in the bloodstream. HSV esophagitis may present similarly in patients not receiving prophylactic acyclovir. Intestinal CMV and HSV infections are now rare. Fungal esophagitis may cause anorexia but not the incessant vomiting often seen with GVHD or herpesvirus infections. Studies of gastric emptying and myeloelectric activity in HCT patients have shown that symptoms of nausea and vomiting are frequently accompanied by retention of radionuclide meals and disordered electrical activity.139 Promotility agents such as metoclopramide, domperidone, and low-dose erythromycin are occasionally useful, but in patients with persistent symptoms, endoscopic evaluation should precede empirical promotility therapy. Anorexia and vomiting may also be manifestations of central nervous system (CNS) disease; other neurologic signs and symptoms usually dominate the clinical picture in patients with CNS disorders. In patients with a history of glucocorticoid exposure, adrenal insufficiency may also cause upper gut symptoms.
Oral medications such as calcineurin inhibitors, MMF, trimethoprim-sulfamethoxazole, itraconazole, posaconazole, voriconazole, imatinib and similar drugs, and high-dose opioids also cause nausea and vomiting.106,107 Mycophenolic acid causes fewer GI symptoms than MMF.64 Parenteral infusions of fat, glucose, and amino acids reduce food intake, slow gastric emptying, and cause nausea. Even after total parenteral nutrition has been stopped, appetite suppression may linger for one to three weeks.140
Jaundice, Hepatomegaly, and Abnormal Liver Biochemical Tests
Development of jaundice following HCT is an ominous prognostic sign, with increased nonrelapse mortality in patients whose total serum bilirubin exceeds 4 mg/dL.141 Fortunately the frequency of severe liver injury after HCT is much lower than it was 10 years ago because of less frequent use of drugs that cause sinusoidal injury and because of prophylaxis of cholestatic injury with ursodiol.142–144 There are multiple causes of jaundice after HCT (Table 34-2).
Sinusoidal Obstruction Syndrome (Veno-occlusive Disease)
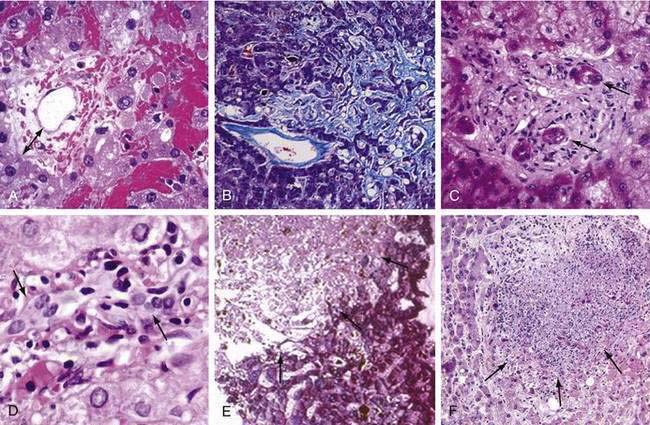
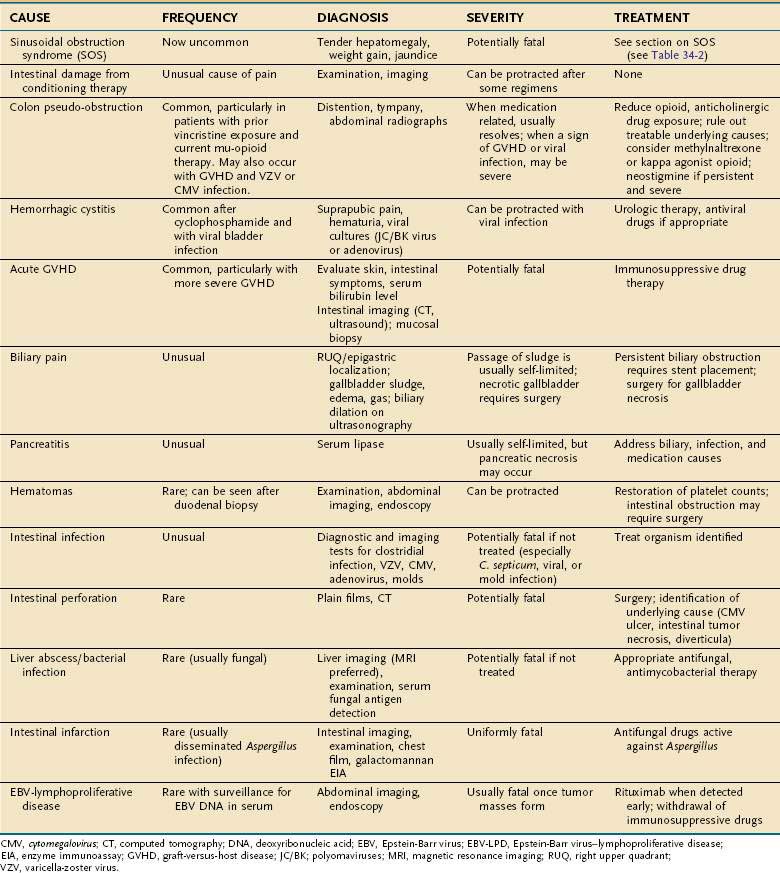
Some myeloablative conditioning regimens may damage hepatic sinusoids, leading to hepatomegaly, fluid retention and weight gain, and elevated serum bilirubin.145 Individual variability in cyclophosphamide metabolism, irradiation dose, use of gemtuzumab ozogamicin, and preexisting liver inflammation and fibrosis are risk factors.121,123,126 Reduced intensity regimens rarely affect hepatic sinusoids.118 A clinical diagnosis of SOS may suffice if typical signs develop before day 20 post transplant, but Doppler ultrasonography, measurement of the wedged hepatic venous pressure gradient, and liver histology may be needed in difficult cases.146–148 Initial histologic changes of SOS are dilation of sinusoids, extravasation of red cells through the space of Disse, necrosis of periventricular hepatocytes, and widening of the subendothelial zone in central veins (Fig. 34-5A).145 The later stages are characterized by extensive collagenization of sinusoids and venules (see Fig. 34-5B). More than 80% of patients with SOS recover completely. A poor prognosis correlates with the degree of bilirubin elevation and weight gain, higher serum aminotransferase enzyme (AST and ALT) values, higher wedged hepatic venous pressure gradient, development of portal vein thrombosis, and multiorgan failure. Treatment of severe SOS is unsatisfactory; the best results are with intravenous defibrotide (25 mg/kg/day), a porcine oligonucleotide that has effects on microvascular endothelial cells.127 SOS can be prevented by identifying patients at risk and altering the transplant regimen using (1) conventional, not transplant therapy; (2) a reduced-intensity conditioning regimen118; (3) a myeloablative regimen that does not contain cyclophosphamide (e.g., targeted busulfan-fludarabine for allogeneic HCT149,150 or BEAM (BCNU [carmustine], etoposide, arabinoside [cytarabine], melphalan) for autologous HCT151); (4) modification of CY-based regimens.152,153 Patients with cirrhosis may decompensate even after a reduced-intensity allograft.118
Cholestatic Liver Diseases
Cyclosporine inhibits canalicular bile transport and commonly causes mild increases in serum bilirubin. Sepsis-associated cholestasis is an important contributor to hyperbilirubinemia in the weeks after HCT, mediated by endotoxins and cytokines such as IL-6 and TNF-α.89 Many drugs used after HCT have been associated with cholestatic liver disease. Acute GVHD is the most common cause of severe cholestatic injury, related initially to cytokines such as IL-6 and later to destruction of small bile ducts caused by alloreactive T cells.154 Hepatic GVHD usually follows cutaneous and/or intestinal GVHD, and is heralded by a gradual rise in serum bilirubin, alkaline phosphatase, and aminotransferases. In allograft recipients on minimal immunosuppression or after donor lymphocyte infusion, GVHD may present as acute hepatitis.155,156 A cholestatic condition identical to GVHD occurs rarely in autologous HCT recipients. Characteristic liver biopsy findings in GVHD include lymphocytic infiltration of small bile ducts with nuclear pleomorphism and epithelial cell dropout (see Fig. 34-5C and D). Because these patients are frequently pancytopenic, inflammatory infiltrates may be minimal. In advanced cases of hepatic GVHD, it may be difficult to identify small bile ducts because they have been destroyed. Only 30% of patients with liver GVHD have resolution of liver abnormalities after initial immunosuppressive treatment. Prophylactic ursodiol reduces the frequency of cholestasis in general and GVHD-related cholestasis specifically, compared with placebo, and should be given routinely through day 80 in allograft recipients.142 More than 50% of patients with acute liver GVHD will develop chronic GVHD.
Acute Hepatocellular Injury
Severe hepatic injury (serum ALT >1500 U/L) is now mostly related to zone three necrosis from circulatory causes such as SOS and hypoxic hepatitis, and not to infection. However, acute hepatitis caused by HSV, VZV, adenovirus or HBV can be fatal after HCT.108,157,158 Except for sporadic cases of adenovirus hepatitis, infections by these other viruses have become rare because of antiviral prophylaxis and preemptive treatment. Hepatic infections caused by CMV and HCV are seldom severe.114 Despite acyclovir, HHV-6 and HHV-8 reactivation have been associated with the development of fever, rash, and hepatitis in HCT recipients.159 Other noninfectious causes of acute hepatocellular injury include a hepatitic presentation of GVHD and drug toxicity.155,156 When there is uncertainty about the cause of rising serum ALT, DNA blood tests for herpesviruses, adenovirus, and HBV; transvenous measurement of the wedged hepatic venous pressure gradient; and liver biopsy are indicated (see Fig. 34-5). If acyclovir is not being given, it should be started empirically, particularly if the patient presents with abdominal complaints typical of VZV infection (see Fig. 34-5E).160 Adenovirus hepatitis should be suspected if the patient has concomitant pulmonary, renal, bladder, or intestinal symptoms (see Fig. 34-5F); the most effective treatment is cidofovir when given early in the course of infection.161,162 Fulminant hepatitis B may develop during immune reconstitution in patients at risk, but can be prevented with prophylactic lamivudine or adefovir.108,163 If severe hepatitis B reactivation does occur, usually because a diagnosis of HBV was not made prior to HCT,117 antiviral therapy with the most potent antiviral drug available should be initiated immediately, although progression to fatal liver failure is not uncommon.164 Fulminant hepatitis B has also occurred after discontinuation of prophylactic antiviral therapy; all HBV-infected patients, particularly those with high pretransplant HBV DNA levels, should be monitored following antiviral drug withdrawal.165,166 HCV infections are seldom severe; asymptomatic elevations of serum ALT are commonly seen from days 60 to 120, frequently coinciding with the tapering of immunosuppressive drugs.114 Therapy directed at chronic HCV infection should be considered once the patient has ceased all immunosuppressive drugs and has no evidence of active GVHD.167 About a third of HCV-infected transplant survivors will develop cirrhosis over 20 to 30 years.
