Gallbladder, Extrahepatic Biliary Tract, and Pancreas Tissue Processing Techniques and Normal Histology
James M. Crawford
Introduction
Disorders of the biliary tract affect a significant portion of the world’s population. More than 95% of biliary tract disease is attributable to cholelithiasis (i.e., gallstones). It is estimated that more than 20 million persons in the United States have gallstones, with a total weight of 25 to 50 tons.1 The annual cost of treatment for cholelithiasis and its complications was estimated in 1998 to exceed $6 billion, representing approximately 0.2% of the national health care budget.2 More than 900,000 cholecystectomies are performed annually in the United States, making it one of the most common abdominal operations performed.3,4
Carcinoma of the pancreas is the fifth most frequent cause of death from cancer in the United States. Its incidence has remained unchanged during the past 50 years. The 5-year survival rate remains a dismal 5%, and there are almost 30,000 deaths per year in the United States from pancreatic cancer.5
This chapter presents an approach to the processing of surgical specimens of the gallbladder, extrahepatic biliary tract, and pancreas. Guidelines for handling intraoperative frozen section specimens are also discussed.
Normal Anatomy and Histology
Gallbladder and Extrahepatic Biliary Tract
The liver secretes as much as 1 L of bile per day. Between meals, bile is stored in the gallbladder, which in adults has a capacity of approximately 50 mL. Bile is concentrated 5- to 10-fold in the gallbladder through active absorption of electrolytes combined with passive movement of water. After ingestion of food that stimulates cholecystokinin and neural activation, the gallbladder contracts and propels the concentrated bile into the tubal gut through the extrahepatic biliary system. The gallbladder is not considered essential for adequate biliary function because humans do not suffer from maldigestion or malabsorption of fat after cholecystectomy.
The gallbladder is an elongated sac that lies in a fossa on the inferior surface of the right hepatic lobe. In adults, it is 7 to 10 cm long and 3 cm at its widest part. It is covered by peritoneum over its inferior aspect, but it is directly apposed to the surface of the liver along its superior aspect. The domelike fundus projects beyond the inferior border of the liver and slightly downward, and it is covered entirely with peritoneum. The body projects upward, backward, and to the left, approaching the porta hepatis. The neck corresponds to the tapered portion of the gallbladder. It curves backward and abruptly downward to enter the delicate connective tissue investment of the porta hepatis.
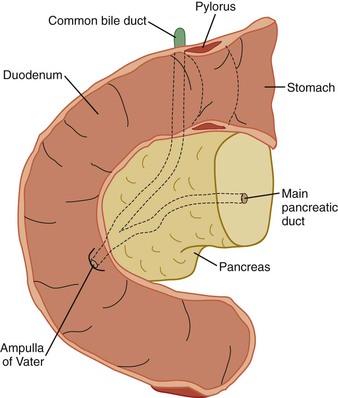
At the most proximal end of the cystic duct, there is a discrete area of narrowing that helps to regulate the flow of bile into and out of the gallbladder. The cystic duct is approximately 3 to 4 cm long and joins the common hepatic duct to form the common bile duct. This junction lies at the lowest region of the porta hepatis. The common bile duct then courses downward about 7 cm, passes behind the superior part of the duodenum, and enters the pancreas. In 80% to 90% of individuals, the common bile duct and main pancreatic duct unite and emerge as a common channel through the ampulla of Vater. However, in a minority of individuals, the common bile duct and pancreatic duct remain separate when they pass through the pancreas and ampulla.6
The common bile duct is the most anterior structure of the three major structures of the porta hepatis: common bile duct, portal vein, and hepatic artery. The common bile duct lies at the edge of the peritoneal reflection, which is the anterior edge of the epiploic foramen, the orifice between the greater and lesser peritoneal cavity located behind the stomach. The portal vein lies posterior to and to the left of the common bile duct. The hepatic artery and peripheral nerves lie most posteriorly. There may be marked variation in the relationship of the biliary tree to the portal vein and hepatic artery where these structures approach the porta hepatis and enter the liver.7
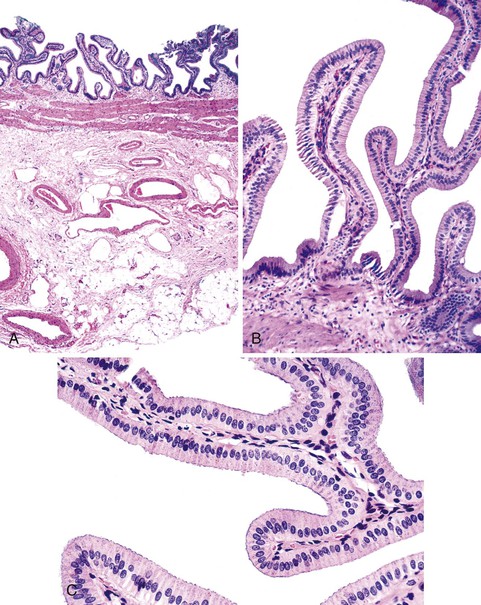
Unlike the remainder of the gastrointestinal tract, the gallbladder lacks a discrete muscularis mucosae and submucosa. It consists only of a mucosal lining with a single layer of simple columnar cells; a fibromuscular layer; a layer of subserosal fat with arteries, veins, lymphatics, nerves, and paraganglia; and a peritoneal covering, except where the gallbladder lies adjacent to or is embedded in the liver (Fig. 33.1). The epithelium is arranged in numerous interlacing folds, which imparts a honeycombed pattern to the surface. In the neck of the gallbladder, these folds coalesce to form the spiral valves of Heister, which extend into the cystic duct. In combination with cystic duct muscle action, the valves of Heister assist in retaining bile between meals. The rapid tapering of the gallbladder neck just proximal to the cystic duct is the primary site of gallstone impaction.
Small tubular channels are occasionally found buried in the gallbladder wall subjacent to the liver. These “subvesical bile ducts” occur in approximately 4% of gallbladders. Although their anatomy is quite variable, approximately half originate in the biliary tree of the right lobe of the liver and drain directly into the gallbladder; the remainder traverse the mesenchyme but do not directly communicate with the gallbladder lumen.
Small outpouchings of the gallbladder mucosa (i.e., Rochitansky-Aschoff sinuses) may penetrate into and occasionally through the muscle layer. Their prominence in the setting of inflammation and gallstone formation suggests that they are a type of acquired herniation.
Scattered along the length of the intrahepatic and extrahepatic biliary tree are mucin-secreting submucosal glands. They become prominent near the terminus of the common bile duct, appearing as microscopic outpouchings that interdigitate with the spiraling smooth muscle of the ampullary sphincter. To the unwary, buried glands may be mistaken for invasive cancer.
Pancreas
In adults, the average length of the pancreas is approximately 15 cm, and the average weight is 60 to 140 g. Embryologically, the pancreas arises from the duodenum as a dorsal bud and a smaller ventral bud. Fusion of the two creates the composite head; the dorsal bud is the primary source of the tapering body and tail. Fusion of the ventral duct and distal portion of the dorsal duct creates the main pancreatic duct (i.e., duct of Wirsung). Occasionally, the proximal portion of the dorsal duct persists as the accessory duct of Santorini.
The head represents the residuum of the embryologic ventral bud of the pancreas, having fused with the dorsal bud that constitutes the body and tail. From the ampulla of Vater, the main pancreatic duct traverses diagonally upward through the pancreatic head to the angle of the pancreatic duct of the body and tail, representing the duct of the embryologic dorsal bud. The bulbous head of the pancreas is situated in the curve of the duodenum and is the widest portion of the pancreas. The uncinate process is the portion of the pancreatic head that is most caudad and imparts a comma shape to the pancreas.
The body of the pancreas normally has a uniform diameter and a prismoid cross section with three surfaces; the posterior surface is the most vertical. The tail of the pancreas consists of the terminal few centimeters. It tapers gently to a point in the splenorenal ligament that contains the splenic artery and vein.
