Chapter 149 Fungal Infections of the Central Nervous System
Surgical infections of the nervous system present with protean clinical manifestations, difficult diagnostic dilemmas, and special therapeutic challenges. The nervous system may be infected by (from most to least common) bacterial, viral, parasitic–protozoal, and fungal organisms. Fungi are common in our environment, but only a few are pathogenic. In general, fungi are organisms of low pathogenicity, emerging as opportunistic organisms thriving in a compromised host. Fungal infection may involve craniospinal axis, meningeal coverings, cerebrospinal fluid (CSF), brain, and spinal cord separately or in various combinations. Although fungal infections (mycoses) of the CNS are uncommon, the spectrum of neurologic manifestations of fungal infections in the CNS is of particular importance to the neurosurgeons.1–18 Therefore, the diagnosis of CNS fungal infection should be considered in appropriate clinical settings.
Historical Aspects
Fungal infections have been recognized since early times, but CNS fungal infections have mainly been recognized since the 19th century. Common fungal infections are relatively benign; serious ones are rare. There are morphologic similarities in various fungi, which create difficulty in differentiating these structurally complex forms.1–19 Generally, Hippocrates is credited with the first description of candidiasis in his book Epidemics, in which he described white patches in the oral cavity of a debilitated patient. The fungal etiology of these thrushes was established in 1840s by Berg and Bennett. Zenkar described the first patient with intracerebral candidiasis, who died in 1861. Smith and Sano were the first to report a case of Candida meningitis in 1933.16,19 Various postmortem studies have established that candidiasis is a more common CNS fungal infection than aspergillosis, zygomycosis, or cryptococcosis.
In 1792 in his book, Micheli (a priest and botanist) described Aspergillus to refer to the nine species of fungi that resembled aspergillum, a perforated globe frequently used to sprinkle holy water during religious ceremonies. In 1856, Virchow had published the first complete microscopic description of Aspergillus. However, it was Pope in 1897 who reported the first case of rhino-orbitocerebral aspergillosis and of cerebral aspergillosis in man due to extension of fungal infection from sphenoid sinusitis.2,14,17 The first case of human zygomycetes was described by Kurchenmeister in 1855 that isolated nonseptate hyphae from a cancerous lung, and Gregory in 1943 described rhinocerebral zygomycosis9 in detail, with presentation of three cases of the disease.3,11,12
In 1905, Van Horseman probably first demonstrated Cryptococcus in spinal fluid, although cerebral Cryptococcus was initially described by Buses in 1894.9 Gonyea19 reported on three patients of blast mycosis meningitis in 1978 without extracranial infection, and Opus was first to report a coccidioidal brain lesion in 1905.3 In 1952, Binford published a case of cerebral abscess due to Cladosporium.17 Histoplasmosis was first described by Darling in 1906 in a patient with disseminated granulomatous infection. Histiocytes were studded with Histoplasma capsulatum.20
Gilchrist described blastomycosis in 1894 and successfully grew the organism in culture.21 Coccidioidomycosis was described by Dosadas and Wernicke in 1892. However, Ophuls was the first to describe coccidioidal meningitis in 1905. Paracoccidioidomycosis was first described in 1908 by Lutz in Brazil. In 1888, Nocard described acid-fast aerobic actinomycetes in cattle, which was called Nocardia farcinica by Trevisan in 1889, and Eppinger in 1891 reported the first case of metastatic (from lung) cerebral nocardiosis.22,23
Antifungal chemotherapy began in 1903 with the successful use of potassium iodide for the treatment of cutaneous–subcutaneous sporotrichosis.22 The first useful polyene drug, nystatin (1953) and second useful polyene drug, amphotericin-B (1956) were introduced for mucosal and systemic mycoses, respectively.8–28 Amphotericin-B remains the standard against which other antifungal drugs are compared.22,27 Although, fungal infections in the CNS have been recognized for more than 100 years, until the antifungal agent amphotericin-B was discovered, fungal infections were regarded as nearly impossible to treat effectively.
Over time, other important antifungal drugs were reported following amphotericin-B, that is, flucytosine (5-fluorocytosine, 1964) and azole drugs (1970s): miconazole (1978), ketoconazole (1981), fluconazole (1990), and itraconazole (1992).22–34 In the last two decades, some potentially beneficial antifungal drugs have been discovered and introduced for their appropriate use. To reduce the toxicity of amphotericin-B, liposomal amphotericin-B, or its combination with lipids, was initially introduced,25,28,33 followed by triazoles (voriconazole, posaconazole, etc.) and, more recently, echinocandins (anidulafungin, caspofungin, micafungin, etc.). These medications are increasingly used in various combinations in seriously ill patients with invasive mycoses with favorable results and giving some hope in improving our results in the future.
Classification and Epidemiology
The phylum Thallophyta includes certain plants (cells that contain chlorophyll and therefore synthesize their own food) and fungi (organisms devoid of chlorophyll that are therefore saprophytic).9 Fungi are ubiquitous microorganisms that may be unicellular or filamentous. The latter produces branching hyphae, which grow only at the apex. The enzymes produced by the complex fungal cell wall break down proteins, carbohydrates, and other macromolecules. The resultant micromolecules are taken up by the fungal cells or hyphae to maintain their life processes.18 One million known fungal species exist in the nature, and around 200 species are known to be pathogenic to humans. Only about 20 fungal species produce invasive systemic infections, including CNS disorders.
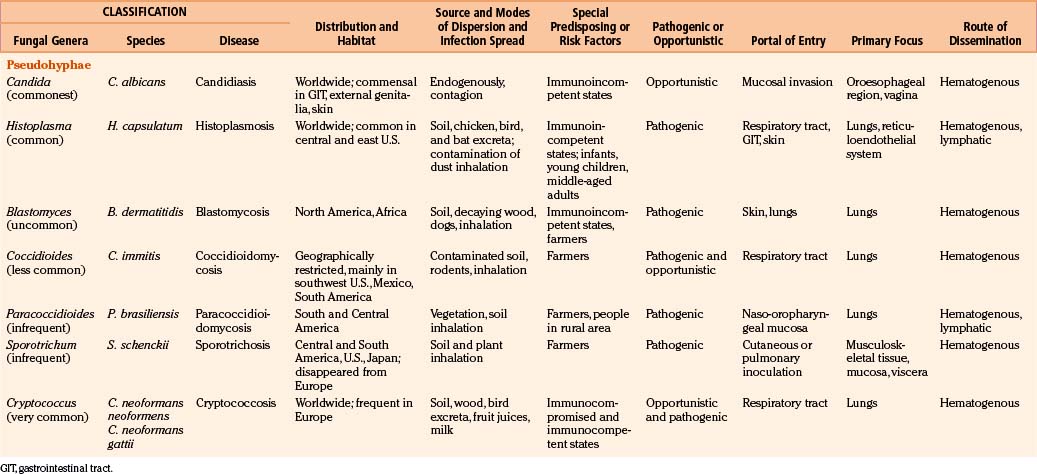
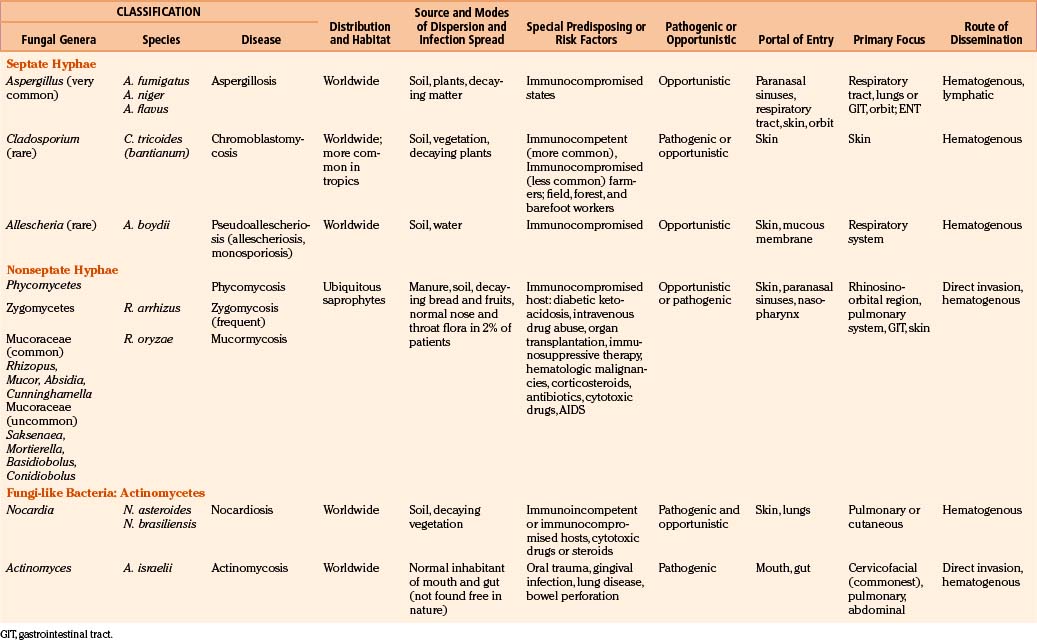
Fungi are classified as follows: pseudomycetes/yeasts (Blastomyces, Candida, Coccidioides, Cryptococcus, Histoplasma, Paracoccidioides, and Sporotrichum), septate mycetes (Aspergillus, Cephalosporium, Cladosporium, Diplorhinotrichum, Hormodendrum, Paecilomyces, and Penicillium), and nonseptate mycetes (Absidia, Basidiobolus, Cunninghamella, Mortierella, Mucor, and Rhizopus).3,6,9,18
Prevalence, Dispersion, and Infection
In general, fungi are ubiquitous, nontransmissible from patient to patient, and rarely pathogenic to humans (Table 149-1). Fungal infections are not notifiable diseases, and precise information on their prevalence throughout the world is not available.3,6,18 However, most fungal infections are not geographically restricted. Fungi are abundant in the environment, including soil and vegetation. Little moisture and organic matter, such as dead plant or animal material, are all that are required for their growth. They have long been recognized as agents of spoilage and destruction. Although only a few mycotic diseases are exclusively tropical, some of them are predominant in regions where they find ideal climatic conditions for their development. In addition, poverty, poor working conditions, and the habit of walking barefoot provide additional conditions for the spread of mycoses.
Fungal spores are common in the air, which is the medium for their dispersion. Man acquires infection by inhalation of airborne spores, implantation of viable fungal elements through the cutaneous puncture wounds, ingestion, and contagion from infected animals or from organisms already present as commensals. Most human mycoses are confined to lining surfaces, such as the skin, lungs, gastrointestinal tract, and female genitalia, and do not involve deep parenchymal organs.19 Usually, the inhaled aerosolized fungi initiate a primary mycotic infection in the lungs or paranasal sinuses, which is usually self-limiting but may spread to the other organs.
Fungal infections in the brain are invariably secondary to infections elsewhere in the body; however, the site of entry may remain unrecognized, and cases have been reported in which the only evidence of fungal infection was in the CNS.19,35,36
Routes of Dissemination
Candida may be endogenous, inhibiting the digestive tract and vagina.37–64 Aspergilli65–87 and zygomycetes88–98 colonize and infect the structures adjacent to the cranial cavity, such as the sinuses, nasopharynx, middle ear cavities, and mastoid air cells. Histoplasmosis99–104 and cryptococcosis105–113 spread by the blood stream to the CNS, meninges, or parameningeal structures from a primary, often subclinical pulmonary focus; only rarely does the organism reach the CNS following direct inoculation after trauma, surgery, or lumbar puncture. Colonization of artificial prosthesis, implants, and other devices—such as intravenous or arterial lines, peritoneal dialysis catheters, or ventriculoperitoneal, ventriculoatrial, or external ventricular drainage systems—by Candida is becoming increasingly common.16,18,35,61,109–116
Host Susceptibility and Fungal Virulence
All fungi may be considered potential pathogens when normal defenses are compromised (Table 149-1). The establishment of infection depends on the host defenses, route of fungal exposure, and size of the inoculum, as well as the virulence of the organism. The presentation for a given organism is fairly consistent, regardless of the underlying host immune status. Nevertheless, the immune status of the host preselects certain organisms. Fungi, which produce systemic infection, fall into two major groups: pathogenic fungi and opportunistic fungi.2–19 In general, pathogenic fungi infect both normal hosts and individuals with reduced host defenses, whereas opportunistic fungi infect mainly immunocompromised patients. Pathogenic fungi are usually yeasts capable of establishing life-threatening CNS mycotic infections: coccidioidomycosis, histoplasmosis, blastomycosis, sporotrichosis, and paracoccidioidomycosis. Cryptococcosis is found in equal numbers of otherwise healthy people and immunosuppressed patients. Many opportunistic fungal infections develop almost exclusively in the immunosuppressed population. Septate mycetes (aspergilli), nonseptate mycetes (zygomycetes), and yeasts (Cryptococcus and Candida mycetes) are mainly opportunistic fungi. However, the distinction between pathogenic and opportunistic fungi is not absolute.
Pathogenesis
Fungi grow profoundly in environments with an abundance of organic matter and water.2,4–9,11,23,37–45,49–51 Enzymes produced by the fungal cell wall act on the organic matter and break down their proteins, carbohydrates, and other macromolecules into micromolecules, which are then easily used by the fungi to maintain their life processes. Generally, the brain and spinal cord are regarded as immunologically privileged sites. The brain has a specialized, relatively impermeable blood–brain barrier (BBB), and its surrounding subarachnoid spaces have effective meningeal barriers. These highly vascular structures provide effective resistance to fungal infections. For immune surveillance, mainly activated T-lymphocytes are usually permitted across the BBB in normal individual. However, in immunocompromised states, these anatomic and functional barriers are easily overcome by common opportunistic and/or pathogenic fungi to produce clinical manifestations. Fungal infections of the CNS also evoke humoral and cellular responses like those in bacterial infections to enable the host to eliminate the pathogen. Activation of the resident brain cells by fungi combined with relative expression of immune-enhancing and immune-suppressing cytokines and chemokines may play a determinant role and partially explain the immunopathogenesis of CNS fungal infections. Activated resident brain cells such as microglia, astrocytes, and endothelial cells express major histocompatibility complex class I and II molecules and therefore act as antigen-presenting cells. In addition, they express complement (C) receptors and produce cytokines, chemokines, and molecules with antifungal activity such as nitric oxide. They are also capable of phagocytosis. Microglia, acting as antigen-presenting cells, stimulate T-cell proliferation and cytokine secretion, which in turn stimulate these microglial cells to ingest and more effectively kill invading fungi. The C system is a key component of innate immune system, playing a central role in host defenses against pathogens. It is also a powerful drive to initiate inflammation and if unregulated can result in pathologic changes that lead to severe tissue damage. It is generally accepted that the C system is essential to mediate cytolysis of fungi. Furthermore, it is well known that C receptors expressed by activated microglial cells are important to mediate phagocytosis. Immunopathogenesis of CNS fungal infections in humans is not completely understood. The exploration of the genomic sequence of most fungal pathogens can help better understand the pathogenesis, virulence, and immune response of host defenses against these pathogens. Further studies in these areas will advance our understanding about CNS mycoses: Fungi produce diseases due to their allergenicity, toxigenicity, pathogenicity, and neurotoxicity, but CNS mycoses are essentially due to serious infective processes.18
CNS fungal infections have been on the increase in the last decade due to many factors, such as the growing number of immunocompromised patients who survive long periods; widespread use of immunosuppressive drugs; a large aging population with an increased number of malignancies; lymphoma and leukemia, especially when associated with leucopenia/granulocytopenia (Candida and Aspergillus); the spread of acquired immune deficiency syndrome (AIDS); poorly controlled diabetes mellitus (zygomycosis); and renal and other transplant patients requiring prolonged immunosuppression.3,6,9,12,18,19
Clinicopathologic Syndromes
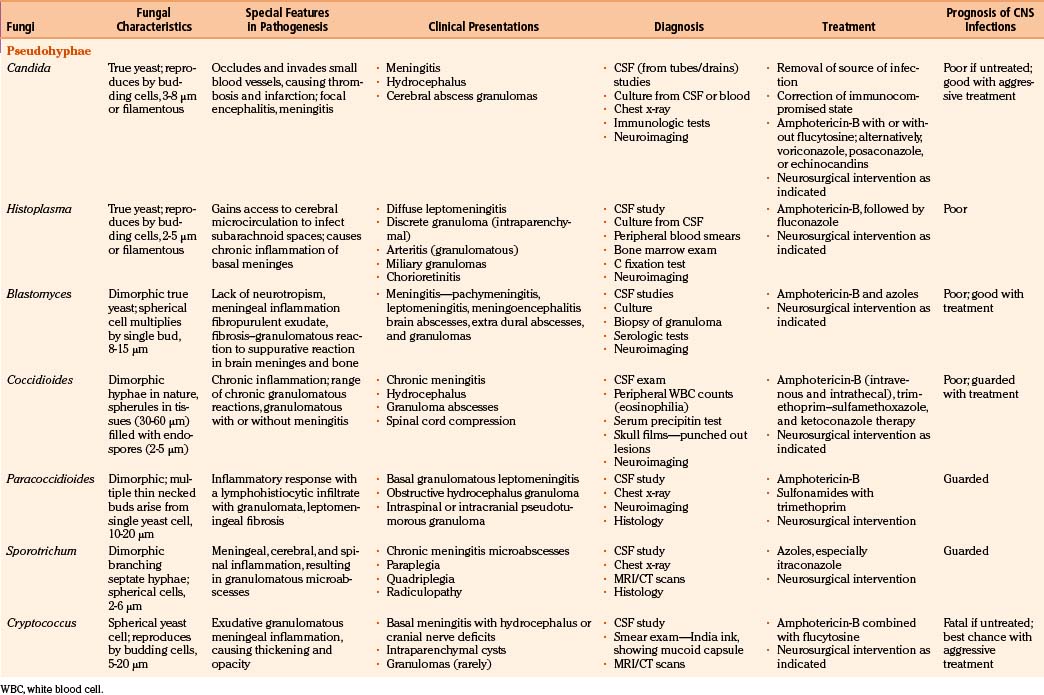
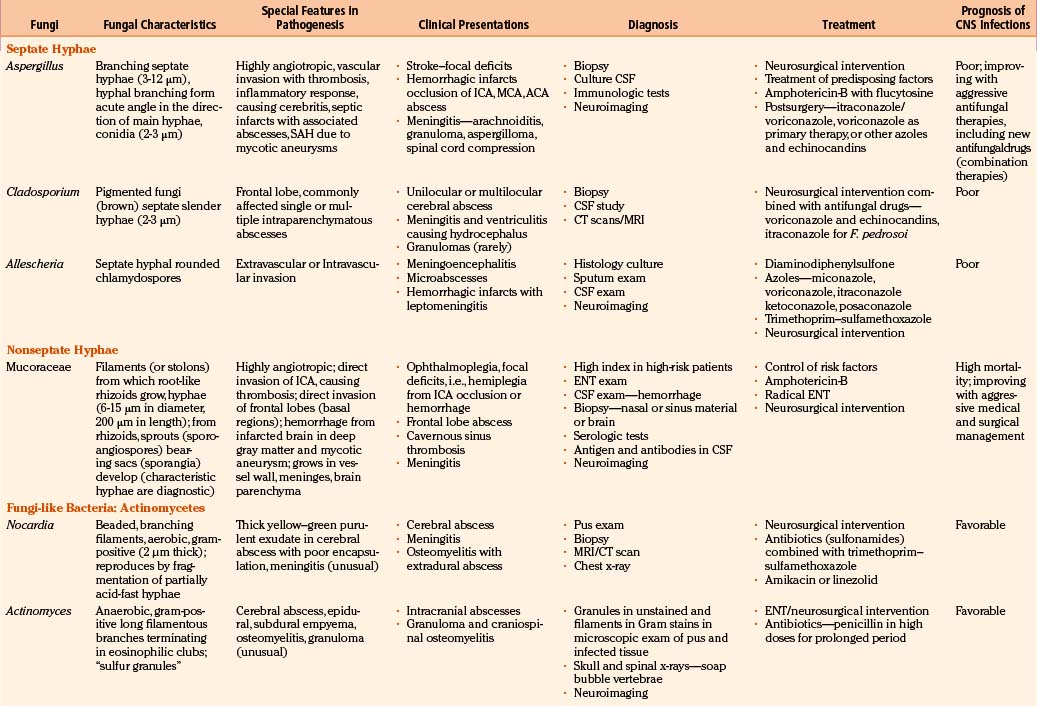
Fungi infecting the CNS are found in three major morphologic forms with distinct clinicopathologic syndromes.6,9,12 The first, leptomeningitis (acute and chronic), is mainly produced by small pure yeasts (pseudomycetes) up to 20 μm in diameter (blastomycosis, cryptococcosis, and histoplasmosis). Because of their small size, these fungi gain access to the cerebral microcirculation, from which they infect the subarachnoid spaces. Next, cerebral abscesses are produced by larger pseudomycetes (candidiasis). These intermediate-sized fungi occlude cerebral arterioles and result in adjacent tissue necrosis that rapidly converts to microabscesses. Persistence of infection causes granulomatous inflammatory reaction in adjacent leptomeninges, neural parenchyma, or both. Finally, very large branched septate (aspergillosis) or nonseptate mycetes (zygomycosis) produce cerebral infarction. These fungi mainly obstruct the intermediate-sized and large cerebral arteries and invade the vessel wall, causing cerebral arterial thrombosis and associated cerebral infarction. The evolving hemorrhagic cerebral infarcts may convert into septic infarcts with associated cerebritis and abscesses.
Clinical Spectrum of CNS Fungal Infections
In fungal infections (mycoses), the organism can invade tissues without an underlying predisposition (pathogenic fungi), but many common systemic mycoses affect patients with abnormalities in structure, immunity, and metabolism (opportunistic fungi) (Table 149-2).
