Chapter 13 Future Directions in Neuromuscular Ultrasound
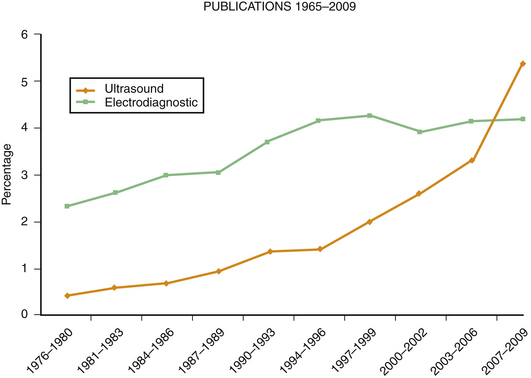
This chapter expands on the future possibilities for neuromuscular ultrasound, including some mentioned briefly in earlier chapters (Table 13.1). Growth in electrodiagnostic technology has been limited in the past few decades, so the emergence of high-resolution ultrasound offers the clinician new ways to explore and perhaps even treat patients with neuromuscular disorders. Many of the other new diagnostic developments in neuromuscular disease have been in serology, genetic testing, and histopathology — fields too specialized for the participation of most clinicians. Newer electrodiagnostic technologies including magnetic stimulation, evoked potentials, singlefiber electromygraphy (EMG),1 and autonomic testing2 are more accessible to clinicians, but tend to have a narrow scope of neuromuscular applications, and are useful in a smaller set of less common diseases. As a result, these technologies are primarily used by acadamic referral centers that specialize in these particular neurologic disorders. Neuromuscular ultrasound, in contrast, is a technology likely to be of use in almost all EMG laboratories as it complements the study of nerve entrapments, peripheral neuropathy, and muscle diseases, as well as being of use in guiding interventional procedures such as chemodenervation and steroid injections for carpal tunnel syndrome. To help physicians decide about the value of purchasing an ultrasound instrument and learning how to use it, this chapter attempts to identify the long-term potential of neuromuscular ultrasound. For those interested in research, the chapter also identifies projects that might be of current or potential importance in the next decade.
Table 13.1 Areas of Future Development in Neuromuscular Ultrasound
| ANATOMIC MEASUREMENTS |
| Location: |
| Detail: |
| Size: |
| Echointensity and Anisotropy: |
| PHYSIOLOGIC MEASUREMENTS |
| Blood Flow: |
| Movement: |
| Elastography: |
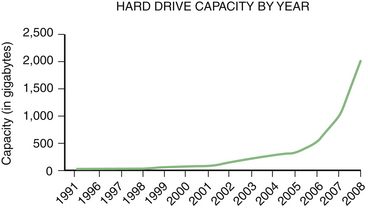
Predicting the future is risky, and it requires sidestepping a general rule of medical writing, which is to never stray from understatement. Nonetheless, promising preliminary studies make it possible to extrapolate about potential uses of neuromuscular ultrasound. Furthermore, there are likely to be additional unexpected applications of this technology as it evolves (Fig. 13.1) and more physicians and investigators explore novel applications. Input from committed users will likely guide manufacturers in the development of better instruments for neuromuscular applications.
Muscle Ultrasound
At first glance, it may seem that secondary, musculoskeletal disorders of muscle, because of their frequency in the general population, might significantly outweigh the prevalence of primary disorders of muscle. However, neuromuscular causes of muscle disease are quite common as well. The most common, although benign, neuromuscular finding detectable by ultrasound is likely to be distal (benign) fasciculations of muscle, which may occur in up to 43% of the general population (see Chapters 3 and 10). Neurogenic changes in distal muscles caused by significant diabetic neuropathy are likely to be detectable by ultrasound in 1.5% of the general population. There are also a variety of other neurogenic causes of muscle atrophy or altered echogenicity including radiculopathies and compression mononeuropathies. Less common are primary diseases of muscle, the myopathies. Regardless of one’s specialty orientation, there is ample demand for both musculoskeletal and neuromuscular ultrasonographers.