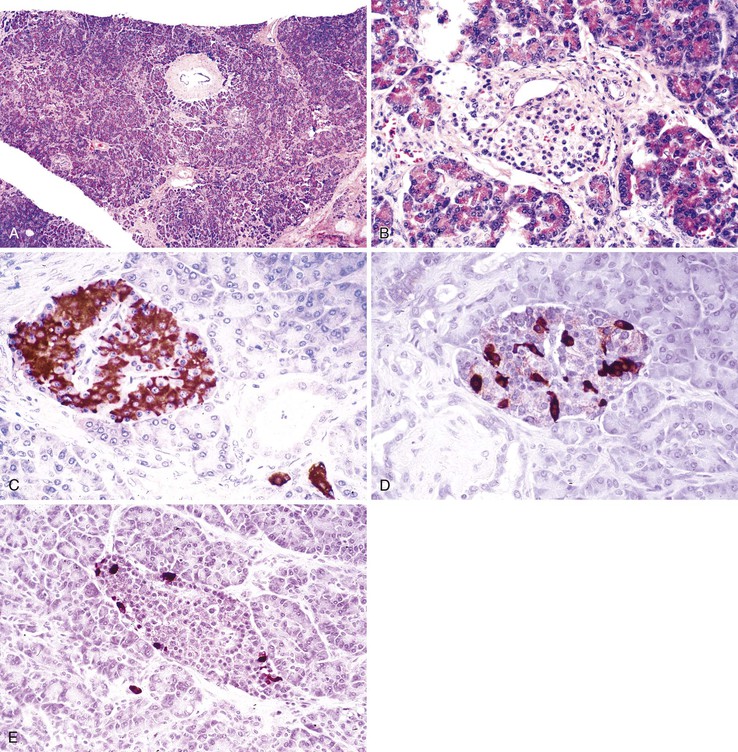
The pancreas is a pinkish-tan organ with grossly distinct, coarse lobulations, which result from delicate collagen septa that subdivide the parenchyma into macroscopic lobules. Histologically, the pancreas has two separate glandular components, the exocrine and endocrine glands (Fig. 33.2). The exocrine portion, which constitutes 80% to 85% of the organ’s volume, is composed of numerous small glands (i.e., acini) that contain columnar to pyramidal epithelial cells oriented in a radial fashion in the individual glands. The fine duct channels that drain each secretory acinus progressively converge to form the main pancreatic ductal system. The epithelium lining the smallest ducts is cuboidal but gradually evolves into a tall, columnar epithelium in the main duct. The main duct’s epithelium secretes electrolytes and mucin. Surrounding the larger pancreatic ducts are numerous accessory mucous glands.
The endocrine pancreas consists of approximately 1 million microscopic clusters of cells, the islets of Langerhans. Most islets are 100 to 200 µm in diameter and consist of four major and two minor cell types. The four main types are beta, alpha, delta, and pancreatic polypeptide (PP) cells. They make up approximately 68%, 20%, 10%, and 2%, respectively, of the adult islet cell population. Beta cells produce insulin, alpha cells secrete glucagon, delta cells produce somatostatin, and PP cells contain a unique type of pancreatic polypeptide. There are rare cell types referred to as D1 cells and enterochromaffin cells. D1 cells elaborate vasoactive intestinal polypeptide. Enterochromaffin cells synthesize serotonin and are the progenitors of pancreatic endocrine tumors that cause the carcinoid syndrome.
The only surfaces of the pancreas that are covered by peritoneum are the most anteroinferior aspect of the head, the anterosuperior surface of the body and tail, and the portion of the anteroinferior surface of the body and tail that is not apposed to the transverse mesocolon. The remainder of the pancreas is devoid of peritoneum and is apposed to some of the most vital structures of the mesenteric root and retroperitoneum.
Depending on the body mass of the host, the pancreas may lie in a bed of adipose tissue. This has relevance in determining where the corpus of the pancreas ends. The mesenchyme of the pancreas corpus consists of delicate connective tissue septa that separate the pancreatic parenchyma into lobules and a delicate sheath of connective tissue, vasculature, and nerves that travels along the pancreatic ductal system. Scattered adipocytes are located within the connective tissue septa, even in the normal pancreas of a lean individual. When the pancreas becomes damaged, inflamed, scarred, or atrophied, the boundaries of residual pancreatic exocrine glandular tissue with the peripancreatic mesenchymal adipose tissue may be difficult to establish with certainty. Pancreatic exocrine atrophy may leave residual islets of Langerhans, giving the impression that they are floating in adipose tissue.
Gallbladder
Gallstones afflict 10% to 20% of the adult population in developed countries. More than 80% of gallstones are clinically silent. Most individuals remain free of biliary pain or gallstone complications for decades, but biliary colic develops in approximately 25% during 10 years.8,9 Each year in the United States, approximately 1,000,000 symptomatic patients are found to have gallstones, ensuring a steady future population for the 900,000 cholecystectomies performed annually.
Gallbladders removed during open laparotomy or laparoscopic cholecystectomy are common specimens. Gallbladder cancer arises most frequently in the setting of long-standing cholecystitis with gallstones. The symptoms associated with gallbladder cancer usually mimic those of chronic cholecystitis, and the cancer may be advanced at the time of diagnosis. Occasionally, previously undetected gallbladder cancer is found only on examination of a resected specimen. Gallbladder lesions identified by endoscopic retrograde cholangiopancreatography (ERCP) may be amenable to endoscopic biopsy,10 and percutaneous fine-needle aspiration of the gallbladder may be performed.11–13 However, most often, gallbladder cancer is encountered in cholecystectomy specimens.
Gallstones
The two main types of gallstones are cholesterol stones and pigment stones. In the West, approximately 80% are cholesterol stones, which contain more than 50% crystalline cholesterol monohydrate. The remainder are pigment stones, which are composed predominantly of bilirubin calcium salts. There is a greater preponderance of pigmented gallstones in Asia because of the high prevalence of fluke infections of the biliary tract.
Cholesterol Stones
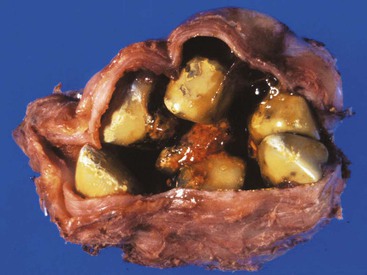
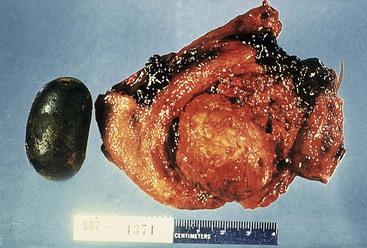
Cholesterol stones develop exclusively in the gallbladder and are composed of various amounts of cholesterol, ranging from 100% (which is rare) to approximately 50%. Pure cholesterol stones are pale yellow and round to ovoid, and they have a finely granular, hard external surface (Fig. 33.3), which on transection reveals a glistening, radiating crystalline palisade. With increasing proportions of calcium carbonate, phosphates, and bilirubin, the stones exhibit discoloration and may be lamellated and grayish-white to black on transection.
Most often, multiple stones may range as large as several centimeters in diameter. Rarely, there is a single, large stone that may fill the entire fundus or obstruct the gallbladder neck. The surfaces of stones may be rounded or faceted; the latter results from tight apposition when they occur in multiples.
Cholesterol stones are gross-only specimens. Regardless of their color, they are hard and cannot be crushed easily between the thumb and forefinger, unlike pigmented gallstones. Larger stones may be crushed intraoperatively during laparoscopic cholecystectomy to minimize the size of the abdominal wall incision required to remove the gallbladder. In this situation, the lamellated interior of the stones is usually evident. Chemical analysis of stones is not required for clinical care and is of value for research purposes only.
