Fair Game
Case 51
 |
1. Specify the examination that is represented on the image above.
2. What is the major finding?
3. What is the probable etiology of this abnormality?
4. Does this abnormality need to be treated and how?
ANSWERS: CASE 51
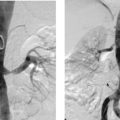
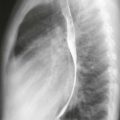
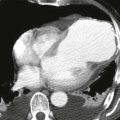
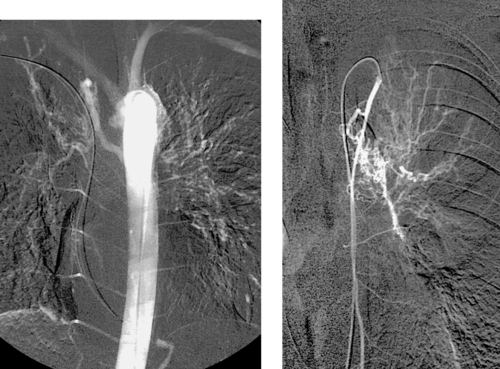
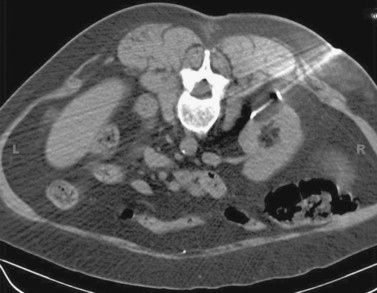
Superior Mesenteric Artery Embolus
1. Selective superior mesenteric arteriogram (SMA).
2. Globular filling defect in the proximal superior mesenteric artery.
3. Embolus from a cardiac source.
4. Yes, by emergent surgical thrombectomy.
Reference
Lee, R.; Tung, H.K.; Tung, P.H.; et al., CT in acute mesenteric ischemia, Clin Radiol. 58 (2003) 279–287.
Cross-Reference
Comment
The image clearly demonstrates partial occlusion of the superior mesenteric artery by a globular filling defect, consistent with an embolus. The remaining SMA branches have a normal appearance.
Acute mesenteric ischemia is associated with a mortality rate of 70%, primarily due to bowel infarction with resultant sepsis. The clinical signs of mesenteric ischemia can include abdominal pain, leukocytosis, hematochezia, and lactic acidosis. Unfortunately, the clinical signs of this disorder are insidious and are often recognized after irreversible bowel ischemia has occurred. Prompt diagnosis and therapy are absolutely imperative in avoiding mortality. The etiologies of acute mesenteric ischemia include embolus (which produces ischemia within a major mesenteric vascular distribution), hypotension in patients with preexisting atherosclerotic stenoses (which can produce ischemia within a watershed distribution, usually near the splenic flexure, which represents the junction of the superior and inferior mesenteric artery territories), and acute aortic dissection.
Surgical therapy, specifically thrombectomy and/or aortomesenteric bypass with bowel resection as needed, is the treatment of choice. Endovascular methods can be used in rare cases where the embolus involves an extremely short segment near the origin of the SMA (enabling the stent to be placed), but stenting has not been shown to be equivalent to surgical therapy in this clinical setting. The special subset of patients with aortic dissection complicated by mesenteric ischemia may also be treated in endovascular fashion, using stent placement and/or percutaneous balloon fenestration of the aortic flap.
Notes
Case 52
 |
1. Where is the tip of the left-sided catheter in these images located?
2. Where should the tip be located?
3. What complication is observed in the second image?
4. Name three other complications of central venous catheter placement.
ANSWERS: CASE 52
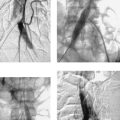
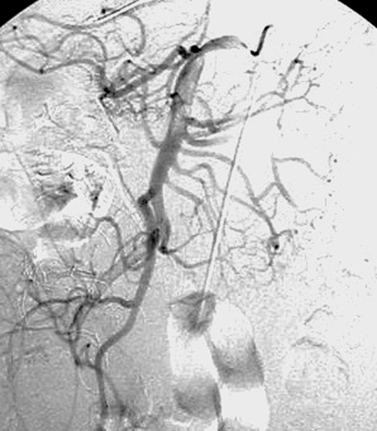
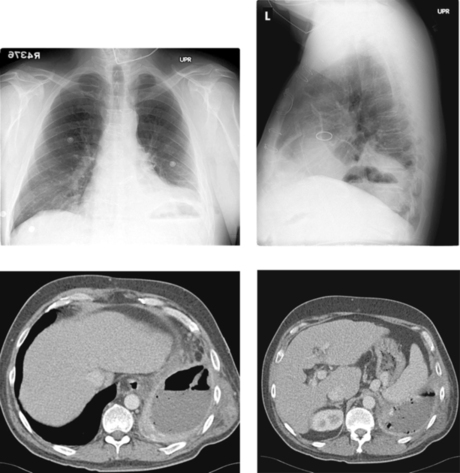
Central Venous Catheter Malposition with Thrombosis
1. Left brachiocephalic vein.
2. Distal superior vena cava.
3. Thrombosis of the left brachiocephalic vein.
4. Infection with or without bacteremia, bleeding after placement, pneumothorax after placement (rare with radiologically guided placement).
Reference
Vesely, T.M., Central venous catheter tip position: A continuing controversy, J Vasc Intervent Radiol. 14 (2003) 527–534.
Cross-Reference
Comment
Radiologists can place the entire gamut of central venous catheters: (1) Peripherally inserted central catheters (PICC lines): These small-caliber catheters are used for short-term (2-6 weeks) venous access and are inserted via an arm vein; (2) Nontunneled or tunneled (for long-term access) catheters for administering blood transfusions, antibiotics, other parenteral medications, and/or total parenteral nutrition; (3) Nontunneled or tunneled pheresis catheters; (4) Nontunneled or tunneled hemodialysis catheters; (5) Implantable ports for intermittent access.
In general, the internal jugular (IJ) vein is the preferred approach for placing tunneled (long-term) catheters and ports. The IJ approach is associated with a lower incidence of catheter migration, pinch-off syndrome, and symptomatic venous occlusion. Furthermore, IJ access spares the subclavian veins; this is extremely important in patients with chronic renal failure for whom upper extremity dialysis access might eventually be needed. For nontunneled catheters, the subclavian vein is generally preferred because chest wall exit sites are associated with a lower infection rate than neck or groin exit sites. However, notable exceptions to this rule include the aforementioned patients with chronic renal failure and those with impaired coagulation, because the subclavian vein is located in a less-compressible location than the IJ.
Although most organizational guidelines recommend placing the catheter tip in the distal superior vena cava, a significant number of physicians are placing hemodialysis and pheresis catheters in the proximal right atrium in order to achieve better flow rates.
Case 53
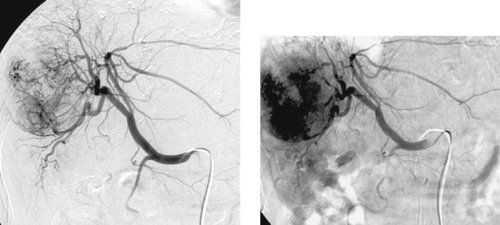 |
1. What abnormalities are apparent on this selective right hepatic arteriogram?
2. What is the most likely diagnosis?
3. What serum marker is commonly elevated in these patients?
4. What percutaneous treatments are available for this disorder?
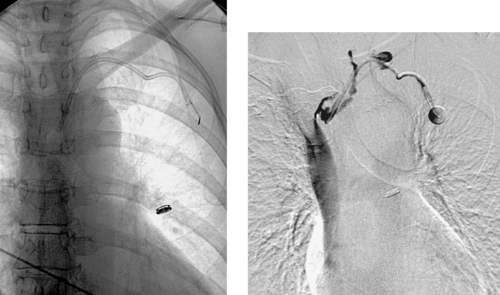
ANSWERS: CASE 53
Hepatocellular Carcinoma
1. Hypervascularity, neovascularity, splaying of arterial branches, dense tumor stain.
2. Hepatocellular carcinoma.
3. Alpha-fetoprotein.
4. Embolization with bland particles and/or chemotherapeutic agents, radioembolization with yttrium-90, hepatic arterial port catheter insertion for chemotherapy, percutaneous ethanol ablation, radiofrequency ablation, and cryoablation.
Reference
Sze, D.Y.; Razavi, M.K.; So, S.K.; et al., Impact of multidetector CT hepatic arteriography on the planning of chemoembolization treatment of hepatocellular carcinoma, AJR Am J Roentgenol. 177 (2001) 1339–1345.
Cross-Reference
Comment
Hepatocellular carcinoma can manifest with a solitary mass, multiple masses, or diffuse hepatic involvement. Commonly found in patients with cirrhosis, it is a highly malignant neoplasm with a poor long-term prognosis. Angiographic features include enlarged feeding arteries, neovascularity, puddling, dense tumor stain, arterioportal shunting, portal vein invasion, and occasionally hepatic vein invasion. A central necrotic area may be present and can splay surrounding abnormal vessels. The uninvolved liver commonly shows arteriographic changes of cirrhosis, including small corkscrew-like vessels.
Arteriography is useful for assessing tumor blood supply before surgery and for providing guidance for hepatic arterial port catheter placement or chemoembolization. It is useful to obtain portal venous images at the time of arteriography to determine the direction of portal venous flow and to detect thrombus in the portal vein.
Because hepatocellular carcinomas derive their blood supply nearly entirely from the hepatic arterial system, they are significantly more sensitive to the ischemic effects of arterial occlusion than normal hepatic parenchyma, which derives more than two thirds of its blood supply from the portal venous system. Chemoembolization, which involves intraarterial infusion of chemotherapy immediately followed by embolization, results in two important effects that constitute the basis for this form of therapy: increased dwell time of the chemotherapeutic agents within the tumor owing to slow flow in and out of the tumor bed, and tumor ischemia.
Case 54
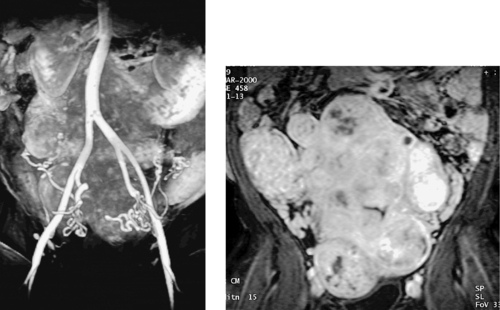 |
1. What study is presented in the images above?
2. Why was this study requested by the interventional radiologist?
3. What common cause of global uterine enlargement may be distinguished from uterine fibroid disease using this study?
4. What common arterial variants can this study help to identify?
ANSWERS: CASE 54
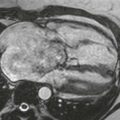
Enlarged Uterine Arteries
1. Magnetic resonance angiogram (MRA) of the uterine arteries.
2. Planning for uterine fibroid embolization (UFE).
3. Adenomyosis.
4. Unilateral absence of the uterine artery, ovarian artery supply to the uterus.
Reference
Spies, J.B.; Roth, A.R.; Jha, R.C.; et al., Leiomyomata treated with uterine artery embolization: Factors associated with successful symptom and imaging outcome, Radiology 222 (2002) 45–52.
Cross-Reference
Comment
The first image demonstrates the characteristic tortuous appearance of enlarged uterine arteries as they descend into the pelvis and then ascend along the lateral uterine wall. The second image demonstrates strong heterogeneous enhancement of the uterus and the individual fibroid tumors on MRI following gadolinium infusion, a typical appearance.
MRI is useful in the preembolization workup of patients with uterine fibroids for several reasons: (1) MRI is extremely accurate in diagnosing uterine fibroids and can distinguish between the diverse causes of global uterine enlargement: (2) MRI can accurately depict the local invasiveness of a mass lesion (triggering suspicion for malignancy); (3) MRI enables accurate and reproducible measurements to be obtained; (4) MRI can accurately classify fibroids as being submucosal, intramural, subserosal, and/or pedunculated. This information can affect the type of therapy employed; (5) Speculated causes of clinical failure of UFE include aberrant uterine artery anatomy, untreated ovarian artery supply to fibroids, and coexistent adenomyosis, all of which can be identified by MRI.
Other MRI findings might also soon be used to predict treatment response to UFE. In recent studies, submucosal fibroid location, small fibroid size, low T1 signal, and hypervascularity have been correlated with greater fibroid volume reduction after UFE, and high T1 signal has been correlated with lesser volume reductions. Hence, although ultrasound is an acceptable diagnostic modality for fibroids, interventional radiologists are increasingly turning to MRI for its superior pretreatment planning capabilities.
Notes
Case 55
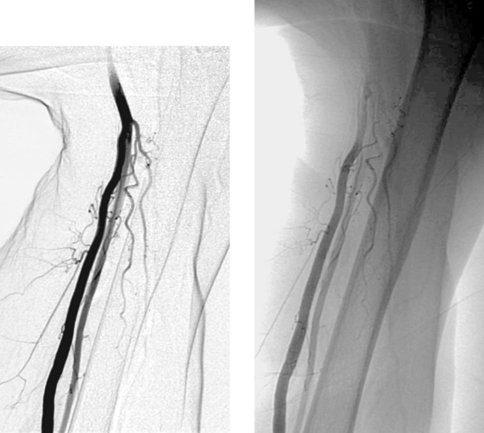 |
1. Name the more medial artery on the left arm images above.
2. Name the more lateral artery on the left arm images above.
3. What symptoms are typically associated with this finding?
4. Where does the more lateral artery normally arise?
ANSWERS: CASE 55
Variant Anatomy: High Radial Artery Origin
1. Left brachial artery.
2. Left radial artery (high origin).
3. None.
4. From the brachial artery below the elbow joint.
Reference
Uglietta, J.P.; Kadir, S., Arteriographic study of variant arterial anatomy of the upper extremities, Cardiovasc Intervent Radiol. 12 (3) (1989) 145–148.
Cross-Reference
Comment
Distal to the elbow joint, the brachial artery normally trifurcates into a radial artery, an ulnar artery, and an interosseous artery. Several anatomic variants of this pattern occur with enough frequency that it is important to be aware of them. For instance, in some patients the brachial artery divides proximally into two limbs that continue in parallel, then reunite distally.
Normally the radial artery arises as the first branch of the brachial artery below the elbow, and the ulnar artery divides into its named branches a few centimeters distally. However, up to 19% of persons have an early bifurcation of the brachial artery, an anomaly more commonly found on the right. Although a high radial artery origin from the brachial artery is the most common upper-extremity arterial variant (7%–8%), the ulnar artery can also arise from the brachial artery above the elbow. Less commonly, the radial artery (1%–3%) or ulnar artery (1%–2%) can arise from the axillary artery. Physiologically these variations are of little significance, but they can be significant when a brachial artery puncture is planned or when a patient suffers trauma to the upper extremity with associated vascular injury.
Notes
Case 56
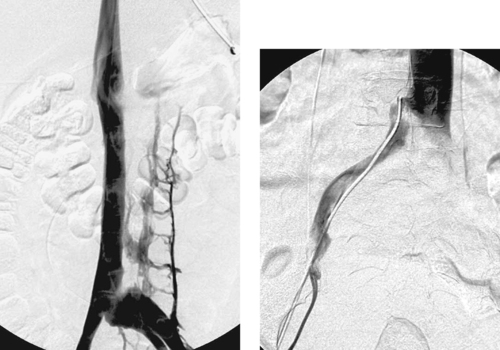 |
1. What findings are present in these two patients and what syndrome is present in the second patient?
2. What is thought to be the etiology of this disorder?
3. What is currently favored as the treatment of choice for this disorder?
4. What is the post-thrombotic syndrome?
ANSWERS: CASE 56
May–Thurner Syndrome
1. Both patients have left common iliac vein stenosis. The second patient has left iliofemoral venous thrombosis, consistent with iliac vein compression (May–Thurner) syndrome.
2. Compression of the left iliac vein by the crossing right common iliac artery.
3. Catheter-directed thrombolysis followed by iliac vein stent placement.
4. The major long-term complication of deep vein thrombosis (DVT), characterized by chronic edema, pain, skin discoloration, varicosities, venous claudication, venous stasis ulcers, and subcutaneous fibrosis.
Reference
Patel, N.H.; Stookey, K.R.; Ketcham, D.B.; et al., Endovascular management of acute extensive iliofemoral deep venous thrombosis caused by MayThurner syndrome, J Vasc Intervent Radiol. 11 (2000) 1297–1302.
Cross-Reference
Comment
Iliofemoral deep venous thrombosis is three to eight times more common in the left leg than in the right leg. This is thought to be due to the relative compression of the left iliac vein at the pelvic brim by the crossing right common iliac artery.
Iliac vein compression (May–Thurner syndrome) is a distinct entity that typically affects women in the second to fourth decades. It is differentiated from bland deep vein thrombosis of the lower extremity by the presence of a fibrous spur or adhesion in the left common iliac vein, thought to represent an inflammatory response to chronic compression of the vein and irritation of its endothelium from adjacent arterial pulsations.
