1 Fundamental concepts in functional neurology
Introduction
Much of the understanding that we have today of how human neurons function was based on the ‘integrate and fire’ concept formed by Eccles in the 1950s which was developed based on studies of spinal motor neurons (Brock et al. 1952). In this model, spinal motor neurons integrate synaptic activity, and when a threshold is reached, they fire an action potential. The firing of this action potential is followed by a period of hyperpolarisation or refraction to further stimulus in the neuron. This early integrate and fire model was then extrapolated to other areas of the nervous system including the cortex and central nervous system which strongly influenced the development of theories relating to neuron and nervous system function (Eccles 1951).
Early in the 1970s, studies that revealed the existence of neurons that operated under much more complex intrinsic firing properties started to emerge. The functional output of these neurons and neuron systems could not be explained by the existing model of integrate and fire for neuron function (Connor & Stevens 1971).
Central integrative state (CIS) of a neuron
The physical state of polarisation existing in the cell at any given moment is determined by the temporal and spatial summation of all the excitatory and inhibitory stimuli it has processed at that moment. The complexity of this process can be put into perspective when you consider that a pyramidal neuron in the adult visual cortex may have up to 12 000 synaptic connections, and certain neurons in the prefrontal cortex can have up to 80 000 different synapses firing at any given moment (Cragg 1975; Huttenlocher 1994).
‘And’ pattern neurons only fire an action potential if two or more specific conditions are met. ‘Or’ pattern neurons only fire an action potential when one or the other specific condition is present (Brooks 1984).
The thalamic relay cells exhibit complex firing patterns. They relay information to the cortex in the usual integrate and fire pattern unless they have recently undergone a period of inhibition. Following a period of inhibition stimulus, in certain circumstances, they can produce bursts of low-threshold spike action potentials referred to as post-inhibitory rebound bursts. This activity seems to be generated endogenously and may be responsible for production of a portion of the activation of the thalamocortical loop pathways thought to be detected in encephalographic recordings of cortical activity captured by electroencephalograms (EEG) (Destexhe & Sejnowski 2003).
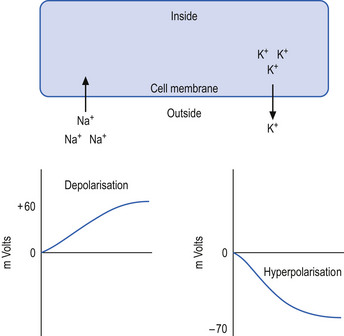
The neuron may be in a state of relative depolarisation, which implies the membrane potential of the cell has shifted towards the firing threshold of the neuron. This generally implies that the neuron has become more positive on the inside and the potential difference across the membrane has become smaller. Alternatively, the neuron may be in a state of relative hyperpolarisation, which implies the membrane potential of the cell has moved away from the firing threshold. This implies that the inside of the cell has become more negative in relation to the outside environment and the potential difference across the membrane has become greater (Ganong 1983) (Fig. 1.1).
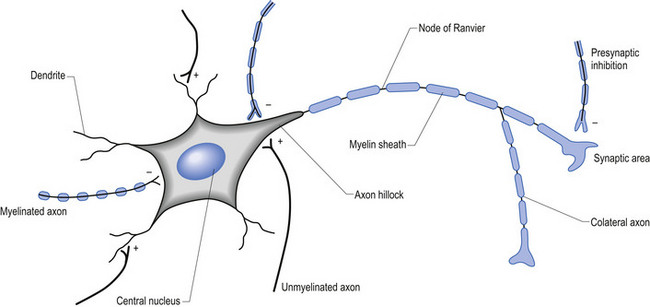
The firing threshold of the neuron is the membrane potential that triggers the activation of specialised voltage gated channels, usually concentrated in the area of the neuron known as the axon hillock or activation zone, that allow the rapid influx of Na into the axon hillock area, resulting in the generation of an action potential in the axon (Stevens 1979) (Fig. 1.2).
Transneural degeneration
1. Adequate gaseous exchange, namely oxygen and carbon dioxide exchange—this includes blood flow and anoxic and ischaemic conditions that may arise from inadequate blood supply;
2. Adequate nutritional supply including glucose, and a variety of necessary cofactors and essential compounds; and
3. Adequate and appropriate stimulation in the form of neurological communication, including both inhibition and activation of neurons via synaptic activation—synaptic activation of a neuron results in the stimulation and production of immediate early genes and second messengers within the neuron that stimulate DNA transcription of appropriate genes and the eventual production of necessary cellular components such as proteins and neurotransmitters.
