CHAPTER 51 Gastritis and Gastropathies
Patients, clinicians, endoscopists, and pathologists have different concepts of what gastritis is. Some think of it as a symptom complex, others as a description of the endoscopic appearance of the stomach, and still others use the term to describe microscopic inflammation of the stomach. This third definition of gastritis is used in this chapter. There is not a close relationship between the presence of microscopic inflammation (histologic gastritis) and gastric symptoms (epigastric pain, nausea, vomiting, bleeding). The correlation between microscopic and gastroscopic abnormalities is also poor.1–2 In fact, most patients with histologic gastritis are asymptomatic and have normal gastroscopic findings. Certain disorders of the gastric mucosa including erosive processes and hyperplastic disorders may be associated with little or no inflammation (gastritis). These conditions collectively are referred to as reactive and hyperplastic gastropathies, respectively.
By the earlier definition, a gastric biopsy must be obtained to be able to diagnose gastritis. Every biopsy represents an excellent opportunity for the clinician and pathologist to communicate to correlate clinical data, endoscopic findings, and pathology. Errors may occur when the pathologist attempts to diagnose biopsies without clinical input. It is important for the pathologist to become familiar with the range of normal gastric biopsy findings because many gastrointestinal biopsies obtained endoscopically show normal mucosa.3
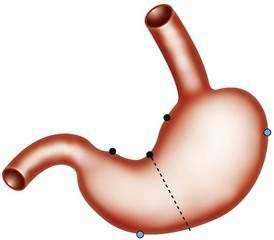
Indications for gastroscopic biopsies include gastric erosion or ulcer, thick gastric fold(s), gastric polyp(s) or mass(es), and diagnosis of Helicobacter pylori infection. A set of five biopsies should be taken from patients in whom clinical or endoscopic findings are suspicious for one of the forms of chronic gastritis (discussed later). Preferred sites for this set of biopsies are shown in Figure 51-1. The location of the biopsy sites should be identified for the pathologist on an accessioning form.
CLASSIFICATION2–7
There is no universally accepted classification of gastritis. The Sydney system was an attempt to unify terminology for endoscopic and histologic gastritis and gastropathy, and it was updated in 1995.4 However, the complexity of the Sydney system precluded widespread use. Failure to obtain adequate numbers of biopsies from various regions of the stomach (see Fig. 51-1) often prevents accurate classification and often precludes a thorough assessment of the distribution of gastritis.5
In this chapter we use a combination of classifications of gastritis by four experts: Rubin,2 Genta,4 Appelman,6 and Montgomery.7 The keystone of the mentioned classification is the fact that H. pylori and nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common causes of gastritis and reactive gastropathies (acute erosive gastritis), respectively. The chapter outline provides an etiology-based classification of gastritis and gastropathies.
CHRONIC GASTRITIS7–9
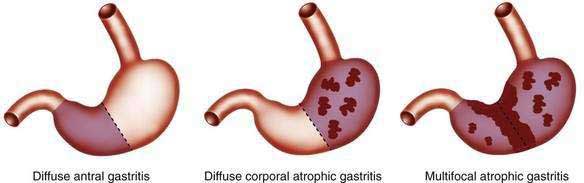
Most forms of chronic gastritis are clinically silent. Their importance relates to the fact that these gastritides are risk factors for other conditions such as peptic ulcer disease, gastric polyps, and benign and malignant gastric neoplasms.8,9 Three types of chronic gastritis are recognized (Figs. 51-2 and 51-3).
Biopsies from the antrum and the incisura are useful for diagnosing H. pylori infection with its diffuse antral-predominant gastritis. However, biopsies from the gastric body mucosa may be more diagnostic for H. pylori infection in some patients treated with proton pump inhibitors. Environmental metaplastic atrophic gastritis (also called multifocal atrophic gastritis, or MAG) is patchy and involves the antrum and body mucosa and sometimes, but not always, is associated with H. pylori infection.7 The diagnosis of autoimmune metaplastic atrophic gastritis, also called diffuse corporal atrophic gastritis (DCAG) and type A gastritis, can be confirmed with multiple biopsies from the gastric body that show atrophy and biopsies from the antrum that do not show atrophy. In most cases biopsies are obtained at the time of endoscopy.
HELICOBACTER PYLORI GASTRITIS2,6,7,10–19
H. pylori gastritis (HPG) is caused by infection of the antral mucosa with H. pylori.2,6,7 In the United States H. pylori gastritis is seen mainly in low socioeconomic and immigrant populations,7 and there is no increased risk of gastric cancer. Most patients with HPG are asymptomatic. In most cases the antrum appears normal to the endoscopist; some patients with active disease in the antrum may demonstrate red streaks. Radiographic differences between antral gastritis due to H. pylori and not due to H. pylori have been described; thickened gastric folds, especially in a polypoid configuration, and enlarged areae gastricae favor H. pylori as the cause, whereas antral erosions favor causes other than H. pylori.10
Histologically, a diffuse, chronic inflammatory infiltrate which includes numerous lymphocytes and plasma cells expands the lamina propria and epithelium (see Fig 51-3).7 The presence of acute inflammatory cells is best designated an active gastritis and not acute gastritis. Additional microscopic changes include injury to the surface and foveolar epithelium with loss of apical mucin and reactive nuclear changes and erosions.6,11 Lymphoid follicles with germinal centers are characteristic of an infection with H. pylori.3,12 H. pylori organisms lie in the superficial mucous layer along the mucosal surface and within the gastric pits. Although the organisms can be seen in routine hematoxylin and eosin–stained tissue when numerous organisms are present, special stains are useful when few organisms are present. Stains that may be used to highlight the organisms are Giemsa stain, Warthin-Starry silver stain, Gram stain, and immunocytochemical stains.13–15 Helicobacter heilmannii spiral bacteria are a less frequent cause of active gastritis.16–18 The organisms originally known as Gastrospirillum hominis are longer than H. pylori and have multiple spirals.16,17 A topographic study of H. pylori density and distribution and the comparison of biopsy sites for the histopathologic diagnosis of H. pylori conclude that two antral biopsy specimens, one from the lesser and one from the greater curvature, have close to 100% sensitivity for detecting H. pylori infection (see Fig. 51-1).19 Biopsy specimens from the corpus increase the diagnostic yield if extensive intestinal metaplasia is present in the antrum.19
ENVIRONMENTAL METAPLASTIC ATROPHIC GASTRITIS4,7,20–32
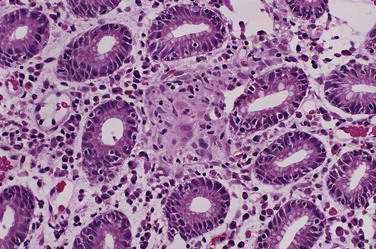
Environmental metaplastic atrophic gastritis (EMAG), also called mutifocal atrophic gastritis (MAG), is characterized by the involvement of the antrum and body with mucosal atrophy and intestinal metaplasia.4,7,20–22 Atrophic gastritis involving the body may be associated with pseudopyloric metaplasia, in which the mucosa resembles antral mucosa but stains for pepsinogen I (PGI), a proenzyme expressed in body mucosa.23 Gastroscopy may show a pale mucosa, shiny surface, and prominent submucosal vessels,24 and magnifying endoscopy is much more sensitive in detecting atrophy.25 The pathogenesis of EMAG is multifactorial. H. pylori plays an important role and has been identified in about 85% of patients with EMAG. EMAG can occur early in life in H. pylori–infected individuals living in developing countries.23 Genetic and environmental factors, especially diet, are also important. Certain population groups are predisposed to EMAG including African Americans, Scandinavians, Asians, Hispanics, Central and South Americans, Japanese, and Chinese.
In patients with EMAG, intestinal metaplasia is a risk factor for dysplasia and gastric cancer, usually the intestinal type (see Chapter 54).2,4,7,22,26–31 Inflammation in EMAG destroys gastric epithelial cells, and eventually the atrophic glands are replaced by metaplastic epithelium.4,7,22 In some cases, especially in patients living in the Pacific basin, metaplastic gastric cells are ciliated, probably due to environmental factors that are more prominent in the Pacific than the Atlantic Ocean basins.32 Because criteria for gastric atrophy among pathologists are debated, intestinal metaplasia is the most reliable marker of atrophy. Intestinal metaplasia of the gastric mucosa can be classified into three types as described in Chapter 54, where their possible associations with the intestinal type of gastric cancer are discussed.
AUTOIMMUNE METAPLASTIC ATROPHIC GASTRITIS6,7,33–55
Autoimmune metaplastic atrophic gastritis (AMAG), also called diffuse corporal atrophic gastritis (DCAG), is an autoimmune destruction of body/fundic glands. AMAG is relatively uncommon, accounting for less than 5% of all cases of chronic gastritis. Endoscopic features of AMAG include effacement of the gastric folds and a thin body/fundic mucosa. AMAG is the pathologic process in patients with pernicious anemia, an autoimmune disorder usually occurring in patients of northern European or Scandinavian background.33 Patients with AMAG exhibit achlorhydria or hypochlorhydria, hypergastrinemia secondary to low or absent gastric acid with antral G-cell hyperplasia, and low serum PGI concentrations, and they often have circulating antibodies to parietal cell antigens and to intrinsic factor.6,7,33 Incomplete (colonic) intestinal metaplasia (type III) may occur in AMAG and be a risk factor for gastric carcinoma in areas of the world that experience a higher incidence of gastric carcinoma than in the United States.34 Metaplastic intestinal Paneth cells in AMAG appear to secrete an antibacterial peptide of the alpha-defensin family, human defensin 5 (HD-5), a peptide not produced in the normal stomach.35 HD-5 could help the atrophic stomach against invasion by indigenous bacterial flora that overgrow in the anacidic stomach (see Chapter 49). Metaplastic pancreatic acinar cells are also a feature of autoimmune gastritis.36
Atrophic glands with extensive intestinal metaplasia are confined to the body/fundic mucosa. Early in the course of this disease, atrophy may be focal and the preserved islands of relatively normal oxyntic mucosa may appear polypoid endoscopically or radiologically.37 Rarely, AMAG progresses to diffuse (complete) atrophy. Hypergastrinemia, a consequence of achlorhydria, is associated with an increase in enterochromaffin-like cell hyperplasia and gastric carcinoid tumors. Cases of gastric carcinoids and simultaneous gastric cancer have been described.38 Gastric carcinoid tumors are discussed further in Chapters 31 and 54.
In one study from Italy, half of 150 patients with AMAG had antibodies to H. pylori and another 25% had H. pylori in their oxyntic mucosa in addition to having antibodies against H. pylori.39 Thus, H. pylori could have contributed to three quarters of the cases of AMAG. Recent studies suggest a role for H. pylori in the early pathogenesis of autoimmune gastritis; evidence of infection early in the course of the disease in individuals with parietal cell antibodies is frequent.40 If gastric atrophy and achlorhydria develop, the incidence of H. pylori infection then decreases. Among 267 H. pylori–infected patients with dyspepsia, 65 had AMAG. Compared with the 202 patients without AMAG, the atrophics were older, more likely to have antibodies against cagA and vacA, more likely to consume alcohol and coffee, more likely to be taking sedative medicines, and less likely to have anxiety.41 Whether H. pylori results in AMAG thus appears to depend on length of infection, as well as bacterial, dietary, and emotional factors.
With regard to bacterial factors promoting atrophy, it appears that cagA+/vacA+ H. pylori are more likely to cause AMAG. These H. pylori are often the s1m1 vacA subtype that also express Lewis blood group antigens X and Y.42 Lewis antigens may help camouflage H. pylori because these antigens are also present on human gastric epithelial cells. It has been suggested that when antibodies to Lewis antigens from H. pylori develop, they cross-react with antigens on epithelial cells such as the H+,K+-ATPase on parietal cells, resulting in autoimmune chronic gastritis.43 Based on uncontrolled studies from Tokyo,44 eradication of H. pylori often leads to a decrease in the amount of gastric atrophy and intestinal metaplasia, whereas failed eradication attempts accomplish neither of these endpoints.
Antibodies to parietal cell antigens, most notably the proton pump (H+,K+-ATPase) are frequently present in autoimmune gastritis.45 These antibodies are frequently detected in patients with various autoimmune diseases including type 1 diabetes mellitus46 and thyroid diseases (Graves’, Hashimoto’s), explaining the association of these conditions with pernicious anemia. The risk of AMAG is increased three- to five-fold in type 1 diabetic individuals, and some authors have suggested screening type 1 diabetics with gastroscopy and mucosal biopsy.47 One in eight patients with chronic hepatitis C treated with interferon-α develops antibodies to parietal cells and to thyroid tissue, and these antibodies recede after therapy is stopped48; the clinical significance of these findings in the stomach is yet to be elucidated.