Patients typically present with acute iliofemoral DVT or chronic DVT with venous insufficiency. Endovascular therapy is the treatment of choice for this disorder. Typically, catheter-directed thrombolysis is used first to remove any acute thrombus, and an endovascular stent is subsequently placed to address the venous stenosis. Surgical thrombectomy that does not also address the underlying left common iliac vein stenosis has a high failure rate.
Notes
Case 57
 |
1. What abnormality is seen in the first image?
2. How did this patient likely present?
3. What are the most common causes?
4. How and why was the second image obtained?
ANSWERS: CASE 57
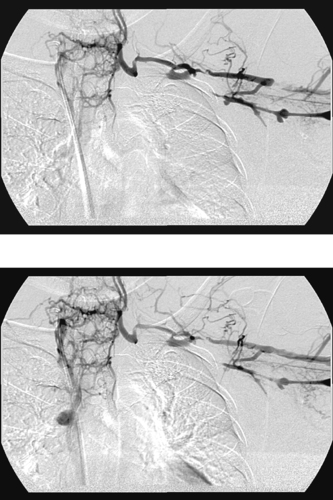
Bronchial Artery Embolization
1. Enlarged right bronchial artery.
2. Massive hemoptysis.
3. In the non-Western world: pulmonary tuberculosis. In the Western world: cystic fibrosis, bronchogenic carcinoma, bronchiectasis, or aspergillosis.
4. Selective angiography of the left internal mammary artery was performed because this vessel is a common source of systemic collateral supply to the lungs.
Reference
Yoon, W.; Kim, J.K.; Kim, Y.H.; et al., Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: A comprehensive review, Radiographics 22 (2002) 1395–1409.
Cross-Reference
Comment
Bronchial artery embolization has become an established procedure for treating life-threatening hemoptysis. Massive hemoptysis is usually defined by the production of 300 to 600mL of blood per day, but the patient’s clinical condition should guide therapy because smaller amounts of bleeding can be life-threatening in some instances.
In 90% of patients, bleeding arises from a bronchial artery. Less-common sources include the pulmonary arteries, aorta, and systemic collaterals to the lungs. Localization of the bleeding site by radiography, bronchoscopy, and/or chest CT before angiography and embolization is important so as to focus therapy. Hypertrophied and tortuous bronchial arteries, neovascularity, hypervascularity, and pulmonary arteriovenous shunting are common angiographic findings. Extravasation of contrast is rarely seen.
The bronchial arteries typically originate from the descending thoracic aorta at the T5-T6 level. In about 40% of patients, there are two arteries on the left and one on the right arising from an intercostobronchial trunk. However, there is extensive variability in number, origin, and branching pattern. It is very important to identify spinal arterial branches that arise from the bronchial and intercostal arteries, because nontarget embolization of these vessels can result in spinal ischemia. In general, embolization with polyvinyl alcohol particles is preferred. Coils are not used because they produce proximal occlusion, precluding repeat embolization should hemoptysis recur.
Notes
Case 58
 |
1. What is the primary abnormality?
2. What symptoms are likely present?
3. What is the best initial invasive treatment option for this patient?
4. If catheter placement did not result in adequate drainage, what adjunctive therapy could be performed?
ANSWERS: CASE 58
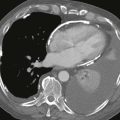
Empyema
1. Left posterior empyema.
2. Fever, leukocytosis, pleuritic chest pain, and possibly dyspnea and/or sepsis.
3. Percutaneous thoracostomy.
4. Intracavitary infusion of a fibrinolytic agent.
Reference
VanSonnenberg, E.; Wittich, G.R.; Goodacre, B.W.; et al., Percutaneous drainage of thoracic collections, J Thorac Imaging. 13 (2) (1998) 74–82.
Cross-Reference
Comment
The images demonstrate a rim-enhancing, gas-containing fluid collection within the left posterior pleural space, consistent with empyema. Imaging-guided chest tube placement is an excellent initial therapy and can be performed under fluoroscopy, CT, or ultrasound guidance. Preprocedural clinical assessment should include an evaluation of whether the patient will be able to tolerate the required position on the procedure table, because many patients with empyema have significant associated pulmonary compromise. Coagulation studies and platelet level should also be obtained.
Under imaging guidance, a needle is placed into the collection and contrast is injected to confirm proper positioning. It is important to avoid placing the needle just under a rib, because this can result in hemorrhage due to traversal of an intercostal artery. Over a guidewire, the tract is dilated and a large-bore drainage catheter is placed and attached to negative pressure. Follow-up CT scans are used to evaluate the progress of drainage. When the patient has improved clinically and the cavity is resolved, the catheter can be incrementally withdrawn to allow gradual healing of the residual cavity and tube tract.
If drainage ceases or stabilizes before the collection completely resolves, the cavity may be loculated and intracavitary fibrinolytic agents can be given. Alternatively, the catheter might be occluded or might not be in optimal position; a repeat CT scan can be used to guide an attempt at repositioning the catheter under fluoroscopy. If the catheter is patent and in optimal position, then a larger catheter may be needed. Infrequently, the fluid may simply be too viscous for percutaneous drainage, and surgery may be required.
Notes
Case 59
 |
1. What vascular problem is present in the first image?
2. What are the common causes of this problem?
3. What intervention was performed between the first two images?
4. What are the two most feared complications of endovascular intervention in the treatment of this problem?
ANSWERS: CASE 59
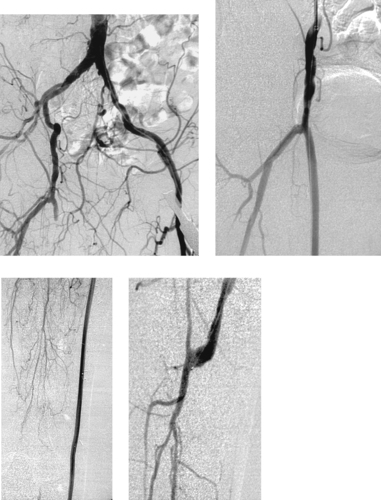
Bypass Graft Occlusion and Thrombolysis
1. Occlusion of a bypass graft originating in the right common femoral artery.
2. Proximal or distal anastomotic stenosis, intragraft stenosis, progression of atherosclerosis proximal or distal to the graft, hypercoagulability, and mechanical compression.
3. Catheter-directed thrombolysis or surgical thrombectomy.
4. Distant hemorrhage and distal embolization of thrombus.
Reference
Ouriel, K., Current status of thrombolysis for peripheral arterial occlusive disease, Ann Vasc Surg. 16 (2002) 797–804.
Cross-Reference
Comment
The first image demonstrates the stump of an occluded right femoropopliteal artery bypass graft. Graft occlusion is commonly the result of stenosis at the anastomosic sites.
The precise role of thrombolysis in the treatment of bypass graft thrombosis is somewhat controversial. However, several published trials support its use for patients with acute (<2 weeks) graft occlusion to achieve the following benefits: (1) It avoids surgical risks in patients who often have vascular comorbidities; (2) It has potential to both declot the graft and treat an underlying stenosis using angioplasty or stent placement; (3) Even if treatment of the underlying disorder cannot be achieved percutaneously, the diagnostic information provided by angiography after thrombolysis often guides definitive surgical therapy and enables reduction of the planned level of surgery. Endovascular therapy tends to be more successful in treating occluded synthetic grafts compared with vein grafts.
Disadvantages of thrombolysis include the longer time to reperfusion compared with surgical thrombectomy, and the risk of complications. Approximately 10% of patients require a transfusion due to bleeding, which most commonly occurs at the arterial access site. Distant bleeding occurs in 1% to 2% of patients, and intracranial bleeding occurs in 0.5% to 1.0%. Distal embolization occurs in 5% to 12% of patients, but it is usually treatable and rarely results in amputation. Compartment syndrome occurs in 2% of patients.
Notes
Case 60
 |
1. What abnormality is present on the images?
2. What is the most common cause of this problem?
3. How long have this patient’s symptoms likely been present?
4. Should endovascular treatment be performed?
ANSWERS: CASE 60
Chronic Axillosubclavian Vein Occlusion
1. Left axillosubclavian vein occlusion.
2. Central venous catheters and pacemakers.
3. More than 2 weeks.
4. No.
Reference
Meissner, M.H., Axillary-subclavian venous thrombosis, Rev Cardiovasc Med. 3 (Suppl 2) (2002) S44–S51.
Cross-Reference
Comment
The images demonstrate occlusion of the left axillosubclavian vein with collateral reconstitution of the superior vena cava. Venographic features that strongly suggest a chronic process include the lack of significant dilation of the occluded veins, the somewhat tapered aspect of the occlusion, the lack of globular filling defects or a meniscus sign, and the presence of abundant collaterals.
Most patients with subclavian vein thrombosis experience initial upper-extremity swelling and/or pain, but these symptoms usually subside as a mature venous collateral network develops. In fact, about 80% of patients with subclavian vein thrombosis eventually become asymptomatic or minimally symptomatic with anticoagulation therapy alone. For these reasons, endovascular interventions to restore patency in patients with subclavian vein thrombosis are reserved for carefully selected patients with primary axillosubclavian thrombosis (i.e., due to thoracic outlet syndrome), for young patients with acute secondary subclavian venous thrombosis who have good functional status, and for rare patients with special concerns such as a continuing need for central venous access where no other access sites are available. In these patients, a trial of thrombolytic therapy may be used, but most patients with chronic secondary subclavian vein thrombosis are treated with anticoagulation and removal of any central venous catheter that is present in the thrombus-containing vein.
Notes
Case 61
 |
 |
1. What is the abnormality in the first image?
2. What procedure is being performed in the second image?
3. What potential complication can this patient develop based on the anatomic location of the lesion?
4. What adjunctive measure was performed in the second image to avoid this complication?
ANSWERS: CASE 61
Liver Metastasis: Radiofrequency Ablation
1. Metastatic liver lesion (from colorectal carcinoma).
2. Radiofrequency ablation (RFA).
3. Thermal injury to the diaphragm.
4. An angiographic catheter has been interposed between the liver surface and the diaphragm; it was used to instill saline during the ablation procedure.
Reference
McGhana, J.P.; Dodd 3rd, G.D., Radiofrequency ablation of the liver: Current status, AJR Am J Roentgenol. 176 (2001) 3–16.
Cross-Reference
Comment
Surgical resection remains the standard of care for metastatic liver lesions; however, only 5% to 15% of patients are eligible for resection. RFA of nonresectable lesions can be performed in a subset of this patient population. Generally accepted selection criteria include fewer than five lesions, each less than 5cm in diameter, and no extrahepatic disease. Anatomic factors that must be taken into consideration include the location of the tumor(s) in relation to intrahepatic structures such as the bile ducts and vessels. Large vessels in close proximity to the lesion can make it difficult to attain good ablation due to a heat sink effect: Relatively high blood flow rates cause a cooling effect on the adjacent tissue. Extrahepatic structures such as the gallbladder, diaphragm, and adjacent bowel should also be evaluated for proximity to the anticipated ablation zone to prevent thermal injury to these structures.
The mechanism of action is the transmission of radiofrequency waves to the tissues, which causes an elevation of tissue temperature leading to coagulative necrosis of the exposed tissues. Image guidance by US, CT, or even MRI is used for correct needle placement through a percutaneous approach. The procedure can be performed under moderate sedation or general anesthesia. Certain adjunctive procedures can be performed to create a safe zone between the lesion and adjacent structures to prevent thermal injury, including patient positioning and hydrodissection (as in this case). Combination therapy with transarterial chemoembolization may also be performed.
Notes
Case 62
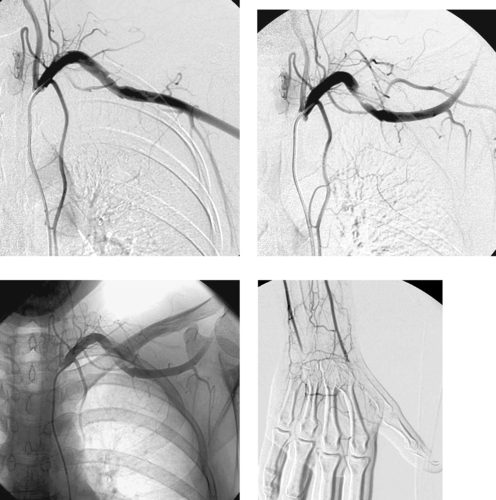 |
1. What positioning maneuver would worsen the abnormality in the first image?
2. What finding in the third image provides an important clue to the diagnosis?
3. What complication has this patient developed?
4. How would this be treated?
ANSWERS: CASE 62
Arterial Thoracic Outlet Compression Syndrome
1. Abduction at the shoulder joint.
2. A cervical rib.
3. Distal embolization of thrombus.
4. Surgical thoracic outlet decompression with embolectomy.
Reference
Demondion, X.; Bacqueville, E.; Paul, C.; et al., Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations, Radiology 227 (2003) 461–468.
Cross-Reference
Comment
Patients with arterial thoracic outlet compression syndrome typically present with symptoms in the hand and fingers including pain, numbness, paresthesias, intermittent claudication, cool skin temperature, and occasionally severe digital ischemia. Nearly half exhibit symptoms of Raynaud’s phenomenon. Symptoms often worsen with arm abduction. Reduction or obliteration of the radial pulse during clinical maneuvers such as passive arm hyperabduction or Adson’s maneuver (deep inspiration with hyperextension of the neck while the head is rotated to the symptomatic side) are highly suggestive of the diagnosis. A systolic bruit can sometimes be heard at the site of compression.
Cervical ribs are present in up to 0.5% of persons in the general population, but less than half of these patients develop symptoms of neurovascular compression. However, of patients with thoracic outlet compression syndrome, about 70% have a cervical rib. Other problems that can result in compression include the presence of a scalenus minimus muscle (seen in one third of normal persons), a wide or abnormal insertion or enlargement of the anterior scalene muscle, an anomalous first rib narrowing of the costoclavicular space, healed fracture deformities of the clavicle or first rib, or a muscular body habitus narrowing the pectoralis minor tunnel.
Arteriography should be performed with selective subclavian artery injection in a neutral position and with passive abduction of the extremity. Arteriographic findings include subclavian artery compression or narrowing with or without post-stenotic dilation, arterial occlusion, aneurysm, mural thrombus formation, and/or distal embolization.
Notes
Case 63
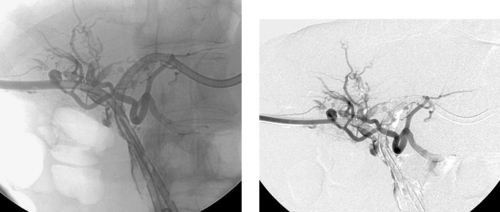 |
 |
1. How did this patient probably present and how commonly does this occur?
2. What is the diagnosis?
3. What was done between the second and third images?
4. Name another major complication that can occur following percutaneous transhepatic cholangiography (PTC) and percutaneous biliary drainage (PBD)?
ANSWERS: CASE 63
Iatrogenic Hepatic Artery Pseudoaneurysm
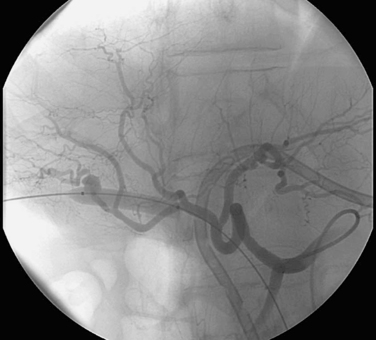
1. Hemobilia. This occurs in 2% to 10% of PTC and PBD procedures.
2. Iatrogenic pseudoaneurysm of a right hepatic artery branch.
3. The catheter was withdrawn over a wire and an angioplasty balloon catheter was inflated at the site of the pseudoaneurysm.
4. Biliary infection in the form of fever or chills, septicemia, and/or cholangitis.
Reference
Winick, A.B.; Waybill, P.N.; Venbrux, A.C., Complications of percutaneous transhepatic biliary interventions, Tech Vasc Intervent Radiol. 4 (3) (2001) 200–206.
Cross-Reference
Comment
Hemobilia following PTC or PBD can be a life-threatening condition, and patients should be instructed to contact the interventional radiologist immediately if they note bloody output from the drainage tube or skin site. Most bleeding, resulting from a catheter side hole within the liver parenchyma or a transient fistula between a bile duct and a portal or hepatic vein branch, is transient and resolves with conservative management. Catheter repositioning or upsizing may be necessary in some of these cases.
Severe hemobilia is usually the result of a bile duct communication with a hepatic artery branch or a major venous branch. Arterial bleeding should be suspected when pulsatile bright red blood drains from the biliary drainage catheter; in these cases, emergent hepatic arteriography is indicated for further evaluation. If arteriography does not demonstrate a site of bleeding, the biliary drainage catheter should be removed over a guidewire and the angiogram should be repeated. A balloon catheter can be replaced to tamponade bleeding while the bleeding vessel is embolized. A variety of embolic agents can be used in this setting, but most interventionalists use coils for this indication. When performing coil embolization of a pseudoaneurysm, it is important to place coils distal to, across, and proximal to the entry site to the pseudoaneurysm sac to prevent recurrent hemorrhage due to backbleeding from peripheral arterial branches.
