Functional neuroanatomy
This chapter examines the functional anatomy of the brain, focusing on the cerebral cortex, basal ganglia, hippocampus and amygdala. The main functional divisions of the thalamic region, brain stem and cerebellum are also discussed. Sensory and motor pathways of the CNS are described in Chapter 4. The blood supply to the brain is discussed in Chapter 10, in the context of stroke.
Cerebral cortex
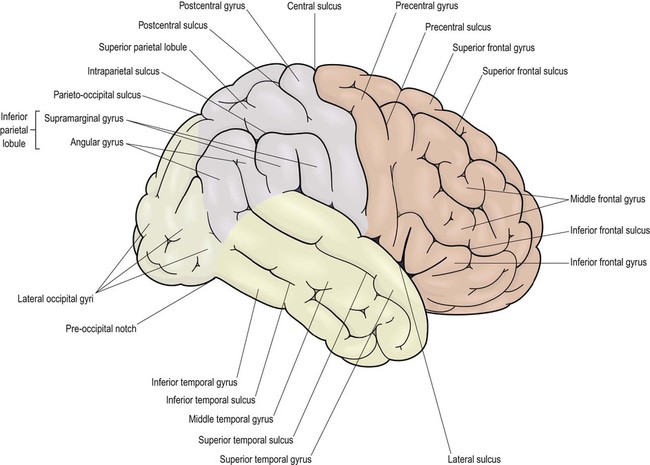
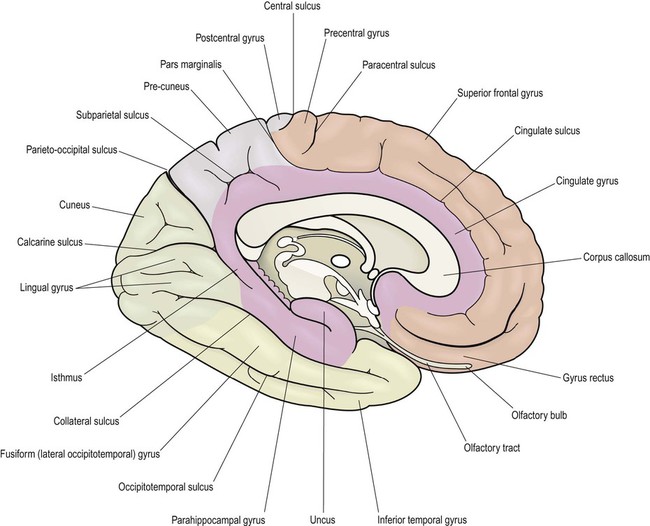
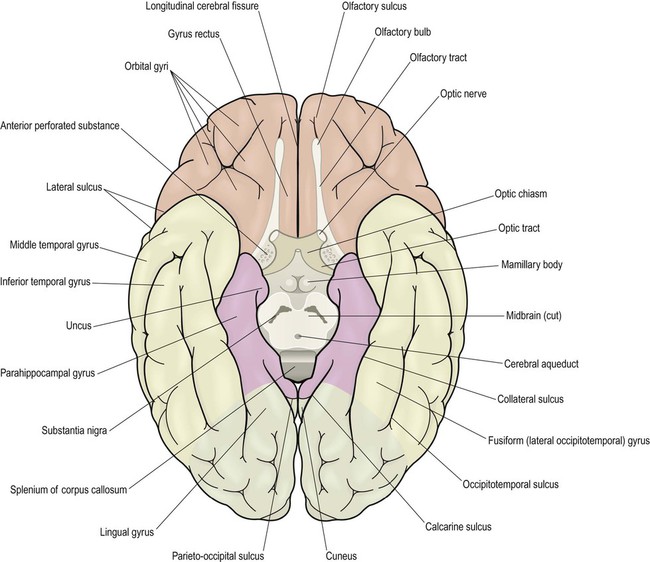
This section describes the gyri and sulci of the cerebral hemispheres (Figs 3.1–3.3) and the main functional areas of the cerebral cortex (Fig. 3.4). Brodmann areas (BA) are indicated in brackets if they have important functional or clinical associations (these are numbered cortical regions, defined by microscopic differences in the structure of the cerebral cortex; see Chapter 5).