A proportion of the CD4+ lymphocytes present in the chronic inflammatory infiltrate within the gastric mucosa proliferate in response to H+,K+-ATPase, and most CD4+ cells secrete Th1 cytokines such as tumor necrosis factor-α (TNF-α); provide help for B cell immunoglobulin production; and enhance perforin-mediated cytotoxicity, as well as Fas ligand–mediated apoptosis.45 These factors in combination may contribute to gland destruction in autoimmune gastritis. An interesting animal model of autoimmune gastritis has been developed in mice in which CD4+ T cells target the β subunit of the H+,K+-ATPase.49
The risk of gastric adenocarcinoma in patients with AMAG is unclear. One recent study suggested a cancer risk of slightly more than 1% per year,50 which would favor periodic endoscopic screening for individuals known to have AMAG. However, other investigators have found cancer much less often and have questioned the cost-effectiveness of cancer screening by endoscopy in AMAG.51,52 The importance of incomplete intestinal metaplasia (type III) as a predictor of gastric cancer also has been questioned.53 Thus, at what intervals AMAG patients should be screened, if at all, remains a matter of debate.54
Molecular events involved in the sequence from AMAG to intestinal metaplasia are beginning to be clarified. For example, the expression of the intestinal transcription factor CDX2 precedes expression of other intestinal-specific genes such as CDX1, alkaline phosphatase, MUC2, HD-5, and sucrase-isomaltase55 and thus may be an early trigger of the metaplastic process that precedes dysplasia and carcinogenesis.
CARDITIS56,57
There has been recent attention to inflammation of the small rim of cardiac glands at the proximal portion of the stomach.56 The pathogenesis of carditis is currently controversial.57 Inflammation of this gland area has been attributed to H. pylori gastritis, EMAG, AMAG, gastroesophageal reflux disease, and other factors. Likewise, atrophy in this area, often accompanied by intestinal metaplasia, has been proposed to be a precursor of adenocarcinoma of the gastroesophageal junction (see Chapters 42 and 44). Der and associates56 reported on 141 patients in whom the cardiac mucosa could be identified in endoscopic biopsies. In this endoscopy population, all biopsies exhibited acute and/or chronic carditis. Nearly 80% of them had no evidence of H. pylori infection on simultaneous biopsies from the gastric body and antrum. H. pylori was present in 20 patients, 17 of whom had pan-gastritis and 15 of whom had H. pylori carditis. The severity of chronic carditis was related directly to 24-hour acid exposure of the lower esophagus, whereas acute carditis was related to H. pylori infection.
INFECTIOUS GASTRITIDES58–123
VIRUSES
Cytomegalovirus60–64
Cytomegalovirus (CMV) is a human herpesvirus that may affect the esophagus, stomach, small bowel, colon, rectum, anus, liver, and gallbladder. CMV infection may occur in an immunocompetent patient.57 However, gastrointestinal CMV infection usually occurs in the immunocompromised patient. Eosinophilic gastroenteritis with cytomeglovirus infection has been reported in an immunocompetent child.62 Patients with malignant disease, immunosuppression (especially due to steroid therapy), transplants, and acquired immunodeficiency syndrome (AIDS) may experience life-threatening CMV infections.
Patients with CMV infection of the stomach may experience epigastric pain, fever, and atypical lymphocytosis. Upper gastrointestinal tract radiographic studies may reveal a rigid and narrowed gastric antrum suggestive of an infiltrating antral neoplasm. Endoscopic studies may reveal a congested and edematous mucosa of the gastric antrum, covered with multiple ulcerations, suggestive of gastric malignancy, submucosal antral mass, or gastric ulcer (Fig. 51-4). A hypertrophic and/or polypoid type of gastritis resembling Ménétrier’s disease with a similar type of protein-losing gastropathy has been described.58,59
Examination of biopsy specimens shows inflammatory debris, chronic active gastritis, and enlarged cells with CMV inclusion bodies indicative of an active infection (see Fig. 51-4). “Owl-eye” intranuclear inclusions are the hallmark of CMV infection in routine hematoxylin and eosin histologic preparations and may be found in vascular endothelial cells, mucosal epithelial cells, and connective tissue stromal cells. Multiple, granular, basophilic, cytoplasmic inclusions may also be present. Usual treatment with intravenous ganciclovir or foscarnet is of uncertain value (see Chapter 33).
Other Herpesviruses65–70
Gastric involvement with herpes simplex and varicella-zoster virus is rare. Infected individuals experience the infection at an early age, and the virus remains dormant until reactivation. Activation has been related to radiation therapy, chemotherapy, lymphoma, and cancer. The typical immunocompromised patient may experience nausea, vomiting, fever, chills, fatigue, cough, and weight loss. An acute abdomen caused by varicella-zoster virus–induced gastritis after autologous peripheral blood stem cell transplantation in a patient with non-Hodgkin’s lymphoma has been reported.67 Barium-air double-contrast radiographs show a cobblestone pattern, shallow ulcerations with a ragged contour, and an interlacing network of crevices filled with barium that corresponds to areas of ulceration. Upper gastrointestinal endoscopy reveals multiple, small, raised, ulcerated plaques or linear, superficial ulcers in a crisscrossing pattern, giving the stomach a cobblestone appearance. Grossly, the ulcers are multiple, small, and of uniform size. Microscopically, cytologic smears and biopsy specimens show numerous single cells and clumps of cells, with ground-glass nuclei and eosinophilic intranuclear inclusion bodies surrounded by halos. Brush cytology and biopsies should be performed at the time of endoscopy. Brush cytology has the advantage of sampling a wider area of mucosa because biopsies may not be representative. Treatment with acyclovir is reasonable but of unproven value.
Human herpesvirus 7, a cause of roseola, is frequently present in the gastric mucosa but does not appear to cause gastritis.68 Epstein-Barr virus (EBV) may cause an acute gastritis with lymphoid hyperplasia.69 There is little evidence that EBV causes chronic gastritis.70
Measles71
Rare cases of morbilliform gastritis with giant cells of the Warthin-Finkeldey type have been described.71
Enterovirus72
Recently, it has been proposed that some patients with chronic fatigue syndrome are chronically infected with a noncytopathic, noncytolyic enterovirus that can be detected by immunostaining or by reverse transcriptase–polymerase chain reaction (PCR) techniques using gastric biopsy samples.72 Confirmatory studies are awaited.
BACTERIA
Helicobacter pylori (see earlier and Chapter 50)
Phlegmonous (Suppurative) and Emphysematous Gastritis73–82
Phlegmonous gastritis is a rare bacterial infection of the submucosa and muscularis propria of the stomach. Acute necrotizing gastritis (gangrene of the stomach) is a rare, often fatal disease that is now thought to be a variant of phlegmonous gastritis. It has been suggested that acute necrotizing gastritis begins as phlegmonous gastritis, producing primary necrosis and gangrene. Acute necrotizing gastritis and phlegmonous gastritis have been associated with a recent large intake of alcohol; upper respiratory tract infection; AIDS and other immunocompromised states; and an infected peritoneojugular venous shunt. Fulminant and fatal gas gangrene of the stomach in a healthy, live liver donor has been reported.76 Patients typically present with acute upper abdominal pain, peritonitis, purulent ascitic fluid, fever, and hypotension. Preoperative diagnosis is possible with plain film, ultrasonography, or computed tomography (CT), and gastroscopy with or without biopsy and culture of gastric contents may establish the diagnosis. Grossly, the stomach wall appears thick and edematous with multiple perforations, and the mucosa may demonstrate a granular, green-black exudate. Microscopically, the edematous submucosa reveals an intense polymorphonuclear infiltrate and numerous gram-positive and gram-negative organisms, as well as vascular thrombosis. The mucosa may demonstrate extensive areas of necrosis.
Emphysematous gastritis is a variant of phlegmonous gastritis in which the infection in the gastric wall is due to gas-forming organisms such as Clostridium welchii. Emphysematous gastritis associated with invasive gastric mucormycosis has been reported.77 Predisposing factors are gastroduodenal surgery, ingestion of corrosive materials, gastroenteritis, or gastrointestinal infarction. Radiographic studies (plain films, CT) show gas bubbles conforming to the contour of the stomach, often in the form of cystic gas pockets.82
Mycobacteria83–85
Gastric infection with Mycobacterium tuberculosis is a rare entity that usually occurs in association with pulmonary tuberculosis. Patients typically present with abdominal pain, nausea and vomiting, gastrointestinal bleeding, fever, and weight loss. Gastric tuberculosis associated with anemia has been reported.85 Gastric tuberculosis may be associated with gastric outlet obstruction or with bleeding from a tuberculous gastric ulcer. Radiographic studies reveal an enlarged stomach with narrowed, deformed antrum with prepyloric ulcerations. Upper endoscopy demonstrates ulcers, masses, or gastric outlet obstruction. Grossly, the stomach may demonstrate multiple small mucosal erosions, ulcers, an infiltrating mass (hypertrophic) form, a sclerosing inflammatory form, acute miliary dissemination, and pyloric obstruction either by extension from peripyloric nodes or by invasion from other neighboring organs. Biopsies show necrotizing granulomas with the presence of acid-fast bacilli, best demonstrated with Kinyoun acid-fast stain. Treatment is discussed in Chapter 107.
Although Mycobacterium avium complex (MAC) is a common opportunistic bacterial infection among patients with AIDS, the stomach is rarely involved. Gastric MAC may be associated with a chronic gastric ulcer refractory to conventional antiulcer therapy. Patients may present with fever, night sweats, anorexia, weight loss, diarrhea, abdominal pain, chylous ascites, severe gastrointestinal hemorrhage, or chronic gastric ulcer. Serial CT scans of the abdomen may show mesenteric lymphadenopathy. Endoscopy may show a chronic gastric ulcer, a coarsely granular duodenal mucosa, or fine white duodenal nodules. Microscopically, the gastric mucosa demonstrates numerous foamy histiocytes containing many acid-fast bacilli. Treatment of MAC is difficult and is discussed in Chapter 33.
Syphilis88–94
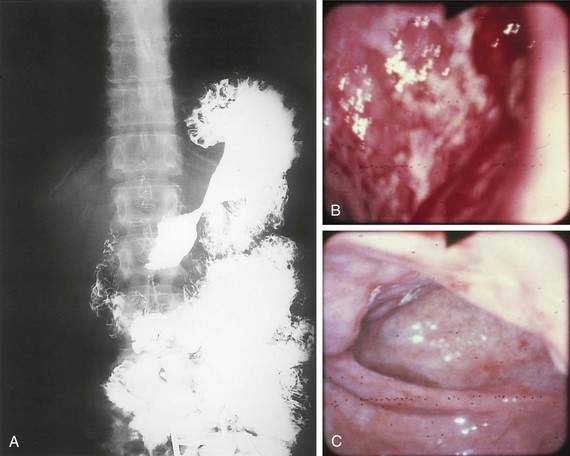
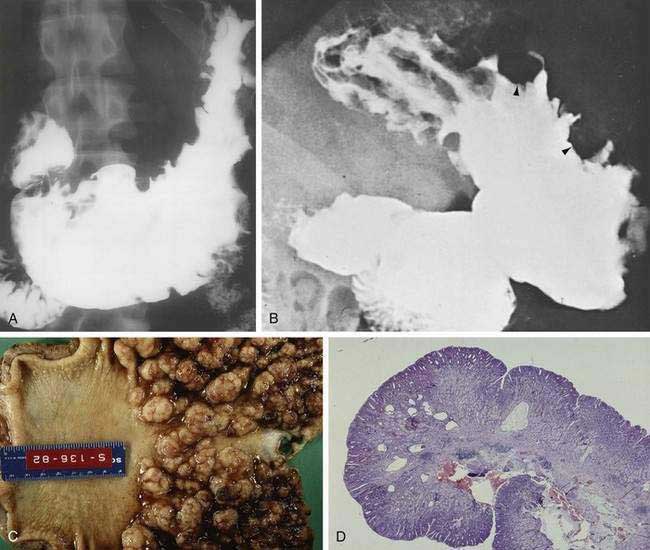
The incidence of syphilis in the United States increased 34% from 13.7 to 18.4 cases per 100,000 persons between 1981 and 1989. Several case reports and small series emphasize the importance of the gastroenterologist and pathologist remaining alert to the protean manifestations of syphilis and familiar with the histopathologic pattern of the disease. Gastric involvement in secondary or tertiary syphilis is rarely recognized clinically, and its diagnosis by examination of endoscopic biopsy specimens has been reported infrequently. The features of syphilis in the stomach should be recognized because they can provide a window of opportunity for effective antibiotic therapy before the disease progresses and causes permanent disability. Patients typically present with symptoms of peptic ulcer disease, and the most common gastric complaint is upper gastrointestinal tract bleeding. Other diseases that may mimic gastric syphilis include benign ulcer disease, gastric carcinoma, gastric lymphoma, tuberculosis, and gastric Crohn’s disease. Gastric syphilis in the setting of human immunodeficiency virus (HIV) has been reported.92 The acute gastritis of early secondary syphilis produces the earliest radiologically detectable sign of the disease. Radiographs show a nonspecific gastritis with diffusely thickened folds that may become nodular with or without detectable ulcers. Strictures in the mid-stomach (“hourglass” stomach) may be present (Fig. 51-5A). Endoscopy shows numerous shallow, irregular ulcers with overlying white exudate and surrounding erythema (see Fig. 51-5B). The surrounding mucosa also demonstrates a nodular appearance. Gastroscopy may also demonstrate prominent, edematous gastric folds.
Grossly, the stomach may be thickened and contracted and may show multiple serpiginous ulcers. Partial gastrectomy specimens may show compact, thick, mucosal rugae and numerous small mucosal ulcers. Microscopically, biopsies show severe gastritis with dense plasma cell infiltrate in the lamina propria, varying numbers of neutrophils and lymphocytes, gland destruction, vasculitis, and granulomas. Warthin-Starry silver stain or modified Steiner silver impregnation stain reveals numerous spirochetes. Serum Venereal Disease Research Laboratory (VDRL) and Treponema immunofluorescence studies may be positive, and the Treponema pallidum gene may be detected by the PCR. Treatment with penicillin is highly effective (see Fig. 51-5C).
