Chapter 80 Gait Analysis in Anterior Cruciate Ligament Deficient and Reconstructed Knees
Introduction
Anterior cruciate ligament (ACL) rupture is a common injury of the knee joint that usually results in surgical reconstruction.1,2 The goal of ACL reconstruction and subsequent rehabilitation is to restore the knee to an acceptable muscular strength and joint stability.3,4 The stability of the knee thought to have an ACL injury is traditionally evaluated with an arthrometer (i.e., KT-1000) while the patient is in a standard static position. The arthrometer provides the clinician with a quantitative measure of the amount of passive movement between the femur and the tibia. A minimal amount of joint laxity during the test is considered to be clinically and functionally acceptable. However, such an evaluation is a measure of passive joint stability and does not provide a measure of the joint’s stability during daily physical activities.5–7 Dynamic functional joint stability is defined as the condition in which the joint is stable during daily physical activities.5 Previous research has indicated that there is lack of a relationship between passive and dynamic functional joint stability.5,8–10
Recently, gait analysis has been used to quantify the dynamic functional knee stability after ACL reconstruction.11–14 Gait analysis can be defined as an advanced laboratory process by which present day electronics (i.e., video cameras) are used to integrate information from a variety of inputs in order to demonstrate and analyze the dynamics of gait (Fig. 80-1). For example, gait analysis can offer a more in-depth evaluation of movement patterns by providing information on each joint. Such information has also become common practice in many other orthopaedic areas where the effects of surgical procedures (i.e., joint arthroplasty, cerebral palsy) are evaluated to identify gains in mobility.15–19
The use of this technology allows the development of normal joint movement profiles that can be used to identify abnormalities, helping in this way to improve diagnosis, treatment, design, and performance of reconstructive surgery and rehabilitation programs. Gait analysis, using advanced computerized systems in conjunction with multiple high-speed (i.e., 200 frames per second) video cameras, can document three-dimensional (3D) knee joint movement profiles.20 Thus all six degrees of freedom of the knee joint can now be discerned, and the dynamic functional levels of individuals performing everyday activities can be objectively measured and evaluated. This is accomplished by obtaining data from surface markers that are placed on specific anatomical bony landmarks. The position of the markers in space is recorded, and then joint movement profiles can be acquired.
A possible limitation of gait analysis is that surface markers may not accurately represent the underlying bone motion during highly dynamic activities,21 as the markers are attached on the skin and not directly on the bone. As skin movement increases, the location of the marker and of the underlying bone differs. As a result, error is introduced.21–26 One way to avoid these limitations is to directly measure skeletal motion with intracortical pins.25 However, the applicability of this method is limited because the implantation of intracortical pins is a highly invasive procedure that may cause discomfort or pain to the patient and result in restriction of movements. In addition, implantation of intracortical pins is a method that is limited by the sample size, as an effective number of volunteers cannot be found.
These suggestions can solidify conclusions drawn from gait analysis. Thus gait analysis is widely accepted at the present time and is considered a well-established and reliable method.28,29 This methodology allows the in vivo evaluation of the ACL deficient and reconstructed knee during dynamic activities (i.e., walking, pivoting), something that static measures (i.e., arthrometer) are unable to do.
Importance of in Vivo Biomechanical Research to Quantify Success of Surgical Techniques
Example 1: Tibial Rotation
Our investigations have examined knee joint rotational movement patterns during high- and low-demand activities in both ACL deficient and reconstructed individuals. In our first study, we evaluated ACL deficient and reconstructed individuals during a low-demand activity such as walking.14 We examined 13 individuals with unilateral ACL deficiency, 21 individuals who had undergone ACL reconstruction, and 10 healthy controls. ACL reconstruction was done arthroscopically using a bone–patellar tendon–bone (BPTB) autograft. We found that the ACL deficient group exhibited significantly increased tibial rotation range of motion during the initial swing phase of the gait cycle when compared with the ACL reconstructed and control groups. Thus our results demonstrated that ACL deficiency produced rotational differences at the knee during walking. These differences did not exist when we compared the ACL reconstructed group with the control. Thus, in this low-demand activity, the surgical reconstruction restored tibial rotation to normal levels.
