CHAPTER 98 FUNGAL INFECTIONS AND ANTIFUNGAL THERAPY IN THE SURGICAL INTENSIVE CARE UNIT
The first clinical description of Candida infection can be traced to Hippocrates, with Parrot recognizing a link to severe illness. Langenbeck implicated fungus as a source of infection, and Berg established causality between this organism and thrush by inoculating healthy babies with aphthous “membrane material.” The first description of a deep infection caused by Candida albicans was made by Zenker in 1861, even though it was not named until 1923 by Berkout. On the other hand, the genus Aspergillus was first described in 1729 by Michaeli, and the first human cases of aspergillosis were described in the mid-1800s.
Fungi are ubiquitous heterotrophic eukaryotes, quite resilient to environmental stress and able to thrive in numerous environments. They may belong to the Chromista or Eumycota kingdom.1 For identification purposes, the separation of taxa is based on the method of spore production, assisted by molecular biology techniques (rRNA and rDNA) that further refine fungal phylogeny and establish new relationships between groups. The most important human pathogens are the yeasts and the molds (from the Norse mowlde, meaning fuzzy). The dual modality of fungal propagation (sexual/teleomorph and asexual/anamorph states) has meant that since the last century there has been a dual nomenclature.
PREDICTORS OF FUNGAL INFECTIONS
The National Nosocomial Infection Surveillance program (NNIS) of the U.S. Centers for Disease Control and Prevention (CDC) has reported that whereas the rate of hospital-acquired fungal infections nearly doubled in the past decade compared with the previous decade, the greatest increase occurred in critically ill surgical patients, making the surgical population in the ICU an extremely high risk group.2 Several conditions (both patient-dependent and disease-specific) have been recognized as independent predictors for invasive fungal complications during critical illness. ICU length of stay was associated with Candida infection as were the degrees of morbidity, alterations of immune response, and the number of medical devices involved. Neutropenia, diabetes mellitus, newonset hemodialysis, total parenteral nutrition, broad-spectrum antibiotic administration, bladder catheterization, azotemia, diarrhea, use of corticosteroids, and cytotoxic drug utilization are also associated with candidemia.2–5
Candida Colonization
Because colonization with Candida spp. is not benign in the context of critical illness, it is desirable to identify and characterize patients further in terms of risk for invasive candidiasis. Screening techniques include routine surveillance cultures in ICU patients. The method proposed by Pittet et al., the colonization index, has been validated in surgical patients. A threshold index of 0.5 has been proposed for the initiation of empiric antifungal therapy in critically ill patients (see following treatment section), although some authorities suggest that the presence of multiple Candida isolates is an epiphenomenon.6
Use of Broad-Spectrum Antibiotics
The use of broad-spectrum antibiotics is one of the bestdocumented risk factors for fungal overgrowth and invasive infections, but the precise mechanism is not understood completely. In evaluating the effect of antibiotic use, one must consider first the complex interrelations between bacteria and fungi in human disease. At least three experimental models have been created to investigate and characterize possible interactions between bacterial and fungal pathogens. In murine models, ticarcillin-clavulanic acid and ceftriaxone (both of which have some antianaerobic therapy) are associated with substantial increases in colony counts of yeast flora of the gut. On the other hand, antibiotics with poor anaerobic activity are less likely to produce this effect (examples are ceftazidime and aztreonam). This observation was validated in a clinical review of the quantitative colonization of stool in immunocompromised patients treated with those antibiotics. However, this interaction between fungi overgrowth and anaerobic suppression is different from the well-studied model of Escherichia coli and Bacteroides fragilis in intra-abdominal abscess formation. The work of Sawyer et al. showed that C. albicans induces bacterial translocation into abscesses, but the relationship is one of direct competency, rather than synergy or cooperation.7,8 This is different than the cooperation between C. albicans and Staphylococcus aureus, Serratia marcescens, and Enterococcus faecalis, where an amplification-type interaction has been documented. A number of immunomodulatory and immunosuppressive viruses have been shown to facilitate superinfections with opportunistic fungi, the most notable examples being CMV and human herpes virus (HHV)-6, because they induce the production of immunosuppressive cytokines. It seems that C. albicans thrives