Other Bacteria95,96
Because approximately 25% of patients with chronic gastritis have no current or past evidence of infection with H. pylori or other Helicobacters such as H. heilmannii, other bacteria have been sought. One gram-negative bacillus, Acinetobacter lwoffi, is a common commensal that is normally not pathogenic in humans but has been proposed to cause gastritis in a manner analogous to H. pylori.84 A case of transient gastritis caused by the gram-positive enterococcus has also been described.85
FUNGI
Histoplasmosis99–101
Progressive disseminated histoplasmosis is rare, occurring most frequently in the very young or the older adult or in those with immunodeficiency. Although disseminated histoplasmosis can involve any portion of the gastrointestinal tract, gastric involvement is rare. Hypertrophic gastric folds or a mass that mimics a gastric carcinoma may be associated with gastric histoplasmosis or disseminated histoplasmosis has been reported.100 Radiographic studies may demonstrate an annular infiltrating lesion of the stomach, and endoscopy may demonstrate enlarged and reddened gastric folds. Biopsy specimens show an intensive infiltration of macrophages containing Histoplasma capsulatum. Gastric histoplasmosis has also been associated with a fatal hemorrhage from a gastric ulcer. Treatment with intravenous amphotericin B is appropriate.
Phycomycosis102–104
Gastric phycomycosis (also called zygomycosis or mucormycosis) is a rare and highly lethal fungal infection. Phycomycosis usually affects the paranasal sinuses, central nervous system, or lungs and is rarely confined to the gastrointestinal tract. Risk factors include malnutrition, immunosuppression, antibiotic therapy, and acidosis, usually diabetic ketoacidosis. A nosocomial outbreak of gastric mucormycosis due to contamination of wooden tongue depressors by Rhizopus microspores has been reported. Most patients presented with upper gastrointestinal bleeding.103 Gastric phycomycosis can be classified as invasive or noninvasive (colonization). The former is characterized by deep invasion of the stomach wall and by blood vessel involvement with the fungus. Abdominal pain is the most frequent presenting complaint. In the noninvasive type, the fungus colonizes the superficial mucosa without causing an inflammatory response.
PARASITES (see also Chapters 109 and 110)
Cryptosporidiosis107–110
Cryptosporidiosis may rarely involve the stomach. Gastric outlet obstruction and antral stricture due to cryptosporidiosis have been reported in patients with AIDS and diarrhea. A case of cryptosporidiosis-associated erosive gastritis in a patient with HIV infection also has been reported. Also, cryptosporidiosis associated with the immunocompromised state and small cell lung cancer has been reported.110
Strongyloidiasis111–115
The stomach is rarely affected by Strongyloides stercoralis. However, the organisms may colonize the intact gastric mucosa and may be associated with a bleeding peptic ulcer. S. stercoralis hyperinfection has been associated with cimetidine therapy in an immunosuppressed patient and was diagnosed by endoscopic gastric biopsy. Diagnosis can be made by endoscopic biopsy, examination of stools, examination of duodenal aspirate, and examination of peripheral smear with elevated eosinophil count. A histologic diagnosis of strongyloidiasis must be taken into consideration when examining gastric and duodenal biopsies in immunocompromised patients.113 Disseminated strongyloidiasis can be rapidly fatal. Treatment is discussed in Chapter 110.
Anisakiasis116–120
Invasive anisakiasis may occur after the ingestion of raw marine fish containing nematode larvae of the genus Anisakis. Hundreds of cases of anisakiasis have been diagnosed in Japan, and the number of reported cases in the United States has also increased. The parasite may migrate into the wall of the stomach, small intestine, or colon. Typically, patients present with sporadic epigastric pain or have no symptoms at all. Gastric perforation due to chronic gastric aniskiasis has been reported.120 Misdiagnosis is common. Some patients may experience a mild peripheral eosinophilia. Endoscopy may show firm, yellowish submucosal masses with erosions.119 Radiographic studies may reveal notched-shadow defects suggestive of a gastric tumor.
GRANULOMATOUS GASTRITIDES
A variety of granulomatous diseases affect the stomach. Crohn’s disease is the most common (Fig. 51-6) and is discussed later and also in Chapter 111. The differential diagnosis of granulomatous gastritis also includes sarcoidosis, as well as rarer conditions such as xanthogranulomatous gastritis, foreign bodies,105 lymphoma,106 Whipple’s disease (see Chapter 106),107 Langerhans cell histiocytosis (gastric eosinophilic granuloma),108 granulomatous vasculitis109 (Churg-Strauss syndrome), and chronic granulomatous disease of childhood.110 An isolated, idiopathic granulomatous gastritis also occurs.111 Some of these latter cases may evolve to Crohn’s disease or sarcoidosis over time. Other cases of “idiopathic” granulomatous gastritis appear to be due to H. pylori infection and may resolve, albeit slowly, following appropriate antibiotic therapy.112,113 Idiopathic granulomatous gastritis can be associated with gastric cancer.114
SARCOIDOSIS124–137
Gastrointestinal manifestations of sarcoidosis are uncommon (see Chapter 35). Sarcoidosis is a systemic disease, frequently involving the lungs, lymph nodes, skin, and eyes. Diagnosis of sarcoidosis of the stomach cannot be made with confidence in the absence of disease in other organs. The stomach (usually the antrum) is the most common part of the gastrointestinal tract affected in sarcoidosis, being involved in 10% of cases.137
XANTHOGRANULOMATOUS GASTRITIS138–139
Xanthogranulomatous gastritis is characterized by inflammation of the gastric wall by foamy histiocytes, inflammatory cells, multinucleated giant cells, and fibrosis. The destructive inflammatory process may extend into adjacent organs and simulate a neoplasm. Xanthogranulomatous gastritis has been associated with xanthogranulomatous cholecystitis. Xanthogranulomatous gastritis with pseudosarcomatous changes mimicking a neoplasm has been reported.139
DISTINCTIVE GASTRITIDES
COLLAGENOUS GASTRITIS140–148
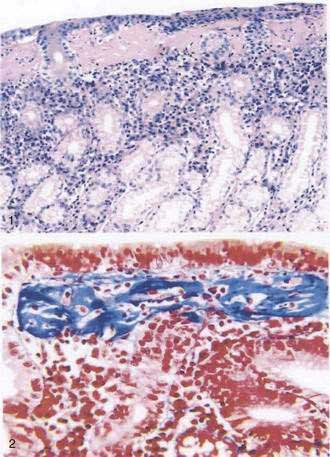
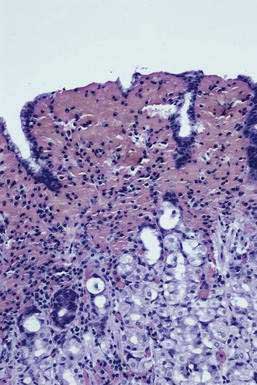
Patients may experience intermittent epigastric abdominal pain, hematemesis, hematochezia, anemia, diarrhea, hypotension, or weight loss. Collagenous gastritis in a Korean child who had been receiving treatment for refractory iron deficiency anemia has been reported.144 Upper gastrointestinal barium radiography may demonstrate an abnormal mucosal surface with a mosaic-like pattern in the body of the stomach, corresponding to mucosal nodularity. Endoscopy may reveal multiple diffusely scattered, discrete submucosal hemorrhages; erosions; and nodularity of the body of the stomach along the greater curvature.
Biopsy specimens from the body and antrum of the stomach reveal a patchy, chronic, superficial gastritis, focal atrophy, and focal deposition of collagen in the subepithelial region of the lamina propria, which measures from 20 to 75 mm thick (Fig. 51-7). Tiny erosions of the surface epithelium are present, and the inflammatory infiltrate consists of mainly plasma cells, intraepithelial lymphocytes and eosinophils, together with marked hypertrophy of muscularis mucosae.144 Little is known about the etiology, natural history, and proper treatment of this rare condition.
LYMPHOCYTIC GASTRITIS149–157
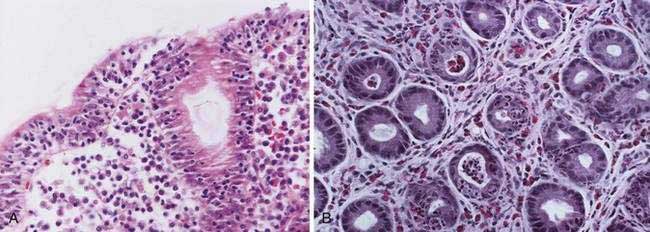
Lymphocytic gastritis is characterized by a dense lymphocytic infiltration of surface and pit gastric epithelium (Fig. 51-8A). Lymphocytic gastritis is related to an endoscopic form of gastropathy known as varioliform gastritis. Lymphocytic gastritis is also seen in H. pylori infection and in celiac disease. Recent findings provide compelling evidence that lymphocytic gastritis may occur as a manifestation of celiac disease, and thus the lymphocytic infiltration of celiac disease may affect gastric epithelial mucous cells. Lymphocytic gastritis in untreated celiac disease may be associated with functional changes such as increased permeability. Gastric biopsies from 10 of 22 patients with diarrhea or malabsorption and small bowel changes characteristic of celiac disease showed striking lymphocytic gastritis. Following institution of a gluten-free diet, lymphocytic gastritis resolves after approximately two years.
Lymphocytic gastritis has also been attributed to an atypical host immune response to H. pylori. H. pylori eradication treatment in patients with lymphocytic gastritis causes significant improvement in the gastric intraepithelial lymphocytic infiltrate, corpus inflammation, and dyspeptic symptoms. H. pylori may be the cause of some cases of protein-losing hypertrophic lymphocytic gastritis. The disease may resolve clinically, endoscopically, and pathologically with therapeutic eradication of H. pylori in some patients. The relationship between lymphocytic gastritis and gastric lymphoid hyperplasia, which also is associated with H. pylori, is not yet clear.154
Patients with gastric lymphoma have a significantly increased prevalence of lymphocytic gastritis due to H. pylori. Because intraepithelial lymphocytes are speculated to have a role in the regulation of normal mucosal inflammatory reaction, they may also participate in the pathogenesis of mucosal lymphoma. In a 10-year follow-up study of lymphocytic gastritis, the patients with lymphocytic gastritis also appeared to have a significant increase in the grade of intestinal metaplasia in the corpus mucosa. In another study, lymphocytic gastritis was more prevalent in patients with gastric adenocarcinoma (16 of 30 cases [12.3%]) than in unselected patients undergoing endoscopy (0.8% to 2.5%). Lymphocytic gastritis with anasarca and venous thrombosis at the confluence of the splenic and mesenteric veins has been reported.153
Endoscopy in lymphocytic gastritis shows thick mucosal folds, nodularity, and aphthous erosion, historically known as varioliform gastritis. Gastric biopsies show expansion of the lamina propria by an infiltrate of plasma cells, lymphocytes, and rare neutrophils. These findings may be seen in the antral mucosa only, body mucosa only, or in antral as well as body mucosa. The surface and superficial pit epithelium shows a marked intraepithelial infiltrate with T lymphocytes, with flattening of the epithelium and loss of apical mucin secretion. Quantification of epithelial lymphocytes revealed 46.5 lymphocytes per 100 epithelial cells in lymphocytic gastritis, compared with 3.5 lymphocytes per 100 cells in normal controls and 5.1 lymphocytes per 100 cells in disease controls including patients with H. pylori gastritis. The immunohistochemistry profile of lymphocytic gastritis in celiac disease and H. pylori infection and the interplay between infection and inflammation has been reported. Only intraepithelial lymphocytes positive for CD3 and CD8 were increased significantly in celiac disease patients with or without H. pylori infection.155
EOSINOPHILIC GASTRITIS158–164
Eosinophilic gastrointestinal disorders are characterized by marked tissue eosinophilia in the absence of known causes for eosinophilia or other gastrointestinal disorders. These disorders include eosinophilic esophagitis, eosinophilic gastritis, eosinophilic enteritis, and eosinophilic colitis. Eosinophilic gastroenteritis is a rare condition of unknown etiology characterized by peripheral eosinophilia, eosinophilic infiltration of the gastrointestinal tract, and gastrointestinal symptomatology. It is discussed in detail in Chapter 27. The gastric mucosa is frequently involved, and thus eosinophilic gastritis is one of the manifestations of eosinophilic gastroenteritis. Eosinophilic gastroenteritis is classified according to the layer of gastrointestinal tract involved (i.e., mucosal layer disease, muscle layer disease, and subserosal disease). Mucosal involvement may result in abdominal pain, nausea, vomiting, diarrhea, weight loss, anemia, protein-losing enteropathy, intestinal perforation, and iron deficiency anemia secondary to gastrointestinal blood loss. Patients with muscular layer disease generally have obstructive symptoms, and patients with subserosal eosinophilic infiltration develop eosinophilic ascites. Patients with gastric involvement frequently present with pyloric obstruction. Radiographic studies may demonstrate thickened mucosal folds, nodularity, or ulcerations. Endoscopy may reveal normal-appearing mucosa or hyperemic edematous mucosa with surface erosions or prominent gastric folds. Eosinophilic gastritis simulating gastric carcinoma has been reported.161
Gastric mucosal biopsies are critical to the diagnosis and show marked eosinophilic infiltration, eosinophilic pit abscesses, necrosis with numerous neutrophils, and epithelial regeneration (see Fig. 51-8B). Abnormal eosinophilic infiltration is defined as at least 20 eosinophils per high-power field either diffusely or multifocally. A full-thickness surgical biopsy is necessary for the diagnosis of muscle layer disease.