Notes
Case 64
 |
1. Describe the findings on the images above.
2. What treatment options are available?
3. If the right superficial femoral artery is also occluded, what symptom would likely be present?
4. If the right superficial femoral artery is also occluded, what therapy is indicated?
ANSWERS: CASE 64
Iliac Artery Occlusion (Atherosclerosis)
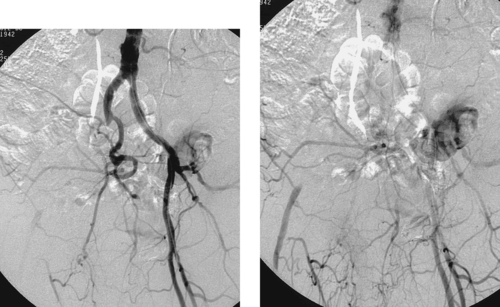
1. Right external iliac artery occlusion with common femoral artery reconstitution.
2. Aortofemoral bypass, femorofemoral bypass, thrombolysis and stent placement.
3. Rest pain.
4. Inflow reconstruction, probably via surgical bypass, is performed first. The patient is reassessed, and if lifestyle-limiting symptoms are still present, then femoropopliteal or femoral–distal bypass could be considered.
Reference
Rzucidlo, E.M.; Powell, R.J.; Zwolak, R.M.; et al., Early results of stent-grafting to treat diffuse aortoiliac occlusive disease, J Vasc Surg. 37 (2003) 1175–1180.
Cross-Reference
Comment
The first image demonstrates an occluded right external iliac artery due to in situ thrombosis of an atherosclerotic stenosis. The second image demonstrates reconstitution of the common femoral artery, information that is critical in planning revascularization.
Aortofemoral bypass is associated with 90% patency at 5 years. In patients who have a normal contralateral iliac artery and in whom medical comorbidities are present, femorofemoral bypass is sometimes used to revascularize the ischemic limb.
Endovascular recanalization of the occluded iliac artery has been performed for many years. In patients with chronic occlusions and short-segment acute occlusions, primary stent placement is considered by many to be the optimal endovascular method. In patients with acute occlusions with longer segments of thrombus, an initial course of thrombolytic therapy is favored by most physicians to eliminate as much thrombus as possible and thereby prevent embolization during later stent placement. Hence, in contrast to iliac artery stenoses, iliac artery occlusions are usually not treated with angioplasty alone; stents are nearly always used. Although stent-based endovascular recanalization is associated with lower primary patency than surgical bypass, the secondary patency (patency with repeat interventions) is comparable to surgical bypass and the overall procedural morbidity is lower. In recent years, stent grafts have shown promise as a way to recanalize the occluded iliac artery with a lower potential for late restenosis.
Notes
Case 65
 |
1. If the infrainguinal arteries are normal, what symptoms would this patient have?
2. Describe the angiographic findings in the first image.
3. What options are there for treatment?
4. What intervention was performed (second image) and why?
ANSWERS: CASE 65
Bilateral Common Iliac Artery Stenoses (Kissing Stents)
1. Bilateral hip and buttock claudication, possibly with impotence.
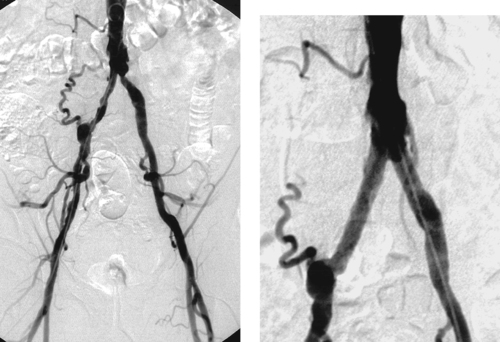
2. Bilateral common iliac artery stenoses, prominent right-sided lumbar collateral.
3. Percutaneous transluminal angioplasty, stent placement, aortobifemoral bypass.
4. Kissing stents were placed. Percutaneous intervention was chosen because of its lower morbidity than surgical therapy. Stenting was chosen over angioplasty because of the eccentric nature of the right-sided stenosis. Kissing stents were placed because of the involvement of the aortoiliac junction.
Reference
Scheinert, D.; Schroder, M.; Balzer, J.O.; et al., Stent-supported reconstruction of the aortoiliac bifurcation with the kissing balloon technique, Circulation 100 (Suppl 19) (1999) II295–II300.
Cross-Reference
Comment
Patients with disease in the aorta or common iliac arteries typically present with hip and buttock claudication and/or impotence, because of limited flow into the internal iliac artery territories. Intermittent claudication generally occurs when ankle–brachial indices are 0.5 to 0.9. When additional levels of disease are present in the femoral and/or tibial vessels, the patient is likely to experience rest pain in the feet. Rest pain signifies limb-threatening ischemia and is associated with a high amputation rate if the vascular abnormality is not treated. Ankle–brachial indices are generally 0.2 to 0.4 in patients with rest pain and no visible tissue loss, and they may be lower in patients with ulcers or gangrene.
Surgical aortofemoral bypass is associated with 90% patency at 5 years. The secondary patency of iliac artery angioplasty with selective stent placement is 80% to 90% at 4 years. Because endovascular therapy is associated with lower mortality and major morbidity, it is the preferred first-line treatment in patients with anatomically suitable iliac lesions.
Patients with aortoiliac junction lesions with or without actual distal aortic stenosis are often treated with kissing stents regardless of whether the contralateral iliac artery is diseased. This is done to effectively cover the aortic bifurcation plaque, to provide support to the other inflated balloon, and to prevent embolization down the contralateral side due to dislodged aortic bifurcation plaque.
Notes
Case 66
 |
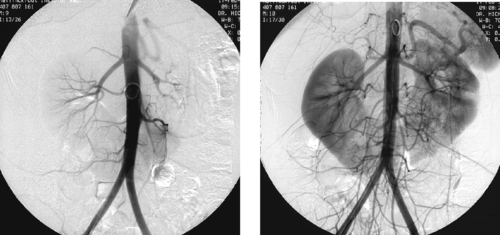
1. What vascular problem is depicted in the first image of this adult patient?
2. Describe the examination depicted in the second image.
3. What is the primary abnormality in the second image?
4. How might this problem be treated?
ANSWERS: CASE 66
Traumatic Splenic Artery Injury
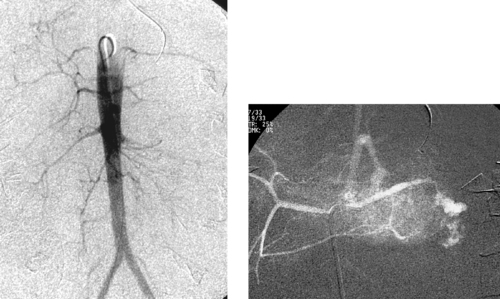
1. The diminutive caliber of the aortic branch vessels indicates systemic vasoconstriction due to hypovolemic shock.
2. Selective celiac arteriogram.
3. Splenic artery injury with pseudoaneurysm formation.
4. Transcatheter embolization or surgical repair.
Reference
Kluger, Y.; Rabau, M., Improved success in nonoperative management of blunt splenic injury: Embolization of splenic artery pseudoaneurysm, J Trauma. 45 (1998) 980–981.
Cross-Reference
Comment
Angiographic findings of visceral arterial trauma can include displacement or splaying of arterial branches due to hematoma formation, pseudoaneurysms, contrast extravasation, arterial filling defects due thrombosis or dissection, and diffuse vasoconstriction due to hypovolemic shock. In the latter instance, the extremely small caliber of the visceral vessels can occasionally render selective catheterization difficult. The patient depicted in these images is in dire need of fluid and blood product resuscitation.
When evaluating the patient with abdominal trauma, a reasonable plan for identifying the bleeding artery can only be developed after reviewing an abdominal CT scan, the results of diagnostic peritoneal lavage, and surgical findings (if the patient has already been explored). Because time is of the essence, the angiographer must direct his or her attention to the most likely source of bleeding. An abdominal aortogram may be performed initially. Although this only detects the grossest forms of branch vessel hemorrhage, it can help in selecting branch vessels in patients with distorted anatomy. The visceral vessels should then be evaluated with selective arteriography, and the vessels most likely to represent the source of bleeding should be studied first.
Traditional treatment of trauma-induced splenic arterial injuries has been splenic artery ligation with splenectomy. In recent years, transcatheter embolization has evolved into a less morbid alternative that might allow the spleen to be conserved. However, because splenic infarcts occur with moderate frequency following embolization, patients should receive appropriate immunizations to prevent later episodes of bacteremia.
Case 67
 |
1. What vascular abnormality is present?
2. Name three clinical sequelae that can result from this lesion.
3. What finding might confound endovascular management of this lesion?
4. What is the standard treatment for this problem?
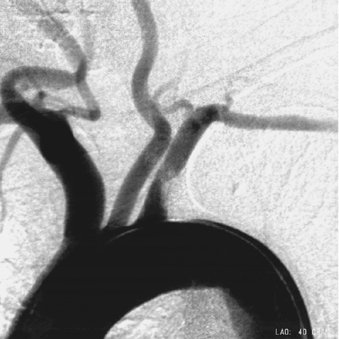
ANSWERS: CASE 67
Subclavian Artery Stenosis with Adherent Thrombus
1. Left subclavian artery stenosis with adherent filling defects.
2. Distal embolization, vertebrobasilar insufficiency due to subclavian steal, left arm claudication.
3. The visualized filling defects probably represent thrombus that could embolize during catheter manipulations.
4. Carotid–subclavian artery bypass.
Reference
Rodriguez-Lopez, J.A.; Werner, A.; Martinez, R.; et al., Stenting for atherosclerotic occlusive disease of the subclavian artery, Ann Vasc Surg. 13 (3) (1999) 254–260.
Cross-Reference
Comment
The image demonstrates a moderate-caliber stenosis of the proximal left subclavian artery. Associated filling defects are present that could indicate the presence of associated thrombus, versus less-likely bulky atherosclerotic plaque. Distal embolization from this lesion is a significant concern.
The standard treatment for a proximal subclavian artery lesion is carotid–subclavian artery bypass, which is associated with extremely high (90%-95%) long-term patency. In selected patients, angioplasty and/or stent placement can be performed with an expectation of good short-term and mid-term results. This particular lesion, with adherent thrombus that could potentially embolize to the vertebrobasilar circulation or to the distal upper-extremity vessels, would be a fairly poor candidate lesion for endovascular management.
Case 68
 |
1. What interventional procedure was previously performed in this patient?
2. Name two common indications for this procedure.
3. Name two important early complications of this procedure.
4. What common cause of late failure of this therapy is depicted in the first image?
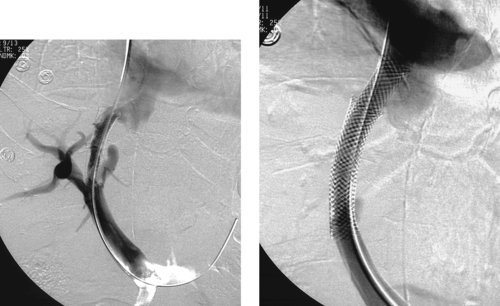
ANSWERS: CASE 68
Post-TIPS Stenosis
1. Transjugular intrahepatic portosystemic shunt (TIPS) placement.
2. Variceal hemorrhage and refractory ascites.
3. Intraperitoneal hemorrhage, development or progression of hepatic encephalopathy.
4. Stenosis at the hepatic vein end of the stent and within the stent.
Reference
Patel, N.H., Portal hypertension, Semin Roentgenol. 37 (4) (2002) 293–302.
Cross-Reference
Comment
TIPS is commonly performed in patients with portal hypertension and recurrent variceal bleeding after failed endoscopic therapy, refractory ascites, and/or hepatic hydrothorax. To perform TIPS, a catheter is placed in a hepatic vein (usually the right) via an internal jugular vein approach. A directional needle is passed through the hepatic parenchyma into the portal venous system (usually the right portal vein). Following portal venography and pressure measurements, the hepatic tract is angioplastied, and a stent is placed bridging the hepatic tract from the portal vein to the hepatic vein.
Major early complications of TIPS include intraperitoneal hemorrhage due to transcapsular needle puncture, worsening of hepatic encephalopathy due to shunting of blood away from the liver via the TIPS, stent infection with sepsis (rare), and early TIPS occlusion. For these reasons, relative contraindications to TIPS include preexisting hepatic encephalopathy, hepatic failure, uncorrected coagulopathy, and active infection.
Early TIPS occlusion is often caused by a fistulous connection with the biliary tree; this can now be treated with placement of a stent graft to exclude the biliary–TIPS fistula.
The 1-year patency rate for TIPS is about 50%. The most common cause of long-term TIPS failure is the development of stenosis along the course of the TIPS, most commonly at the hepatic vein end but also at the portal vein end or within the stent. When this occurs, variceal bleeding or ascites can recur. For this reason, post-TIPS duplex ultrasound surveillance is performed at regular intervals in order to identify developing stenoses. When abnormally low or high flow velocities are present within or adjacent to the TIPS, the patient is referred for TIPS venography. TIPS stenoses or occlusions can be effectively treated with balloon angioplasty or repeat stent placement.
Notes
Case 69
 |
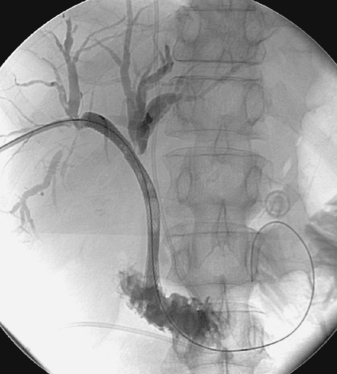
1. What arteriographic abnormality is present?
2. What is the endovascular treatment of choice?
3. If superselective catheterization is not successful, what endovascular pharmacologic therapy could be recommended?
4. What are the contraindications to this form of therapy?
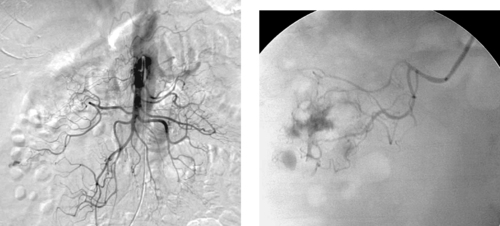
ANSWERS: CASE 69
Lower Gastrointestinal (Diverticular) Bleed
1. Active extravasation from a branch of the right colic artery.
2. Coil embolization of the bleeding branch.
3. Vasopressin infusion.
4. Severe coronary artery disease, dysrhythmia, cerebrovascular disease, severe hypertension.
Reference
Darcy, M., Treatment of lower gastrointestinal (LGI) bleeding: Vasopressin infusion versus embolization, J Vasc Intervent Radiol. 14 (2003) 535–543.
Cross-Reference
Comment
Catheter-directed vasopressin infusion has traditionally been the first-line treatment for LGI bleeding. This is based on findings in older literature in which infarction often complicated LGI embolization. With modern embolization techniques, clinically significant bowel ischemia has become an uncommon complication. Although the efficacies of vasopressin and embolization are fairly comparable, embolotherapy has advantages in terms of quicker completion of therapy and decreased likelihood of systemic complications. Although vasopressin is still probably preferable for diffuse lesions and cases in which superselective catheterization is not technically possible, embolization should be considered a primary option for treating LGI bleeding.
Vasopressin is a natural hormone that reduces pulse pressure and blood flow via constriction of smooth muscle in splanchnic blood vessels and bowel wall, allowing clot to form.
After a bleeding site is identified, a catheter is secured with its tip in a central vessel supplying the bleeding site; in this case, the tip would be located in the proximal superior mesenteric artery. Vasopressin infusion is started at 0.2U/min. Follow-up arteriography is performed in 20 to 30 minutes to assess response. If active extravasation is still present, the infusion is increased to 0.4U/min, and arteriography is again repeated in 20 to 30 minutes. If active extravasation is still observed, alternative therapies (such as embolization) should be pursued. If vasopressin infusion does result in cessation of bleeding, the infusion is continued for 12 to 24 hours and the patient is closely monitored in an intensive care unit. Mild abdominal discomfort at the initiation of infusion is common, but persistent pain might indicate ischemia, signaling the need to reduce the infusion rate. If continued cessation of bleeding is observed clinically, then the vasopressin infusion is tapered over the next 12 to 24 hours.
Initial success rates for vasopressin infusion therapy in controlling LGI bleeding range from 60% to 90%, but rebleeding occurs in approximately 40%. Vasopressin is not effective in the treatment of upper gastrointestinal bleeding, and it is rarely used in that setting.
Notes
Case 70
 |
1. What anatomic variant is present?
2. How common is this anomaly?
3. Why is this significant during placement of an inferior vena cava (IVC) filter?
4. What potential solutions exist?
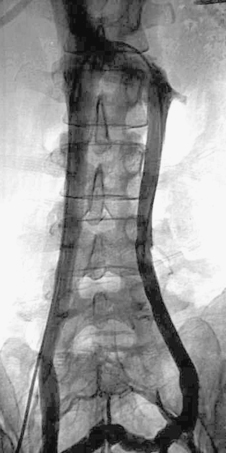
ANSWERS: CASE 70
Variant Anatomy: Duplicated Inferior Vena Cava
1. Duplicated IVC.
2. Approximately 2%.
3. Failure to recognize the vascular duplication and insertion of an infrarenal IVC filter into the right-sided cava alone can permit pulmonary embolism from a left lower extremity thrombus.