The involvement of the CNS in fungal infection may be disseminated (cryptococcosis and coccidioidomycosis), focal (aspergillosis), or multifocal (candidiasis). Due to their protean manifestations in various clinical settings, CNS fungal infections present a difficult diagnostic and therapeutic challenge to neurosurgeons. Because these organisms are uncommon and often manifest indolently, diagnosis tends to be difficult and at times missed. Expected mortality rates are high under the best circumstances, even with rapid diagnosis, aggressive medical therapy, and operative approach. The clinicopathologic findings and results of a combined series of verified cases of fungal and fungi-like bacterial (pathologic and actinomycetic) infections of the nervous system are presented from major institutions where the principal author (Sharma) either had worked (King Edward Memorial Hospital, Bombay, India, and Royal Preston Hospital, Preston–Lancashire, UK) or is working (Khoula Hospital, Muscat, Oman) or from major institutions associated with other authors (Table 149-3).
Pseudomycetes Causing Infections of the Nervous System
Candidiasis
As a complication of disseminated candidiasis, the brain parenchyma and meninges get involved, with fatal outcome in many patients. In autopsy studies, cerebral mycosis was noted to be most commonly caused by candidiasis.3–19
Candidiasis commonly presents as thrush in the oral cavity or vagina; less commonly, skin and visceral organs are involved.51 Candida organisms are found worldwide. Their pseudohyphae are associated with 2- to 3-μm spherical or oval blastospores. Candidiasis arises when the balance between Candida species and the host is altered in favor of the yeast.64 The C. albicans originally infects the gastrointestinal tract (oral cavity and esophagus) after the antibiotic treatment, then invades submucosal blood vessels, and finally disseminates hematogenously to the CNS. Organisms also reach the CNS via colonization of ventricular drains, shunt tubing, and central venous lines.16,18,35,61,114–116 Recently, AIDS has been recognized as a predisposing factor for Candida meningitis.
Neuropathology
In CNS, Candida invades small blood vessels, causing thrombosis and infarction. Disseminated granulomatous lesions may be scattered throughout the meninges and brain, causing meningitis or focal encephalitis.6,9,18 Candida meningitis can occur spontaneously, as a complication of disseminated candidiasis, or as a complication of an infected wound or ventriculostomies via direct inoculation of the organism into the CNS.61 At autopsy, gross lesions may not be apparent. Microscopically, multiple microabscesses, small macroabscesses, and microgranulomas in the distribution of anterior and middle cerebral vessels are found.6,7,9,18 The abscesses are composed of neutrophils, lymphocytes, and macrophages that evolve to a granuloma after a week. On histology, they are faintly basophilic when stained with hematoxylin and eosin (H&E) but are intensely stained with periodic acid-Schiff (PAS) and methenamine silver reaction.6,7,9,11,13,14
Clinical Presentation
The clinical symptomatology of CNS candidiasis is that of low-grade meningitis.55,62 A marked basal infiltrate may cause multiple cranial nerve palsies and deterioration in the level of consciousness, which is frequent in later stages of the disease, together with hydrocephalus.51,64 In acute meningitis, the signs of meningeal irritation are present. In newborns, particularly a low-birth-weight neonate, the diagnosis is difficult and delayed, leading to permanent neurologic sequelae. Symptoms and signs in these cases are nonspecific. Mortality is very high in untreated cases, whereas with antifungal therapy, it is reduced substantially nowadays.
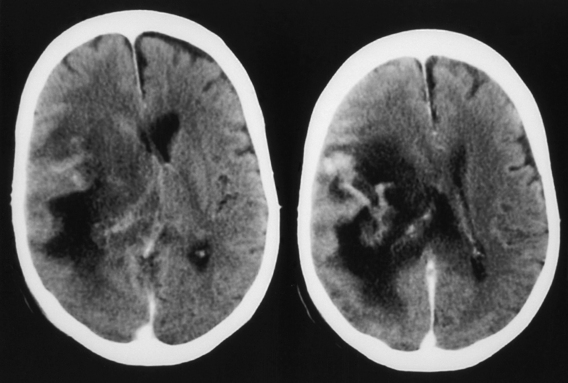
Spinal candidiasis is rare. It can involve the vertebral body46,49 and/or the disc space60 by hematogenous spread in patients with Candida sepsis. It may occur by local invasion and as a postoperative complication of spinal surgery. Persistent low back pain is a common complaint; however, 50% of cases may have significant neurologic deficits. Neuroimaging studies show nonspecific spondylitis and discitis. Among 12 patients of CNS candidiasis in our series, 8 patients presented with meningitis, 1 patient presented with encephalitis–cerebritis, 2 patients presented with cerebral abscesses (treated with aspirations), and the remaining patient presented with upper cervical cord syndrome (diffuse cord changes at autopsy) associated with cervical vertebral osteomyelitis and discitis. One patient with meningitis also had a cerebral abscess, which was successfully drained by computed tomography (CT)–guided stereotactic surgery, with good outcome (Fig. 149-1).
Diagnosis
Candidiasis diagnosis should be suspected in patients with external ventricular drains or blocked shunts. CSF examination, including cultures, should be routine. The CSF may show a pleocytosis, with either neutrophils or lymphocytes predominating, and glucose is often low. Serial serum examinations (double diffusion, counterimmunoelectrophoresis, immunofluorescence, and latex agglutination tests) are helpful. Positive blood and CSF cultures provide definitive diagnosis. Funduscopic examination is invaluable, detecting endophthalmitis before permanent loss of vision occurs. Bone or disc biopsy, histology, and culture principally make the diagnosis of spinal candidiasis.5,9,18
Magnetic resonance imaging (MRI) in fungal spinal osteomyelitis usually shows hypointensity of the vertebral bodies on T1-weighted images, with enhancement on contrast administration. There is lack of hyperintensity within the discs on T2-weighted images with preservation of the intranuclear cleft, which is in contrast with pyogenic osteomyelitis, where the intervertebral disc may be hyperintense with loss of intranuclear cleft on T2-weighted images.117 The diagnosis may be made by percutaneous needle aspiration of the involved area.
Treatment
Amphotericin-B, flucytosine, fluconazole, miconazole, and ketoconazole have been used successfully in spinal candidiasis. Decompressive surgery is usually required in patients with significant compressive spinal lesions. If there is an evidence of pus collection on spinal MRI, then preoperative image-guided aspirations to prove the fungal nature of the lesion should be followed by preoperative antifungal medications; surgical débridement and fixation should be carried out with a full postoperative course of antifungal medications to achieve good results in many cases.118 Fluconazole (with amphotericin-B in the initial phases) for 3 to 6 months postoperatively may lead to healing of fungal osteomyelitis with osseous consolidation.119 The overall prognosis is better than in earlier times due to combined medical and surgical therapies.
Histoplasmosis
Histoplasmosis is present throughout the world. It is the most frequently observed pulmonary mycotic infection in the eastern and central United States, whereas in Europe its incidence is low. It is known to invade the reticuloendothelial system.3,6,9,13,14,18 Lesions are found in the spleen, liver, and lymph nodes, as well as the lungs. CNS involvement is rare. The causative organism (2- to 5-μm yeast) is H. capsulatum, a dimorphic fungus. It is commonly found in soil. Organisms are inhaled with dust contaminated by chicken, bird, or bat excreta. The primary focus is formed in the lungs, which become calcified, but may occur in the mouth, gastrointestinal tract, or skin. In immunocompetent individuals, CNS histoplasmosis is extremely rare,99 but many patients with CNS involvement are immunocompromised by burns, antibiotics, steroids, and AIDS.6,101 Even though about 25% of the population in the United States have positive histoplasmin skin tests, CNS involvement is rare.18 Hematogenous dissemination may spread to the CNS. Neurologic involvement occurs in 10% to 20% of patients with disseminated histoplasmosis.
Neuropathology
Involvement of the CNS in histoplasmosis occurs in less than 1% of all patients with active histoplasmosis and includes diffuse leptomeningitis, discrete granuloma, periventricular granulomata, parenchymal granulomatosis, choroid plexus granulomata, and granulomatous arteritis.6,9,13,18,101,104 In diffuse basilar leptomeningitis, there is thickening of the leptomeninges, thick yellow exudate with miliary granulomas along the blood vessels. In chronic cases, meningeal fibrosis with hydrocephalus develops. Meningitis is proportionally less prominent in histoplasmosis than in cryptococcosis and coccidioidomycosis. Histoplasmosis granulomas mimic sarcoidosis, other fungal, or tubercular mass lesions consisting of central noncaseating granulomas or small caseous areas surrounded by macrophages, lymphocytes, plasma cells, and Langhans giant cells. It is well shown with PAS. In sections stained with H&E, shrinkage of the organisms produces a halo and gives an impression of a capsule.9 Methenamine silver stain demonstrates macrophages packed with organisms, reactive gliosis, and fibrosis.
Clinical Presentation
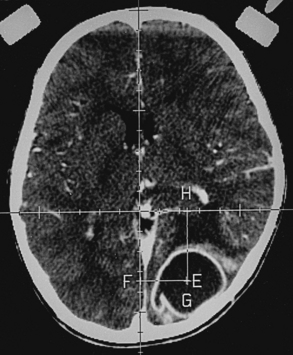
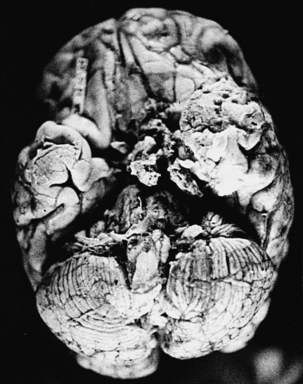
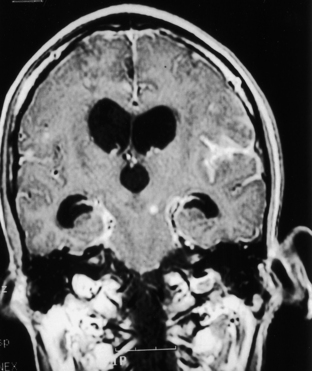
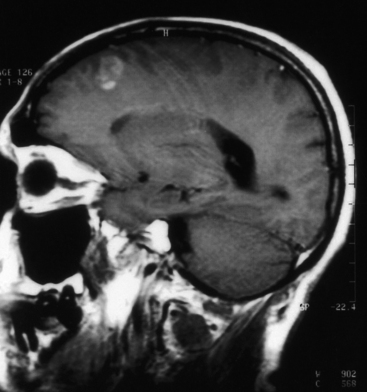
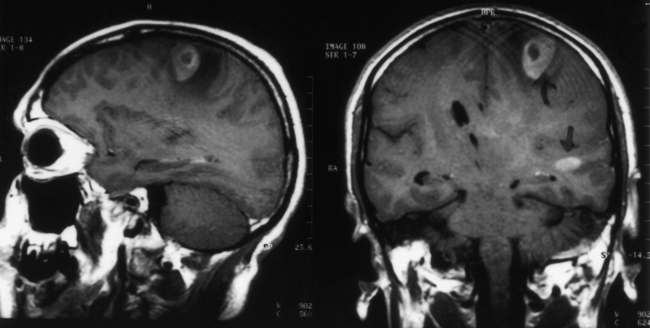
Histoplasmosis has two peaks of incidence: one in early childhood and the other in middle age.104 Four forms are described as leptomeningitis, cerebritis, military granulomas, and spinal cord lesions. Leptomeningitis is common, whereas other CNS lesions in histoplasmosis are rare. The condition usually presents as chronic meningitis with or without hydrocephalus. Mass lesions are rare (Fig. 149-2), and occasionally chorioretinitis is seen. The skull and vertebrae may be involved by osteomyelitis with secondary spinal cord compression, especially with H. duboisii.5,104
Diagnosis
The clinician should maintain a high index of suspicion with patients who are from any area endemic for histoplasmosis. Definite diagnosis is made by demonstration of organism by culture of sputum, CSF, and serum or on histology.99–104 C fixation, agar gel, diffusion testing, and radioimmunoassay (urine or serum) are helpful. Biopsy supplies a more definitive answer. The CSF is usually under pressure, shows a moderate pleocytosis up to 300 cells per cubic millimeter, and is more often mononuclear than polymorphonuclear in type. Proteins are elevated, glucose is somewhat reduced, and the organism is cultured from the CSF samples in about 50% of cases. Peripheral blood smear and bone marrow examination may yield early diagnosis. MRI shows enhancing mass lesions in the brain. On T1-weighted images, Histoplasma lesions appear as hypointense rims, and they show surrounding edema on T2-weighted images. CT scans show ring-enhancing lesions.
Blastomycosis (North American Blastomycosis)
Blastomycosis is endemic in the southeastern regions of the United States and widely reported in Africa.17,18,58 The causative agent, B. dermatitidis, is found in the soil, may have a natural reservoir in dogs, and has worldwide distribution. The fungus is a spherical cell 8 to 15 μm in diameter that multiplies by a single bud, which is attached to the parent cell by a broad base. Blastomycosis is mainly a pyogranulomatous (blastomycoma) disease; it begins as an initial subclinical pulmonary lesion and then hematogenously spreads to other organ systems. The yeasts are phagocytized by pulmonary macrophages, which may disseminate to produce secondary lesions in the skin, bone, and genitourinary system, but the CNS rarely gets involved. In agricultural workers, the primary focus is in the lungs or skin.17–1958 Hematogenous spread of infection to the CNS occurs rarely (in about 5% of patients). In endemic areas, blastomycosis is seen in about 40% of AIDS patients.6,9,18 However, it is a less common mycotic infection when involving the CNS compared to histoplasmosis and coccidioidomycosis.120–127
Neuropathology
Primary CNS blastomycosis is extremely rare, whereas secondary CNS involvement occurs in 3% to 33% of cases of disseminated blastomycosis. Typically, cerebral blastomycosis produces leptomeningitis, meningoencephalitis, brain abscesses, paradural abscesses, and adjacent granulomas. Grossly, two fifths of cases present with chronic leptomeningitis, one third with granulomas and abscesses, and one fourth with spinal epidural granulomas and abscesses. Epidural abscesses occur rarely secondary to craniovertebral osteomyelitis. Fibrosis in subarachnoid spaces can cause hydrocephalus.125,126 Abscesses may be extradural, subdural, or intraparenchymatous in location. No region in the CNS or peripheral nervous system is immune. It may be either focal or disseminated basal leptomeningitis with fibrinopurulent exudate. This can block subarachnoid spaces, causing hydrocephalus. Pachymeningitis may result. Histologic features elicit a mixed granulomatous and suppurative reaction. The center of an abscess contains caseous necrotic material with cells (lymphocytes, neutrophils, plasma cells, macrophages, and Langhans giant cells) and organisms. H&E unstained wet preparation, PAS, and methenamine silver stain demonstrate the organism. Bone and vertebral disc destruction with paraspinal abscess closely simulates tuberculous disease of the spine.5,58,121,123,127
Clinical Presentation
CNS blastomycosis presents with headaches and neck stiffness; intracranial blastomycotic abscesses or granulomas result in increased intracranial pressure (ICP) with or without localized signs.120,125,126 Eventually, these patients develop convulsions, mental deterioration, confusion, and lethargy. Plain CT scan may show iso- or hyperattenuation lesions. However, small solitary lesions show homogeneous enhancement on CT scanning with surrounding edema. Slightly bigger lesions may show ring-enhancing lesions with surrounding edema. In our series, all six patients with CNS blastomycosis presented with signs and symptoms of raised ICP, mainly due to granulomas. Motor impairment, cranial nerve palsies, and visual symptoms were also encountered in varying degrees. One patient had rhino-orbital blastomycosis, and another patient had blastomycosis of mastoid sinus. Initial clinical impression in four cases was a progressive space-occupying lesion, and in two cases, a space-occupying lesion was seen with meningeal signs. Neurosurgical interventions for the intraparenchymatous lesions as seen on CT scans resulted in good outcome in five patients with histologic diagnosis of blastomycotic granuloma. One patient died who had postmortem evidence of meningitis, subarachnoid hemorrhage (SAH), cerebral abscess, and cerebral infarction, in addition to a cerebral granuloma. Blastomycosis in African countries commonly affects the thoracic spine, ribs, and sternum by direct extension from the lungs.121,123,127 Osteomyelitis and discitis are associated with paraspinal abscess formation.5,123 Clinical and radiologic features are similar to tuberculosis, with which blastomycosis sometimes coexists.5
Coccidioidomycosis (Modeling Valley Fever)
Coccidioidomycosis is caused by C. immitis, which is probably the most virulent fungi, causing human mycoses accounting for about 100,000 cases per year in the United States, with 70 to 80 deaths annually.128–132
It is a geographically restricted mycosis, which includes regions of semiarid climate. Its distribution corresponds to areas where warm temperatures and dry conditions exist. It is endemic in the southwestern United States (especially in San Joaquin Valley, California, and in Arizona, where 85% of the population is skin test positive), Mexico, and South America, particularly Argentina and Paraguay.133,134 Both mycelia and spores are carried a considerable distance by wind or transported by rodents. The causative organism C. immitis is a nonbudding spherical structure measuring 20 to 70 μm in diameter with a double refractile capsule. Mature forms are filled with numerous small (two to five) endospores. C. immitis is a dimorphic fungus producing hyphae and arthroconidia (arthrospores) in its saprophytic (soil) environment and spherules (sporangia) in infected tissues. Pulmonary infection is contracted by inhalation of the airborne arthroconidia in immunocompetent or immunocompromised individuals. Most infections are self-limited. About two thirds of cases of pulmonary infections are asymptomatic, while one third of cases develop mild through severe pulmonary disorders. However, the majority of patients recover and develop strong, specific immunity against infection.135–138 The fungus is therefore considered both a pathogen and an opportunist. Hematogenous spread to the CNS occurs in about 50% of cases as a terminal event.6,18
Neuropathology
With coccidioidomycosis, meningeal inflammation results in accumulation of exudate, opacification of leptomeninges, and obliteration of sulci with caseous granulomatous nodules at the base of brain and in the cervical region.6,9,18,131,134 Extensive fibrosis causes obstructive hydrocephalus. Invasion of blood vessels leads to multiple aneurysms.131 Unusually large granulomatous lesions and frank abscesses can occur in the brain or spinal cord parenchyma. The microscopic picture mimics tuberculous meningitis. Meningeal fibrosis, granulomas (organisms surrounded by epithelioid cells, giant cells, lymphocytes, and plasma cells), small abscesses with caseous necrosis, and vascular invasion are seen. On H&E stains, Coccidioides are basophilic, and they are well demonstrated with methenamine silver stains.