in situations where immunocompromise is present and adds virulence and mortality to existent bacterial infections in a species-specific manner. This hypothesis has been validated from clinical observations, where antifungal treatment adds little to the therapeutic effect of antibacterial agents alone. Thus, the use of antibiotics (three or more), especially those with anti-anaerobic properties, constitute a risk factor for fungal colonization and overgrowth, which in turn is a predictor for systemic fungal infections. The precise mechanism of action for this observation is unknown but is probably related to fungi-to-microbe competence and growth suppression. Candida may enhance the pathogenicity of certain bacteria, but not others, and this interaction remains to be elucidated.7
Duration of ICU Care and Invasive Mechanical Ventilation
Epidemiological observations correlating the duration of mechanical ventilation and the amount of intensive care required correlate with the occurrence of both systemic fungal infections and fungal colonization. Other factors involved in the pathogenesis and susceptibility of systemic candidiasis are total parenteral nutrition, use of H2 blockers, acquired immunodeficiency syndrome (AIDS), radiation therapy, previous bacteremia, abdominal surgery, hemodialysis, extremes of age, recurrent mucocutaneous candidiasis, and duration of cardiopulmonary bypass greater than 120 minutes.2
PATHOGENIC ORGANISMS
Candida albicans
The most common fungal pathogen both in the United States and abroad, and ranked among the most common sources of ICU sepsis, C. albicans is a common cause of human disease.9 Candida albicans accounts for 59% of Candida isolates, followed by C. glabrata (15%–25% of all Candida infections). Both colonization and invasive candidiasis can be focal or disseminated. Multifocal candidiasis is the simultaneous isolation of Candida from two or more of the following locations: respiratory, digestive, urinary, wounds, or drainage. Disseminated candidiasis is microbiological evidence of yeast in fluids from normally sterile sites such as cerebrospinal, pleural, pericardial, or peritoneal fluid, histologic samples from deep organs, or diagnosis of endophthalmitis or candidemia with negative catheter-tip cultures. The incidence of candidemia has increased over the past 30 years, with mortality rates reported in some series to be as high as 80%. The NNIS system of the CDC found Candida species responsible for 8%–15% of all nosocomial bloodstream infection episodes in the United States in 1993, which ranked fourth among commonly isolated pathogens in bloodstream infections.
It is well established that a morphological transition in C. albicans, from yeast to hyphal forms, is the most important determinant of dissemination, because the mycelial phase is invasive.10 Both host and pathogen play a role on this dimorphism. The fungus produces several proteins during the hyphal transition, which are currently the focus of research. The thiol-specific antioxidant, or TSA-1, has shown an increased survival capability in an antioxidant environment created by host cells. Host recognition molecules (adhesins), secreted aspartyl proteases and phospholipases, and phenotypic switching accompanied by changes in antigen expression, colony morphology, and tissue affinities are other recognized virulence factors. The inducer mechanisms and the multiple stimuli that trigger this change are unknown.
Disseminated candidiasis and fungemia can lead to septic shock, similar to that seen with other microorganisms. The dimorphic transition generates shock and end-organ failure in susceptible individuals, and these events are independent of TNF-α. The diagnosis of fungemia as the cause of a patient’s sepsis depends on a strong clinical suspicion. Only 50% of blood cultures for invasive candidiasis are positive and bacterial pathogens may interfere with the recovery of Candida. There are no reliable laboratory tests to differentiate between Candida colonization and invasive candidiasis, and no single site of isolation is superior to others in predicting which patients are likely to have developed systemic infection. The diagnostic criteria for fungemia are a combination of positive tissue cultures (including burn excision cultures and peritoneal cultures), endophthalmitis, osteomyelitis, and candiduria. Purpura fulminans and unexplained myalgias are suggestive of candidiasis in the appropriate clinical context. The presence of three or more colonized sites or two positive blood cultures at least 24 hours apart, with one obtained after the removal of any central venous catheters are strong indicators of fungemia.10 Whereas asymptomatic recovery of Candida in urine rarely requires therapy, candiduria should be treated in symptomatic patients, neutropenic patients, renal transplant patients, and after instrumentation. The removal or at least changing of the Foley catheter is required.