As discussed in Chapter 27, patients with disabling symptoms can be effectively treated with glucocorticoids (after other systemic disorders associated with peripheral eosinophilia have been excluded) or possibly with oral sodium cromoglycate. Surgical intervention may be required in patients with obstructive complications or refractory disease. Collagenous colitis associated with eosinophilic gastritis in a four-year-old girl has been reported.
MISCELLANEOUS FORMS OF GASTRITIS
GASTRITIS IN INFLAMMATORY BOWEL DISEASE (CROHN’S AND ULCERATIVE COLITIS)165–177
Crohn’s disease is the most common disease associated with granulomatous gastritis.111 Crohn’s disease involving the stomach is uncommon, however, and almost always occurs together with lower intestinal disease (see Chapter 111). Cases may be isolated to the stomach or the stomach and duodenum. The diagnosis of isolated Crohn’s disease of the stomach should be made with caution,165 and close follow-up is indicated for the subsequent development of Crohn’s disease elsewhere in the gastrointestinal tract or of other granulomatous diseases such as sarcoidosis.
The microscopic features of surgical specimens of gastric Crohn’s disease can be, but are not always, similar to those in the ileum or colon (see Chapter 111). They include granulomas, transmural chronic inflammation, ulcers, and marked submucosal fibrosis (see Fig. 51-6). Granulomas may be present in endoscopically normal antral mucosa.
In the past few years it has been recognized that although Crohn’s patients may have granulomatous gastritis, H. pylori–associated gastritis (HAG) or focal active antral gastritis is even more common.166–175 The majority of pediatric inflammatory bowel disease (IBD) patients (Crohn’s or ulcerative colitis [UC]) have HAG and/or focal active gastritis, although the latter is more prevalent in Crohn’s patients than in UC patients.174 The focal active gastritis is not due to H. pylori and is accompanied by macrophages in the center of the focal lesion and mast cells at its periphery.169 It is as yet unclear whether the type of gastritis identified in pediatric IBD patients can reliably distinguish Crohn’s colitis from UC (except for granulomatous gastritis, which favors Crohn’s)168 and even less is certain in adult IBD patients.
Therapy of gastritis in Crohn’s disease should be driven by gastric symptoms and not solely by demonstration of gastritis on mucosal biopsy. Double-blinded, randomized, controlled clinical trials of pharmacologic agents are lacking in gastric and gastroduodenal Crohn’s disease. Proton pump inhibitors should be the first therapy for symptomatic patients.176,177 The effectiveness of glucocorticoids, immunosuppressive medications, and anti–TNF-α drugs such as infliximab has not been clearly demonstrated. Gastric outlet obstruction refractory to medical therapy can be treated by gastroenterostomy, ideally laparoscopically. Treatment of Crohn’s disease is discussed in more detail in Chapter 111.
GASTRITIS CYSTICA PROFUNDA178–182
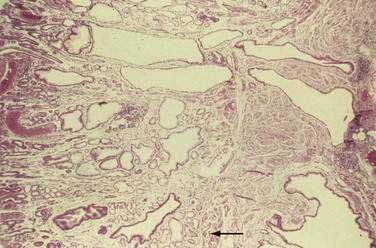
Gastritis cystica profunda is a rare complication of partial gastrectomy with gastrojejunostomy for benign peptic ulcer disease and typically occurs at the site of the gastroenterostomy. Gastritis cystica profunda may also develop in the unoperated stomach, and chronic atrophic gastritis may be a risk factor for it. Radiography and endoscopy typically demonstrate multiple exophytic gastric masses that simulate a malignancy. Endoscopic ultrasound may assist in the diagnosis. Grossly, the gastric mucosal surface demonstrates multiple nodules and exophytic masses. On section, the gastric wall is thick and multiple cysts are present. Microscopically, the mucosa is characterized by foveolar hyperplasia, and cystic glands extend through a disrupted muscularis mucosae into the submucosa and, rarely, the muscularis propria (Fig. 51-9). Gastritis cystica profunda may be associated with gastric stump adenocarcinoma.
Removal of this lesion by snare polypectomy after submucosal injection to elevate the lesion has been reported.179 The disease may also coexist with gastric inverted hyperplastic polyp, and the latter may in fact be a variant of gastritis cystica profunda.182 Gastritis cystica profunda, if present, should lead to a thorough examination for a synchronous or metachronous gastric cancer, although exact recommendations for surveillance interval are not clear.
ALLERGIC GASTRITIS188–190
Whether allergies to certain foods can lead to gastritis and whether the gastritis correlates with upper gastrointestinal symptoms are unclear (see Chapter 9). Children with food allergy as diagnosed by an open elimination challenge test have no higher incidence of gastritis than children without food allergy.188 An exception may be infants who are allergic to cow’s milk protein, in whom hematemesis and endoscopic signs of gastritis are common.189
REACTIVE GASTROPATHIES (ACUTE EROSIVE GASTRITIS)
Grossly, most erosions and acute ulcers appear as well-defined hemorrhagic lesions 1 to 2 mm in diameter. If the insult is severe, the mucosa between the lesions is intensely hemorrhagic. Microscopically, an erosion demonstrates superficial lamina propria necrosis. An acute ulcer is an area of necrosis that extends to the muscularis mucosa. Foveolar hyperplasia, the reactive epithelial changes secondary to regeneration of the mucosa (Fig. 51-10), is often associated with glands with atypical nuclei that can be misdiagnosed as dysplasia or carcinoma. The diagnosis of neoplasia in a background of necrosis, cellular debris, and granulation tissue should be made with utmost caution. The biopsy procedure itself may induce tissue hemorrhage; thus, subepithelial hemorrhage should involve more than one fourth of a biopsy specimen to be considered significant.17
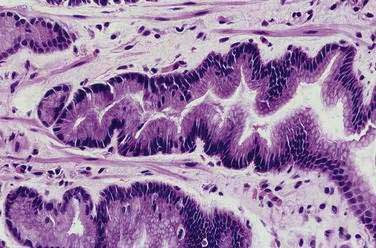
Figure 51-10. Histopathology of foveolar hyperplasia. The gastric pits show an elongated, corkscrew appearance.
MEDICATIONS AND TOXINS191–201
Aspirin (even in low daily or less-than-daily doses) and NSAIDs (including cyclooxygenase [COX]-2 selective inhibitors) are the most common causes of reactive gastropathy (see Chapter 52). Oral iron therapy may rarely cause mild endoscopic abnormalities consisting of erythema, small areas of subepithelial hemorrhage, and erosions.191 Oral potassium chloride may also be associated with endoscopic erosions.192 Endoscopic petechiae, erosions, and erythema have been associated with long-term fluoride ingestion.195 Bisphosphonates for osteoporosis or Paget’s disease can also cause gastric erosions, although their clinical significance is uncertain.196,197 These drugs exacerbate gastric damage from NSAIDs such as naproxen as well. Various intensive cancer chemotherapy drugs given to children with leukemia, lymphoma, or solid tumors are associated with a hemorrhagic or erosive gastropathy and histologic evidence of inflammation, but cause and effect have not been clearly established in these ill individuals.198
Reactive gastric epithelial atypia and gastric ulceration may be associated with hepatic arterial infusion chemotherapy for metastatic disease to the liver.199,200 The marked epithelial atypia that results may erroneously be interpreted as carcinoma and lead to unnecessary surgery.
Toxin ingestion of heavy metals such as mercury sulfate poisoning may cause an erosive or ulcerative gastropathy with hematemesis.201 Corrosive gastric injuries are discussed in Chapter 25.
ALCOHOL202–209
After acute alcohol ingestion, subepithelial hemorrhages are seen frequently at endoscopy, typically without prominent mucosal inflammation on biopsy specimens (Fig. 51-11).202 Gastric biopsy specimens obtained from patients with chronic alcoholism have shown a higher prevalence of chronic antral gastritis due to H. pylori, with almost complete normalization of histologic findings after treatment.203,204
The combined effects of alcohol and the NSAID ibuprofen were associated with more gastric mucosal damage by endoscopic assessment than with either agent alone. The combination of alcohol and aspirin also caused more damage in the stomach than either agent alone, though not to a significant degree.205 Alcohol appeared to be an acute triggering factor in 35% of patients admitted to an intensive care unit for massive upper gastrointestinal bleeding in Sweden.206 Chronic alcohol ingestion was related to an increased risk of chronic atrophic gastritis and hypochlorhydria in a study from Poland.207 Recent alcohol ingestion was also found to be a risk factor for gastric erosions and ulcers in cirrhotic patients referred for upper endoscopy.208
Other alcohols, besides ethanol, can injure the stomach. Even the topical application of isopropyl alcohol (rubbing alcohol) used to cool a child with fever has resulted in hemorrhagic gastropathy with hematemesis.209
PORTAL HYPERTENSIVE GASTROPATHY210–214
As discussed in more detail in Chapters 19 and 90, gastric mucosal lesions are common in portal hypertension, occurring in up to 65% of cirrhotic patients, and represent an important cause of gastrointestinal blood loss.210 Portal hypertensive gastropathy was found in 33% of children after surgery for biliary atresia. Risk factors for portal hypertensive gastropathy were frequent endoscopic treatment of gastroesophageal varices, liver dysfunction, and hypersplenism.213 Biopsies show vascular ectasia and congestion in the mucosal layer without a significant degree of inflammatory infiltrate. Portal hypertensive gastropathy (PHG) is a risk factor not only for upper gastrointestinal bleeding but also for gastroduodenal erosions and ulcers in cirrhotic patients.208 It has been suggested that perturbations in the tissue levels of TNF-α, prostaglandins, endothelin, and nitric oxide/peroxynitrite participate in the vascular congestion and mucosal damage characteristic of PHG.211 As discussed in Chapter 90, lowering portal pressure pharmacologically or by creation of a portosystemic shunt effectively treats PHG and reduces bleeding.
Some patients with cirrhosis and portal hypertension have gastric antral vascular ectasia (GAVE), which can bleed and is sometimes difficult to distinguish from PHG involving the gastric antrum.212 GAVE does not respond as readily as PHG to measures that reduce portal pressure.212
COCAINE215–217
Gastrointestinal hemorrhage due to diffuse exudative erosion throughout the gastric fundus, body, antrum, and duodenal bulb has been reported with crack cocaine use. Gastrointestinal hemorrhage or pyloric perforation due to cocaine is well described.215–217
STRESS218
Erosions of the gastric mucosa may occur rapidly after major physical or thermal trauma, shock, sepsis, or head injury. These are often referred to as stress ulcers and are discussed in Chapters 52 and 53.
RADIATION218–219 (see also Chapter 39)
Radiation effects on the stomach depend on the cell kinetics of the gastric mucosa, as well as the dose of the radiation. The gastric mucosal response to radiation is unique, however, in that the most radiosensitive epithelial cells are the differentiated cells (parietal and chief cells) rather than the germinative cells in the mucous neck region. Radiation injury to the stomach can be classified into acute (less than six months) and chronic (more than one year) phases. It is thought that the tolerance level for radiation-induced gastric ulceration is approximately 4500 cGy. After a gastric dose of 5500 cGy or more, 50% of patients will develop clinical evidence of gastric ulcer formation.218 Radiation-induced gastric ulcers are usually solitary, from 0.5 to 2 cm in diameter, and located in the antrum. Massive hemorrhagic gastropathy requiring endoscopically administered therapy to control the bleeding has been reported.219
BILE REFLUX220–231
Bile reflux into the stomach is common after partial gastrectomy with anastomosis to the duodenum (Billroth I) or jejunum (Billroth II) and after truncal vagotomy and pyloroplasty for peptic ulcer (see Chapter 53). It has even been reported after parietal cell vagotomy.220 Bile reflux gastropathy also may occur after cholecystectomy or sphincteroplasty, which allows the continuous exposure of bile to the duodenum with the potential for duodenogastric reflux. Occasionally, bile reflux gastropathy is observed in adult or pediatric patients who have not had surgery.221,223 Interleukins, particularly IL-8 and perhaps IL-6, may participate in the gastric damage.224,225 Bile reflux contributes to muscosal lesions in the stomach and may facilitate H. pylori colonization in the corpus region.222
Endoscopy shows swelling, redness, erosions, and bile staining of the gastric mucosa. Biopsy specimens show foveolar hyperplasia, dilated cystic glands, atypical glands that may be misdiagnosed as dysplasia or carcinoma, and a paucity of acute and chronic inflammatory cells. Gastric atrophy may result and increase the risk of carcinoma in the gastric stump (see Chapter 54). In fact, bile reflux into the unoperated stomach has been proposed to be a risk factor for intestinal metaplasia in the distal stomach, at the gastroesophageal junction (cardia), and in the distal esophagus (Barrett’s esophagus).226–228 Unfortunately, bile-diverting procedures performed because of severe bile gastropathy do not reverse gastric atrophy or intestinal metaplasia.229 It may be worthwhile, at the time of the original gastric surgery for gastric cancer or peptic ulcer, to construct a 30-cm Roux-en-Y limb176 or perform a 10- to 12-cm isoperistaltic jejunal interposition230 to prevent bile gastropathy and subsequent metaplastic changes.