Next, we wanted to identify whether this is also the case in a higher-demand activity that can apply increased rotational loading at the knee. Therefore we examined 18 ACL reconstructed individuals and 15 controls during a high-demand activity (descending stairs and subsequent pivoting).27 The ACL reconstruction was done arthroscopically, again using a BPTB autograft. The evaluation was performed at an average of 12 months after reconstruction. The individuals were asked to descend three steps and then immediately pivot on the landing leg at 90 degrees and walk away from the stairway while kinematic data were collected. The tibial rotation range of motion during the pivoting period was found to be significantly larger in the ACL reconstructed leg compared with the contralateral intact leg and the healthy control. No significant differences were found between the healthy control leg and the intact leg of the ACL reconstructed group. Therefore our results demonstrated that tibial rotation remained abnormal and significantly increased 1 year after ACL reconstruction during high-demand activities such as pivoting after descending from stairs.
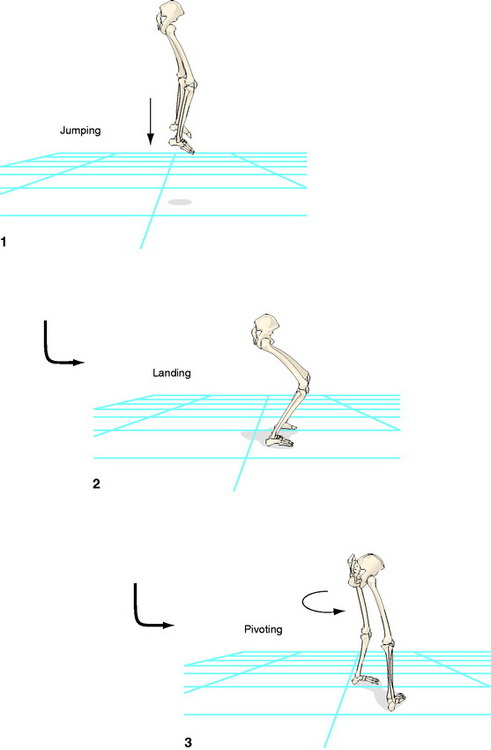
To verify our findings, we performed an additional experiment in which we evaluated another high-demand activity.30 Data were collected while the subjects jumped off a 40-cm platform and landed on the ground; following foot contact, they immediately pivoted at 90 degrees and walked away from the platform. We chose this activity because landing from a jump is a task that places higher demands on the knee than walking or even stepping down.31,32 We combined landing with a subsequent pivoting to create rotational loads on the knee. The subjects were 11 patients, all ACL reconstructed with the same arthroscopic technique using a BPTB autograft, 1 year after the surgery; 11 ACL deficient subjects who had sustained the injury more than 1 year prior to testing; and 11 controls. The same dependent variable was evaluated as in the previous study.27 Both the reconstructed leg of the ACL group and the deficient leg of the ACL deficient group had significantly larger tibial rotation values than in the healthy control group. We also found no significant differences between the deficient leg of the ACL deficient group and the reconstructed leg of the ACL reconstructed group. It was concluded that current ACL reconstruction using the BPTB autograft is inadequate to restore excessive tibial rotation during an activity such as landing and subsequent pivoting, which practically simulates sport activities.
Next, we wanted to identify whether tibial rotation remains excessive for a longer period: 2 years following the reconstruction. We speculated that it is possible adaptations will set in and the patients will compensate. Thus we performed a follow-up evaluation33 in nine ACL reconstructed subjects who had participated in our previous study.30 We examined them with the same methodology and for both activities that we used in our previous work.27,30We also incorporated a control group of 10 individuals. We found that tibial rotation remained significantly excessive even 2 years after the reconstruction. This result was verified with comparisons conducted with both the intact contralateral knees of our patient group and with the healthy controls. Furthermore, we found that tibial rotation of the intact knee of our patient group was similar to those recorded from the healthy control group.
In all of our previous work, ACL reconstruction was performed with a BPTB autograft. Thus it was logical to question whether tibial rotation will remain excessive if an alternative autograft is used. Such an autograft is the quadrupled hamstring tendon (semitendinous and gracilis [ST/Gr]). Originally we hypothesized that the ST/Gr autograft would be able to restore tibial rotation during our experimental protocols due to its superiority in strength and linear stiffness34–37 and because it is closer morphologically to the anatomy of the natural ACL.34–36 We examined 11 individuals who were ACL reconstructed with an ST/Gr autograft, 11 individuals who were ACL reconstructed with a BPTB autograft, and 11 healthy controls.38,39 The experimental protocol was identical to our previous studies. Tibial rotation was found to be significantly larger in both ACL reconstructed groups when compared with the healthy controls. Therefore our hypothesis was refuted, and we concluded that ACL reconstruction using the ST/Gr autograft is as inadequate as the one using the BPTB autograft in terms of restoring excessive tibial rotation.