Non–albicans Candida
The incidence of non-Candida fungemia and sepsis syndrome has been increasing in recent years, accounting for up to one-half of non–albicans Candida adult ICU infections. The reasons for this are likely multifactorial. Undoubtedly, one explanation for the emergence of C. glabrata and C. krusei is the selection of less-susceptible species by the pressure of antifungal agents.11 Other species of yeast are related to specific events, such as the presence of an indwelling central venous catheter and C. parapsilosis. The increased incidence of C. tropicalis in oncology patients is secondary to the increased invasiveness of the organism, especially in damaged gastrointestinal mucosa. The clinical features of this infection are indistinguishable from C. albicans.
Aspergillus
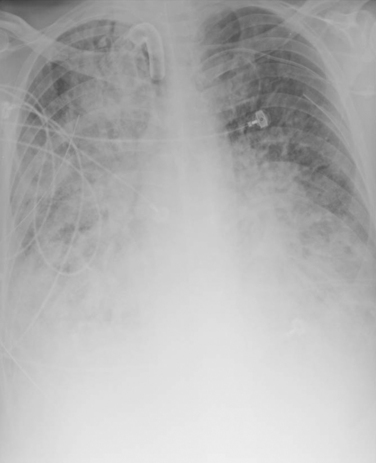
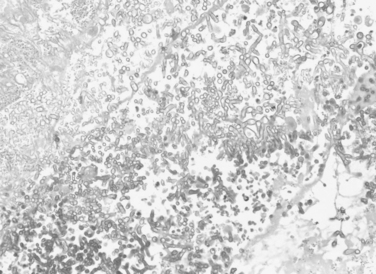
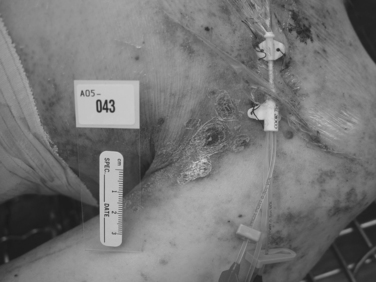
The noninvasive types of aspergillosis include allergic bronchopulmonary aspergillosis (a form of hypersensitivity reaction in asthmatics) and aspergilloma. These entities, without tissue invasion, usually do not require antifungal therapy. Invasive aspergillosis has experienced an increased incidence over the last decade, and has become a major cause of death among patients with liquid tumors. Although invasive Aspergillus infections usually occur via inhalation of conidia, the fungus is also frequently present on food (i.e., pepper, regular and herbal tea bags, fruits, corn, and rice). The thermotolerant spores of Aspergillus and other fungi present are difficult to eradicate, and represent a threat to the immunocompromised host. Conidia that fail to be cleared by alveolar macrophages germinate in the alveolar space, and hyphal forms invade the pulmonary parenchyma, with prominent vascular invasion and early dissemination (Figures 1 through 3).12
PRINCIPLES OF THERAPY
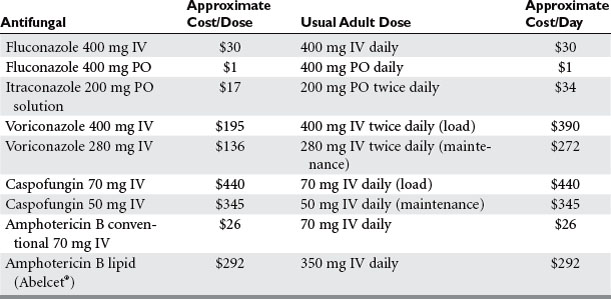
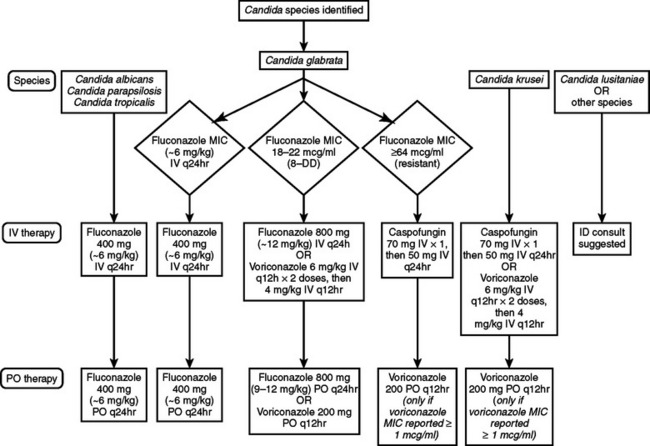