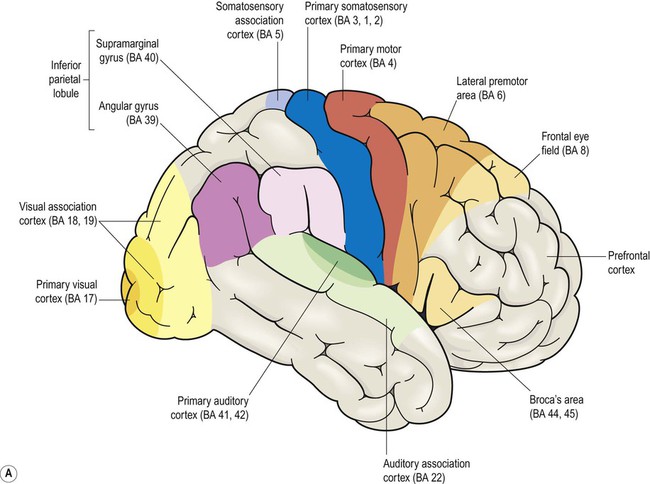
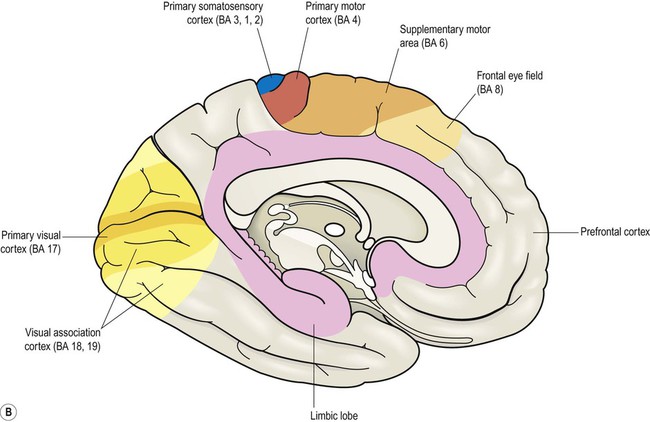
Brodmann areas are indicated in brackets (apart from the prefrontal cortex and limbic lobe, which are large regions that incorporate numerous cortical functional zones).
Frontal lobe
Lateral aspect (Figs 3.1 and 3.4A)
The precentral gyrus ribbons forward over the cerebral convexity, immediately anterior to the central sulcus. It corresponds to the primary motor cortex (BA 4) which contains an inverted, point-to-point or somatotopic map of the opposite half of the body (Greek: soma, body; topos, place). The representation of each body part in the motor strip is proportional to the precision of movement control. This means that the areas for the hands, face and tongue are disproportionately large (Fig. 3.5). A useful landmark for identifying the motor cortex is the motor hand area which resembles an inverted capital omega (Fig. 3.6).
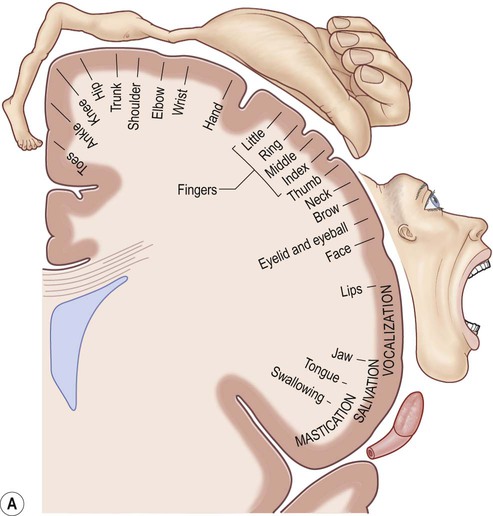
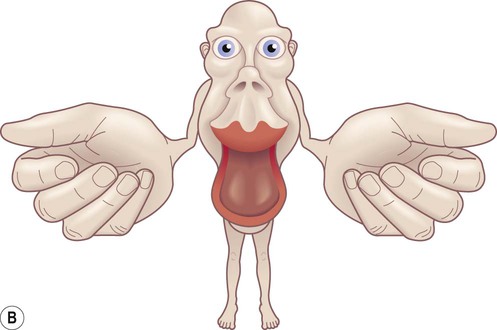
(A) The motor strip contains an orderly somatotopic (point-to-point) representation of the contralateral half of the body, from the toes (medially) to the face and tongue (laterally). The size of the cortical representation for each body part reflects the precision of motor control, so that the areas for the hands, face and tongue are disproportionately large; (B) This can be represented graphically as a ‘homunculus’. Adapted from Matthew Levy, Bruce Koeppen, Bruce Stanton: Berne & Levy Principles of Physiology 4e (Mosby 2006) with permission.
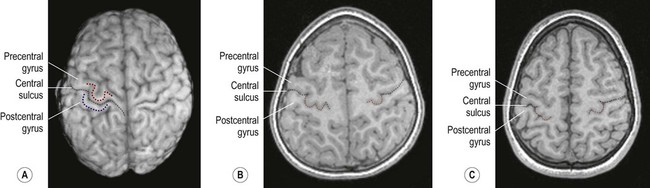
(A) On the superior surface of the brain, the convolution corresponding to the ‘hand area’ of the primary motor cortex often resembles an inverted capital omega (Ω) as shown here in a surface rendering of the brain (obtained from a volumetric MRI scan). The motor hand area (in the precentral gyrus) is indicated by the red dashed line. The sensory hand area (of the postcentral gyrus) is shown in blue; (B) In axial sections (in this case, a T1-weighted MRI scan in the same individual) the ‘hand area’ of the primary motor cortex can be seen projecting backwards (red dashed line). Its shape often resembles a door-knob, as seen on the right-hand side in this individual; (C) Another example (in a different person). [Note: it is more common for the ‘hand-knob’ to be single (Ω-shaped) rather than double (ω-shaped).] Courtesy of Dr Gemma Northam.