Fungal and Bacterial Infections
Antifungal prophylaxis has significantly reduced the incidence of invasive fungal disease in HCT recipients, particularly in those requiring ongoing immunosuppression for treatment of GVHD.106,168,169 If invasive fungal disease does occur, infection with resistant Candida species or molds is likely. Signs are fever and tender hepatomegaly, with increased serum alkaline phosphatase levels. High-resolution CT or MRI may demonstrate multiple fungal abscesses, and serological tests for fungal antigens may be useful for diagnosis. Antifungal drugs and return of neutrophil function after HCT can lead to resolution of previously treatment-refractory mold infection.105 Bacterial liver abscesses are rare in HCT recipients, probably because of the high use of systemic antibiotics; however, latent mycobacterial infection may reactivate within the liver with prolonged immunosuppressive therapy. Disseminated bacille Calmette-Guérin (BCG) infection with liver involvement has been reported. Disseminated clostridial infection and gallbladder infection with gas-producing organisms may lead to air in the liver and biliary system.
Gallbladder and Biliary Disease
Gallbladder sludge (calcium bilirubinate) is almost universally present in HCT patients. Although sludge is usually asymptomatic, passage through the bile duct may cause epigastric pain, nausea, and elevated serum liver enzymes. Endoscopic papillotomy is rarely indicated. Biliary sludge may be a cause of acute “acalculous” cholecystitis, acute pancreatitis, and bacterial cholangitis.170,171 Acute cholecystitis is uncommonly seen in HCT recipients and is frequently acalculous. Cholecystitis in this setting may also be due to leukemic relapse with gallbladder involvement or infection by CMV or fungi. Diagnosis is difficult because of the high frequency of gallbladder abnormalities on ultrasonography following HCT. Pericholecystic fluid, gallbladder wall necrosis, or localized tenderness suggests cholecystitis. A radionuclide bile excretion study, with morphine infusion to enhance gallbladder filling, can be useful; nonvisualization of the gallbladder suggests cholecystitis. Biliary obstruction is a rare event, caused by a variety of disorders (e.g., lymphoblastic infiltration of the bile duct and gallbladder in EBV-LPD; CMV-related biliary disease; dissecting duodenal hematoma complicating endoscopic biopsy; inspissated biliary sludge; and leukemic relapse [chloroma] in the head of the pancreas).170,171 Therapeutic endoscopic cholangiopancreatography may be needed if there is cholangitis or persistent obstruction.
Idiopathic Hyperammonemia and Coma
A syndrome of hyperammonemia and coma has been described in patients who received high dose chemotherapy, including conditioning for HCT.172 Patients present with progressive lethargy, confusion, weakness, incoordination, vomiting and hyperventilation. The diagnosis is confirmed when the plasma ammonia exceeds 200 µmol/L and there is no evidence of liver failure. This syndrome is rare, but is associated with a high mortality. Its pathogenesis involves the unmasking of a latent genetic disorder similar to ornithine transcarbamylase deficiency.173,174
Gastrointestinal Bleeding
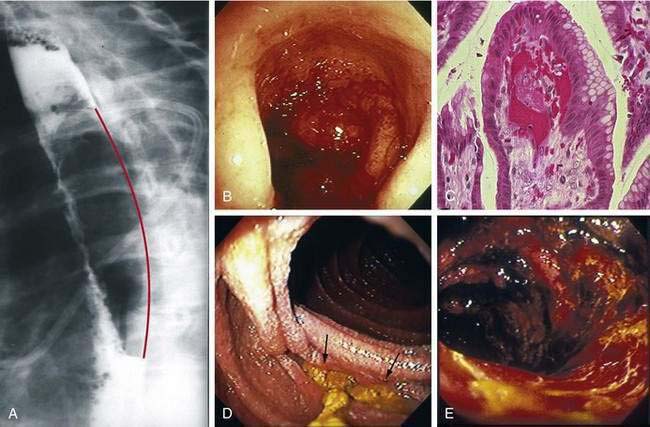
Bleeding that does not require transfusion is very common after HCT, particularly when platelet counts are low. Causes include retching-induced trauma to the esophageal or gastric mucosa (Fig. 34-6A), mucosal injury from conditioning therapy, peptic esophagitis, C. difficile colitis, anal fissures and hemorrhoids, and mild acute GVHD. The incidence of severe GI bleeding, particularly in patients with adequate platelet counts, is less than 1% because of effective prophylaxis against viruses, fungi, and acute GVHD.175 Mortality from severe intestinal bleeding, however, remains at 40%.175,176 The most common cause of severe bleeding is refractory acute GVHD, which can result in bleeding from extensive ulceration in the small intestine and cecum (see Fig. 34-4). In some patients with GVHD, bleeding may appear to be coming from specific areas of the mucosa, but when such patients are operated on or come to autopsy, diffuse rather than focal mucosal ulceration is the rule. Ulcers in the stomach or duodenum that develop after HCT are usually caused by acute GVHD or CMV infection, but with preemptive ganciclovir therapy, bleeding CMV ulcers have become rare.175 Gastric ulcerations also may be caused by infection by VZV, bacteria (phlegmonous gastritis), or EBV (lymphoproliferative disease) (see Chapter 51). Gastric antral vascular ectasia, is also a cause of severe upper intestinal bleeding in HCT recipients who received oral busulfan as part of conditioning therapy.177,178 Diffuse areas of hemorrhage are seen in the gastric antrum and proximal duodenum, but the underlying mucosa is intact (see Fig. 34-6B). Histology is diagnostic, revealing abnormal dilated capillaries, thromboses, and fibromuscular hyperplasia in the lamina propria (see Fig. 34-6C). Endoscopic laser therapy is the treatment of choice to control bleeding, but multiple laser treatments may be required to obliterate ectatic lesions.178 Rare gastroduodenal causes of bleeding post HCT include ulcers caused by molds (see Fig. 34-6D), Dieulafoy lesions, Curling (stress) ulcers, duodenal biopsy sites, adenovirus colitis (see Fig. 34-6E), and C. septicum infection (typhlitis).179 There is no effective therapy for mucosa that is diffusely oozing blood other than raising the platelet count and treating the underlying condition. In GVHD, re-epithelialization of ulcerated intestinal mucosa occurs very slowly (see Fig. 34-4F). Focal bleeding lesions, especially those caused by mucosal infection, can be treated with endoscopic cautery, heater probe, or epinephrine injection provided platelet counts are adequate. Unless the underlying disease process is eliminated, these endoscopic methods will not cure the bleeding problem. Attempts to resect large segments of diffusely bleeding intestine involved with GVHD have not been successful.
Dysphagia
Mucositis, acid-peptic esophagitis, and pill esophagitis are the leading causes of dysphagia. Infections of the esophagus have largely disappeared because of antiviral and antifungal prophylaxis. Desquamation of oropharyngeal epithelium caused by conditioning therapy may lead to pain on initiating a swallow and inability to move a bolus past the cricopharyngeus. Rarely, non-healing esophageal ulcerations, strictures, and dysphagia result from conditioning therapy. The abrupt onset of severe retrosternal pain, hematemesis, and painful swallowing suggests a hematoma in the wall of the esophagus, a result of retching when platelet counts are very low (see Fig. 34-6A).180 Endoscopy is relatively contraindicated because many intramural hematomas represent contained perforations. The course of intramural hematomas is one of slow resolution over one or two weeks. In patients with severe GVHD, esophageal edema, erythema, and a peeling epithelium lead to ulcerations (see Fig. 34-4A).181 Pill esophagitis occurs after ingestion of medications that might be used after HCT, such as phenytoin, foscarnet, captopril, oral bisphosphonates, ascorbic acid, ciprofloxacin, clindamycin, and oral potassium chloride.