The results of our studies were also supported by in vitro research work in which the biomechanical efficiency of the ACL reconstruction has also been questioned.40–43 These studies showed that ACL reconstruction was successful in limiting anterior tibial translation in response to an anterior tibial load but was insufficient to control a combined rotatory load of internal and valgus torque. Furthermore, our tibial rotational values were in close agreement with the in vitro work.40
Example 2: Dynamic Functional Knee Stability Using Nonlinear Analysis
Biomechanists have recently proposed that the use of stride-to-stride variability, defined as fluctuations on the walking movement patterns from one stride to the next, provides a quantitative measure of functional joint stability.44–47 This proposal is based on scientific evidence that neuromuscular pathology is related to an increased amount of stride-to-stride variability.44–47 Hence a “biomechanical” hypothesis has been formed in which neuromuscular pathology is related to an increased amount of variability and deterioration of functional stability. However, this biomechanical hypothesis lacks support in other medical domains. Numerous studies in diverse medical areas have shown that a decreased amount of variability is related to pathology. These investigations include medical domains such as heart rate irregularities, sudden cardiac death syndrome, blood pressure control, brain ischemia, and epileptic seizures.48–55 Hence a contradictory hypothesis has been proposed in which variability is described as “healthy flexibility.”56–58 These investigations indicate that variations in the behavior of the biological system may be necessary to provide flexible adaptations to everyday stresses placed on the human body. Alternatively, a lack of healthy flexibility is associated with rigidity and inability to adapt to stresses. Based on this logic, it is possible that injury or pathology can result in a loss of healthy flexibility that may not be regained despite surgical treatment (loss of complexity hypothesis).
This contradiction in the literature may be due to the usage of linear tools (i.e., standard deviation) to assess stride-to-stride variability.44–47 Linear tools only provide a measure of the amount of variability that is present in the gait pattern and may mask the true structure of motor variability. Masking occurs when strides are averaged to generate a “mean” picture of the subject’s gait. This averaging procedure may lose the temporal variations of the gait pattern. Additionally, the statistical processing of linear measures requires random and independent variations between subsequent strides.
Recent studies have overcome the problems of linear measures by using nonlinear tools such as the Approximate Entropy.59–62 These studies have determined that variations in the gait pattern are distinguishable from noise and have a deterministic origin. A deterministic origin indicates that stride-to-stride variations are neither random nor independent. Rather, these variations have a meaningful pattern that characterizes the behavior of the locomotive system. Linear tools are not able to provide such information. Thus the ability to quantify the characteristic features of these variations has been the strength of using nonlinear tools to support the “loss of complexity” hypothesis.
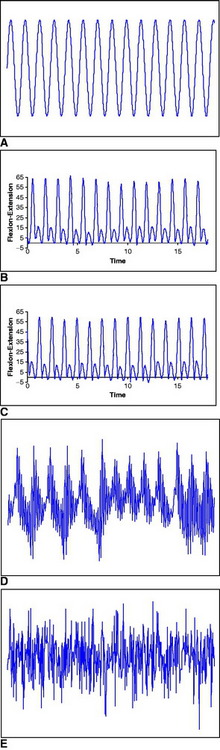
In our research work, we wanted to quantify knee joint stride-to-stride variability in ACL deficient and reconstructed individuals during a common daily activity such as walking. We used nonlinear analysis to explore whether the “loss of complexity” hypothesis can also be generalized to orthopaedic-related problems. In our first study62 we examined ten subjects with unilateral ACL deficiency who walked on a treadmill at different speeds while kinematic data were collected for 80 consecutive strides for each speed. The Approximate Entropy of the resultant knee joint flexion–extension kinematic data was calculated (Fig. 80-2). The ACL deficient knee had significantly smaller values than the intact contralateral knee. This indicated more regular and repeatable movement patterns for the injured knee and a decrease in healthy flexibility, as mentioned previously. Therefore nonlinear measures such as Approximate Entropy could prove to be of great importance in orthopaedics, providing the clinician with a mean of dynamical assessment of the effect of the pathology on movement and of the results of various therapeutic interventions. In addition, we believe that the “loss of complexity” hypothesis may be more universal than its proponents suggested. Pathologies of biorhythms are similar no matter whether one deals with the cardiovascular, nervous, or musculoskeletal system.