Clinical Presentation
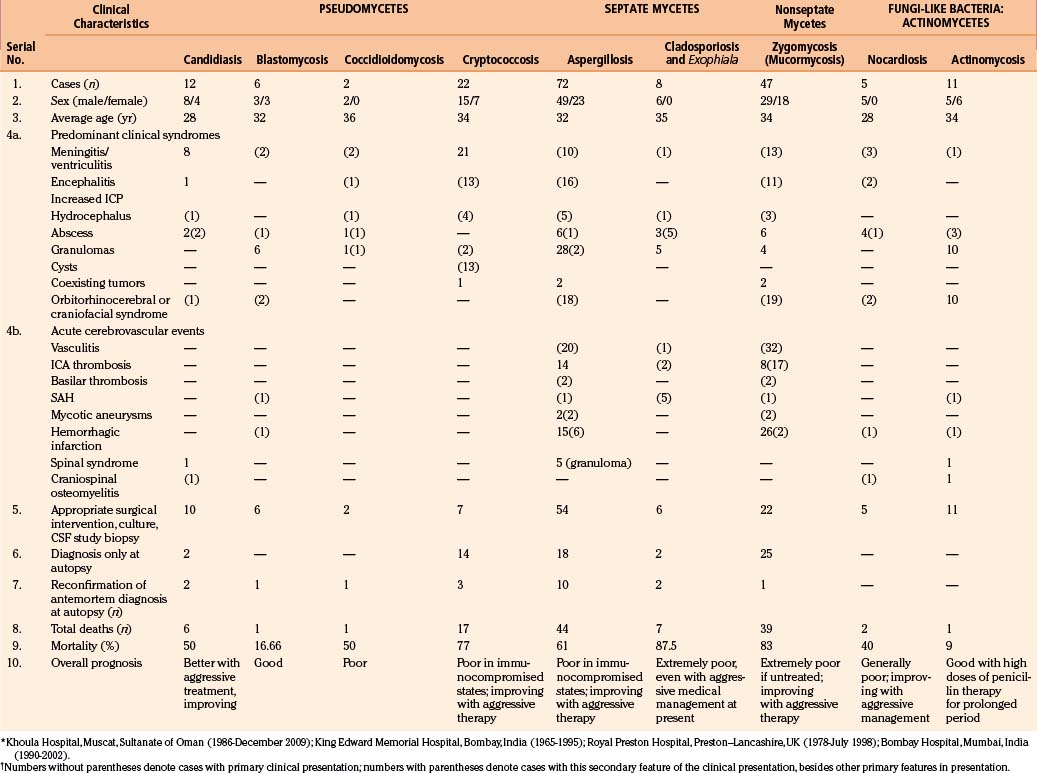
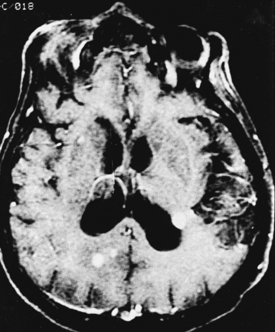
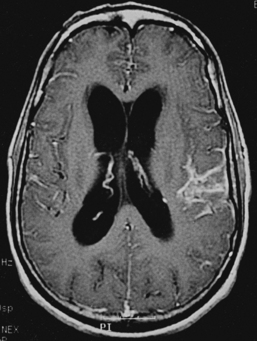
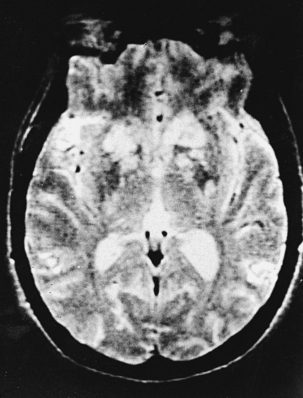
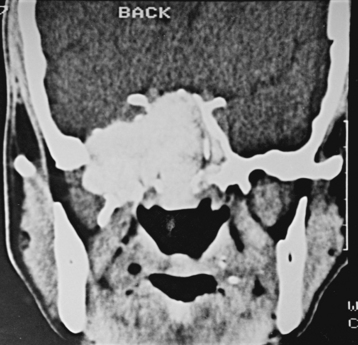
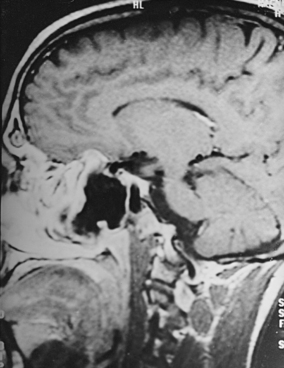
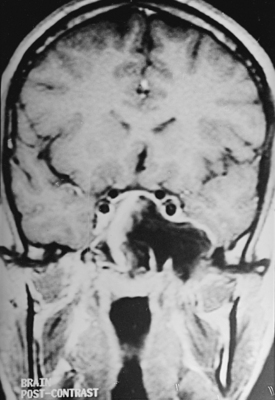
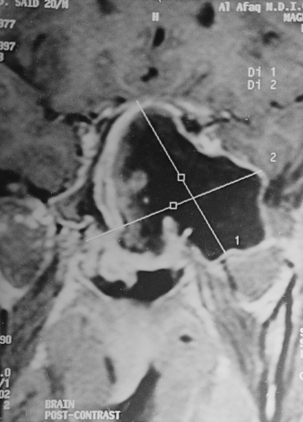
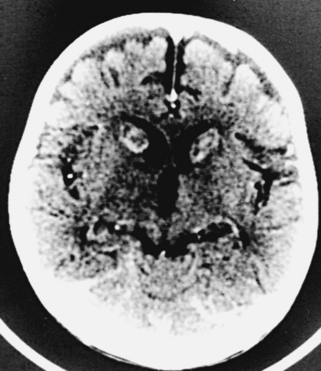
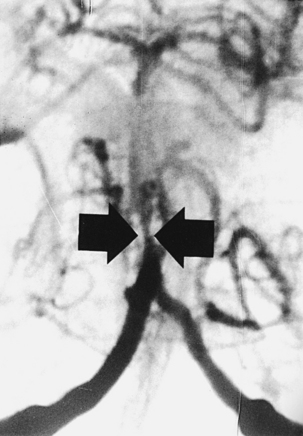
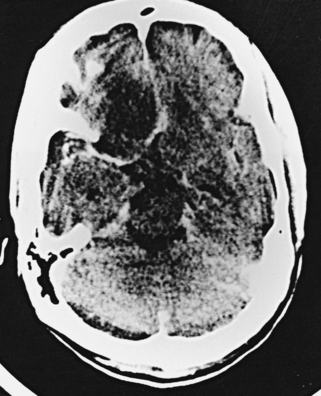
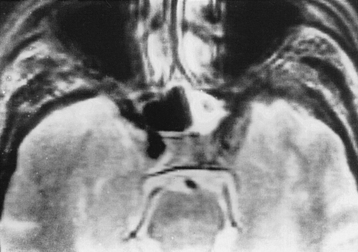
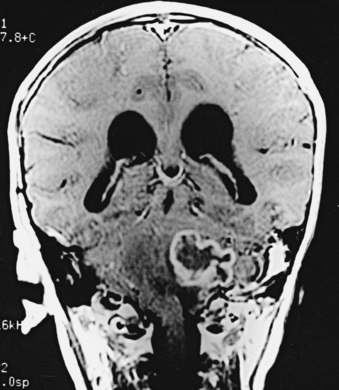
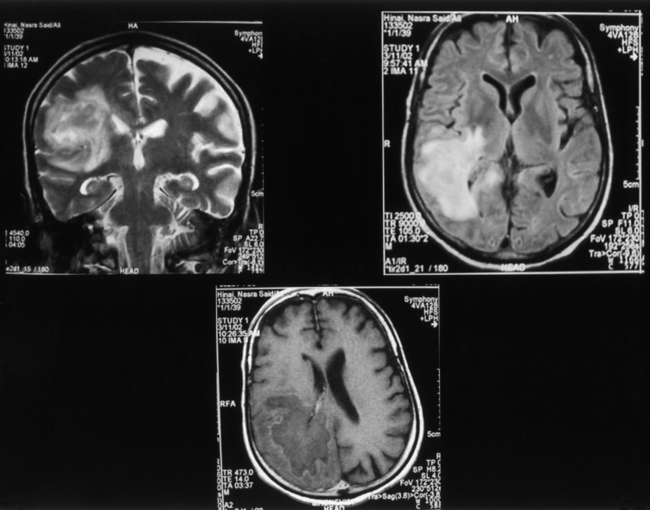
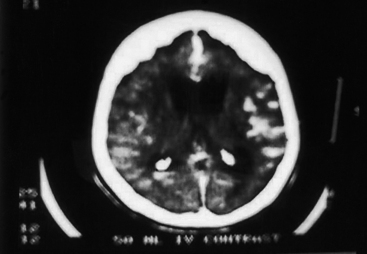
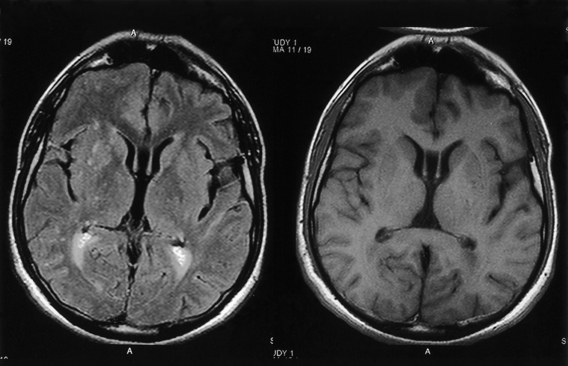
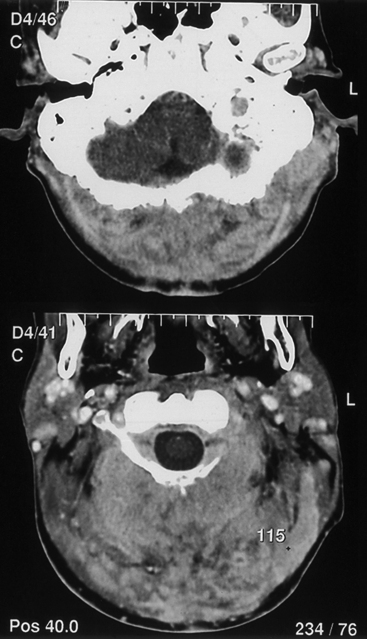
CNS coccidioidomycosis presents as acute, subacute, or chronic symptomatic meningitis; transient focal deficits (aphasia and hemiparesis); confusion; restlessness; and depression. Multiple cranial nerve palsies, raised ICP, and hydrocephalus occur as complications of basal meningitis, and/or mass lesion.128,133–138 Donor-related coccidioidomycosis in organ transplant recipients is also described. In our series, one fatal case of CNS coccidioidomycosis presented with signs of raised ICP, multiple cranial nerve palsies, and rapidly progressive sensory motor impairment in limbs. MRI studies showed evidence of leptomeningitis, intraparenchymatous brain stem granulomatous lesions, and hydrocephalus (Figs. 149-3 and 149-4). A nonfatal case with cerebral granulomas and leptomeningitis was biopsied and then treated with antifungal medications. Myelopathy may result from adhesive meningitis or from a mass lesion or an extradural abscess associated with a vertebral osteomyelitis. Spinal coccidioidomycosis may present as acute or chronic spondylitis in the thoracic and lumbar regions, especially in cases of disseminated coccidioidomycosis.5,130 Apart from vertebral bodies, the pedicles, transverse processes, laminae, spinous processes, and contiguous ribs may be affected; however, intervertebral discs are relatively spared. Coccidioidomycosis usually presents as progressive spinal cord compression.5,130,134 Paraspinal masses and sinuses are commonly seen, and meningitis rarely occurs, with fatal outcome.
Diagnosis
With coccidioidomycosis, CSF is usually under increased pressure but occasionally shows evidence of a spinal block. Raised proteins, reduced glucose, and a persistent pleocytosis may be present, and the organisms can be recognized in wet preparations of the CSF. A high index of suspicion for raised erythrocyte sedimentation rate, calcified nodes on chest x-ray, and craniospinal bone lesions on CT scans/MRI of the cerebrospinal axis (mass lesion and hydrocephalus) form the mainstays of investigations. Osteomyelitis of the skull or vertebrae may be visible on the plain x-ray film. Osteomyelitis may also present as causing punched-out lesions on skull films and an underlying abscess on CT/MRI studies. Radiolucent vertebral lesions devoid of surrounding sclerosis in early cases and dense sclerotic vertebrae with normal disc spaces in late states are features seen on plain x-ray films. However, bone changes and paraspinal lesions are well delineated on CT/MRI studies. Differential diagnosis includes multiple myeloma, sarcoidosis, histiocytosis, and metastases. It is unusual for coccidioidomycosis to present as a solitary bone lesion, which may mimic a primary bone tumor. Fine-needle aspiration biopsy helps the clinician differentiate between inflammatory and neoplastic processes involving bone by acquiring material for cytologic studies and cultures.135
Treatment
Antifungal drugs effective against coccidioidomycosis include amphotericin-B and azoles (ketoconazole, fluconazole, and itraconazole). Intravenous amphotericin-B is the most promising drug, with intrathecal administration via lumbar puncture or a subcutaneous reservoir in seriously ill or deteriorating cases. In cases of severe pulmonary compromise, where an early response is highly desired, amphotericin-B is used for its rapid onset of action. Azoles are used for the chronic processes. When localized mass lesion is causing compression of the brain/spinal cord, appropriate neurosurgical intervention is carried out and intrathecal azoles have been used. Coccidioidomycosis osteomyelitis remains a rare but difficult disease to treat, with a lifelong risk of recurrence. Spinal decompression and stabilization where indicated may be required, along with antifungal drugs (amphotericin-B/ketoconazole).5,6,17,18,130,134
Paracoccidioidomycosis (South American Blastomycosis)
Paracoccidioidomycosis is a chronic progressive granulomatous disease primarily spreading from external nares to the lungs and thereby to local lymph nodes.139–143 It is present from Mexico to Argentina and in South American countries—Brazil, Venezuela, and Colombia.6,9,18 It is especially endemic in South America and Mexico and is caused by P. brasiliensis, a dimorphic fungus. Multiple thick, walled buds arise from single yeast. The organism lives in the soil or vegetation, and farmers are most affected. Lesions of the mucous membranes, especially of the mouth, nasal passages, pharynx, and lungs, are the primary foci in previously healthy patients. Disseminated disease involves many organs, including liver, spleen, and bones. The anatomicoclinical manifestations of the disease have been classified as follows: tegumentary forms (mucocutaneous), lymphoid forms, visceral forms, and mixed forms.
Neuropathology
Three main types of pathologic CNS lesions are seen: (1) granulomas—South American blastomycomas/paracoccidioidomycomas (commonest)—(2) meningitis with or without hydrocephalus (uncommon), and (3) an abscess (rare).3,6,12–14,18,140–143 The pseudotumors or granulomas may be intraparenchymatous (common) or meningeal (uncommon). They may occur in the dura mater with clinical characteristics of meningiomas.140,141,143 Leptomeningitis is typically of the basal granulomatous type. Spread to the brain parenchyma occurs through the Virchow-Robin spaces, especially at the level of the hypothalamus and the lateral cerebral fissures. Granulomas are formed by epithelioid cells: Langhans giant cells and lymphohistiocytic inflammatory infiltrate. Nodules may resemble tubercles.
Treatment
Surgical excision of the mass lesions, together with therapy with amphotericin-B (the drug of choice), offers the best chance of paracoccidioidomycosis treatment. Sulfonamides, sulfamethoxazole, trimethoprim, and ketoconazole are also helpful. Trimethoprim–sulfamethoxazole is one of the best combinations, with good outcome. Therapy may also consist of long-term administration of itraconazole. Early diagnosis and adequate therapy may prevent extensive tissue destructions. Long-term follow-up is mandatory. Clinical improvement may be accompanied by diminishing P. brasiliensis antigen and antibody titers in cases of neuroparacoccidioidomycosis.139–147
Sporotrichosis
Sporotrichosis is caused by S. schenckii, which is found in the soil and on the plants.4,6,9,18 Domestic cats may be an important carrier of agents of sporotrichosis to humans.148–150 The infection usually starts when the fungus is inoculated peripherally at the site of cutaneous injury or is inhaled. Lymphangitis or granulomatous pneumonitis is produced. The fungus may then disseminate hematogenously to other organs, including the CNS.
Neuropathology
Cerebral sporotrichosis shows features of chronic meningitis, microabscesses, and granulomas.6,18 Microscopically, widespread cortical granulomatous microabscesses in the brain, spinal cord, or spinal nerve rootlets are seen.
Cryptococcosis (European Blastomycosis)
Cryptococcosis is one of the commonest CNS fungal infections in immunocompromised patients.151,152 It is ubiquitous but more common in Europe than elsewhere.18 It is a generalized systemic visceral mycosis affecting previously healthy people66; however, in 50% of cases, it has been reported in immunocompromised subjects, children, and middle-aged and older males.24 Pigeon breeders are at special risk. The causative agent, C. neoformans, is a spherical budding capsulated yeast (5-20 μm) (Fig. 149-5). It is found in soil and wood contaminated with bird excreta. It has been isolated from fruit juices and milk. C. neoformans neoformans causes disease in immunocompromised hosts, and C. neoformans gattii is the cause in immunocompetent hosts6; the portal of entry is the respiratory system. The primary focus lies in the lungs, from which secondary systemic dissemination occurs via hematogenous spread. There is a strong neurotropic tendency to involve meninges and the brain.105–113143 CNS cryptococcal infection commonly presents with meningitis (subacute or chronic) or meningoencephalitis. Acute fatal meningitis occurs rarely in cryptococcosis. Usually, chronic meningitis present with the periods of remission and relapses. Cryptococcosis is one of the common CNS fungal infections in immunocompromised patients: nearly one tenth of patients with human immunodeficiency virus (HIV) develop cryptococcosis, and many HIV patients have cryptococcal meningitis as their initial clinical presentation.

FIGURE 149-5 India ink preparation of the CSF showing the mucoid capsule of the cryptococcal spherules.
Neuropathology
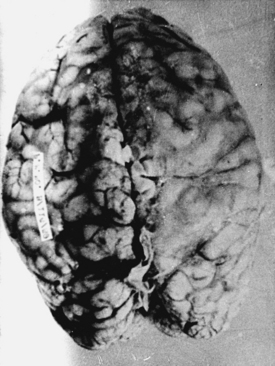
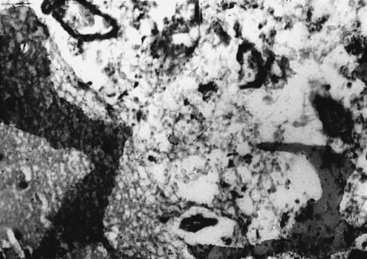
With cryptococcosis, the leptomeninges become infiltrated, thickened, and opaque (Fig. 149-6).3,8,17,18,143 The Virchow-Robin spaces around penetrating vessels are distended with organisms (Fig. 149-7).6,18 Granulomatous lesions can be found in the cerebral or spinal parenchyma. Spinal arachnoiditis may also be present. Chronic fibrosing leptomeningitis may cause hydrocephalus. Less commonly, intraparenchymatous cysts are seen (basal ganglionic regions), related to exuberant mucinous capsular material produced by the proliferating cryptococci.7,105,109,131 Rarely, fungi aggregate in an inflammatory lesion and produce small or large granulomas (cryptococcomas or torulomas) in the meninges, parenchyma, ependymal surfaces, or choroid plexuses.
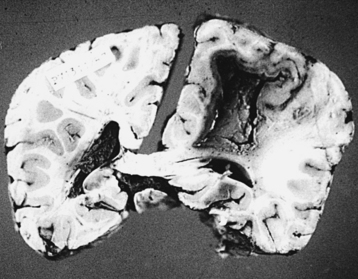
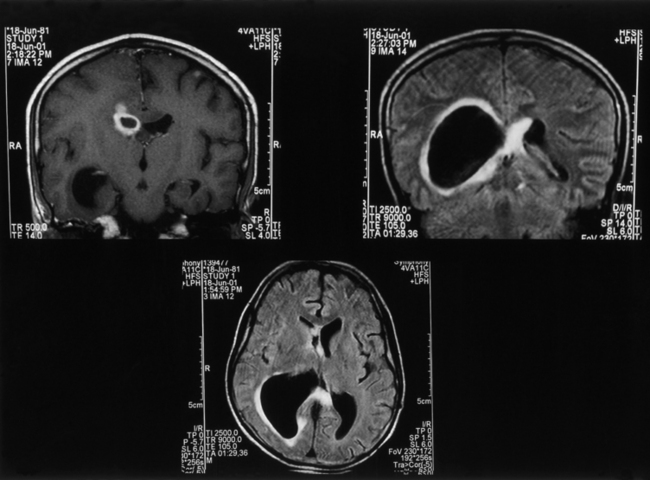
Microscopic examination shows three types of tissue reactions: (1) disseminated leptomeningitis, (2) granulomas, and (3) intraparenchymal cysts.3,6,18,107,143 In meningitis, there is minimal inflammatory response. The capsule of the fungus seems to impede inflammation by masking surface antigen. Inflammatory response consists of lymphocytes (mainly), plasma cells, eosinophils, fibroblasts, and multinucleated giant cells (studded with cryptococci).6,9,143 Glial reaction and associated cerebral edema are minimal. Granulomas are rarer late tissue reactions mimicking tubercles. They are composed of fibroblasts, giant cells (with fungal organisms), and necrotic areas. Multiple intraparenchymal cysts related to exuberant capsular material produced by the proliferating cryptococci create honeycomb-like cystic cerebral changes, especially in the basal ganglia. No membrane or capsule surrounds these cysts, which are well delineated from the surrounding tissue.9,143,151,152 Inflammatory response (macrophages with fungi and giant cells) around these cystic lesions is minimal. In our series of 22 cases, 21 cases presented with meningitis, and in an autopsy study in 17 cases, meningitis was confirmed in the form of thickened, hazy meninges with a characteristic slimy exudate over the superolateral surfaces and base of the brain. Among the secondary features, only 3 cases showed multiple granulomas in the cerebral hemispheres, hypothalamus, and, in 2 of these 3 cases, in the brain stem. Tiny cryptococcal cysts in the brain parenchyma containing plasma-like coagulated material were seen in 13 cases, and multiple areas of cystic degeneration destroying the brain parenchyma extensively and containing cryptococci were found in 13 cases (Fig. 149-8). In 1 case, multiple areas of demyelination with coexisting cerebral edema were seen, and in 4 cases, dilated ventricles were noted. In 2 biopsy cases, the structure of a granuloma consisted of inflammatory granulation tissue with foreign body giant cells and cryptococci. In 1 case of cerebral astrocytoma grade II, cryptococcosis was present within the tumor tissue and was well managed following surgery.
Clinical Presentation
CNS cryptococcosis commonly presents with nonspecific manifestations. At onset, patients usually present with headaches, nausea, vomiting, visual impairment, and papilledema; at a later stage, neck stiffness develops, followed by fever, personality changes, seizures, deterioration in sensorium, cranial nerve palsies, and hydrocephalus.7,108 In many patients, there are no physical signs. Periods of remission and relapses are noted. Cryptococcomas may produce prolonged, unresolving, focal neurologic deficits. Acute fatal meningitis in cryptococcosis is extremely rare.6,7 In our series, all 21 cases of CNS cryptococcosis presented with signs and symptoms of meningitis with raised ICP. Fever, headache, and vomiting were the commonest presenting symptoms. Altered sensorium, cranial nerve palsies, and visual symptoms were seen in 6 cases, and fatal meningitis occurred in 2 cases. Spinal cryptococcal arachnoiditis may present with progressive myelopathy or myeloradiculopathy.105–113143
Diagnosis
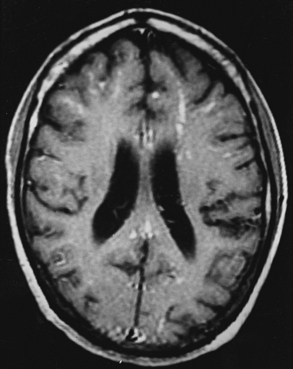
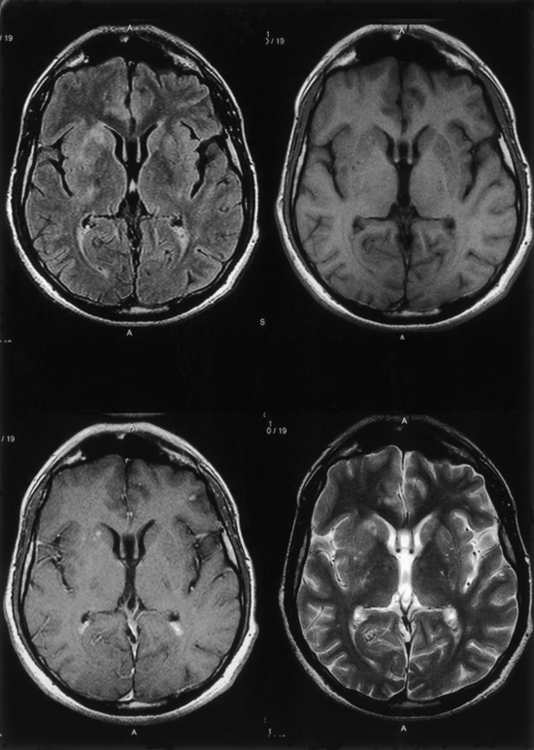
The fungal capsule is transparent with cryptococcosis; therefore, the CSF appears clear, although it is mildly xanthochromic and under high pressure. The cell count may go up to 100 cells per cubic millimeter (mainly lymphocytes and polymorphs). The sugar and chloride levels are reduced, and total proteins may be raised. As the fungal capsule is transparent on routine microscopy, India ink preparation of the CSF can demonstrate a mucoid capsule. Organisms can be seen in tissues with PAS and methenamine silver stains.6,9,18 On mucicarmine and alcian blue stains, the fungal capsule is well recognized. However, routinely, CNS cryptococcosis is diagnosed by positive cryptococcal antigen titer and India ink staining of the CSF film preparation. Chest x-ray (pulmonary lesion) and CT/MRI brain scans (brain edema, hydrocephalus, basal meningitis, granuloma, and intraparenchymal cysts) are helpful (Figs. 149-9 and 149-10). The commonest patterns of CNS cryptococcosis are ventricular dilation in CT scans and Virchow-Robin space dilation in MRI. MRI is more sensitive in detecting CNS cryptococcal infection, such as Virchow-Robin space dilation and leptomeningeal enhancement. There is no significant pattern difference between immunocompromised and nonimmunocompromised patients with CNS cryptococcosis.142 The CSF culture should be done at 30°C for 5 days. This organism can be isolated in 95% of cryptococcal meningitis cases. Positive latex agglutination testing with rising serum titers of polysaccharide capsular antigen is of prognostic value.