Diarrhea (see Table 34-3)
Diarrhea caused by mucosal damage from myeloablative conditioning therapy is seldom severe, usually resolving by days 12 to 15. Cytarabine-containing regimens, high-dose melphalan, and multiple alkylating regimens cause more severe, protracted diarrhea. Intravenous octreotide and oral loperamide at (4 mg by mouth every six hours) may be effective for severe diarrhea associated with conditioning therapy. Acute GVHD is the most common cause of diarrhea after day +15.176,182 The onset of diarrhea can be sudden, with daily stool volumes in excess of 2 L in severe cases. The diarrheal fluid is watery and green, with ropy strands of mucoid material that reflect transmucosal protein loss. In an allografted patient with skin and liver abnormalities typical of acute GVHD, this diarrheal syndrome is almost diagnostic of intestinal GVHD, particularly when there is falling serum albumin and negative stool studies for infection. In GVHD, abdominal imaging (CT, positron-emission tomography [PET]-CT, or focused ultrasonography) may reveal intestinal edema, but this finding does not differentiate acute GVHD from CMV infection.148,183,184 Pneumatosis intestinalis, which may be associated with GVHD or CMV enteritis, may be seen by plain x-ray, CT, or MRI. A definitive diagnosis of GVHD in problematic cases requires mucosal biopsy. In mild cases, the gastroduodenal and rectosigmoid mucosa are grossly normal, but moderately severe GVHD causes diffusely edematous and erythematous mucosa (see Fig. 34-4).134 Severe GVHD may lead to ulcerations and large areas of mucosal sloughing in the stomach, small intestine and colon (see Fig. 34-4F). Even when the endoscopic appearance is normal, biopsies often reveal intestinal crypt cell necrosis and apoptotic bodies diagnostic of acute GVHD (see Fig. 34-4D). In severe cases of GVHD, whole crypts are destroyed, then adjacent crypts, and finally whole segments of intestinal mucosa (see Fig. 34-4E and F). Other histologic findings that support the diagnosis of GVHD include pericapillary hemorrhage,185 infiltrating neutrophils,186 or eosinophils.187 The use of capsule endoscopy for diagnosis of GVHD can provide visual inspection of the small intestine that cannot be seen with routine endoscopy, but does not allow biopsy.188 The negative predictive value of a capsule endoscopy examination of the small intestine appears to be high and is thus useful for excluding more severe acute GVHD.188 Bleeding often accompanies diarrhea in patients with mucosal ulceration.175 Successful treatment of acute GVHD with immunosuppressive therapy results in a dramatic reduction in stool volume, with resolution of accompanying symptoms of abdominal pain, nausea, and vomiting. The management of patients whose diarrhea and other symptoms of intestinal GVHD persist after 7 to 14 days of immunosuppressive therapy is unsatisfactory because the rate of failure of secondary therapy is high.189 Overall prognosis in patients with GVHD can be estimated by use of the acute GVHD Activity Index.190
In allograft recipients, infectious causes of diarrhea are far less common than GVHD, accounting for only 10% to 15% of diarrheal episodes.176,182 In countries where intestinal parasitism and bacterial contamination of water are endemic, the spectrum of infections may be wider.98 C. difficile colitis is usually a relatively mild, treatable disease when diagnosed at the onset of diarrhea, but the recent emergence of more virulent strains of C. difficile has changed the natural history of this infection. The inappropriate use of proton pump inhibitors for marginal indications191 increases the risk of C. difficile colitis twofold.192 Other relatively common causes of infectious diarrhea include astrovirus, norovirus, rotavirus, CMV, and adenovirus.99,182,193,194 Some serotypes of adenovirus cause necrotizing enteritis and rapidly fatal multiorgan failure involving the gut, liver, lungs, and kidneys (see Fig. 34-6E).157,158,195 There should be a sense of urgency in identifying adenovirus as a cause of enteritis, as early treatment with cidofovir appears to be effective.161,162 CMV is the only cause of enteritis after HCT that requires an intestinal biopsy for diagnosis.182 Otherwise the negative predictive value of a stool examination (including PCR) for other viruses, bacteria, fungi, and parasites is high. Watery diarrhea secondary to intestinal parasite infection (Cryptosporidium, G. lamblia, and E. histolytica) and mycobacteria infection are rare outside of endemic areas.100,194,196,197 Diarrhea may also result from carbohydrate malabsorption (particularly in patients on antibiotics), oral magnesium salts, tacrolimus (a motilin agonist), metoclopramide, and MMF.198,199 MMF gut toxicity can be addressed by switching to mycophenolic acid.64
Abdominal Pain
It is extremely important to distinguish abdominal pain as an indicator of a rapidly progressive, fatal condition from illnesses with a benign natural history that require only conservative management. The causes of abdominal pain after HCT are listed in Table 34-4. The illnesses that may progress rapidly include intestinal perforation, some infections (e.g., typhlitis caused by C. septicum, adenovirus, and VZV), gallbladder necrosis, liver abscess, and acute GVHD presenting only as abdominal pain.200 Fortunately, these disorders are far less common than intestinal pseudo-obstruction, hepatic pain related to SOS, multisystem acute GVHD, and hemorrhagic cystitis. Intestinal perforation may develop in the setting of lysis of a transmural lymphoma or metastatic carcinoma shortly after conditioning therapy, or later on, from CMV ulcers or diverticular perforation. Perforation may present with only mild to moderate abdominal pain and pneumoperitoneum on plain abdominal x-ray. Dilation of the bowel in the absence of a mechanical obstruction is the most common cause of moderate to severe abdominal pain. Most patients with pseudo-obstruction have an underlying intestinal disease, such as enteritis from conditioning therapy, GVHD, or infection, but frequently the acute presentation is related to increasing use of mu-opioid medications. Pseudo-obstruction is more frequent among patients with lymphoma, a result of intestinal neuropathy from repeated use of vincristine (see Chapter 120). To allow pain relief without affecting colon motility, methylnaltrexone can be used.77 Alternatively, colon distention may decrease after switching from a mu-opioid to a kappa-opioid agonist (for example, to butorphanol). Neostigmine (2 mg intravenously) has been successfully used in patients with acute colonic pseudo-obstruction after HCT.201 In visceral VZV infection, abdominal distention, severe pain, fever, and rising serum ALT may precede cutaneous manifestations by up to 10 days.160 In rare instances, a skin rash never develops. Acyclovir should be started on clinical suspicion while serum is analyzed by PCR for VZV DNA.160
More severe acute intestinal GVHD may present with nausea, anorexia, periumbilical crampy abdominal pain, and diarrhea. The sudden onset of intestinal edema (see Fig. 34-4B and C) can cause a rigid abdomen with rebound tenderness preceding the development of a skin rash or diarrhea. The decision to treat a patient empirically with prednisone when definitive evidence of GVHD is not at hand can be difficult, but when the pretest probability of GVHD is high (e.g., an HLA-mismatched or unrelated donor; engraftment; a nascent skin rash) and that of perforation or infection low, treatment should be started while GVHD is sought by endoscopic mucosal biopsy (see Fig. 34-4).
Pancreatitis is an uncommon cause of abdominal pain in HCT patients, but in a study of autopsied patients the prevalence of acute pancreatitis was 28%.202 Symptoms of pancreatitis had been absent in many of the patients who were found to have florid pancreatitis at autopsy, suggesting that symptoms had been masked by immunosuppressive drugs. Patients with low platelet counts or prolongation of blood clotting may rarely bleed into the retroperitoneum, abdominal wall, or intra-abdominal viscera, particularly after duodenal biopsy, causing significant pain.
Intestinal infections presenting with significant pain are listed in Table 34-4. Typhlitis (C. septicum infection) occurs in granulocytopenic patients but is not common after HCT. Symptoms include fever, right lower quadrant pain, nausea and vomiting, diarrhea, occult blood in stool, and shock.102 Typhlitis is usually diagnosed using imaging studies; laparotomy is rarely necessary.203 If typhlitis is a possibility, imipenem and oral vancomycin therapy should be started along with coverage for luminal bacteria and fungi.204
Perianal Pain
Perianal pain after HCT can be caused by an anal fissure, a thrombosed external hemorrhoid, cellulitis related to tissue maceration, fistulas, and abscesses. In patients with granulocytopenia, infections in the perineum or perianal spaces are usually polymicrobial, arising either from anal crypts or from tears in the anal canal. After HCT these infections can be difficult to recognize because they may not produce abscesses but rather a spreading cellulitis. Extensive supralevator and intersphincteric abscesses may be present without being apparent on external examination. CT, MRI, or endoscopic ultrasonography can give a clear view of the anatomy involved, particularly if there is pus present.205 When antibiotics covering anaerobic and aerobic bacteria are given to patients with incipient perianal infection, far fewer patients require surgical drainage than in the past.