Next, we wanted to examine the effect of an ACL reconstruction on knee joint stride-to-stride variability.63 Again, we used the same nonlinear analysis, the Approximate Entropy. We examined six individuals who were ACL reconstructed with an ST/Gr autograft, seven individuals who were ACL reconstructed with a BPTB autograft, and 12 healthy controls. All subjects walked on a treadmill at a self-selected pace while kinematic data were collected from 120 consecutive strides. The control group had the smallest Approximate Entropy values, whereas the ST/Gr group had the largest. Significant differences were found only between the control and the ST/Gr reconstructed knees. We concluded that the ST/Gr reconstructed knee flexion/extension movement patterns during walking are less regular and repeatable than in the healthy control knee. However, the BPTB reconstructed knee seems to exhibit properties similar to the control. In addition, the results are also quite intriguing because they showed that the ACL reconstruction led to increased “flexibility” in the system. In the next section, we will present a theoretical explanation for this research outcome.
Advanced Theoretical Considerations
Development of Osteoarthritis Due to Excessive Tibial Rotation
Degeneration of the knee joint and eventual development of osteoarthritis have been associated with ACL deficiency. Longitudinal follow-up studies have shown that ACL deficiency leads to the development of chondral injuries, meniscal tears, degeneration of the articular cartilage, and eventually posttraumatic arthritis.64–68 However, similar problems have also been found longitudinally in the ACL reconstructed knee.69 Even more disturbingly, such findings have been seen shortly after the reconstruction as well.70 Therefore ACL reconstruction cannot protect the knee from progressing to degenerative change.
Based on our research results presented earlier, we would like to propose that excessive tibial rotation may be an abnormal movement mechanism that degenerates soft tissues (i.e., cartilage), resulting in osteoarthritis. We hypothesize that because current ACL reconstruction procedures cannot exactly replicate normal ACL anatomical complexity, they cannot restore normal tibiofemoral kinematics at the knee joint, thus leading to pathological movement patterns. These patterns also exist in ACL deficient knees. The abnormal rotational movements of the articulating bones at the knee could result in the applications of loads at areas of the cartilage that are not commonly loaded in a healthy knee. It has been shown that normal functional loading results in increased resistance of the cartilage by improving the mechanical stiffness and the proteoglycan content of the tissue.71–74 Furthermore, in joints that are prone to arthrosis, it has been found that the best-preserved cartilage areas are those of higher loading.75 Therefore in a healthy knee there are areas that are commonly loaded and others that are not. These latter areas, due to lack of sufficient cartilage, may not be able to withstand the newly introduced loading that is the result of the abnormal rotational movements of the articulating bones. Over time this could lead to knee osteoarthritis.
A Modified Complexity Hypothesis Model
Changes in the system’s variability have been associated with pathology in several medical areas. Using few examples from cardiology, Kleiger et al (1987)76 showed a correlation between decreased heart rate variability (greater rigidity) and increased mortality in subjects who had suffered an acute myocardial infarction. Kaplan et al (1991)77 showed decreases in cardiovascular variability with age and concluded that variability as measured with nonlinear tools may be a useful physiological marker. Similarly, decreases in variability have been reported in electroencephalographic (EEG) tracings during seizures when compared with resting EEG recordings.78 Our research work explored another physiological biorhythm, stride-to-stride variability, which can be mapped to heart rate variability. We showed that musculoskeletal pathology (i.e., ACL rupture) can also lead to similar results as in other medical areas where the “loss of complexity” hypothesis has been proposed.
Our previously discussed results supported the “loss of complexity” hypothesis in the ACL deficient knee. However, they also provide ground for an even more interesting hypothesis regarding musculoskeletal variability. It is possible that changes in knee stride-to-stride variability may in fact be the consequence of modifications, not only in the deterministic operation of the adaptive complex control systems, but also in intrinsic stochasticity (noise). It is possible that musculoskeletal variability can actually be represented by a continuum. The two ends of the continuum are complete periodicity and complete randomness (see also Fig. 80-2). A “healthy” optimal variability or “complexity” by a motor system is somewhere between the two ends. Decreases or losses can make the system more rigid/periodic and less adaptable, as in the ACL deficient knee. Thus an individual with ACL deficiency is more cautious in the way that he or she walks, trying to eliminate any extra movements, and thus is more rigid. On the other hand, increases can make the system more noisy, as in the ACL reconstructed knee with the ST/Gr autograft. Thus an individual, knowing that now the ACL is reconstructed, feels secure in increasing and adding extra movements. However, because the proper proprioceptive channels are not exactly present, more noise enters in the system, resulting in excess movements. These deviations from the healthy optimal variability may result in a knee more susceptible to acute and chronic injury. If the knee is more rigid as in ACL deficiency or noisier as in ACL reconstruction, it may reduce the capability of the joint to respond to different perturbations and adapt to the changing environment. This may in turn increase susceptibility to injury and future pathology, such as the development of degenerative knee arthritis.