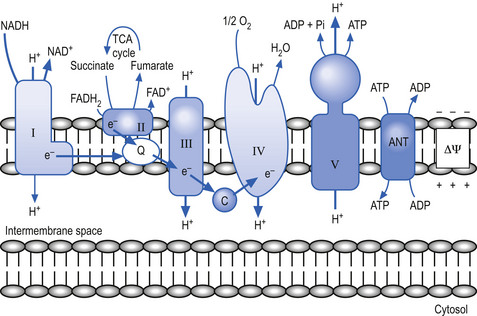
The mitochondria utilise a process called chemiosmotic coupling to harness energy from the food obtained from the environment for use in metabolic and cellular processes. The energy obtained from the tightly controlled slow chemical oxidation of food is used by membrane-bound proton pumps in the mitochondrial membrane that transfer H ions from one side to the other, creating an electrochemical proton gradient across the membrane. A variety of enzymes utilise this proton gradient to power their activities including the enzyme ATPase that utilises the potential electrochemical energy created by the proton gradient to drive the production of ATP via the phosphorylation of adenosine diphosphate (ADP) (Alberts et al. 1994). Other proteins produced in the mitochondria utilise the proton gradient to couple transport metabolites in, out of, and around the mitochondria (Fig. 1.3).
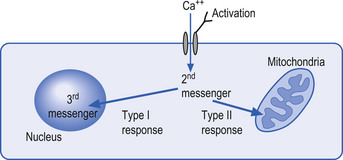
The mechanisms by which extracellular signals communicate their message across the neuron membrane to alter the protein production are discussed in Chapter 3. Here it will suffice to say that special transmission proteins called immediate early genes (IEG) are activated by a variety of second messenger systems in the neuron in response to membrane stimulus (Mitchell & Tjian 1989). Type 1 IEG responses are specific for the genes in the nucleus of the neuron and type 2 IEG responses are specific for mitochondrial DNA (Fig. 1.4).
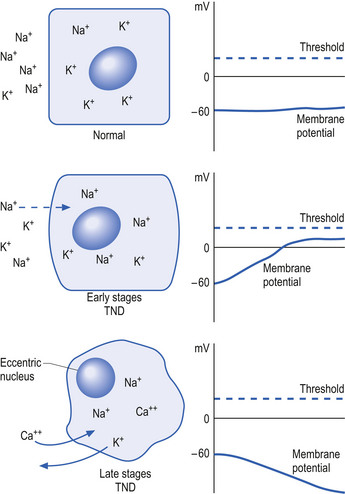
Initially, the neuron response to this down-regulation is to increase its sensitivity to stimulus so that less stimulus is required to stimulate protein production. This essentially means that the neuron alters its membrane potential so that it is closer to its threshold potential; in other words, it becomes more depolarised and becomes more irritable to any stimulus it may receive.
After a period of time if the neuron does not receive the deficient component in sufficient amounts, it can no longer sustain its state of depolarisation and starts to drastically downgrade the production of protein as a last ditch effort to conserve energy and maintain survival. At this stage, the neuron will still respond to stimulus but only for short periods as it consumes its available protein and ATP stores very quickly. In this state the neuron is vulnerable to overstimulation that may further exhaust and damage the neuron (Fig. 1.5).
The process of transneural degeneration may be one approach that determines the survival or death of neurons during embryological development where it has become quite clear that neurons that do not receive adequate stimulus do not usually survive (see Chapter 2).
Time to activation of a neuron or neuron system
The time to activation (TTA) of a neuron is a measure of the time from which the neuron receives a stimulus to the time that an activation response can be detected. Obviously, in clinical practice the response of individual neurons cannot be measured but the response of neuron systems such as the pupil response to light can be. As a rule, the TTA will be less (faster) in situations where the neuron system has maintained a high level of integration and activity and greater (slower) in situations where the neuron has not maintained a high level of integration and activity or is in the late stages of transneural degeneration. Again, an exception to this rule can occur in situations where the neuron system is in the early stages of transneural degeneration and is irritable to stimulus and responds quickly. This response will be of short duration and cannot be maintained for more than a short period of time.