In selected, previously unoperated patients with primary bile gastropathy, surgery using a Roux-en-Y choledochojejunostomy without gastric resection has been successful.221 In unoperated patients with bile gastropathy following cholecystectomy, the proton pump inhibitor rabeprazole and sucralfate were equally effective in relieving symptoms and improving gastroscopic evidence of mucosal damage as compared with observation alone (no placebo was given).231 At present, lacking definitive studies, medical therapy should precede surgical therapy for bile gastropathy occurring in the unoperated stomach such as it does spontaneously or after cholecystectomy or biliary sphincterotomy.
ISCHEMIA232–237
Chronic ischemic gastropathy may occur secondary to chronic mesenteric insufficiency and can be reversed after a revascularization operation.191,192 Chronic ischemic gastropathy, as well as chronic ischemic gastric ulcers, may also occur in association with atheromatous embolization.193,194 Athletes involved in intense physical activity, especially long-distance running, may experience recurrent ischemic gastropathy and chronic gastrointestinal bleeding with anemia.195,196
PROLAPSE238–239
The mucosa of the gastric cardia may prolapse into the esophageal lumen during retching and vomiting.238 The resulting mechanical injury to the cardia has been proposed to be a cause of upper gastrointestinal hemorrhage, but this association has been questioned.239 Esophagoscopy may demonstrate the prolapsed gastric mucosa. The congested mucosa may show erosions and superficial ulcerations.
LINEAR EROSIONS IN A HIATAL HERNIA (CAMERON LESIONS)240–241
Linear gastric erosions in a hiatal hernia are discussed in Chapters 19, 24, and 52.
AGING GASTROPATHY242,243
Normal aging is associated with impaired gastric mucosal defense against injury in animals and humans.242 Two proteins have been implicated in aging gastropathy: PTEN (phosphatase and tensin homolog deleted on chromosome 10), which is overexpressed with aging, and survivin, which is underexpressed.243
HYPERPLASTIC GASTROPATHIES244–251
MÉNÉTRIER’S DISEASE AND HYPERPLASTIC, HYPERSECRETORY GASTROPATHY
Hyperplastic gastropathy is a rare condition characterized by giant gastric folds associated with epithelial hyperplasia. Two clinical syndromes have been identified: Ménétrier’s disease and a variant of it referred to as hyperplastic, hypersecretory gastropathy, and Zollinger-Ellison syndrome, which is discussed in Chapter 32. Figure 51-12A and B demonstrates enlarged gastric folds in these conditions.
Ménétrier’s disease is typically associated with protein-losing gastropathy (see Chapter 28) and with hypochlorhydria, whereas the hyperplastic, hypersecretory variant is associated with increased or normal acid secretion and parietal and chief cell hyperplasia, with or without excessive gastric protein loss.
Other more common conditions can also cause enlarged gastric folds or protein-losing gastropathy including gastric neoplasm (lymphoma, carcinoma), granulomatous gastritides, gastric varices, infectious gastritis (particularly H. pylori and CMV58), eosinophilic gastritis, and Zollinger-Ellison syndrome. The enlarged gastric folds in Ménétrier’s disease are due to foveolar cell hyperplasia, edema, and variable degrees of inflammation. Patients may present with weight loss, epigastric pain, vomiting, anorexia, dyspepsia, hematemesis, and positive fecal occult blood tests. Most patients with a clinical syndrome associated with hyperplastic gastritis showed histology typical for the syndrome; however, clinical-histologic concordance was not absolute.247 The mechanism responsible for the low gastric acid secretion is unclear, but it could be related to overexpression of transforming growth factor-α (TGF-α), a ligand for epidermal growth factor receptor (EGFR), a receptor tyrosine kinase.244 Although mucus hypersecretion is often seen in Ménétrier’s disease, abnormalities in mucins have not been consistent in a few cases that have been examined.245,246
The mucosa of patients with Ménétrier’s disease demonstrates irregular hypertrophic folds that involve the entire gastric body. The mucosa also demonstrates a swollen, spongy appearance subdivided by creases, creating a picture similar to cerebral convolutions. A polypoid variant of Ménétrier’s disease that resembles multiple hyperplastic gastric polyps has been described (see Chapter 54).
Gastric resections from patients with Ménétrier’s disease typically show large polypoid gastric folds or large cerebriform gastric folds with antral sparing (see Fig. 51-12C). In the absence of a gastrectomy, a full-thickness gastric mucosal biopsy is required to adequately assess the gastric histology in patients with hyperplastic gastropathy. The predominant microscopic feature of Ménétrier’s disease and hyperplastic, hypersecretory gastropathy is foveolar hyperplasia with cystic dilation (see Fig. 51-12D). The parietal and chief cells may be decreased and replaced by mucous glands. Inflammation in hyperplastic gastropathies is variable and may be absent.
The etiology of Ménétrier’s disease is unknown, although some cases have undoubtedly been infections with CMV or H. pylori. Genetic factors have recently been emphasized after the report of the disorder in identical twin men who presented at ages 29 and 35, respectively.247 Hyperplasia of surface mucous cells may be due to enhanced EGFR signaling in the gastric mucosa due to local overproduction of TGF-α.244
Ideal treatment of hyperplastic gastropathy is unclear because the condition is rare and controlled trials are lacking. Spontaneous resolution may occur, especially in children. It is likely that some cases, particularly in children, were actually cases of CMV gastritis (see earlier discussion). Ganciclovir has been used successfully in children with Ménétrier’s disease associated with CMV gastritis.249 H. pylori infection should be treated, if present, and the entire syndrome may resolve.250 Symptoms may improve with antisecretory agents (histamine-2 [H2] receptor antagonists, anticholinergic agents, proton pump inhibitors), especially if the patient has Zollinger-Ellison syndrome or normogastrinemic hyperplastic, hypersecretory gastropathy. It has been suggested that H2 blockers and anticholinergics reduce gastric protein loss by strengthening intercellular tight junctions. Some patients with Ménétrier’s disease have responded to glucocorticoids, octreotide, antifibrinolytic agents, or monoclonal antibody against the EGFR.251 Partial or total gastric resection is reserved for severe complications such as refractory or recurrent bleeding, obstruction, severe hypoproteinemia, or cancer development.
DIFFERENTIAL DIAGNOSIS OF GASTRITIS AND GASTROPATHY
The most important disorders that can simulate gastritis and gastropathy are gastric polyps (non-neoplastic and neoplastic) and gastric neoplasms such as adenocarcinoma and lymphoma (see Chapter 54).252,253 Although CT criteria have been useful in distinguishing benign gastritis or gastropathy from gastric malignancy,254 endoscopy and gastric biopsy with review by an expert pathologist are the most useful diagnostic procedures. B cell clonality using advanced PCR technology can also help distinguish gastric marginal zone lymphomas from chronic gastritis.255 At the other end of the spectrum, many patients with gastritis have a normal endoscopic appearance,256 so the differential diagnosis of gastritis also includes functional dyspepsia (see Chapter 13), in which case the gastric biopsy is usually normal.
TREATMENT AND PREVENTION OF GASTRITIS AND GASTROPATHY257
The treatment of these disorders depends on the underlying etiology (if one can be identified). In countries where the incidence of H. pylori infection is declining, the prevalence of chronic gastritis will decline as well (see Chapter 50). It has been shown in a case-control study performed in a region of southeastern China with a very high prevalence of chronic gastritis and gastric cancer that ingestion of green tea reduced the risk of gastritis and gastric cancer by close to 50%.257
Carlson AP, Chan JWH, Ketai LH, Demarest GB. Emphysematous gastritis in a severely burned patient: Case report and literature review. J Trauma. 2007;62:765-7. (Ref 82.)
Chia JKS, Chia AY. Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach. J Clin Pathol. 2008;61:43-8. (Ref 72.)
Coffey RJ, Washington MK, Corless CL, Heinrich MC. Ménétrier disease and gastrointestinal stromal tumors: Hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70-80. (Ref 244.)
D’Elios MM, Bergman MP, Azzurri A, et al. H+,K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120:377-86. (Ref 45.)
DeBlock CEM, DeLeeuw IH, VanGaal LF. Autoimmune gastritis in type 1 diabetes: A clinically oriented review. J Clin Endocrinol Metab. 2008;93:363-71. (Ref 47.)
Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-9. (Ref 9.)
Hummel M, Oeschger S, Barth TFE, et al. Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut. 2006;55:782-7. (Ref 255.)
Insko EK, Levine MS, Birnbaum BA, et al. Benign and malignant lesions of the stomach: Evaluation of CT criteria for differentiation. Radiology. 2003;228:166-71. (Ref 254.)
Kaur G, Raj SM. A study of the concordance between endoscopic gastritis and histological gastritis in an area with a low background prevalence of Helicobacter pylori infection. Singapore Med J. 2002;43:90-2. (Ref 1.)
Marshall JK, Thabane M, James C. Randomized active and placebo-controlled endoscopy study of a novel protected formulation of oral alendronate. Dig Dis Sci. 2006;51:864-8. (Ref 196.)
Redeen S, Peterson F, Jonsson KA, et al. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-50. (Ref 256.)
Ricuarte O, Gutierrez O, Cardona H, et al. Atrophic gastritis in young children and adolescents. J Clin Pathol. 2005;58:1189-93. (Ref 23.)
Rubio CA, Nesi G, Zampi GC, et al. Gastric ciliated metaplasia. A study of 3406 gastrectomy specimens from dwellers of the Atlantic and the Pacific basins. J Clin Pathol. 2005;58:605-10. (Ref 32.)
Tarnawski A, Pai R, Deng X, et al. Aging gastropathy-novel mechanisms: Hypoxia, up-regulation of multifunctional phosphatase PTEN, and proapoptotic factors. Gastroenterology. 2007;133:1938-47. (Ref 243.)
1. Kaur G, Raj SM. A study of the concordance between endoscopic gastritis and histological gastritis in an area with a low background prevalence of Helicobacter pylori infection. Singapore Med J. 2002;43:90-2.
2. Rubin C. Are there three types of Helicobacter pylori gastritis? Gastroenterology. 1997;112:2108.
3. Genta R, Hamner H. The significance of lymphoid follicles in the interpretation of gastric biopsy specimens. Arch Pathol Lab Med. 1994;118:740.
4. Dixon M, Genta R, Yardley J, et al. Classification and grading of gastritis. Am J Surg Pathol. 1996;20:1161.
5. Stolte M, Meining A. The updated Sydney system: Classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591.
6. Appelman H. Gastritis: Terminology, etiology, and clinicopathological correlations-another biased view. Hum Pathol. 1994;25:1006.
7. Bhattacharya B, Montgomery E, et al. Non-neoplastic disorders of the stomach. In: Iacobuzio-Donahue CA, Montgomery EA, editors. Gastrointestinal and Liver Pathology. Philadelphia: Elsevier; 2005:66-125.
8. Borch K, Skarsgard J, Franzén L, et al. Benign gastric polyps. Morphological and functional origin. Dig Dis Sci. 2003;48:1292.
9. Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-9.
10. Dheer S, Levine MS, Redfern RO, et al. Radiographically diagnosed antral gastritis: Findings in patients with and without Helicobacter pylori infection. Br J Radiol. 2002;75:805.
11. Chan Y, Hui P, Chan J, et al. Epithelial damage by Helicobacter pylori in gastric ulcers. Histopathology. 1991;19:47.
12. Rosh J, Kurfist L, Benkov K, et al. Helicobacter pylori and gastric lymphonodular hyperplasia in children. Am J Gastroenterol. 1992;87:135.
13. Peterson W, Lee E, Feldman M. Relationship between Campylobacter pylori and gastritis in healthy humans after administration of placebo or indomethacin. Gastroenterology. 1988;95:1185.
14. Montgomery E, Martin D, Peura D. Rapid diagnosis of Campylobacter pylori by Gram’s stain. Am J Clin Pathol. 1988;90:606.
15. Madan E, Kemp J, Westblom T, et al. Evaluation of staining methods for identifying Campylobacter pylori. Am J Clin Pathol. 1988;90:450.
16. Morris A, Ali M, Thomsen L, et al. Tightly spiral-shaped bacteria in the human stomach: Another cause of active chronic gastritis? Gut. 1990;31:139.
17. Heilmann K, Borchard F. Gastritis due to spiral-shaped bacteria other than Helicobacter pylori: Clinical, histological, and ultrastructural findings. Gut. 1991;32:137.
18. Hilzenrat N, Lamoureux E, Weintrub I, et al. Helicobacter heilmannii–like spiral bacteria in gastric mucosal biopsies. Arch Pathol Lab Med. 1995;119:1149.
19. Genta R, Graham D. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: A topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342.
20. Satoh K, Kimura K, Taniguchi Y, et al. Biopsy sites suitable for the diagnosis of Helicobacter pylori infection and the assessment of the extent of atrophic gastritis. Am J Gastroenterol. 1998;93:569.
21. Urakami Y, Kimura M, Seki H, et al. Gastric metaplasia and Helicobacter pylori. Am J Gastroenterol. 1997;92:795.
22. Genta R. Recognizing atrophy: Another step toward a classification of gastritis. Am J Surg Pathol. 1996;20:S23.
23. Ricuarte O, Gutierrez O, Cardona H, et al. Atrophic gastritis in young children and adolescents. J Clin Pathol. 2005;58:1189-93.