4. Either place one filter in each IVC (two filters total) or place a suprarenal filter.
Reference
Hicks, M.E.; Malden, E.S.; Vesely, T.M.; et al., Prospective anatomic study of the inferior vena cava and renal veins: Comparison of selective renal venography with cavography and relevance in filter placement, J Vasc Intervent Radiol. 6 (1995) 721–729.
Cross-Reference
Comment
Normally a single IVC drains the lower extremities and iliac veins. However, several congenital anomalies of venous development exist and can affect IVC filter placement. Therefore, an inferior vena cavogram should always be performed before deploying a filter.
In patients with duplication of the IVC, the left-sided IVC accepts drainage from the left renal and adrenal veins and subsequently drains into the right-sided IVC at the L1-L2 level. Therefore, to achieve adequate protection against potential pulmonary emboli originating in both limbs, it is necessary either to place two filters (one in each IVC) or to place a single (suprarenal) filter above the confluence of the two IVCs.
A solitary left-sided IVC is less common, occurring in 0.2% of persons. Similar to the duplicated IVC, this vessel drains to the right at the level of the renal veins.
Congenital absence of the IVC is seen in 0.6% of patients with congenital heart disease but is more common with cyanotic heart disease. Typically, the hepatic segment of the IVC is interrupted and lower-extremity venous drainage occurs via the azygous and hemiazygous systems. The hepatic veins drain directly to the right atrium.
Notes
Case 71
 |
 |
 |
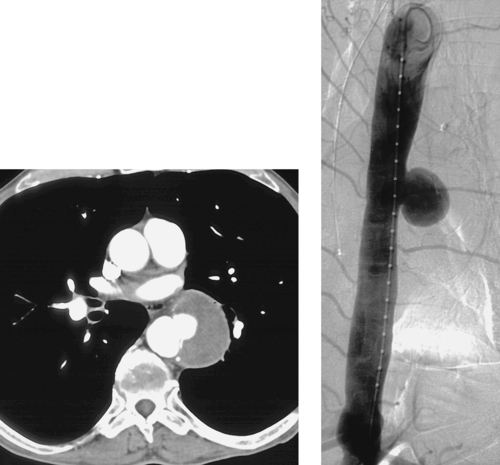
1. What is the diagnosis in this patient with a history of colon cancer?
2. What procedure is being performed in the second and third images?
3. What potential complications are associated with this procedure?
4. What other local therapies can be used to treat these lesions?
ANSWERS: CASE 71
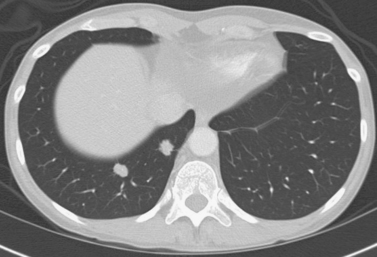
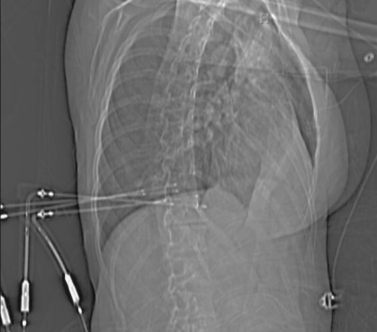
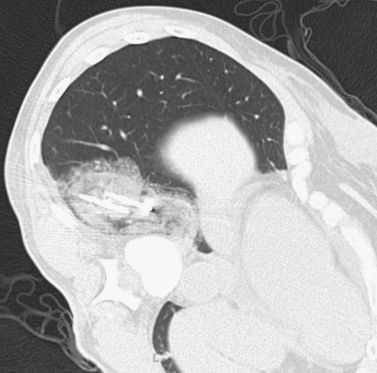
Lung Metastases: Cryoablation
1. Metastatic lung nodules.
2. Cryoablation.
3. Hemoptysis, pneumothorax, hemothorax.
4. Wedge resection, radiofrequency ablation, and external beam radiation.
References
Wang, H.; Littrup, P.J.; Duan, Y.; et al., Thoracic masses treated with percutaneous cryotherapy: Initial experience with more than 200 procedures, Radiology 235 (2005) 289–298.
Kawamura, M.; Izumi, Y.; Tsukada, N.; et al., Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates, J Thorac Cardiovasc Surg. 131 (2006) 1007–1013.
Goldberg, S.N.; Charboneau, J.W.; Dodd 3rd, G.D.; et al., International Working Group on Image-Guided Tumor Ablation: Image-guided tumor ablation: Proposal for standardization of terms and reporting criteria, Radiology 228 (2003) 335–345.
Comment
The standard of care for metastatic lung nodules with no extrapulmonary involvement is surgical resection: wedge resection, lobectomy, or even pneumonectomy. Many patients with metastatic lung disease are not candidates for surgery owing to poor pulmonary reserve or general health condition. Less-invasive therapies such as cryoablation, radiofrequency ablation, or external beam radiation can be offered to these patients. Local ablative therapies have a further advantage over external beam radiation in that they can be repeated as needed for lesions that develop further along in the course of the disease, as is typically seen in patients with metastatic disease.
Cryoablation involves freezing and rapid thawing of tissue that leads to cell membrane disruption, resulting in cell death. The application of cryoablation includes tumors in many locations including lung, kidney, liver, bone, soft tissues, and others. Image guidance with CT or US is used for placement of the cryoprobes, and the freezing and thawing is monitored by CT imaging. Lung consolidation is clearly seen in the area exposed to freezing in the third image.
Hemoptysis occurs commonly; however, this is minimal in the majority of patients and requires no further intervention aside from patient reassurance. Owing to the relatively large size of the cryoprobes, pneumothorax is a common procedural complication, with rates reported at 50% to 62%. Chest tube placement is only needed in 12% of these patients.
Notes
Case 72
 |
1. What is the diagnosis in this patient with recurrent gastrointestinal (GI) bleeding?
2. What procedure did this patient likely undergo in the past?
3. What structure is opacified in the second image?
4. How are these patients usually treated?
ANSWERS: CASE 72
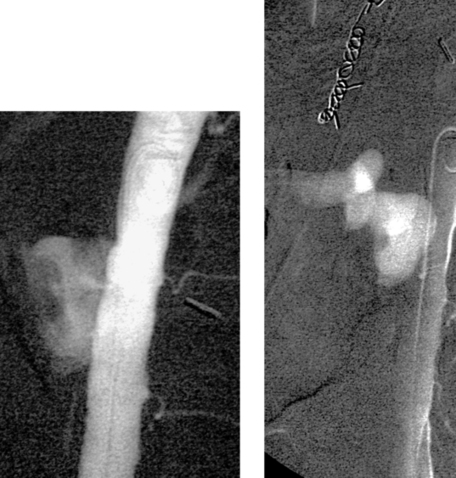
Aortoenteric Fistula
1. Aortoenteric fistula.
2. An aortic vascular procedure, such as repair of an abdominal aortic aneurysm.
3. Duodenum.
4. Graft removal with closure of the aortic stump, repair of the bowel defect, interposition of viable tissue (e.g., omentum) between the aorta and the bowel, and extraanatomic bypass grafting for lower-extremity revascularization.
Reference
Lemos, J.D.; Raffetto, T.C.; et al., Primary aortoduodenal fistula: A case report and review of the literature, J Vasc Surg. 37 (2003) 686–689.
Cross-Reference
Comment
Formation of an aortoenteric fistula is much more common as an infrequent (0.4%-2.4%) complication of aortic vascular procedures (termed secondary) than as a complication of aneurysm rupture (termed primary). In either situation, this disorder carries a significant risk of death or limb loss despite treatment. Patients with a direct communication between the anastomotic suture line of the aortic graft and the adjacent bowel lumen (graft–enteric fistula) often present with intermittent hematemesis or melena days to weeks after the procedure. Patients with an erosion remote from the suture line with exposure of a portion of the graft prosthesis (graft–enteric erosion) commonly present with chronic GI bleeding. Patients with lesser degrees of bleeding can present with septicemia resulting from graft infection.
Most cases occur two or more years after the aortic surgery. For this reason, although aortography is not routinely performed in the angiographic evaluation of all GI bleeding patients, those with a suitable history should be studied with aortography. Most cases involve the third or fourth portions of the duodenum (as in this case) owing to the proximity of the graft anastomosis to this fairly fixated portion of bowel, but in 10% to 20% the more distal small bowel or colon is involved. Endoscopy is the preferred first step in evaluation because it can diagnose many other more-common causes of GI bleeding, and rarely it can provide a definitive diagnosis by visualizing a focus of exposed graft.
Notes
Case 73
 |
1. What congenital variant is present?
2. How common is this variant?
3. Is the renal arterial anatomy usually normal?
4. Is this more common in men or women?
ANSWERS: CASE 73
Horseshoe Kidney
1. Horseshoe kidney.
2. 1 in 500 to 700 autopsies.
3. Usually not.
4. More common in men (2:1 ratio).
References
Raj, G.V.; Auge, B.K.; Weizer, A.Z., Percutaneous management of calculi within horseshoe kidneys, J Urol. 170 (2003) 48–51.
Cross-Reference
Comment
In a horseshoe kidney, the two kidneys are joined at either the lower pole (90%) or upper pole (10%) by a parenchymal–fibrous isthmus. Typically, the long axis of each kidney is oriented somewhat more medially than normal, and the renal pelves and ureters exit their respective kidneys anteriorly. The vascular supply is often aberrant and often there are multiple renal arteries (six or more renal arteries are possible). In the case presented here, at least three renal arteries supply the right kidney, one left renal artery is present, and one more artery can be seen to supply at least part of the isthmus that joins the lower poles of the kidneys.
A primary complication of horseshoe kidney is the development of renal calculi. Other associations include genitourinary abnormalities such as caudal ectopia, vesicoureteral reflux, hydronephrosis, ureteral duplication, hypospadias, undescended testis, and anomalies of the cardiovascular, skeletal, central nervous, and gastrointestinal systems.
Case 74
 |
1. What device has been inserted?
2. Is this a temporary or permanent device?
3. Is this device typically used for benign or malignant disorders?
4. In the case above, will the entire biliary ductal system be adequately drained?
ANSWERS: CASE 74
Metallic Biliary Stent Placement
1. Wallstent.
2. Permanent.
3. Malignant.
4. Probably not, but unilateral drainage will probably be sufficient to palliate obstructive symptoms.
Reference
Schmassmann, A.; Von Gunten, E.; Knuchel, J.; et al., Wallstents versus plastic stents in malignant biliary obstruction: Effects of stent patency of the first and second stent upon patient compliance and survival, Am J Gastroenterol. 91 (1996) 654–659.
Cross-Reference
Comment
There are a wide variety of causes of biliary ductal obstruction or stricture. Benign causes include operative trauma, biliary ductal stones, scarring from prior passage of biliary stones, chronic pancreatitis, external compression, and nonoperative trauma. Malignant causes include cholangiocarcinoma, gallbladder cancer, pancreatic head carcinoma, ampullary neoplasms, and metastatic porta hepatis lymphadenopathy.
Completely internalized biliary stents are often preferred to external or internal–external drainage catheters. However, because of the viscous nature of biliary secretions, all biliary stents must be changed regularly to prevent occlusion of the stent and/or formation of calculi. For this reason, the vast majority of internal stents and all stents placed for benign causes are made of catheter-type materials such as silicone rubber (Silastic). Permanent metallic stents should only be inserted for palliation of malignant obstructions in patients with short anticipated life expectancy.
The imaging in this case demonstrates extension of a metallic stent from the right main hepatic duct into the duodenum. A focal narrowing and obstruction persists at the distal left main bile duct, with contrast stasis in the left bile ducts from the cholangiogram. Fortunately, it is not necessary to drain the entire biliary ductal system to obtain symptom relief from pain, jaundice, and pruritus, so the obstructed left ducts can be left undrained unless infection later occurs in the left system.
Case 75
 |
1. What is the diagnosis?
2. Where does this problem occur most often?
3. Name two major complications of this problem.
4. Why was the angiogram obtained?
ANSWERS: CASE 75
Penetrating Aortic Ulcer
1. Penetrating ulcer of the thoracic aorta.
2. Descending thoracic aorta.
3. Dissection and rupture.
4. To plan endovascular therapy.
Reference
Ganaha, F.; Miller, D.C.; Sugimoto, K.; et al., Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: A clinical and radiological analysis, Circulation 106 (3) (2002) 342–348.
Cross-Reference
Comment
The first image demonstrates a focal outpouching of contrast from the thoracic aorta with an associated pseudoaneurysm. The second image confirms the presence of a focal contrast collection with a narrow neck that protrudes from the descending thoracic aorta. The findings in this patient, who presented with acute chest pain, are consistent with a penetrating atherosclerotic aortic ulcer.
Penetrating atherosclerotic ulcer represents one entity within a spectrum of aortic disorders, which also encompasses aortic intramural hematoma and aortic dissection. A significant percentage of patients with symptomatic penetrating atherosclerotic ulcer do progress to aortic dissection, and some patients progress to aortic rupture. The presence of left-sided hemothorax on a CT scan is therefore of significant concern.
The conventional treatment for this disorder is surgical aortic repair. However, thoracotomy and descending thoracic aortic repair are associated with considerable risk of death, paraplegia, and other major morbidities. For this reason, selected patients with this disease are now treated with placement of an endovascular stent graft. The patient depicted here would be an excellent candidate for this procedure, because the aorta is straight and not excessively enlarged, the ulcer defect is short, and the surrounding aorta appears reasonably free of bulky atherosclerotic plaque.
Notes
Case 76
 |
 |
1. What abnormality is depicted in the first image?
2. Is this likely to become symptomatic?
3. What do you recommend?
4. What device is most commonly used for this procedure?
ANSWERS: CASE 76
Foreign Body Retrieval
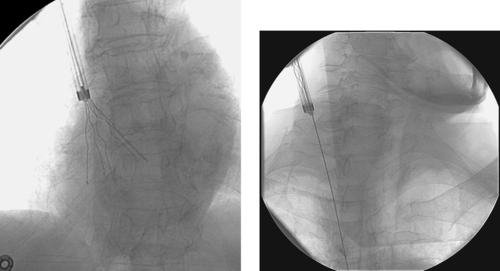
1. Foreign body (a Greenfield inferor vena cava [IVC] filter) within the right atrium.
2. Yes.
3. Transcatheter retrieval.
4. Amplatz nitinol gooseneck snare (Microvena, White Bear Lake, Minn).
Reference
Gabelmann, A.; Kramer, S.; Gorich, J., Percutaneous retrieval of lost or misplaced intravascular objects, Am J Roentgenol. 176 (2001) 1509–1513.
Cross-Reference
Comment
A variety of intravascular foreign bodies have been described, including catheter and guidewire fragments, filters, stents, coils, and balloon and bullet fragments. Objects lodge in a location corresponding to their size, flexibility, and shape. Intravenous objects commonly lodge within the superior vena cava, right heart, or pulmonary artery.
Large intravascular foreign bodies (as in this case) and small foreign bodies that have been present for a short time are typically removed to eliminate the potential complications of arrhythmia, thrombosis, bacteremia, and vascular or cardiac perforation. Small objects that have been present for prolonged periods can become incorporated into the vessel wall or endocardium, and they are often left alone unless they are causing complications.
Transcatheter retrieval is the preferred treatment. The site of percutaneous entry is selected based upon the location and orientation of the foreign body. A sheath should be inserted to minimize trauma to the access vessel when the object is removed. Typically, a guiding catheter and snare device are used. Preferably, the foreign body is snared near its tip, the snare loop is snugged around the fragment, and the object is withdrawn into the sheath and out of the vessel in a single motion. On occasion, a cutdown may be necessary to remove large objects or objects with sharp edges.
Notes
Case 77
 |
1. What vessel has been selected for the angiogram in the first image?
2. From what parent artery does this vessel arise?
3. What symptoms is this patient likely experiencing?
4. Why is arteriography being performed?
ANSWERS: CASE 77
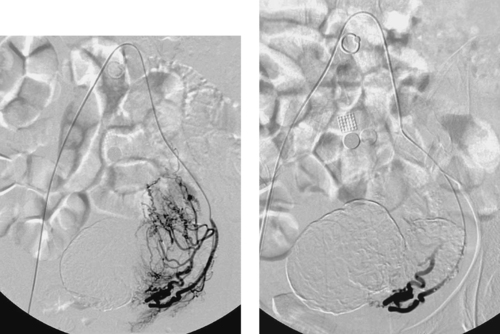
Uterine Fibroid Embolization
1. Left uterine artery.
2. Anterior division of the left internal iliac artery.
3. Menorrhagia, pelvic pain or discomfort, and/or urinary frequency.
4. To facilitate uterine artery embolization.
Reference
Pron, G.; Bennett, J.; Common, A.; et al., The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids, Fertil Steril. 79 (2003) 120–127.
Cross-Reference
Comment
Uterine arteriography and embolization therapy can be performed for a variety of indications: (1) To control postpartum bleeding refractory to medical management; (2) To control postoperative bleeding after cesarean section or other procedures; (3) To treat trauma-related pelvic hemorrhage; (4) To improve symptoms in women with uterine fibroids (uterine fibroid embolization, or UFE); (5) To palliate bleeding pelvic malignancies (although bleeding commonly recurs in this situation).