Constant and non-constant neural pathways
Constant stimulus pathways are neural receptive systems that supply constant input into the neuraxis that are integrated throughout all multimodal systems to provide the stimulus necessary for the development and maintenance of the systems. Examples of constant stimulus pathways include receptors that detect the effects of gravity or constant motion, namely the joint and muscle position receptors of joint capsules and muscle spindles of the midline or axial structures including the ribs and spinal column. Certain aspects of the vestibulocerebellar system receive constant input and are constantly active. Several neural systems contain groups of neurons that exhibit innate pacemaker depolarisation mechanisms such as cardiac pacemaker cells, certain thalamic neurons, and selective neurons of the basal ganglia.
Neural plasticity
Neural plasticity results when changes in the physiological function of the neuraxis occur in response to changes in the internal or external milieu (Jacobson 1991). In other words, the development of synapses in the nervous system is very dependent on the activation stimulus that those synapses receive. The synapses that receive adequate stimulation will strengthen and those that do not receive adequate stimulation will weaken and eventually be eliminated (see Chapter 2).
Cerebral asymmetry (hemisphericity)
The fact that the human brain is asymmetric has been fairly well established in the literature (Geschwind & Levitsky 1968; LeMay & Culebras 1972; Galaburda et al. 1978; Falk et al. 1991; Steinmetz et al. 1991). The exact relationship between this asymmetric design and the functional control exerted by each hemisphere remains controversial.
The concept of hemispheric asymmetry or lateralisation involves the assumption that the two hemispheres of the brain control different asymmetric aspects of a diverse array of functions and that the hemispheres can function at two different levels of activation. The level at which each hemisphere functions is dependent on the central integrative state of each hemisphere, which is determined to a large extent by the afferent stimulation it receives from the periphery as well as nutrient and oxygen supply. Afferent stimulation is gated through the brainstem and thalamus, both of which are asymmetric structures themselves, and indirectly modulated by their respective ipsilateral cortices (Savic et al. 1994).
Traditionally, the concepts of hemisphericity were only applied to the processing of language and visuospatial stimuli. Today, the concept of hemisphericity has developed into a more elaborate theory that involves cortical asymmetric modulation of such diverse constructs as approach versus withdrawal behaviour, maintenance versus interruption of ongoing activity, tonic versus phasic aspects of behaviour, positive versus negative emotional valence, asymmetric control of the autonomic nervous system, and asymmetric modulation of sensory perception, as well as cognitive, attentional, learning, and emotional processes (Davidson & Hugdahl 1995).
Embryological homological relationships
The term embryological homologues is used to describe the functional relationships that exist between neurons born at the same time in the cell proliferation phase of development. These cells born at the same time along the length of neuraxial ventricular area develop and retain synaptic contact with each other, many of which remain in the mature functional state. This cohort of cells that remain functionally connected after migration results in groups of neurons that may be unrelated in cell type or location but fire as a functional group when brought to threshold. Dorsal root ganglion cells detecting joint motion and muscle contraction and postsynaptic neurons in the sympathetic ganglia controlling blood flow to the joints and muscles illustrate the concept. Another example includes the motor column of the cranial nerves III, IV, VI, and XII in the brainstem which act functionally as a homologous column. This concept also applies to functional areas of the neuraxis that developed from the same embryological tissues. Neuron systems that have developed from the same embryological tissues usually maintain reciprocal connections throughout their life span. For example, in the mesencephalon there is an area of the neuraxis that develops in an undifferentiated fashion, all of the functional structures would be considered embryological homologues and as such be expected to maintain reciprocal connections throughout life. This would imply that the structures in the mesencephalon such as the red nucleus, the substantia nigra, the oculomotor nucleus, the Edinger–Westphal nucleus, and the reticular neurons all maintain close functional relationships. This is in fact the case. Another spin-off from this concept is that all neuron systems developing from the same tissue remain in close reciprocal contact even after further differentiation; for example, the cortex and the thalamus, which develop from prosencephalon but further differentiate to telencephalon and diencephalon respectively, would still be considered embryological homologues. These two structures do indeed retain reciprocal connectivity throughout life. Other examples include the structures that have developed from the rhombencephalon: the cerebellum, pons, and brainstem.
Neurophysiological excitation and inhibition in neural systems
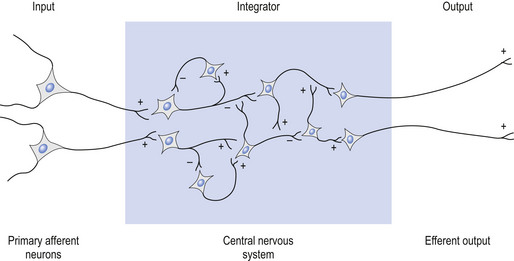
Components of a neuron system usually involve an input stimulus, a series of integration steps, and an output (Williams & Warwick 1980).