Medial aspect (Figs 3.2 and 3.4B)
The supplementary motor area (SMA) is just in front of the paracentral lobule. It contains a map of both sides of the body and tends to be recruited with its counterpart in the opposite hemisphere (e.g. when the hands are working together to manipulate an object). In contrast to the lateral premotor area, the SMA (or ‘medial premotor area’) is particularly concerned with internally generated (self-initiated) actions, rather than those that occur in response to an external event. The SMA is underactive in Parkinson’s disease, in which voluntary movements are initiated with effort and performed slowly (Ch. 13). Just anterior to the SMA, there is a small medial extension of the frontal eye field.
Prefrontal cortex (Figs 3.3 and 3.4)
 The dorsolateral prefrontal cortex (DLPFC) is concerned with organizing and planning behaviour in pursuit of short-, medium- and long-term goals.
The dorsolateral prefrontal cortex (DLPFC) is concerned with organizing and planning behaviour in pursuit of short-, medium- and long-term goals.
 The orbitofrontal cortex (OFC) has a predominantly inhibitory role, preventing inappropriate behaviour (e.g. during social interactions) and facilitating moderation, restraint and tact.
The orbitofrontal cortex (OFC) has a predominantly inhibitory role, preventing inappropriate behaviour (e.g. during social interactions) and facilitating moderation, restraint and tact.
 The medial prefrontal cortex (mPFC) is concerned with mood, motivation and emotion. It is part of the so-called ‘default network’ of the brain (areas that are active during quiet contemplation).
The medial prefrontal cortex (mPFC) is concerned with mood, motivation and emotion. It is part of the so-called ‘default network’ of the brain (areas that are active during quiet contemplation).
The prefrontal cortex includes Broca’s area (discussed below, in the context of language) and the frontal eye fields (see above). The effects of prefrontal cortex lesions are discussed in Clinical Box 3.1.
Parietal lobe
Lateral aspect (Figs 3.1 and 3.4A)
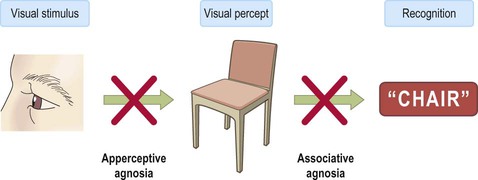
The remainder of the lateral parietal lobe is divided into superior and inferior parietal lobules by the intraparietal sulcus. This is a deep cleft at right angles to the central sulcus. The somatosensory association cortex (BA 5) is a small area in the superior parietal lobule, just behind the sensory strip. Lesions here may lead to astereognosia: the inability to recognize objects by touch (Greek: a-, without; stereos, solid; gnosis, knowledge). The posterior parietal cortex (BA 7) has close links with the occipital lobe and is concerned with visuospatial perception and attention (Clinical Box 3.2). This includes the representation and manipulation of objects (e.g. using a knife and fork) and the perception of movement (e.g. judging the approach of a moving vehicle). Certain semi-automatic movements are initiated by projections from the parietal cortex to the lateral premotor area (Clinical Box 3.3).
Central visual pathways (Figs 3.7 and 3.8)
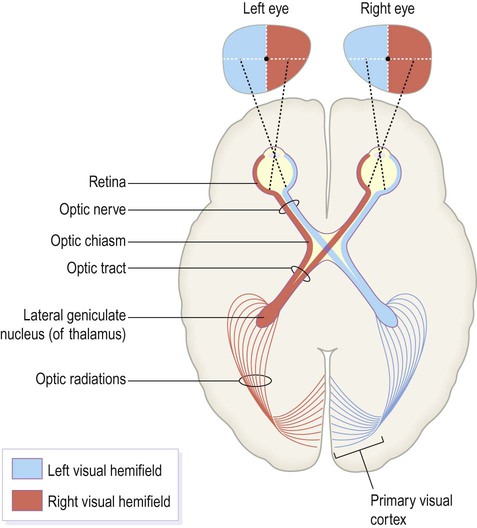
The visual fields of each eye are illustrated separately in this figure, but in reality they overlap extensively to allow for binocular vision (and contributing to depth perception).
The optic chiasm (Fig. 3.7)
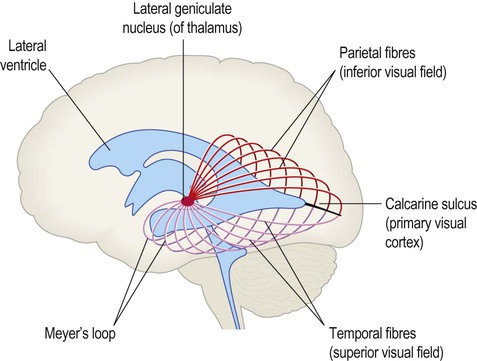
The central visual pathways are crossed. This means that the right visual field is represented in the left occipital lobe and vice versa. Since light travels in straight lines and enters the eye via the small aperture of the pupil, objects in the right visual field project to the left half of each retina (coloured red in Fig. 3.7). Axons originating from the left half of each retina must therefore project to the left cerebral hemisphere. For this to happen, nerve fibres from the inner half of the retina must cross the midline to enter the opposite optic tract. This takes place at the optic chiasm, named for its resemblance to the Greek letter chi.