Uterine fibroid tumors occur in 20% to 40% of women older than 35 years, but they cause significant symptoms only in a minority of patients. The symptoms are usually initially treated with hormonal agents, but long-term use of these agents can be associated with unfavorable side effects. Hysterectomy has long been the mainstay of therapy, but in recent years myomectomy (removal of one or more fibroids) has been used in patients desiring to preserve the uterus. Myomectomy, however, is associated with a fairly high incidence of bleeding complications when performed for multiple fibroids, and a high recurrence rate has been observed.
For this reason, UFE has evolved into an excellent alternative therapy for women with symptomatic uterine fibroids who desire to preserve the uterus. UFE is associated with 85% to 90% success in producing significant improvement in symptoms of menorrhagia and/or pelvic pain, and it has been associated with improvements in health-related quality of life. Compared with hysterectomy, UFE is associated with less blood loss, decreased hospital stay, and decreased major complications.
Notes
Case 78
 |
 |
1. What is the primary abnormality seen on the first image?
2. What symptoms is this patient likely experiencing?
3. What procedure was performed in the second image?
4. What is the potential long-term complication of this procedure?
ANSWERS: CASE 78
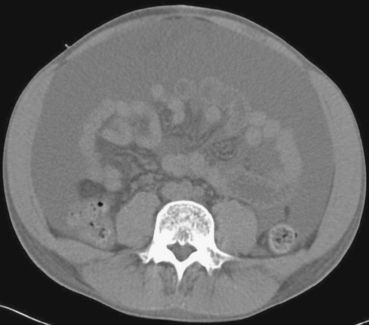
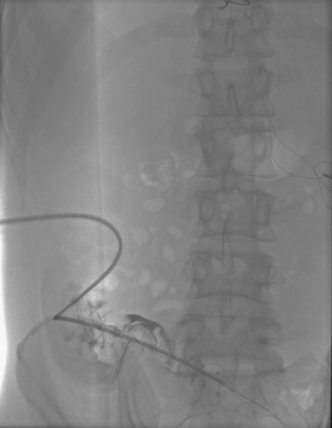
Massive Ascites: Tunneled Peritoneal Catheter Placement
1. Massive ascites.
2. Abdominal distention, dyspnea, and fatigue.
3. Tunneled peritoneal catheter placement.
4. Bacterial peritonitis.
Reference
O’Neill, M.J.; Weissleder, R.; Gervais, D.A.; et al., Tunneled peritoneal catheter placement under sonographic and fluoroscopic guidance in the palliative treatment of malignant ascites, AJR Am J Roentgenol. 177 (2001) 615–618.
Comment
Massive ascites can develop secondary to a variety of etiologies including portal hypertension, malignant ascites, and right-sided heart failure (as in this case). The accumulation of fluid in the peritoneal cavity causes abdominal distention, which in extreme cases can result in increased pressure on the diaphragm, leading to dyspnea and easy fatigability. This can greatly affect the patient’s quality of life.
Medical management with diuretics is the first-line treatment. Patients might also require therapeutic paracentesis to remove the accumulated ascites. In patients who fail to get relief of their symptoms from these measures, those who require frequent large-volume paracentesis, and those who suffer adverse effects of diuretic therapy (impaired renal function), other therapies are indicated. Transjugular intrahepatic portosystemic shunt (TIPS) can be performed for patients with portal hypertension; however, for patients with malignant ascites or ascites secondary to severe right-sided heart failure, placement of a tunneled peritoneal catheter is the procedure of choice.
The procedure is performed similar to tunneled central venous catheter placement with the exception that the catheter is placed within the peritoneal cavity and tunneled through the soft tissues of the anterior abdominal wall. The patient can then easily drain the ascitic fluid by opening the clamp intermittently as needed. Bacterial peritonitis is a rare but serious potential complication.
Case 79
 |
1. Describe the abnormality.
2. What is the differential diagnosis?
3. How is the definitive diagnosis of this lesion made?
4. What imaging modalities could be used to assist image-guided biopsy?
ANSWERS: CASE 79
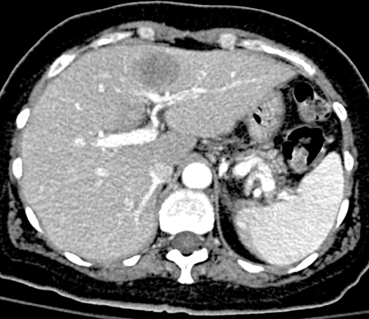
Percutaneous Transabdominal Liver Biopsy (Focal Lesion)
1. Hypoattenuating lesion in the left liver lobe, likely a solid mass.
2. Hepatocellular carcinoma, focal nodular hyperplasia, hepatic adenoma, metastasis.
3. Biopsy.
4. Ultrasound, CT, MRI.
Reference
Shankar, S.; Van Sonnenberg, E.; Silverman, S.G.; et al., Interventional radiology procedures in the liver. Biopsy, drainage, and ablation, Clin Liver Dis. 6 (2002) 91–118.
Cross-Reference
Comment
Noninvasive imaging of solid hepatic lesions is often unable to exclude the possibility of primary or metastatic malignant disease. For this reason, tissue biopsy is often indicated. For focal liver lesions, biopsy is performed from a percutaneous transabdominal route. Fine-needle aspiration and core biopsy specimens may be obtained.
The potential complications of liver biopsy include severe intraperitoneal hemorrhage, hemobilia, and pneumothorax. Accordingly, preprocedure precautions should ensure that coagulation parameters and platelet level are within normal limits, particularly in patients with hepatic cirrhosis. In addition, for lesions located high within the liver parenchyma, the route of access should be carefully planned to avoid traversing the lateral sulcus of the right pleural space. For left-sided lesions, the proximity of the pericardium should be similarly kept in mind.
Ultrasound or CT are used for the vast majority of image-guided biopsies. Ultrasound provides the advantage of real-time needle visualization and is less costly than using CT. On the other hand, many lesions are not clearly visible on ultrasound, making CT a better choice in these cases. MRI guidance is being used in some centers, primarily for hepatic dome lesions in which its multiplanar capability can be advantageous.
Case 80
 |
1. What is the probable underlying diagnosis causing biliary obstruction?
2. What symptoms does this patient most likely have?
3. How might the interventionalist contribute to obtaining a definitive diagnosis?
4. Are bilateral drainage catheters needed?
ANSWERS: CASE 80
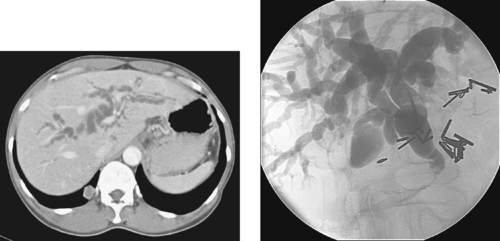
Malignant Common Bile Duct Stricture
1. Pancreatic head adenocarcinoma or cholangiocarcinoma.
2. Painless jaundice, weight loss.
3. Biliary brush biopsy.
4. No. The right and left biliary systems communicate in this case, so only one catheter is needed.
Reference
Govil, H.; Reddy, V.; Kluskens, L.; et al., Brush cytology of the biliary tract: Retrospective study of 278 cases with histopathologic correlation, Diagn Cytopathol. 26 (5) (2002) 273–277.
Cross-Reference
Comment
Malignant biliary obstruction may be produced by pancreatic carcinoma, cholangiocarcinoma, gallbladder cancer, intrahepatic metastatic disease, or portal hepatic nodal disease causing extrinsic common bile duct (CBD) compression. Although the clinical context must always be taken into account, the irregular rat-tail appearance of this distal CBD stricture is highly suggestive of a malignant etiology.
The interventional radiologist might have several tasks to complete in managing the patient with malignant CBD obstruction: (1) Percutaneous transhepatic cholangiography can outline the extent of the biliary abnormality and allow selection of a duct to use for definitive biliary access; (2) Biliary drainage can be performed to treat cholangitis, prevent episodes of biliary sepsis, and relieve obstructive symptoms including jaundice and pruritus; (3) Although its diagnostic sensitivity for pancreatic or biliary adenocarcinoma is only 50% to 70%, biliary brush biopsy can sometimes provide a definitive tissue diagnosis; (4) If the malignancy is unresectable and the patient has short life expectancy, a metallic (permanent) stent can be placed to facilitate internal drainage and thereby allow removal of the externally protruding biliary drainage catheter(s).
Case 81
 |
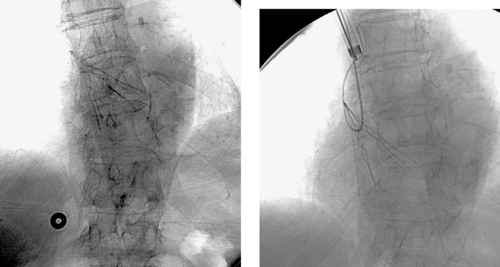
1. What vascular device is shown in the images?
2. What problem is identified and how did this occur?
3. Name two other mechanical problems of these devices.
4. For what type of patient is this device typically used?
ANSWERS: CASE 81
Port Separation
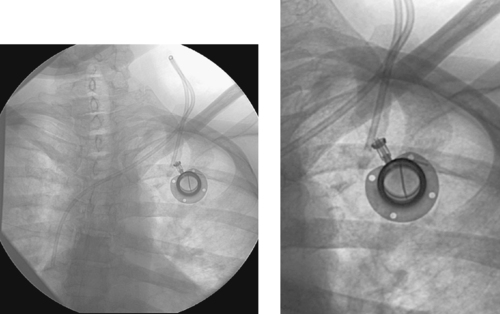
1. Implantable port catheter.
2. Separation of the two components of an attachable-type port.
3. Flipping of the port within its pocket, kinking, proximal migration.
4. Patients in need of long-term, intermittent access.
Reference
Funaki, B.; Szymski, G.X.; Hackworth, C.A.; et al., Radiologic placement of subcutaneous infusion chest ports for long-term central venous access, Am J Roentgenol. 169 (1997) 1431–1434.
Cross-Reference
Comment
In addition to the general complications of central venous access described earlier, a variety of device-specific complications can occur. This case demonstrates one device-specific complication of a port catheter: separation of the port and catheter components of the device. In addition, this particular catheter has been placed with an unfavorable upward curve, which may be partly extravascular.
Several types of port catheter devices are available. In many institutions, single-piece devices are used, and in other institutions, double-piece or attachable ports are used. The attachable ports are typically assembled by the physician when the port is placed. In occasional instances, presumably related to poor initial technique in attaching the pieces and/or to local trauma, the two components can separate. This is important, because any fluid injected into the port will extravasate into the chest wall. The separation can be fixed by reopening the port incision, dissecting down to the port and catheter, and reattaching the components. Alternatively, the port can be replaced with a completely new device.
Case 82
 |
1. What problem is identified on injection of this hemodialysis catheter?
2. Which is more likely to be present: problems with aspiration or with injection?
3. Is catheter exchange over a guidewire likely to result in long-term improvement?
4. Why was this long-term dialysis catheter placed from the common femoral vein?
ANSWERS: CASE 82
Pericatheter Fibrin Sheath
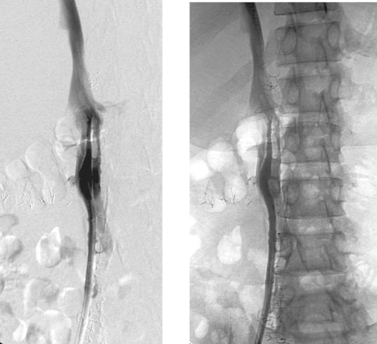
1. Fibrin sheath.
2. Aspiration.
3. No.
4. The internal jugular veins are probably occluded.
Reference
Gray, R.J.; Levitin, A.; Buck, D.; et al., Percutaneous fibrin sheath stripping versus transcatheter urokinase infusion for malfunctioning well-positioned tunneled central venous dialysis catheters: A prospective, randomized trial, J Vasc Interv Radiol. 11 (2000) 1121–1129.
Cross-Reference
Comment
Central venous catheters are prone to problems including infection, end-hole occlusion, venous thrombosis, malposition, migration, kinking, vessel wall damage, and catheter fracture. One of the most common and difficult problems is the development of a fibrin sheath around the catheter tip. Shortly after placement, virtually all catheters become covered by a layer of fibrin, which eventually forms a sleeve around the catheter. When this obstructs the catheter end hole and/or side holes, the usual clinical finding is impaired ability to aspirate blood but preserved ability to inject. Dialysis catheters often demonstrate diminished flow rates in this situation. The typical venographic appearance is observed in this case: Contrast flows retrograde along the catheter within the fibrin sheath, outlining the catheter.
Treatment options vary. Catheter exchange over a guidewire can be performed, but it is unlikely to produce long-term improvement because the catheter is typically reinserted into the existing fibrin sheath. Alternatively, low-dose thrombolytic agents can be used, either via instillation in the catheter for 30 to 60 minutes or via infusion for several hours. Some advocate disrupting the fibrin sheath using guidewire or angioplasty balloon manipulation or stripping the fibrin sheath using a snare device inserted from another access vein. Unfortunately, most treatments lead to only temporary relief because the fibrin sheath tends to re-form over time.
Case 83
 |
1. What treatment options are available for the abnormality shown?
2. What routes of access can be used to drain a pelvic fluid collection?
3. For each of these routes of access, what imaging modalities are typically used to facilitate drainage?
4. Name two potential undesirable sequelae of transgluteal abscess drainage.
ANSWERS: CASE 83
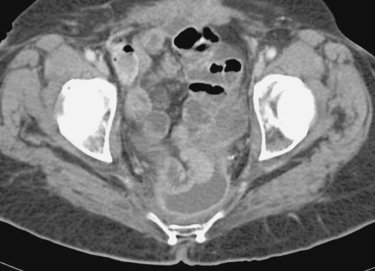
Percutaneous Drainage of Deep Pelvic Abscess
1. Surgical abscess drainage or percutaneous image-guided abscess drainage.
2. Transabdominal, transgluteal, transvaginal, transrectal, and transperineal.
3. Transvaginal, transrectal, and transperineal drainage are generally performed under combination US/fluoroscopic guidance. Transabdominal and transgluteal abscess drainage can be performed under CT or ultrasound/fluoroscopy. CT is commonly used for transgluteal drainage.
4. Pelvic arterial hemorrhage, pain.
Reference
Hovsepian, D.M., Transrectal and transvaginal abscess drainage, J Vasc Intervent Radiol. 8 (1997) 501–515.
Cross-Reference
Comment
The first-line management of deep pelvic abscesses is usually percutaneous imaging-guided drainage. Because of the presence of small bowel, bladder, and other structures within the pelvis, a simple transabdominal access route is often not available.
In choosing a potential access route, the first question to ask is whether a draining sinus is present. If so, then the abscess cavity may be defined by contrast injection directly into the skin defect; in this situation a catheter can usually be advanced under fluoroscopy into the abscess without the need for needle puncture. When this option does not exist, careful examination of the CT scan is critical in planning therapy.
When a direct transabdominal route into the collection is not present, the transvaginal and transrectal access routes are usually considered next in the absence of contraindications (e.g., a surgical bowel anastomosis in the rectal area). The advantages of the transvaginal and transrectal routes are their usual close proximity to the fluid collection and the lower risk of complications (bleeding and pain) compared with transgluteal drainage. Additionally, transgluteal drainage is occasionally technically difficult when the collection is small, because the window into the collection must be crossed with pinpoint accuracy to avoid traversing surrounding bowel and pelvic organs.
Case 84
 |
 |
 |
 |
1. What abnormalities are present on the first two images?
2. What is the most likely diagnosis?
3. What treatment options are available for this patient?
4. What procedure is being performed in the last image?
ANSWERS: CASE 84
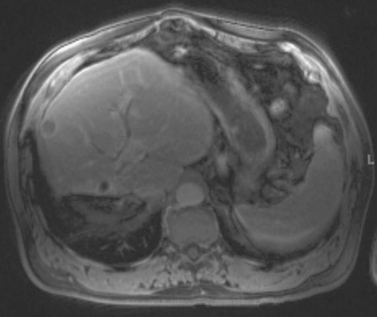
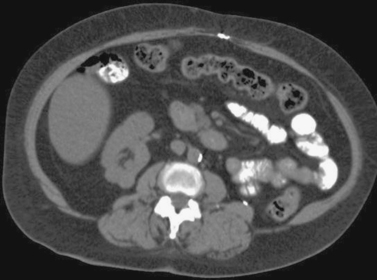
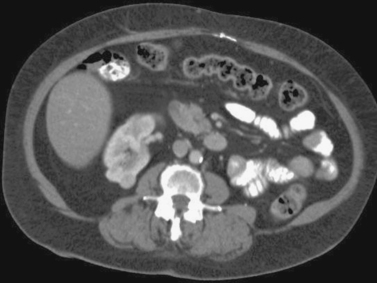
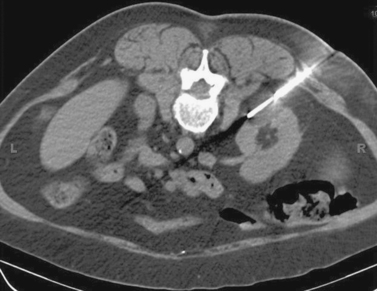
Renal Cell Carcinoma: Percutaneous Cryoablation
1. Solid enhancing partially exophytic mass arising from the superior pole of the right kidney and nonvisualization of the left kidney with the presence of a surgical clip in the left retroperitoneum.