PROBLEMS IN LONG-TERM TRANSPLANT SURVIVORS
Liver Disease Caused by Graft-Versus-Host Disease
Long-term survivors of transplant who have hepatic GVHD usually have other evidence of chronic GVHD, a pleomorphic immune disorder characterized by oral and ocular sicca; ulceration in squamous epithelium of the skin, mouth, esophagus, and vagina; subcutaneous fibrosis; contractures and myositis; immunodeficiency; and other manifestations of immune dysregulation. Liver involvement in these patients, like gut mucosal inflammation, is considered to be a protracted form of acute GVHD because clinical and histologic changes in these organs are identical to those in patients with acute GVHD that occurs in the months following HCT.206 Patients with isolated elevations of serum alkaline phosphatase or ALT related to GVHD should be followed closely, but may not require high-dose immunosuppressive therapy. By the time that jaundice develops in patients with chronic GVHD, liver biopsy shows extensive damage to small bile ducts and, in severe cases, ductopenia. In patients receiving no, or tapering doses of, immunosuppression, liver GVHD may also present as an acute hepatitis, with abrupt elevations of serum aminotransferase levels to more than 2000 U/L.155 A similar presentation can be seen after donor lymphocyte infusion (DLI).156 In patients presenting with an acute hepatitis, blood samples for viral DNA or ribonucleic acid (RNA) or liver biopsy are essential in excluding acute viral hepatitis due to a herpesvirus (HSV or VZV) or a hepatitis virus and to make a definitive diagnosis of hepatic GVHD (see Fig. 34-5D and E). A serum autoantibody test for CYP1A2 may prove diagnostically useful in the diagnosis of hepatitic GVHD because this enzyme appears to be a target antigen in GVHD.207 Immunosuppressive drug treatment of chronic GVHD is successful in 50% to 80% of patients with extensive multiorgan disease, but refractory chronic GVHD is a fatal illness. The addition of ursodeoxycholic acid (15 mg/kg/day) may result in biochemical improvement.208 Ductopenic GVHD is potentially reversible if ongoing immunologic destruction of biliary epithelium ceases, but this process may take months before resolution of jaundice.155 Liver transplantation, including living-donor transplantation from the original stem cell donor,209 has been performed for patients with liver failure due to chronic hepatic GVHD, although frequently there are contraindications to this approach.210
Chronic Viral Hepatitis and Cirrhosis
HCV infection in HCT survivors almost always results in chronic hepatitis.114,115 In the first 10 years of HCV infection after HCT, there is little liver-related morbidity.114 However, a third of patients transplanted before the 1990s will develop cirrhosis related to chronic HCV infection over a 20- to 40-year time frame.115 The reasons for more rapid progression of fibrosis after HCT may be related to concomitant liver involvement with GVHD, immunosuppression, and iron overload.211 Iron overload is particularly severe in thalassemic patients who have undergone HCT.212 Patients with chronic HCV should be offered therapy with combination pegylated INF-α plus ribavirin, unless there are contraindications.167,213 Pegylated interferons, with their longer half-lives, should be administered with caution because some HCT patients experience rapid falls in platelet and granulocyte counts. INF-α may also activate chronic GVHD. Liver transplantation should be considered in any HCT survivor with incipient liver decompensation; in some cases, the original allogeneic cell donor can be a partial liver donor.214
The prevalence of chronic HBV infection among HCT survivors varies widely depending on the country. Patients who remain viremic or HBsAg positive after HCT are at risk of flares of hepatitis B at times of reduction of immunosuppression, such as during tapering or cessation of treatment for chronic GVHD. All long-term survivors with chronic hepatitis B should be regularly monitored to assess the need for antiviral therapy. Hepatitis B e antigen and antibody status should be determined in all patients, and liver enzymes monitored every 6 to 12 months. The need for antiviral treatment with an oral nucleoside or nucleotide analog (for instance, entecavir, telbivudine, adefovir, tenofovir) is based on the ALT and HBV DNA levels and the severity of hepatic fibrosis.215 These levels may change over time, emphasizing the need to continually reassess patients who are not on treatment. As in the peritransplant period, patients with viremia or positive HBsAg should receive an antiviral agent whenever they receive immunosuppressive or cytotoxic therapy.216
Hemosiderosis
Iron overload is caused by a combination of multiple red cell transfusions (e.g., for thalassemia) and dyserythropoiesis leading to increased iron transport by the intestine. After HCT, iron accumulation stops and body iron stores fall slowly over time.217 The consequences of extreme iron overload in HCT survivors are primarily those of cardiac, pituitary, and endocrine pancreatic dysfunction. Iron overload may be an important cofactor in liver disease in long-term survivors of HCT and should be part of a screening panel.218,219 In the past, liver biopsy with liver iron determination was required, but increasingly noninvasive methods (e.g., MRI129) are being used to provide assessments of liver iron concentration and distribution. Patients with liver iron content greater than 15,000 µg/g dry weight should be treated aggressively with phlebotomy and chelation; when liver iron content is 7000 to 15,000 µg/g dry weight, phlebotomy is indicated; when liver iron content is less than 7000 µg/g dry weight, treatment is indicated only if there is evidence of liver disease.220 Mobilization of iron from heavily overloaded patients improves cardiac function, normalizes serum ALT levels, and results in improved liver histology.219–222
Hepatic Drug Toxicity
Drug-induced liver injury may be related to drugs in common use by transplant survivors, including antihypertensive drugs, lipid-lowering agents, hypoglycemic agents, NSAIDs, antidepressants, antibiotics, and herbal preparations.223 Some drug reactions may result in chronic liver disease.224
Fungal Liver Infections
Fungal abscesses can recur after apparently successful antifungal therapy when high-dose immunosuppressive drugs are started for GVHD. Oral, nonsterile herbal remedies contaminated by molds may lead to liver abscesses in immunosuppressed HCT survivors.225
Liver Cancer
Compared with the general population, patients who survive more than 10 years post HCT have an eightfold risk of developing a new solid malignancy. The risk of hepatocellular carcinoma is particularly elevated.226 Transplant survivors with risk factors for hepatocellular carcinoma (HCV or HBV infection, obesity, diabetes, low platelet count) should be screened at yearly intervals (see Chapter 94).227,228 Chronic hepatitis C may also be a risk factor for development of lymphoma229 and other lymphoproliferative disorders230 after transplant.
Other Hepatobiliary Disorders
There is a higher than expected incidence of gallstones and stone-related biliary problems after HCT than in an age-matched population, probably related to earlier formation of biliary sludge. Chronic cyclosporine dosing also may lead to gallstones and biliary symptoms. Patients who have experienced SOS from either chemotherapy or a myeloablative conditioning regimen may rarely develop hepatic nodularity caused by atrophy of zone three and hypertrophy of zone one hepatocytes, without fibrosis.231 This process (nodular regenerative hyperplasia) is usually clinically silent unless portal hypertension develops, manifested by variceal bleeding, ascites, splenomegaly, and thrombocytopenia, but with preserved liver function. Focal nodular hyperplasia (nodules readily seen by liver imaging, with characteristic central scars) has been described as an incidental finding in 12% of a cohort of HCT survivors, up to 14 years after transplant.232 These lesions are likely the result of sinusoidal liver injury caused by myeloablative conditioning regimens.
Esophageal Disorders
Some patients with extensive chronic GVHD have esophageal desquamation, webs, submucosal fibrous rings, bullae, and long, narrow strictures in the upper and mid-esophagus.233–236 Although the most common symptom is dysphagia, some patients present with insidious weight loss, retrosternal pain, and aspiration of gastric contents. The diagnosis is made by barium contrast radiography and endoscopy,236 which should be done with caution because perforations have been reported.233 Tight strictures are difficult to dilate safely, but failure to dilate strictures may lead to progressive esophageal narrowing. Esophageal involvement can be prevented by prompt treatment of chronic GVHD at its early stages. Therapy with proton pump inhibitors should be considered if there is uncontrolled acid reflux. Myasthenia gravis may also complicate chronic GVHD, with dysphagia as its presenting complaint.237 Sporadic cases of fungal and rarely viral esophagitis may occur in patients with chronic GVHD on immunosuppressive and antibiotic therapy. Esophageal strictures may be sequelae of earlier herpesvirus infection or mucositis. Squamous cell carcinoma of the esophagus has been reported in HCT survivors, usually with concomitant chronic GVHD of the oropharynx.238
Gastrointestinal Disorders
The incidence of diarrhea falls sharply after day 100 except in patients who have received allografts following reduced intensity conditioning therapy239 and in those whose acute GVHD has never resolved. Patients with protracted acute GVHD, however, often have symptoms that wax and wane with intensity of immunosuppressive therapy for up to 15 years after HCT, with each exacerbation similar to the presenting signs of GVHD that occurred earlier after HCT (satiety, poor appetite, nausea, episodic diarrhea, and weight loss).240,241 The endoscopic and histologic appearance of intestinal mucosa is identical to that seen in acute GVHD (see Fig. 34-4). Use of oral beclomethasone dipropionate can be effective in treating patients with protracted acute GVHD involving the GI tract.242 Before the introduction of more effective immunosuppressive drugs, chronic GVHD resulted in extensive collagen deposition in submucosal and subserosal areas of the intestinal tract, resulting in refractory malabsorption243; this process has not been seen in recent years. There are sporadic cases of C. difficile, CMV,138 and rarely G. lamblia and cryptosporidiosis in long-term survivors. Chronic intestinal viral infection can be seen in patients who remain on immunosuppressive drugs, including rotavirus, norovirus, and adenovirus. Intestinal diseases in donors have been reported in their recipients, such as idiopathic inflammatory bowel disease and celiac disease.244
Pancreatic Disease
Acute pancreatitis has been described in transplant survivors, usually related to passage of gallstones or sludge. Cyclosporine and tacrolimus also may cause pancreatitis.245–247 Diarrhea, steatorrhea, and weight loss secondary to pancreatic insufficiency have developed in some HCT survivors.248,249 The cause is unclear; previous pancreatic necrosis, prolonged glucocorticoid exposure, and tacrolimus toxicity are the leading possibilities.202 Transient pancreatic insufficiency has been noted in patients with gut and liver GVHD.250 Chronic GVHD and extreme iron overload also may contribute to pancreatic damage.
Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: A decade of experience at a single center. Ann Surg. 2001;234:404-16. (Ref 61.)
Appelbaum FR, Forman SJ, Negrin RS, Blume KG. Thomas’ Hematopoietic Cell Transplantation, 4th ed. Oxford, UK: Wiley-Blackwell Publishing; 2009. (Ref 94.)
Assi MA, Pulido JS, Peters SG, et al. Graft-vs.-host disease in lung and other solid organ transplant recipients. Clin Transplant. 2007;21:1-6. (Ref 66.)
Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? J Hepatol. 2005;42:448-56. (Ref 46.)
Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108:1602-7. (Ref 52.)
Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557-63. (Ref 135.)
Hogan WJ, Maris M, Storer B, et al. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: A study of 193 patients. Blood. 2004;103:76-82. (Ref 118.)
Lau GK, Suri D, Liang R, et al. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002;122:614-24. (Ref 112.)
McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043-8. (Ref 121.)
Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-vs.-host disease after marrow transplantation. Gastrointest Endosc. 1999;49:612-21. (Ref 134.)
Ponticelli C, Passerini P. Gastrointestinal complications in renal transplant recipients. Transplant Int. 2005;18:643-50. (Ref 16.)
Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977-83. (Ref 142.)
Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: A meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006;43:869-80. (Ref 11.)
St Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855-61. (Ref 129.)
van Burik JA, Lawatsch EJ, DeFor TE, Weisdorf DJ. Cytomegalovirus enteritis among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2001;7:674-9. (Ref 138.)
1. Kathuria P, Sakhuja V, Gupta KL, et al. Gastrointestinal complications after renal transplantation. 10 year data from a North Indian Transplant Center. ASAIO J. 1995;41:M698-703.
