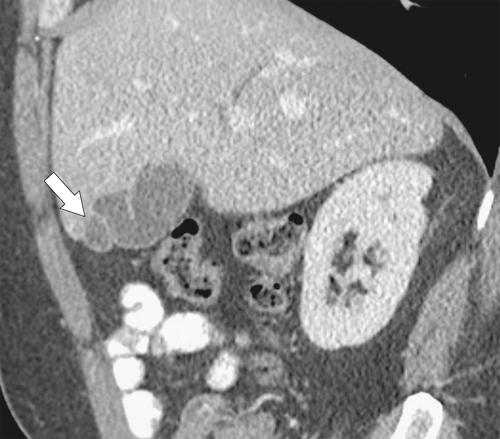
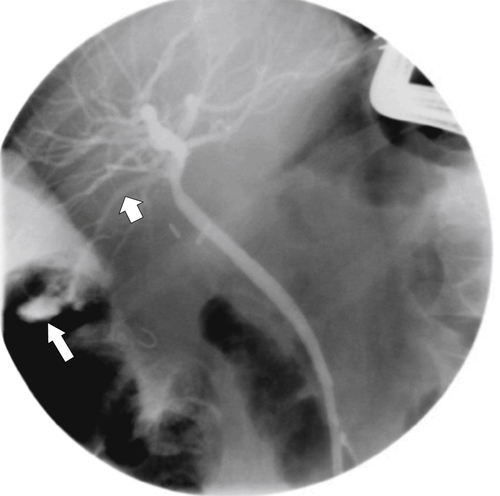
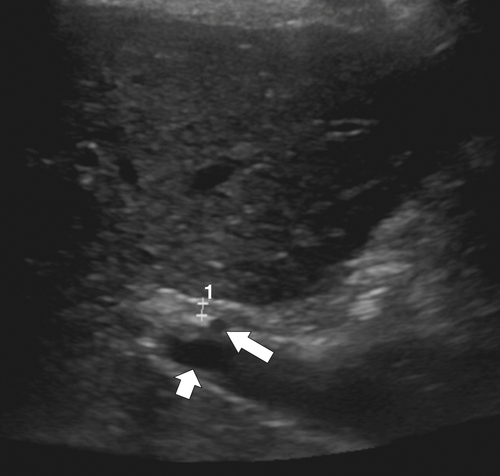
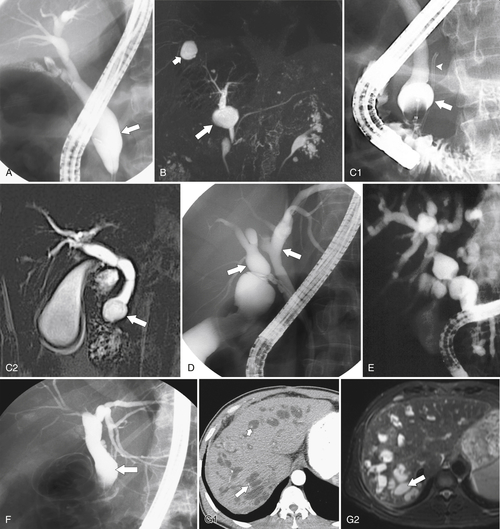
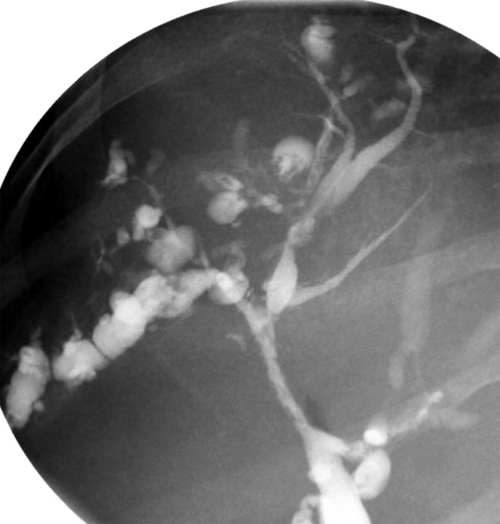
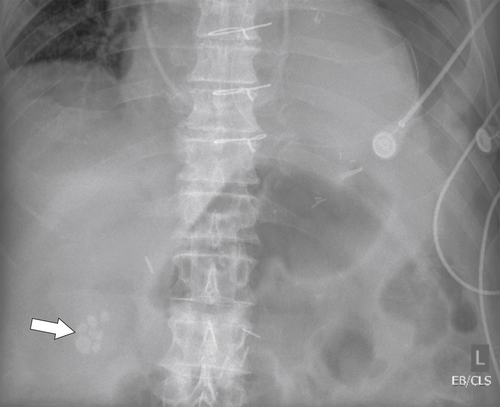
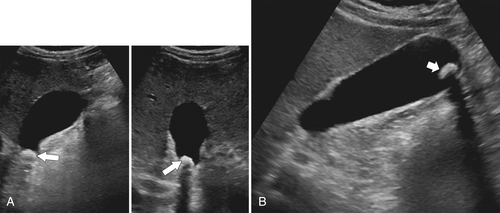
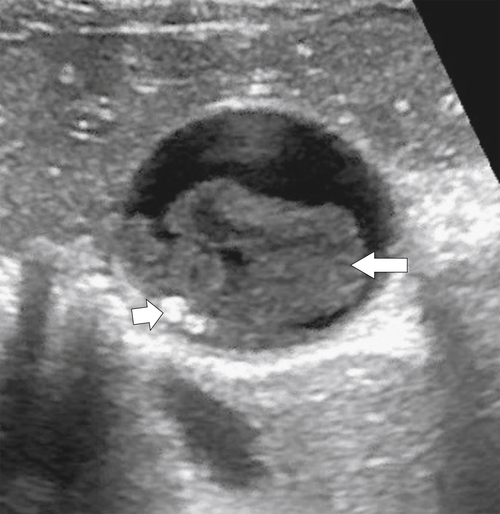
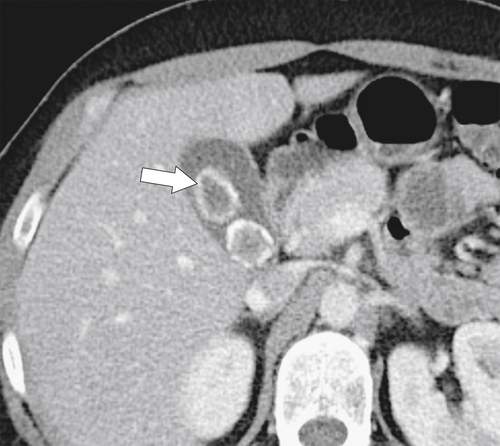
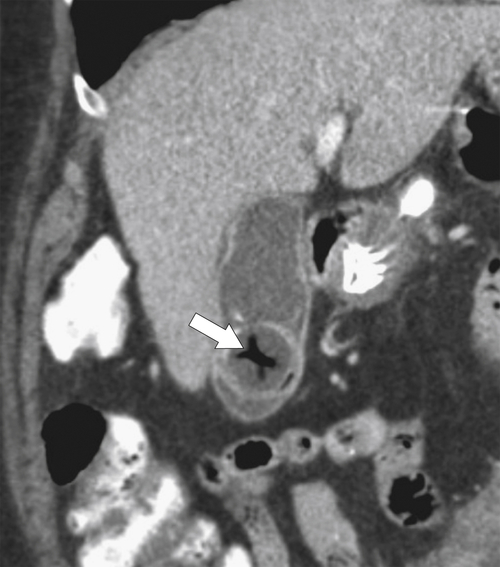
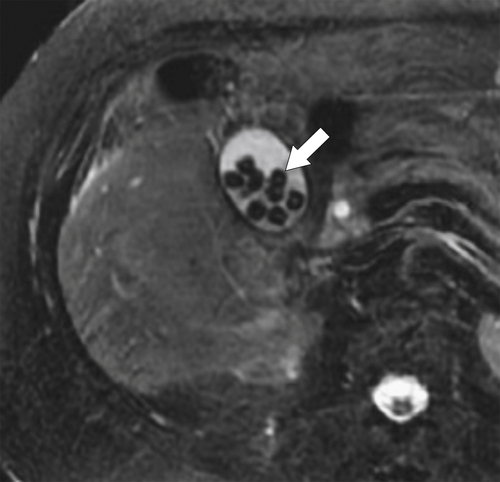
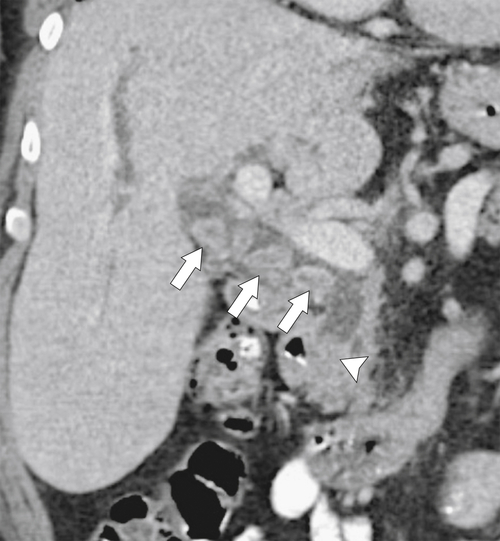
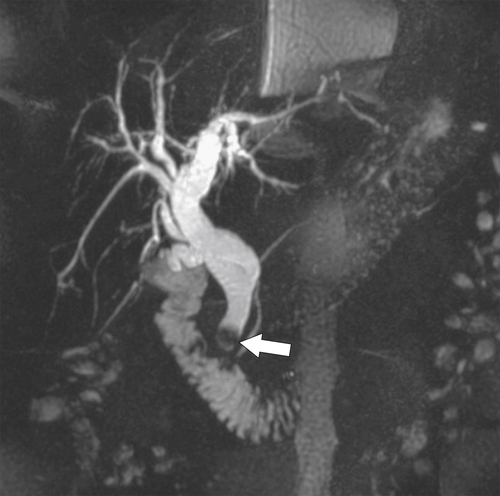