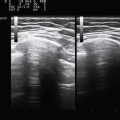
Ultrasound is likely to change the evaluation of muscle disease in several ways. Based on trends in the literature, ultrasound will become an increasingly quantitative tool in the study of muscle (Fig. 13.2). Its simplest and most obvious use will be measuring muscle size.3 Currently normative data are available for muscle thickness,4–7 and it seems likely that accurate estimates of muscle volume, either by the development of nomograms, linear measures, or by improved experience using three-dimensional (3D) ultrasound, will further enhance the ability to assess normal muscle size, atrophy, and hypertrophy. As effective therapies for wasting disorders of nerve or muscle are discovered, ultrasound measurements of muscle size may become a useful biomarker of disease progression.8
One interesting paper has suggested that the thickness of distal muscles in patients with diabetic neuropathy correlates robustly with motor response amplitudes.9 One implication of this study, and the common experience of finding low compound muscle action potential amplitudes in atrophic distal muscles, is that axon loss, currently measured by loss of compound muscle action potential amplitude, may also be measurable by relative atrophy in distal muscles by ultrasound. If so, it may be possible that ultrasound imaging could reduce the need for uncomfortable nerve conduction testing in situations in which demyelination is unexpected or in patients who cannot tolerate electrical stimulation, particularly if serial nerve conduction studies are needed for follow-up.
A number of lines of research have established that, in addition to atrophy, increased echogenicity is also a sensitive marker of muscle disease.10–13 Increased echogenicity is helpful in the descriptive classification of some myopathies (see Chapter 10), and further refinement in this approach, perhaps by using quantitative measures, is likely to expand such applications. Echogenicity may also be a particularly robust indicator of disease severity in spinal muscular atrophy (SMA), a disorder primarily found in young adults and children, individuals who may be reluctant to undergo electrodiagnostic testing, particularly serial testing.14 It can be hoped that user-friendly methods of measuring muscle echogenicity will be available on the next generation of ultrasound instruments to simplify clinical studies investigating muscle disease. Of perhaps greater interest to those not specializing in neuromuscular medicine, muscle echogenicity may also relate to muscle fat content. With aging, muscle storage of fat may be an indicator of susceptibility to vascular disease, and ultrasound of muscle may be a simple noninvasive technique to evaluate patients and improve systemic health through dietary change.
Another area of development in muscle imaging, one that fits in with an evolving field of electrodiagnosis, is the use of ultrasound in the evaluation of pelvic floor muscles.15–17 Ultrasound can not only assess thickness and echogenicity of pelvic floor muscles but also provide information regarding needle placement, muscle movement, muscle injury, residual urine volumes, bladder neck mobility, urethral integrity, prolapse, and other structural problems. It is likely this technique will prove complementary to electrodiagnosis in the evaluation of patients with bowel and bladder dysfunction.
Considerable interest has focused on measuring the thickness of truncal and paraspinal muscles,18–22 in large part in an attempt to assess exercise programs that focus on core strengthening and their role in managing chronic back pain. For those interested in measuring the outcomes of certain types of physical therapy, ultrasound offers a noninvasive tool for quantifying exercise effects.
More advanced capabilities of ultrasound allow for the observation of muscle movement. Current instruments are sufficiently sensitive to detect fasciculations.23–26 Optimal parameters for identifying fibrillations have not yet been worked out27–28 but it seems likely that instrument-specific enhancements of frame rate, resolution, and display will make it possible to better image fibrillation potentials in muscles, reducing the need for electromyography in select patients.
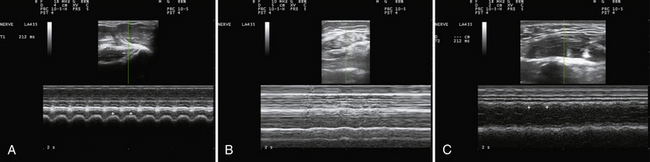
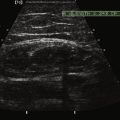
Muscle movement and size are also relevant in the evaluation of movement disorders. M-mode proves to be a simple and reliable way to evaluate tremor frequency and amplitude, one that avoids some of the problematic harmonics and sub-harmonics that sometimes contaminate EMG recordings. Although not routinely studied (but see Reimers and coworkers),29 hypertrophy is sometimes a manifestation of focal and segmental dystonia. Its absence, as measured by ultrasound, may also turn out to be useful in identifying some cases of psychogenic dystonia. It remains to be seen if M-mode ultrasound could provide better precision to the clinical differentiation of assorted types of tremors, tics, chorea, ballismus, dystonia, and myoclonus (Fig. 13.3A and B). Because the mechanical contraction of muscle lasts several hundred milliseconds longer than the electrical activity associated with movement (see Fig. 1.21) ultrasound can better capture the duration and patterns of movement than EMG. And, unlike standard clinical video recordings, that can only assess surface movement that reflects the sum of forces acting across a joint, ultrasound offers the added ability of studying the contractile behavior of individual muscles, independent of the behavior of other agonists or antagonists. Experience in the EMG laboratory indicates that much of the “lore” of movement disorders, derived from clinical observations, sometimes has surprisingly little evidence base. For example, older neurology textbooks30 used to assign the frequency range of essential tremor to 6 to 8 Hz; but, more recent studies have confirmed a broader range of frequencies (4 to 11 MHz), a finding that is immediately apparent when studying essential tremor of the head with ultrasound (Fig. 13.3C). Although selectivity problems in assessing movement by electromyography can be addressed in part with needle, as opposed to surface, electromyography, needle or wire insertion is limited by pain, and does not exclude “crosstalk” from nearby muscles.