2. Helderman JH, Goral S. Gastrointestinal complications of transplant immunosuppression. J Am Soc Nephrol. 2002;13:277-87.
3. Logan AJ, Morris-Stiff GJ, Bowrey DJ, Jurewicz WA. Upper gastrointestinal complications after renal transplantation: A 3-yr sequential study. Clin Transplant. 2002;16:163-7.
4. Schnitzler MA, Lowell JA, Hardinger KL, et al. The association of cytomegalovirus sero-pairing with outcomes and costs following cadaveric renal transplantation prior to the introduction of oral ganciclovir CMV prophylaxis. Am J Transplant. 2003;3:445-51.
5. Robinson L-G, Hilinski J, Graham F, et al. Predictors of cytomegalovirus disease among pediatric transplant recipients within one year of renal transplantation. Pediatr Transplant. 2002;6:111-18.
6. Hibberd PL, Snydman DR. Cytomegalovirus infection in organ transplant recipients. Infect Dis Clin North Am. 1995;9:863-77.
7. Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation—challenging the status quo. Clin Transplant. 2007;21:149-58.
8. Hodson EM, Jones CA, Webster AC, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: A systematic review of randomised controlled trials. Lancet. 2005;365:2105-15.
9. Strippoli GFM, Hodson EM, Jones C, Craig JC. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation. 2006;81:139-45.
10. Kalil AC, Levitsky J, Lyden E, et al. Meta-analysis: The efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143:870-80.
11. Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: A meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006;43:869-80.
12. Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611-20.
13. Richardson M, Lass-Florl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008;14(Suppl 4):5-24.
14. Sarkio S, Halme L, Kyllonen L, Salmela K. Severe gastrointestinal complications after 1,515 adult kidney transplantations. Transpl Int. 2004;17:505-10.
15. Gil-Vernet S, Amado A, Ortega F, et al. Gastrointestinal complications in renal transplant recipients: MITOS study. Transplant Proc. 2007;39:2190-3.
16. Ponticelli C, Passerini P. Gastrointestinal complications in renal transplant recipients. Transpl Int. 2005;18:643-50.
17. Neri L, Rey LAR, Pinsky BW, et al. Increased risk of graft failure in kidney transplant recipients after a diagnosis of dyspepsia or gastroesophageal reflux disease. Transplantation. 2008;85:344-52.
18. McBeth BD, Stern SA. Lower gastrointestinal hemorrhage from an arterioenteric fistula in a pancreatorenal transplant patient. Ann Emerg Med. 2003;42:587-91.
19. Green BT, Tuttle-Newhall J, Suhocki P, et al. Massive gastrointestinal hemorrhage due to rupture of a donor pancreatic artery pseudoaneurysm in a pancreas transplant patient. Clin Transplant. 2004;18:108-11.
20. Moghaddam SM, Alavian SM, Kermani NA. Hepatitis C and renal transplantation: A review on historical aspects and current issues. Rev Med Virol. 2008;18:375-86.
21. Mahmoud IM, Elhabashi AF, Elsawy E, et al. The impact of hepatitis C virus viremia on renal graft and patient survival: A 9-year prospective study. Am J Kidney Dis. 2004;43:131-9.
22. Ghafari A, Sanadgol H. Impact of hepatitis B and hepatitis C virus infections on patients and allograft outcomes in renal transplant recipients: A single center study. Transplant Proc. 2008;40:196-8.
23. Fabrizi F, Martin P, Dixit V, et al. HBsAg seropositive status and survival after renal transplantation: Meta-analysis of observational studies. Am J Transplant. 2005;5:2913-21.
24. Ridruejo E, Cusumano A, Diaz C, et al. Hepatitis C virus infection and outcome of renal transplantation. Transplant Proc. 2007;39:3127-30.
25. San Juan R, Aguado JM, Lumbreras C, et al. RESITRA network of the Spanish study group of infection in transplantation. Impact of current transplantation management on the development of cytomegalovirus disease after renal transplantation. Clin Infect Dis. 2008;47:875-82.
26. Ahsan N, Rao KV. Hepatobiliary diseases after kidney transplantation unrelated to classic hepatitis virus. Semin Dial. 2002;15:358-65.
27. Lowell JA, Stratta RJ, Taylor RJ, et al. Cholelithiasis in pancreas and kidney transplant recipients with diabetes. Surgery. 1993;114:858-63.
28. Tokat Y, Zeytunlu M, Kilic M, et al. Gastrointestinal complications in renal transplantation. Transplant Proc. 1996;28:2351-2.
29. Sarkio S, Rautelin H, Kyllonen L, et al. Should Helicobacter pylori infection be treated before kidney transplantation? Nephrol Dial Transplant. 2001;16:2053-7.
30. Dee SL, Butt K, Ramaswamy G. Intestinal ischemia. Arch Pathol Lab Med. 2002;126:1201-4.
31. Andreoni KA, Pelletier RP, Elkhammas EA, et al. Increased incidence of gastrointestinal surgical complications in renal transplant recipients with polycystic kidney disease. Transplantation. 1999;67:262-6.
32. Sterling RK. Management of gastrointestinal disease in liver transplant recipients. Gastrointest Endosc Clin North Am. 2001;11:185-97.
33. Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: A review. Scand J Gastroenterol (Suppl). 2006;243:89-101.
34. Shah JN, Haigh WG, Lee SP, et al. Biliary casts after orthotopic liver transplantation: Clinical factors, treatment, biochemical analysis. Am J Gastroenterol. 2003;98:1861-7.
35. Buck DG, Zajko AB. Biliary complications after orthotopic liver transplantation. Tech Vasc Interv Radiol. 2008;11:51-9.
36. Lautenschlager I, Halme L, Hockerstedt K, et al. Cytomegalovirus infection of the liver transplant: Virological, histological, immunological, and clinical observations. Transplant. Infect Dis. 2006;8:21-30.
37. Vivarelli M, De Ruvo N, Lazzarotto T, et al. Abstension from treatment of low-level pp65 cytomegalovirus antigenemia after liver transplantation: A prospective study. Transplantation. 2000;70:1183-7.
38. Shi S-h, Lu A-w, Shen Y, et al. Spectrum and risk factors for invasive candidiasis and non-Candida fungal infections after liver transplantation. Chinese Med J. 2008;121:625-30.
39. Kawazu M, Kanda Y, Nannya Y, et al. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-beta-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J Clin Microbiol. 2004;42:2733-41.
40. Marr KA, Balajee SA, McLaughlin L, et al. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: Variables that affect performance. J Infect Dis. 2004;190:641-9.
41. Jacob DA, Neumann UP, Bahra M, et al. Long-term follow-up after recurrence of primary biliary cirrhosis after liver transplantation in 100 patients. Clin Transplant. 2006;20:211-20.
42. Campsen J, Zimmerman MA, Trotter JF, et al. Clinically recurrent primary sclerosing cholangitis following liver transplantation: A time course. Liver Transplant. 2008;14:181-5.
43. Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: A systematic review. Liver Transplant. 2006;12:1813-24.
44. Samuel D, Feray C. Recurrent hepatitis C after liver transplantation: Clinical and therapeutical issues. J Viral Hepat. 2000;7:87-92.
45. Forman LM, Lewis JD, Berlin JA, et al. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889-96.
46. Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? J Hepatol. 2005;42:448-56.
47. Diaz B, Gonzalez Vilchez F, Almenar L, et al. MITOS study group, Gastrointestinal complications in heart transplant patients: MITOS study. Transplant Proc. 2007;39:2397-400.
48. Bravo C, Gispert P, Borro JM, et al. Prevalence and management of gastrointestinal complications in lung transplant patients: MITOS study group. Transplant Proc. 2007;39:2409-12.
49. Paik HC, Kim DH, Lee DY, et al. Gastric ulcer perforation in heart-lung transplant patient: A successful case of early surgical intervention and management. Yonsei Med J. 2003;44:1094-7.
50. Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689-93.
51. Hadjiliadis D, Duane Davis R, Steele MP, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant. 2003;17:363-8.
52. Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108:1602-7.
53. Sodhi SS, Guo JP, Maurer AH, et al. Gastroparesis after combined heart and lung transplantation. J Clin Gastroenterol. 2002 Jan;34:34-9.
54. Verleden GM, Besse T, Maes B. Successful conversion from cyclosporine to tacrolimus for gastric motor dysfunction in a lung transplant recipient. Transplantation. 2002;73:1974-6.
55. Akindipe OA, Faul JL, Vierra MA, et al. The surgical management of severe gastroparesis in heart/lung transplant recipients. Chest. 2000;117:907-10.
56. Lau CL, Palmer SM, Howell DN, et al. Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc. 2002;16:1674-8.
57. Davis RDJr, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533-42.
58. Gilljam M, Chaparro C, Tullis E, et al. GI complications after lung transplantation in patients with cystic fibrosis. Chest. 2003;123:37-41.
59. Delmonico FL. Cadaver donor screening for infectious agents in solid organ transplantation. Clin Infect Dis. 2000;31:781-6.
60. Lunel F, Cadranel JF, Rosenheim M, et al. Hepatitis virus infections in heart transplant recipients: Epidemiology, natural history, characteristics, and impact on survival. Gastroenterology. 2000;119:1064-74.
61. Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: A decade of experience at a single center. Ann Surg. 2001;234:404-16.
62. Abu-Elmagd KM, Zak M, Stamos JM, et al. De novo malignancies after intestinal and multivisceral transplantation. Transplantation. 2004;77:1719-25.
63. Rubin RH. Gastrointestinal infectious disease complications following transplantation and their differentiation from immunosuppressant-induced gastrointestinal toxicities. Clin Transplant. 2001;4:11-22.