2. This patient previously underwent a radical left nephrectomy for renal cell carcinoma. A contralateral enhancing solid renal mass is identified consistent with renal cell carcinoma.
3. Nephron-sparing therapies include partial nephrectomy (open or laparoscopic), laparoscopic cryoablation, and percutaneous cryoablation or radiofrequency ablation.
4. Percutaneous cryoablation.
Reference
Atwell, T.D.; Farrell, M.A.; Callstrom, M.R.; et al., Percutaneous cryoablation of large renal masses: Technical feasibility and short-term outcome, AJR Am J Roentgenol. 188 (2007) 1195–2000.
Comment
Renal tumors are being found incidentally at an increasing rate. This has allowed detection of these tumors at earlier stages where tumors are much smaller. Surgical resection of these tumors remains the standard of care. This comprises radical and partial nephrectomy, which may be performed in an open manner or laparoscopically.
Nephron-sparing therapies have gained favor in recent years for preservation of renal function. These treatments include partial nephrectomy, laparoscopic cryoablation, and percutaneous image-guided cryoablation and radiofrequency ablation. Percutaneous ablations have obvious advantages over open or laparoscopic surgical procedures including decreased morbidity and the potential for being performed under moderate sedation.
Percutaneous cryoablation was the chosen therapy for the patient in this case to treat the tumor in her solitary kidney for its nephron-sparing potential and the lack of need for general anesthesia because she had many comorbid conditions, placing her at increased risk for morbidity from a surgical procedure. Ultrasound or CT guidance can be used for placement of the cryoprobes, and the freezing and thawing of the tumor is monitored with CT surveillance, which clearly demonstrates the formation of the ice ball around the cryoprobe (low-attenuation area seen in the fourth image).
Notes
Case 85
 |
1. What abnormality is present on this chest CT scan?
2. Is this problem likely acute or chronic?
3. What are the potential advantages of CT in evaluating this clinical problem?
4. What is the primary pitfall of CT in evaluating this clinical problem?
ANSWERS: CASE 85
Acute Pulmonary Embolism on CT Angiography
1. Bilateral pulmonary embolism (PE).
2. Acute.
3. CT is noninvasive compared with angiography, it provides more direct visualization of thrombus than a ventilation–perfusion scan, and it can provide information on other potential causes of pulmonary symptoms.
4. Subsegmental emboli are often misdiagnosed on CT angiography.
Reference
Saad, W.E.; Saad, N., Computer tomography for venous thromboembolic disease, Radiol Clin North Am. 45 (2007) 423–445.
Cross-Reference
Comment
Although the clinical symptoms, electocardiographic findings, and chest radiography of patients with PE have been well characterized, these findings are nearly always nonspecific. Because the medical and procedural interventions for PE (e.g., anticoagulation and/or IVC filter placement) can have substantial morbidity, the diagnosis of pulmonary embolism requires imaging confirmation. Traditionally, ventilation–perfusion scanning has been used as the initial screening test for acute pulmonary embolism, but in recent years CT angiography has been investigated as a potentially superior screening examination.
To achieve optimal results with CTA, several factors must be present. There must be power IV injection of contrast into an arm vein at 3 to 5mL/s. There must be helical scanning capability with reconstruction into small increments. There must be meticulous review of the scans by a radiologist with abundant experience in interpreting thoracic CT scans.
The sensitivity of multidetector CT scanning for detecting PE is 83% to 94%, with a specificity of 94% to 100% and a reader confidence of 90%. Furthermore, CT angiography provides a diagnosis of not PE in 70% of patients in whom PE was initially suspected but who were found not to have PE (>50% of patients in whom PE was initially suspected).
Case 86
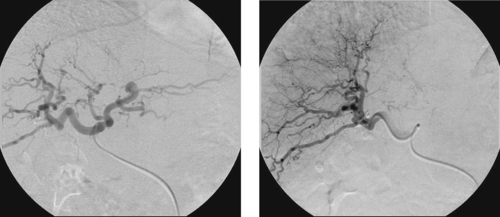 |
1. Which artery is being injected in the first image?
2. How is this arteriogram abnormal?
3. What diagnosis is likely?
4. Is flow through this vessel increased or decreased in this condition?
ANSWERS: CASE 86
Hepatic Cirrhosis
1. Proper hepatic artery.
2. Corkscrew tortuosity of branch vessels.
3. Hepatic cirrhosis.
4. Increased.
Reference
Hori, M.; Murakami, T.; Kim, T.; et al., Diagnosis of hepatic neoplasms using CT arterial portography and CT hepatic arteriography, Tech Vasc Interv Radiol. 5 (3) (2002) 164–169.
Cross-Reference
Comment
Arteriographic changes of cirrhosis are not typically apparent until there is substantial loss of liver parenchyma and the liver is fibrotic and contracted. Therefore, cirrhosis may be present long before the arteriographic changes are obvious. Early liver disease often results in hepatic swelling and enlargement, which can give a stretched appearance to the small arterial branches. As cirrhosis worsens and portal hypertension develops, portal venous return to the liver decreases. To compensate for this, hepatic arterial flow increases. This increased flow is the likely cause of these intrahepatic arterial changes. Ultimately, as fibrosis develops and worsens, the peripheral branches exhibit a characteristic corkscrew configuration. Occasionally, telangiectasias, aneurysms, or arterioportal venous shunting can be demonstrated.
Other vascular changes are notable in cirrhotic livers as well. The parenchymal phase of liver enhancement typically demonstrates a mottled appearance with nonuniform enhancement. Hypervascular masses within the parenchyma are highly suspicious for hepatocellular carcinoma. Portal venous flow can be hepatopedal or hepatofugal, and in some instances the portal vein can be occluded by thrombus or tumor.
Case 87
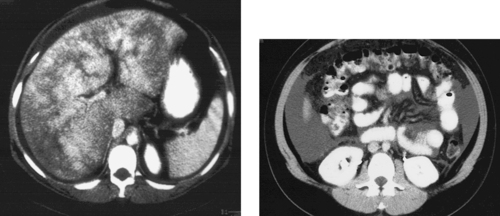 |
1. How can the definitive diagnosis of the hepatic pathology pictured above be obtained?
2. What factor visualized on the images would predispose this patient to complications of liver biopsy?
3. How would you obtain tissue from this liver if the patient’s coagulation parameters were impaired?
4. How is this procedure performed?
ANSWERS: CASE 87
Transjugular Biopsy of Diffuse Liver Disease
1. Image-guided biopsy.
2. The presence of ascites.
3. Transjugular liver biopsy.
4. The right hepatic vein is selected and a biopsy needle is passed into the hepatic parenchyma using a metallic guiding cannula.
Reference
Wallace, M.J.; Narvios, A.; Lichtiger, B.; et al., Transjugular liver biopsy in patients with hematologic malignancy and severe thrombocytopenia, J Vasc Interv Radiol. 14 (2003) 323–327.
Cross-Reference
Comment
In most patients with diffuse liver lesions, tissue diagnosis may be safely obtained by transabdominal percutaneous biopsy. However, patients with impaired coagulation and those with ascites are at significant risk for intraperitoneal bleeding following needle traversal of the liver capsule. Liver biopsy from a transjugular route is associated with a lower rate of bleeding complications and represents a safer option in these situations.
To perform transjugular liver biopsy, the right internal jugular vein is percutaneously accessed under ultrasound guidance. The right hepatic vein is selected and contrast venography may be used to confirm catheter position. The catheter is then exchanged for a metallic cannula. This cannula is directed anteriorly (away from the posterior liver capsule) under fluoroscopic guidance, and a specially designed long biopsy needle is used to obtain one or more core biopsy specimens from the hepatic parenchyma. If the middle hepatic vein is used instead of the right hepatic vein, then the cannula may be directed in a posterolateral direction to avoid the anterior liver capsule. The cannula is then removed and the jugular entry site is compressed for hemostasis. Hemostasis within the liver occurs quickly provided the hepatic capsule was not breached.
Notes
Case 88
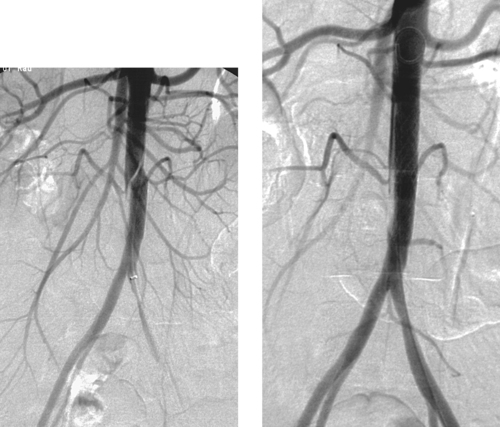 |
1. What abnormality is present in the first image?
2. What are the potential etiologies of this abnormality?
3. What treatment has been performed?
4. Are the indications for aortic stent placement different from those of iliac artery stent placement?
ANSWERS: CASE 88
Iatrogenic Aortic Dissection
1. Abdominal aortic dissection.
2. Iatrogenic injury, trauma, hypertension, connective tissue disease.
3. Stent placement.
4. No.
Reference
Bariseel, H.; Batt, M.; Rogopoulos, A.; et al., Iatrogenic dissection of the abdominal aorta, J Vasc Surg. 27 (1998) 366–370.
Cross-Reference
Comment
The first image demonstrates a diagonally oriented intimal defect within the infrarenal abdominal aorta, consistent with a dissection flap. This patient had undergone angioplasty of a distal aortic stenosis 1 week earlier but did not experience improvement in her symptoms or in her femoral pulses.
The indications for bare stent placement in the abdominal aorta are identical to those for iliac artery stent placement: technical failure of percutaneous balloon angioplasty (>30% residual stenosis or >10mm Hg systolic pressure gradient), recurrent stenosis following angioplasty, arterial dissection, and treatment of eccentric or heavily calcified stenoses.
Notes
Case 89
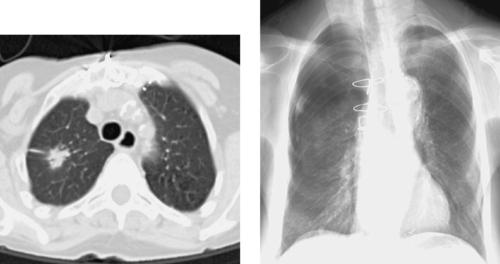 |
1. By what methods can a lung lesion be biopsied?
2. Can core biopsy be safely performed in the lung?
3. What complication of lung biopsy is visualized in the images?
4. Name two other complications of lung biopsy.
ANSWERS: CASE 89
CT-Guided Lung Biopsy with Pneumothorax
1. Percutaneous CT-guided, bronchoscopic, and surgical.
2. Yes.
3. Pneumothorax.
4. Hemothorax, severe hemoptysis.
Reference
Ohno, Y.; Hatabu, H.; Takenaka, D.; et al., CT-guided transthoracic needle aspiration biopsy of small solitary pulmonary nodules, AJR Am J Roentenol. 180 (2003) 1665–1669.
Cross-Reference
Comment
Percutaneous lung biopsy is a safe procedure when performed by radiologists who are capable of managing its potential complications. For spiculated masses for which primary lung cancer is suspected, multiple fine-needle-aspiration specimens often suffice to make the diagnosis. For more complex lesions and when lymphoma is a diagnostic possibility, core biopsy is needed. Preprocedure planning should include careful evaluation of the CT scan and a review of the patient’s overall cardiopulmonary status.
It is common (25%) for patients to experience a small amount of hemoptysis following the procedure, but massive hemoptysis and significant hemothorax are rare. Pneumothorax occurs in 15% to 30% of patients undergoing lung biopsy, but it only requires chest tube placement in 2% to 5%. Patients with chronic obstructive pulmonary disease, however, have a significantly higher risk of post-biopsy pneumothorax, with a more significant fraction requiring chest tube placement. In general, when chest tube placement is required, a small-bore tube (10-12F) may be used and the tube can usually be removed within 1 to 3 days.
The incidence of pneumothorax may be reduced by the use of coaxial technique; by minimizing the number of pleural surfaces crossed by the needle, minimizing needle path length; and by avoiding oblique passes across pleural surfaces. A cytopathologist should be present to verify that sufficient tissue is obtained for confident diagnosis with a minimum number of needle passes.
Case 90
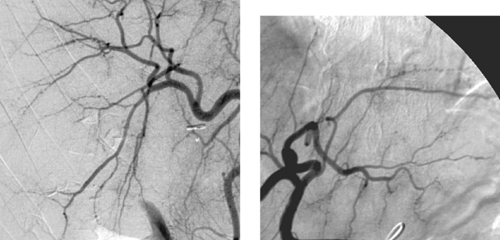 |
1. What is the most likely diagnosis?
2. Is the aorta likely to also be involved with this process?
3. Are the renal arteries likely to also be involved with this process?
4. Can these lesions be treated in endovascular fashion?
ANSWERS: CASE 90
Polyarteritis Nodosa
1. Polyarteritis nodosa.
2. No.
3. Yes.
4. No.
Reference
Stanson, A.W.; Friese, J.L.; Johnson, C.M.; et al., Polyarteritis nodosa: Spectrum of angiographic findings, Radiographics 21 (2001) 151–159.
Cross-Reference
Comment
The images demonstrate multifocal irregularity, stenoses, and small aneurysms in the small and medium-sized arteries of the hepatic circulation. This appearance is most likely due to polyarteritis nodosa. Another condition that can produce an identical appearance is necrotizing angiitis due to drug abuse (often methamphetamines). When microaneurysms are present, the possibility of mycotic aneurysms must also be considered in the appropriate clinical context (e.g., fever and/or signs of sepsis), but the angiographic appearance shown here is much less likely to represent infection.
Polyarteritis nodosa is a systemic necrotizing vasculitis of small and medium-sized muscular arteries and arterioles. Patients can experience low-grade fever, myalgias and arthralgias, tender subcutaneous nodules, peripheral neuropathy, hematuria, and symptoms related to vascular involvement. The most commonly involved visceral vascular beds are the renal arteries (85%) and the hepatomesenteric circulation (50%). The large vessels, such as the aorta or major mesenteric trunks, are not likely to be involved.
Case 91
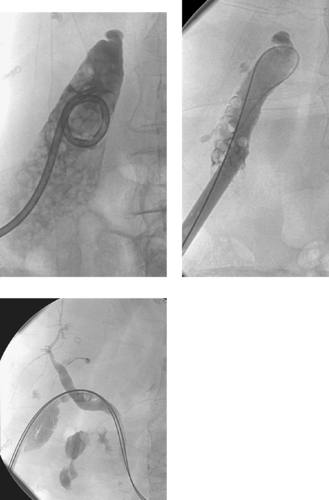 |
1. What procedure has been performed after the first image?
2. Is this procedure the treatment of choice for this condition?
3. Can the drainage catheter be removed immediately after the procedure?
4. Name two complications of this procedure.
ANSWERS: CASE 91
Percutaneous Gallstone Removal
1. Percutaneous gallstone removal.
2. No. Surgical cholecystectomy is the treatment of choice.
3. No.
4. Bile peritonitis and sepsis.
Reference
Courtois, C.S.; Picus, D.; Hicks, M.E., Percutaneous gallstone removal: Long-term follow-up, J Vasc Intervent Radiol. 7 (1996) 229–234.
Cross-Reference
Comment
Patients with acute cholecystitis are often managed with catheter drainage during the initial phase. Once the acute infection and inflammation have resolved, a decision needs to be made as to what the appropriate definitive management should be. In most patients, surgical cholecystectomy should be performed, either using an open transabdominal approach or using laparoscopic technology.
Patients with significant contraindications for surgical resection are considered candidates for percutaneous methods of gallstone removal. After a mature transperitoneal tract has formed around the percutaneous cholecystostomy catheter (usually 2-4 weeks), the catheter is removed over a guidewire, the tract is dilated, and a large sheath is placed. Calculi are usually ultrasonically fragmented under endoscopic visualization, and the stone fragments are removed by aspiration and a variety of grasping devices. Following this, a drainage catheter is left in place. Once all stones are removed (as demonstrated by cholangiography), the cystic and common bile ducts are patent, and the patient is clinically well, the tube can be capped and subsequently removed.
The recurrence rate of gallstones following percutaneous removal is extremely high (perhaps 40% within 3 years). For this reason, this procedure is generally reserved for elderly patients with significant surgical contraindications.