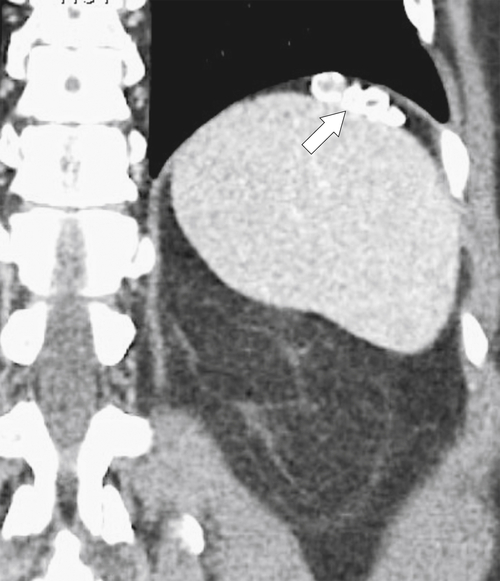
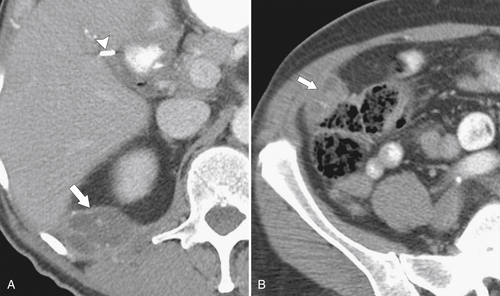
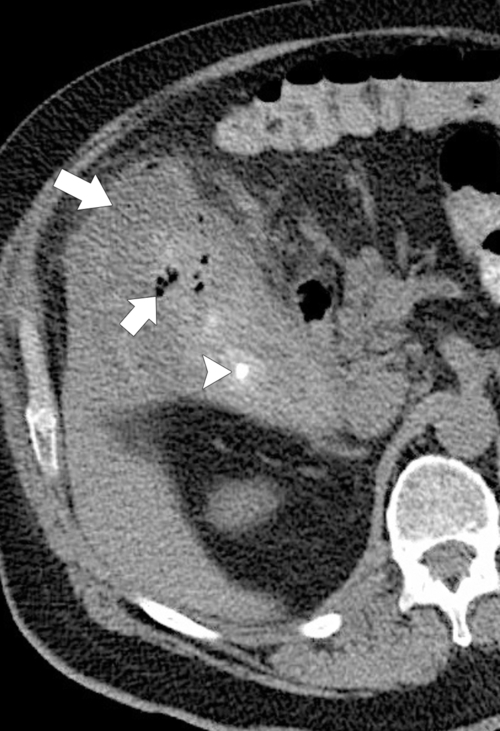
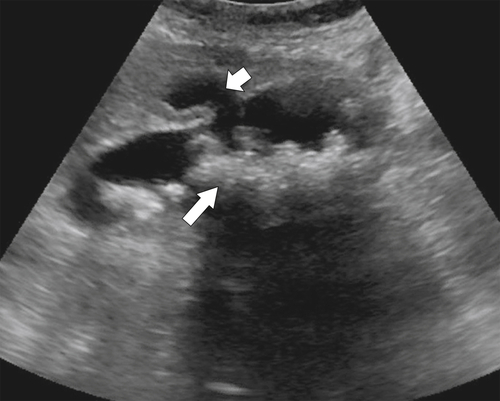
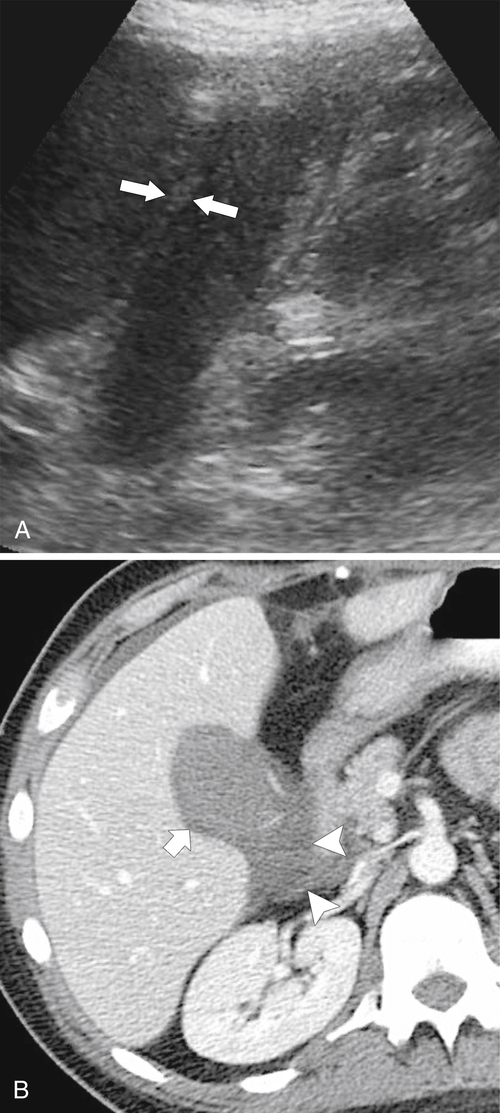
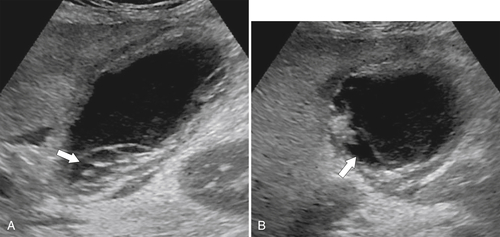
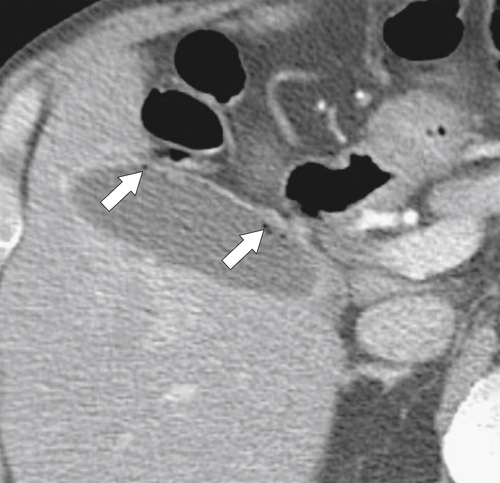
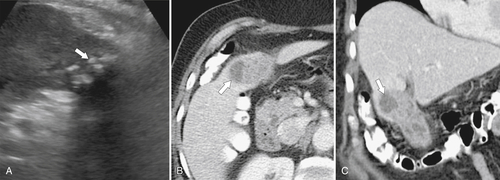
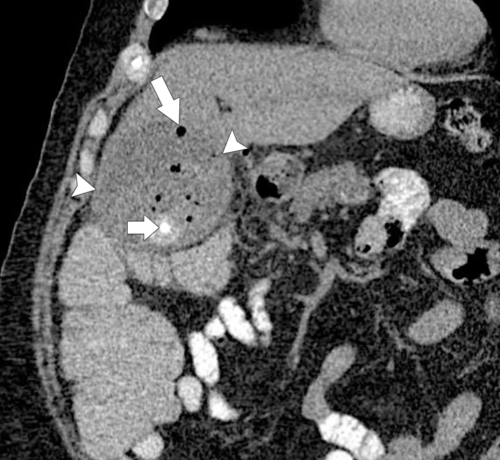
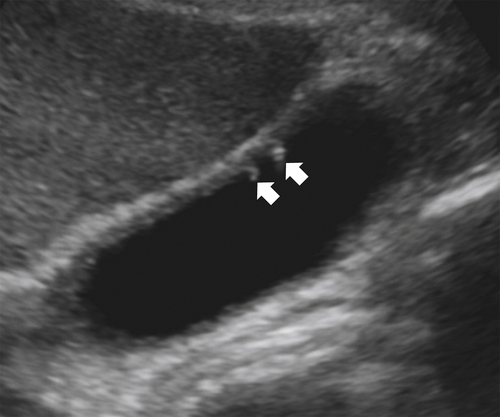
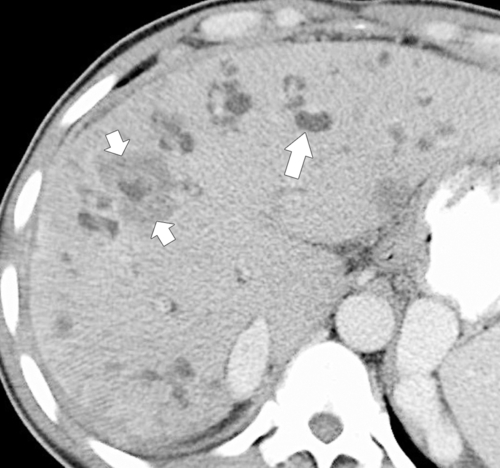
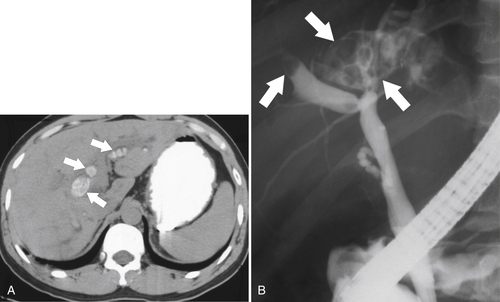
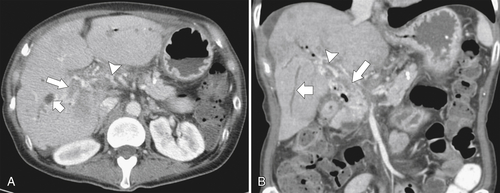