The past 10 years has seen a major expansion in the repertoire of antifungal agents with the introduction of less-toxic formulations of amphotericin B, improved triazoles, echinocandins, and other agents that target the fungal cell wall. As described by Flanagan and Barnes, therapy for fungal infections in the ICU can be directed using four different strategies: prophylactic, preemptive, empiric, and definitive. Some data suggest a decrease in invasive fungal infections with prophylactic antifungal therapy in non-neutropenic critically ill surgical patients with Candida isolates from sites other than blood and the presence of risk factors mentioned previously. Others have suggested that use of antifungal prophylaxis in unselected SICU patients increases mortality, length of stay, and the appearance of resistance in previously susceptible fungi, not to mention the increase in cost this approach generates.13 Prophylactic fluconazole treatment in the SICU leads to secondary mycoses, with up to 80% of the pathogens resistant to fluconazole.14,15 Tables 1 and 2 and Figures 4 and 5 show one schema used in the SICU at NewYork-Presbyterian Hospital-Weill Cornell Medical Center. Independent of the species, infection by fluconazole-resistant Candida doubles the mortality rate. The colonization index developed by Pittet et al. and Ostrosky-Zeichner suggests that high-risk patients are those who remain in the ICU for 4 days or more and who either have a central venous catheter in place or are treated with antibiotics in addition to two of the following: use of total parenteral nutrition, need for dialysis, recent major surgery, diagnosis of pancreatitis, and treatment with systemic corticosteroids or other immunosuppressive agents.15,16 Studies have documented the lack of benefit for fluconazole prophylaxis in unselected trauma patients, and in ICU patients, for whom the contribution of mortality by candidemia is surpassed by that of age and severity of illness.17,18
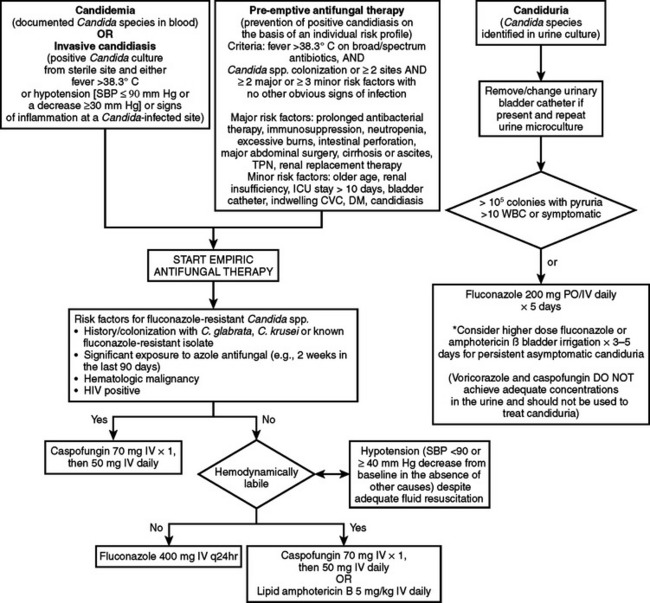
Figure 4 Antifungal therapy for the treatment of infections caused by Candida species in adult patients.