The primary visual cortex
Visual information is segregated into three separate ‘channels’ (concerned with form, motion and colour) by the primary visual cortex (V1) and visual association cortices (V2, V3… and higher). Two parallel visual ‘streams’ dealing with different aspects of visual perception arise from the occipital lobe. The dorsal or parietal lobe stream is concerned with the location and movement of objects and their positions relative to the body (the ‘where’ pathway). The ventral or temporal lobe stream synthesizes information about form and colour, allowing objects to be recognized (the ‘what’ pathway). Central visual pathway lesions are discussed in Clinical Box 3.4.
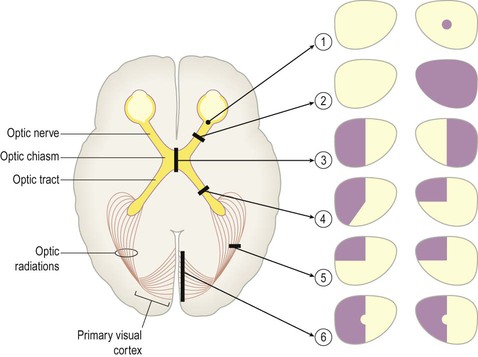
The effects of lesions in various parts of the central visual pathway are illustrated. The visual fields of the left and right eyes are shown in pale yellow and the areas of reduced or absent vision are shown as purple shading. [The defects depicted are as follows: (1) = small scotoma (blind spot) in the right eye; (2) = monocular blindness, right eye; (3) = bitemporal hemianopia or ‘tunnel vision’; (4) = heteronymous left-sided field defect; (5) = left superior quadrantanopia, homonymous field defect; (6) = left hemianopia with macular (central) sparing.]
Temporal lobe
Superior aspect (Fig 3.10)
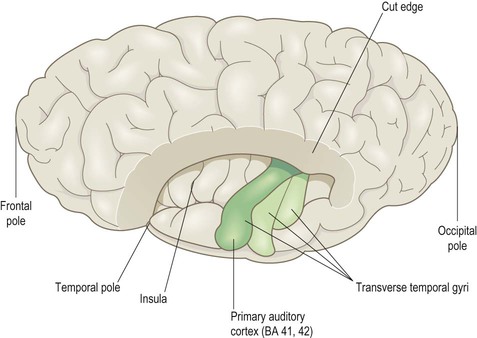
This view shows the superolateral aspect of the left cerebral hemisphere with the frontal and parietal opercula dissected away to reveal the superior surface of the temporal lobe (including the transverse temporal gyri and primary auditory cortex). Part of the insula can also be seen.
Inferior aspect (Fig. 3.3)
On the inferior aspect of the hemisphere, the occipital and temporal lobes form a continuous, uninterrupted surface that is composed of the medial and lateral occipitotemporal gyri. The medial occipitotemporal gyrus is medial to the collateral sulcus. The portion lying within the occipital lobe is also known as the lingual gyrus, whereas the part contained in the temporal lobe is the parahippocampal gyrus. The lateral occipitotemporal gyrus runs alongside, between the collateral sulcus medially and occipitotemporal sulcus laterally. The anterior and posterior ends of the lateral occipitotemporal gyrus are usually tapered to give it a ‘spindle’ shape. It is therefore also known as the fusiform gyrus (Latin: fusiform, shaped like a spindle). It receives projections from the occipital lobe (part of the ‘what pathway’) and appears to be involved in the recognition of complex visual patterns. It contributes to reading (in the language-dominant hemisphere) and face recognition (in the non-dominant hemisphere) (Clinical Box 3.5).
Language areas (Fig. 3.12)
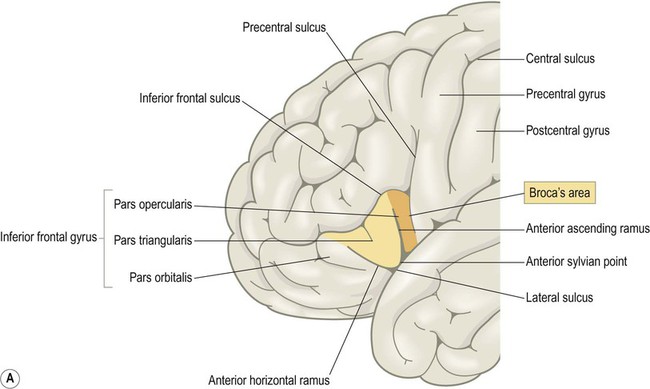
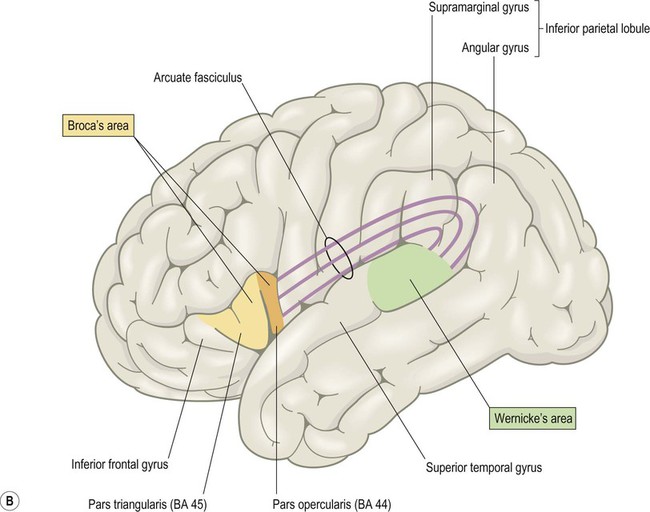
(A) Broca’s area corresponds to the opercular and triangular parts of the inferior frontal gyrus (i.e. the pars opercularis and pars triangularis, coloured orange and yellow in the figure). These are defined by the ascending and horizontal branches (rami) of the lateral sulcus, which arise from an enlargement of the lateral sulcus called the anterior sylvian point; (B) The anterior and posterior language areas are linked by the arcuate fasciculus, which is the main language-associated white matter bundle.