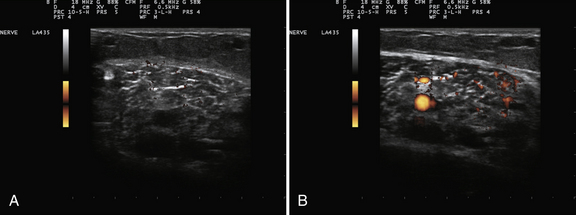
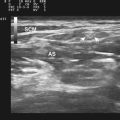
Muscle blood flow is an area in which there has been too little investigation with respect to muscle pathology.31–33 It is well known that ultrasound can be used to measure volume changes in muscle after exercise, which are presumably secondary to enhanced blood flow, and quantitative techniques for estimating a number of arteriolar parameters of blood flow are routinely available on most instruments. Too little is known about muscle blood flow in either degenerative or inflammatory myopathies at rest or with muscle activation, so further study is needed (Fig. 13.4).
Finally, with the advent of new therapies, ultrasound’s capability as an interventional tool may be of particular value. To this day it remains unknown if steroids are beneficial in inflammatory myopathies because of a local protective effect or a systemic effect on inflammatory cells, and conducting studies of intramuscular steroids (with ultrasound guidance) in such patients may help answer this question. Ultrasound might also be useful for testing the theory that fibrillations in myopathy result from loss of continuity of distal portions of muscle fibers that have been disconnected from more central portions in contact with the innervating motor axon. If this were to be the case, more fibrillations should occur as one samples muscle at increasing distances from the endplate zone. Another potential avenue of intervention involves ultrasound ablative techniques, which may someday be helpful in treating heterotopic calcifications34 in muscle or small neoplasms.35 Sonoporation and microbubble techniques may even be useful for selective delivery of gene therapy.36,37
Nerve Ultrasound
The use of ultrasound to study nerves came more than one decade after the initial reports of its use for muscle because of the delay in developing sufficiently high resolution ultrasound transducers. The wide variety and distribution of peripheral nerves have made it difficult to acquire a sufficient number of reference value studies with nerve ultrasound. Evidence suggests that body size and age influence the cross-sectional areas of nerves,38 and studies that help discriminate between normal and pathologic variations in nerve size will enhance the ability of this measure to identify disease. As in the case of muscle disorders, better ways of measuring echogenicity will also likely be helpful in evaluating nerve disorders; however, unlike what is found in muscle, reduced echogenicity is the more common indicator of pathologic changes in nerve (see Chapter 5) and there currently is no simple way to quantify this. Also, for years interest has been expressed in nerve shape, particularly in the form of the measurement of a flattening ratio of the median nerve at the wrist in carpal tunnel syndrome.39,40 It may be that in the future, nerve pathology will not just be identified by size, but also by certain shape parameters that help identify areas of abnormality. In like manner, close attention to fascicular architecture may be informative.41–43 Certain neoplasms seem to limit themselves to specific fascicles, and in some patients with Charcot-Marie-Tooth disease there is prominent fascicular enlargement (Videos 13.1 and 13.2); it may be that other disorders also routinely manifest as selective fascicle involvement on ultrasound.
Increasing interest is focusing on the usefulness of ultrasound in imaging cranial nerves and cranial nerve–supported functions. Optic nerve imaging by ultrasound is routine in ophthalmology, and even in some emergency departments44; with proper safety precautions and equipment, this can be an informative area for additional study. Cranial nerves 1, 3 through 9, and 12 are not easily visualized, but for the motor nerves, the muscles they innervate are visible with ultrasound imaging. The tenth and eleventh cranial nerves can be directly imaged with ultrasound.45
Nerve movement (e.g., sliding; Video 13.3) is an area of increasing interest in the literature. It seems likely that better techniques for identifying normal and pathologic nerve movement, 46–50 particularly in areas subject to entrapment, may shed more light on how to better diagnose and treat compression neuropathies. Less well studied is the phenomenon of nerve retraction (nerve gap) after nerve transection.51 Nerve gap refers to the distance between visible ends of a nerve after transection (Video 13.4). The larger the gap, the easier it is to assess ultrasonographically but the more difficult it is to repair the nerve. The distal-proximal tension on a nerve is what causes the gap to occur after nerve transection; better ways of measuring tension on the nerve (perhaps with elastography, discussed later) may be of use in assessing nerve mobility and the potential for nerve regeneration after severe nerve injury.