Recommendations for Future Work: How Gait Analysis can Guide the Development of Surgical Techniques
Double Bundle
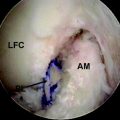
In the past few years, the rotational role of the ACL has been studied more thoroughly. Recent cadaveric studies of the ACL have shown that it consists of two major components, the anteromedial (AM) bundle and the posterolateral (PL) bundle (Fig. 80-3). The two-bundle description of the ACL has been accepted as a basis for understanding the function of the ACL. The ACL does not function as a simple band of fibers with constant tension as the knee moves; the two bundles seem to exhibit different tension patterns, and they seem to be susceptible to different forces. When the knee is extended, the PL bundle is tight and the AM bundle is moderately lax. As the knee is flexed, the femoral attachment of the ACL takes a more horizontal orientation, causing the AM bundle to tighten and the PM bundle to loosen.79 However, it seems that this structural morphology of the ACL cannot be restored with the common ACL reconstruction techniques. Therefore recent techniques have been developed to better approximate the actual anatomy and physiology of the ACL. One very promising technique is the two-bundle ACL reconstruction.
Fig. 80-3 The posterolateral (PL) and anteromedial (AM) bundles of the anterior cruciate ligament (ACL).
The advantage of two-bundle reconstruction is that it can better replicate the function of the ACL. This is accomplished due to the reinstatement of the two-bundle anatomy of the ligament.80 It is generally agreed that current ACL reconstruction techniques using BPTB or ST/Gr grafts, anchored in one femoral and one tibial tunnel, achieve this goal partially because they replicate mostly the AM bundle of the ACL. The role of this bundle has been well documented as resisting anterior translational loads.41 However, the PL bundle has received limited attention. A recent in vitro study81 has revealed that the PL bundle is important for the stabilization of the knee against rotational loads. Thus it is possible that the lack of restoration of tibial rotation after an ACL reconstruction is related to the lack of proper replication of the two ACL bundles and specifically of the PL bundle. Recent studies in both human and animals have demonstrated similar results with the two-bundle reconstruction technique.80,82–86 However, this conclusion needs to be verified in vivo using gait analysis, as described earlier in our research work. Our experimental protocols can determine whether the double-bundle technique is truly superior in restoring tibial rotation during physical activities.
Tunnel Positioning
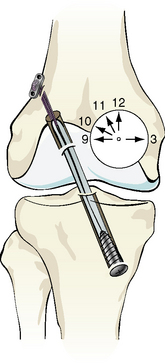
Another very promising technique that has been developed recently to better approximate the actual anatomy and physiology of the ACL is the more oblique femoral tunnel placement. A more oblique placement of the femoral tunnel can also affect rotational stability.40,87,88 The basic advantages of this technique are: (1) it is not as surgically demanding as others (i.e., a two-bundle reconstruction) and (2) the only difference from the current techniques is in the setting of the femoral tunnel in a more oblique location (between 9 and 10 o’clock for a right knee). Current techniques use a vertical orientation approximately at the 11-o’clock position (Fig. 80-4). Several studies used in vitro methodology to examine the more oblique placement of the femoral tunnel using either the BPTB40,87 or the ST/Gr autograft.88 They found that the more oblique placement of the femoral tunnel more effectively resisted rotational loads. This can be attributed to the fact that the PL bundle of the ACL is located more horizontally and toward the 9-o’clock position of the femur (for the right leg) and is important for the stabilization of the knee against rotational loads. Thus a more oblique placement can better replicate the PL bundle and result in increased resistive ability to rotational forces. In our studies, the femoral tunnel was placed at the 11-o’clock position.
1 Heier KA, Mack DR, Moseley JB, et al. An analysis of anterior cruciate ligament reconstruction in middle-aged patients. Am J Sports Med. 1997;25:527-532.
2 Noyes FR, Barber-Westin SD. A comparison of results in acute and chronic anterior cruciate ligament ruptures of arthroscopically assisted autogenous patellar tendon reconstruction. Am J Sports Med. 1997;25:460-471.
3 Chmielewski TL, Rudolph KS, Fitzgerald GK, et al. Biomechanical evidence supporting a differential response to acute ACL injury. Clin Biomech. 2001;16:586-591.