Broca’s area
Broca’s area is involved in the expressive aspects of spoken and written language (production of sentences constrained by the rules of grammar and syntax). It corresponds to the opercular and triangular parts of the inferior frontal gyrus (BA 44 and 45) (Fig. 3.12A). As illustrated in the figure, these are defined by two rami (branches) of the lateral sulcus (one ascending, one horizontal) which ‘slice into’ the inferior frontal gyrus. In keeping with its role in speech and language, Broca’s area is immediately anterior to the motor and premotor representations of the face, tongue and larynx. A homologous area in the opposite hemisphere is involved in non-verbal communication such as facial expression, gesticulation and modulation of the rate, rhythm and intonation of speech.
Wernicke’s area
Wernicke’s area (pronounced: VER-nikker) corresponds to the posterior third of the superior temporal gyrus and is part of the auditory association cortex (Fig. 3.12B). It lies at the junction of the visual and auditory cortices and is involved in transforming the visual impression of letters (graphemes) into mental representations of speech sounds (phonemes). It is therefore important for speech comprehension and reading. The non-dominant homologue of Wernicke’s area is involved in understanding intonation and emphasis (the ‘music’ of speech) which can alter the meaning of words considerably. Some patients with non-dominant temporal lobe lesions may therefore have monotone, ‘robotic’ speech or fail to grasp nuances of intonation (termed aprosodia).
Language pathways
The two main language areas are connected by the arcuate fasciculus, a large white matter bundle that arches around the lateral sulcus (Latin: arcus, bow) (Fig. 3.12B).
A second language-associated pathway interconnects the anterior and lateral temporal lobe with the inferior frontal lobe via the hook-shaped uncinate fasciculus (Latin: uncus, hook) (see Ch. 1, Fig. 1.18). This has been referred to as the ventral language stream and is more concerned with semantic aspects of language (the meaning of words and concepts). Language disorders are discussed in Clinical Box 3.6.
Limbic lobe and insula
The limbic lobe is a ring-shaped convolution surrounding the medial border of the cerebral hemisphere (Latin: limbus, border) (Fig. 3.13). It is primarily concerned with emotion and memory. The anterior insula, posterior orbitofrontal cortex and temporal pole have similar functional roles and are referred to as paralimbic areas. The hippocampus (a cortical region that belongs to the limbic lobe) and the amygdala (a subcortical structure involved in emotional responses) are discussed separately below. The outdated term ‘limbic system’ is sometimes used as vague shorthand for ‘emotional brain’ but it has no proper definition and is best avoided.
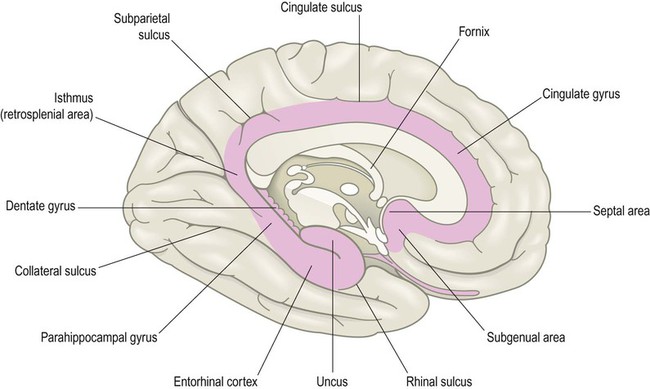
The brain stem has been removed and the ‘teeth’ of the dentate gyrus can be seen (Latin: dentālis, bearing teeth). The septal area is also indicated. This is connected to numerous brain regions that are involved in mood, emotion and reward-based learning.