Notes
Case 92
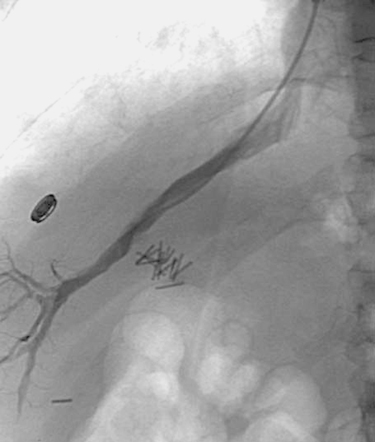 |
1. How has this study been performed?
2. Why might a balloon catheter be used diagnostically in this vein?
3. What major diagnosis can be made from hepatic vein pressure measurements?
4. Name two other reasons to catheterize this vein.
ANSWERS: CASE 92
Hepatic Venography
1. The right hepatic vein has been selected from a jugular vein approach.
2. To obtain wedged hepatic vein pressures.
3. Portal hypertension.
4. To perform transjugular intrahepatic portosystemic shunt (TIPS); to evaluate for anatomic evidence of Budd–Chiari syndrome.
Reference
Pieters, P.C.; Miller, W.J.; DeMeo, J.H., Evaluation of the portal venous system: Complementary roles of invasive and noninvasive imaging strategies, Radiographics 17 (1997) 879–895.
Cross-Reference
Comment
Hepatic vein catheterization is most easily accomplished from the right internal jugular vein, but it may also be performed from the left internal jugular vein or a femoral vein. The most common reasons to perform hepatic venography are to obtain pressure measurements to estimate the portal vein to systemic pressure gradient, to define the portal vein target site for TIPS via carbon dioxide portography, to perform the TIPS procedure, and to make the diagnosis of Budd–Chiari syndrome.
The corrected hepatic sinusoidal pressure is the difference between the wedged hepatic vein pressure (obtained with the balloon inflated and wedged distally) and the free hepatic vein pressure (the hepatic vein pressure obtained with the balloon deflated). The corrected hepatic sinusoidal pressure, which should be confirmed by measurement in at least two hepatic veins, provides a fairly accurate estimate of the portosystemic gradient except in the presence of severe parenchymal hepatic disease involving the presinusoidal venules. The normal corrected hepatic sinusoidal pressure is less than 5mm Hg; a gradient greater than 6mm Hg represents indirect evidence of portal hypertension, and a gradient of greater than 12mm Hg is thought to correlate with an increased risk of variceal bleeding.
Notes
Case 93
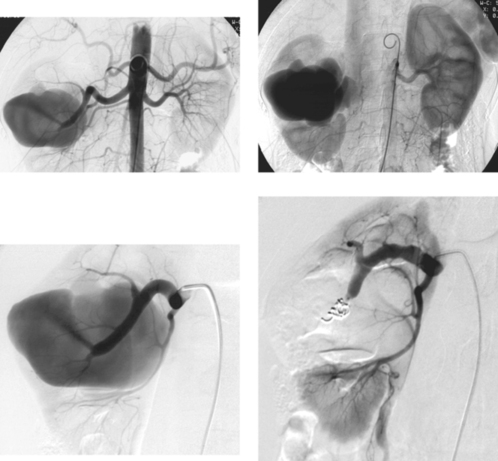 |
1. Describe the angiographic findings on the first two images.
2. Name three symptoms that may be present.
3. In this case, does the nidus require embolization?
4. In this case, is embolization likely to be a durable therapy?
ANSWERS: CASE 93
Renal Arteriovenous Malformation
1. Enlarged right renal artery, arteriovenous malformation from a right renal artery branch vessel with early filling of the right renal vein and inferior vena cava.
2. Hematuria, hypertension, high-output cardiac failure.
3. No. This lesion is a simple arteriovenous fistula and no nidus is present.
4. Yes. This lesion has a single feeding vessel that can be embolized permanently.
Reference
Dinkel, H.P.; Danauser, H.; Triller, J., Blunt renal trauma: Minimally invasive management with microcatheter embolization experience in nine patients, Radiology 223 (2002) 723–730.
Cross-Reference
Comment
The selective right renal angiogram demonstrates opacification of a large arteriovenous malformation. Early filling of the venous system is a characteristic finding of such lesions and emphasizes the importance of considering whether all opacified structures are appropriate for the particular phase of the angiogram being evaluated. Opacification of venous structures should never be apparent on arterial phase images.
Renal arteriovenous malformations are rare lesions that may be congenital or acquired. When they are acquired, the most common cause is iatrogenic injury secondary to a renal biopsy or other intervention. The lesions often manifest with recurrent gross hematuria, and they can even produce renovascular hypertension. Successful endovascular treatment of the abnormality generally results in regression of these symptoms.
Endovascular therapy for simple renal arteriovenous malformations (like the one pictured here) usually consists of coil embolization of the feeding artery (as seen in the last image). If the lesion is more complex, then particle embolization may be used to treat the abnormality while preserving the kidney for as long as possible; however, such lesions often recur following embolization, and total or partial nephrectomy is likely to be needed eventually.
Notes
Case 94
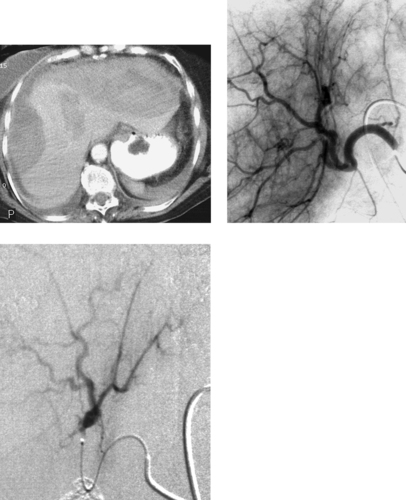 |
1. What abnormalities are seen on the images above?
2. What is the likely etiology?
3. What materials are commonly used for embolization in this setting?
4. What preprocedural CT findings would indicate a patient at higher risk for developing hepatic ischemia following embolization therapy?
ANSWERS: CASE 94
Traumatic Hepatic Laceration
1. Two large subcapsular hematomas, a liver laceration, and a pseudoaneurysm arising from a left hepatic artery branch.
2. Trauma.
3. Coils and Gelfoam.
4. Portal vein thrombosis, other findings of severe portal hypertension including enlarged hepatic artery and the presence of varices.
Reference
Hagiwara, A.; Murata, A.; Matsuda, T.; et al., The efficacy and limitations of transarterial embolization for severe hepatic injury, J Trauma. 52 (2002) 1091–1096.
Cross-Reference
Comment
Patients with blunt abdominal trauma are initially evaluated with contrast–enhanced CT scanning. Hepatic injuries are typically categorized on CT into five categories, and in many institutions patients with CT evidence of contrast extravasation and most patients with grade 3 to 5 injuries (grade 3 = laceration 3–10cm in length or hematoma 3–10cm in thickness) typically undergo arteriography to search for arterial bleeding. When bleeding is seen, embolization can be performed with a high likelihood of success.
Hepatic ischemia following embolization is common because approximately 70% of hepatic blood supply is derived from the portal venous system. For these reasons, when a laceration involves a large part of the hepatic parenchyma and multiple bleeding arteries are present, Gelfoam can be used to embolize an entire hepatic lobe rather than attempting to coil embolize each individual branch. However, patients with portal venous thrombosis and those with portal hypertension are at increased risk for hepatic ischemia, and embolization in these patients should be performed judiciously (portal hypertension patients) or not at all (portal vein thrombosis patients).
Notes
Case 95
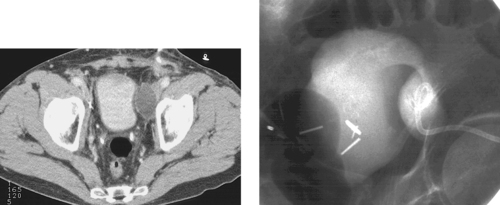 |
1. What abnormality is seen on the CT scan above?
2. What is the differential diagnosis in this patient who is status post pelvic lymph node dissection?
3. What laboratory studies should be performed on the aspirated fluid?
4. If persistently high drainage catheter outputs are seen several days after drainage and no fistula is present, does the patient require surgical management?
ANSWERS: CASE 95
Pelvic Lymphocele
1. Left pelvic fluid collection.
2. Seroma, hematoma, urinoma, abscess, lymphocele.
3. Gram stain and bacterial culture, creatinine level, cell count, and differential.
4. No. The patient can undergo ethanol sclerosis if the lesion is a lymphocele.
Reference
Zuckerman, D.A.; Yeager, T.D., Percutaneous ethanol sclerotherapy of postoperative lymphoceles, AJR Am J Roentgenol. 169 (1997) 433–437.
Cross-Reference
Comment
The images demonstrate the typical appearance of a postoperative pelvic lymphocele. If the patient is symptomatic (commonly pain, fever, or leg swelling due to iliac vein compression), the intervention of first choice is percutaneous drainage.
The aspirated fluid should be sent for laboratory analysis as described, because the differential diagnosis is broad. If the collection is sterile and has an extremely high concentration of lymphocytes, the diagnosis of lymphocele is made.
Although catheter drainage is sometimes successful as stand-alone therapy, a significant percentage of lymphoceles do not completely resolve with catheter drainage alone because lymphatic channels within the wall of the collection continue to produce fluid. In this situation, if the collection is not infected and no fistula is present, then periodic (usually 2-3 times per week) instillation of absolute ethanol can produce cessation of fluid output from the collection, eventually allowing removal of the catheter. In general, at least several sessions of sclerosis are needed, and the entire process can take up to several months in a minority of cases.
Notes
Case 96
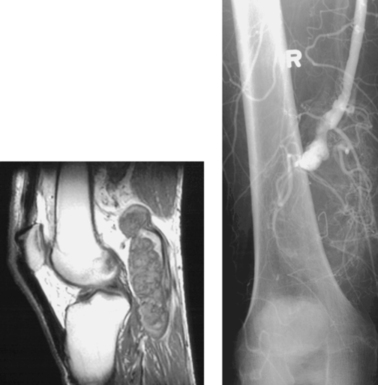 |
1. What diagnosis is evident on the angiogram?
2. Is this likely to be an isolated lesion?
3. What complication of this lesion is apparent?
4. Is rupture a common complication of this lesion?
ANSWERS: CASE 96
Popliteal Artery Aneurysm
1. Popliteal artery aneurysm.
2. No.
3. Popliteal artery thrombosis.
4. No.
Reference
Ascher, E.; Markevich, N.; Schutzer, R.W.; et al., Small popliteal artery aneurysms: Are they clinically significant?J Vasc Surg. 37 (2003) 755–760.
Cross-Reference
Comment
Popliteal artery aneurysms are commonly bilateral, and they often coexist with aneurysms in another location, most commonly abdominal aortic aneurysms and common femoral artery aneurysms. The most common presenting symptoms include lower–extremity ischemia and compression of adjacent nerves or other structures. Although rupture of popliteal aneurysms can occur, this complication is quite uncommon (<5% of patients). More commonly, popliteal aneurysms manifest with limb–threatening ischemia due to distal embolization (20%) or popliteal artery thrombosis (40%) (as seen in this case). Hence, even small aneurysms can produce limb–threatening ischemia.
The treatment of choice is surgical aneurysm resection with distal bypass. Stent grafts have been used to treat a small number of selected patients with popliteal artery aneurysms, but early stent–graft occlusion is very common. Hence, this approach can only be recommended for rare patients with very strong contraindications to surgical management.
Notes
Case 97
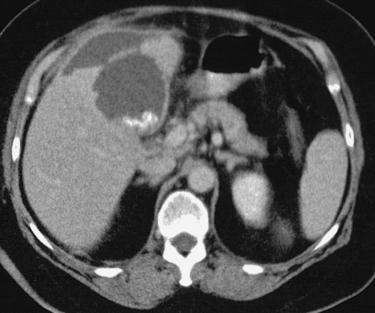 |
1. What is the diagnosis?
2. What is the etiology of this condition?
3. Does the visualized complication of this condition mandate immediate surgery?
4. What clinical criteria will be used to determine the timing of tube removal?
ANSWERS: CASE 97
Gallbladder Rupture with Pericholecystic Abscess
1. Acute cholecystitis with gallbladder rupture and pericholecystic abscess formation.
2. Gallstones with subsequent cystic duct obstruction.
3. No. Percutaneous cholecystostomy can still be performed provided the pericholecystic abscess is drained, too.
4. The tube remains in place until surgical gallbladder resection is performed. Alternatively, if percutaneous gallstone removal is performed, the tube should remain in place until a mature tract is present around the tube, no residual gallstones are seen, the cystic and common bile ducts are patent, and the patient is clinically well, with the tube capped.
Reference
Ahkan, O.; Akinsi, D.; Ozmen, M.N., Percutaneous cholecystostomy, Eur J Radiol. 43 (3) (2002) 229–236.
Cross-Reference
Comment
Complications of acute cholecystitis can include free rupture into the peritoneum or contained rupture with pericholecystic abscess formation. Historically, the presence of gallbladder rupture was an absolute indication for emergency cholecystectomy. In modern practice, however, most surgeons much prefer to operate after the acute infection and pericholecystic inflammation have subsided. For these reasons, percutaneous cholecystostomy may still be performed. However, the abscess must also be drained, either using the same drainage catheter with additional side holes created to drain the abscess (as was done in this case) or via a separate (second) catheter.
Case 98
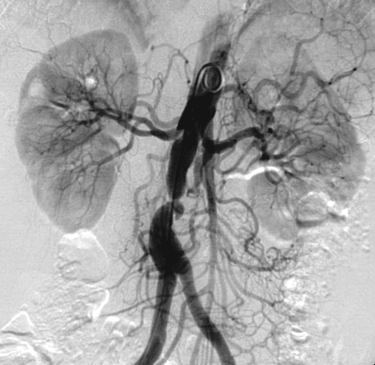 |
1. What symptoms are present in this female patient if the remainder of the lower extremity angiogram is normal?
2. What risk factor for atherosclerotic disease does this patient probably have?
3. Is this lesion likely to respond to angioplasty?
4. Does the patient require anticoagulation following percutaneous intervention with a good technical result?
ANSWERS: CASE 98
Abdominal Aortic Stenosis
1. Bilateral hip and buttock claudication.
2. Smoking history.
3. Yes.
4. No.
Reference
Sandhu, C.; Belli, A.M., Abdominal aortic stenting: Current practice, Abdom Imaging. 6 (2001) 453–460.
Cross-Reference
Comment
Although hypertension, diabetes, and hyperlipidemia are all associated with the development of atherosclerotic lesions, patients with a history of smoking are particularly prone to develop occlusive disease in the aorta and iliac arteries. Although aortofemoral bypass grafting is associated with high patency rates, percutaneous interventions are usually used first for appropriate lesions because of their lower morbidity rates.
Invasive therapy is only performed in patients with lifestyle–limiting claudication or rest pain. For focal, concentric, short lesions (as in this case), percutaneous balloon angioplasty is an appropriate first treatment. Patencies following aortic angioplasty are comparable to those associated with iliac artery lesions.
Postangioplasty stent placement may be performed if flow–limiting dissection occurs following angioplasty, if angioplasty fails to reduce the stenosis to less than 30% diameter, if a persistent pressure gradient (>10mm Hg systolic) is present across the lesion after angioplasty, or if the stenosis recurs following successful angioplasty. Primary stent placement (placement of a stent without preceding angioplasty) is generally used for extremely calcified or eccentric lesions.
Case 99
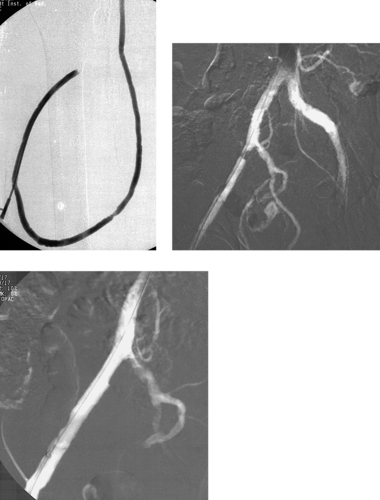 |
1. What diagnostic study has been performed and why?
2. Assuming that the iliac and common femoral veins are normal, what is the cause of the low flows seen in this patient?
3. How often is arterial inflow the major factor in hemodialysis graft malfunction?
4. What is the most common anatomic cause of hemodialysis graft malfunction or occlusion?
ANSWERS: CASE 99
Iliac Artery Stenosis Above a Hemodialysis Graft
1. Right femoral dialysis fistulogram was performed to evaluate the cause of hemodialysis graft malfunction.
2. Right external iliac artery stenosis.
3. From 5% to 10% of cases.
4. Stenosis at the venous anastomosis.
Reference
Surlan, M.; Popovic, P., The role of interventional radiology in management of patients with end-stage renal disease, Eur J Radiol. 46 (2) (2003) 96–114.
Cross-Reference
Comment
Although hemodialysis grafts are most often placed in the upper extremity, the presence of central venous occlusion with subsequent graft failure often necessitates a search for alternative access sites. The common femoral veins are usually used for dialysis access, but they are prone to the same complications as upper-extremity dialysis access.
The vast majority of culprit stenoses occur at the venous anastomosis of the graft, within the graft, within the peripheral or central venous outflow veins, or at the arterial anastomosis. However, when the angiographic appearance of these regions does not explain the clinical findings of graft malfunction, then the entire arterial inflow to the graft should be studied arteriographically. When a culprit stenosis is identified, treatment with angioplasty or stent placement (as seen in the last image) may be performed.