Nerve blood flow is another area of particular interest. The presence of a persistent median artery is readily detected by color flow Doppler (Video 13.5). Of pathologic significance, an increase in nerve blood flow within the median nerve at the wrist is seen in some patients with carpal tunnel syndrome52 (Video 13.6), and a better understanding of why this occurs and its significance may provide critical insights into the pathophysiology of entrapment neuropathies. Nerve blood flow has not been studied in other entrapment neuropathies. It is also not clear how to best evaluate blood flow with current instrumentation, or with ultrasound contrast agents. The ability of ultrasound to alter the blood-nerve barrier and to enhance local delivery of systemically administered therapy by sonoporation and microbubbles36,37 may someday provide a unique therapeutic approach to the treatment of certain kinds of neuropathies.
Interventional use of ultrasound with respect to nerves is in its infancy. As outlined in the Chapter 11, ultrasound likely would be of preoperative value in mapping the course of nerves subject to injury during surgical procedures.53,54 The challenge of establishing the validity of this type of research involves studying a large enough population to have reasonable power for detecting a statistically reduced incidence of nerve injury because it is a rather rare complication of most surgical procedures.
In an analogous fashion to steroid-responsive inflammatory disorders of muscle, it remains unclear how steroids mediate beneficial effects in certain types of inflammatory neuropathies. Focal injections of steroids might provide additional insight into this process, by separating local versus systemic effects. This is of particular interest in disorders with focal conduction block, sometimes visible as focal nerve enlargement by ultrasound.55 The use of agents such as phenol56 to destroy nerves, for pain control, or for the treatment of spasticity seems an area appropriate for future research and development as well. Although botulinum toxin represents a significant advance in this area, the longer duration of action, and potentially more complete efficacy of phenol, may be a desirable outcome for those with chronic irreversible conditions such as spasticity, particularly when injecting nerves that are predominantly motor such as the obturator or musculocutaneous nerves. For dystonia, phenol may be less attractive, because experience has shown a tendency for dystonia to recruit different agonist muscle groups over time and for there to be possibly greater sensitivity to sensory side effects. Of interest, high-intensity focused ultrasound also may be able to induce nerve conduction block for treating pain and spasticity.57,58
The development of intravascular ultrasound catheters for studying the walls of blood vessels,59 given the close association of nerves to arteries and veins in multiple areas of the body, could provide high resolution ultrasound access to nerves in sites that were deep or otherwise difficult to image with surface ultrasound.
Small Fiber and Autonomic Nerves
Although ultrasound is not likely to provide sufficient resolution to selectively identify the autonomic fibers directly, the technology offers unique approaches for assessing the function of autonomically innervated structures. Chief among these are vascular structures, including the heart, arteries, arterioles, and veins. Sophisticated technology exists for imaging blood flow, and alterations in blood flow are intimately mediated by autonomic innervations of these structures. Of particular interest is the ability of ultrasound to look for asymmetry in autonomic innervation, and to study the innervations of different types of vascular structures.2 For example, the control of blood flow to extremity muscles likely involves a different subset of autonomic fibers than those that control thermoregulation, and those that control the blood flow to gastrointestinal structures, such as the mesenteric artery. In some patients with orthostatic hypotension, for example, the electrophysiologic assessment of cardiac autonomic function by the Valsalva ratio and variation in heart rate to deep breathing is normal. In such cases the ability to evaluate venous tone in the lower extremities on standing, for example, may provide insight into autonomic dysfunction that would be missed by currently available tests. One explanation for difficulty in diagnosing and quantifying autonomic neuropathies may reflect the limited number of meaningful tests available for assessing the many different aspects of this complicated system. With better use of ultrasound technology, it may be possible to begin to classify these disorders on parameters other than the assumed size of involved nerve fibers, and instead use features such as segmental distribution of deficits, type of autonomic function subserved, or type of autonomic end-organ involved (as end-organ visualization is also possible with ultrasound). There are ample research opportunities for exploring the role of ultrasound in evaluating different types of autonomic function (Video 13.7).