Treatment
Untreated cryptococcal meningitis is generally fatal. Early aggressive therapy with combined amphotericin-B and flucytosine offers best chances of cure. Alternatively a 6-week course of amphotericin-B followed by maintenance oral fluconazole gives good results. Granulomas, cysts, and hydrocephalus are treated on their own merits. Predisposing factors should be carefully corrected. Failure of treatment raises the possibility of an underlying medical disorder, such as AIDS. Patients receiving treatment before CNS complications have a good prognosis.
Septate Hyphae Causing Infections of the Nervous System
Aspergillosis
However, routinely, the majority of cases of aspergillosis are reported from countries with a temperate climate, where constant exposure to the high spore content of pathogenic Aspergillus species is present in the moldy work environment. Several species of Aspergillus can cause CNS infection, but most cases are due to A. fumigatus (primary pulmonary infection), A. Niger (primary otitic infections), A. flavus (primary paranasal sinus infection), and A. terreus.15,83 These are saprophytic, opportunistic, ubiquitous fungi found in soil, plants, and decaying matter and consisting of branching septate hyphae varying from 4 to 12 μm in width (Fig. 149-11). Aspergillus species have a worldwide distribution. The primary portal of entry for Aspergillus is the respiratory tract. Infection either reaches the brain directly from the paranasal sinuses via vascular channels or is bloodborne from the lungs and gastrointestinal tract.66–87 The infection may also be airborne, contaminating the operative field during a neurosurgical procedure.15
Neuropathology
In the majority of cases, CNS aspergillosis occurs due to sinocranial origin, where skull base syndromes are the presenting features. The primary focus lies commonly in the paranasal sinuses. Chronic mycoses of the paranasal sinuses result in orbital, cranial, and intracranial (extradural, dural, and intradural) fungal lesions.153–157 Aspergillus has a marked tendency to invade arteries and veins (angiotropic), producing a necrotizing angiitis, secondary thrombosis, and hemorrhage.3,6,9,18,83 Onset of cerebral aspergillosis may be heralded by acute manifestations of focal neurologic deficits in middle cerebral artery (MCA) and anterior cerebral artery (ACA) distributions.3,6,9,66–87 In our series of 72 cases, 28 cases had granulomas causing neurologic deficits, 6 cases had intracranial abscesses (five intraparenchymatous and one interhemispheric), 9 cases had primary presentation as internal carotid artery (ICA) thrombosis with rhinosinoaspergillosis, and in 7 other cases, ICA/basilar artery thrombosis was an associated feature. However, 15 patients presented with an acute stroke due to hemorrhagic infarction, and in 6 other patients, infarction developed during the course of management. The evolving hemorrhagic infarcts may convert into septic infarcts with associated cerebritis and abscesses. The fungal hyphae are found in large, intermediate-sized, and small blood vessels with invasion through vascular walls into the adjacent tissue; invasion in the reverse direction can also occur.9 Purulent lesions may be chronic and have a tendency to fibrosis and granuloma formation (Figs. 149-12 and 149-13). Microscopically, the most striking feature is the intensity of the vascular invasion with thrombosis, as was seen in our 20 cases as vasculitis; in 14 cases as ICA, MCA, and ACA thrombosis; and in 2 cases as coexisting basilar thrombosis. In purulent lesions (7 cases in our series), the pus is seen in the center of the abscesses with abundant polymorphs at the periphery. Granulomas consist of lymphocytes, plasma cells, and fungal hyphae as seen in 28 cases in the present study.
Clinical Presentation
CNS aspergillosis may present with a variety of clinical syndromes. Distinctly, Aspergillus meningitis is rare. The clinical presentation of cerebral aspergillosis is nonspecific and is characterized by recurrent headaches, changes in mental faculties, appearance of focal neurologic deficits, and deterioration in the level of consciousness. It should be considered in severely immunocompromised patients with pulmonary or paranasal sinus aspergillosis who are manifesting with acute onset of focal neurologic deficits due to a suspected secondary infective, vascular, or space-occupying lesion.3,9,69,71,83,158,159
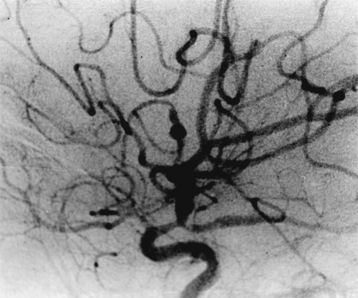
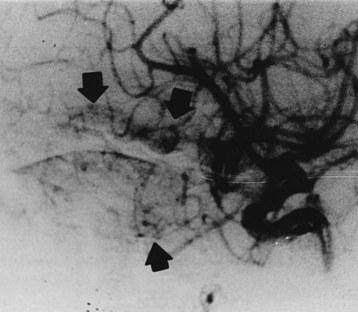
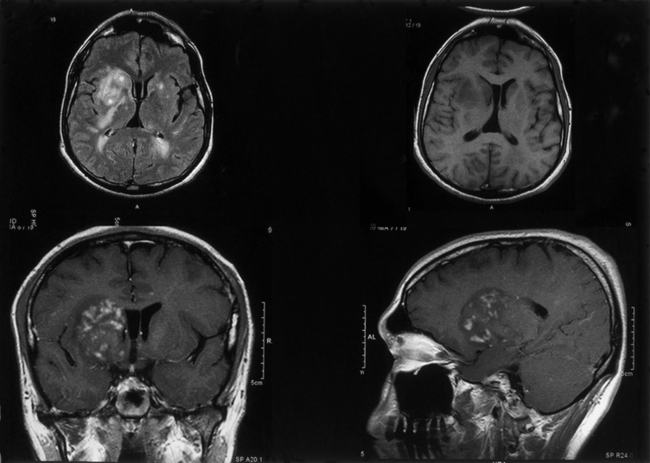
In patients with paranasal sinus aspergillosis, orbital extension with proptosis, ocular palsies, visual deterioration, and chemosis may occur.69,83 Van Landeghem et al.157 reported an interesting, rare fatal case of cerebral aspergilloma located in the midbrain, causing aqueductal stenosis and obstructive hydrocephalus. There were 44 cases of rhinosinoaspergillosis, which were treated in the ear, nose, and throat (ENT) department, and 9 of these had orbitorhinosinocerebral syndrome. There were 9 other cases of orbitorhinosinocerebral syndrome in this series. The symptomatologies frequently encountered were headache, vomiting, convulsions, hemiparesis, fever, cranial nerve deficits, papilledema, and coma. In our series, the majority of patients with CNS aspergillosis presented with signs and symptoms of an intracranial space-occupying lesion with raised ICP. Altered sensorium, acute stroke syndrome–like hemiparesis/monoparesis, fever, visual symptoms, cranial nerve palsies, and progressive motor and sensory impairment of varying degree in the limbs were the most commonly encountered symptoms. Only a few patients were suspected of meningitis. Among our 8 cases of cerebral Aspergillus abscess, 4 died, and in 4 cases, aspiration with or without craniotomy and aggressive antifungal therapy resulted in a successful outcome (Fig. 149-13). Features typical of meningitis and SAH due to mycotic aneurysms may manifest.160,161 We encountered 2 fatal cases of mycotic peripheral aneurysm of the MCA branch, and in 2 other cases of aspergillosis, mycotic aneurysms of the ICA territory were noted at autopsy. In 2 fatal cases with primary brain tumor (glioblastoma multiforme in one and anaplastic astrocytoma in the other), aspergillosis was present within the tumor mass. One patient had SAH without a detectable vascular lesion. Spinal aspergillosis may present as lumbago and/or sciatica.68,70,72,81,85,87 Spinal cord invasion may also occur but rarely. Vertebral body involvement is common, and paradural granulomas are frequent.75 We had 5 cases of spinal extradural granulomas, which were operated upon successfully, with mortality in 1 patient after long-term follow-up. Paraspinal abscesses are rare due to aspergillosis.
Diagnosis
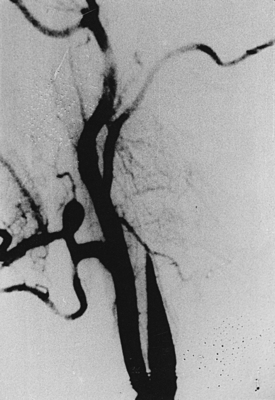
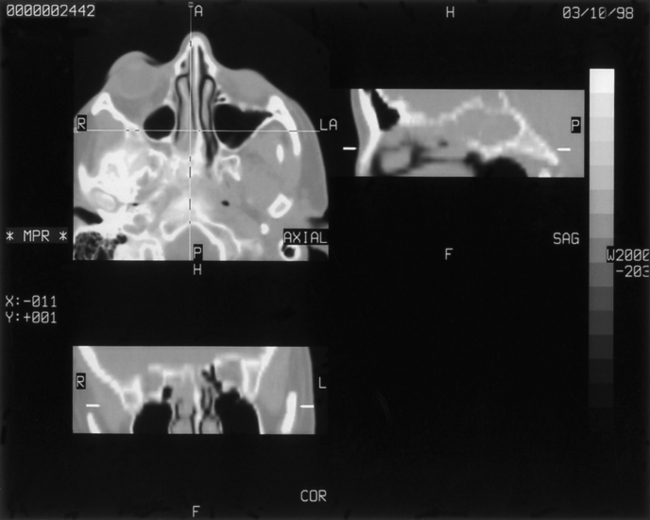
Aspergillosis is diagnosed on direct examinations and culture of aspirates/swabs/biopsy material. CSF pleocytosis (600 cells per cubic millimeter) and moderately elevated CSF proteins are present, but CSF sugar is usually normal. Organisms are rarely found in CSF. Organisms are faintly visible with H&E stain and PAS but most readily seen with methenamine silver stains. Plain x-rays, CT scans/MRI (Figs. 149-14 to 149-18), angiography (Figs. 149-19 to 149-21), and serial serologic tests—that is, double diffusion counterimmunoelectrophoresis, immunofluorescence, and enzyme-linked immunosorbent assays—significantly help in arriving at the diagnosis. On CT brain scan, the Aspergillus brain abscess appears as a peripherally enhancing ring with a hypoattenuating cavity within it and extensive surrounding cerebral edema. These findings are nonspecific, as in other infective disorders. MRI brain scans may pick additional small lesions and show more details of nonspecific pathologic changes. These changes result in a long list of differential diagnoses. In patients without a clear diagnosis of cerebral aspergillosis, the biopsy procedure is mandatory to establish the diagnosis firmly and treat the patient aggressively, with improved outcome. In spinal aspergillosis, image-guided aspirations, vertebral biopsy, histologic examination, and culture usually establish the diagnosis.5,6,9,11,18
Treatment
Aggressive neurosurgical intervention for surgical removal of Aspergillus abscesses, granulomas, and focally infarcted brain; correction of underlying risk factors; amphotericin-B combined with flucytosine; and the treatment of the source of infection should form the mainstays of management. After extensive intracranial surgery, the residual aspergillosis may be, alternatively, treated with a high dosage of itraconazole (880 mg/day for 4 months, followed by 400 mg/day for 5 months). This high dosage of itraconazole is about 16 mg/kg/day for the adults. The outcome of this infection has been universally fatal, although in recent trials voriconazole has been associated with favorable response in 30% of cases.154 Voriconazole has potent fungicidal activity against various Aspergillus species, including A. terreus. Voriconazole has been recommended as primary therapy based on a randomized clinical trial comparing voriconazole with amphotericin-B deoxycholate. The results were in favor of voriconazole (response rate of 52%) over amphotericin-B (response rate of 31%) in adult patients undergoing bone marrow transplant surgery or extrapulmonary aspergillosis, clearly demonstrating a survival benefit of voriconazole. There were also obvious benefits in pediatric patients (43%), as well as patients with CNS aspergillosis (34%) and osteomyelitis (52%). Caution must be exercised in patients with potential hepatic toxicity, and the clinician must watch for the risk of reversible transient visual disturbances. Caspofungin is also approved for refractory infections, alone or in combination with voriconazole, with promising results. In patients with extensive vascular invasion/involvement in aspergillosis, the disease spread may be beyond surgical control; therefore, heavy reliance has to be placed on extended aggressive antifungal therapy with supportive care.
Chromoblastomycosis (Phaeohyphomycosis, Cladosporiosis)
Chromoblastomycosis is a rare chronic mycosis involving corneal, cutaneous, subcutaneous, or paranasal sinus areas and prominently presents as verrucoid, warty, or cauliflower-like, raised, scaly, and colored or crusted cutaneous lesions or as invasive fungal sinusitis.6 It usually results when thorns or sharp vegetation inoculates the fungus into the subcutaneous tissue, especially in the lower extremity.162–168 It is caused by many pigmented fungi, but the common ones are Cladosporium, Hormodendrum, Exophiala, and Phialophora.9,18,164 These fungi have dark-walled cells, dematiaceous pathognomonic muriform cells (also called “copper pennies”) are well seen on histologic examination. Cerebral infection is the commonest form of systemic phaeohyphomycosis, apart from arthritis and endocarditis. Many isolated case reports on this rare cerebral mycosis have been published.169–175 C. trichoioides (bantianum) is the most frequently isolated fungus from these brain abscesses.165 It is saprophytic in soil and decaying vegetables and affects barefoot workers and farmers. It is considered a pathogen, but cases have been recorded in association with immunosuppressive treatment. Hyphae of C. trichoioides are slender, from 2 to 3 μm in thickness, and septations occur at every 3- to 15-μm interval. Single-cell conidia are 3 to 4 μm in thickness. Cerebral chromoblastomycosis arises from a non-neural infected site (cutaneous and/or respiratory system) and spreads hematogenously to the brain.
Neuropathology
Any part of the brain may be involved in chromoblastomycosis; however, the frontal lobes are the commonest sites of Cladosporium infection. Typically, they produce intraparenchymatous abscesses associated with acute and chronic inflammatory changes with focal multinucleated giant cells.3,6,9,18,163–165 These multilocular abscesses can extend into the subarachnoid spaces or toward cerebral ventricles, resulting in leptomeningitis and ventriculitis (Fig. 149-22). Rarely, the CNS may be the only site of infection and meningitis may be the sole presentation. Brain abscesses (unilocular or multilocular) are commonly seen in cladosporiosis. Granulomatous reaction is rare in this mycosis, and in many cases, it is minimal when it occurs. Microscopically, primary intraparenchymatous abscesses consist of lymphocytes, polymorphs, histiocytes, giant cells, and the Cladosporium fungus, with surrounding fibrosis and reactive gliosis. In our autopsy studies of cladosporiosis, all five cases showed blackish–brownish granulation tissue at the base of the brain and multiple areas of acute suppurative breakdown, with areas of hemorrhage in places. These coexisting abscesses were multiple and scattered in different parts of the brain. Among the three patients with primary brain abscesses, one patient who had a single, localized abscess to the occipital lobe survived following radical surgery, while the two other patients with brain abscess (one in the left frontal and the other in the left parietal region) died. The case of left parietal brain abscess was due to Exophiala species. In our series, there was associated meningitis in one case and mild to moderate hydrocephalus in the other.
Clinical Presentation
Most cases of cerebral phaeohyphomycosis have been reported among immunocompetent patients and less commonly transplant recipients.171–175 Headache, vomiting, convulsions, altered sensorium with fever, and raised ICP due to mass effect of cerebral lesions were present in our eight cases. The clinical impression was of an intracranial space-occupying lesion in five cases and an intracranial abscess in three cases. Focal neurologic deficits, raised ICP, and CT/MRI evidence of brain granulomas, basal meningitis, hemorrhages, and abscesses are the main presenting cliniconeuroimaging features.
Diagnosis
With chromoblastomycosis, the characteristic brown of fungi is recognized macroscopically.6,12,18,164,167 However, pigment may not be apparent in stained preparations with PAS and methenamine silver stains; therefore, unstained preparations are more helpful. Cladosporium is brown in cultures and tissue lesions. Biopsy of the abscess wall and culture of the pus are diagnostic.
Pseudoallescheriosis (Monosporiosis, Allescheriosis, Scedosporiosis)
Pseudoallescheriosis or allescheriosis is a rare cerebral mycosis affecting mainly the immunocompromised hosts.9 It is the commonest cause of mycetoma in United States.6,176,177 The fungus Pseudoallescheria boydii occurs in tissues as septate hyphae. It is a saprophyte that has a worldwide distribution and is found in soil and water. The organism gains access to deeper tissues through the skin. The primary focus is in the skin or respiratory system, and hematogenous spread occurs to the CNS. Pseudoallescheriosis is usually associated with chronic bronchitis, emphysema, and sarcoidosis. The fungus grows as a mold within tissues, causing necrosis and abscess formation.6
Systemic scedosporiosis due to the anamorphic or asexual form, Scedosporium apiospermum (P. boydii), has become an important cause of an opportunistic mycosis, especially in patients undergoing high-risk hematopoietic stem cell transplantation.178–180 A case of rapidly progressive cerebellar hyalohyphomycosis due to S. apiospermum in an allogeneic marrow graft recipient receiving treatment for severe graft-versus-host disease was reported by Safdar et al.180 A rare fatal case of multiple true mycotic aneurysms in a child with brain abscess due to P. boydii was reported by Messori et al.179
Neuropathology
Typically, mass lesions resulting from pseudoallescheriosis are hemorrhagic infarcts with associated leptomeningitis.6,9,18,177 These hemorrhagic infarcts may be converted into cerebral abscesses. However, pseudoallescheriosis is a rare cause of multiple brain abscesses or meningitis. In the wall of the abscesses are numerous hyphae and rounded chlamydospores. Intravascular hyphae, hallmarks of aspergillosis, may also be found in pseudoallescheriosis.
Nonseptate Hyphae Causing Infections of the Nervous System (Phycomycosis, Zygomycosis, Mucormycosis)
Phycomycosis encompasses zygomycosis in its broader group.9,181 Zygomycosis is a term that includes entorhinomucormycosis and mucormycosis.6,9,181 The former is a tropical infection of subcutaneous tissue or paranasal sinuses caused by Basidiobolus and Conidiobolus species following trauma-related implantation. The principal family causing CNS zygomycosis is Mucoraceae (mucormycosis has three main genera: Rhizopus, Mucor, and Absidia).88–94 R. arrhizus and R. oryzae are responsible for 95% of cases. The other members of this group, Saksenaea and Mortierella, usually result in extra-CNS mycosis. The organisms are ubiquitous; they are found in the soil, manure, and decaying vegetation and are frequently airborne. The spores are deposited in the nasal turbinates and may be inhaled into the pulmonary alveoli. Mucormycosis occurs initially in the rhinosino-orbital region, respiratory system, gastrointestinal tract, and skin.3,6,9,12,18,92,181,182 These fungi gain entry to the body tissues most commonly through the respiratory tract, less commonly via direct extension from paranasal sinuses, and only rarely by other routes. CNS mucormycosis results from either direct invasion or hematogenous spread of nonseptate irregular hyphae with right-angle branches measuring 6 to 15 μm in diameter and 200 μm in length.183–185 From these hyphae, the root-like rhizoids grow. From rhizoids, sporoangiospores bearing sporangia develop, which contain brown spores (7 μm). Although these organisms are considered opportunistic, CNS mucormycosis has been described in previously healthy individuals.186–190 It is widely observed in the United States and often associated with diabetic mellitus, particularly in the presence of acidosis (ketoacidosis), intravenous drug abuse, renal transplantation, malignancy, and steroid therapy.183–185 Mucormycosis ranks as the fourth most common CNS fungal infection in immunocompromised hosts; it is surpassed in frequency by candidiasis, aspergillosis, and cryptococcosis. Six clinical syndromes have been described: cutaneous, pulmonary, gastrointestinal, rhinocerebral, CNS, and disseminated. Pulmonary, rhinocerebral, and cerebral forms are common.186–190 It may also present with endophthalmitis, with fatal outcome.186
Initially, the infection develops in the nasal mucosa, which becomes swollen and dark red–brown or blackish–brown.88,181,182 Subsequent invasions of the paranasal structures (maxillary antrum) occur. The orbital invasion results in marked conjunctival edema, loss of ocular movements, loss of vision due to retinal artery thrombosis, and then spread of infection to the cavernous sinus, with involvement of the trigeminal nerve and petrous involvement with facial and lower cranial nerves. Invasion of the skull base leads to thrombosis of cavernous sinus and internal carotid and vertebrobasilar vessels, which may progress to coma and death.