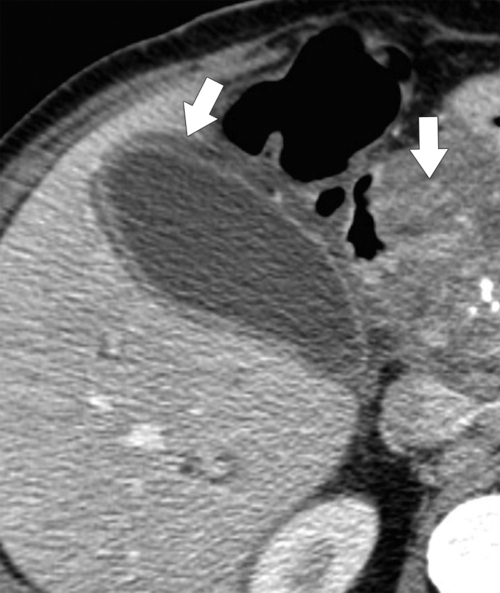
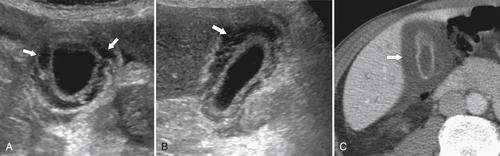
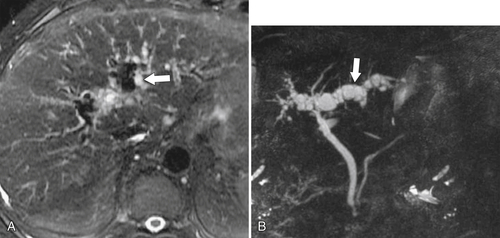
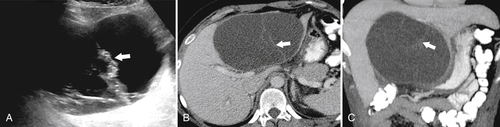
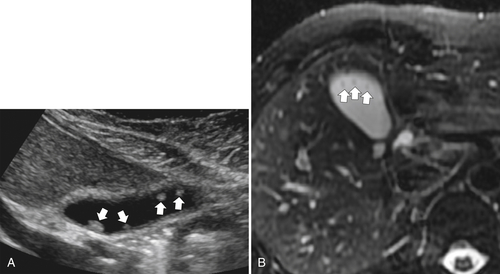
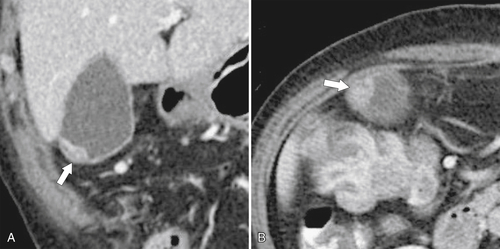
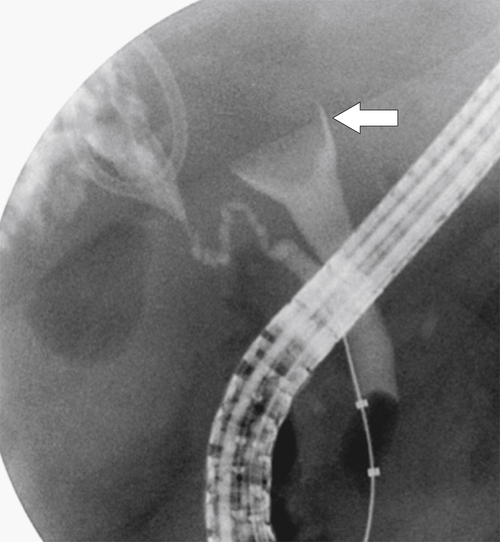
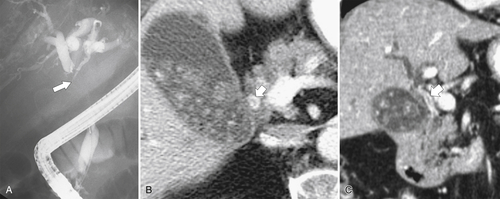
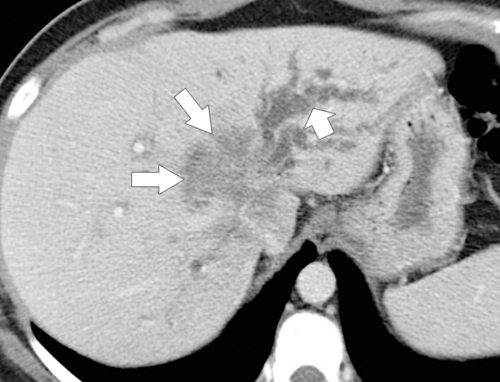
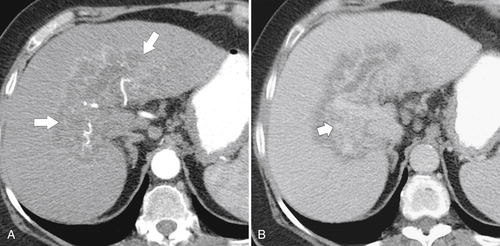
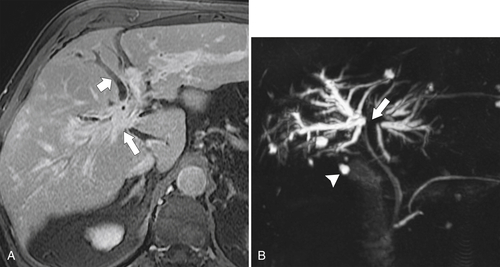
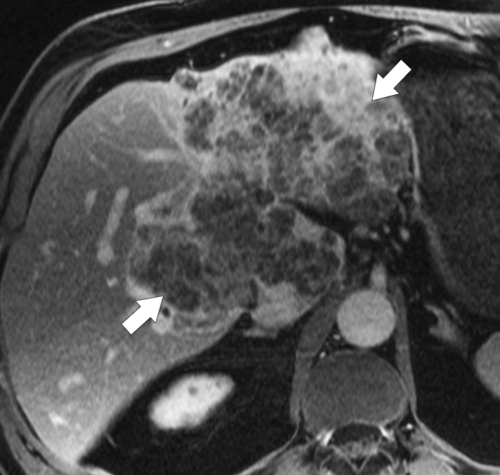
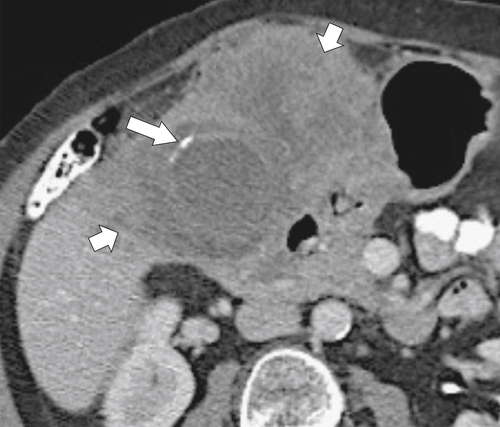