One other potential use of ultrasound in evaluating autonomic function may come from the use of particularly high-resolution instruments, similar to the technique of ultrasound biomicroscopy used for studying intraocular structures. With sufficient resolution it is conceivably possible to image sweat glands (or for that matter, other terminal structures such as Meissner’s corpuscles) percutaneously.60,61 Recent advances have made this possible using high-resolution optics, so it seems plausible to assume that similar results might be achievable with ultrasound of sufficiently high resolution. In like manner, other small receptors (e.g., muscle spindles), located in deeper tissues, may be amenable to intraoperative observation with the same technology. Such approaches will depend on continued technological advancement and clinical trial and error.
Emerging Ultrasound Technologies
Ultrasound technology continues to evolve in a variety of different directions. Elastography, a measure of tissue stiffness, may be of particular interest in studying nerve and muscle disease. The use of this technique has been primarily for differentiating tumor from healthy tissue, particularly in fat-laden tissues like the breast.62 However, it is conceivable that the technique could be informative about a variety of conditions that alter the stiffness of muscle,63 either via histologic change, such as in myopathic disorders, or via changes in muscle tone and the supportive tissues of muscle as occur in movement disorders and spasticity. The real-time and noninvasive nature of this technique makes it particularly attractive for research.
The potential for 3D64–65 and 4D ultrasound imaging may have some role in furthering neuromuscular research, but at this time, the applications are more theoretic than real. The technology shows promise for true muscle volume measurement but it may be that simpler estimates of muscle volume might be just as useful.
Novel developments in interventional technology also could enhance the use of ultrasound. Specially treated markers (wires, clips, or beads) that are easily identified by ultrasound, CT, or MRI, can be inserted under ultrasound guidance to help direct subsequent surgery, biopsy, radiation, or imaging. Such technology is particularly helpful with the management of tumors, but it can also be used to monitor changes in any type of nerve or muscle tissue as well. Needles that have been treated to enhance back-scatter, or that emit ultrasound directly from their tips have been developed to assist in needle guidance with ultrasound. Finally, ultrasound contrast agents66–68 may be a useful way to better study blood flow in a variety of neuromuscular disorders and for the development of sonoporation and related therapeutic technologies.
There is also increasing interest in using ultrasound to visualize structures in the central nervous system. The open fontanelle in infants has long been used as a portal of entry for ultrasound imaging of the brain in the perinatal period. Similarly in small infants, the spinal cord can be imaged in small segments if the spine is flexed and the probe is angled to look just below the spinous process. It now turns out that it is possible to directly image the brain through the skull with low-frequency probes using ultrasound. The findings have shown that there are identifiable changes in the substantia nigra in Parkinson’s disease and other movement disorders.69–71 The relationship of such imaging to manifestations of typical neuromuscular disorders remains to be elucidated
Practice Issues
The author believes that individuals of any medical speciality, if interested and appropriately trained, should be able to perform and interpret neuromuscular ultrasound. The American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM), a major society dedicated to neuromuscular medicine, has outlined the nature of training and knowledge essential for neuromuscular ultrasound.72 As with the case of most ultrasound procedures, there is usually a mixture of specialists in practice performing ultrasound, typically specialists in a field of interest (such as cardiologists for cardiac ultrasound imaging) and imaging specialists such as radiologists. In neuromuscular ultrasound, primary specialties include neurology and physical medicine and rehabilitation; but it is also clear that neurosurgeons and orthopedic surgeons share an interest in the technique, as do rheumatologists and plastic surgeons; radiologists also are a core group of involved physicians. An interesting question arises regarding clinical neurophysiologists and their use of neuromuscular ultrasound. Traditionally, clinical neurophysiologists have not been actively involved in imaging, but with the progress of technology, this has slowly changed. Electroencephalography, evoked potentials, and magnetoencephalography have the capacity of generating images in the form of brain mapping. Clinical neurophysiologists should also note that ultrasound evolved from oscilloscopes, a technology fundamental to the most basic procedures in clinical neurophysiology. Of course, ultrasound captures non-electrophysiologic elements of neurophysiology, in the form of anatomy, kinesiology, blood flow and other physical properties of nerve and muscle. As such, it may be prudent for clinical neurophysiologists to include neuromuscular ultrasound in their repertoire of laboratory skills. Ultimately, time will tell which specialties incorporate neuromuscular ultrasound in their practice, but it seems likely that a collaborative approach from multiple specialties will be the most fruitful in identifying practical applications of the technology and ensuring that it is available to patients through physicians in their communities.