The frontal lobes are involved by direct venous invasion through the orbital plate. When brain involvement results from hematogenous dissemination from pulmonary, gastrointestinal, or cutaneous sites, thrombosis of the ICA and/or cavernous sinuses commonly occurs, with associated hemorrhagic infarction in deep basal ganglionic structures.90,95,190
Neuropathology
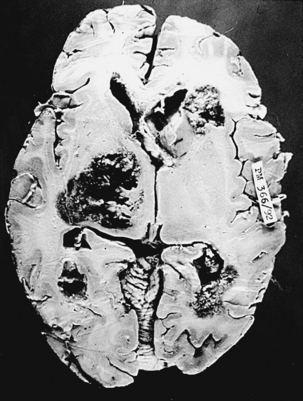
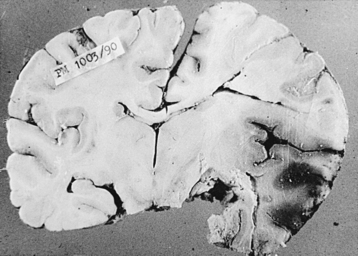
Fungus causing phycomycosis excites a mixed inflammatory response.92 The hyphae occlude and penetrate vascular walls, causing thrombosis and associated infarction.18 Features of acute, as well as chronic, cerebritis are usually present and lead to abscess formations at these sites. Hemorrhage into the infarcted brain or from mycotic aneurysms is typical.3,6,9,18 These highly angiotropic fungi invade the walls of vessels in the brain and meninges. Predominantly, a neutrophilic response and multinucleate giant cells are seen on microscopic examination. Granulomas are not typically seen. Mucor has a strong tendency to proliferate along and through the vascular structures, producing arteritis with formation of aneurysms, pseudoaneurysms, vascular occlusion, and cerebral infarction.90,95,190 In our series of mucormycosis, the brain was examined in 33 cases at autopsy. Multiple hemorrhagic infarctions (Figs. 149-23 and 149-24) were seen in 28 cases, meningeal exudate in 13 cases, localized brain abscess in 6 cases, and marked cerebral edema in most cases. Areas of suppurative necrosis with hemorrhage in different parts of the brain were commonly noted. Complete thrombosis of one or more blood vessels of the circle of Willis could be seen in 8 cases, whereas partial occlusion was noted in 19 other cases and mycotic aneurysms was seen in 2 cases. In 2 patients with primary brain tumors (1 case of malignant astrocytoma and 1 case of primary CNS lymphoma), mucormycosis was present within the brain tumor, which was operated on via stereotactic surgery. Surgical biopsy showed 4 cases with typical features of a mucormycotic granuloma with distinctive fungal hyphae.
Clinical Presentation
Phycomycotic fungal infections of the CNS are fortunately rare and remain challenging problems occurring mostly in immunocompromised individuals with protean clinical manifestations, unpredictable course, and unfavorable outcome in many cases, despite aggressive neurosurgical interventions and recent antifungal drugs. Rhino-orbitocerebral mucormycosis is a potentially lethal opportunistic fungal infection, with rapid progression and high mortality. It occurs less commonly in an apparently normal host.190
In rhinocerebral mucormycosis, the pace of the illness is rapid and includes periorbital pain, nasal discharge, proptosis, chemosis, progressive visual loss, ophthalmoplegia with loss of sensation over the forehead, retro-orbital venous obstruction (cavernous sinus thrombosis), and involvement of the ICA, producing contralateral hemiplegia (occlusive or hemorrhagic stroke).92,181,182 In the present study, among 47 cases of cerebral mucormycosis, there were 19 cases with orbitorhinocerebral syndrome. Among 34 cases presented as acute stroke, in 26 cases an acute hemorrhagic infarct and in 8 cases complete ICA thrombosis/occlusions were noted. In 17 other cases, varying grades of ICA occlusion were seen, and in 2 cases, basilar thrombosis was demonstrated at autopsy. We had 10 patients who presented with a subacute space-occupying lesion, 6 patients with cerebral abscesses and 4 patients with primary granulomas that had small areas of cerebral abscess formation. There were 2 cases of true zygomycotic fungal aneurysm, and in 1 case, only SAH was detected. In 3 patients, some degree of hydrocephalus was seen. Nasal passages and nasopharynx may show black areas of the mucosa and underlying bone. Frontal lobe abscess and/or cerebral infarct may result. Headaches, neck stiffness, convulsions, and hydrocephalus may occur from initial meningeal involvement; later, the vascular invasion results in acute focal neurologic deficits: aphasia, hemiplegia, disorientation, and altered sensorium.18,92 Once intracranial infection is established, the clinical course is acute in onset, and a rapidly fulminating course in progression results in high mortality within a few days.6,190
Diagnosis
Plain x-rays of the paranasal sinuses and the orbits show sinusoidal mucosal thickening with or without air–fluid levels. Features on CT scan of the rhino-orbitonasopharyngeal mucormycosis include opacification with bony destruction of the paranasal sinuses; orbital extension from the ethmoid sinuses, producing proptosis and chemosis; and obliteration of the nasopharyngeal tissue planes. When this virulent fungus extends intracranially, low attenuation abnormalities are noted on CT scans—particularly in the anterior cranial fossa, but they may be in any part of the brain (Fig. 149-25). These regions show mass effect and contrast enhancement. Mucor may also cause large vessel (ICA and vertebrobasilar system) obstruction and thereby cerebral infarction90,92,95,96 (Figs. 149-26 and 149-27) and abscess formation (Fig. 149-28).
On CT scanning, nonenhancing hypoattenuation lesions may represent areas of infarction or cerebritis. These lesions may be homogeneous or have a peripheral enhancement. Thrombosis of the cavernous sinus causes nonenhancement of the sinuses. On scanning MRI, hyperintense secretions are seen in the paranasal sinuses, with hyperintense thickening of the nasal mucosa on long T2 time-reduced images. A mixed picture of cerebritis and cerebral infarction as a hyperintense lesion is well outlined on proton density and T2-weighted images, especially in the frontotemporal–basal regions. Flow void is absent in cases of carotid or basilar artery and cavernous sinus thrombosis. In such cases, a mixed signal inflammatory tissue is seen replacing the usual carotid flow void.189
Fairley et al.187 reported survival and long-term follow-up of a case of rhino-orbitocerebral mucormycosis in an immunocompetent patient after extensive orbital exenteration and débridement of involved adjacent structures, along with liposomal amphotericin.
Treatment
Early diagnosis and control of risk factors, especially diabetes mellitus (hyperglycemia and acidemia), are the most important measures for treating phycomycotic fungal infections.6,18,92 Reduction or stoppage of steroids, immunosuppressive drugs, and so on, and correction of neutropenia should be undertaken on timely basis. The standard medical treatment for rhinocerebral/CNS mucormycosis is amphotericin-B deoxycholate at dosages of 1.0 to 1.5 mg/kg/day while keeping an eye on the renal functions. If renal functions are of concern, then lipid formulations of amphotericin-B (AmBisome or Abelcet in dosages of 5 mg/kg/day) are used.
Early aggressive investigations, including surgical biopsies, should be undertaken. Intensive treatment with amphotericin-B is effective, but penetration of the drug is poor due to vascular occlusions and local gangrene of the bone. Radical surgical procedures for débridement of necrotic tissue are performed repeatedly as the necrotic tissue appears along with the adjunctive hyperbaric oxygen therapy. Major reconstructive surgical procedures are required later in surviving patients. Treatment should be individualized, because no single approach is preferred over another option. Exenteration may be required in the orbits, paranasal sinuses, and other skull base areas to reduce the infective mass but are major undertakings in such ill patients. Irrigation of paranasal sinuses with antifungal agents should be undertaken. Even though the prognosis in this infection is usually poor, aggressive surgical extirpation combined with antifungal agents have helped many patients. Intravenous amphotericin-B is of great value in mucormycosis.187 The maximum tolerated dose is given until progression is halted. The drug is continued for 10 to 12 weeks, and hyperbaric oxygen therapy is simultaneously used. Although 50% of craniofacial infections may be cured, survival of patients with cerebral, gastrointestinal, or disseminated mucormycosis is extremely rare, because this virulent fungus causes death within a few weeks, especially if untreated. The overall mortality is about 50% to 80%. A team approach to management is recommended for early, appropriate surgery and systemic antifungal agents.
Fungi-Like Bacteria: Actinomycetes Causing Infections of the Nervous System
Nocardiosis
Nocardiosis has a worldwide distribution. Nocardia species are aerobic actinomycetic, a large, diverse group of gram-positive bacteria that appear on microscopy as branching filamentous cells. Nocardia therefore appears as thin, gram-positive, branching filament that is less than 1 μm thick. It is focally acid fast. N. asteroides causes most human infections (90%), but N. brasiliensis is also a pathogen (7%) found particularly in Central and South America.191–194 In contrast, N. otitidiscaviarum rarely (3%) causes nocardiosis. About 1000 cases are reported in the United States each year. Nocardia is ubiquitous, common in soil, decaying vegetation, and water. The primary infection is pulmonary or cutaneous or occurs as a chronic granulomatous disease of the bone.192 It may develop in immunocompetent or immunocompromised hosts (receiving steroids or cytotoxic drugs or having pulmonary alveolar proteinosis, sarcoidosis, ulcerative colitis, intestinal lipodystrophy, etc.).195–198 Therefore, Nocardia is considered an opportunistic pathogen. CNS nocardiosis occurs in more than 44% of systemic nocardiosis cases. The disease spreads in the CNS insidiously, and then slowly progressive symptoms develop, giving an impression of a brain tumor. There are often no clinical symptomatologies of bacterial inflammation. In such situations, the diagnosis of nocardiosis and its treatment become difficult. Hematogenous or contiguous spread of infection to the CNS occurs from the lungs, paranasal sinuses, and oral cavity.
Neuropathology
Nocardia produces multiloculated solitary or multiple brain abscesses, which are poorly encapsulated.6,9,18 Central thick exudate is yellowish–green. Meningitis infrequently coexists with parenchymal abscesses. An atypical case of Nocardia abscess of the choroid plexus was reported by Mogilner et al.196 CNS granulomas are extremely rare in nocardiosis. Involvement of cranial or vertebral bones can cause osteomyelitis with secondary extradural abscesses. Microabscesses in the CNS are surrounded by dark red margins and contain neutrophiles, mononuclear cells, liquefactive necrosis, reactive gliosis, and fibrosis. Chronic granulomatous changes when present adjacent to an abscess may show occasional giant cells.3,6,9,18,152 Durmaz et al. reported a case of multiple nocardial abscesses of cerebrum, cerebellum, and spinal cord.195
Clinical Presentation
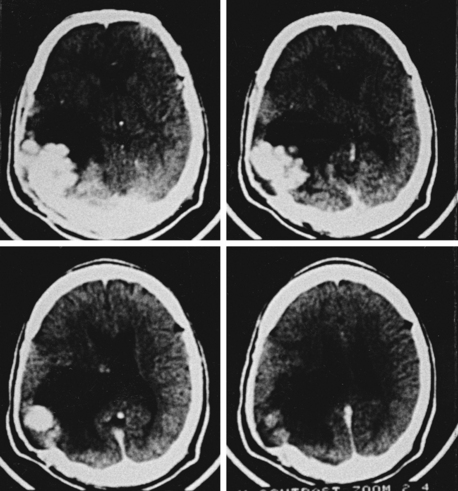
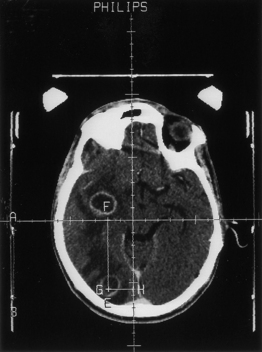
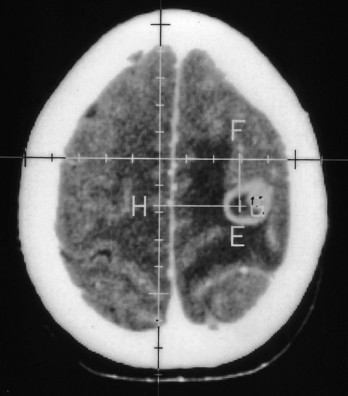
Nocardiosis usually progresses over months to years with focal neurologic deficits. Frontal and/or temporal nocardiosis presents with personality changes, behavioral disorders, and gross psychiatric manifestations. Some cases of large cerebral abscesses or granulomas have a rapidly progressive clinical course with focal neurologic deficits depending on the location of the brain lesions. Suddenly, raised ICP may develop, which may result in rupture of the nocardial brain abscess into the subarachnoid spaces, causing purulent meningitis. In our series of five cases of cerebral nocardiosis, one presented with frontal sinusitis, cranial osteomyelitis, extradural abscess, and bifrontal multiple intraparenchymatous abscesses in the frontal lobes (Fig. 149-29). Surgical excision of the abscesses was performed, but the patient died and no autopsy was performed. Our second patient with nocardiosis had a renal transplant and was having a pulmonary lesion. He had developed multiple cerebral abscesses, which were aspirated stereotactically, and then he was treated successfully with appropriate long-term drug therapy. Two cases of brain abscesses had initial frontal sinus infection, which was treated with antibiotics prior to the diagnosis and later with antifungal medications. These developed frontal brain abscesses later, which were aspirated stereotactically, and the diagnosis of nocardiosis was established. Aggressive management of the nocardial sinusitis, as well as cerebral abscess, resulted in a complete cure in three cases. One case of nocardiosis typically presented with meningitis/ventriculitis, which later progressed to hydrocephalus.
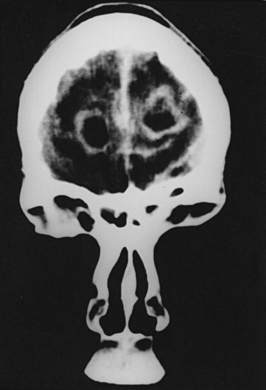
FIGURE 149-29 Contrast CT scan showing two ring-enhancing abscesses, one in each frontal lobe with right frontal sinusitis.
(Courtesy of Sanjeev D. Athale, MD.)
Spinal cord compression following vertebral osteomyelitis and intramedullary spinal abscess occurs rarely in nocardiosis.191,193,194 Spinal nocardiosis occurs commonly in cervical and thoracic regions. It usually develops by hematogenous spread from a pulmonary focus and presents as localized pain, radiculopathy, and/or myelopathy. Intramedullary spinal cord abscess has been described, although rarely. Vertebral bodies are commonly involved, and paraspinal abscesses may be present.191,193,194
Diagnosis
CSF shows few abnormalities unless meningitis coexists with nocardial abscesses. The organism is difficult to culture, and multiple lumbar punctures may fail to isolate it. CSF findings are nonspecific. CT scanning and MRI show ring enhancement in nocardial cerebral abscesses following administration of contrast material. In cases of intraventricular rupture of nocardial brain abscess, fluid-attenuated inversion recovery (FLAIR)–MRI may reveal a clear hyperintense component in the ventricle and a niveau formation inside the intracerebral lesion, indicating the state of intraventricular rupture of the nocardial brain abscess.197 CT demonstrates hypoattenuation lesions with contrast-enhancing peripheral rims and surrounding edema. Indium-111–labeled leukocyte scanning may differentiate abscesses from neoplasms. The diagnosis depends predominantly on obtaining specimens of pus from the lesions, usually at craniotomy or burr hole biopsy. These organisms are better seen on Gram stain or methenamine silver stain than on H&E sections. These are acid-fast aerobic bacteria. They are easily cultured on Sabouraud or beef infusion glucose agar in 1 to 4 weeks.
Treatment
Successful treatment of nocardiosis is achieved with a combination of the most effective antinocardial medications (trimethoprim–sulfamethoxazole, amikacin, or linezolid) with timely CT-guided, stereotactic surgical drainage of the abscess. Extensive excisional débridement of the infected granulomatous tissues via craniotomy or laminectomy should be performed wherever possible. In contrast to actinomycosis, nocardiosis tends to be penicillin resistant. Recommended primary treatment is trimethoprim with sulfamethoxazole, 4 to 8 g/day in divided doses for 6 to 12 months. In allergic patients, amikacin, linezolid, clindamycin, minocycline, amoxicillin, ceftriaxone, erythromycin, or imipenem should be used, depending on drug sensitivity testing. Progress is best monitored by interval CT scanning/MRI in appropriate settings, and the general clinical state of the patient is monitored by periodic assessments. Mortality in disseminated nocardiosis approaches 80%. Abscess management (aspiration/excision) and appropriate chemotherapy form the basis of treatment. Although most cases of spinal nocardiosis can be managed with medical therapy (sulfonamides, trimethoprim, aminoglycoside, linezolid, amoxicillin, ceftriaxone, erythromycin, etc.), a surgical decompressive procedure may be required in patients with spinal cord and/or nerve root compression. Syringomyelia may occur as a delayed complication of treatment for nocardial brain abscess.198 Neuronocardiosis is still associated with significant morbidity and mortality rates, although the results are better than before. The cure rate in cases of nocardial brain abscess is about 50%. Mortality in patients with nocardial brain abscess diagnosed antemortem is about 30% but increases to 40% in cases of multiple abscesses and more than 50% in immunocompromised patients.
Actinomycosis
CNS actinomycosis is a rare disease most frequently forming cerebral abscesses and infrequently granulomatous lesions.199–200 Actinomycosis is worldwide in distribution. It is an indolent, slowly progressive, chronic infection caused by anaerobic or microaerophilic bacteria belonging to the genus Actinomyces. A. israelii (commonly) and A. bovis (less commonly) usually produce most human infections.201 A. israelii is a normal inhabitant of the mouth, colon, and vagina and is not found free in nature. It requires devitalized tissue to provide an anaerobic environment for growth.202–204 The portal of entry is the damaged mucosal lining. Actinomycosis is basically a chronic suppurative and granulomatous infection characterized by multilobulated sulfur granules. The sulfur granules consist of thin (less than 1 μm), branching, gram-positive, actinomycetic filaments in matted tangles. The disease has three primary forms: cervicofacial (mandible), pulmonary, and abdominal (ileocecal region). CNS involvement occurs in 2% to 5% of all cases by direct tissue invasion or hematogenous dissemination.205–206 The organism is uninhibited by the tissue planes and usually spreads to contiguous structures without difficulty.
The risk factors are dental pathology, intrauterine device, alcohol abuse, and Rendu-Osler-Weber disease.199
Neuropathology
The commonest form of CNS actinomycosis is intracerebral abscess.201–206 The abscess is typically a single, thick, walled multilobulated lesion. In cervicofacial actinomycosis, the meninges are invaded, causing epidural and subdural empyemas associated with cranial osteomyelitis. Involvement of the spine in pulmonary actinomycosis occurs as a result of contiguous spread of infection with the production of osteomyelitis and epidural abscess.203–206 Microscopically, actinomycotic abscesses have central liquefactive necrosis, neutrophils, mononuclear cells, granulation tissue, fibrosis, and bacterial filaments. Lymphocytes, plasma cells, and monocytes are present in the abscesses with multinucleated giant cells. Granulomas are unusually seen.205
Clinical Presentation
Although no age is immune, actinomycosis occurs in middle age with male preponderance. Commonly, CNS actinomycosis develops through direct continuity of orocervicofacial disease and less commonly via hematogenous spread from the lungs and colon. Intracranial actinomycotic abscess is the commonest presentation. This lesion commonly presents with progressive symptoms and signs of raised ICP and focal neurologic deficits appropriate to the location.205 There may be single or multiple brain abscesses. Meningitis is rare, and granulomas are unusual.204,205 Chronic meningitis in actinomycosis may develop due to spread from the parameningeal infections in the paranasal sinuses and middle ear.
Spinal actinomycotic infection may occur from direct extension/continuity of infection in the cervical, thoracic, and abdominal regions. Spinal osteomyelitis with paradural abscesses may result.202 The infection runs a relentless course over many years, with destruction of contiguous bones and soft tissues. At Khoula Hospital, Muscat, 11 cases of CNS actinomycosis with cerebral complications were seen. These patients were treated with multiple courses of antibiotics. In 10 cases, the patients had osteomyelitis and intracranial granulomas, and in 3 of these cases, cerebral abscesses were associated with granulomas (Fig. 149-30).One patient presented with an acute fatal SAH, and another presented with spinal actinomycosis. In 9 cases, the cranial/intracranial involvement was due to contiguous spread from the scalp (Figs. 149-31 and 149-32), the facial structures, and the spine, while 1 case had an extradural granuloma following a dental extraction. With neurosurgical management and medications, 10 patients are asymptomatic and 1 patient expired due to extensive SAH. In this case, no autopsy was performed.
FIGURE 149-31 Lateral skull radiograph showing extensive cranial osteomyelitis in a case of craniocerebral actinomycosis.
Spinal actinomycosis (Fig. 149-33) results from direct extension from a pulmonary or abdominal actinomycosis focus and involves vertebral bodies, related ribs, pedicles, laminate, and spinous processes, but intervertebral discs are commonly spared in actinomycosis.203,206 The anterior vertebral body surface may show a sawtooth appearance. Paraspinal abscess and pus-draining sinuses are common occurrences, whereas the spinal cord compression is rare. One of our cases, as mentioned previously, had craniocervical actinomycosis with marked swelling of the cervical soft tissues and destruction of the occipital bone and posterior elements of the upper cervical spine. She was diagnosed with biopsy and treated effectively with long-term medical therapy. There was no spinal instability.
Diagnosis
A high index of suspicion of actinomycosis is needed in patients who have history of dental extraction, appendectomy, or pulmonary actinomycotic infection. Involvement of the jaw may show painless swelling, woody induration, and sinus tracts, which discharging pus intermittently.205 The diagnosis requires demonstration of sulfur granules (which are actually tightly clumped colonies of the causative agents, A. israelii) present in the pus in the draining sinus or in biopsy sections. Similar to actinomycosis, sulfur granules may be seen in infection with other actinomycetes (i.e., maduromycosis).9 Gram staining shows positive, long, branching filaments, and on H&E sections, basophilic filaments are seen terminating in eosinophilic clubs. Plain skull films may demonstrate osteomyelitis or sinusitis.206 Soap bubble vertebrae (area of rarefaction at active and area of sclerosis at long-standing infection) may be seen on spinal x-rays.5 A CT brain scan usually demonstrates a solitary, thick, walled ring-enhancing abscess with surrounding edema. The abscess wall may be irregular or nodular. Multiloculations with extensive cerebral edema may be present. Less commonly, solid nodules or masses, granulomas (actinomycotic granuloma), are seen as slightly hyperattenuation lesions with contrast enhancement. MRI with gadolinium–diethylenetriamine penta-acetic acid enhancement shows an irregularly enhancing mass lesion mimicking a mass lesion such as meningioma.200 MRI reveals extensive involvement of the neighboring dura, falx, and subdural spaces in cases of craniospinal osteomyelitis and epidural/subdural empyema. The organism grows on microaerophilic or anaerobic cultures. CSF studies tend to be normal or nonspecific.