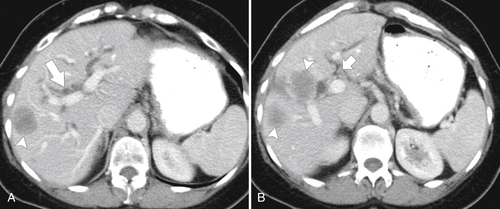
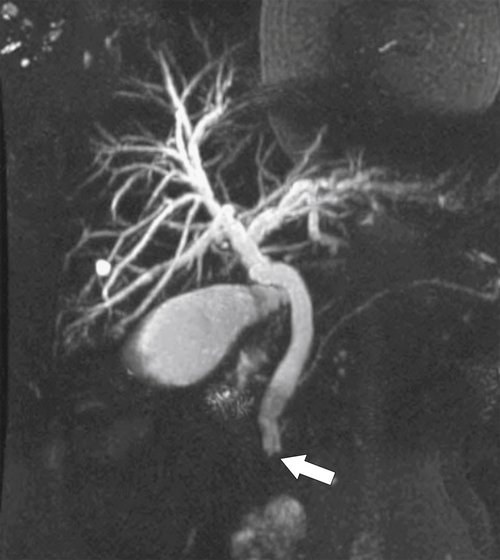
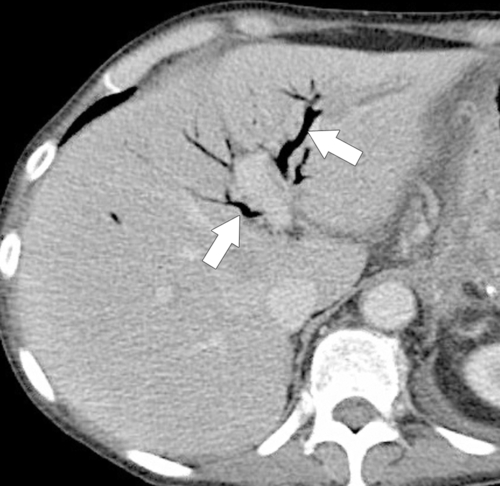
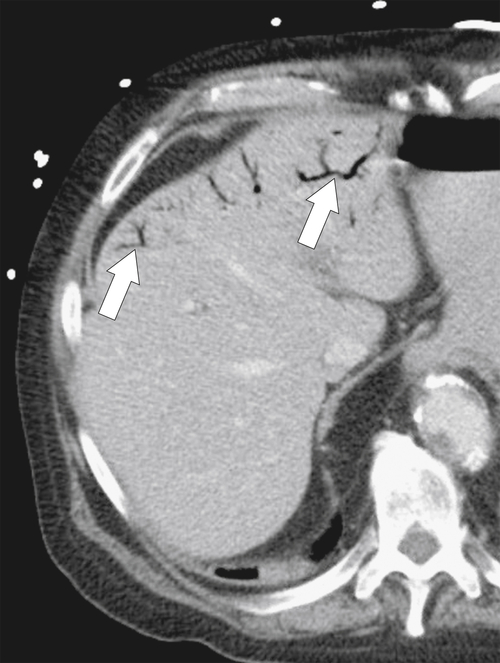
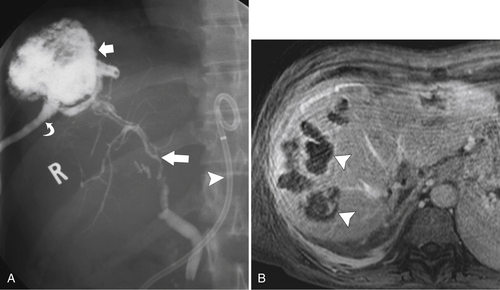
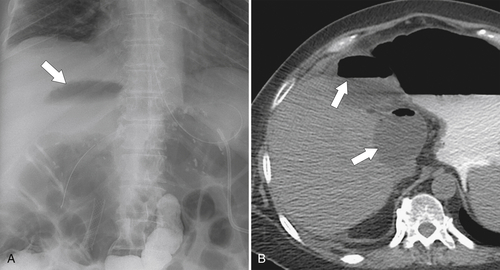
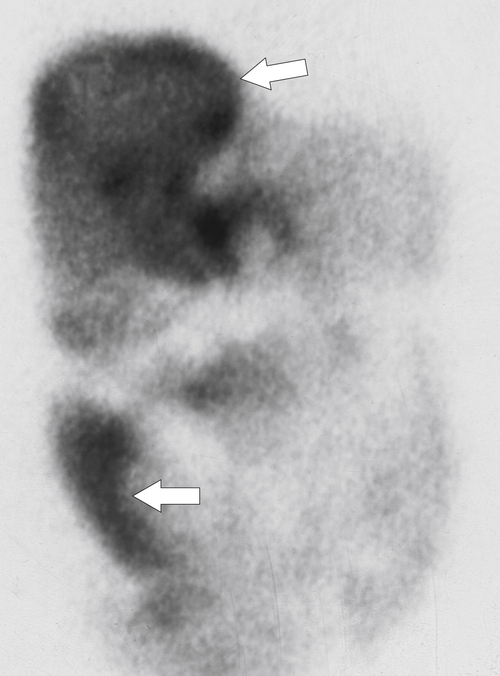
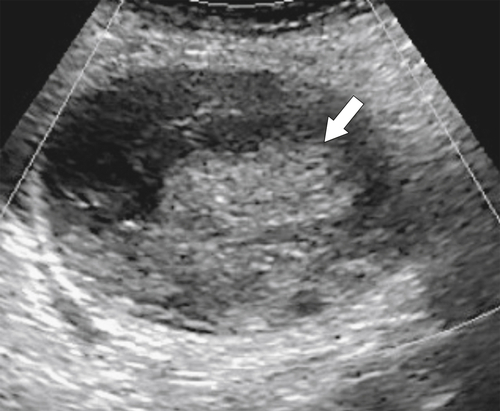
Ahualli J. The double duct sign. Radiology 2007;244(1):314–315.
Altun E. et al. Acute cholecystitis: MR findings and differentiation from chronic cholecystitis. Radiology 2007;244(1):174–183. doi:10.1148/radiol.2441060920.
Anderson S.W. et al. Accuracy of MDCT in the diagnosis of choledocholithiasis. AJR 2006;187(1):174–180.
Anderson S.W. et al. Detection of biliary duct narrowing and choledocholithiasis: accuracy of portal venous phase multidetector CT. Radiology 2008;247(2):418–427.
Arai K. et al. Dynamic CT of acute cholangitis: early inhomogeneous enhancement of the liver. AJR 2003;181(1):115–118.
Bader T.R. et al. MR imaging features of primary sclerosing cholangitis: patterns of cirrhosis in relationship to clinical severity of disease. Radiology 2003;226(3):675–685.
Bennett G.L. et al. CT findings in acute gangrenous cholecystitis. AJR 2002;178:275–281.
Bennett G.L. et al. Ultrasound and CT evaluation of emergent gallbladder pathology. Radiol Clin North Am 2003;41(6):1203–1216.
Berk R.N. et al. Carcinoma in the porcelain gallbladder. Radiology 1973;106:29–31.
Bilgin M. et al. Hepatobiliary and pancreatic MRI and MRCP findings in patients with HIV infection. AJR 2008;191(1):228–232.
Brancatelli G. et al. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics 2005;25(3):659–670.