24. Meshkinpour H, Orlando R, Arguello J, et al. Significance of endoscopically visible blood vessels as an index of atrophic gastritis. Am J Gastroenterol. 1979;71:376.
25. Yang J, Chen L, Fan Y, et al. Endoscopic patterns of gastric mucosa and its clinicopathological significance. World J Gastroenterol. 2003;9:2552.
26. Sipponen P. Intestinal metaplasia and gastric carcinoma. Ann Clin Res. 1981;13:139.
27. Antonioli D. Precursors of gastric carcinoma: A critical review with a brief description of early (curable) gastric cancer. Hum Pathol. 1994;25:994.
28. Stemmermann G. Intestinal metaplasia of the stomach. Cancer. 1994;74:556.
29. Ramesar K, Sander D, Hopwood D. Limited value of type III intestinal metaplasia in predicting risk of gastric carcinoma. J Clin Pathol. 1987;40:1287.
30. Fox J, Correa P, Taylor N, et al. Campylobacter pylori–associated gastritis and immune response in a population at increased risk of gastric carcinoma. Am J Gastroenterol. 1989;84:775.
31. Sipponen P. Intestinal metaplasia and gastric carcinoma. Ann Clin Res. 1981;13:139.
32. Rubio CA, Nesi G, Zampi GC, et al. Gastric ciliated metaplasia. A study of 3406 gastrectomy specimens from dwellers of the Atlantic and the Pacific basins. J. Clin Pathol. 2005;58:605-10.
33. Lewin K, Dowling F, Wright J, et al. Gastric morphology and serum gastrin levels in pernicious anemia. Gut. 1976;17:551.
34. Hsing A, Hansson L, McLaughlin J, et al. Pernicious anemia and subsequent cancer: A population-based cohort study. Cancer. 1993;71:745.
35. Cunliffe RN. α-Defensins in the gastrointestinal tract. Mol Immunol. 2003;40:463.
36. Jhala NC, Montemor M, Jhala D, et al. Pancreatic acinar cell metaplasia in autoimmune gastritis. Arch Pathol Lab Med. 2003;127:854.
37. Krasinskas AM, Abraham SC, Metz DC, et al. Oxyntic mucosa pseudopolyps: A presentation of atrophic autoimmune gastritis. Am J Surg Pathol. 2003;27:236.
38. Kitago M, Inada T, Igarashi S, et al. Multiple gastric carcinoid tumors with type A gastritis concomitant with gastric cancer: A case report. Oncol Rep. 2001;8:343.
39. Annibale B, Negrini R, Caruana P, et al. Two-thirds of atrophic body gastritis patients have evidence of Helicobacter pylori infection. Helicobacter. 2001;6:225.
40. Presotto F, Sabini B, Cecchetto A, et al. Helicobacter pylori infection and gastric autoimmune diseases: Is there a link? Helicobacter. 2003;8:578.
41. The Eurohepygast Study Group. Risk factors for atrophic chronic gastritis in a European population: Results of the Eurohepygast study. Gut. 2002;50:779.
42. Broutet N, Moran A, Hynes S, et al. Lewis antigen expression and other pathogenic factors in the presence of atrophic chronic gastritis in a European population. J Infect Dis. 2002;185:503.
43. Moran AP, Prendergast MM. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: Contribution of gastrointestinal infections to autoimmunity. J Autoimmun. 2001;16:241.
44. Toshifumi O, Fujiki K, Takashimizu I, et al. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Ann Intern Med. 2001;5:380.
45. D’Elios MM, Bergman MP, Azzurri A, et al. H+,K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120:377-86.
46. De Block CE, De Leeuw IH, Bogers JJ, et al. Autoimmune gastropathy in type 1 diabetic patients with parietal cell antibodies: Histological and clinical findings. Diabetes Care. 2003;26:82.
47. DeBlock CEM, DeLeeuw IH, VanGaal LF. Autoimmune gastritis in type 1 diabetes: A clinically oriented review. J Clin Endocrinol Metab. 2008;93:363-71.
48. Fabbri C, Jaboli MF, Giovanelli S, et al. Gastric autoimmune disorders in patients with chronic hepatitis C before, during and after interferon-alpha therapy. World J Gastroenterol. 2003;9:1487.
49. Driel IR, Baxter AG, Laurie KL, et al. Immunopathogenesis, loss of T cell tolerance and genetics of autoimmune gastritis. Autoimmunity Rev. 2002;1:290.
50. Whiting JL, Sigurdsson A, Rolands DC, et al. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378.
51. Kang JY, Finlayson C, Maxwell JD, et al. Risk of gastric carcinoma in patients with atrophic gastritis and intestinal metaplasia. Gut. 2002;50:899.
52. Capurso G, Lahner E, Delle Fave G, et al. Timing and sampling in surveillance of premalignant gastric lesions. Gut. 2002;50:896.
53. Petersson F, Borch K, Franzen LE. Prevalence of subtypes of intestinal metaplasia in the general population and in patients with autoimmune chronic atrophic gastritis. Scand J Gastroenterol. 2002;37:262.
54. Lahner E, Caruana P, D’Ambra G, et al. First endoscopic-histologic follow-up in patients with body-predominant atrophic gastritis: When should it be done? Gastrointest Endosc. 2001;53:443.
55. Eda A, Osawa H, Yanaka I, et al. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002;37:147.
56. Der R, Tsao-Wei DD, Demeester T, et al. Carditis: A manifestation of gastroesophageal reflux disease. Am J Surg Pathol. 2001;25:245.
57. Zentilin P, Mastracci L, Dulbecco P, et al. Carditis in patients with gastro-esophageal reflux disease: Results of a controlled study based on both endoscopy and 24-hr esophageal pH monitoring. Aliment Pharmacol Thera. 2004;19:1285-92.
58. Jang TJ, Kim NI, Yang CH. Carditis is associated with Helicobacter pylori–induced gastritis and not reflux esophagitis. J Clinical Gastroenterol. 2003;36:26-29.
59. Owen DA. Gastritis and carditis. Mod Pathol. 2003;16:325-41.
60. Bonnet F, Neau D, Viallard JF, et al. Clinical and laboratory findings of cytomegalovirus infection in 115 hospitalized non-immunocompromised adults. Ann Med Interne (Paris). 2001;152:227.
61. Xiao Sy, Hart J. Marked gastric foveolar hyperplasia associated with active cytomegalovirus infection. Am J Gastroenterol. 2001;96:223.
62. Takeyama J, Abukawa D, Miura K. Eosinophilic gastroenteritis with cytomegalovirus infection in an immunocompetent child. World J Gastroenterol. 2007;13:4653-4.
63. Pederiva C, Ruscitto A, Bruneti I, et al. Cytomegalovirus-induced protein-losing gastropathy. Pediatr Med Chir. 2006;28:42-4.
64. Yoshioka M, Ishiguro N, Ma X, et al. Protein-losing cytomegalovirus gastritis in a patient with Stevens-Johnson syndrome. Digestion. 2002;65:234.
65. Hong J, Elgart M. Gastrointestinal complications of dermatomal herpes zoster successfully treated with famciclovir and lactulose. J Am Acad Dermatol. 1998;38:279.
66. Rivera-Vaquerizo PA, Gomez-Garrido J, Vicente-Gutierrez M, et al. Varicella zoster gastritis 3 years after bone marrow transplantation for treatment of acute leukemia. Gastrointest Endosc. 2001;53:809.
67. Scholl S, Hocke M, Hoffken K, Sayer HG. Acute abdomen by varicella zoster virus induced gastritis after autologous peripheral blood stem cell transplantation in a patient with non-Hodgkin’s lymphoma. Acta Hematol. 2006;116:58-61.
68. Gonelli A, Boccia S, Boni M, et al. Human herpes virus 7 is latent in gastric mucosa. J Med Virol. 2001;63:277.
69. Zhang Y, Molot R. Severe gastritis secondary to Epstein-Barr viral infection. Unusual presentation of infectious mononucleosis and associated diffuse lymphoid hyperplasia in gastric mucosa. Arch Pathol Lab Med. 2003;127:478.
70. Hungermann D, Muller S, Spieker T, et al. Low prevalence of latently Epstein-Barr virus–infected cells in chronic gastritis. Microsc Res Tech. 2001;53:409.
71. Vieth M, Dirshmid K, Oehler U, et al. Acute measles gastric infection. Am J Surg Pathol. 2001;25:259.
72. Chia JKS, Chia AY. Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach. J Clin Pathol. 2008;61:43-8.
73. Dharap SB, Ghag G, Biswas A. Acute necrotizing gastritis. Indian J Gastroenterol. 2003;22:150.
74. Staroverov VV, Kisel AT, Sumarokov UA, et al. A case of phlegmonous gastritis diagnosed by echography. Eur J Ultrasound. 2001;13:197.
75. van Mook WN, van der Geest S, Goessens ML, et al. Gas within the wall of the stomach due to emphysematous gastritis: Case report and review. Eur J Gastroenterol Hepatol. 2002;14:1155.
76. Miller C, Florman S, Schluger LK, et al. Fulminant and fatal gas gangrene of the stomach in healthy live liver donor. Liver Transplantation. 2004;10:1315-19.
77. Jung JH, Choi HJ, Yoo J, Kang SJ, et al. Emphysematous gastritis associated with invasive gastric mucormycosis. J Korean Med Sci. 2007;22:923-7.
78. Shipman PJ, Drury P. Emphysematous gastritis: Case report and literature review. Australas Radiol. 2001;45:64.
79. Buyl L, Smeets P, Verstraete K. Infectious emphysematous gastritis in multiple sclerosis. JBR-BTR. 2003;86:148.
80. Gutierrez O, Cantalapiedra A, Tabuyo MI, et al. Emphysematous gastritis and severe aplastic anemia. Hematol J. 2003;4:82.
81. Yalamanchili M, Cady W. Emphysematous gastritis in a hemodialysis patient. South Med J. 2003;96:84.
82. Carlson AP, Chan JWH, Ketai LH, Demarest GB. Emphysematous gastritis in a severely burned patient: Case report and literature review. J Trauma. 2007;62:765-7.
83. Marshall J. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989.
84. Benson C. Disease due to the Mycobacterium avium complex in patients with AIDS: Epidemiology and clinical syndrome. Clin Infect Dis. 1994;18:S218.
85. Lopez Calaya JF, Martin Rodrigo L, Mohammad Mourad F, et al. Gastritis tuberculosis. Review apropos of a case. Gastroenterol Hepatol. 2007;30:334-7.
86. Yang S, Li A, Lin J. Colonoscopy in abdominal actinomycosis. Gastrointest Endosc. 2000;51:236.
87. Berardi R. Abdominal actinomycosis. Surg Gynecol Obstet. 1990;149:257.
88. Rolfs R, Nakashima A. Epidemiology of primary and secondary syphilis in the United States, 1981 through 1989. JAMA. 1990;264:1432.
89. Atten M, Altar B, Teopengco E, et al. Gastric syphilis: A disease with multiple manifestations. Am J Gastroenterol. 1994;89:2227.
90. Fyfe B, Poppiti R, Lubin J, et al. Gastric syphilis—primary diagnosis by gastric biopsy: Report of four cases. Arch Pathol Lab Med. 1993;117:820.
91. Choi Y-L, Han JJ, Lee DK, et al. Gastric syphilis mimicking adenocarcinoma: A case report. J Korean Med Sci. 2006;21:559-62.
92. Guerrero AF, Straight TM, Eastone J, Spooner K. Gastric syphilis in a HIV-infected patient. AIDS Patient Care STDS. 2005;19:281-5.
93. Jones B, Lichtenstein J. Gastric syphilis: Radiologic findings. Am J Radiol. 1993;160:59.
94. Inagaki H, Kawai T, Miyata M, et al. Gastric syphilis: Polymerase chain reaction of treponemal in DNA pseudolymphomatous lesions. Hum Pathol. 1996;27:763.
95. Rathinavelu S, Zavros Y, Merchant J. Acinetobacter lwoffii infection and gastritis. Microbes Infect. 2003;5:651.
96. El-Zimaity HM, Ramchatesingh J, Clarridge JE, et al. Enterococcus gastritis. Hum Pathol. 2003;34:944.
97. Zwolinska-Wcisto M, Budak A, Bogdal J, et al. Fungal colonization of gastric mucosa and its clinical relevance. Med Sci Monit. 2001;7:982.
98. Loffeld R, Loffeld B, Arends J, et al. Fungal colonization of gastric ulcers. Am J Gastroenterol. 1988;83:730.
99. Sanguino J, Rodrigues B, Baptista A, et al. Focal lesion of African histoplasmosis presenting as a malignant gastric ulcer. Hepatogastroenterology. 1996;43:771.
100. Hindupur S, Despotovic V. Gastric histoplasmosis. Lancet Infect Dis. 2006;6:60.
101. Jayalakshmi P, Soo-Hoo T, Goh K, et al. Disseminated histoplasmosis presenting as penile ulcer. Aust N Z J Med. 1990;20:175.
102. Cherney C, Chutuape A, Fikrig M. Fatal invasive gastric mucormycosis occurring with emphysematous gastritis: Case report and literature review. Am J Gastroenterol. 1999;94:252.