Conclusion
For those with experience practicing electrodiagnostic medicine, ultrasound offers a unique opportunity to enhance one’s own, limited, conceptualizations of nerves and muscles with accurate 3D representations of their spatial relationships, function, and blood flow. It is a superlative tool for educating oneself about how nature has constructed the peripheral nervous system and to identify some blind spots in our understanding of such function. Ultrasound may be a particularly valuable tool for teaching those new to the field of neuromuscular medicine, providing them with anatomic information as well as skills in imaging and image reconstruction, which are generalizable to MRI and CT as well. For all of us who work in clinical laboratories, real-time imaging can reinfuse its users with the thrill of discovery and promote the kind of inquisitiveness that leads to new discoveries and better ways of caring for patients.
1. Stalberg E. Single fiber EMG, macro EMG, and scanning EMG: new ways of looking at the motor unit. CRC Crit Rev Clin Neurobiol. 1986;2:125-131.
2. Walker F.O. Autonomic testing. In: Kimura J., editor. Peripheral nerve diseases. New York: Elsevier; 2006:487-510.
3. Walker F.O. Normal neuromuscular sonography. In: Tegler C.H., Babikian V.L., Gomez C.T., editors. Neurosonology. New York: Mosby; 1996:396-405.
4. Arts I.M., Pillen S., Schelhaas H.J., et al. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41:32-41.
5. Pillen S., van Alfen N., Zwarts M.J. Muscle ultrasound: a grown-up technique for children with neuromuscular disorders. Muscle Nerve. 2008;38:1213-1214.
6. Pillen S., Arts I.M., Zwarts M.J. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37:679-693.
7. Pillen S., Verrips A., Van Alfen N., et al. Quantitative skeletal muscle ultrasound: diagnostic value in childhood neuromuscular disease. Neuromuscul Disord. 2007;17:509-516.
8. Hamjian J.A., Walker F.O. Serial neurophysiological studies of intramuscular botulinum: a toxin in humans. Muscle Nerve. 1994;17:1385-1392.
9. Severinsen K., Andersen H. Evaluation of atrophy of foot muscles in diabetic neuropathy: a comparative study of nerve conduction studies and ultrasonography. Clin Neurophysiol. 2007;118:2172-2175.
10. Pillen S., van Dijk J.P., Weijers G., et al. Quantitative gray-scale analysis in skeletal muscle ultrasound: a comparison study of two ultrasound devices. Muscle Nerve. 2009;39:781-786.
11. Pillen S., Tak R.O., Zwarts M.J., et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echogenicity. Ultrasound Med Biol. 2009;35:443-446.
12. Pillen S., van Keimpema M., Nievelstein R.A., et al. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32:1315-1321.
13. Pillen S., van Engelen B., van den Hoogen F., et al. Eosinophilic fasciitis in a child mimicking a myopathy. Neuromuscul Disord. 2006;16:144-148.
14. Wu J.S., Darras B.T., Rutkove S.B. Assessing spinal muscular atrophy with quantitative ultrasound. Neurology. 2010;75:526-531.
15. Dietz H.P. Pelvic floor ultrasound: a review. Am J Obstet Gynecol. 2010;202:321-333.
16. Falkert A., Endress E., Weigl M., Seelbach-Gobel B. Three-dimensional ultrasound of the pelvic floor 2 days after first delivery: influence of constitutional and obstetric factors. Ultrasound Obstet Gynecol. 2010;35:583-588.
17. Timor-Tritsch I.E. Appearance of the levator ani muscle subdivisions in endovaginal three-dimensional ultrasonography. Obstet Gynecol. 2009;114:1145-1146.
18. Stetts D.M., Freund J.E., Allison S.C., Carpenter G. A rehabilitative ultrasound imaging investigation of lateral abdominal muscle thickness in healthy aging adults. J Geriatr Phys Ther. 2009;32:60-66.
19. Brown S.H., McGill S.M. A comparison of ultrasound and electromyography measures of force and activation to examine the mechanics of abdominal wall contraction. Clin Biomech. 2010;25:115-123.
20. Costa L.O., Maher C.G., Latimer J., Smeets R.J. Reproducibility of rehabilitative ultrasound imaging for the measurement of abdominal muscle activity: a systematic review. Phys Ther. 2009;89:756-769.
21. Hebert J.J., Koppenhaver S.L., Parent E.C., Fritz J.M. A systematic review of the reliability of rehabilitative ultrasound imaging for the quantitative assessment of the abdominal and lumbar trunk muscles. Spine. 2009;34:E848-E856.