Notes
Case 100
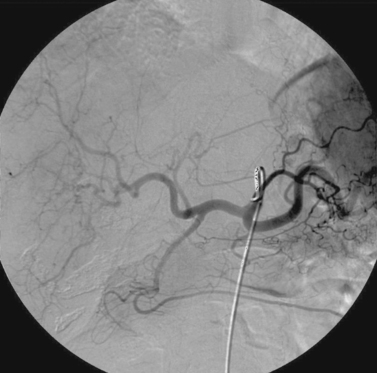 |
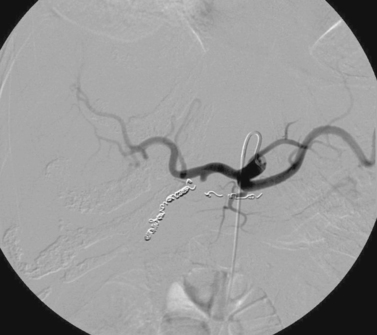 |
 |
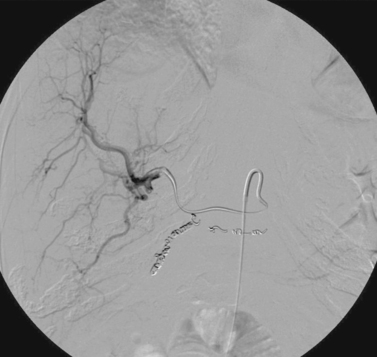 |
1. Which artery is being selectively injected on the first image?
2. Which arteries have been selectively embolized on the second image?
3. Name the radiotracer used to obtain the images in the third image.
4. Name the radioisotope used for treatment of this patient with metastatic colorectal cancer in the fourth image.
ANSWERS: CASE 100
Colorectal Metastases to Liver: Radioembolization
1. Celiac axis.
2. Gastroduodenal and right gastric arteries.
3. Technetium-99m macroaggregated albumin (99mTc-MAA).
4. Yttrium-90 microspheres (90Y microspheres).
Reference
Salem, R.; Thurston, K.G., Radioembolization with 90Yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations, J Vasc Interv Radiol. 17 (2006) 1251–1278.
Comment
Radioembolization with 90Y microspheres is an FDA-approved therapy for treating unresectable hepatocellular carcinoma and metastatic colorectal carcinoma to the liver. The microspheres are delivered to the tumors in a transcatheter approach through the hepatic artery and, once in place, emit radiation that acts on the tumor cells. Careful patient selection is necessary to obtain optimal results. Owing to the variation in hepatic arterial anatomy and the potential complications that can result from nontarget embolization of microspheres to the bowel, gallbladder, pancreas, and other nearby structures that derive part of their arterial supply from branches closely associated with those that supply the liver, careful mapping of the arterial supply to the intended target lesion(s) and optimization of the arterial anatomy by selective embolization of vessels is mandatory.
After optimizing the arterial anatomy, the microcatheter tip is placed in the planned position for delivery of the radioembolic microspheres and approximately 5mCi of 99mTc-MAA is delivered through the microcatheter. This is followed by CT SPECT imaging to assess the activity within the liver and to detect any activity in extrahepatic abdominal structures and the lungs to evaluate for nontarget delivery and tumor arteriovenous shunting, respectively.
Once the planning images are reviewed and the patient is deemed a candidate for treatment, dosimetry data are calculated. Dosimetry calculation methods vary; however, some factors that are considered include body surface area, liver volume, tumor burden, and underlying liver cirrhosis. The dose delivery must be carefully monitored under fluoroscopy to ensure that stasis is not reached.
Notes
Case 101
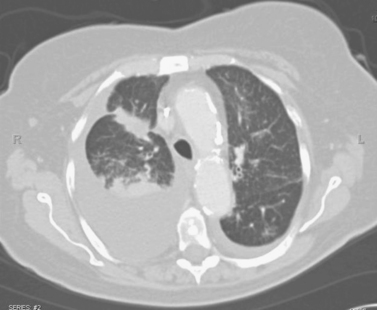 |
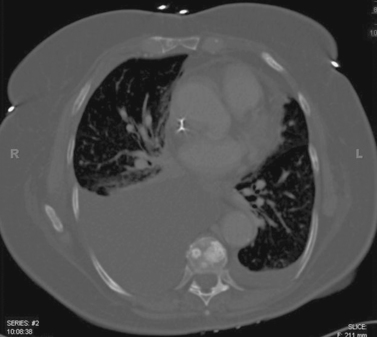 |
 |
1. Name the three main findings in the first two images.
2. What is the diagnosis?
3. What procedure has been performed in the third image?
4. What symptom is likely to be relieved by this procedure?
ANSWERS: CASE 101
Malignant Pleural Effusion: Tunneled Pleural Catheter Placement
1. Spiculated right lung mass, large right pleural effusion, and vertebral body metastases.
2. Metastatic lung cancer.
3. Placement of a tunneled pleural catheter (TPC).
4. Dyspnea.
References
Pollak, J.S.; Burdge, C.M.; Rosenblatt, M.; et al., Treatment of malignant pleural effusions with tunneled long-term drainage catheters, J Vasc Interv Radiol. 12 (2001) 201–208.
Pollak, J.S., Malignant pleural effusions: Treatment with tunneled long-term drainage catheters, Curr Opin Pulm Med. 8 (2002) 302–307.
Comment
Pleural effusions often complicate the course of malignant disease and are poor prognostic indicators. The impact on patients’ quality of life is significant, and the most common symptom is dyspnea. The approach to management is a palliative one and has traditionally been by thoracostomy and pleurodesis. More recently, management has been trending toward minimally invasive procedures by placement of TPCs.
Ultrasound or fluoroscopic guidance is used for accessing the pleural cavity, and the catheter is placed through a peel-away sheath and subsequently tunneled in the subcutaneous tissues of the chest. The catheter has a one-way valve that prevents leakage of pleural fluid from the catheter when it is not connected to a drainage flask and also prevents the entry of air into the pleural cavity through the catheter.
Ninety percent of patients experience relief of dyspnea within 48 hours. Spontaneous pleurodesis can occur in up to 46% of patients, usually within a month of placement. Chemical sclerotherapy through the catheter can be used to increase the rate of pleurodesis. If pleurodesis is achieved, the catheter may be removed.
Notes
Case 102
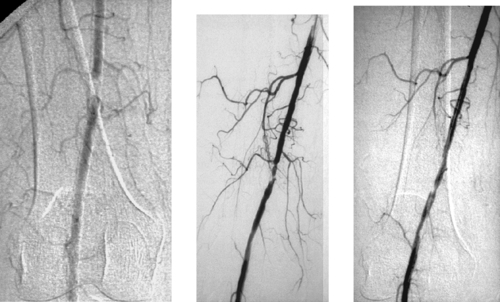 |
1. Name three methods of treating the lesion seen in the first image.
2. What has probably been done between the first and second images?
3. Describe the findings on the final image, which was obtained after angioplasty.
4. Is this an abnormal physiologic response to angioplasty?
ANSWERS: CASE 102
Iatrogenic Superficial Femoral Artery Dissection
1. Femoropopliteal bypass, thrombolysis with subsequent angioplasty (this was done in this case), primary stent placement.
2. Thrombolysis.
3. Extensive arterial dissection, which is likely to limit flow.
4. Not really. The primary mechanism by which angioplasty works is via creation of controlled intimal dissection.
Reference
Lipsitz, E.C.; Ohki, T.; Veith, F.J.; et al., Does subintimal angioplasty have a role in the treatment of severe lower extremity ischemia?J Vasc Surg. 37 (2003) 386–391.
Cross-Reference
Comment
Repeat angiography is always performed immediately following angioplasty in order to assess whether the stenosis has been adequately treated and to evaluate for any complications. It is wise to perform angiography in two projections because intimal dissection flaps oriented in the coronal plane might not be appreciated on a single anteroposterior view. Postangioplasty pressure measurements across the lesion may also be obtained: If an unexplained significant (>10mm Hg) systolic pressure gradient is still present, the presence of occult dissection should be suspected. Intravascular ultrasound, when available, can be extremely helpful in clarifying whether this is indeed the case and in assessing the entire extent of the dissection process.
Dissections caused by retrograde passage of a guidewire in a false channel often resolve spontaneously. Anterograde dissections are more likely to extend and to occlude flow.
Flow-limiting dissections require urgent treatment to prevent thrombosis. Most iliac artery dissections that do not extend into the common femoral artery can be managed with stent placement. Because patency rates for superficial femoral artery stents are comparatively poor, surgical bypass may be a better choice if the patient is thought to be able to tolerate a surgical procedure.
Case 103
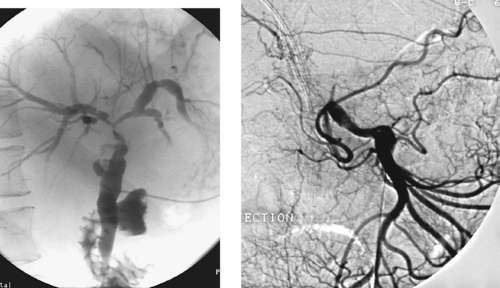 |
1. What abnormality is present in the biliary system of this liver transplant patient?
2. What is the likely etiology?
3. How would this be managed?
4. If the arterial problem was stenosis rather than occlusion, could angioplasty be performed?
ANSWERS: CASE 103
Hepatic Artery Thrombosis with Biliary Strictures
1. Extensive stricturing of the central biliary ducts.
2. Ischemia due to hepatic artery thrombosis.
3. Surgical revascularization of the arterial supply to the transplant, and biliary drainage.
4. Yes.
Reference
Saad, W.E.; Saad, N.E.; Davies, M.G.; et al., Transhepatic balloon dilation of anastomotic biliary strictures in liver transplant recipients: The significance of a patent hepatic artery, J Vasc Interv Radiol. 16 (2005) 1221–1228.
Cross-Reference
Comment
In the case shown, tight stricturing of the right and left central biliary ducts is evident on cholangiography, and the angiogram demonstrates thrombosis of the transplant hepatic artery that was anastomosed to a native replaced right hepatic artery.
A variety of anatomic complications can occur in patients who have undergone liver transplantation: biliary anstomotic leak, stenosis of the biliary anastomosis, stenosis at the inferior vena caval anastomosis, and hepatic artery anastomotic stenosis with subsequent thrombosis. Patients who experience hepatic artery thrombosis are prone to develop biliary strictures and/or necrosis. When this occurs, the strictures are usually centrally located and may be quite extensive and irregular in appearance.
Patients with evidence of transplant malfunction usually undergo sonographic evaluation. When elevated velocities are observed near the arterial anastomosis, a stenosis is suspected and the patient is usually referred for confirmatory angiography. Angioplasty can be performed to treat such stenoses, or surgical anastomotic revision may be performed. When hepatic artery thrombosis is present, surgical therapy is needed.
Case 104
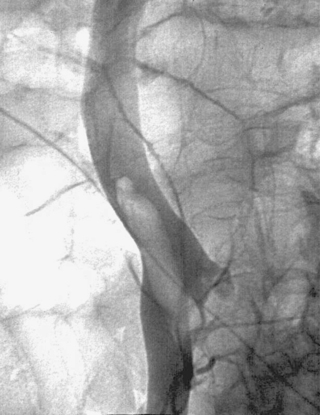 |
1. Is this abnormality acute or chronic?
2. If the patient has no contraindication to anticoagulation, should an inferior vena cava (IVC) filter be placed anyway?
3. If an IVC filter is being placed, what access site should be used?
4. Has catheter-directed thrombolysis been shown to be a better treatment for this problem?
ANSWERS: CASE 104
Free-Floating Inferior Vena Cava Thrombus
1. Acute.
2. Yes.
3. Right internal jugular vein (best choice) or left common femoral vein.
4. Not yet. However, if the patient has no contraindications to thrombolysis and is reasonably healthy otherwise, catheter-directed thrombolysis probably does offer a lower risk of later developing post-phlebitic symptoms.
Reference
Proctor, M.C., Indications for filter placement, Semin Vasc Surg. 13 (3) (2000) 194–198.
Cross-Reference
Comment
The images demonstrate a large ovoid filling defect within the right iliac vein extending into the inferior vena cava, consistent with a large free-floating acute thrombus. The venographic appearance of this abnormality suggests a predilection for easy migration and embolization. This necessitates extreme care if catheter manipulations are performed in this region, either for IVC filter placement or for thrombolytic therapy. Free-floating iliac vein and/or IVC thrombus is considered an indication for filter placement, even if no other contraindications to anticoagulation exist.
Notes
Case 105
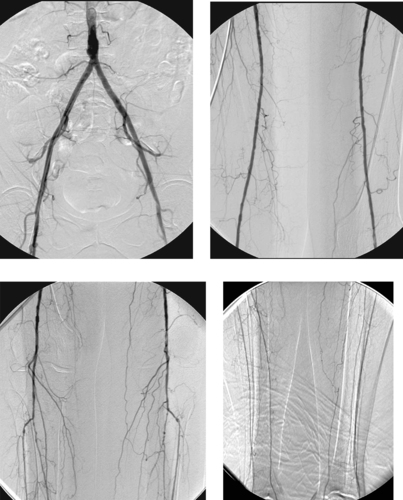 |
1. What are the primary findings of the angiogram depicted above?
2. What is the probable cause of the observed abnormalities?
3. What surgical treatment might be performed in such a patient?
4. What endovascular options exist for this patient?
ANSWERS: CASE 105
Diabetes-Related Tibial Atherosclerosis
1. Bilateral occlusion of the posterior tibial arteries and severe stenosis of the peroneal arteries.
2. Diabetes mellitus.
3. Femoral–distal arterial bypass graft placement.
4. None.
Reference
Kellicut III, D.C.; Sidawy, A.N.; Arora, S., Diabetic vascular disease and its management, Semin Vasc Surg. 16 (1) (2003) 12–18.
Cross-Reference
Comment
Although smoking, hypertension, and hyperlipidemia also promote atherosclerosis, a particular pattern of arterial involvement is often seen in patients with diabetes mellitus. The primary feature of this pattern is multifocal severe stenoses and/or occlusions of the distal popliteal artery, tibioperoneal trunk, and the proximal segments of the anterior tibial, posterior tibial, and peroneal arteries. This typical pattern of disease often occurs in the presence of normal or near-normal iliac and femoral arteries in diabetic patients, as seen in the images here, although diabetic patients are certainly prone to atherosclerotic lesions in the more proximal arteries.
The clinical manifestations of diabetic arteriopathy are often more severe than the angiogram might indicate, because of the presence of microvascular disease not appreciated on angiography. Because patients with diabetic neuropathy are prone to pedal trauma, the combination of arterial insufficiency and trauma leads to frequent ulceration with difficulty in healing. Surgical arterial revascularization is commonly performed to help with ulcer healing in these patients. Unfortunately, limb amputation owing to vascular insufficiency is a fairly common event in this patient subset.
Notes
Case 106
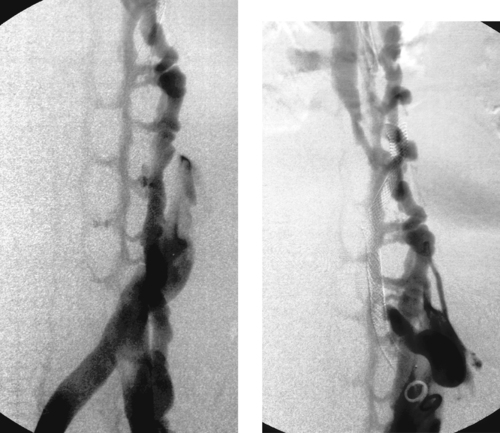 |
1. What is the primary abnormality?
2. Is this finding acute or chronic?
3. What symptoms might this patient have?
4. What vascular systems are serving as collateral networks?
ANSWERS: CASE 106
Chronic Inferior Vena Cava Occlusion
1. Chronic inferior vena cava (IVC) occlusion.
2. Chronic.
3. Resting or ambulatory lower-extremity edema, venous claudication, lower-extremity hyperpigmentation and/or ulcerations.
4. Lumbar venous plexus and azygos/hemiazygos system.
Reference
Razavi, M.K.; Hansch, E.C.; Kee, S.T.; et al., Chronically occluded inferior venae cavae: Endovascular treatment, Radiology 214 (2000) 133–138.
Cross-Reference
Comment
The images demonstrate occlusion of the distal IVC and reconstitution of the suprarenal IVC by collaterals from the lumbar venous plexus and azygos and hemiazygos system. Given the prominent visualization of the collateral network, the occlusion has undoubtedly been present for at least a few weeks and possibly years. Occlusion of the IVC can be caused by extension of iliofemoral deep vein thrombosis, IVC filters, and abdominal masses.
Patients with chronic IVC occlusion are typically treated with anticoagulation and lower-extremity compression stockings. Although this management is generally sufficient to prevent pulmonary embolism in all but a few cases, these patients tend to experience severe long-term post-thrombotic symptoms. For this reason, in carefully selected patients with severe symptoms, aggressive endovascular therapy may be attempted. Aggressive endovascular therapy consists of stent placement with a course of preceding thrombolysis if any acute thrombus is thought to be present.
Notes