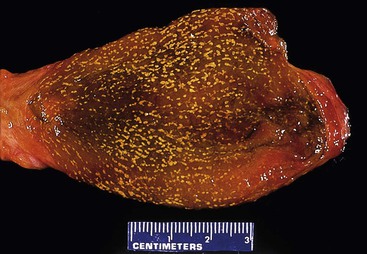
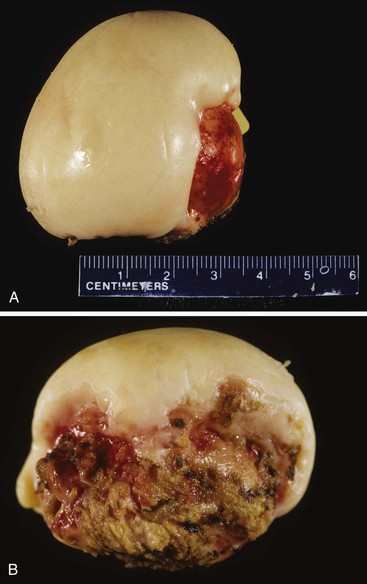
Cholesterolosis is an incidental finding pertinent to cholesterol biology but not directly related to gallstone formation. Cholesterol normally enters the gallbladder mucosa by free exchange with the lumen and is esterified by acyl-coenzyme A : cholesterol acyltransferase. Cholesterol hypersecretion by the liver promotes excessive accumulation of cholesterol esters in the lamina propria of the gallbladder. The mucosal surface may be studded with minute, yellow flecks, giving a strawberry appearance to the gallbladder mucosa (Fig. 33.4).
Pigment Stones
Pigment gallstones are a complex mixture of insoluble calcium salts of unconjugated bilirubin combined with inorganic calcium salts. Pigment gallstones are classified as black or brown. Black pigment stones usually are found in sterile gallbladder bile, and brown stones are found in infected intrahepatic or extrahepatic ducts. Black pigment stones contain oxidized polymers of calcium salts of unconjugated bilirubin; lesser amounts of calcium carbonate, calcium phosphate, and mucin glycoprotein; and a modicum of cholesterol monohydrate crystals. Brown pigment stones contain pure calcium salts of unconjugated bilirubin, mucin glycoprotein, a substantial cholesterol fraction, and calcium salts of palmitate and stearate.
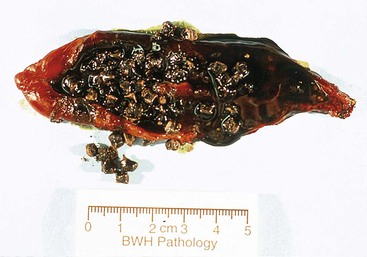
Black stones are rarely greater than 1.5 cm in diameter and are almost invariably occur in large numbers (with an inverse relationship between size and number) (Fig. 33.5). Their contours are usually spiculated and molded. Brown stones tend to be laminated and soft, and they may have a soaplike or greasy consistency, resembling fecal material.
Pigment stones are gross-only specimens. Black and brown stones crumble or crush easily between the thumb and forefinger, which constitutes the primary confirmatory finding for distinguishing true pigment stones from darkly pigmented cholesterol stones, which do not crush.
Processing Gallbladder Specimens
Cholecystectomy is performed for gallstone disease, cholecystitis, cancer or presumed neoplasia, or incidental findings. Gallbladders removed at open laparotomy usually are intact. Because those removed during laparoscopic cholecystectomy may be torn, spillage of bile and gallstones into the abdominal cavity is a complication of this procedure.
Cholecystitis
Acute cholecystitis may be acalculous (without stones) or calculous (with stones). Chronic cholecystitis almost always occurs in the setting of gallstones. In acute cholecystitis, the gallbladder is usually enlarged and tense, and it may assume a bright red or blotchy, violaceous to green-black discoloration because of subserosal hemorrhages. The serosal covering is frequently layered by fibrin and, in severe cases, by a suppurative, coagulated exudate. There are no specific morphologic differences between acute acalculous and calculous cholecystitis, except for the absence of macroscopic stones in the former. In the latter, an obstructing stone is usually found in the neck of the gallbladder or the cystic duct.
In addition to gallstones, the gallbladder lumen is usually filled with cloudy or turbid bile that may contain large amounts of fibrin, pus, and blood. When the exudate is composed of pure pus, the condition is referred to as empyema of the gallbladder. In mild cases of cholecystitis, the gallbladder wall is thickened, edematous, and hyperemic. In more severe cases, the organ is green-black and necrotic, with or without perforation; this condition is called gangrenous cholecystitis. The inflammatory reactions are not histologically distinctive and consist of the usual patterns of acute inflammation (i.e., edema, leukocytic infiltration, vascular congestion, frank abscess formation, or gangrenous necrosis).
The morphologic changes in chronic cholecystitis are extremely varied and sometimes minimal. The serosa is usually smooth and glistening, but it may be dulled by subserosal fibrosis. Dense fibrous adhesions may remain as a sequela of preexistent acute inflammation. On tissue sectioning, the wall is thickened to some degree but rarely to more than three times normal. The wall of the gallbladder has an opaque gray-white appearance and may be less flexible than normal. In uncomplicated cases, the lumen contains fairly clear, greenish-yellow, mucoid bile and usually contains stones. The mucosa typically is well preserved.
In rare instances, extensive dystrophic calcification in a chronically inflamed gallbladder wall may produce a porcelain gallbladder, which is associated with a markedly increased incidence of cancer. Xanthogranulomatous cholecystitis is a rare condition in which the gallbladder is shrunken, nodular, and chronically inflamed with foci of necrosis and hemorrhage. Gallstones are usually found. This rare condition can be confused macroscopically with a malignant neoplasm. An atrophic, chronically obstructed gallbladder may contain only clear secretions, a condition known as hydrops of the gallbladder.
Carcinoma of the Gallbladder
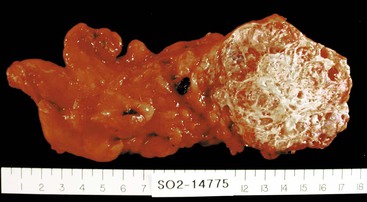
Carcinomas of the gallbladder exhibit two patterns of growth: infiltrating or exophytic. The infiltrating pattern is more common and usually appears as a poorly defined area of diffuse thickening and induration of the gallbladder wall that may cover several square centimeters or may involve the entire gallbladder. Deep ulceration may cause direct penetration of the gallbladder wall or fistula formation into adjacent viscera. These tumors are scirrhous and have a very firm consistency.
The exophytic pattern grows into the lumen as an irregular, cauliflower-shaped mass, but it may also invade the underlying gallbladder wall. The luminal portion may be necrotic, hemorrhagic, and ulcerated. The most common sites of involvement are the fundus and neck; approximately 20% of cases involve the lateral walls. By the time these neoplasms are discovered, most have invaded the liver centrifugally, and many have extended to the cystic duct, adjacent bile ducts, and portohepatic lymph nodes.
Specimen Appearance
Perforations or tears in the gallbladder specimen should be documented. The serosa should be evaluated for its color and consistency. Inflammation usually imparts a dull and irregular appearance to the serosa. Adhesions may be evident, particularly in the region adjacent to the liver bed. In many cases, adherent cauterized liver tissue may be included with the gallbladder specimen. The finding of a fibrinous or purulent exudate points to a severe form of acute cholecystitis. Gangrenous gallbladders are blue-black, typically with a green bile discoloration; gross necrosis and perforation also are seen. The wall of a gallbladder with chronic cholecystitis may be markedly thickened. With extensive calcification, the gallbladder may take on a shiny, white, and hard porcelain appearance (Fig. 33.6).