Treatment
The use of penicillin in actinomycosis had revolutionized its management. It also clarified that medical management should be with high doses and for a prolonged period. Management basically includes combined medical and surgical therapy, including surgical aspiration or excision of intracranial actinomycotic lesions.205 However, a cure should be attempted initially with medical therapy, and periodic assessment with neuroimaging studies should be used to monitor progress. Large dosages of penicillin (at least 18 million to 24 million units per day) given over 3 to 4 months and followed by oral therapy with penicillin or amoxicillin for 6 to 12 months, or more as needed, should be given. Erythromycin, doxycycline, clindamycin, imipenem, ceftriaxone, ciprofloxacin, rifampicin, and lincomycin are used in patients allergic to penicillin. In pregnant women with penicillin hypersensitivity, erythromycin is a safe alternative. Actinomycotic abscess may be aspirated with image-guided techniques. Appropriate craniotomy or laminectomy procedures must be undertaken wherever needed as per the urgency of the case. Penicillin may effect a cure in spinal actinomycosis; however, surgical drainage, spinal decompression, and stabilization procedures may have to be undertaken where indicated. Because of optimal therapy, the prognosis is far better than that for true fungal infections. In some cases, complete resolution of the infection may be achieved by means of surgical treatment and prolonged antibiotic therapy in higher doses.
Clinical Syndromes of CNS Fungal Infections
Meningeal Syndrome
Acute fungal meningitis is distinctively rare except in severely immunocompromised patients and is usually seen in ICUs. Chronic fungal meningitis is fairly common. Patients present clinically with headaches, nausea, vomiting, visual impairment, and papilledema initially and then show features of cerebral irritation (seizures) or raised ICP with or without generalized or focal neurologic deficits. Because of their smaller size, pseudomycetes pass through the BBB or blood–CSF barrier easily and infect Virchow-Robin spaces and CSF. Therefore, these produce meningitis or meningoencephalitis. Such occurrences are commonly seen in cryptococcosis, candidiasis, and coccidioidomycosis.18,19,55,134,143 Tuberculous meningitis must be ruled out, and the fungal infection should be detected by careful CSF studies (routine examination, Gram stain, India ink preparation, detection of fungal capsular antigen, C fixation test for CSF antibody to the fungus, and culture). In our combined series, 8 cases of CNS candidiasis and 21 cases of CNS cryptococcosis presented with meningeal syndrome; however, meningitis as a secondary feature was coexisting with associated manifestations in cases of blastomycosis, coccidioidomycosis (Fig. 149-34), aspergillosis, Cladosporium, zygomycosis, nocardiosis, and actinomycosis (Table 149-3).
Intravenous drug therapy alone is generally successful in treating cryptococcal (amphotericin-B with flucytosine) and Candida (amphotericin-B) meningitis. Coccidioidal meningitis is a chronic infection that also requires intraventricular or intrathecal (cisternal/lumbar routes) administration of amphotericin-B for a successful outcome.18,24 Patients presenting with chronic leptomeningitis due to blastomycosis and histoplasmosis are treated with amphotericin-B and fluconazole, because the azole drugs (itraconazole and ketoconazole) have poor CSF penetration but are recommended as primary drugs for nonmeningeal systemic blastomycosis and histoplasmosis.9,24,26,29
Encephalitis–Cerebritis
Fungal infection of the brain parenchyma is usually associated with meningeal involvement in cases of small pseudomycetes (blastomycosis, cryptococcosis, histoplasmosis, etc.), but meningeal involvement may or may not be present in cases of larger pseudomycetes (candidiasis), as well as in larger mycetes (aspergillosis and zygomycetes). The process ranges from diffuse inflammation in brain parenchyma to multiple focal cerebral lesions. Superficial cortical and subcortical mass lesions, as well as deep-seated basal ganglionic and thalamic mass lesions, are best treated with stereotactic-guided biopsy and/or excision and, in patients with extracraniospinal mycosis, with ultrasound-guided tissue sampling. Appropriate management is then instituted once the results of the biopsy materials are available. In the present series, one case of CNS candidiasis initially had encephalitis (Fig. 149-35) and later developed multiple cerebral abscesses. Encephalitis was mainly seen as a coexisting feature in our cases of CNS coccidioidomycosis, cryptococcosis, aspergillosis, zygomycosis, and nocardiosis (Table 149-3). Management includes débridement of infected and devitalized tissue, control of underlying factors/diseases, and systemic amphotericin-B (or lysosomal amphotericin-B) and/or flucytosine. Candidiasis and mucormycosis exemplify diffuse fungal encephalitis in immunocompromised hosts, with poor outcome.6,18,64,92
Raised ICP
Symptomatic Hydrocephalus: Meningitis/Ventriculitis
As mentioned earlier, usually chronic but infrequently subacute, basal fungal meningitis causes obliteration of intracranial subarachnoid spaces and results in obstruction of smooth flow of CSF; therefore, symptomatic hydrocephalus develops secondary to arachnoid scarring, particularly in the basal regions, or obstruction within the ventricular system (Fig. 149-36). In these cases, CSF shunting may be necessary. A ventriculoperitoneal shunt, unilateral or bilateral, may be required to relieve raised ICP. In our series, hydrocephalus as a secondary phenomenon was seen in 12 patients, and these cases were managed with ventricular tapping and/or ventriculoperitoneal shunt surgery where indicated. Cryptococcal meningitis or meningoencephalitis may appear as pseudotumor cerebri, with slit ventricles requiring bilateral subtemporal decompressions. However, an unusually high incidence of shunt obstruction due to fungal organisms and the additional risk of introducing fungal infection intraperitoneally have been reported.6,18,114–116 A high index of suspicion, prompt diagnosis, and vigorous therapies are needed to improve the prognosis.
Fungal Abscesses
Candidiasis, aspergillosis, cryptococcosis, cladosporiosis, mucormycosis, and fungi-like bacterial diseases—nocardiosis (Fig. 149-37) and actinomycosis—commonly produce CNS fungal abscesses.59,64,97,130,165 As fungal organisms disseminate hematogenously from an extracranial site of infection, they cause multiple areas of infection within the brain. Initially, meningoencephalitis occurs with vasculitis–thrombosis; later, hemorrhagic cerebral infarction develops; and then finally, abscess forms. Abscess-forming organisms may progress fulminantly, leading rapidly to death. Extremely preterm neonates and newly born neonates with predisposing conditions such as congenital or acquired immunodeficiency are at high risk for systemic fungal infections. Abscess formation in the brain is a serious complication, occurring in about 70% of neonates with systemic fungal infection. Ultrasonography or helical 64-slice CT scanning can be used to promptly diagnose abscesses in the brain in these cases. Candidal abscesses are multiple small, round, hypoechoic lesions with echogenic rims in both cerebral hemispheres, while aspergillotic abscesses may be a few large, echogenic areas in periventricular locations. In our series, 22 patients presented with cerebral abscesses; in another 14 cases, brain abscesses were associated as a secondary feature with other presenting primary clinical entities. Initially, stereotactical (or CT/ultrasound-guided) drainage is indicated for most solitary or multiple fungal abscesses, followed by appropriate antifungal drug therapy; later, surgical excision of the abscess is performed whenever possible. The progress of the treatment is best monitored periodically by neuroimaging studies (CT scans/MRI), as well as clinical reassessments of patients.
CNS Fungal Granulomas
CNS fungal granulomas are commonly produced by aspergillosis, histoplasmosis, blastomycosis, paracoccidioidomycosis, cladosporiosis, cryptococcosis, mucormycosis, and fungi-like bacterial lesions (actinomycosis).3,6,9,76,92,203,204 Aspergillosis and mucormycosis of the paranasal sinuses infect the subjacent meninges and brain parenchyma to produce frontal and temporal granulomas, whereas hematogenous spread of other fungi from elsewhere produce parietal granulomas in general. Clinical symptomatology depends on the severity of fungal infection at the primary location (lung, colon, and paranasal sinuses) and the site of brain lesion, as well as associated meningitis, edema, and mass effect. Fungal granulomas may resemble tuberculomas, except that they have a more fibrous consistency. There were 53 cases of CNS fungal granulomas as primary presenting fungal lesions in our series (Figs. 149-38 and 149-39); in 6 other cases, granulomas were present as associated secondary lesions. Surgical excisions were performed where indicated. Infective masses should be surgically removed wherever possible in the first instance. Fungal granulomas often need to be cut with a knife or scissors, because they resist curetting. The clear plane of cleavage seen with tuberculomas or meningiomas is not present, and adherence to the dura is firmer. Unlike tuberculomas, a fungal granuloma should be completely excised whenever possible and then followed by an appropriate antifungal drug therapy to best treat these cases.
Intraparenchymal Cerebral Cysts
Intraparenchymal cerebral cysts (Figs. 149-40 to 149-42), especially in the basal ganglia, may sometimes develop in cryptococcosis,6,9,18,143 which may require stereotactic or ultrasound-guided drainage. In the present series, there were 13 cases of intraparenchymal (basal ganglionic–thalamus) cysts as secondary features in cryptococcosis. However, none of the cysts needed surgical aspiration.
Fungal Infections Coexisting with Brain Tumors
Cerebrospinal or craniospinal tumors may harbor fungal infections (Figs. 149-43 and 149-44). In such cases, mycoses thrive within the cellular matrix of the intraparenchymal tumors. In our series, there were five cases of mycoses associated with brain tumors. In two cases, aspergillosis was coexisting with cerebral gliomas (one case of glioblastoma multiforme and one case of malignant astrocytoma). Both of these patients were operated on via stereotactic surgery. Initially, in the postoperative period, these patients were clinically well; however, 72 hours later, they suddenly worsened in sensorium and repeat CT scans showed extensive cerebral edema and cerebral infarction, with fatal outcome. In one case of cerebral astrocytoma grade II, cryptococcosis was harboring within the tumor tissue and was well managed actively following surgery. In two cases, mucormycosis was coexisting with brain tumors (in one case within the astrocytoma and in another case within the malignant CNS lymphoma). Both of these patients were operated on via stereotactic trephine craniotomy, and the lesions were excised. One patient with mucormycosis developed delayed ependymitis and unilateral hydrocephalus due to blockage of the foramen of Monro. He was referred to the oncology department in another hospital after insertion of an Ommaya reservoir. The second patient with lymphoma was also referred to the oncology department in another hospital. Both of these patients succumbed within 4 months following surgery. In our experience, mycoses coexisting within the malignant brain tumors portend a very poor prognosis; out of five patients, only one of our patients survived more than a year.
Orbitorhinocerebral Syndrome
Fungal infections involving the nasal cavities, paranasal sinuses, orbits, cranial bones, and mandible may be the source of intracranial infection181–185 (Fig. 149-45), because these infections may extend to cranial and intracranial structures, especially basifrontal and basitemporal cerebral lobes. Long-standing fungal sinusitis due to invasive fungi such as Aspergillus, Cladosporium, and Zygomycetes (mucormycosis), as well as fungus-like bacteria such as actinomycetes and Nocardia, are mainly responsible. According to the involvement of the anatomic structures (paranasal sinuses, orbits, optic nerves, and cranial bone penetration with involvement of intracranial space and the brain parenchyma) in the skull base region, many types and combinations of clinical syndromes are named: sinocranial (commonest), sino-orbital, sino-orbitocranial, orbitocranial, cavernous sinus, orbital apex, purely orbital, and more extensive orbitorhinocerebral syndromes with associated specific and nonspecific symptoms and signs. Some patients present purely with cranial mono- or polyneuropathy. Each patient must be clinicoradiologically assessed according to that patient’s symptomatology to define the anatomic extent of the fungal involvement.
Acute Cerebrovascular Events
In CNS fungal infections, acute cerebrovascular events take the form of either ischemic (commonly) or hemorrhagic (uncommonly) strokes. Aspergillosis, zygomycosis, candidiasis, coccidioidomycosis, histoplasmosis, cryptococcosis, Penicillium infection, and so on, are all known fungal infections presenting, although rarely, with acute cerebrovascular events (Fig. 149-46).
Ischemic strokes occur due to gradual contiguous involvement of the skull base structures in cases of prolonged paranasal fungal sinusitis (commonly aspergillosis, zygomycosis, cladosporiosis, etc.), leading to angioinvasion, fungal vasculitis, and thrombotic occlusions in the major branches of the cerebral vasculature at the skull base (ICAs and/or vertebrobasilar system). In these cases, the fungal hyphae are directly invading the vessel walls, causing cerebral arterial thrombosis and cerebral infarction. The evolving hemorrhagic infarcts may convert into septic infarcts. Aspergillosis and zygomycosis mainly obstruct the large and intermediate-sized cerebral arteries.83,90,95 In our series, 17 cases primarily presented as ICA thrombosis, whereas in another 24 cases and 4 cases ICA thrombosis and basilar artery thrombosis, respectively, were noted as secondary features in association with other presenting primary syndromes. Ischemic cerebral strokes also result from cardiac emboli in patients with fungal endocarditis. Organisms frequently involved in fungal endocarditis include Candida and Aspergillus. However, Candida is the commonest causative organism of fungal endocarditis in both immunocompetent and immunocompromised patients. In cases of major cerebral fungal arteritis and thrombosis, where direct surgery is inappropriate, reliance has to be placed heavily on antifungal medications and supportive therapies.
Fungal aneurysms commonly present with severe sudden SAH, which is fatal in most cases. The rarity of fungal aneurysm is obvious: fewer than 50 cases have been described in the literature.207–215 Aspergillus is the commonest causative organism of cerebral fungal aneurysm, but uncommonly, Penicillium, Coccidioides, Zygomycetes, Candida, and other organisms cause single or multiple aneurysms.50,160,184,207,208 The warning symptoms or signs are absent in most cases; therefore, the disease progresses rapidly and is quite lethal, as in our cases. Intracranial fungal mycotic aneurysms in the proximal cerebral vasculature (internal carotid vessels and vertebrobasilar branches) usually result from the direct fungal angioinvasion in cases of long-standing fungal paranasal sinusitis (aspergillosis), whereas emboli lodging in the peripheral cerebral vasculature lead to peripheral cerebral fungal aneurysms, solitary or multiple, which may present with extremely rare and usually fatal complications of SAH/intracerebral hematoma with and without cerebral infarctions. Apart from antifungal drug therapy and supportive treatment, a peripheral cerebral fungal aneurysm should be excised, if recognized, whenever possible. However, direct surgical treatment is usually not possible in proximal cerebral fungal aneurysms, except that rarely endovascular therapy, surgical wrapping, or peripheral ligations can be used. The prognosis remains poor in these cases of symptomatic fungal aneurysms.
Spinal Fungal Infections
Fungal infections of the spine present commonly as vertebral, less commonly as extradural, and rarely as intradural lesions. Spinal fungal infections per se are relatively rare and usually occur in immunocompromised patients. The spinal column may be affected from the upper cervical region to the sacrum; however, the upper thoracic spine is most commonly affected by contiguous spread of fungal infections/lesions within the lungs (the endemic respiratory fungal pathogens Aspergillus, Blastomyces, Coccidioides, etc.) and uncommonly involved due to hematogenous spread (Aspergillus, C. albicans, etc.). Commonly, coccidioidomycosis,130 blastomycosis,123 histoplasmosis,104 aspergillosis72 (4 cases of extradural granulomas in our series), rarely candidiasis46 (1 case in our study), and exceptionally cryptococcosis5,143 (spinal intramedullary lesion) may present with spinal symptomatology. Fungi-like bacteria (A. israelii and N. asteroides) also rarely present with spinal lesions.159,206 One of our patients had extensive craniocervical actinomycosis and was treated effectively with medications. About 100 cases of spinal actinomycosis and fewer than 12 cases of spinal nocardiosis have been reported in the literature. Spinal fungal infections closely mimic mycobacterial lesions and should be considered in the differential diagnoses of the various osteolytic, granulomatous, and/or abscess-producing spinal lesions.
Plain x-rays of the spine show osteolysis, spinal deformities, and instability, as well as soft tissue shadow of the paraspinal abscesses and granulomas. The spinal CT scan demonstrates the findings seen on the plain x-rays more clearly, as well as showing the surrounding soft tissue involvement more precisely. MRI of the fungal spondylitis (aspergillosis/candidiasis, etc.) shows discal hypointensity with relative preservation of the intranuclear cleft on T2-weighted images, compared to hyperintensity of the intervertebral discs with loss of intranuclear cleft in pyogenic spondylitis in these images. In general, fungal and tubercular granulomatous lesions cause more pronounced destructive vertebral lesions with relative sparing of the intervertebral discs in the initial stages of infection, whereas pyogenic infection destroys both the vertebral bodies and the discs. Nonspecific findings of the fungal spondylitis, paraspinal abscesses, and granulomas on CT scans/MRI are usually seen (Fig. 149-47).
Once the fungal infection establishes within the spine, the destruction of the vertebrae occurs quite rapidly initially; then, the intervertebral discs are involved. Multilevel spinal lesions, spinal column deformities, significant paravertebral lesions, fungal lesions at junctional regions, spinal cord deformation/compression, and progressive neurologic deficits are frequent consequences needing active neurosurgical management. Neurosurgical armamentarium consists of percutaneous CT-guided biopsy, aggressive surgical débridement of the infected tissues, excision of the granulomatous lesions, drainage of abscesses, fusion reconstructions in cases of spinal destructive deformities, and stabilization with instrumentations in patients with spinal instabilities to maintain normal vertebral alignment, along with antifungal drug therapy and supportive care. The prognosis is far better than in earlier times due to recent antifungal medications and the aforementioned aggressive neurosurgical interventions for paraspinal abscesses, spinal granulomas, and vertebral lesions as needed.
Antifungal Chemotherapy
In recent times, the incidence of systemic fungal infections, including CNS mycoses, has increased significantly due to widespread proliferation of ICUs, transplant procedures and concomitant use of immunosuppressive therapies, oncologic units, pandemic spread of HIV infection, and so on.37–45 Increasingly, amphotericin-B is found to be ineffective against many forms of invasive mycoses. Fortunately, in the recent past, useful antifungal drugs have been introduced24–36216: initially lipid-based formulations of amphotericin-B, then the new triazoles, and most recently echinocandins. Recent advances in antifungal pharmacotherapy are attempting to provide drugs with greater efficacy and lesser toxicity, especially in such invasive CNS fungal infections. Evidence-based studies using new antifungal drugs, alone or in combination, suggest greater roles for these medications in improved management strategies of serious fungal infections. Currently, the following classes of natural and synthetic antifungal drugs are commonly used:
• Polyenes: amphotericin-B deoxycholate (Fungizone, Bristol-Myers Squibb), nystatin, lipid formulations of amphotericin-B, such as the following:
• Pyrimidines: 5-fluorocytosine (flucytosine)
• Triazole drugs: fluconazole, itraconazole, posaconazole, voriconazole
• Echinocandins: caspofungin, anidulafungin, micafungin
• Miscellaneous: imidazoles (clotrimazole, ketoconazole, etc.), griseofulvin, and so on, for dermal, oropharyngeal infections, as well as oral polyenes (amphotericin, nystatin); terbinafine for nail and ring worm infections
Amphotericin-B
Amphotericin-B is the broad spectrum antifungal drug active against most fungi, molds, and yeasts. Amphotericin-A and amphotericin-B are isolated byproducts from a fermentation process of Streptomyces nodosus, a soil actinomycete.24 Amphotericin-B is useful in human fungal infections, because it binds to ergosterol in the fungal cell membrane and causes increased membrane permeability, leakage of cell components, and cell death. Drug auto-oxidation may also cause cell damage.
Amphotericin-B is poorly absorbed orally, with less than 5% bioavailability, necessitating intravenous administration as a colloidal suspension with sodium deoxycholate. Because it precipitates in normal saline, it must be given in solution with 5% dextrose in water. Peak levels occur during the first hour of a 4- to 6-hour infusion, with high levels persisting for 6 to 8 hours. Hepatic or renal failure and hemodialysis do not affect drug clearance.26–30 In the blood circulation, it is highly protein bound, with less penetration in the tissues and body fluids; hence, it behaves like a highly toxic substance with profound nephrotoxicity.