The insula
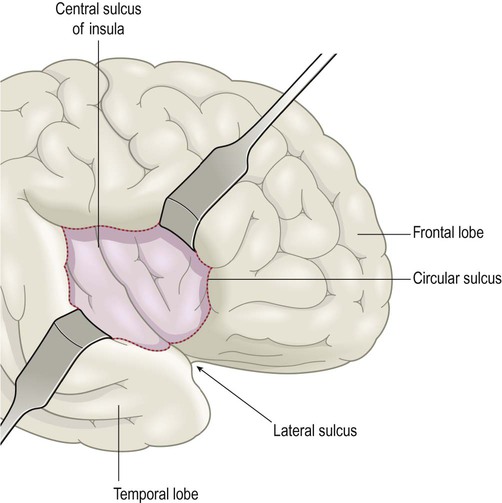
The insula is an ‘island’ of cortex hidden in the depths of the lateral sulcus (Latin: insula, island) which can be exposed by retracting the overhanging opercula of the frontal, parietal and temporal lobes (Latin: operculum, lid) (Fig. 3.14; see also Ch. 2, Fig. 2.10). It is divided into anterior (visceral) and posterior (somatic) parts by the central sulcus of the insula. The anterior insula receives projections from the olfactory bulb and is part of the primary olfactory cortex. It is also involved in nausea, vomiting, disgust and pain perception, including the accompanying visceral and autonomic phenomena. Studies using functional magnetic resonance imaging (fMRI) suggest that the anterior insula, which is immediately adjacent to Broca’s area, may also be involved in language. The posterior insula integrates non-visceral (somatic) information related to touch, vision and hearing.
Hippocampus and amygdala
Hippocampus
The hippocampus occupies the temporal horn of the lateral ventricle. It is therefore ‘submerged’ in cerebrospinal fluid and forms a longitudinal bulge in the ventricular floor (Fig. 3.15A). The hippocampus is covered by a thin sheet of white matter called the alveus, which derives from the Latin for ‘river bed’. The name hippocampus continues the aquatic theme, reflecting its resemblance to a sea horse (Fig. 3.15B). The term derives ultimately from a creature in Greek mythology that is part horse, part fish (Greek: hippos, horse; kampos, sea monster).
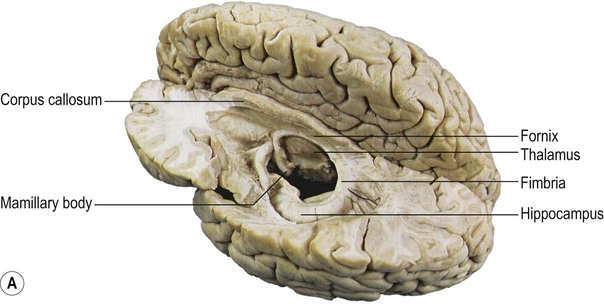
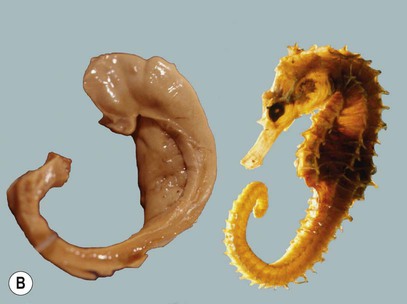
(A) Dissection of the left cerebral hemisphere showing the hippocampus in the floor of the temporal horn of the lateral ventricle. The fornix, a major outflow pathway of the hippocampus, can be seen arching up towards the midline (below the corpus callosum) before terminating in the mamillary body in the floor of the third ventricle. From Standring: Gray’s Anatomy 40e (2008: Churchill Livingstone) with permission; (B) When the hippocampus and fornix are dissected free from the brain they resemble a seahorse (Hippocampus spp). Courtesy of Professor László Seress.
Parts of the hippocampus
The hippocampus is formed by an S-shaped fold of relatively simple three-layered cortex that is continuous with the neighbouring parahippocampal gyrus (Greek: para-, beside). It is composed of allocortex (see Ch. 5) which is thinner and less complex than the six-layered neocortex that is found in 90% of the cerebral hemisphere. Its relationship to the lateral ventricle and parahippocampal gyrus are illustrated in Figure 3.16A. The hippocampus consists of the dentate gyrus and Ammon’s horn (within the ventricle) together with the subiculum below (Latin: subicere, to support) (Fig. 3.16B). Ammon’s horn (also referred to as the hippocampus proper) contains large pyramidal neurons arranged in three zones called CA1, CA2 and CA3. The terminology derives from the Latin form of Ammon’s horn, cornu ammonis (CA) and refers to an Egyptian deity with ram’s horns.
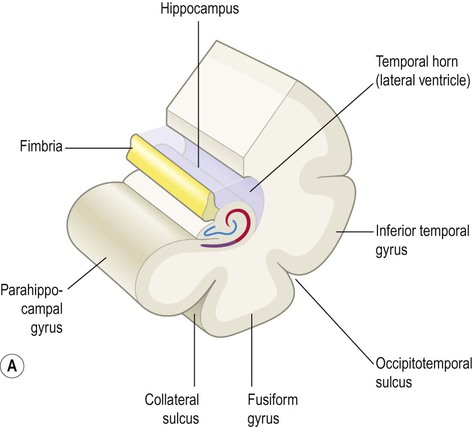
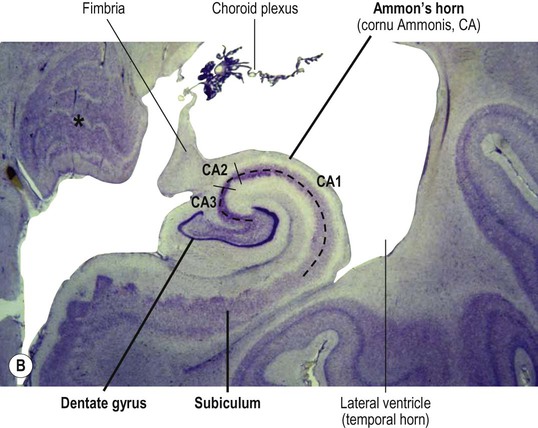
(A) Illustration of the hippocampus (viewed from the medial aspect) and its relationship to the parahippocampal gyrus and temporal horn of the lateral ventricle [blue = dentate gyrus, red = Ammon’s horn, purple = subiculum]; (B) Microscopic structure of the hippocampus showing the main parts, including the subsectors of Ammon’s horn, CA1-CA3 [Nissl stain]. From Weller, R: Advances in Clinical Neuroscience and Rehabilitation, Vol 7 (Whitehouse Publishing 2008) with permission. *Indicates the lateral geniculate nucleus (LGN) of the thalamus.