Carcinoma in a gallbladder specimen may be evident on external examination as a dense mass bulging out of or penetrating into the gallbladder wall. Given the frequent advanced nature of gallbladder cancer at the time of diagnosis or resection, the tumor may be grossly transected at the specimen margin. Lymph nodes in the vicinity of the cystic duct should be identified and evaluated for metastasis. Serosal tumor implants from elsewhere in the abdominal cavity may also be found by the surgeon.
Specimen Dissection
The length of the gallbladder should be measured, and it should be opened longitudinally. The luminal contents are documented, including their volume and color, mucin content, and the presence or absence of sludge or stones. Biliary sludge consists of microcrystalline aggregates suspended in the mucinous fluid. The gallbladder wall thickness and circumference should be measured. The cystic duct is often tortuous and difficult to open, but an attempt should be made to examine the cystic duct margin at minimum. Gallstones in the gallbladder lumen, gallbladder neck, or cystic duct should be reported. Their gross features should be specifically described.
Occasionally, gallstones are returned to the patient. This should be done in a sealed container without formalin because formalin is a chemical biohazard. If the gallstones have been immersed in formalin, they should be rinsed in water before release to the patient. If chemical analysis is requested, the gallstones should be placed in a dry, sterile container and forwarded to the appropriate laboratory.
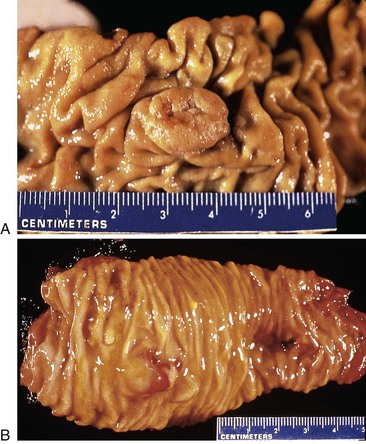
The mucosa of the gallbladder should be inspected and described. Normal mucosa has a honeycomb appearance and is tan and velvety. In cases of acute or chronic cholecystitis, the mucosa becomes effaced and loses its velvety appearance; it may be ulcerated and friable or flattened and atrophic, respectively. Cholesterolosis produces diffuse, small, yellow flecks on the mucosal surface.
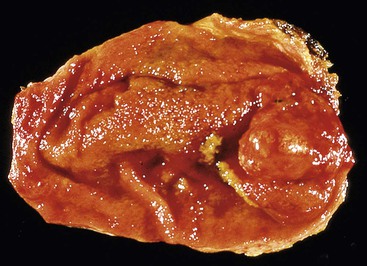
Mucosal polyps are usually delicate, protuberant, papillary structures that are easily disrupted. Alternatively, flat areas of mucosal dysplasia or nascent adenocarcinoma may appear as firm, plaquelike elevations of the mucosa or exophytic tumors (Fig. 33.7) with effacement of the layers of the gallbladder wall in cases of invasive carcinoma. Invasive carcinoma should not be confused with adenomyosis, which is displacement of benign glandular epithelium into the gallbladder wall and which is usually associated with marked muscular hypertrophy. Adenomyosis may appear as a dense, focal, tumor-like thickening of the gallbladder wall (Fig. 33.8). Massively dilated Rokitansky-Aschoff sinuses in chronic cholecystitis may impart tumor-like thickening of the gallbladder wall.
Specimen Sectioning
Cross sections of the gallbladder fundus and lateral wall should be submitted along with a section through the neck of the gallbladder and cystic duct, including its margin. Gross lesions, whether non-neoplastic or neoplastic, should be sampled thoroughly, with particular attention paid to the surgical margins of resection. Accompanying lymph nodes should be submitted and evaluated histologically.
If a neoplastic lesion is identified on gross examination, that entire portion of the gallbladder should be submitted for histologic evaluation. If neoplasia is identified on histologic review of standard sections obtained from a gallbladder specimen, the specimen should be revisited and the entire area of interest submitted for histologic evaluation, which often involves submission of the entire gallbladder specimen.
Extrahepatic Biliary Tract
The extrahepatic biliary tract consists of the common bile duct, cystic duct, gallbladder, and common hepatic duct. In approximately 60% to 70% of individuals, the common hepatic duct bifurcates into the right and left hepatic ducts before entry into the liver.14 The most common anatomic variation is congenital absence of the right hepatic duct. Instead, the posterior and anterior branches of the bile ducts that supply the right portion of the liver arise from a hilar confluence with the left hepatic bile duct. This occurs in the form of a three-way branch point with the left hepatic bile duct or by a two-way confluence of the anterior or posterior branches with the left hepatic duct.14 Although the common hepatic duct and its branches lie ventral to the portal vein system, the right posterior bile duct may be situated in an inferior-ventral or superior-dorsal fashion around the right portal vein.
In two situations, the extrahepatic biliary tract should be submitted for surgical pathology analysis. The first is for resection of lesions that are primary to the biliary tree, particularly bile duct cancer15 and choledochal cysts. The second is for resection of the biliary tree as part of a pancreas or liver resection specimen. Pancreaticoduodenectomy (Whipple) specimens are discussed later.
Processing Biliary Tract Specimens
Isolated resection of the extrahepatic biliary tree is performed as part of procedures designed to reconstruct the biliary system. Biliary tract reconstruction in situ is not usually possible except in cases of small perforations or strictures, such as those that occur accidentally during laparoscopic cholecystectomy.16
The most common surgical procedure of the extrahepatic biliary tract is resection combined with the performance of a Roux-en-Y choledochojejunostomy. In this procedure, a blind loop of jejunum is anastomosed end to side or end to end with the remaining common bile duct. This type of reconstructive procedure is a routine part of pancreaticoduodenectomy. Because patients with primary sclerosing cholangitis are not treated with surgery, except for those undergoing liver transplantation, the primary indications for biliary tract extirpation and choledochojejunostomy are biliary tract cancer and bypass and resection of a choledochal cyst.
Biliary Tract Cancer
For patients with bile duct cancer, the resection specimen usually consists of the extrahepatic biliary tree, gallbladder, and accompanying soft tissue with lymph nodes. The surgeon should provide anatomic orientation by applying suture tags at the proximal and distal duct margins. Consultation with the surgeon is mandatory if there is any uncertainty with regard to anatomic orientation. En face sections should be obtained of the proximal (toward the liver) and distal (toward the pancreas) bile duct margins. The soft tissue margins should be marked with ink. However, disruption of anatomic planes during operative dissection may make identification of the true soft tissue margins difficult. The biliary system should be opened in the fresh state and examined for the intraluminal extent of tumor. The specimen should then be fixed in formalin overnight.
After fixation, optimal tissue sections for histologic evaluation include cross sections along the length of the biliary tree and all soft tissue margins: anterior, left lateral, posterior, and right lateral. Depending on the amount of soft tissue, lymph nodes may be included with the cut sections through the biliary tree or may be separately dissected from the soft tissue and submitted for histologic evaluation. Lymph node groups should be submitted in separate cassettes as follows: periductal (i.e., along the common bile duct), perihilar, peripancreatic, subpyloric, and celiac and preaortic.
Choledochal Cyst
Congenital cystic dilations of the common bile duct (i.e., choledochal cyst) are nonheritable, extrahepatic lesions (see Chapter 36). Although they may be associated with cystic dilation of the intrahepatic biliary tree, extrahepatic choledochal cysts do not arise from ductal plate malformations. Cystic dilations of the extrahepatic biliary tract are classified as cylindrical dilations of the common bile duct, cystic diverticula, intrapancreatic dilations, or lesions protruding into the gut lumen at the ampulla of Vater.