The input stimulus or output, as well as any steps in the integration portion, may be either excitatory or inhibitory in nature (Fig. 1.6).
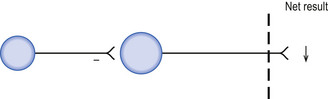
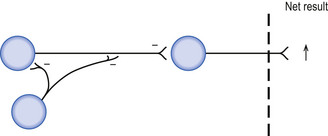
Direct inhibition involves a hyperpolarising stimulus to the target neuron, which results in a decreased probability that the target neuron will be brought to threshold potential and fire and action potential per unit stimulus (Fig. 1.7).
Feedforward inhibition involves the linking of an inhibitory interneuron into a pathway that causes relative hyperpolarisation of the next neuron in the pathway, resulting in a decreased probability of output activation of that pathway (Fig. 1.8).
Feedback inhibition involves an inhibitory interneuron that receives stimulus from the neuron that it projects to and thus also inhibits (Fig. 1.9).
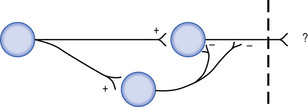
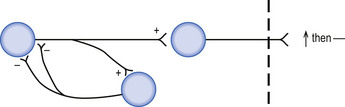
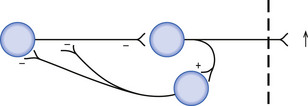
Disinhibition (inhibition of inhibition) involves two inhibitory interneurons linked in series with each other so that stimulation of the first neuron results in inhibition of the second neuron, which in turn results in decreased inhibitory output of the second interneuron to the target. The overall effect of disinhibition is an increased probability that the effector will reach threshold potential per unit stimulus (Fig. 1.10).
Feedback disinhibition involves a series of inhibitory interneurons that receive their original stimulus from the neuron that they project to; however, in this case the net result is an increase in the probability that the target neuron will reach threshold per unit stimulus (Fig. 1.11).
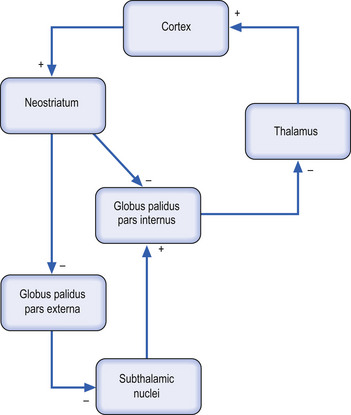
To illustrate these concepts the corticostriate-basal ganglio-thalamocortical neuron projection system in the neuraxis will be examined. (See Chapter 11 for more detailed descriptions.)
Selective pyramidal output neurons, in wide areas of cortex, project to the neostriatum (caudate and putamen) via the corticostriatal projection system. The cortical neurons are excitatory in nature to the neurons in the caudate and putamen. The neurons of the caudate and putamen project to neurons in both regions of the globus pallidus, the globus pallidus pars interna, and globus pallidus pars externa, and are inhibitory in nature. For the purposes of this example we will only consider the projections to the globus pallidus pars interna. This nucleus comprises the final output nucleus of the basal ganglia to the thalamus. (For a more complete description, see Chapter 11.)
Following the flow of stimulus through the system (Fig. 1.12), it can be noted that the end result is a disinhibition of the thalamus and an increased likelihood of cortical activation by thalamic neurons.
Neurological ‘wind-up’ in a system
1. Overactivation of glutamate N-methyl-D-aspartate (NMDA) receptors can result in excitotoxicity in neurons because of increased intracellular Ca++ ion concentrations.
2. Free radical formation due to anaerobic energy production pathways may lead to damage to membrane and membrane receptor structures, or to mutations in DNA.
3. Transneural degeneration may result when intracellular protein and energy stores become inadequate to support the increased demands of hyperexcitability.
The thalamus contains neurons that tonically generate innate excitatory potentials that project to the cortex and result in excitation of cortical neurons. Under normal conditions, modulation of this tonic excitation occurs via the inhibitory output of the globus pallidus pars interna (GPi) of the basal ganglionic circuits. However, in certain circumstances, such as an increased output of the neostriatum, which can occur with loss of inhibition from the substantia nigra pars compacta, the globus pallidus receives an increased inhibitory input from the neostriatum. This increased inhibition to the GPi reduces the inhibition received by the thalamus from the GPi. This results in an increase in the innate tonic excitation to the cortex from the thalamus, which in turn results in a hyperexcitation or wind-up of cortical neurons. This example demonstrates how inhibition of inhibition can result in hyperexcitation of a neuron system. The concept of inhibition of inhibition is very important clinically in understanding the symptoms produced in multimodal integrative systems and is encountered frequently in clinical practice.