Afferent and efferent connections
Fimbria and fornix
Hippocampal efferent fibres gather in the fimbria, which runs along the medial border of the hippocampus (Latin: fimbria, fringe) (see Fig. 3.16). The fimbria emerges from the posterior aspect of the hippocampus to be renamed the fornix. This is a white, cord-like structure that sweeps up to the midline and forms an arch below the corpus callosum (Latin: fornix, arch) (see Fig. 3.15A). The fornix terminates in the mamillary bodies (of the hypothalamus) in the floor of the third ventricle (Latin: mamilla, nipple). The mamillary bodies project in turn to the cingulate gyrus (via a ‘relay station’ in the anterior thalamus) and a complete loop can be traced back to the entorhinal cortex via the cingulum bundle (Fig. 3.17).
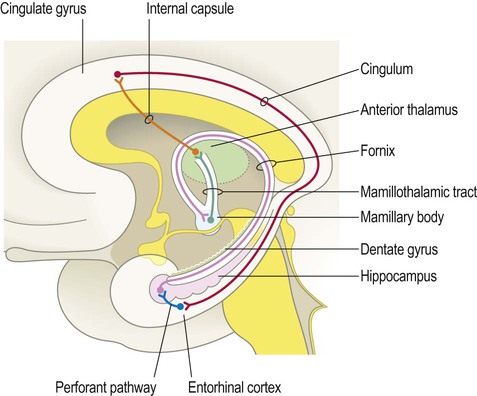
The main afferent projection into the hippocampus is the perforant path (blue) which arises from the entorhinal cortex in the anterior part of the parahippocampal gyrus; the main outflow projection is the fornix (pink) which arches up towards the midline before terminating in the mamillary body of the hypothalamus.
Functions of the hippocampus
The hippocampus is involved in the formation of new memories and is particularly important for the recollection of personal experiences, termed episodic memory; this is in contrast to semantic memory which has to do with abstract knowledge and facts (Clinical Box 3.7). The hippocampus also stores spatial maps of the environment (e.g. familiar towns, streets and buildings) that we use to navigate. In keeping with this role, it has been found to be significantly larger in licensed London taxi drivers. The effects of hippocampal lesions are discussed in Clinical Boxes 3.8 and 3.9.
Amygdala
The amygdala is an almond-shaped mass of grey matter in the anterior part of the medial temporal region that is concerned with emotional responses (Greek: amygdalum, almond). It lies just in front of the hippocampus, close to the temporal pole, and blends with the medial temporal cortex (see Fig. 3.21). Although the amygdala is involved in all types of emotional response (both ‘positive’ and ‘negative’), it is particularly important in situations that elicit anxiety, fear or rage. The amygdala has three main nuclear groups (Fig. 3.18):
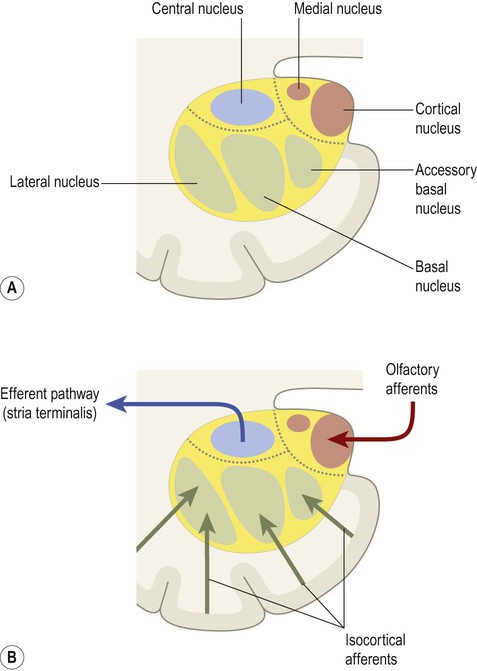
(A) The three major nuclear groups of the amygdala are illustrated (corticomedial, basolateral and central) with their main subnuclei labelled; (B) The corticomedial and basolateral nuclei receive olfactory and non-olfactory afferents, respectively. The central nucleus gives rise to a major efferent projection, the stria terminalis.
 The basolateral group is the largest division in the human brain. It receives particularly strong projections from the visual and auditory association areas of the temporal lobe.