High concentrations of amphotericin-B are usually found in the liver, spleen, kidneys, and lungs. In adults, the CSF concentration is 2% to 4% of the serum concentrations. Initial half-life is 12 to 18 hours, and late phase half-life is about 15 days. It is used as a first-line drug active against Candida, Cryptococcus, Aspergillus, Mucorales, and so on, and as a second choice or alternative drug for blastomycosis, histoplasmosis, and coccidioidomycosis. However, Candida lusitaniae, Trichosporon beigelli, and P. boydii are usually resistant to amphotericin-B.24
Liposomal Amphotericin-B
Encapsulating amphotericin-B into liposomal vesicles or binding of amphotericin-B to other lipid carriers is reported to result in a significant reduction in toxicity and possibly an increased therapeutic index of the drug.27 Three formulations are AmBisome, ABCD (Amphocil), and ABLC (Abelcet).24,27,30 These preparations accumulate in the reticuloendothelial system and have low nephrotoxicity in patients with life-threatening mycoses. At present, these compounds are used only in cases of intolerance to or failure of conventional amphotericin-B therapy. ABLC is used in invasive aspergillosis in dosages as high as 7 mg/kg/day. Liposomal amphotericin-B also appears to be safe, effective, and well tolerated in infants with a very low birth weight.24,27
Flucytosine
Flucytosine is a chlorine analogue of cytosine. It is useful in combination with amphotericin-B in management of deep mycoses (i.e., candidiasis, cryptococcosis, aspergillosis, and chromoblastomycosis).24,29,32,36 Absorption from the gut is rapid and complete. Spinal fluid concentrations reach up to 75% of the serum concentration. The dosage is 50 to 150 mg/kg/day given orally at 6-hour intervals, and 90% of the drug is excreted unchanged in urine. Hepatic and renal toxicities are less frequent than myelotoxicity. The drug is readily cleared with hemodialysis, as well as with peritoneal dialysis. In about 65% of cases, diarrhea, nausea, and vomiting are common side effects, which are treated symptomatically. The 2-hour postdosage levels are usually maintained between 50 and 70 μm/ml. Levels above 100 μm/ml are associated with a higher incidence of myelosuppression and enterocolitis. Flucytosine is contraindicated in pregnancy because of its teratogenic effects in rats.24
Azoles
These drugs interfere with the biosynthesis of sterols and fungal cell membrane lipids, thereby causing increased permeability and cellular damage. Ketoconazole, although less expensive as a long-term therapy, is less well tolerated and has more toxic effects than either fluconazole or itraconazole. However, these three drugs are effective alternatives to amphotericin-B and flucytosine for selected systemic mycoses: blastomycosis, coccidioidomycosis, and histoplasmosis.24,28,34,36 Itraconazole is effective in sporotrichosis and aspergillosis, and fluconazole is effective in cryptococcal and coccidioidal meningitis and in candidiasis. Ketoconazole has poor penetration into the CSF, especially of noninflamed meninges. Fluconazole appears to be superior to amphotericin-B for maintenance therapy in cryptococcal meningitis in AIDS patients. To improve efficacy and to reduce side effects, as well as to decrease the duration of therapy, antifungal drug combinations are often used, such as amphotericin-B and flucytosine in systemic candidiasis and cryptococcal meningitis.24
Echinocandins
The most recently introduced antifungal drugs are the echinocandins (caspofungin, anidulafungin, and micafungin). These are showing promising results in combination antifungal therapies for serious, opportunistic, invasive fungal infections. At present, their spectrum of activity is mainly limited to candidiasis and aspergillosis (caspofungin only).
Al-Mohsen I., Hughes W.T. Systemic Antifungal Therapy: past, Present and Future. Annals of Saudi Medicine. 1997;18:28-38.
Anaissic E.J., Meginnie M.R., Pfaller M.A. Clinical Mycology. New York: Churchill Livingstone, 2003.
Bauserman S.C., Schochet S.S.Jr. Bacterial, fungal and parasitic diseases of the central nervous system. In: Nelson J.S., Parisi J.E., Schochet s.s.Jr. Principles and Practice of Neuropathology. London: Mosby; 1993:42-74.
Bazan C., Rinaldi M.G., Rauch R.R., Jinkins R. Fungal infections of the brain. Neuroimaging clin of North Am. 1991;1:57-88.
Bell W.E. Treatment of fungal infection of the central nervous system. Ann Neurol. 1981;9:417-422.
Bennett J.E.. Introduction to Mycoses, Mandell G.L., Bennett J.E., Dolin R., Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 6th ed Churchill Livingstone, Elsevier, 2005:2935-2938
Carey M.E.. Infections of the Spine and Spinal cord, Youmans J.R., editor, Youmans Neurological surgery 4th ed, Philadelphia, WB Saunders Co, 1996;vol. 5:3270-3304.
Chimelli L., Mahler-Araujo M.B. Fungal infections. Brain Pathology. 1997:613-627.
Darling T.S. A protozoan general infection producing pseudotubercles in the lungs and focal necrosis in the liver, spleen and lymph nodes. JAMA. 1906;46:1283-1285.
Friedman A.H., Bullitt E. Fungal infections. In: Wilkins R.H., Rengachary S.S. Neurosurgery. Newyork: McGraw Hill; 1985:2002-2010.
Gilchrist T.C. Protozoan dermatitis. J Cutan Dis. 1894;12:406.
Gonyea E.F. The spectrum of primary blastomycotic meningitis: a review of central nervous system blastomycosis. Ann Neurol. 1978;3:26-39.
Jorgensen J.H., Rinaldi M.G. A Clinicians Directory of Bacteria and Fungi. Indianapolis: Eli Lilly; 1987. 70–72
Kirkpatrick J.B.. Neurologic Infections due to Bacteria, Fungi and Parasites, Davis R.L., Robertson D.M., Textbook of Neuropathology, 2nd ed Baltimore, Williams & Wilkins, 1991:719-803
Kissane J.M., editor. Anderson’s Pathology. St. Louis: CV Mosby, 1990.
Mori T., Ebe T. Analysis of cases of central nervous system fungal infections reported in Japan between January 1979 and June 1989. Int Med. 1992;31(2):174-179.
Rippon J.W. Medical mycology: the Pathogenic Fungi and The Pathogenic Actinomycetes. Philadelphia: WB Saunders; 1988.
Salaki J.S., Louria D.B., Chmel H. Fungal and yeast infections of the central nervous system. Medicine (Baltimore). 1984;63:103-132.
Scaravilli F. Parasitic and fungal infections of the nervous system. In: Adams J.H., Corsellis Duchen L.W. Greenfield’s Neuropathology. London: Edward Arnold; 1984:304-337.
Scaravilli F. Parasitic and fungal infections of the nervous system. In: Hume-Adams J.H., Corsellis J.A.N., Duchen L.W. Greenfield’s Neuropathology. London: Edward Arnold; 1992:400-446.
Sharma R.R., Gurusinghe N.T., Lynch P.G. Cerebral infarction due to Aspergillus arteritis following glioma surgery. Brit J Neurosurg. 1992;6:485-490.
Sunde N. Fungal Infections. In: Palmar J.D., editor. Manual of Neurosurgery. Newyork: Churchill Livingstone; 1996:896-898.
Walsh T.J., Gonzalez C., Lymon C.A., et al. Invasive fungal infections in Children: recent advances in diagnosis and treatment. Adv Pediatr Infect Dis. 1996;11:187-290.
Wiles C.M., Mackenzie D.W.R. Fungal Diseases of The Central Nervous System. In: Kennedy P.G.E., Johnson R.T. Infections of the Nervous system. London: Butterworths; 1987:93-117.
Wood M., Anderson M. Neurological Infections. Philadelphia: WB Saunders, 1988.
1. Anaissic E.J., Meginnie M.R., Pfaller M.A. Clinical Mycology. New York: Churchill Livingstone, 2003.
2. Bauserman S.C., Schochet S.S.Jr. Bacterial, fungal and parasitic diseases of the central nervous system. In: Nelson J.S., Parisi J.E., Schochet S.S.Jr. Principles and Practice of Neuropathology. London: Mosby; 1993:42-74.
3. Bazan C., Rinaldi M.G., Rauch R.R., Jinkins R. Fungal infections of the brain. Neuroimaging Clin of North Am. 1991;1:57-88.
4. Bennett J.E. Introduction to Mycoses. In: Mandell G.L., Bennett J.E., Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Churchill Livingstone: Elsevier; 2005:2935-2938.
5. Carey M.E.. Infections of the Spine and Spinal cord, Youmans J.R., editor, Youmans Neurological surgery Fourth Edition, Philadelphia, WB Saunders Co, 1996;Vol. 5:3270-3304.
6. Chimelli L., Mahler-Araujo M.B. Fungal infections. Brain Pathology. 1997:613-627.
7. Friedman A.H., Bullitt E. Fungal infections. In: Wilkins R.H., Rengachary S.S. Neurosurgery. New York: McGraw-Hill; 1985:2002-2010.
8. Jorgensen J.H., Rinaldi M.G. A Clinicians Directory of Bacteria and Fungi. Indianapolis: Eli Lilly; 1987. 70-72
9. Kirkpatrick J.B. Neurologic Infections due to Bacteria, Fungi and Parasites. In: Davis R.L., Robertson D.M. Textbook of Neuropathology. Second edition. Baltimore: Williams & Wilkins; 1991:719-803.
10. Mori T., Ebe T. Analysis of cases of central nervous system fungal infections reported in Japan between January 1979 and June 1989. Int Med. 1992;31(2):174-179.
11. Rippon J.W. Medical mycology: the Pathogenic Fungi and The Pathogenic Actinomycetes. Philadelphia: WB Saunders; 1988.
12. Salaki J.S., Louria D.B., Chmel H. Fungal and yeast infections of the central nervous system. Medicine (Baltimore). 1984;63:103-132.
13. Scaravilli F. Parasitic and fungal infections of the nervous system. In: Adams J.H., Corsellis Duchen L.W. Greenfield’s Neuropathology. London: Edward Arnold; 1984:304-337.
14. Scaravilli F. Parasitic and fungal infections of the nervous system. In: Hume-Adams J.H., Corsellis J.A.N., Duchen L.W. Greenfield’s Neuropathology. London: Edward Arnold; 1992:400-446.
15. Sharma R.R., Gurusinghe N.T., Lynch P.G. Cerebral infarction due to Aspergillus arteritis following glioma surgery. Brit J Neurosurg. 1992;6:485-490.
16. Sunde N. Fungal Infections. In: Palmar J.D., editor. Manual of Neurosurgery, Newyork. Churchill Livingstone; 1996:896-898.
17. Walsh T.J., Gonzalez C., Lymon C.A., et al. Invasive fungal infections in Children: recent advances in diagnosis and treatment. Adv Pediatr Infect Dis. 1996;11:187-290.
18. Wiles C.M., Mackenzie D.W.R. Fungal Diseases of The Central Nervous System. In: Kennedy P.G.E., Johnson R.T. Infections of the Nervous system. London: Butterworths; 1987:93-117.
19. Gonyea E.F. The spectrum of primary blastomycotic meningitis: a review of central nervous system blastomycosis. Ann Neurol. 1978;3:26-39.
20. Darling T.S. A protozoan general infection producing pseudotubercles in the lungs and focal necrosis in the liver, spleen and lymph nodes. JAMA. 1906;46:1283-1285.
21. Gilchrist T.C. Protozoan dermatitis. J Cutan Dis. 1894;12:406.
22. Kissane J.M., editor. Anderson’s Pathology. St. Louis: CV Mosby, 1990.
23. Wood M., Anderson M. Neurological Infections. Philadelphia: WB Saunders, 1988.
24. Al-Mohsen I., Hughes W.T. Systemic Antifungal Therapy: past, Present and Future. Annals of Saudi Medicine. 1997;18:28-38.
25. Bell W.E. Treatment of fungal infection of the central nervous system. Ann Neurol. 1981;9:417-422.
26. Camarata P.J., Dunn D.L., Farney A.C., et al. Continuous intracavitary administration of amphotericin-B as an adjunct in the treatment of Aspergillus brain abscess. Case report and review of the literature. Neurosurgery. 1992;31:575-579.
27. De Marie S. Liposomal and lipid-based formulations of amphotericin-B. Leukemia. 1996;10(suppl 2):93-96.
28. Goodpasture H.C., Hershberger R.E., Barnett A.M., Peteric J.D. Treatment of central nervous system fungal infections with ketoconazole. Arch Int Med. 1985;145:879-880.
29. Graybill J.R. The future of antifungal therapy. Clin. Infect. Dis. 1996;22(suppl 2):166-178.
30. Hiemenz J.W., Walsh T.J. Lipid formulations of amphotericin-B: recent progress and future direction. Clin Infect Dis. 1996;22(suppl 2):133-144.
31. Joly V., Aubry P., Ndayiragide A., et al. Randomized comparison of amphotericin-B deoxycholate dissolved in dextrose or intralipid for treatment of AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1996;23:556-562.
32. Medoff G., Brajtburg J., Kobayashi G.S. Antifungal agents useful in therapy of systemic fungal infections. Am Rev Pharmacol Toxicol. 1983;23:303.
33. Slavoski L.A., Tunkel A.R. Therapy of fungal meningitis. Clin Neuropathol. 1995;18(2):95-112.
34. Van de Velde V.J., Van Peer A.P., Heykants J.J., Woestenborghs R.J., et al. Effect of food on the pharmacokinetics of a new hydroxy propyl-beta-cyclodextrin formulation of itraconazole. Pharmacotherapy. 1996;16:424-428.
35. Walsh T.J., Hiemenz J.W., Anaissie E. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin North Am. 1996;10:365-400.
36. Walsh T.J., Peter J., Mc Gough D.A., et al. Activities of amphotericin-B and antifungal azoles alone and in combination against Pseudoallescheria boydii. Antimicrob Agents Chemother. 1995:1361-1364.
37. Almekinders L.E., Greene W.B. Vertebral Candida infections: a case report and review of the literature. Clin Orthop. 1991;267:174.
38. Basma R., Barada G., Ojaimi N., Khalaf R.A. Susceptibility of Candida albicans to common and novel antifungal drugs and relationship between the mating type locus and resistance, in Lebanese hospital isolates. Mycoses. 2009;52(2):141-148.
39. Clark T.A., Hajjeh R.A. Recent trends in the epidemiology of invasive mycoses. Curr Opin Infect Dis. 2002;15:569-574.
40. Davis L.E. Fungal infections of the central nervous system. Neurol Clin. 1999;17:761-781.
41. de Almeida S.M., Rebelatto C.L., Telles Q.F., Wernecka L.C. Major histocompatibility complex nervous system involvement by paracoccidioidomycosis. J Infect. 2005;51:140-143.
42. Dotis J., Roilides E. Immunopathogenesis of central nervous system fungal infections. Neurol India. 2007;55:216-220.
43. Edwards J.E. Candida species. In: Mandell G.L., Bennett J.E., Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Churchill Livingstone: Elsevier; 2005:2939-2957.
44. Friedman B.C., Simon G.L. Candida vertebral osteomyelitis. Report of three cases and a review of the literature. Diagn Microbiol Infect Dis. 1987;8:31.
45. Goldman J.A., Fleischer A.S., Leifer W., et al. Candida albicans mycotic aneurysms associated with systemic lupus erythematosus. Neurosurgery. 1979;4:325-328.
46. Hostetter M.K. New insights into Candida infections. Adv Pediatri. 1996:209-230.
47. Khazim R.M., Debnath U.K., Fares Y. Candida albicans osteomyelitis of the spine: progressive clinical and radiological features and surgical management in three cases. Eur Spine J. 2006;15(9):1404-1410.
48. Klein J., Sato A. The HLA system. Second of two parts. N Engl J Med. 2000;343:782-786.
49. Lockhart S.R., Joly S., Vargas K., et al. Natural defences against Candida colonization breakdown in the oral cavities of the elderly. J Dent Res. 1999;78:857-868.
50. Levitz S.M. Overview of host defences in fungal infections. Clin Infect Dis. 1992;14:S37-S42.
51. Marr K.A., Carter R.A., Crippa F., et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipient. Clin Infect Dis. 2002;34:909-917.
52. Miller D.J. Diagnosis and management of Candida and other fungal infections of the head and neck. Current Infectious Disease Reports. 2002;4(3):194-200.
53. Nguyen M.H., Yu V.L. Meningitis caused by Candida species: an emergency problem in neurosurgical patients. Clin Infect Dis. 1995;21:323-327.
54. Olson J.K., Miller S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916-3924.
55. Osmund J.D., Schweitzer G., Dunbar J.M., et al. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45:431.
56. Parker J.C., Mc Closkey J.J., Lee R.S. The emergence of candidiosis: the dominant postmortem cerebral candidosis. Am J Clin Pathol. 1978;70:31-38.
57. Pennisi A.K., Davis D.O., Wiesel S., et al. CT appearance of Candida diskitis. J Comput Assist Tomogr. 1985;9:1050.
58. Rex J.H., Walsh T.J., Sobel J.D., et al. Practice Guidelines for the treatment of candidiasis. Clin Infec Dis. 2000;30:662-678.
59. Sanchez-Portocarrero J.M., Martin-Rabadan P., Saldana C.J., Pareza-Cecilia E. Candida cerebrospinal fluid shunt infection. Report of two new cases and review of the literature. Diagn Microbiol Infect Dis. 1994;20:33-40.
60. Spellberg B.J., Filler S.G., Edwards J.E.Jr. Clinical Practice: current Treatment Strategies for Disseminated Candidiasis. Clin Infect Dis. 2006;42(2):244-251.
61. Speth C., Dierich M.P., Gasque P. Neuroinvasion by pathogens: a key role of the complement system. Molecular Immun. 2001;38:669-679.
62. Sugarman B., Massanari R.M. Candida meningitis in patients with CSF shunts. Arch Neurol. 1980;37:180-181.
63. Takeda S., Wakabayashi K., Miyakawa T., Arai H. Intracranial fungal aneurysm caused by Candida endocarditis. Clin Neuropathol. 1998;17:199-203.
64. Tventen L.. Candidiasis, Vinken P.J., Bruyn G.W., Klawans H., editors, Handbook of Clinical Neurology; Infections of the Nervous system, Amsterdam, Elsevier North-Holland, 1978;vol. 35:413-442.
65. Akhaddar A., Gazzaz M., Albouzidi A., et al. Invasive Aspergillus terreus sinusitis with orbito-cranial extension: case report. Surg Neurol. 2008;69(5):490-495.
66. Artico M., Pastore F.S., Polosa M., et al. Intracerebral Aspergillus abscess: case report and review of the literature. Neurosurg Rev. 1997;20:135-138.
67. Beluffi G., Bernardo M.E., Meloni G., et al. Spinal osteomyelitis due to Aspergillus flavus in a child: a rare complication after haematopoietic stem cell transplantation. Paed Radiol. 2008;38(6):709-712.
68. Byrd B., Weiner M.H., McGee Z.A. Aspergillus spinal epidural abscess. JAMA. 1982;248:3138.
69. Casey A.T., Wilkeins P., Uttley D. Aspergillosis infection in neurosurgical practice. Brit J Neurosurg. 1994;8:31-39.
70. Chee Y.C., Poh S.C. Aspergillus epidural abscess in a patient with obstructive airway disease. Postgrad Med J. 1983;59:43.
71. Deshpande D.H., Desai A.P., Dastur H.M. Aspergillosis of the central nervous system. Neurol India. 1975;23:167-175.
72. D’Hoore K., Hoogmartens M. Vertebral aspergillosis: a case report and review of the literature. Acta Orthop. Belg. 1993;59:306.
73. Freely M., Steinberg M. Aspergillus infection complicating trans-sphenoidal yttrium-90 pituitary implant. Report of two cases J. Neurosurg. 1977;46:530-532.
74. Galassi E., Pozzati E., Poppi M., Vinci A. Cerebral aspergillosis following intracranial surgery, Case report. J Neurosurg. 1978;49:308-311.
75. Gupta R., Singh A.K., Bishnu P., Malhotra V. Intracranial Aspergillus granuloma simulating meningioma on MR imaging. J Comput Assist Tomogr. 1990;14:467-469.
76. Haran R.P., Chandy M.J. Intracranial Aspergillus granuloma. Br J Neurosurg. 1993;7:383-388.
77. Ho C.L., Deruytter M.J. CNS aspergillosis with mycotic aneurysm, cerebral granuloma and infraction. Acta Neurochir (Wien). 2004;146:851-856.
78. Hurst R.W., Judkins A., Bolger W., et al. Mycotic aneurysm and cerebral infarction resulting from fungal sinusitis: imaging and pathologic correlation. AJNR Am J Neuroradiol. 2001;22:858-863.
79. Jinkins J.R., Siqueira E., Al-Kawi M.Z. Cranial manifestations of aspergillosis. Neuroradiology. 1987;29:181-185.
80. Mawk J.R., Erickson D.L., Chou S.N., et al. Aspergillus infections of the lumbar disc spaces. J Neurosurg. 1983;58:270.
81. Nadkarni T., Goel A. Aspergilloma of the Brain: an overview. JPGM. 2005;51(5):37-41.
82. Savaria-Gomez J.. Aspergillosis of the Central Nervous System, Vinken P.J., Bruyn G.W., Klawans H., editors, Handbook of Clinical Neurology: infections of the Nervous System, Amsterdam, Elsevier North Holland, 1978;Vol. 35:395-400.
83. Schwartz S., Thiel E. Clinical presentation of invasive aspergillosis. Mycoses. 1997;40(suppl 2):21-24.
84. Seth N.K., Varkey B., Wagner D. Spinal cord Aspergillus invasion: complication of an aspergilloma. Am J Clin Pathol. 1985;84:763.
85. Turgut M., Ozsunar Y., Oncu S., et al. Invasive fungal granuloma of the brain caused by Aspergillus fumigatus: a case report and review of literature. Surg Neurol. 2008;69:169-174.
86. Wagner D.K., Varkey B., Seth N.K., et al. Epidural abscess, vertebral destruction and paraplegia caused by extending infection from an aspergilloma. Am J Med. 1985;78:518.
87. Watson J.C., Myseros J.S., Bullock M.R. True fungal aneurysm of the basilar artery: a clinical and surgical dilemma. Cerebrovas Dis. 1999;9:50-53.
88. Bhattacharya A.K., Deshpande A.R., Nayak S.R., et al. Rhinocerebral mucormycosis: an unusual case presentation. J Laryngotol. 1992;106(1):48-49.
89. Bichile L.S., Abhyankar S.C., Hase N.K. Chronic mucormycosis manifesting as hydrocephalus. J. Neurol Neurosurg Psychiatry. 1985;48:1188.
90. Carpenter D.F., Brubaker L.H., Powell R.O., et al. Phycomycotic thrombosis of the basilar artery. Neurology. 1968;18:807-812.
91. Chen F., Lu G., Kang Y., et al. Mucormycosis spondylo-discitis after lumbar disc puncture. Eur Spine J. 2006;15(3):370-376.
92. Dehmy P.. Phycomycosis (mucormycosis), Vinken P.J., Bruyn G.W., editors, Infections of the Nervous System. Part III Handbook of Clinical Neurology, Amsterdam, North Holland, 1978;vol. 35:541-556.
93. Escobar A., Del Brutto O.H. Multiple brain abscesses from isolated cerebral mucormycosis. J Neurol Neurosurg Psychiatry. 1990;53:431-433.
94. Hopkins R.J., Rothman M., Fiore A., Goldblum S.E. Cerebral mucormycosis associated with intravenous drug use: three case reports and review. Clin Infect. Dis. 1994;19:1133-1137.