Fundamental functional projection systems
About 90% of the output axons of the cortex are involved in modulation of the neuraxis. About 10% of the cortical output axons of the cortex are involved in motor control and form the corticospinal tracts.
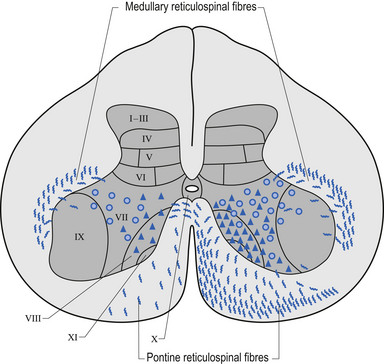
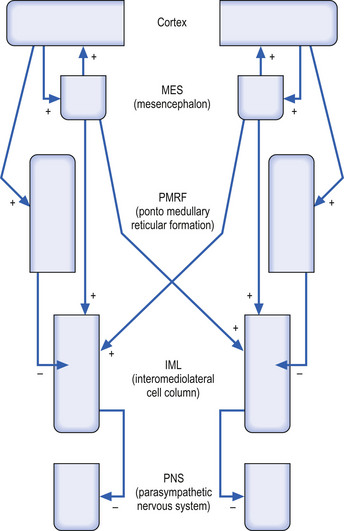
Of the 90% output dedicated to neuraxis modulation about 10% projects bilaterally to the reticular formation of the mesencephalon (MRF) and 90% projects ipsilaterally to the reticular formation of the pons and medulla or pontomedullary reticular formation (PMRF). The cortical projections to both the MRF and the PMRF are excitatory in nature. The neurons in the MRF and some of those in the PMRF project bilaterally to excite neurons in the intermediolateral (IML) cell columns located between T1 and L2 spinal cord levels in the grey matter of the spinal cord; however, the majority of the PMRF remain ipsilateral (Fig. 1.13) (Nyberg-Hansen 1965). These neurons in the IML form the presynaptic output neurons of the sympathetic nervous system, and project to inhibit neurons in the sacral spinal cord regions that form the pelvic or sacral output of the parasympathetic nervous system.
Following the stimulus flow through the functional system, it can be seen that high cortical output results in high PMRF output, which results in strong inhibition of the IML, which in turn results in disinhibition of the sacral parasympathetic output. The bilateral excitatory output of the MRF is overshadowed by the powerful stimulus from the cortex to the PMRF (Fig. 1.14).
1. Inhibition of pain ipsilaterally;
2. Inhibition of the inhibitory interneurons which project to ventral horn cells (VHCs) ipsilaterally which acts to facilitate muscle tone—this is another example of inhibition in the neuraxis as discussed above; and
3. Inhibition of the ipsilateral anterior muscles above T6 and the posterior muscles below T6.
• Increased blood pressure systemically or ipsilaterally to the side of decreased PMRF stimulation, which results in differences in blood pressure between right and left sides of the body;
• Increased vein-to-artery ratio, which is most apparent on examination of the retina;
• Increased sweating globally or ipsilaterally to the side of decreased PMRF stimulation;
• Decreased skin temperature globally or ipsilaterally to the side of decreased PMRF stimulation;
• Arrhythmia if decreased left PMRF stimulation occurs or tachycardia if decreased right PMRF stimulation occurs;
• Large pupil (also due to decreased mesencephalic integration) to the side of decreased PMRF stimulation;
• Ipsilateral pain syndromes to the side of decreased PMRF stimulation;
• Global decrease in muscle tone ipsilaterally to the side of decreased PMRF stimulation;
• Flexor angulation of the upper limb ipsilaterally to the side of decreased PMRF stimulation; and
• Extensor angulation of the lower limb ipsilaterally to the side of decreased PMRF stimulation.
The fundamental projection systems (see Fig. 1.14) presented above are simplified for the purposes of introduction and will be discussed more thoroughly throughout the rest of the text.
Longitudinal level of a lesion in the neuraxis
The concepts of direct linearity and singularity
Monosynaptic connections between two structures suggest an important functional relationship between the two structures in question. Polysynaptic connections may be important as well but are not as well understood. For example, the existence of monosynaptic connections between the hypothalamus and the preganglionic sympathetic neurons in the IML suggests an important functional relationship between the output of the sympathetic nervous system and the CIS of the hypothalamus.