4 Chmielewski TL, Rudolph KS, Snyder-Mackler L. Development of dynamic knee stability after acute ACL injury. J Electromyogr Kinesiol. 2002;12:267-274.
5 Johansson H, Sjolander P, Sojka P. A sensory role for the cruciate ligaments. Clin Orthop. 1991;268:161-175.
6 Rudroff T. Functional capability is enhanced with semitendinosus than patellar tendon ACL repair. Med Sci Sports Exerc. 2003;35:1486-1492.
7 Li G, DeFrate LE, Rubash HE, et al. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23:340-344.
8 Harter RA, Osternig LR, Singer KM, et al. Long-term evaluation of knee stability and function following surgical reconstruction for anterior cruciate ligament insufficiency. Am J Sports Med. 1988;16:434-443.
9 Tegner Y. Strength training in the rehabilitation of cruciate ligament tears. Sports Med. 1990;9:129-136.
10 Baker D, Wilson G, Carlyon B. Generality versus specificity: a comparison of dynamic and isometric measures of strength and speed-strength. Eur J Appl Physiol Occup Physiol. 1994;68:350-355.
11 Devita P, Hortobagyi T, Barrier J. Gait biomechanics are not normal after anterior cruciate ligament reconstruction and accelerated rehabilitation. Med Sci Sports Exerc. 1998;30:1481-1488.
12 Timoney JM, Inman WS, Quesada PM, et al. Return of normal gait patterns after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:887-889.
13 Berchuck M, Andriacchi TP, Bach BR, et al. Gait adaptations by individuals who have a deficient anterior cruciate ligament. J Bone Joint Surg. 1990;72A:871-877.
14 Georgoulis AD, Papadonikolakis A, Papageorgiou CD, et al. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75-79.
15 Andriacchi TP. Dynamics of pathological motion: applied to the anterior cruciate deficient knee. J Biomech. 1990;23:99-105.
16 Andriacchi TP. Functional analysis of pre and post-knee surgery: total knee arthroplasty and ACL reconstruction. J Biomech Eng. 1993;115:575-581.
17 DeLuca PA, Davis RB, Ounpuu S, et al. Alterations in surgical decision making in patients with cerebral palsy based on three dimensional gait analysis. J Pediatric Orthopedics. 1997;17:608-614.
18 Lee E, Goh J, Bose K. Value of gait analysis in the assessment of surgery in cerebral palsy. Arch Phys Med Rehabil. 1992;73:642-646.
19 Winter D, Patla A, Frank J, et al. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Therapy. 1990;70:340-347.
20 Harris GF, Wertsch JJ. Procedures for gait analysis. Arch Phys Med Rehab. 1994;75:216-225.
21 Reinschmidt C, van den Bogert AJ, Nigg BM, et al. Effect of skin movement on the analysis of skeletal knee joint motion during running. J Biomech. 1997;30:729-732.
22 Lafortune MA, Cavanagh PR, Sommer HJ, et al. Three-dimensional kinematics of the human knee during walking. J Biomech. 1992;25:347-357.
23 Ishii Y, Terajima K, Terashima S, et al. Three-dimensional kinematics of the human knee with intracortical pin fixation. Clin Orthop. 1997;343:144-150.
24 Lafortune MA. Three-dimensional acceleration of the tibia during walking and running. J Biomech. 1991;24:877-886.
25 Cappozzo A, Catani F, Leardini A, et al. Position and orientation in space of bones during movement: experimental artefacts. Clin Biomech. 1996;11:90-100.
26 Lucchetti L, Cappozzo A, Cappello A, et al. Skin movement artefact assessment and compensation in the estimation of knee-joint kinematics. J Biomech. 1998;31:977-984.
27 Ristanis S, Giakas G, Papageorgiou CD, et al. The effects of anterior cruciate ligament reconstruction on tibial rotation during pivoting after descending stairs. Knee Surg Sports Traumatol Arthrosc. 2003;11:360-365.
28 Gage JR. Gait analysis. An essential tool in the treatment of cerebral palsy. Clin Orthop. 1993;288:6-34.
29 Chambers HG, Sutherland DH. A practical guide to gait analysis. J Am Acad Orthop Surg. 2002;10:222-231.
30 Ristanis S, Stergiou N, Patras K, et al. Excessive tibial rotation during high demanding activities is not restored by ACL reconstruction. Arthroscopy. 2005;21:1323-1329.