Table 3 presents a list of available antifungal agents. Amphotericin B is a natural polyene macrolide that binds primarily to ergosterol, the principal sterol in the fungal cell membrane, leading to disruption of ion channels, production of oxygen free radicals, and apoptosis. It is active against most fungi, including in cerebrospinal fluid. Due to its high level of protein binding, tissue concentrations are not usually affected by hemodialysis. Infusion-related reactions can occur in up to 73% of patients with the first dose and often diminish during continued therapy. Amphotericin B–associated nephrotoxicity can lead to azotemia and hypokalemia, although acute potassium release with rapid infusion can occur and lead to cardiac arrest. Amphotericin B lipid formulations allow for higher dose administration with lessened nephrotoxicity, but whether outcomes are enhanced is unproved. Nystatin is a polyene similar in structure to amphotericin B, and is currently used topically for C. albicans. A parenteral formulation is under investigation. Flucytosine is a fluorinated pyrimidine analog that is converted to 5-fluorouracil, which causes RNA miscoding and inhibits DNA synthesis. It is available in the United States in oral form only and has been used with amphotericin B for synergism against Candida spp.
| Antifungal Agent | Indications | Routes/Dosage |
|---|---|---|
| Amphotericin B |
Second-generation antifungal triazoles include posaconazole, ravuconazole, and voriconazole. They are active against Candida spp., including fluconazole-resistant strains, and Aspergillus spp. For the latter, voriconazole is emerging as the treatment of choice.19,20
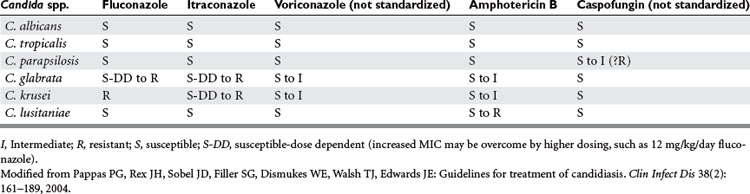
The echinocandins include caspofungin, micafungin, and anidulafungin, each of which is approved therapy for candidiasis and candidemia, but third-line treatment for invasive aspergillosis. Due to their distinct mechanism of action, disrupting the fungal cell wall by inhibiting β(1,3)-D-glucan synthesis, the echinocandins can theoretically be used in combination with other standard antifungal agents. The echinocandins have activity against Candida spp. and Aspergillus spp., but are not reliably active against other fungi. Echinocandin activity is excellent against most Candida spp., but moderate against C. parapsilosis, C. guillermondi, and C. lusitaniae. Echinocandins exhibit no cross-resistance with azoles or polyenes.21
Neutropenic Patients and Preemptive Therapy
A novel approach in the use of antifungal therapy in patients who do not exhibit clinical evidence of systemic candidiasis is the concept of preemptive therapy.16 Being more than just semantics, prophylaxis is defined as treatment triggered by risk stratification (thus directed at patients with “possible” fungal infection), whereas preemptive therapy is the early treatment of identified infection, without clinical signs, detected by the use of surrogate markers or non–culture-based methods. The appeal of preemptive therapy (for patients with “probable” fungal infection) is that it theoretically combines the best of the evidence for fungal prophylaxis with the benefits of early treatment, mitigating against the increase in fungal resistance and recovery of resistant strains. Given that there is little evidence that prophylactic use of fluconazole confers benefit in the management of nonfebrile neutropenic patients, the development of new protocols using frequent surveillance and screening (instead of therapy) for patients at high risk is imperative.
Therapy Tailored to Specific Risk Factors and Likely Offending Organisms
The New York–Presbyterian Hospital, Weill Cornell Medical Center has developed algorithms and guidelines for the use of antifungal agents (see Tables 1 and 2, Figures 4 and 5) based on epidemiological considerations. Table 3 lists the antifungal agents most commonly in use in the United States. Several studies have demonstrated that azole antifungals have immunosuppressive activity in that imidazoles interfere with neutrophil and lymphocyte function. Ketoconazole has been used to attempt to reduce the frequency of the acute respiratory distress syndrome in high-risk patients, possibly as a thromboxane synthase inhibitor. Itraconazole and miconazole are potent inhibitors of 5-lipoxygenase. Fluconazole, which has no effect on plasma thromboxane and leukotriene concentrations, has been shown in animals and in vitro to augment the bactericidal activity of neutrophils, suggesting a possible role in treating patients with sepsis.