The physiological ‘blind spot’ as a measure of cortical activation
It would be expected that when one eye is closed the visual field should now have an area not represented by visual input and one should be aware of the absence of vision over the area of the blind spot. However, this does not occur. The cortical neurons responsible for the area of the blind spot must receive stimulus from other neurons that create the illusion that the blind spot is not there. This is indeed the case and is accomplished by a series of horizontal projection neurons located in the visual striate cortex that allow for neighbouring hypercolumns to activate one another. The horizontal connections between these hypercolumns allow for perceptual completion or ‘fill in’ to occur (Gilbert & Wiesel 1989; McGuire et al. 1991).
Perceptual completion refers to the process whereby the brain fills in the region of the visual field that corresponds to a lack of visual receptors. This explains why one generally is not aware of the blind spot in everyday experience. The size and shape of the blind spots can be mapped utilising simple procedures as outlined in Chapter 4.
The stimulus utilised by Professor Carrick in his study was a manipulation of the upper cervical spine which is known to increase the FOF of multimodal neurons in areas of the thalamus and brainstem that project to the visual striate cortex. These reciprocal connections lower the threshold for activation of neurons in the visual cortex. By decreasing the threshold for firing of neurons in the visual cortex the blind spot became smaller because the area surrounding the permanent geometric blind spot zone is more likely to reach threshold and respond to the receptor activation that occurs immediately adjacent to the optic disc on the contralateral side. The size and shape of the blind spot will also be associated with the degree of activation of neurons associated with receptors adjacent to the optic disc. The receptors surrounding the optic disc underlie the neurons that form the optic nerve exiting by way of the optic disc. The amplitude of receptor potentials adjacent to the optic disc may therefore also be decreased due to interference of light transmission through the overlying fibres even though they should have lost their myelin coating during development; otherwise, interference would be even greater. This interference results in a decreased receptor amplitude, which in turn results in decreased FOF of the corresponding primary afferent nerve. This may result in a blind spot physiologically larger than the true anatomical size of the blind spot.
Therefore, muscle stretch and joint mechanoreceptor potentials will alter the FOF of primary afferents that may have an effect on visual neurons associated with the cortical receptive field of the blind spot when visual afferents are in a steady state of firing. Professor Carrick proposed that ‘A change in the frequency of firing of one receptor-based neural system should effect the central integration of neurons that share synaptic relationships between other environmental modalities, resulting in an increase or decrease of cortical neuronal expression that is generally associated with a single modality’ (Carrick 1997).
• Multiple evanescent white dot syndrome;
• Acute macular neuroretinopathy;
• Acute idiopathic blind spot enlargement (AIBSE) syndrome;
• Pseudo presumed ocular histoplasmosis;
An ophthalmoscopical examination is therefore an important component of the functional neurological examination. There are several other valuable ophthalmoscopic findings discussed in Chapter 4 that can assist with estimating the CIS of various neuronal pools.
Upper and lower motor neurons
Lower motor neuron lesions will produce flaccid muscle weakness, muscular atrophy, fasciculations, and hyporeflexia. Upper motor neuron lesions will produce spastic muscle weakness, and hyperreflexia. Upper motor neuron dysfunction involving the corticospinal tracts usually produces a classic reflex sign referred to as Babinski’s sign or reflex. Under normal conditions, stroking the plantar aspect of the foot will produce a reflex flexion of the toes. In cases of corticospinal tract dysfunction, stroking the plantar surface of the foot produces an ‘up-going’ or extended big toe and fanning action of the rest of the toes. This is referred to as a positive or present Babinski sign or up-going plantar reflex. Other reflex signs of upper motor neuron dysfunction will be discussed in Chapter 4. Initially, in the acute stages, the signs of upper motor neuron dysfunction may mimic lower motor neuron dysfunction exhibiting flaccid muscle weakness and hyporeflexia. These signs change progressively over hours or days to the true signs of upper motor neuron dysfunction. In the case of long-standing upper motor neuron dysfunction, the involved muscles may atrophy due to disuse and give the appearance of a lower motor neuron involvement.
References
Brooks V.B. The neural basis of motor control. Oxford: Oxford University Press, 1984.
Davidson R.J., Hugdahl K. Brain asymmetry. Cambridge, MA/London: MIT Press; 1995.
Jacobson M. Developmental Neurobiology, third ed. New York/London: Plenum Press, 1991.