31 Decker M, Torry M, Noonan T, et al. Landing adaptations after ACL reconstruction. Med Sci Sports Exerc. 2002;34:1408-1413.
32 McNair P, Marshall R. Landing characteristics in subjects with normal and anterior cruciate ligament deficient knee joints. Arch Phys Med Rehabil. 1994;75:584-589.
33 Ristanis S, Stergiou N, Patras K, et al. Follow-up evaluation 2 years after ACL reconstruction with a BPTB graft shows that excessive tibial rotation persists. Clin J Sports Med. 2006;16:111-116.
34 Chen L, Cooley V, Rosenberg T. ACL reconstruction with hamstring tendon. Orthop Clin N Am. 2003;34:9-18.
35 Hamner DL, Brown CH, Steiner ME, et al. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Boint Joint Surg. 1999;81A:549-557.
36 Rowden N, Sher D, Rogers G, et al. Anterior cruciate ligament graft fixation. Initial comparison of patellar tendon and semitendinosus autografts in young fresh cadavers. Am J Sports Med. 1997;25:472-478.
37 Fu FH, Bennett CH, Lattermann C, et al. Current trends in anterior cruciate ligament reconstruction. Part I: biology and biomechanics of reconstruction. Am J Sports Med. 1999;27:821-830.
38 Chouliaras V, Ristanis S, Moraiti C, et al. The effectiveness of reconstruction of the ACL with quadrupled hamstrings and bone-patellar tendon-bone autografts. An in-vivo study comparing tibial internal-external rotation. Am J Sports Med. 2007;35:189-196.
39 Georgoulis AD, Ristanis S, Chouliaras V, et al. Tibial rotation is not restored after ACL reconstruction with a hamstring graft. Clin Orthop Relat Res. 2007;454:89-94.
40 Loh JC, Fukuda Y, Tsuda E, et al. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. Arthroscopy. 2003;19:297-304.
41 Kanamori A, Woo SL, Ma CB, et al. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: a human cadaveric study using robotic technology. Arthroscopy. 2000;16:633-639.
42 Yoo JD, Papannagari R, Park SE, et al. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33:240-246.
43 Woo SL, Kanamori A, Zeminski J, et al. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon. A cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg. 2002;84A:907-914.
44 Winter DA. Biomechanics of normal and pathological gait: implications for understanding human locomotion control. J Motor Behav. 1989;21:337-355.
45 Yack J, Berger RC. Dynamic stability in the elderly: identifying a possible measure. J Gerontol Med Sci. 1993;48:225-230.
46 Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriat Soc. 1997;45:313-320.
47 Hausdorff JM, Cudkowicz ME, Firtion R, et al. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington disease. Mov Disord. 1998;13:428-437.
48 Amato R. Chaos breaks out at NIH, but order may come out of it. Science. 1992;257:1763-1764.
49 Buchman TG, Cobb JP, Lapedes AS, et al. Complex systems analysis: a tool for shock research. Shock. 2001;16:248-251.
50 Goldberger AL, Rigney DR, Mietus J, et al. Nonlinear dynamics in sudden cardiac death syndrome: heart rate oscillations and bifurcations. Experentia. 1988;44:983-987.
51 Goldstein B, Toweill D, Lai S, et al. Uncoupling of the automatic and cardiovascular systems in acute brain injury. Am J Physiol. 1998;257:R1287-R1292.
52 Lanza GA, Guido V, Galeazzi MM, et al. Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am J Cardiol. 1998;82:1323-1328.
53 Slutzky MW, Cvitanovic P, Mogul DJ. Deterministic chaos and noise in three in vivo hippocampal models of epilepsy. Ann Biomed Eng. 2001;29:607-618.
54 Toweill DL, Goldstein B. Linear and nonlinear dynamics and the pathophysiology of shock. New Horiz. 1998;6:155-168.
55 Wagner CD, Nafz B, Persson PB. Chaos in blood pressure control. Cardiovasc Res. 1996;31:380-387.
56 Pool R. Is it healthy to be chaotic? Science. 1989;243:604-607.
57 Lipsitz LA, Goldberger AL. Loss of complexity and aging. J Am Med Assoc. 1992;267:1806-1809.
58 Goldberger AL, Amaral LAN, Hausdorff JM, et al. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci. 2002;99:2466-2472.
59 Hausdorff JM, Peng CK, Landin Z, et al. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J Appl Physiol. 1995;78:349-358.
60 Buzzi UH, Stergiou N, Kurz MJ, et al. Nonlinear dynamics indicates aging affects variability during gait. Clin Biomech. 2003;18:435-443.