22. Lin Y.J., Chai H.M., Wang S.F. Reliability of thickness measurements of the dorsal muscles of the upper cervical spine: an ultrasonographic study. J Orthop Sports Phys Ther. 2009;39:850-857.
23. Reimers C.D., Ziemann U., Scheel A., et al. Fasciculations: clinical, electromyographic, and ultrasonographic assessment. J Neurol. 1996;243:579-584.
24. Walker F.O., Donofrio P.D., Harpold G.J., Ferrell W.G. Sonographic imaging of muscle contraction and fasciculations: a correlation with electromyography. Muscle Nerve. 1990;13:33-39.
25. Scheel A.K., Toepfer M., Kunkel M., et al. Ultrasonographic assessment of the prevalence of fasciculations in lesions of the peripheral nervous system. J Neuroimaging. 1997;7:23-27.
26. Fermont J., Arts I.M., Overeem S., et al. Prevalence and distribution of fasciculations in healthy adults: effect of age, caffeine consumption and exercise. Amyotroph Lateral Scler. 2009;11:181-186.
27. van Baalen A., Stephani U. Fibration, fibrillation, and fasciculation: say what you see. Clin Neurophysiol. 2007;118:1418-1420.
28. Pillen S., Nienhuis M., van Dijk J.P., et al. Muscles alive: ultrasound detects fibrillations. Clin Neurophysiol. 2009;120:932-936.
29. Reimers C.D., Schlotter B., Eicke B.M., Witt T.N. Calf enlargement in neuromuscular diseases: a quantitative ultrasound study in 350 patients and review of the literature. J Neurol Sci. 1996;143:46-56.
30. Victor M., Adams R.D. Principles of neurology, ed 4. New York: McGraw-Hill; 1989.
31. Weber M.A., Krix M., Delorme S. Quantitative evaluation of muscle perfusion with CEUS and with MR. Eur Radiol. 2007;17:2663-2674.
32. Weber M.A., Jappe U., Essig M., et al. Contrast-enhanced ultrasound in dermatomyositis and polymyositis. J Neurol. 2006;253:1625-1632.
33. Adler R.S., Garolfalo G., Paget S., Kagen L. Muscle sonography in six patients with hereditary inclusion body myopathy. Skeletal Radiol. 2008;37:43-48.
34. Poliachik S.L., Khokhlova T.D., Bailey M.R. High intesnity focused ultrasound as a potential treatment modality for heterotopic ossification. J Acoust Soc Am. 2010;127:1759.
35. Palussiere J., Salomir R., Le B.B., et al. Feasibility of MR-guided focused ultrasound with real-time temperature mapping and continuous sonication for ablation of VX2 carcinoma in rabbit thigh. Magn Reson Med. 2003;49:89-98.
36. Liang H.D., Tang J., Halliwell M. Sonoporation, drug delivery, and gene therapy. Proc Inst Mech Eng H. 2010;224:343-361.
37. Lentacker I., Geers B., Demeester J., et al. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther. 2010;18:101-108.
38. Cartwright M.S., Passmore L.V., Yoon J.S., et al. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve. 2008;37:566-571.
39. Yesildag A., Kutluhan S., Sengul N., et al. The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol. 2004;59:910-915.
40. El-Karabaty H., Hetzel A., Galla T.J., et al. The effect of carpal tunnel release on median nerve flattening and nerve conduction. Electromyogr Clin Neurophysiol. 2005;45:223-227.
41. Kuo Y.L., Yao W.J., Chiu H.Y. Role of sonography in the preoperative assessment of neurilemmoma. J Clin Ultrasound. 2005;33:87-89.
42. Boppart S.A., Bouma B.E., Pitris C., et al. Intraoperative assessment of microsurgery with three-dimensional optical coherence tomography. Radiology. 1998;208:81-86.
43. Peer S., Harpf C., Willeit J., et al. Sonographic evaluation of primary peripheral nerve repair. J Ultrasound Med. 2003;22:1317-1322.
44. Major R., al-Salim W. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary, BET 3: ultrasound of optic nerve sheath to evaluate intracranial pressure. Emerg Med J. 2008;25:766-767.
45. Gruber H., Kovacs P. Sonographic anatomy of the peripheral nervous system. In: Peer S., Bodner G., editors. High-resolution sonography of the peripheral nervous system. Berlin: Springer; 2003:13-37.
46. Coppieters M.W., Hough A.D., Dilley A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: an in vivo study using dynamic ultrasound imaging. J Orthop Sports Phys Ther. 2009;39:164-171.