Choledochal cysts are more common in Japan than in Western countries,17 and they are more common among females. Approximately 60% of patients are diagnosed before the age of 10 years, but cyst identification in adulthood also occurs. Ascending cholangitis and obstruction are possible underlying causes of symptoms. Complications other than obstruction include stone formation (approximately 8% of patients), pancreatitis (infrequent), carcinoma (approximately 4% of patients), and perforation (rare).18 Surgical resection of choledochal cysts is the preferred method of treatment because of the risk of carcinoma, which increases progressively with age.19–21
Dissection of the resection specimen requires careful attention to the anatomy and the sites and nature of the cystic dilation. The cyst walls may be thin and delicate. The specimen’s anatomy may be easily disrupted by the surgeon or pathologist. Because of the risk of carcinoma, the mucosal surfaces of the cyst should be examined carefully, and areas exhibiting plaquelike thickening; a thickened, velvety texture; or effacement of the underlying tissue layers should be submitted for histologic examination. The proximal and distal bile duct margins (including the region of the ampulla if a Whipple procedure was performed) should be sampled, along with soft tissue margins adjacent to suspect areas.
Extrahepatic Biliary Atresia
The salient features of extrahepatic biliary atresia include inflammation and fibrosing strictures of the common hepatic duct and common bile duct, with secondary periductal inflammation of intrahepatic bile ducts and progressive destruction of the intrahepatic biliary tree. There is considerable variation in the anatomy of patients with biliary atresia. When the disease is limited to the common (type I) or hepatic (type II) bile ducts with patent proximal branches, it is considered surgically correctable. Unfortunately, 90% of patients have type III biliary atresia, in which there also is obstruction of bile ducts at or above the porta hepatis. The latter group includes early, severe biliary atresia, in which the intrahepatic biliary tree does not form properly, and there is no patency to the intrahepatic biliary tree.21 Cases with severe obliteration of the intrahepatic biliary tree are not surgically correctable except by liver transplantation. In most patients with biliary atresia, bile ducts in the liver may be patent initially, but they are progressively destroyed with time.
In infants with type I or type II extrahepatic biliary atresia, biliary tract resection with a portoenterostomy (i.e., Kasai procedure) is the first surgical option. Biliary drainage is reestablished by connecting a portion of the jejunum with the hilum of the liver by a Roux-en-Y procedure.22 The primary responsibility of the pathologist is to obtain cross sections of the resected biliary tree, with particular attention to the most proximal portion. The diameter of the residual bile duct lumina at the porta hepatis correlates with long-term functionality of the portoenterostomy.23 If a liver transplantation is performed some time after a Kasai procedure, the resected liver specimen usually reveals a segment of jejunum anastomosed to the porta hepatis.
Hepatectomy Specimens
Whether the biliary tract accompanies complete liver explants or partial hepatectomy specimens, the fundamental goal of dissection is to identify the anatomy of the extrahepatic biliary tree, including its vasculature. Livers explanted for diseases that affect the extrahepatic biliary tree, particularly biliary atresia or primary sclerosing cholangitis, likely have a partially or completely obliterated extrahepatic bile duct system. Cross sections of the biliary tree are the best method to document the extent of disease. In patients with primary sclerosing cholangitis, attention should be given to the possibility of a coexistent bile duct carcinoma. Sections should be submitted of hilar or extrahepatic areas that are diffusely effaced by white, sclerotic tissue. In all instances, a cross section of the most distal margin of the extrahepatic biliary tree should be obtained.
Partial hepatectomy specimens for known biliary tract cancer, especially for those involving the confluence of the right and left hepatic bile ducts (i.e., Klatskin tumor), should be examined carefully.24 This examination includes obtaining sections of the distal surgical margins (i.e., bile duct, portal vein, and hepatic artery samples), all regional lymph nodes, soft tissue margins, and the liver margin. The main tumor mass should be well sampled, with specific attention to its relationship to the major bile ducts. The major ramifications of the intrahepatic portal tree should be sampled because cancer cells can track for a considerable distance up the vasculature, lymphatics, bile ducts, and mesenchyme of portal tracts. This may be evident grossly as white, thickened portal tracts extending well into the liver corpus. As a result, hepatic resection margins may contain invasive cancer at sites where portal tracts have been transected (Fig. 33.9). Major portal tracts at the hepatic resection margin should be sampled generously. The liver parenchyma well away from the tumor also should be sampled to gain insight into the obstructive changes in the liver tissue.
Distal carcinomas of the extrahepatic biliary tree up to and including the ampulla of Vater are uncommon and are most often treated with a pancreaticoduodenectomy. A subgroup of biliary tract carcinomas arises in the immediate vicinity of the ampulla of Vater. Tumors of this region also include adenomas of the duodenal mucosa and pancreatic carcinoma. Collectively, these tumors are referred to as periampullary carcinomas, and all are treated by surgical resection.
Pancreas
Surgical resection of the pancreas typically generates two classes of specimens: pancreaticoduodenectomy specimens and distal pancreatectomy specimens. Needle biopsies may be obtained during surgery by ERCP (often as brushings), through the gastric wall during endoscopy, or during radiographic localizing procedures.25,26 Biopsy of biliary or pancreatic tissue during ERCP uses standard or specially designed small biopsy forceps.27
Processing Pancreaticoduodenectomy Specimens
Pancreaticoduodenectomy (i.e., Whipple procedure) usually is performed for resection of neoplasms of the pancreas, distal bile duct, ampulla of Vater, and duodenum.28 The intent of the operation (palliative versus curative) is a key prognostic indicator.29 If it is meant to be curative, the intraoperative goal is to obtain surgical margins that are free of tumor.
Whipple specimens consist of duodenum from the pylorus to the ligament of Treitz, the head of the pancreas, and the distal extrahepatic biliary tract (Fig. 33.10). The most distal portion of the stomach also may be included. The gallbladder is usually submitted as a separate specimen. Proper anatomic orientation must be maintained at all times during processing of a Whipple resection specimen. Carcinomas of the common bile duct, ampulla of Vater, and head of the pancreas are removed to effect a cure.
On initial examination of the fresh specimen, several anatomic structures should be identified: common bile duct margin; pancreatic tissue margin (with main pancreatic duct); proximal tubal gut margin (stomach or duodenum); and the distal duodenal margin. The common bile duct, pancreatic, and selected peripancreatic soft tissue margins should be inked before processing. At least two en face sections of the pancreatic resection margin, including the pancreatic duct, should be performed at the outset to assess the pancreatic margin. If tumor appears close to the pancreatic margin, sampling of the pancreatic margin can be delayed until after overnight fixation. This approach allows perpendicular margins, which help to establish the precise distance of tumor to the pancreatic margin. The common bile duct margin, which is usually located posterior to the duodenum, should be cut in cross section and submitted en face for microscopic examination of the tissue margin.
For further dissection of the fresh specimen, the following protocol is recommended. The specimen should be opened along the greater curvature of the stomach, the anterior wall of the pylorus, and the greater curvature of the duodenum. The contents of the lumen should be rinsed with saline, and any mucosal lesions, particularly those surrounding the ampulla, should be documented. Photographs of lesions should be obtained after the specimen is opened (Fig. 33.11).
A probe should then be placed in the common bile duct (proximal end) and advanced carefully toward the ampulla. Similarly, a probe should be placed in the main pancreatic duct at the pancreatic margin and advanced gently and carefully toward the ampulla. The ducts may then be opened along the longitudinal paths of the probes with fine blunt scissors. This method of dissection permits evaluation of the relationship of biliary or intrapancreatic tumors to the ductal system and provides an opportunity for specimen photography (Fig. 33.12).
Snap-freezing a portion of tumor should be considered because molecular analysis of pancreatic cancer can provide useful prognostic information.30 Samples of tumors also should be placed in glutaraldehyde-based fixative for subsequent electron microscopy.