Bucceri A.M. et al. Common bile duct caliber following cholecystectomy: two-year sonographic survey. Abdom Imaging 1994;19:251–258.
Catalano O. et al. Complications of biliary and gastrointestinal stents: MDCT of the cancer patient. AJR 2012;199:W187–W196. doi:10.2214/AJR.11.7145.
Catalano O.A. et al. MR imaging of the gallbladder: a pictorial essay. Radiographics 2008;28(1):135–155: quiz 324.
Chan W.C. et al. Gallstone detection at CT in vitro: effect of peak voltage setting. Radiology 2006;241(2):546–553.
Chan Y.L. et al. Choledocholithiasis: comparison of MR cholangiography and endoscopic retrograde cholangiography. Radiology 1996;200:85–91.
Chavhan G.B. et al. Pediatric MR cholangiopancreatography: principles, techniques, and clinical applications. Radiographics 2008;28(7):1951–1962.
Chen R.C. et al. Value of ultrasound measurement of gallbladder wall thickness in predicting laparoscopic operability prior to cholecystectomy. Clin Radiol 1995;50:570–578.
Choi E. et al. Duplication of the extrahepatic bile duct with anomalous union of the pancreaticobiliary ductal system revealed by MR cholangiopancreatography. Br J Radiol 2007;80(955):e150–154.
Choi J.Y. et al. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR 2008;191(5):1448–1457.
Chung Y.E. et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29(3):683–700. doi:10.1148/rg.293085729.
Corwin M.T. et al. Incidentally detected gallbladder polyps: is follow-up necessary? Long-term clinical and US analysis of 346 patients. Radiology 2011;258(1):277–282: Published online August 9, 2010, doi: 10.1148/radiol.10100273.
Darge K. et al. MR imaging of the abdomen and pelvis in infants, children, and adolescents. Radiology 2011;261(1):12–29. doi:10.1148/radiol.11101922.
Dave M. et al. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology 2010;256:387–396.
Dohke M. et al. Anomalies and anatomic variants of the biliary tree revealed by MR cholangiopancreatography. AJR 1999;173(5):1251–1254.
Elsayes K.M. et al. Gastrointestinal manifestation of diabetes mellitus: spectrum of imaging findings. J Comput Assist Tomogr 2009;33(1):86–89.
Fuks D. et al. Acute cholecystitis: preoperative CT can help the surgeon consider conversion from laparoscopic to open cholecystectomy. Radiology 2012;263(1):128–138: Published online February 13, 2012, doi: 10.1148/radiol.12110460.
Furlan A. et al. Gallbladder carcinoma update: multimodality imaging evaluation, staging, and treatment options. AJR 2008;191(5):1440–1447.
Ginat D. et al. Incidence of cholangitis and sepsis associated with percutaneous transhepatic biliary drain cholangiography and exchange: a comparison between liver transplant and native liver patients. AJR 2011;196:W73–W77. doi:10.2214/AJR.09.3925.
Grand D. et al. CT of the gallbladder: spectrum of disease. AJR 2003;183:163–170.
Gupta R.T. et al. Dynamic MR imaging of the biliary system using hepatocyte-specific contrast agents. AJR 2010;195:405–413. doi:10.2214/AJR.09.3641.
Haller J.O. Sonography of the biliary tract in infants and children. AJR 1991;157:1051–1058.
Han J.K. et al. Cholangiocarcinoma: pictorial essay of CT and cholangiographic findings. Radiographics 2002;22(1):173–187.
Hashimoto M. et al. Evaluation of biliary abnormalities with 64-channel multidetector CT. Radiographics 2008;28(1):119–134. doi:10.1148/rg.281075058.
Heffernan E.J. et al. Recurrent pyogenic cholangitis: from imaging to intervention. AJR 2009;192(1):W28–35.
Hoeffel C. et al. Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. Radiographics 2006;26(6):1603–1620. doi:10.1148/rg.266055730.
Jutras J.A. Hyperplastic cholecystoses. AJR 1960;83:795–827.
Kiewiet J.J.S. et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology July 12, 2012: Published online. http://dx.doi.org/10.1148/radiol.12111561.
Kim J.H. et al. MR cholangiography in symptomatic gallstones: diagnostic accuracy according to clinical risk group. Radiology 2002;224(2):410–416: Published online May 17, 2002, doi: 10.1148/radiol.2241011223.
Kim J.H. et al. CT findings of cholangiocarcinoma associated with recurrent pyogenic cholangitis. AJR 2006;187:1571–1577. doi:10.2214/AJR.05.0486.
Kim J.Y. et al. Differentiation between biliary cystic neoplasms and simple cysts of the liver: accuracy of CT. AJR 2010;195:1142–1148. doi:10.2214/AJR.09.4026.
Kim J.Y. et al. Spectrum of biliary and nonbiliary complications after lapatoscopic cholecystectomy: radiologic findings. AJR 2008;191(3):783–789.
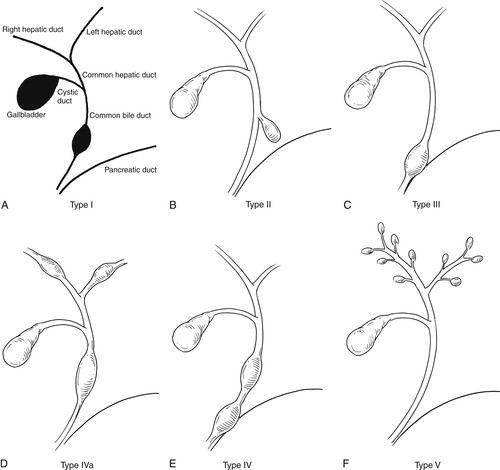
Kim O.H. et al. Imaging of the choledochal cyst. Radiographics 1995;15:69–88.