103. Maravi Poma E, Rodriquez-Tudela JL, de Jalon JG, et al. Outbreak of gastric mucormycosis associated with the use of wooden tongue depressors in critically ill patients. Intens Care Med. 2004;30:724-8.
104. Corley D, Lindeman N, Ostroff J. Survival with early diagnosis of invasive gastric mucormycosis in a heart transplant patient [letter]. Gastrointest Endosc. 1997;46:452.
105. Sanders DL, Pfeiffer RB, Hashimoto LA, et al. Pseudomembranous gastritis: A complication from aspergillus infection. Am Surg. 2003;69:536.
106. Konstantopoulos K, Agapitos E, Komninaka V, et al. Acute aspergillosis gastritis in a case of fatal aplastic anemia. Scand J Infect Dis. 2002;34:148-9.
107. Forester G, Sidhom O, Nahass R, et al. AIDS-associated cryptosporidiosis with gastric stricture and a therapeutic response to paromomycin. Am J Gastroenterol. 1994;89:1096.
108. Garone M, Winston B, Lewis J, et al. Cryptosporidiosis of the stomach. Am J Gastroenterol. 1986;81:465.
109. Kourda N, Biel A, Ben Jilani SB, et al. Gastric cryptosporidiosis revealing a small cell lung carcinoma. Bull Soc Pathol Exot. 2008;101:22-3.
110. Clemente CM, Caramori CA, Padula P, Rodrigues MA. Gastric cryptosporidiosis as a clue for the diagnosis of the acquired immunodeficiency syndrome. Arg Gastroenterol. 2000;37:180-2.
111. Wurtz R, Mirot M, Fronda G, et al. Gastric infection by Strongyloides stercoralis. Am J Trop Med Hyg. 1994;51:339.
112. Dees A, Batenburg P, Umar H, et al. Strongyloides stercoralis associated with a bleeding gastric ulcer. Gut. 1990;31:1414.
113. Ainley C, Clarke D, Timothy A, et al. Strongyloides stercoralis hyperinfection associated with cimetidine in an immunosuppressed patient: Diagnosis by endoscopic biopsy. Gut. 1986;27:337.
114. Rivasi F, Pampiglione S, Boldorini R, Cardinale L. Histopathology of gastric and duodenal strongyloides stercoralis locations in fifteen immunocompromised subjects. Arch Pathol Lab Med. 2006;130:1792-8.
115. Kim J, Joo H, Kim D, et al. A case of gastric strongyloidiasis in a Korean patient. Korean J Parasitol. 2003;41:63.
116. Takeuchi K, Hanai H, Iida T, et al. A bleeding gastric ulcer on a vanishing tumor caused by anisakiasis. Gastrointest Endosc. 2000;52:549.
117. Testini M, Gentile A, Lissidini G, et al. Splenic anisakiasis resulting from a gastric perforation: An unusual occurrence. Int Surg. 2003;88:126.
118. Okanobu H, Hata J, Haruma K, et al. Giant gastric folds: Differential diagnosis at US. Radiology. 2003;226:686.
119. Kim SG, Jo YJ, Park YS, et al. Four cases of gastric submucosal mass suspected as anisakiasis. Korean J Parasitol. 2006;44:81-6.
120. Ito Y, Ikematsu Y, Yuzawa H, et al. Chronic gastric anisakiasis presenting as pneumoperitonium. Asian J Surg. 2007;30:67-71.
121. Choudhuri G, Saha S, Tandon R. Gastric ascariasis. Am J Gastroenterol. 1986;81:788.
122. Jacob G, Nakib A, Ruwaih A, et al. Ascariasis producing upper gastrointestinal hemorrhage. Endoscopy. 1983;15:67.
123. Dumont A, Seferian V, Barbier P, et al. Endoscopic discovery and capture of Necator americanus in the stomach. Endoscopy. 1983;15:65.
124. Belleza N, Lowman R. Suture granuloma of the stomach following total colectomy. Radiology. 1978;127:84.
125. Leach I, Maclennan K. Gastric lymphoma associated with mucosal and nodal granulomas: A new differential diagnosis in granulomatous gastritis. Histopathology. 1990;17:87.
126. Ectors N, Geboes K, Wynants P, et al. Granulomatous gastritis and Whipple’s disease. Am J Gastroenterol. 1992;87:509.
127. Grosman GM, Rosh JR, Harpaz N. Langerhans cell histiocytosis of the stomach: A cause of granulomatous gastritis and gastric polyposis. Arch Pathol Lab Med. 1994;118:1232.
128. Maeng L, Lee A, Choi K, et al. Granulomatous gastritis: A clinicopathologic analysis of 18 biopsy cases. Am J Surg Pathol.. 2004;28:941-5.
129. O’Donovan C, Murray J, Staunton H, et al. Granulomatous gastritis: Part of a vasculitic syndrome. Hum Pathol. 1991;22:1057.
130. Chin T, Stiehm R, Falloon J, et al. Corticosteroids in treatment of obstructive lesions of chronic granulomatous disease. J Pediatr. 1987;111:349.
131. Shapiro J, Goldblum J, Petras R. A clinicopathologic study of 42 patients with granulomatous gastritis: Is there really an “idiopathic” granulomatous gastritis? Am J Surg Pathol. 1996;20:462.
132. Miyamoto M, Haruma K, Yoshihara M, et al. Isolated granulomatous gastritis successfully treated by Helicobacter pylori eradication: A possible association between granulomatous gastritis and Helicobacter pylori. J Gastroenterol. 2003;38:371.
133. Koyama S, Nagashima F. Idiopathic granulomatous gastritis with multiple aphthoid ulcers. Intern Med. 2003;42:691.
134. Bigotti G, Coli A, Magistrelli P, et al. Gastric adenocarcinoma associated with granulomatous gastritis. Case report and review of the literature. Tumori. 2002;88:163.
135. Croxon S, Chen K, Davidson A. Sarcoidosis of the stomach. Digestion. 1987;38:193.
136. Kawaura K, Takahashi T, Kusaka K, et al. Spontaneously identified gastric sarcoidosis: A report of three cases. J Int Med Res. 2003;31:239.
137. Friedman M, Ali MA, Borum ML. Gastric sarcoidosis: A case report and review of the literature. South Med J. 2007;1:301-3.
138. Guarino M, Reale D, Micoli G, et al. Xanthogranulomatous gastritis: Association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46:88.
139. Kubosawa H, Yano K, Oda K, et al. Xanthogranulomatous gastritis with pseudosarcomatous changes. Pathol Int. 2007;57:291-5.
140. Colletti R, Trainer T. Collagenous gastritis. Gastroenterology. 1989;97:1552.
141. Pulimood A, Ramakrishna B, Mathan M. Collagenous gastritis and collagenous colitis: A report with sequential histological and ultrastructural findings. Gut. 1999;44:881.
142. Vesoulis Z, Lazanski G, Ravichandran P, et al. Collagenous gastritis: A case report, morphologic evaluation, and review. Mod Pathol. 2000;13:591.
143. Lagorce-Pages C, Fabiani B, Bouvier R, et al. Collagenous gastritis: A report of six cases. Am J Surg Pathol. 2001;25:1174.
144. Park S, Kim DH, Choe YH, et al. Collagenous gastritis in Korean child: A case report. J Korean Med Sci. 2005;20:146-9.
145. Freeman HJ. Topographic mapping of collagenous gastritis. Can J Gastroenterol. 2001;15:475.
146. Stancu M, De Petris G, Palumbo TP, et al. Collagenous gastritis associated with lymphocytic gastritis and celiac disease. Arch Pathol Lab Med. 2001;125:1579.
147. Wang HL, Shah AG, Yerian LM, et al. Collagenous gastritis: An unusual association with profound weight loss. Arch Pathol Lab Med. 2004;128:229.
148. Côté J, Handark G, Faure C, et al. Collagenous gastritis revealed by severe anemia in a child. Hum Pathol. 1998;28:883.
149. Haot J, Hamichi L, Wallez L, et al. Lymphocytic gastritis—a newly described entity: A retrospective endoscopic and histological study. Gut. 1988;29:1258.
150. Miettinen A, Karttunen T, Alavaikko M. Lymphocytic gastritis and Helicobacter pylori infection in gastric lymphoma. Gut. 1995;37:471.
151. Haot J, Jouret A, Willette M, et al. Lymphocytic gastritis—prospective of its relationship with varioliform gastritis. Gut. 1990;31:282.
152. Feeley K, Heneghan M, Stevens F, et al. Lymphocytic gastritis and coeliac disease: Evidence of a positive association. J Clin Pathol. 1998;51:207.
153. Tissot B, Tropet AL, Blanchi A, et al. Lymphocytic gastritis with anasarca and venous thrombosis. Presse Med. 2006;35:974-6.
154. Verkarre V, Asnafi V, Lecomte T, et al. Refractory coeliac sprue is a diffuse gastrointestinal disease. Gut. 2003;52:205.
155. Broide E, Sandbank J, Scape E, et al. The immunohistochemistry profile of lymphocytic gastritis in celiac disease and Helicobacter pylori infection: Interplay between infection and inflammation. Mediat Inflam. 2007;2007:81838.
156. Torigian DA, Levine MS, Gill NS, et al. Lymphoid hyperplasia of the stomach: Radiographic findings in five adult patients. AJR Am J Roentgenol. 2001;177:71.
157. Muller H, Volkholz H, Stolte M. Healing of lymphocytic gastritis by eradication of Helicobacter pylori. Digestion. 2001;63:14.
158. Talley N, Shorter R, Phillips S, et al. Eosinophilic gastroenteritis: A clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54.
159. Lee M, Hodges W, Huggins T, et al. Eosinophilic gastroenteritis. South Med J. 1996;89:189.
160. Rothenberg ME. Molecular mechanisms in allergy and clinical immunology: Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immun. 2004;113:11.
161. Hogan SP, Rothenberg ME. Eosinophil function in eosinophil-associated gastrointestinal disorders. Curr Allergy Asthma Rep. 2006;6:65-71.
162. Benchimol EI, Kirsch R, Viero S, et al. Collagenous colitis and eosinophillic gastritis in a 4-year old girl: A case report and review of the literature. Acta Paediatr. 2007;96:1365-7.
163. Bori R, Cserni G. Eosinophilic gastritis simulating gastric carcinoma. Orv Hetil. 2003;144:529-31.
164. Nagy F, Molnar T, F Kiss Z, Tiszlavicz L, Lonovics J. Ulcerative colitis and eosinophilic corpus gastritis. Orv Hetil. 2004;145:2241-6.
165. Oren R, Harats N, Polak A, et al. Granulomatous colitis 10 years after presentation with isolated Crohn’s gastritis. Am J Gastroenterol. 1989;84:449.
166. Weinstein WM. Emerging gastritides. Curr Gastroenterol Rep. 2001;3:523.
167. Sharif F, McDermott M, Dillon M, et al. Focally enhanced gastritis in children with Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2002;97:1415.
168. Tobin JM, Sinha B, Ramani P, et al. Upper gastrointestinal mucosal disease in pediatric Crohn disease and ulcerative colitis: A blinded, controlled study. J Pediatr Gastroenterol Nutr. 2001;32:443.
169. Furusu H, Murase K, Nishida Y, et al. Accumulation of mast cells and macrophages in focal active gastritis of patients with Crohn’s disease. Hepatogastroenterology. 2002;49:639.
170. Kundhal PS, Stormon MO, Zachos M, et al. Gastral antral biopsy in the differentiation of pediatric colitides. Am J Gastroenterol. 2003;98:557.
171. Ando T, Nobata K, Watanabe O, et al. Abnormalities in the upper gastrointestinal tract in the inflammatory bowel disease. Inflammopharmacology. 2007. Apr 28
172. Kuriyama M, Kato J, Morimoto N, et al. Specific gastroduodenoscopic finding in Crohn’s disease: Comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig Liver Dis. 2008;40:468-75.
173. Ikeuchi H, Hori K, Nishigami T, et al. Diffuse gastroduodenitis and pouchitis associated with ulcerative colitis. World J Gastroenterol. 2006;12:5913-15.
174. Hendrickson BA. Gastric inflammation as a feature of ulcerative colitis. J Pediatr Gastroenterol Nutr. 2003;37:228.
175. Pascasio JM, Hammond S, Qualman SJ. Recognition of Crohn disease on incidental gastric biopsy in childhood. Pediatr Dev Pathol. 2003;6:209.
176. Tremaine WJ. Gastroduodenal Crohn’s disease: Medical management. Inflamm Bowel Dis. 2003;9:127.
177. Grübel P, Choi Y, Schneider D, et al. Severe isolated Crohn’s-like disease of the gastroduodenal tract. Dig Dis Sci. 2003;48:1360.
178. Fonde E, Rodning C. Gastritis cystica profunda. Am J Gastroenterol. 1986;81:459.
179. Tuncer K, Alkanat M, Musoglu A, et al. Gastritis cystica polyposa found in an unoperated stomach: An unusual case treated by endoscopic polypectomy. Endoscopy. 2003;35:882.
180. Emna EJ, Haiffa T-G, Moussaddak A, et al. Gastric cystic polyposa: Report of 7 cases and literature review. Tunis Med. 2005;83:562-71.
181. Zamecnik M, Sosna B, Chlumska A. Gastrointestinal stromal tumor (GIST) with glandular component. A report of an unusual tumor resembling adenosarcoma. Cesk Patol. 2005;41:150-6.