Delineation of the anatomy of the common bile duct and pancreatic duct is done to determine the precise anatomic location and origin of the tumor.31 After initial dissection, it is best to fix the specimen in 10% buffered formalin overnight before further dissection of the tumor, intrapancreatic ductal system, extrapancreatic common bile duct, and the ampulla of Vater. Attention also should be given to possible patency of the accessory duct of Santorini.
The cut sections of the fixed pancreas should be evaluated for color, consistency, and texture and for tumors, nodules, cysts, and dilation of the pancreatic duct system. In addition to the initial tissue sections obtained from the fresh specimen (e.g., pancreatic margin, common bile duct margin), recommended sections for histologic analysis include the relationship of the tumor to the pancreas and representative sections of the common bile duct and pancreatic ducts, ampulla, duodenum, pancreatic tissue margin, and common bile duct margin. Perpendicular sections also should be obtained through the nearest superior, posterior, inferior, and anterior soft tissue margins. The uninvolved pancreas, ampulla in longitudinal section (if not previously sampled), and proximal gastric and distal duodenal margins (i.e., en face and full thickness) should be sampled.
All regional lymph nodes should be submitted. This includes lymph nodes superior, anterior, inferior, and posterior to the pancreas; retroperitoneal lymph nodes; and lymph nodes from the lateral aortic, hepatic artery, superior mesenteric, subpyloric, and celiac areas.
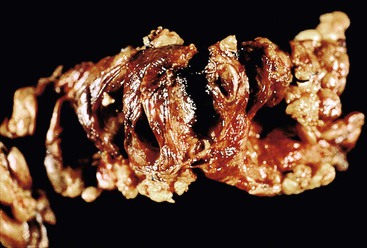
Pancreatic adenocarcinomas of ductal or acinar origin may be difficult to appreciate on gross examination. In some instances, the predominant indicator of tumor extent is effacement of the lobular architecture of the pancreas by dense, white fibrous tissue. However, chronic pancreatitis may obscure the macroscopic relationship of invasive tumor to the surrounding pancreatic parenchyma. Some infiltrative tumors may secrete mucin. Many desmoplastic lesions therefore appear as gritty, gray-white, hard masses. Early-stage tumors are confined to the pancreas. In more advanced stages, tumor extends into the peripancreatic soft tissue and into adjacent peripancreatic structures. The goal in obtaining samples for microscopic sections is to maximize the pathologist’s ability to characterize the histologic features of the tumor, determine its relationship to the surrounding pancreatic parenchyma and soft tissues, and establish its anatomic origin and extent.
For endocrine tumors, cystic tumors, and other mass lesions of the pancreas, the same dissection principles usually apply. Cystadenomas and cystadenocarcinomas are usually multicystic and contain serous or mucinous fluid. For cystic lesions, cystic cavities should be opened and examined closely for areas of thickening, nodularity, ulceration, tumors, or effacement of adjacent tissue. Cyst walls should be generously sampled up to and including the entire epithelial lining in mucinous tumors.
Intraductal Papillary Mucinous Neoplasms
Intraductal papillary mucinous neoplasms (IPMNs) are a unique category of pancreatic neoplasia as defined and classified by the World Health Organization32 (WHO) (see Chapter 40). They may contain dysplasia ranging from minimal mucinous hyperplasia to invasive carcinoma,33 and they may involve the main pancreatic duct (MD-IPMNs), its branch ducts (BD-IPMNs), or a mixed pattern.
In the most florid form, the IPMN manifests as a massive dilation of the intrapancreatic ductal system, and this appearance may extend throughout the length of the pancreatic ductal system. This type of neoplasm may be suspected on the basis of cytology or radiographic imaging.34 The type of surgical procedure often is chosen preoperatively: a pancreaticoduodenectomy for tumors located in the head, neck, or uncinate process of the pancreas or a distal pancreatectomy or partial pancreatectomy for tumors located in the body or tail. Rarely, total pancreatectomy may be considered.
Preoperative findings and gross examination of the resected specimen are critical in evaluating possible IPMNs, although the final diagnosis is ultimately established by histologic evaluation.35 Adequate tissue sampling of the specimen includes the pancreatic resection margin, extensive samples along the pancreatic duct system (i.e., main duct and its branches), and any cystic lesions or mural nodules identified in the pancreatic parenchyma. When mural nodules are identified outside of the duct system, their relationship to the duct system should be described carefully.36
The postoperative prognosis is significantly better for patients with tumors located in the branches of the main pancreatic duct (BD-IPMNs) compared with MD-IPMNs.35 The rate of postoperative recurrence is considerably higher among patients who have an invasive IPMN compared with those without invasion.37 When a cystic tumor is identified grossly, the cyst lumen and pancreatic duct should be cannulated carefully to determine whether these two structures are connected. Continuity between the duct system and the cyst lumen usually indicates an IPMN. A cyst lumen not connected to the main pancreatic duct provides more evidence for a mucinous cystic neoplasm or BD-IPMN.
Multicentric tumor with intervening normal pancreatic parenchyma and ducts is a concern when evaluating IPMNs. The gross examination should be sharply focused on whether there is evidence of invasion.38 The entire resected pancreatic tissue should be inspected carefully on gross examination for intraductal and invasive cancer. Unless there is grossly identifiable invasive carcinoma, which can be sampled, the entire lesional area affected by the IPMN should be submitted for histologic examination.38 A focus of invasive carcinoma may be missed if a resection specimen is not well sampled.
For patients with negative pancreatic tumor margins who have subsequent recurrence, metachronous multicentric tumor is often considered a plausible explanation. These tumors are more likely to occur after distal pancreatectomy for an IPMN compared with proximal pancreaticoduodenectomy.35 Discontinuous IPMNs have been documented in 6% of specimens in two surgical series.39,40
Distal Pancreatectomy for Tumor
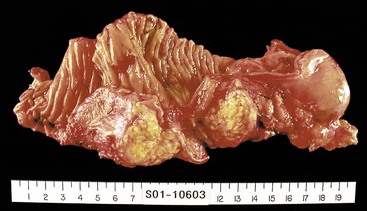
When neoplasia is located in the body or tail of the pancreas, a distal pancreatectomy usually is performed. It typically includes the tail and some portion of the body of the pancreas, the spleen, and the accompanying splenic vein along the superior aspect of the pancreas and peripancreatic fat (Fig. 33.13). Ductal carcinomas of the body and tail of the pancreas do not impinge on the biliary tract and often remain clinically silent for some time. At the time of discovery, these tumors may be large and widely disseminated, often with extension into the retroperitoneal soft tissue and adjacent nerves. Occasionally, they may invade the spleen, adrenal glands, vertebral column, transverse colon, and stomach. Peripancreatic, gastric, mesenteric, omental, and portohepatic nodes are frequently involved.
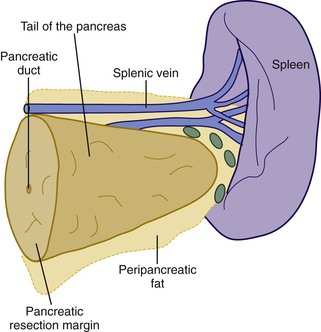
Other pancreatic tumors in the body and tail with life-limiting potential include acinar cell carcinoma and cystic or solid epithelial neoplasms of the exocrine pancreas. Islet cell tumors of the endocrine pancreas (i.e., endocrine tumors) in the body or tail also are resected by distal pancreatectomy.
The following guidelines describe appropriate dissection and sampling of a distal pancreatectomy specimen.13
• The proximal pancreatic resection margin should be sectioned en face for histologic processing.
• Cystic tumors (Fig. 33.14) should be sampled as described for Whipple specimens.