95. Martin F.P., Lukeman J.M., Ranson R.F., et al. Mucormycosis of the central nervous system associated with thrombosis of the internal carotid artery. J Paediatr. 1954;44:437-442.
96. Parfrey N.A. Improved diagnosis and prognosis of mucormycosis. Medicine (Baltimore). 1986;65:113.
97. Pierce P.F.Jr., Solomon S.L., Kaufman L., et al. Zygomycetes brain abscesses in narcotic addicts with serological diagnosis. JAMA. 1982;248:2881.
98. Price D.L., Wolpow E.R., Richardson E.P. Intracranial Phycomycosis: a clinicopathological and radiological study. J Neurol Sci. 1971;14:359-375.
99. Azizirad O., Clifford D.B., Groger R.K., et al. Histoplasmoma: isolated central nervous system infection with Histoplasma capsulatum in a patient with AIDS—case report and brief review of the literature. Clin Neurol Neurosurg. 2007;109(2):176-181.
100. Goodwin R.A., Loyd J.E., Des Prez R.M. Histoplasmosis in normal hosts. Medicine (Baltimore). 1981;60:231.
101. Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol. 2008;10(3):161-167.
102. Vakili S.T., Eble J.N., Richmond B.D., et al. Cerebral histoplasmoma. J Neurosurg. 1983;59:332-336.
103. Weidenheim K.M., Nelson S.J., Kure K., et al. Unusual patterns of Histoplasma capsulatum meningitis and progressive multifocal leukoencephalopathy in a patient with the acquired immunodeficiency virus. Hum Pathol. 1992;23:581-586.
104. Wheat L.J., Batteiger B.E., Sathapatayavangs B. Histoplasma capsulatum infections of the central nervous system. A clinical review. Medicine (Baltimore). 1990;69:244-260.
105. Al-Soub H. Bilateral visual loss due to cryptococcal meningitis. Annals of Saudi Medicine. 1996;16:84-86.
106. Blackie J.D., Danta G., Sorrell T., Collignon P. Ophthalmological complications of cryptococcal meningitis. Clin Exp Neurol. 1985;21:263-270.
107. Chan K.H., Mann K.S., Yue C.P. Neurosurgical aspects of cerebral cryptococcosis. Neurosurgery. 1989;25:44-47.
108. Diamond R.D.. Cryptococcus neoformans, G.L. Mandell, J.E. Bennett, R. Dolin, New York, Churchill Livingstone Inc, 1995:2331-2339
109. Diamond R.D., Bennett J.E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974;80:176.
110. Ellis D.H., Pieiffer T.J. Ecology life cycle and infectious propagule of Cryptococcus neoformans. Lancet. 1990;336:923-925.
111. Leenders A.C., Reiss P., Portegies P., et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B followed by fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS. 1997;11:1463-1471.
112. Mathews M., Pare L., Hasso A. Intraventricular cryptococcal cysts masquerading as racemose neurocysticercosis. Surg Neurol. 2007;67(6):647-649.
113. Mitchell D.H., Sorrell T.C., Allworth A.M., et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis. 1995;20:611-616.
114. Chiou C.C., Wong T.T., Lin H.H. Fungal infections of ventriculoperitoneal shunts in children. Clin Infect Dis. 1994;19:1049-1053.
115. Tang L.M. Ventriculoperitoneal shunt in cryptococcal meningitis with hydrocephalus. Surg Neurol. 1990;33:314-319.
116. Yadav S.S., Perfect J., Friedman A.H. Successful treatment of cryptococcal ventriculoatrial shunt infection with systemic therapy alone. Neurosurgery. 1988;23:372-373.
117. Williams R.L., Fukui M.B., Meltzer C.C., et al. Fungal Spinal Osteomylitis in the immunocompromised patients. MR findings in three cases. Am J Neuroradiol. 1999 Mar;20(3):381-385.
118. El-Zaatari M.M., Hulten K., Faresy, et al. Successful treatment of Candida albicans osteomylitis of the spine with fluconazole and surgical debridement: case report. J Chemother, 2002 Dec 14;6:627-630
119. Rosler-Meier D., Brunner-La Rocca H.P., Senn P., Schlumpf U. Candida albicans spondylitis: successful treatment with fluconazole. Two case reports. Schweiz Reindsch Med Prax. 1996 Apr 16;85(16):520-525.
120. Bradsher R.W. Blastomycosis. Clin Infect Dis. 1992;14(suppl 1):582-590.
121. Detrisac D.A., Harding W.G., Greiner A., et al. Vertebral North American Blastomycosis. Surg Neurol. 1980;13:311.
122. Frean J., Blumberg L., Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26:203.
123. Gottileb J.R., Eismont F.J. Nonoperative treatment of vertebral osteomyelitis associated with paraspinal abscess and cord compression. A case report. JBJS Am. 2006;88(4):854-856.
124. Krarup C., Davis C.H., Symon L. Spinal blastomycosis: case report. J Neurol Neurosurg Psychiatry. 1984;47:217.
125. Leers W.D.. North American Blastomycosis, Vinken P.J., Bruyn G.W., Klawans H., editors, Handbook of Clinical Neurology: infections of the Nervous System, Amsterdam, Elsevier North Holland, 1978;vol. 35:401-411.
126. Roos K.L., Bryan J.P., Maggio W.W., et al. Intracranial blastomycoma. Medicine (Baltimore). 1987;66:224-235.
127. Titrud L.A. Blastomycosis of the cervical spine. Minn Med. 1975;58:729.
128. Cortez K., Walsh T.J., Bennett J.E. Coccidioidal meningitis successfully treated with voriconazole. Clin Infect Dis. 2003;36:1619-1622.
129. Deresinski S.C., Stevens D.A. Coccidioidomycosis in compromised host. Experience at Stanford University Hospital. Medicine (Baltimore). 1974;54:377.
130. Deresinski S.C. Coccidioidomycosis of Bone and Joints. In: Stevens D.A., editor. Coccidioidomycosis: A text. New York: Plenum Medical Block Company; 1980:195-209.
131. Mischel P.S., Vinters H.V. Coccidioidomycosis of the central nervous system: neuropathological and vasculopathic manifestations and clinical correlates. Clin Infect Dis. 1995;20:400-405.
132. Nakazawa G., Lulu R.E., Koo A.H. Intracerebellar coccidioidal granuloma. AJNR. 1983;4:1243-1244.
133. Saviers M.L. Disseminated coccidioidomycosis among Southwestern American Indians. Am Rev Respir Dis. 1974;109:602.
134. Sobel R.A., Ellis W.C., Nielsen S.L., et al. Central nervous system coccidioidomycosis: a clinicopathological study of treatment with and without amphotericin-B. Hum Pathol. 1984;15:980-995.
135. Caraway N.P., Fanning C.V., Stewart J.M., et al. Coccidioidomycosis osteomyelitis masquerading as a bone tumor. A report of 2 cases. Acta Cytol. 2003;47(5):777-782.
136. Holley K., Muldoon M. Taskers. Coccidioides immitis osteomyelitis: a case series review. Orthopaedics. 2002;25(8):827-832.
137. Vincent T., Galgiani J.N., Huppert M., et al. The natural history of coccidioidal meningitis: VA–Armed Forces Cooperative Studies, 1955-1958. Clin Infect Dis. 1993;16:247-254.
138. Wright P.W., Pappagianis D., Wilson M., et al. Donor related coccidioidomycosis in organ transplant recipient. Clin Infect Dis. 2003;37(9):1265-1269.
139. Colli B., Assirati J.Jr., Machado H.R., et al. Intramedullary spinal cord paracoccidioidomycosis. Report of two cases. Arq Neuropsiquiatr. 1996;54:466-473.
140. de Almeida S.M., Queiroz-Telles F., Teive H.A., et al. Central Nervous System Paracoccidioidomycosis: clinical features and laboratory findings. J Infect. 2004:48.
141. Giraldo R., Restrepo A., Gutierrez F. Pathogenesis of paracoccidioidomycosis: a model based on the study of 46 patients. Mycopathologia. 1976;58:63-70.
142. Londero A.T., Ramos C.D. Paracoccidioidomycosis: a clinical and mycotic study of forty-one cases observed in Santa Maria, RS. Brazil Medicine. 1972;52:771-775.
143. Minguetti G., Madalozzo L.E. Paracoccidioidal granulomatosis of the brain. Arch Neurol. 1983;40:100-102.
144. Forjaz M.H., Fischman O., de Camargo Z.P., et al. Paracoccidioidomycosis in Brazilian Indians of the Surui tribe: clinical laboratory study of 2 cases. Rev Soc Bras Med Trop. 1999;32(5):571-575.
145. Godoy H., Reichart R.A. Oral manifestations of paracoccidioidomycosis. Report of 21 cases from Argentina. Mycoses. 2003;46(9-10):412-417.
146. Villa L.A., Tobon A., Restrepo A., et al. Central Nervous System paracoccidioidomycosis. Report of a case successfully treated with itraconazole. Rev Inst Med Trop Sao Paulo. 2000;42(4):231-234.
147. Weenink H.R., Bruyn G.W.. Cryptococcosis of the Nervous System, Vinken P.J., Bruyn G.W., editors, Infections of The Central Nervous System Part III. Hand book of Clinical Neurology, Amsterdam, North Holland, 1978;Vol. 35:459-502.
148. Fleury R.N., Taborda P.R., Gupta A.K., et al. Zoonotic sporotrichosis. Transmission to humans by infected domestic cat scratching: report of four cases in San Paulo, Brazil. Int J Dermatol. 2001;40(5):318-322.
149. Naqvi S.H., Becherer P., Gudipati S. Ketoconazole treatment of a family with zoonotic sporotrichosis. Scand J Infect Dis. 1993;25(4):543-545.
150. Rafal E.S., Rasmussen J.E. An unusual presentation of fixed cutaneous sporotrichosis: a case report and review of the literature. J Am Acad Dermatol. 1991;25:928-932.
151. Barenfanger J., Lawhorn J., Drake C. Nonvalue of culturing cerebrospinal fluid for fungi. J Clin Microbiol. 2004;42(1):236-238.
152. Cheng Y.C., Ling J.F., Chang F.C., et al. Radiological manifestations of cryptococcal infection in central nervous system. J Chin Med Assoc. 2003;66(1):19-26.
153. Choi M.Y., Bae I.H., Lee J.H., Lee S.J. Aspergillosis presenting as an optic neuritis. Korean J Ophthalmol. 2002;16(2):119-123.
154. Herbrecht R., Denning D.W., Patterson T.F., et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Eng J Med. 2002;347:408-415.
155. Murthy J.M., Sundaram C., Prasad V.S., et al. Sino-cranial aspergillosis: a form of central nervous system aspergillosis in South India. Mycoses. 2001;44(5):141-145.
156. Sharma R.R., Pawar S.J., Ravi R.R., et al. A solitary primary Aspergillus brain abscess in an immunocompetent host: CT-guided stereotaxy with an excellent outcome. Pan Arab Journal of Neurosurgery. 2002;6(2):62-65.
157. Van Landeghem F.K., Stiller B., Lehmann T.N., et al. Aquaductal stenosis and hydrocephalus in an infant due to Aspergillus infection. Clin Neuropathol. 2000;19(1):26-29.
158. Goodman M.L., Coffey R.J. Stereotactic drainage of Aspergillus brain abscess with long term survival: case report and review. Neurosurgery. 1989;24:96-99.
159. Nov A.A., Cromwell L.D. Computed tomography of neuraxis aspergillosis. J Comput Assist Tomogr. 1984;9:413.
160. Chou S.M., Chong Y.Y., Kinkel R., et al. A proposed pathogenetic process in the formation of Aspergillus mycotic aneurysm in the central nervous system. Ann Acad Med Singapore. 1993;22(suppl 3):518-528.
161. Poitrowski W.P., Pilz P., Chuang I.H. Subarachnoid hemorrhage caused by a fungal aneurysm of the vertebral artery as a complication of intracranial aneurysm clipping. J Neurosurg. 1990;73:962-964.
162. Aviv J.E., Lawson W., Boltone E.J., et al. Multiple intracranial mucoceles associated with phaeohyphomycosis of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 1990;116:1210-1213.
163. Bennett J.E., Bonner H., Janning A.E., Lopez R.I. Chronic meningitis caused by Cladosporium trichoides. Am J Clin Pathol. 1973;59:398.
164. Dixon D.M., Walsh T.J., Merz W.G., McGinnis M.R. Infections due to Xylohypha bantiana (Cladosporium trichoides). Rev Infect Dis. 1989;11(4):515-525.
165. Goel A., Satoskar A., Desai A.P., Pandya S.K. Brain abscess caused by Cladosporium trichoides. Brit J Neurosurg. 1992;6:591-593.
166. Kullu S., Onol B., Kustimor S., et al. Cerebral cladosporiosis. Surg Neurol. 1985;24:437-440.
167. McGinnis M.R., Borelli D., Padhye A.A., et al. Reclassification of Cladosporium bantianum in the genus Xylohypha. J Clin Microbiol. 1986;23:1148-1151.
168. Salem F.A., Kannangara D.W., Nachum R. Cerebral chromomycosis. Arch Neurol. 1983;40:173.
169. Hart A.P., Sutton D.A., McFeeley P.J., Kornfeld M. Cerebral phaeomycosis caused by a dematiaceous Scopulariopsis species. Clin Neuropathol. 2001;20(5):224-228.
170. Keyser A., Schmid F.X., Linde H.J., et al. Disseminated Cladophialophora bantiana infarction in a heart transplant recipient. J Heart Lung Transplant. 2002;21(4):503-505.
171. Nobrega J.P., Rosemberg S., Adami A.M., et al. Fonsecaea pedrosoi cerebral phaeohyphomycosis (“chromoblastomycosis”): first human culture–proven case reported in Brazil. Rev Inst Med Trop Sao Paulo. 2003;45(4):217-220.
172. Singh S., Singh P., Sarkar C., et al. Fungal granuloma of the brain caused by Cladosporium bantianum: a case report and review of literature. J Neurol Sci. 2005;228(1):109-112.
173. Trinth J.V., Steinback W.J., Schell W.A., et al. Cerebral phaeohyphomycosis in an immunodeficient child treated medically with combination antifungal therapy. Med Mycol. 2003;41(4):339-345.
174. Umabala P., Lakshmi V., Murthy A.R., et al. Isolation of a Nodulisporium species from a case of cerebral phaeohyphomycosis. J Clin Microbiol. 2001;39(11):4213-4218.
175. Vieira M.R., Milheiro A., Pacheco F.A. Phaeohyphomycosis due to Cladosporium cladosporioides. Medical Mycology. 2001;39(1):135-137.
176. Kershaw P., Freeman R., Templeton D., et al. Pseudoallescheria boydii infection of the central nervous system. Arch Neurol. 1990;47:470-472.
177. Selby R. Pachymeningitis secondary to Allescheria boydii. Case report. J Neurosurg. 1972;36:225.
178. Marcinkowski M., Bauer K., Stoltenburg-Didinger G., Versmold H. Fungal brain abscesses in neonates: sonographic appearances and corresponding histopathologic findings. J Clin Ultrasound. 2001 Sept;29(7):417-421.
179. Messori A., Lanza C., DeNicola M., et al. Mycotic aneurysms as lethal complication of brain pseudoallescheriasis in a near-drowned child: a CT demonstration. AJNR Am J Neuroradiol. 2002;23(10):1697-1699.
180. Safdar A., Papadopoulos E.B., Young J.W. Breakthrough Scedosporium apiospermum (Pseudoallescheria boydii) brain abscess during therapy for invasive pulmonary aspergillosis following high risk allogeneic hematopoietic stem-cell transplantation. Scedosporiosis and recent advances in antifungal therapy. Transpl Infect Dis. 2002;4(4):212-217.
181. Ellis C.J.K., Daniel S.E., Kennedy P.G., et al. Rhino-orbital zygomycosis. J. Neurol Neurosurg Psychiatry. 1985;48:455-458.
182. Hussain S., Salahudin N., Ahmad I., et al. Rhinocerebral invasive mycosis: occurrence in immunocompetent individuals. Eur J Radiol. 1995;20:151-155.
183. Gupta S.K., Manjunath-Prasad K.S., Sharma B.S., et al. Brain abscess in renal transplant recipients: report of three cases. Surg Neurol. 1997;48(3):284-287.
184. Kikuchi K., Watanabe K., Sugawara A., et al. Multiple fungal aneurysms: report of a rare case implicating steroids as predisposing factor. Surg Neurol. 1985;24:253-259.
185. Nampoory M.R., Khan Z.U., Johny K.V., et al. Invasive fungal infections in renal transplant patients. J Infection. 1996;33(2):95-101.
186. Bhansali A., Sharma A., Kashyap A., et al. Mucor endophthalmitis. Acta Ophthalmol Scand. 2001;79(1):88-90.
187. Fairley C., Sullivan T.J., Bartley P., et al. Survival after rhino-orbital mucormycosis in an immunocompetent patient. Ophthalmology. 2000;107(3):555-558.
188. Georgopoulou S., Kounougeri E., Katsenos C., et al. Rhinocerebral mucormycosis in a patient with cirrhosis and chronic renal failure. Hepatogastroenterology. 2003;50(51):843-845.
189. Mathi M., Chaouir S., Amil T., et al. Rhino-orbito-cerebral mucormycosis. J Radiol. 2002;83:165-167.
190. Sharma R.R., Pawar S.J., Delmendo A., et al. Fatal rhino-orbito-cerebral mucormycosis in an apparently normal host: case report and literature review. J Cln Neurosci. 2001;8(6):583-586.
191. Laurin J.M., Resnik C.S., Wheeler D., et al. Vertebral osteomyelitis caused by Nocardia asteroides: report and review of the literature. J Rheumatol. 1991;18:455.
192. Palmer D.L., Harvey R.L., Wheeler J.K. Diagnosis and therapeutic considerations in Nocardia asteroides infection. Medicine (Baltimore). 1974;53:391-401.
193. Peterson J.M., Awad I., Ahmed M., et al. Nocardia osteomyelitis and epidural abscess in the nonimmunosuppressed host. Cleve Clin Q. 1983;50:453.
194. Siao P., McCabe P., Yagnik P. Nocardial spinal epidural abscess. Neurology. 1989;39:996.
195. Durmaz R., Atasoy M.A., Durmaz G., et al. Multiple nocardial abscesses of cerebrum, cerebellum and spinal cord causing quadriplegia. Clin Neurol Neurosurg. 2001;103(1):59-62.
196. Mogilner A., Jallo G.I., Zagzag D., Kelly P.J. Nocardia abscess of the choroids plexus: clinical and pathological case report. Neurosurgery. 1998;43(4):949-952.
197. Oshiro S., Ohnishi H., Ohta M., Tsuchimochi H. Intraventricular rupture of Nocardia brain abscess: case report. Neurol Med Chir (Tokyo). 2003;43(7):360-363.
198. Young W.F. Syringomyelia presenting as a delayed complication of treatment for Nocardia brain abscess. Spinal cord. 2000;38(4):265-269.
199. Benito Leon J., Munoz A., Leon P.G., et al. Actinomycotic brain abscess. Neurologia. 1998;13(7):357-361.
200. Ushikoshi S., Koyanagi I., Hida K., et al. Spinal intrathecal actinomycosis: a case report. Surg Neurol. 1998;50(3):221-225.
201. Alday R., Lopez-Ferro M.O., Fernandez-Guerrero M., et al. Spinal intrathecal empyema due to Actinomyces israelii. Acta Neurochir (Wien). 1989;101:159.
202. Bolton C.F., Ashenhurst E.M. Actinomycosis of the brain. Can Med Ass J. 1964;90:922-928.
203. David C.V., Jayalakshmi P. Actinomycosis of the Spine: two case reports. Med J Malaysia. 1983;38:161.
204. Khosla V.K., Banerjee A.K., Chopra J.S. Intracranial actinomycoma with osteomyelitis simulating meningioma. Case report. J. Neurosurg. 1984;60:204-207.
205. Lad S.D., Chandy M.J. Craniofacial actinomycosis. Brit J Neurosurg. 1991;5:361-370.
206. Nolan R.L., Ross J.D., Chapman S.W. Thoracic actinomycosis presenting as spinal cord compression. J Miss State Med Assoc. 1990;31:41.
207. Ahuja G.K., Jain N., Vijayaraghavan M., Roy S. Cerebral mycotic aneurysm of fungal origin. J Neurosurg. 1978;49:107.
208. Hadley M.N., Martin N.A., Spetzler R.F., Johnson P.C. Multiple intracranial aneurysms due to Coccidioides immitis infection. J. Neurosurg. 1987;66:453-456.
209. Lu D.C., Wang V., Chou D. The use of allograft or autograft and expandable titanium cages for the treatment of vertebral osteomyelitis. Neurosurgery. 2009;64(1):122-129.
210. Mahiquez M., Bunton K.L., Caney G., et al. Nonsurgical treatment of lumbosacral blastomycosis involving L2-S1: a case report. Spine (Phila Pa 1976). 2008;33(13):E442-E446.
211. Cross S.A., Scott L.J. Micafungin. Drugs. 2008;68:2225-2255.
212. DiNubile M.J., Strohmaier K.M., Lupinacci R.J., et al. Efficacy and safety of caspofungin therapy in elderly patients with proven or suspected invasive fungal infections. Eur J Clin Microbio Infect Dis. 2008;27(8):663-670.
213. Dismukes W.E. Guidelines from the infectious diseases society of America. Introduction to Antifungal Drugs. Clin Infect Dis. 2000;30:653-657.
214. Lai C.C., Tan C.K., Huang Y.T., et al. Current challenges in the management of invasive fungal infection. J Infection and Chemotherapy. 2008;14(2):77-85.
215. Maschmeyer G., Haas A. Voriconazole: a broad spectrum triazole for the treatment of serious and invasive fungal infections. Future Microbiology. 2007;1(4):365-385.
216. del Brutto O.H. Central nervous system mycotic infections. Rev Neurol (Spanish). 2000;30(5):447-459.