64. Hardinger KL, Hebbar S, Bloomer T, Murillo D. Adverse drug reaction driven immunosuppressive drug manipulations: A single-center comparison of enteric-coated mycophenolate sodium vs. mycophenolate mofetil. Clin Transplant. 2008;22:555-61.
65. Smith DM, Agura E, Netto G, et al. Liver transplant-associated graft-versus-host disease. Transplantation. 2003;75:118-26.
66. Assi MA, Pulido JS, Peters SG, et al. Graft-vs.-host disease in lung and other solid organ transplant recipients. Clin Transplant. 2007;21:1-6.
67. Gulbahce HE, Brown CA, Wick M, et al. Graft-vs-host disease after solid organ transplant. Am J Clin Pathol. 2003;119:568-73.
68. Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-16.
69. Pescovitz MD, Navarro MT. Immunosuppressive therapy and post-transplantation diarrhea. Clin Transplant. 2001;4:23-8.
70. Altiparmak MR, Trablus S, Pamuk ON, et al. Diarrhoea following renal transplantation. Clin Transplant. 2002;16:212-16.
71. Underwood JCE, Corbett CL. Persistent diarrhea and hypoalbuminemia associated with cytomegalovirus enteritis. BMJ. 1978;1:1029-30.
72. Remzi FH. Colonic complications of organ transplantation. Transplant Proc. 2002;34:2119-21.
73. Keven K, Basu A, Re L, et al. Clostridium difficile colitis in patients after kidney and pancreas-kidney transplantation. Transpl Infect Dis. 2004;6:10-14.
74. Cesaro S, Chinello P, Rossi L, Zanesco L. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharomyces boulardii. Supp Care Cancer. 2000;8:504-5.
75. Wahbeh G, Hupertz V, Hallowell S, Patel R, Chrisant MR. Idiopathic colitis following cardiac transplantation: Three pediatric cases. Pediatr Transplant. 2003;7:464-8.
76. Bunnapradist S, Neri L, Wong W, et al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis. 2008;51:478-86.
77. Yuan C-S, Israel RJ. Methylnaltrexone, a novel peripheral opioid receptor antagonist for the treatment of opioid side effects. Expert Opin Investig Drugs. 2006;15:541-52.
78. Kaplan B, Meier-Kriesche HU, Jacobs MG, et al. Prevalence of cytomegalovirus in the gastrointestinal tract of renal transplant recipients with persistent abdominal pain. Am J Kidney Dis. 1999;34:65-8.
79. Hoekstra HJ, Hawkins K, de Boer WJ, et al. Gastrointestinal complications in lung transplant survivors that require surgical intervention. Brit J Surg. 2001;88:433-8.
80. Gupta D, Sakorafas GH, McGregor CG, et al. Management of biliary tract disease in heart and lung transplant patients. Surgery. 2000;128:641-9.
81. Behrend M. Adverse gastrointestinal effects of mycophenolate mofetil: Aetiology, incidence and management. Drug Saf. 2001;24:645-63.
82. Verran DJ, Gurkan A, Chui AK, et al. Pancreatitis in adult orthotopic liver allograft recipients: Risk factors and outcome. Liver Transplant. 2000;6:362-6.
83. Hosotani Y, Kawanami C, Hasegawa K, et al. A role of Helicobacter pylori infection in the development of duodenal ulcer after adult living-related liver transplantation. Transplantation. 2003;76:702-4.
84. Andreone P, Gramenzi A, Lorenzini S, et al. Posttransplantation lymphoproliferative disorders. Arch Intern Med. 2003;163:1997-2004.
85. Ganne V, Siddiqi N, Kamaplath B, et al. Humanized anti-CD20 monoclonal antibody (Rituximab) treatment for post-transplant lymphoproliferative disorder. Clin Transplant. 2003;17:417-22.
86. Shehab TM, Hsi ED, Poterucha JJ, et al. Helicobacter pylori–associated gastric MALT lymphoma in liver transplant recipients. Transplantation. 2001;71:1172-5.
87. Haagsma EB, Hagens VE, Schaapveld M, et al. Increased cancer risk after liver transplantation: A population-based study. J Hepatol. 2001;34:84-91.
88. Loftus EVJr, Aguilar HI, Sandborn WJ, et al. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis following orthotopic liver transplantation. Hepatology. 1998;27:685-90.
89. Chand N, Sanyal AJ. Sepsis-induced cholestasis. Hepatology. 2007;45:230-41.
90. Yilmaz N, Shiffman ML, Stravitz RT, et al. A prospective evaluation of fibrosis progression in patients with recurrent hepatitis C virus following liver transplantation. Liver Transplant. 2007;13:975-83.
91. Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274-87.
92. Chan SE, Schwartz JM, Rosen JR. Treatment of hepatitis C in solid organ transplantation. Drugs. 2004;64:489-98.
93. Vela CG, Cristol JP, Descomps B, Mourad G. Prospective study of lipid disorders in FK506-versus cyclosporine-treated renal transplant patients. Transpl Proc. 2000;32:398.
94. Appelbaum FR, Forman SJ, Negrin RS, Blume KG. Thomas’ Hematopoietic Cell Transplantation, 4th ed. Oxford, UK: Wiley-Blackwell Publishing; 2009.
95. Fries BC, Riddell SR, Kim HW, et al. Cytomegalovirus disease before hematopoietic cell transplantation as a risk for complications after transplantation. Biol Blood Marrow Transplant. 2005;11:136-48.
96. Otero Lopez-Cubero S, Sullivan KM, McDonald GB. Course of Crohn’s disease after allogeneic marrow transplantation. Gastroenterology. 1998;114:433-40.
97. Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128:552-63.
98. Kang G, Srivastava A, Pulimood AB, et al. Etiology of diarrhea in patients undergoing allogeneic bone marrow transplantation in South India. Transplantation. 2002;73:1247-51.
99. McLauchlin J, Amar CFL, Pedraza-Diaz S, et al. Polymerase chain reaction-based diagnosis of infection with Cryptosporidium in children with primary immunodeficiencies. Pediatr Infect Dis J. 2003;22:329-35.
100. Dimicoli S, Bensoussan D, Latger-Cannard V, et al. Complete recovery from Cryptosporidium parvum infection with gastroenteritis and sclerosing cholangitis after successful bone marrow transplantation in two brothers with X-linked hyper-IgM syndrome. Bone Marrow Transplant. 2003;32:733-7.
101. Rao A, Kamani N, Filipovich A, et al. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood. 2007;109:383-5.
102. McCullough KD, McDonald GB. Neutropenic enterocolitis. Curr Treat Options Infect Dis. 2003;5:367-75.
103. Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654-9.
104. Mennink-Kersten MASH, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004;4:349-57.
105. Marotta G, Tozzi M, Sammassimo S, et al. Complete resolution of hepatic aspergillosis after non-myeloablative hematopoietic stem cell transplantation in a patient with acute myeloid leukemia. Hematology. 2005;10:383-6.
106. van Burik J-AH. Role of new antifungal agents in prophylaxis of mycoses in high risk patients. Curr Opin Infect Dis. 2005;18:479-83.
107. Ullmann AJ, Cornely OA, Burchardt A, et al. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother. 2006;50:658-66.
108. Lau GKK, Strasser SI, McDonald GB. Hepatitis virus infections in patients with cancer. In: Wingard JR, Bowden RA, editors. Management of infection in oncology patients. London: Martin Dunitz; 2003:321-42.
109. Hui C-k, Lie A, Au W-y, et al. Effectiveness of prophylactic anti-HBV therapy in allogeneic hematopoietic stem cell transplantation with HBsAg positive donors. Am J Transplant. 2005;5:1437-45.
110. Piekarska A, Zaucha JM, Hellman A, McDonald GB. Prevention of hepatitis B virus (HBV) transmission from an infected stem cell donor. Bone Marrow Transplant. 2007;40:399-400.
111. Deschenes M, Laneuville P. Pre-emptive use of lamivudine in bone marrow transplantation with chronic hepatitis B virus infection. Hepatology. 2004;39:867-8.
112. Lau GK, Suri D, Liang R, et al. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002;122:614-24.
113. Surapaneni SN, Hari P, Knox J, et al. Suppressive anti-HCV therapy for prevention of donor to recipient transmission in stem cell transplantation. Am J Gastroenterol. 2007;102:449-51.
114. Strasser SI, Myerson D, Spurgeon CL, et al. Hepatitis C virus infection after bone marrow transplantation: A cohort study with 10 year follow-up. Hepatology. 1999;29:1893-9.
115. Peffault de Latour R, Levy V, Asselah T, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618-24.
116. Picardi M, De Rosa G, Selleri C, et al. Clinical relevance of intrahepatic hepatitis B virus DNA in HBsAg-negative HBcAb-positive patients undergoing hematopoietic stem cell transplantation for hematological malignancies. Transplantation. 2006;82:141-2.
117. Carpenter PA, Huang ML, McDonald GB. Activation of occult hepatitis B from a seronegative patient after hematopoietic cell transplant: A cautionary tale. Blood. 2002;99:4245-6.
118. Hogan WJ, Maris M, Storer B, et al. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: A study of 193 patients. Blood. 2004;103:76-82.
119. Jacobsohn DA, Emerick KM, Scholl P, et al. Nonmyeloablative hematopoietic stem cell transplant for X-linked hyper-immunoglobulin m syndrome with cholangiopathy. Pediatrics. 2004;113:e122-7.
120. McDonald GB, Frieze D. A problem-oriented approach to liver disease in oncology patients. Gut. 2008;57:987-1003.
121. McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043-8.
122. McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: Pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853-62.
123. McDonald GB. Management of hepatic disease following hematopoietic cell transplant. Aliment Pharmacol Ther. 2006;24:441-52.
124. Ohyashiki K, Kuriyama Y, Nakajima A, et al. Imatinib mesylate-induced hepato-toxicity in chronic myeloid leukemia demonstrated focal necrosis resembling acute viral hepatitis. Leukemia. 2002;16:2160-1.