Distal Pancreatectomy for Pancreatitis
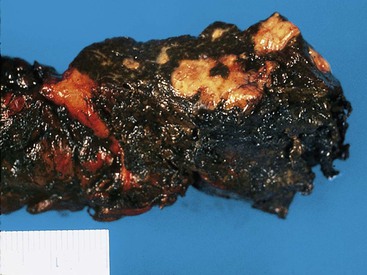
Partial resection of the pancreas for acute pancreatitis is considered a final, desperate procedure for patients with severe, hemorrhagic, acute pancreatitis. In these cases, the pancreatic parenchyma is extensively necrotic and hemorrhagic (Fig. 33.15). Dissection and tissue processing are done primarily to document the severity of pancreatic injury and the presence or absence of microorganisms.
Partial resection of the pancreas for chronic pancreatitis is an established procedure for alleviation of intractable pain. The main goals of dissection and tissue processing are to establish an accurate diagnosis and to exclude the possibility of unsuspected pancreatic carcinoma. Accordingly, attention should be given to the surgical margins of resection as described previously.
Frozen Sections and Intraoperative Consultation
For extrahepatic bile duct cancer and pancreatic cancer, positive surgical margins constitute a strong risk factor for postoperative recurrence.41–44 Frozen section confirmation of lymph node metastasis at the time of surgery may help the surgeon to decide against resection.44,45 The pathologist commonly plays a key role in intraoperative decision making in the following situations:
• Proximal and distal bile duct margins of a limited resection of an extrahepatic biliary tract tumor
• Pancreatic margin of a pancreaticoduodenectomy or distal pancreatectomy procedure
The main intraoperative decision is whether to extend the surgical resection. False-negative rates for intraoperative interpretation of surgical margins range as high as 10% in some series.42
Occasionally, an intraoperative frozen section is requested to confirm a biliary or pancreatic lesion as malignant.46–48 Specimens may be obtained by an intraoperative incisional biopsy or, in the case of the pancreas, a needle biopsy. The intraoperative decision in these instances (e.g., biliary tract cancer versus benign lesions, chronic pancreatitis versus pancreatic carcinoma) depends on the need for definitive resection.
Bile Duct Margins
Hilar Resections or Partial Hepatectomy
Distal Bile Duct Margin
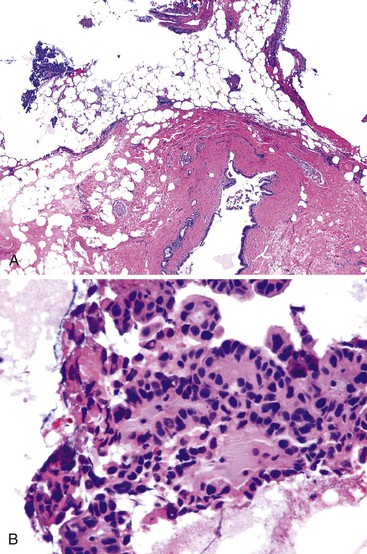
Intraoperative frozen section evaluation of the distal bile duct stump is frequently performed for resections of a carcinoma of the proximal extrahepatic (i.e., Klatskin tumor) or intrahepatic biliary tract.49 A distal tumor-free surgical margin of at least 0.5 cm is desirable.50 Because the distal portion of the biliary tract is normally not obstructed in patients with Klatskin tumor, obstructive changes of the bile duct epithelium and peribiliary glands are normally not found.
One task is to determine the presence or absence of a malignant tumor (Fig. 33.16). Well-differentiated tumors may be difficult to identify because they exhibit well-formed glandular structures with ample mucin and have regularly shaped, inconspicuous nuclei.49,51–54 The location of glandular structures in the stroma and wall of the bile duct is critical to help determine malignancy. In contrast, moderately differentiated tumor exhibits a predominantly cribriform growth pattern of tumor cells. Poorly differentiated tumors exhibit solid strands of tumor cells or single, infiltrating malignant cells. In moderately and poorly differentiated tumors, the nucleus-to-cytoplasm ratio is high, and the nucleus is large, irregularly shaped, and hyperchromatic. A signet ring configuration may be prominent. Specific attention should be given to identifying perineural invasion, which always implies malignancy.
Proximal Bile Duct Margin
The surgeon may request frozen section evaluation of the proximal resection margin of hilar cholangiocarcinoma (i.e., Klatskin tumor). The same criteria for malignancy should be applied, and the invasive carcinoma within the wall of a bile duct should be distinguished from dysplasia.
The ultimate value of frozen sections for proximal bile duct margins is controversial,55 particularly because accuracy, sensitivity, and specificity for invasive carcinoma have been reported as only 57%, 75%, and 47%, respectively.56 In 9% of cases, proximal margins deemed negative for invasive carcinoma at the time of frozen section evaluation are found to have invasive carcinoma in the permanent histologic section.57 Moreover, extension of a proximal margin of resection found to be positive for invasive cancer on frozen section is technically difficult, regardless of whether the resection is of a proximal extrahepatic tumor or is a partial hepatectomy with sacrifice of a major portion of the liver. Even if the new margin of resection is negative, there is inconsistent evidence about whether survival is improved compared with that of patients for whom a positive proximal margin was not extended.58,59
Prognostic Considerations
Invasive carcinoma in the wall of a distal bile duct resection margin is considered a negative prognostic indicator, although not all reports concur with this assumption.60 High-grade dysplasia at the distal bile duct resection margin is of uncertain significance. Carcinoma in situ,61,62 which is encompassed in the spectrum of high-grade dysplasia, at the bile duct resection margin does not have a strong adverse effect on patient survival.63 Carcinoma in situ may be papillary or nonpapillary and shows nuclear pseudostratification, a high nuclear-to-cytoplasmic ratio, and enlarged atypical nuclei, but it remains confined to the basement membrane and does not invade the underlying stroma.
Extension of the proximal or distal surgical resection margins for a frozen section finding of high-grade dysplasia (i.e., carcinoma in situ) may not be justified, because progression of in situ lesions seems to take several years.63 Residual carcinoma in situ may cause late local disease recurrence, whereas residual invasive carcinoma causes early local recurrence.
Surgical intervention for highly suspect hilar masses is justified because there are patients for whom a definitive preoperative diagnosis of malignancy cannot be made with certainty.46,64–67 In this instance, intraoperative frozen sections may instead document fibrosing cholangitis, erosive inflammation, a benign granular cell tumor, granulomatous disease, or lymphoplasmacytic sclerosing pancreatitis and cholangitis.68–70
Pancreaticoduodenectomy (Whipple) Procedure
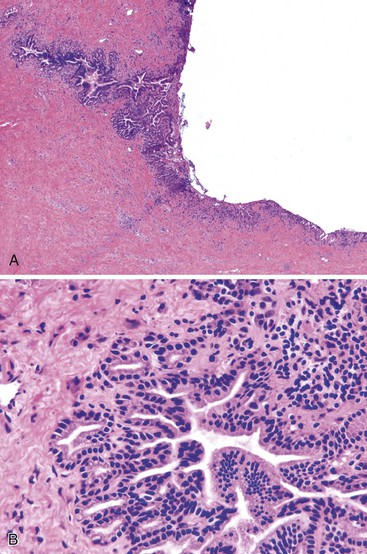
Intraoperative frozen section evaluation of proximal bile duct margins is common during resection of tumors of the middle or distal biliary tract, pancreas, or ampullary region.55 Unfortunately, obstructing tumors in the middle and distal biliary tract often lead to dilation and inflammation of the proximal common bile duct. The bile duct epithelium may become markedly reactive and inflamed, showing loss of maturation, elongation and stratification of cell nuclei, and increased mitoses (Fig. 33.17). If the common bile duct has been stented before surgery, ulceration, epithelial metaplasia, increased nuclear atypia, increased wall thickness, nerve entrapment, and smooth muscle hyperplasia may be seen.71 The peribiliary glands also may show hyperplasia, inflammation, and edema.