61 Stergiou N, Buzzi UH, Kurz MJ, et al. Nonlinear tools in human movement. In: Stergiou N, editor. Innovative analyses of human movement. Champaign, IL: Human Kinetics; 2004:63-90.
62 Georgoulis AD, Moraiti C, Ristanis S, et al. A novel approach to measure variability in the anterior cruciate ligament deficient knee during walking: the use of Approximate Entropy in orthopaedics. J Clin Monit Comput. 2006;20:11-18.
63 Moraiti C, Vasiliadis H, Tzimas V, et al. Patellar tendon vs hamstrings graft: variability changes in knee flexion/extension movement patterns during walking. Presented at the 12th meeting of ESSKA 2000 Congress. Innsbruck, Austria, May 24–27, 2006. 2006.
64 Mankin HJ. The response of articular cartilage to mechanical injury [current concepts]. J Bone Joint Surg. 1982;64A:460-466.
65 Finsterbush A, Frankl U, Matan Y, et al. Secondary damage to the knee after isolated injury of the anterior cruciate ligament. Am J Sports Med. 1990;18:475-479.
66 McDaniel WJ, Dameron TJ. The untreated anterior cruciate ligament rupture. Clin Orthop. 1983;172:158-163.
67 Noyes F, Matthews D, Mooar P, et al. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg. 1983;65A:163-174.
68 Noyes F, Mooar P, Matthews D, et al. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg. 1983;65B:154-162.
69 Daniel DM, Stone ML, Dobson BE, et al. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med. 1994;22:632-644.
70 Asano H, Muneta T, Ikeda H, et al. Arthroscopic evaluation of the articular cartilage after anterior cruciate ligament reconstruction: a short-term prospective study of 105 patients. Arthroscopy. 2004;20:474-481.
71 Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1-13.
72 Jurvelin J, Kiviranta I, Tammi M, et al. Effect of physical exercise on indentation stiffness of articular cartilage in the canine knee. Int J Sports Med. 1986;7:106-110.
73 Saamanen AM, Tammi M, Kiviranta I, et al. Running exercise as a modulatory of proteoglycan matrix in the articular cartilage of young rabbits. Int J Sports Med. 1988;9:127-133.
74 Kiviranta I, Tammi M, Jurvelin J, et al. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6:188-195.
75 Bullogh PG. The pathology of osteoarthritis. In: Moskowitz R, Howell D, Goldberg V, et al, editors. Osteoarthritis: diagnosis and medical/surgical management. Philadelphia: Saunders; 1992:36-69.
76 Kleiger RE, Miller JP, Bigger JT, et al. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256-262.
77 Kaplan DT, Furman MI, Pincus SM, et al. Aging and the complexity of cardiovascular dynamics. Biophys J. 1991;59:945-949.
78 Bhattacharya J. Complexity analysis of spontaneous EEG. Acta Neurobiol Exp (Wars). 2000;60:495-501.
79 Amis AA, Dawkins GP. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg. 1991;73B:260-267.
80 Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660-666.
81 Gabriel MT, Wong EK, Woo SL, et al. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22:85-89.
82 Radford WJ, Amis AA, Kempson SA, et al. A comparative study of single- and double-bundle ACL reconstructions in sheep. Knee Surg Sports Traumatol Arthrosc. 1994;2:94-99.
83 Muneta T, Sekiya I, Yagishita K, et al. Two-bundle reconstruction of the anterior cruciate ligament using semitendinosus tendon with endobuttons: operative technique and preliminary results. Arthroscopy. 1999;15:618-624.
84 Zaricznyj B. Reconstruction of the anterior cruciate ligament of the knee using a doubled tendon graft. Clin Orthop. 1987;220:162-175.
85 Mae T, Shino K, Miyama T, et al. Single- versus two-femoral socket anterior cruciate ligament reconstruction technique: biomechanical analysis using a robotic simulator. Arthroscopy. 2001;17:708-716.
86 Guardamagna L, Seedhom BB, Ostell AE. Double-bundle reconstruction of the ACL using a synthetic implant: a cadaveric study of knee laxity. J Orthop Sci. 2004;9:372-379.
87 Scopp JM, Jasper LE, Belkoff SM, et al. The effect of oblique femoral tunnel placement on rotational constraint of the knee reconstructed using patellar tendon autografts. Arthroscopy. 2004;20:294-299.
88 Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions. Effect on kinematics of the ACL reconstructed knee. Am J Sports Med. 2005;33:1-7.