125. Ayoub WS, Geller SA, Tran T, et al. Imatinib (Gleevec)-induced hepatotoxicity. J Clin Gastroenterol. 2005;39:75-7.
126. McKoy JM, Angelotta C, Bennett CL, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): An overview from the research on adverse drug events and reports (RADAR) project. Leukemia Res. 2007;31:599-604.
127. Wadleigh M, Richardson PG, Zahrieh D, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102:1578-82.
128. Safford SD, Safford KM, Martin P, et al. Management of cholelithiasis in pediatric patients who undergo bone marrow transplantation. J Pediatr Surg. 2001;36:86-90.
129. St Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855-61.
130. Lucarelli G, Galimberti M, Polchi P, et al. Marrow transplantation in patients with thalassemia responsive to iron chelation therapy. N Engl J Med. 1993;329:840-4.
131. Malone FR, Leisenring WM, Storer BE, et al. Prolonged anorexia and elevated plasma cytokine levels following myeloablative allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2007;40:765-72.
132. DiBaise JK, Lyden E, Tarantolo SR, et al. A prospective study of gastric emptying and its relationship to the development of nausea, vomiting, and anorexia after autologous stem cell transplantation. Am J Gastroenterol. 2005;100:1571-7.
133. Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320-7.
134. Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-vs.-host disease after marrow transplantation. Gastrointest Endosc. 1999;49:612-21.
135. Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557-63.
136. Castilla C, Perez-Simon JA, Sanchez-Guijo FM, et al. Oral beclomethasone dipropionate for the treatment of gastrointestinal acute graft-versus-host disease (GVHD). Biol Blood Marrow Transplant. 2006;12:936-41.
137. Holmberg L, Kikuchi K, Gooley TA, et al. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: Incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2006;12:226-34.
138. van Burik JA, Lawatsch EJ, DeFor TE, Weisdorf DJ. Cytomegalovirus enteritis among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2001;7:674-9.
139. DiBaise JK, Brand RE, Lyden E, et al. Gastric myoelectrical activity and its relationship to the development of nausea and vomiting after intensive chemotherapy and autologous stem cell transplantation. Am J Gastroenterol. 2001;96:2873-81.
140. Charuhas PM, Fosberg KL, Bruemmer B, et al. A double-blind randomized trial comparing outpatient parenteral nutrition with intravenous hydration: Effect on resumption of oral intake after marrow transplantation. J Parenter Enteral Nutr. 1997;21:157-61.
141. Gooley TA, Rajvanshi P, Schoch HG, McDonald GB. Serum bilirubin levels and mortality after myeloablative allogeneic hematopoietic cell transplantation. Hepatology. 2005;41:345-52.
142. Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977-83.
143. Tay J, Tinmouth A, Fergusson D, et al. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:206-17.
144. McDonald GB, Schoch H, Gooley T. Liver complications of allogeneic hematopoietic cell transplant: Declining incidence related to changes in practice (Abstract). Hepatology. 2008;48(Supplement):318A-19A.
145. Deleve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002;22:27-41.
146. Lassau N, Auperin A, Leclere J, et al. Prognostic value of Doppler-ultrasonography in hepatic veno-occlusive disease. Transplantation. 2002;74:60-6.
147. Erturk SM, Mortele KJ, Binkert CA, et al. CT features of hepatic venoocclusive disease and hepatic graft-versus-host disease in patients after hematopoietic stem cell transplantation. AJR Am J Roentgenol. 2006;186:1497-501.
148. Coy DL, Ormazabal A, Godwin JD, Lalani T. Imaging evaluation of pulmonary and abdominal complications following hematopoietic stem cell transplantation. Radiographics. 2005;25:305-17.
149. Bornhauser M, Storer B, Slattery J, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820-6.
150. de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: Clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857-64.
151. Puig N, de la Rubia J, Remigia MJ, et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma. 2006;47:1488-94.
152. McCune JS, Batchelder AL, Guthrie KA, et al. Personalized dosing of cyclophosphamide in the Total Body Irradiation–cyclophosphamide conditioning regimen: A phase II trial in patients with hematologic malignancy. Clinical Pharmacol Ther. 2009;85:615-22.
153. Salinger DH, McCune JS, Ren AG, et al. Real-time dose adjustment of cyclophosphamide in a preparative regimen for hematopoietic cell transplant: A Bayesian pharmacokinetic approach. Clin Cancer Res. 2006;12:4888-98.
154. Shulman HM, McDonald GB. Hepatic complications of hematopoietic cell transplantation. In: Gershwin ME, Vierling JM, Manns M, editors. Liver immunology: Principles and practice. Totowa, NJ: Humana Press; 2007:409-21.
155. Strasser SI, Shulman HM, Flowers ME, et al. Chronic graft-vs-host disease of the liver: Presentation as an acute hepatitis. Hepatology. 2000;32:1265-71.
156. Akpek G, Boitnott JK, Lee LA, et al. Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood. 2002;100:3903-7.
157. Bruno B, Gooley T, Hackman RC, et al. Adenovirus infection in hematopoietic stem cell transplantation: Effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9:341-52.
158. Walls T, Shankar AG, Shingadia D. Adenovirus: An increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect Dis. 2003;3:79-86.
159. Kuribayashi K, Matsunaga T, Iyama S, et al. Human herpesvirus-6 hepatitis associated with cyclosporine-A encephalitis after bone marrow transplantation for chronic myeloid leukemia. Intern Med. 2006;45:475-8.
160. Yagi T, Karasuno T, Hasegawa T, et al. Acute abdomen without cutaneous signs of varicella zoster virus infection as a late complication of allogeneic bone marrow transplantation: Importance of empiric therapy with acyclovir. Bone Marrow Transplant. 2000;25:1003-5.
161. Neofytos D, Ojha A, Mookerjee B, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant. 2007;13:74-81.
162. Muller WJ, Levin MJ, Shin YK, et al. Clinical and in vitro evaluation of cidofovir for treatment of adenovirus infection in pediatric hematopoietic stem cell transplant recipients. Clin Infect Dis. 2005;41:1812-16.
163. Lau GK, He M-L, Fong DYT, et al. Preemptive use of lamivudine reduces hepatitis B exacerbation after allogeneic hematopoietic cell transplantation. Hepatology. 2002;36:702-9.
164. Tsang SW, Chan HL, Leung NW, et al. Lamivudine treatment for fulminant hepatic failure due to acute exacerbation of chronic hepatitis B infection. Aliment Pharmacol Ther. 2001;15:1737-44.
165. Hui CK, Cheung WWW, Au WY, et al. Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut. 2005;54:1597-603.
166. Lin P-C, Poh S-B, Lee M-Y, et al. Fatal fulminant hepatitis B after withdrawal of prophylactic lamivudine in hematopoietic stem cell transplantation patients. Int J Hematol. 2005;81:349-51.
167. Peffault de Latour R, Asselah T, Levy V, et al. Treatment of chronic hepatitis C virus in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 2005;36:709-13.
168. Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335-47.
169. Winston DJ, Maziarz RT, Chandrasekar PH, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med. 2003;138:705-13.
170. Murakami CS, Louie W, Chan GS, et al. Biliary obstruction in hematopoietic cell transplant recipients: An uncommon diagnosis with specific causes. Bone Marrow Transplant. 1999;23:921-7.
171. Alnusair MM, DeMagalhaes-Silverman M, Silverman WB. The role of ERCP in patients with pancreatico-biliary problems in the setting of hematopoietic stem cell transplant. Gastrointest Endosc. 2006;63:655-9.
172. Frere P, Canivet JL, Gennigens C, et al. Hyperammonemia after high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant. 2000;26:343-5.
173. Chiong MA, Bennetts BH, Strasser SI, Wilcken B. Fatal late-onset ornithine transcarbamylase deficiency after coronary artery bypass surgery. Med J Aust. 2007;186:418-9.
174. Summar ML, Barr F, Dawling S, et al. Unmasked adult-onset urea cycle disorders in the critical care setting. Crit Care Clin. 2005;21:S1-8.
175. Schwartz JM, Wolford JL, Thornquist MD, et al. Severe gastrointestinal bleeding after marrow transplantation, 1987-1997: Incidence, causes, and outcome. Am J Gastroenterol. 2001;96:385-93.
176. Barker CC, Anderson RA, Sauve RS, Butzner JD. GI complications in pediatric patients post-BMT. Bone Marrow Transplant. 2005;36:51-8.
177. Tobin RW, Hackman RC, Hayashi A, et al. Bleeding from gastric antral vascular ectasia in marrow transplant patients. Gastrointest Endosc. 1996;44:223-29.
178. Selinger RRE, McDonald GB, Hockenbery DM, et al. Efficacy of neodymium:YAG laser therapy for gastric antral vascular ectasia (GAVE) following hematopoietic cell transplant. Bone Marrow Transplant. 2006;37:191-7.
179. Pinto-Marques P, Hockenbery DM, Hackman RC, et al. Successful medical treatment of intestinal ulceration caused by Rhizopus microsporus. Bone Marrow Transplant. 2003;32:739-40.
180. Hiller N, Zagal I, Hadas-Halpern I. Spontaneous intramural hematoma of the esophagus. Am J Gastroenterol. 1999;94:2282-4.
181. Otero Lopez-Cubero S, Sale GE, McDonald GB. Acute graft-versus-host disease of the esophagus. Endoscopy. 1997;29:S35-6.
182. Cox GJ, Matsui SM, Lo RS, et al. Etiology and outcome of diarrhea after marrow transplantation: A prospective study. Gastroenterology. 1994;107:1398-407.
183. Kalantari BN, Mortele KJ, Cantisani V, et al. CT features with pathologic correlation of acute gastrointestinal graft-versus-host disease after bone marrow transplantation in adults. Am J Roentgenol. 2003;181:1621-5.