Fungi as an Epiphenomenon
Recent advances in critical care have produced a selected population of very ill individuals that in previous years would have succumbed to their disease processes. Many of these improvements in survival have preceded (and in some cases paralleled) the explosive growth of fungal colonization and infection in ICU patients, and the availability of antifungal therapy. As antibiotic choice has become more complex and resistance has developed, so too has the complexity of fungal infections and the expanded multimodality therapy. Whereas there is little argument that invasive fungal infections are associated with increased mortality, morbidity, and length of stay (both in the ICU and hospital), the mortality attributable to these infections remains controversial. More problematic is the fact that, although antifungal prophylaxis is effective in preventing fungal colonization and invasive infections, this does not translate into a difference in mortality. Data on length of stay are also contradictory, with staunch supporters for both sides of the debate. Localized fungal infections and colonization have different natural histories depending on the severity of illness, but they remain predictors of invasive infection in very ill patients as defined by higher APACHE scores.
1 Guarro J, Gene J, Stchigel AM, et al. Developments in fungal taxonomy. Clin Microbiol Rev. 1999;12:454-500.
2 Vincent JL, Anaissie E, Bruining H, et al. Epidemiology, diagnosis and treatment of systemic Candida infections in surgical patients under intensive care. Intensive Care Med. 1998;24:206-216.
3 Cornwell EE, Belzberg H, Berne TV, et al. The pattern of fungal infections in critically ill surgical patients. Am Surg. 1995;61:847-850.
4 Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis. 2001;33:177-186.
5 Paphitou NI, Ostrosky-Zeichner L, Rex JH. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycol. 2005;43:235-243.
6 Piarroux R, Grenouillet F, Balvay P, et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med. 2004;32:2443-2449.
7 Holzheimer RG, Dralle H. Management of mycoses in surgical patients: review of the literature. Eur J Med Res. 2002;7:200-226.
8 Sawyer RG, Adams RB, May AK, et al. Development of candida albicans and C. albicans/escherichia coli/bacteroides fragilis intraperitoneal abscess models with demonstration of fungus-induced bacterial translocation. J Med Vet Mycol. 1995;33:49-52.
9 Lipsett PA. Clinical trials of antifungal prophylaxis among patients in surgical intensive care units: concepts and considerations. Clin Infect Dis. 2004;39:S193-S199.
10 Dean DA, Burchard KW. Surgical perspective on invasive Candida infections. World J Surg. 1998;22:127-134.
11 P Eggimann, J Garbino & D Pittet Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 3: 685–702
12 Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621-625.
13 Rocco TR, Reinert SE, Simms HH. Effects of fluconazole administration in critically ill patients: analysis of bacterial and fungal resistance. Arch Surg. 2000;135:160-165.
14 Gleason TG, May AK, Caparelli D, et al. Emerging evidence of selection of fluconazole-tolerant fungi in surgical intensive care units. Arch Surg. 1997;132:1197-1201.
15 Shorr AF, Chung K, Jackson WL, et al. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit Care Med. 2005;33:1928-1935.
16 Ostrosky-Zeichner L. New approaches to the risk of Candida in the intensive care unit. Curr Opin Infect Dis. 2003;16:533-537.
17 Groll AH, Gea-Banacloche JC, Glasmacher A, et al. Clinical pharmacology of antifungal compounds. Infect Dis Clin North Am. 2003;17:159-191.
18 Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662-678.
19 Herbrecht R, Denning DW, Patterson TF, et al. Vooriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408-415.
20 Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366:1435-1442.
21 Anidulafung in. Med Lett Drugs Ther. 2006;48:43-44.
22 Cochran A, Morris SE, Edelman LS, et al. Systemic Candida infection in burn patients: a case-control study of management patterns and outcomes. Surg Infect. 2002;4:367-374.