Kim S.J. et al. Peripheral mass–forming cholangiocarcinoma in cirrhotic liver. AJR 2007;189:1428–1434. doi:10.2214/AJR.07.2484.
Knowlton J.Q. et al. Imaging of biliary tract inflammation: an update. AJR 2008;190(4):984–992.
Kumar A. et al. Carcinoma of the gallbladder: CT findings in 50 patients. Abdom Imaging 1994;19:304–310.
Lee W.J. et al. Radiologic spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics 2001;21:S97–S116: Spec No.
Levy A.D. et al. Caroli’s disease: radiologic spectrum with pathologic correlation. AJR 2002;179(4):1053–1057.
Lim J.H. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR 2003;181:819–827.
Lim J.H. et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics 2004;24(1):53–66: discussion 66-67.
Lim J.H. et al. Parasitic diseases of the biliary tract. AJR 2007;188(6):1596–1603.
Lim J.H. et al. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom Imaging 2008;33(2):157–165.
Lim J.H. et al. Intraductal papillary mucinous tumors of the bile ducts. Radiographics 2004;24:53–67.
Malet P.F. et al. Gallstone composition in relation to buoyancy at oral cholecystography. Radiology 1990;177:167–169.
Mall J.C. et al. Caroli’s disease associated with congenital hepatic fibrosis and renal tubular ectasia. Gastroenterology 1974;66:1029–1035.
Martel J.P. et al. Melanoma of the gallbladder. Radiographics 2009;29(1):291–296.
Menias C.O. et al. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics 2008;28(4):1115–1129. doi:10.1148/rg.284075148.
Miller F.H. et al. Contrast-enhanced helical CT of choledocholithiasis. AJR 2002;181:125–130.
Miller W.J. et al. Imaging findings in Caroli’s disease. AJR 1995;165:333–340.
Mortelé K.J. et al. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics 2006;26(3):715–731. doi:10.1148/rg.263055164.
O’Connor O.J. et al. Imaging of cholecystitis. AJR 2011;196:W367–W374. doi:10.2214/AJR.10.4340.
O’Connor O.J. et al. Structured review: imaging of biliary tract disease. AJR 2011;197:W551–W558. doi:10.2214/AJR.10.4341.
Patel H.T. et al. MR cholangiopancreatography at 3.0 T. Radiographics 2009;29:6. doi:10.1148/rg.296095505: 1689-1706.
Reinhold C. et al. MR cholangiopancreatography: potential clinical applications. Radiographics 1996;16:309–331.
Rizzo R.J. et al. Congenital abnormalities of the pancreas and biliary tree in adults. Radiographics 1995;15:49–68.
Rybicki F.J. The WES sign. Radiology 2000;214(3):881–882.
Sainani N.I. Cholangiocarcinoma: current and novel imaging techniques. Radiographics 2008;28(5):1263–1287.
Santiago I. et al. Congenital cystic lesions of the biliary tree. AJR 2012;198:825–835. doi:10.2214/AJR.11.7294.
Savader S.J. et al. Choledochal cysts: classification and cholangiographic appearance. AJR 1991;156:327–331.
Schuster D.M. et al. Magnetic resonance cholangiography. Abdom Imaging 1995;20:353–361.
Shakespear J.S. et al. CT findings of acute cholecystitis and its complications. AJR 2010;194:1523–1529. doi:10.2214/AJR.09.3640.
Shanbhogue A.K.P. et al. Benign biliary strictures: a current comprehensive clinical and imaging review. AJR 2011;197:W295–W306. doi:10.2214/AJR.10.6002.
Shanmugam V. et al. Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging? Br J Radiol 2005;78(934):888–893.
Sheng R. et al. Cholangiographic features of biliary strictures after liver transplantation for primary sclerosing cholangitis: evidence of recurrent disease. AJR 1996;166:1109–1116.
Shin S.M. et al. Biliary abnormalities associated with portal biliopathy: evaluation on MR cholangiography. AJR 2007;188:W341–W347. doi:10.2214/AJR.05.1649.
Silva A.C. et al. MR cholangiopancreatography: improved distention with intervenous morphine administration. Radiographics 2004;24:677–687.
Silverthorn K. Sonographic follow-up of patients with gallbladder polyps. AJR 2001;177:467.
Smith E.A. et al. Cross-sectional imaging of acute and chronic gallbladder inflammatory disease. AJR 2009;192(1):188–196.
Sugita R. et al. Periampullary tumors: high-spatial-resolution MR imaging and histopathologic findings in ampullary region specimens. Radiology 2004;231(3):767–774.
Taourel P. et al. Anatomic variants of the biliary tree: diagnosis with MR cholangiopancreatography. Radiology 1996;199:521–527.
Valls C. et al. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR 2005;184:812–820.
Vermani N. et al. MR cholangiopancreatographic demonstration of biliary tract abnormalities in AIDS cholangiopathy: report of two cases. Clin Radiol 2009;64(3):335–338.
Ward J. et al. Bile duct strictures after heptobiliary surgery: assessment with MR cholangiography. Radiology 2004;231:101–108.
Weltman D.I., Zeman R.K. Acute diseases of the gallbladder and biliary ducts. Radiol Clin North Am 1994;32:933–954.
Wiot J.F., Felson B. Gas in the portal venous system. AJR 1961;86:920–929.
Yeh B.M. et al. MR imaging and CT of the biliary tract. Radiographics 2009;29:6. doi:10.1148/rg.296095514: 1669-1688.
Yu J. et al. Congenital anomalies and normal variants of the pancreaticobiliary tract and the pancreas in adults: part 1, biliary tract. AJR 2006;187(6):1536–1543.
Yun E.J. et al. Gallbladder carcinoma and chronic cholecystitis: differentiation with two-phase spiral CT. Abdom Imaging 2003;29(1):102–108.