In contrast, malignant cells may be identified in the stroma or muscle layer of the wall of the bile duct. The criteria for infiltration of malignant glands into the bile duct wall are similar to those used to examine the distal bile duct margin of a proximal tumor resection. Although outcome data are limited, the finding of in situ carcinoma or high-grade dysplasia at the proximal bile duct margin of a pancreaticoduodenectomy specimen may not necessarily impart a significant negative impact on postoperative outcome.55
Pancreatic Margins
The most powerful independent predictors of favorable long-term survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma are a tumor diameter less than 3 cm, negative resection margins, absence of lymph node metastases, well-differentiated histology, and lack of postoperative complications.28 The surgical pancreatic margin should be assessed intraoperatively to look for adenocarcinoma.72,73
At the time of intraoperative frozen section consultation, when special immunohistochemical or molecular studies are not readily available, several features help to determine whether a proliferation of glands detected at a parenchymal margin is malignant74,75 (see Chapter 40). Even in the setting of fibrosis and inflammation, non-neoplastic glands retain a lobular architecture, exhibit a maximum nuclear size variation of 3 : 1, and reveal an absence of mitoses and enlarged, irregular nucleoli. Cancerous glands are usually arranged in a haphazard fashion. Cancerous glands are larger than benign ducts, are more irregularly distributed, may show irregular branching, and may be located adjacent to muscular arteries without intervening pancreatic parenchyma.
Perineural or vascular invasion always implies malignancy. Marked variability in nuclear size, exceeding 4 : 1 among cells and the finding of necrotic debris in the lumen of a gland are features of malignancy. Incomplete gland lumens (i.e., glands that not completely lined by an epithelial layer) and glands that are in direct contact with adipose tissue without intervening stroma indicate malignancy.
Three grades of pancreatic intraepithelial neoplasia (PanIN) are recognized on the basis of architectural and nuclear atypia (see Chapter 40). At the time of frozen section evaluation, PanIN may be identified in more than 50% of resections for pancreatic ductal adenocarcinoma.76 Authorities have substantially agreed that extension of the pancreatic surgical resection is not indicated when low-grade lesions (PanIN-1 or PanIN-2) are identified at a surgical margin.77 However, it has been demonstrated that even the finding of high-grade PanIN (PanIN-3) at a frozen section parenchymal margin does not negatively affect postoperative survival.76 Only invasive carcinoma at a frozen section margin is a negative prognostic indicator of postoperative survival, and it may justify extension of the surgical resection margin.
Intraductal Papillary Mucinous Neoplasms
IPMNs of the pancreas may be suspected before surgery on the basis of endoscopic, cytologic, or radiographic findings. However, preoperative imaging is not reliable for precise evaluation of tumor extension, and preoperative biopsies often underestimate tumor grade.78–80
At the time of pancreaticoduodenectomy or distal pancreatectomy, intraoperative frozen section consultation is often performed. On receiving a request to perform a frozen section analysis of a tissue margin in a patient with an IPMN, the pathologist should determine whether the ductal epithelium is normal or exhibits adenomatous, borderline, or carcinoma in situ features33 (see Chapter 40). The pancreatic parenchyma should also be examined for the presence or absence of invasive carcinoma. Long-term survival of patients without invasive tumor is excellent, but invasive carcinoma carries a 5-year survival rate of approximately 65% and a 10-year survival rate of approximately 35%.35
The histologic classification of IPMNs is provided in Chapter 40. In brief, four types are recognized: intestinal type, pancreaticobiliary type, gastric foveolar type, intraductal papillary oncocytic neoplasm, and intraductal tubulopapillary neoplasm.38,81 The most common type, the intestinal type, shows a villous growth pattern similar to that of villous adenoma of the colon. When this type of IPMN becomes invasive, the invasive component resembles mucinous (colloid) carcinoma, with pools of extracellular mucin containing single cells or strands of neoplastic glandular epithelium with or without signet ring cells. The pancreaticobiliary type of IPMN shows complex, arborizing intraductal papillae, and its invasive component resembles conventional pancreatic ductal adenocarcinoma. The oncocytic type of IPMN shows complex intraductal papillae similar to the pancreaticobiliary type, but the lining cells have a granular eosinophilic cytoplasm. Numerous goblet cells may be seen. The gastric type exhibits papillary projections lined by epithelial cells resembling gastric surface epithelial cells and shows pyloric gland–like structures at the base of the papillae. The gastric type has been reported only in BD-IPMNs.81 The fifth type of intraductal tubulopapillary neoplasm is the most recently recognized, associated with dilated pancreatic ducts and predominant tubulopapillary growth of neoplastic cells.
IPMNs may exhibit low-, intermediate-, or high-grade dysplasia, or they may harbor invasive adenocarcinoma. The criteria for invasive carcinoma are the same as those stated previously for ductal adenocarcinoma. MD-IPMNs have a higher risk of associated invasive adenocarcinoma than BD-IPMNs. However, if untreated, both forms of IPMN may progress to invasive carcinoma and eventually metastasize. Complete surgical resection of the IPMN is considered the best approach to prevent the development of an invasive cancer.38 Frozen section evaluation of the pancreatic resection margin is performed to secure complete resection of the IPMN lesion.
The pancreatic surgical resection margin should be extended if there is high-grade dysplasia or invasive carcinoma.82–88 Extension of the margin occurs in approximately 30% of cases examined by frozen section.89–91 However, there are no management standards for IPMNs with low- or intermediate-grade dysplasia (also referred to as adenoma or borderline in the literature) found at frozen section evaluation of the pancreatic resection margin38 because the frequency of recurrence of invasive cancer in the remnant pancreas is low (0% to 8%).92–94 The decision to resect more pancreas if the resection margin shows only low- or intermediate-grade dysplasia may rest on clinical factors such as patient comorbidities and age and the preferences of the surgeon and patient.
A second consideration is the discontinuous nature of IPMNs. Intraoperative pancreatoscopy (i.e., wirsungoscopy)—endoscopic examination of the intact pancreatic duct—is performed in some highly specialized medical centers.95–97 Staged biopsies of duct epithelium are performed, permitting detection of discontinuous intraductal lesions. However, this technique is feasible only when the main pancreatic duct is dilated. A more feasible technique is intraoperative cytology, for which samples are obtained from residual pancreas even if the frozen section margin is histologically negative for IPMN.98 Cytologic positivity for carcinoma in a sample obtained from residual pancreatic tissue is justification for extension of the surgical resection, potentially up to a total pancreatectomy.98,99
The pathologist should be aware that PanIN is a common lesion in the older adult patient. An incidental PanIN lesion identified on frozen section should not be confused with pursuit of high-grade lesions in an IPMN. The gross features of the specimen can be helpful in distinguishing whether an intraepithelial mucinous lesion is an incidental PanIN or part of a more worrisome IPMN lesion.38 The goal of intraoperative frozen section evaluation of pancreatic margins is not to overreact, but to guide resection of grossly visible lesions that exhibit a high-grade IPMN or invasive carcinoma at the surgical resection margin.
Intraoperative Pancreatic Biopsy
Differentiating pancreatic carcinoma from chronic pancreatitis may be requested for an intraoperative specimen obtained in a cutting-needle biopsy or an incisional biopsy. Distinguishing benign from malignant glands in frozen section specimens is discussed in Chapters 39 and 40.48,100,101
Gallbladder
Frozen section assessment of the gallbladder is rare and indicated only for the unexpected finding of a polypoid mucosal lesion or a suspicious thickening of the gallbladder wall.102 Identification of a malignant lesion determines conversion from a laparoscopic cholecystectomy to an open procedure to allow lymph node exploration and resection of the liver bed.103 Frozen section diagnosis is most reliable for mural lesions, with concurrence with permanent section findings in more than 95% of cases.104 Frozen section assessment of polypoid or exophytic lesions for carcinoma is less reliable.104,105 Fortunately, simple cholecystectomy should be curative for superficial lesions. Determining the depth of invasion is best done by permanent sections.