182. Yamashita M, Hirokawa M, Nakasono M, et al. Gastric inverted hyperplastic polyp. Report of four cases and relation to gastritis cystica profunda. APMIS. 2002;110:717.
183. Snover D, Weisdorf S, Vercellotti G, et al. A histopathologic study of gastric and small intestinal graft-versus-host disease following allogeneic bone marrow transplant. Hum Pathol. 1985;16:387.
184. Snover D. Graft-versus-host disease of the gastrointestinal tract. Am J Surg Pathol. 1990;14:101.
185. Welch DC, Wirth PS, Goldenring JR, et al. Gastric graft-versus-host disease revisted: Does proton pump inhibitor therapy affect endoscopic gastric biopsy interpretation? Am J Surg Pathol. 2006;30:444-9.
186. Martin P, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host diseases after allogenic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320-27.
187. Snover D. Acute and chronic graft-versus-host disease: Histopathological evidence for two distinct pathogenetic mechanisms. Hum Pathol. 1984;15:202.
188. Kokkonen J, Ruuska T, Karttunen TJ, et al. Mucosal pathology of the foregut associated with food allergy and recurrent abdominal pains in children. Acta Paediatr. 2001;90:16.
189. Yimyaem P, Chongsriswawat V, Vivatvakin B, et al. Gastrointestinal manifestations of cow’s milk protein allergy during the first year of life. J Med Assoc Thai. 2003;86:116.
190. Oerda G, Vivenza D, Rapa A, et al. Increased interleukin-10 in Helicobacter pylori infection could be involved in the mechanism protecting from allergy. J Pediatr Gastroenterol Nutr. 2007;45:301-5.
191. Laine L, Bentley E, Chandrasoma P, et al. Effect of oral iron therapy on the upper gastrointestinal tract: A prospective evaluation. Dig Dis Sci. 1988;33:172.
192. Moore J, Alsop W, Freston J, et al. The effect of oral potassium chloride on upper gastrointestinal mucosa in healthy subjects: Healing of lesions despite continuing treatment. Gastrointest Endosc. 1986;32:210.
193. Jeremy R, Parfit MD, Driman DK. Pathological effects of drugs on the gastrointestinal tract: A review. Hum Pathol. 2007;38:527-36.
194. Walker J, Robinson J, Stewart J, Jacob S. Does enteric coated aspirin results in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovascular Thoracic Surg. 2007;6:519-22.
195. Das T, Susheela A, Gupta I, et al. Toxic effects of chronic fluoride ingestion on the upper gastrointestinal tract. J Clin Gastroenterol. 1994;18:194.
196. Marshall JK, Thabane M, James C. Randomized active and placebo-controlled endoscopy study of a novel protected formulation of oral alendronate. Dig Dis Sci. 2006;51:864-8.
197. Graham DY. What the gastroenterologist should know about the gastrointestinal safety profiles of bisphosphonates. Dig Dis Sci. 2002;47:1665.
198. Kokkonen J, Möttönen M, Karttunen TJ, et al. Mucosal pathology of the upper gastrointestinal tract associated with intensive chemotherapy in children: Vitamin A supplements do not prevent lesions. Ped Hem Oncol. 2002;19:181.
199. Petras R, Hart W, Bukowski R. Gastric epithelial atypia associated with hepatic arterial infusion chemotherapy: Its distinction from early gastric carcinoma. Cancer. 1985;56:745.
200. Weidner N, Smith J, LaVanway J. Peptic ulceration with marked epithelial atypia following hepatic arterial infusion chemotherapy: A lesion initially misinterpreted as carcinoma. Am J Surg Pathol. 1983;7:261.
201. Dargan PI, Giles LJ, Wallace CI, et al. Case report: Severe mercuric sulphate poisoning treated with 2,3-dimercaptopropane-1-sulphonate and haemodiafiltration. Crit Care. 2003;7:R1.
202. Laine L, Weinstein W. Histology of alcoholic hemorrhagic “gastritis”: A prospective evaluation. Gastroenterology. 1988;94:1254.
203. Parl F, Lev R, Thomas E, et al. Histologic and morphometric study of chronic gastritis in alcoholic patients. Hum Pathol. 1979;10:45.
204. Uppal R, Lateef S, Korsten M, et al. Chronic alcoholic gastritis: Roles of alcohol and Helicobacter pylori. Arch Intern Med. 1991;151:760.
205. Lanza F, Royer G, Nelson R, et al. Ethanol, aspirin, ibuprofen, and the gastroduodenal mucosa: An endoscopic assessment. Am J Gastroenterol. 1985;80:767.
206. Borch K, Jansson L, Sjodahl R, et al. Haemorrhagic gastritis. Incidence, etiological factors, and prognosis. Acta Chir Scand. 1987;154:211.
207. Bienia A, Sodolski W, Luchowska E. The effect of chronic alcohol abuse on gastric and duodenal mucosa. Ann Univ Mariae Curie Sklodowska. 2002;57:570.
208. Auroux J, Lamarque D, Roudot-Thoraval F, et al. Gastroduodenal ulcer and erosions are related to portal hypertensive gastropathy and recent alcohol intake in cirrhotic patients. Dig Dis Sci. 2003;48:1118.
209. Dyer S, Mycyk MB, Ahrens WR, et al. Hemorrhagic gastritis from topical isopropanol exposure. Ann Pharmacother. 2002;36:1733.
210. Thuluvath PJ, Yoo HY. Portal hypertensive gastropathy. Am J Gastroenterol. 2002;97:2973.
211. Ohta M, Yamaguchi S, Gotoh N, et al. Pathogenesis of portal hypertensive gastropathy: A clinical and experimental review. Surgery. 2002;131:165.
212. Burak KW, Lee SS, Beck PL. Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE) syndrome. Gut. 2001;49:866.
213. Sasaki T, Hasegawa T, Shimizu Y, et al. Portal hypertension gastropathy after surgery for biliary atresia. Surg Today. 2005;35:385-8.
214. Bresci G. Portal hypertension: The management of esophageal/gastric varices, portal hypertensive gastropathy or hypertensive colopathy. Fut Med. 2007;4:91-6.
215. Kodali V, Gordon S. Gastrointestinal hemorrhage secondary to crack cocaine. Gastrointest Endosc. 1995;41:604.
216. Fennell D, Gandhi S, Prichard B. Gastrointestinal haemorrhage associated with free-base (crack) cocaine. Postgrad Med J. 1995;71:377.
217. Arrillaga A, Sosa JL, Najjar R. Laparoscopic patching of crack cocaine-induced perforated ulcers. Am Surg. 1996;62:1007.
218. Cohen J. Surgical treatment of recalcitrant radiation-induced gastric erosions. Head Neck. 2000;22:303.
219. Wada S, Tamada K, Tomiyama T, et al. Endoscopic hemostasis for radiation-induced gastritis using argon plasma coagulation. J Gastroenterol Hepatol. 2003;18:1215.
220. Eriksson B, Szego T, Emås S. Duodenogastric bile reflux before and after selective proximal vagotomy with and without pyloroplasty. Scand J Gastroenterol. 1990;25:161.
221. Madura JA. Primary bile reflux gastritis: Diagnosis and surgical treatment. Am J Surg. 2003;186:269.
222. Chen SL, Mo JZ, Cao ZJ, et al. Effects of bile reflux on gastric mucosal lesions in patients with dyspepsia or chronic gastritis. World J Gastroenterol. 2005;11:2834-7.
223. Hermans D, Sokal EM, Collard JM, et al. Primary duodenogastric reflux in children and adolescents. Eur J Pediatr. 2003;162:598.
224. Cichoz-Lach H, Slomka M, Celinski K, et al. Level of serum cytokines in biliary gastritis and erosive gastritis with Helicobacter pylori coinfection. Ann Univ Mariae Curie Sklodowska. 2001;56:271.
225. Fukuhara K, Osugi H, Takada N, et al. Quantitative determinations of duodenogastric reflux, prevalence of Helicobacter pylori infection, and concentrations of interleukin-8. World J Surg. 2003;27:567.
226. Nakamura M, Haruma K, Kamada T, et al. Duodenogastric reflux is associated with antral metaplastic gastritis. Gastrointest Endosc. 2001;53:53.
227. Dixon MF, Mapstone NP, Neville PM, et al. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut. 2002;51:351.
228. Dixon MF, Neville PM, Mapstone NP, et al. Bile reflux gastritis and Barrett’s oesophagus: Further evidence of a role for duodenogastro-oesophageal reflux? Gut. 2001;49:359.
229. Johannesson KA, Hammar E, Stael von Holstein C. Mucosal changes in the gastric remnant: Long-term effects of bile reflux diversion and Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2003;15:35.
230. Nakane Y, Michiura T, Inoue K, et al. Jejunal interposition helps prevent reflux gastritis. Hepatogastroenterology. 2002;49:1461.
231. Santarelli L, Gabrielli M, Candelli M, et al. Post-cholecystectomy alkaline reactive gastritis: A randomized trial comparing sucralfate versus rabeprazole or no treatment. Eur J Gastroenterol Hepatol. 2003;15:975.
232. Højgaard L, Krag E. Chronic ischemic gastritis reversed after revascularization operation. Gastroenterology. 1987;92:226.
233. Force T, MacDonald P, Eade O, et al. Ischemic gastritis and duodenitis. Dig Dis Sci. 1980;25:307.
234. Karalis D, Quinn V, Victor M, et al. Risk of catheter-related emboli in patients with atherosclerotic debris in the thoracic aorta. Am Heart J. 1996;131:1149.
235. Hendel R, Cuenoid H, Giansiracusa D, et al. Multiple cholesterol emboli syndrome: Bowel infarction after retrograde angiography. Arch Intern Med. 1989;149:2371.
236. Cooper B, Douglas S, Firth L, et al. Erosive gastritis and gastrointestinal bleeding in a female runner. Gastroenterology. 1987;92:2019.
237. Choi SC, Choi SJ, Kim JA, et al. The role of gastrointestinal endoscopy in long-distance runners with gastrointestinal symptoms. Eur J Gastroenterol Hepatol. 2001;14:1089.
238. Chen Y. Mechanical gastritis as cause of upper gastrointestinal hemorrhage. Scand J Gastroenterol. 1993;28:512.
239. Yoruk G, Aksoz K, Buyrac Z, et al. Is prolapse gastropathy a cause of upper gastrointestinal bleeding? Turk J Gastroenterol. 2003;14:106.
240. Cameron AJ, Higgins JA. Linear gastric erosion: A lesion associated with large diaphragmatic hernia and chronic blood loss anemia. Gastroenterology. 1986;91:338.
241. Moskovitz M, Fadden R, Min T, et al. Large hiatal hernias, anemia, and linear gastric erosion: Studies of etiology and medical therapy. Am J Gastroenterol. 1992;87:622.
242. Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology. 2008;135:41-60.
243. Tarnawski A, Pai R, Deng X, et al. Aging gastropathy-novel mechanisms: Hypoxia, up-regulation of multifunctional phosphatase PTEN, and proapoptotic factors. Gastroenterology. 2007;133:1938-47.
244. Coffey RJ, Washington MK, Corless CL, Heinrich MC. Ménétrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70-80.
245. Mall AS, Dent DM, McLeod H, et al. Extraction, isolation, and SDS-PAGE analysis of purified gastric mucin in a patient with Menetrier’s disease. Am J Gastroenterol. 2002;97:752.
246. Mall AS, Taylor K, Barnard R, et al. Expression of gastric mucin in the stomachs of two patients with Menetrier’s disease: An immunohistochemical study. J Gastroenterol Hepatol. 2003;18:876.
247. Richard A, Komorowski JG. Hyperplastic gastropathy. Am J Surg Pathol. 1999;15:577-85.
248. Ibarrola C, Rodriguez-Pinilla M, Valino C, et al. An unusual expression of hyperplastic gastropathy (Menetrier type) in twins. Eur J Gastroenterol Hepatol. 2003;15:441.
249. Hoffer V, Finkelstein Y, Balter J, et al. Ganciclovir treatment in Menetrier’s disease. Acta Paediatr. 2003;92:983.
250. Di Vita G, Patti R, Aragona F, et al. Resolution of Menetrier’s disease after Helicobacter pylori eradicating therapy. Dig Dis. 2001;19:179.
251. Burdick J, Chung E, Tanner G, et al. Treatment of Ménétrier’s disease with a monoclonal antibody against the epidermal gross factor receptor. N Engl J Med. 2000;344:1697.
252. Tran T, Hung P, Laucirica R, et al. The clinical significance of thickened gastric folds found on upper gastrointestinal series. J Clin Gastroenterol. 2002;35:138.
253. Gencosmanoglu R, Sen-Oran E, Kurtkaya-Yapicier O, et al. Gastric polypoid lesions: Analysis of 150 endoscopic polypectomy specimens from 91 patients. World J Gastroenterol. 2003;9:2236.
254. Insko EK, Levine MS, Birnbaum BA, et al. Benign and malignant lesions of the stomach: Evaluation of CT criteria for differentiation. Radiology. 2003;228:166-71.
255. Hummel M, Oeschger S, Barth TFE, et al. Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut. 2006;55:782-7.
256. Redeen S, Peterson F, Jonsson KA, et al. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-50.
257. Setiawan VW, Zhang ZF, Yu GP, et al. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int J Cancer. 2001;92:600.