184. Gorg C, Wollenberg B, Beyer J, et al. High-resolution ultrasonography in gastrointestinal graft-versus-host disease. Ann Hematol. 2005;84:33-9.
185. Ertault-Daneshpouy M, Leboeuf C, Lemann M, et al. Pericapillary hemorrhage as criterion of severe human digestive graft-versus-host disease. Blood. 2004;103:4681-4.
186. Socie G, Mary J-Y, Lemann M, et al. Prognostic value of apoptotic cells and infiltrating neutrophils in graft-versus-host disease of the gastrointestinal tract in humans: TNF and Fas expression. Blood. 2004;103:50-7.
187. Janin A, Ertault-Daneshpouy M, Socie G. Eosinophils and severe forms of graft-versus-host disease. Blood. 2003;101:2073.
188. Yakoub-Agha I, Maunoury V, Wacrenier A, et al. Impact of small bowel exploration using video-capsule endoscopy in the management of acute gastrointestinal graft-versus-host disease. Transplantation. 2004;78:1697-701.
189. Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: Significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177-81.
190. Leisenring W, Martin P, Petersdorf E, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749-55.
191. Pham CQD, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40:1261-6.
192. Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile–associated disease. JAMA. 2005;294:2989-95.
193. Sebire NJ, Malone M, Shah N, et al. Pathology of astrovirus associated diarrhoea in a paediatric bone marrow transplant recipient. J Clin Pathol. 2004;57:1001-3.
194. Nicholson O, Feja K, LaRussa P, et al. Nontuberculous mycobacterial infections in pediatric hematopoietic stem cell transplant recipients: Case report and review of the literature. Pediatr Infect Dis J. 2006;25:263-7.
195. Baldwin A, Kingman H, Darville M, et al. Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplant. 2000;26:1333-8.
196. Muller CI, Zeiser R, Grullich C, et al. Intestinal cryptosporidiosis mimicking acute graft-versus-host disease following matched unrelated hematopoietic stem cell transplantation. Transplantation. 2004;77:1478-9.
197. Cordonnier C, Martino R, Trabasso P, et al. Mycobacterial infection: A difficult and late diagnosis in stem cell transplant recipients. Clin Infect Dis. 2004;38:1229-36.
198. Papadimitriou JC, Drachenberg CB, Beskow CO, et al. Graft-versus-host disease–like features in mycophenolate mofetil-related colitis. Transplant Proc. 2001;33:2237-8.
199. Maes BD, Dalle I, Geboes K, et al. Erosive enterocolitis in mycophenolate mofetil–treated renal-transplant recipients with persistent afebrile diarrhea. Transplantation. 2003;75:665-72.
200. Jones AD, Maziarz R, Gilster J, et al. Surgical complications of bone marrow transplantation. Am J Surg. 2003;185:481-4.
201. Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137-41.
202. Ko CW, Gooley T, Schoch HG, et al. Acute pancreatitis in marrow transplant patients: Prevalence at autopsy and risk factor analysis. Bone Marrow Transplant. 1997;20:1081-6.
203. Cartoni C, Dragoni F, Micozzi A, et al. Neutropenic enterocolitis in patients with acute leukemia: Prognostic significance of bowel wall thickening detected by ultrasonography. J Clin Oncol. 2001;19:756-61.
204. Schlatter M, Snyder K, Freyer D. Successful nonoperative management of typhlitis in pediatric oncology patients. J Pediatr Surg. 2002;37:1151-5.
205. Schwartz DA, Harewood GC, Wiersema MJ. EUS for rectal disease. Gastroint Endosc. 2002;56:100-9.
206. Filipovich A, Weisdorf D, Pavletic S, et al. National Institutes of health consensus developement project on criteria for clinical trials in chronic graft-versus-host disease. I Diagnosis of staging working group report. Biol Blood Marrow Transplant. 2005;11:945-55.
207. Mullighan CG, Bogdanos DP, Vergani D, Bardy PG. Cytochrome P450 1A2 is a target antigen in hepatitic graft-versus-host disease. Bone Marrow Transplant. 2006;38:703-5.
208. Arat M, Idilman R, Soydan EA, et al. Ursodeoxycholic acid treatment in isolated chronic graft-vs.-host disease of the liver. Clin Transplant. 2005;19:798-803.
209. Shimizu T, Kasahara M, Tanaka K. Living-donor liver transplantation for chronic hepatic graft-versus-host disease. N Engl J Med. 2006;354:1536-7.
210. Orlando G, Ferrant A, Schots R, et al. Liver transplantation for chronic graft-versus-host disease: Case report with 10-year follow-up. Transpl Int. 2005;18:125-9.
211. Angelucci E, Muretto P, Nicolucci A, et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100:17-21.
212. Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327-31.
213. Dienstag JL, McHutchison JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006;130:225-30.
214. Andreoni KA, Lin JI, Groben PA. Liver transplantation 27 years after bone marrow transplantation from the same living donor. N Engl J Med. 2004;350:2624-5.
215. Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-39.
216. Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699-712.
217. Lucarelli G, Angelucci E, Giardini C, et al. Fate of iron stores in thalassaemia after bone-marrow transplantation. Lancet. 1993;342:1388-91.
218. Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: Joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2006;37:249-61.
219. Kamble R, Mims M. Iron-overload in long-term survivors of hematopoietic transplantation. Bone Marrow Transplant. 2006;37:805-6.
220. Angelucci E, Muretto P, Lucarelli G, et al. Phlebotomy to reduce iron overload in patients cured of thalassemia by bone marrow transplantation. Italian Cooperative Group for Phlebotomy Treatment of Transplanted Thalassemia Patients. Blood. 1997;90:994-8.
221. Tomas JF, Pinilla I, Garcia-Buey ML, et al. Long-term liver dysfunction after allogeneic bone marrow transplantation: Clinical features and course in 61 patients. Bone Marrow Transplant. 2000;26:649-55.
222. Muretto P, Angelucci E, Lucarelli G. Reversibility of cirrhosis in patients cured of thalassemia by bone marrow transplantation. Ann Intern Med. 2002;136:667-72.
223. Kaplowitz N, DeLeve LD, editors. Drug induced liver disease, 2nd ed, New York: Informa Healthcare USA, 2007.
224. Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581-8.
225. Oliver MR, Van Voorhis WC, Boeckh M, et al. Hepatic mucormycosis in a bone marrow transplant recipient who ingested naturopathic medicine. Clin Infect Dis. 1996;22:521-4.
226. Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464-71.
227. Ioannou GN, Splan MF, Weiss NS, et al. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-45.
228. Bruix J, Sherman M, Practice Guidelines Committee AASLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-36.
229. Nieters A, Kallinowski B, Brennan P, et al. Hepatitis C and risk of lymphoma: Results of the European multicenter case-control study EPILYMPH. Gastroenterology. 2006;131:1879-86.
230. Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Systematic review: Regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Thera. 2005;21:653-62.
231. Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: A report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787-97.
232. Sudour H, Mainard L, Baumann C, et al. Focal hepatic hyperplasia following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2009;43:127-32.
233. McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-21.
234. McDonald GB, Sullivan KM, Plumley TF. Radiographic features of esophageal involvement in chronic graft-versus-host disease. Am J Roentgenol. 1984;142:501-6.
235. Minocha A, Mandanas RA, Kida M, Jazzar A. Bullous esophagitis due to chronic graft-versus-host disease. Am J Gastroenterol. 1997;92:529-30.
236. Sakai M, McDonald GB. Gastrointestinal and hepatic manifestations of chronic GVHD. In: Vogelsang G, Pavletic S, editors. Chronic graft-versus-host disease: Principles and practice of interdisciplinary management. New York: Cambridge University Press; 2008:216-226.
237. Mackey JR, Desai S, Larratt L, et al. Myasthenia gravis in association with allogeneic bone marrow transplantation: Clinical observations, therapeutic implications and review of literature. Bone Marrow Transplant. 1997;19:939-42.
238. Shimada K, Yokozawa T, Atsuta Y, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: Incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36:115-21.
239. Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756-62.
240. Patey-Mariaud de Serre N, Reijasse D, Verkarre V, et al. Chronic intestinal graft-versus-host disease: Clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplant. 2002;29:223-30.
241. Akpek G, Chinratanalab W, Lee LA, et al. Gastrointestinal involvement in chronic graft-versus-host disease: A clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46-51.
242. Iyer RV, Hahn T, Roy HN, et al. Long-term use of oral beclomethasone dipropionate for the treatment of gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:587-92.
243. Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 29 Seattle patients. Am J Med. 1980;69:204-17.
244. Borgaonkar MR, Duggan PR, Adams G. Differing clinical manifestations of celiac disease transmitted by bone marrow transplantation. Dig Dis Sci. 2006;51:210-12.
245. Lorber MI, Van Buren CT, Flechner SM, et al. Hepatobiliary and pancreatic complications of cyclosporine therapy in 466 renal transplant recipients. Transplantation. 1987;43:35-40.
246. Sastry J, Young S, Shaw PJ. Acute pancreatitis due to tacrolimus in a case of allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:867-8.
247. Ogunseinde BA, Wimmers E, Washington B, et al. A case of tacrolimus (FK506)-induced pancreatitis and fatality 2 years postcadaveric renal transplant. Transplantation. 2003;76:448.
248. Ljungman P, Johansson N, Aschan J, et al. Long-term effects of hepatitis C virus infection in allogeneic bone marrow transplant recipients. Blood. 1995;86:1614-18.
249. Akpek G, Valladares JL, Lee L, et al. Pancreatic insufficiency in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2001;27:163-6.
250. Grigg AP, Angus PW, Hoyt R, Szer J. The incidence, pathogenesis and natural history of steatorrhea after bone marrow transplantation. Bone Marrow Transplant. 2003;31:701-3.