47. Dilley A., Lynn B., Greening J., DeLeon N. Quantitative in vivo studies of median nerve sliding in response to wrist, elbow, shoulder and neck movements. Clin Biomech. 2003;18:899.
48. Dilley A., Summerhayes C., Lynn B. An in vivo investigation of ulnar nerve sliding during upper limb movements. Clin Biomech. 2007;22:774-779.
49. Dilley A., Greening J., Lynn B., et al. The use of cross-correlation analysis between high-frequency ultrasound images to measure longitudinal median nerve movement. Ultrasound Med Biol. 27, 2001. 1211–121
50. Erel E., Dilley A., Greening J., et al. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J Hand Surg Br. 2003;28:439-443.
51. Cartwright M.S., Chloros G.D., Walker F.O., et al. Diagnostic ultrasound for nerve transection. Muscle Nerve. 2007;35:796-799.
52. Mallouhi A., Pulzl P., Trieb T., et al. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240-1245.
53. Flavin R., Gibney R.G., O’Rourke S.K. A clinical test to avoid sural nerve injuries in percutaneous Achilles tendon repairs. Injury. 2007;38:845-884.
54. Ricci S., Moro L. Antonelli Incalzi R: Ultrasound imaging of the sural nerve: ultrasound anatomy and rationale for investigation. Eur J Vasc Endovasc Surg. 2010;39:636-641.
55. Granata G., Pazzaglia C., Calandro P., et al. Ultrasound visualization of nerve morphological alteration at the site of conduction block. Muscle Nerve. 2009;40:1068-1070.
56. Lee J., Lee Y.S. Percutaneous chemical nerve block with ultrasound-guided intraneural injection. Eur Radiol. 2008;18:1506-1512.
57. Foley J.L., Little J.W., Vaezy S. Effects of high-intensity focused ultrasound on nerve conduction. Muscle Nerve. 2008;37:241-250.
58. Foley J.L., Little J.W., Vaezy S. Image-guided high-intensity focused ultrasound for conduction block of peripheral nerves. Ann Biomed Eng. 2007;35:109-119.
59. Vogt M., Opretzka J., Perrey C., Ermert H. Ultrasonic microscanning. Proc Inst Mech Eng H. 2010;224:225-240.
60. Nolano M., Provitera V., Santoro L., et al. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology. 2008;71:536-537.
61. Provitera V., Nolano M., Caporaso G., et al. Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology. 2010;74:50-56.
62. Regini E., Bagnera S., Tota D., et al. Role of sonoelastography in characterising breast nodules: preliminary experience with 120 lesions. Radiol Med. 2010;115:551-562.
63. Drakonaki E.E., Allen G.M. Magnetic resonance imaging, ultrasound and real-time ultrasound elastography of the thigh muscles in congenital muscle dystrophy. Skeletal Radiol. 2010;39:391-396.
64. Min L., Lai G., Xin L. Changes in masseter muscle following curved ostectomy of the prominent mandibular angle: an initial study with real-time 3D ultrasonograpy. J Oral Maxillofac Surg. 2008;66:2434-2443.
65. Pyun S.B., Kang C.H., Yoon J.S., et al. Application of 3-dimensional ultrasonography in assessing carpal tunnel syndrome. J Ultrasound Med. 2011;30:3-10.
66. Stride E.P., Coussios C.C. Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc Inst Mech Eng H. 2010;224:171-191.
67. Lonnebakken M.T., Gerdts E. Impact of ultrasound contrast agents in echocardiographic assessment of ischemic heart disease. Recent Pat Cardiovasc Drug Discov. 2010;5:103-112.
68. Tinkov S., Winter G., Coester C., Bekeredjian R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy, part I: formulation development and in-vitro characterization. J Contr Rel. 2010;143:143-150.
69. Krogias C., Eyding J., Postert T. Transcranial sonography in Huntington’s disease. Int Rev Neurobiol. 2010;90:237-257.
70. Behnke S., Schorder U., Berg D. Transcranial sonography in the premotor diagosis of Parkinson’s disease. Int Rev Neurombiol. 2010;90:93-106.
71. Godau J., Hanz A., Wevers A.K., et al. Sonographic substantia nigra hypoechogencity in polyneuropathy and restless legs syndrome. Move Disord. 2009;14:133-137.
72. Walker F.O., Alter K.E., Boon A.J., et al. Qualifications for practitioners of neuromuscular ultrasound: position statement of the American Association of Neuromuscular and Electrodiagnostic Medicine. Muscle Nerve. 2010;42:442-444.