The basolateral group is the largest division in the human brain. It receives particularly strong projections from the visual and auditory association areas of the temporal lobe.
 The corticomedial group mainly receives sensory afferents from the olfactory bulb. It is therefore more important in macrosmatic animals (those with a keen sense of smell).
The corticomedial group mainly receives sensory afferents from the olfactory bulb. It is therefore more important in macrosmatic animals (those with a keen sense of smell).
 The central nucleus elicits emotional responses by projecting to the hypothalamus and autonomic nuclei of the brain stem via the stria terminalis (Fig. 3.19).
The central nucleus elicits emotional responses by projecting to the hypothalamus and autonomic nuclei of the brain stem via the stria terminalis (Fig. 3.19).
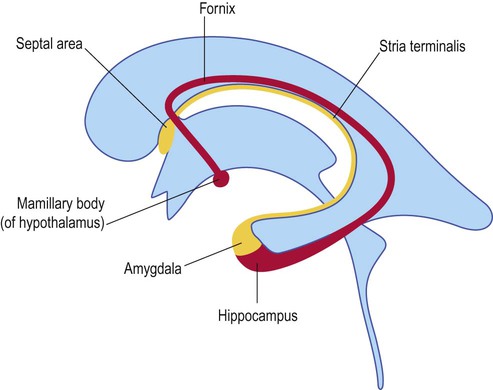
The targets of the stria terminalis and fornix are similar, including the hypothalamus, brain stem and limbic (‘emotion-related’) part of the basal ganglia. Both pathways also project to the septal area (illustrated here; see also Fig. 3.13) which is involved in mood, emotion and reward-based learning.
The amygdala integrates diverse sensory, cognitive and other information to help determine the emotional significance of a particular situation. An important role is the identification of potentially harmful circumstances and triggering appropriate autonomic responses (e.g. a ‘fight or flight’ reaction) via projections to the hypothalamus and brain stem. It has therefore been described as a danger detector. The orbital region of the prefrontal cortex exerts a moderating influence on the amygdala which can alter or inhibit emotional responses based on context or previous experience (e.g. fleeing from a snake encountered on a forest floor, but not in a zoo or pet shop).
Basal ganglia
The basal ganglia are a collection of deep hemispheric nuclei (strictly speaking, the term ‘ganglia’ is a misnomer) that contribute to voluntary movement, cognition and behaviour. Their involvement in movement control is illustrated by Parkinson’s disease, the most common basal ganglia disorder (Ch. 13). The largest component of the basal ganglia is the corpus striatum.
Corpus striatum (Fig. 3.20)
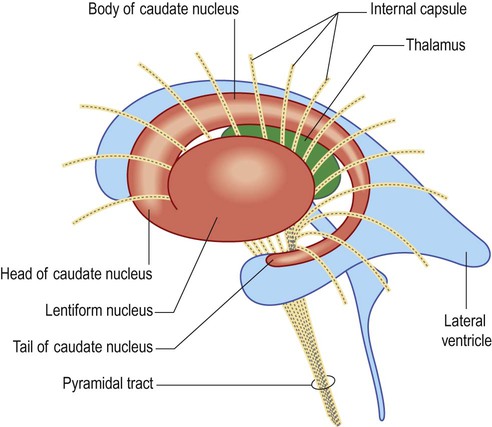
These two nuclei are largely divided by the white matter of the internal capsule, but are fused anteriorly (as shown in the figure). Note that in this view only the base of the cone-shaped lentiform nucleus can be seen; its blunt apex is pointing towards the midline (compare with coronal view in Fig. 3.21).
Lentiform nucleus
The lentiform nucleus lies beneath the insula. It is said to resemble a lens (Latin: lentiform, lens-shaped) but is best regarded as a cone with the base underlying the insula and a blunt apex pointing towards the midline (Fig. 3.21). The outer portion of the lentiform nucleus, immediately beneath the insula, is the putamen (Latin: putamen, husk or shell). The inner part is the globus pallidus which has internal and external segments. The globus pallidus is so named because of its pallid (pale) appearance in comparison to the dark grey colour of the caudate nucleus and putamen. This is due to the presence of myelinated fibres forming the internal connections of the basal ganglia.
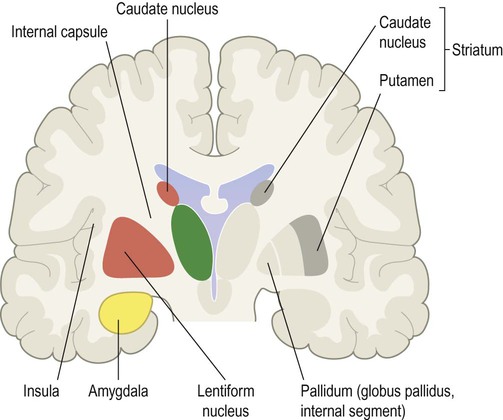
The left side of the figure shows the caudate and lentiform nuclei, separated by the white matter of the internal capsule; the amygdala can be seen below the cone-shaped lentiform nucleus, in the medial temporal region. The right side of the figure shows the functional sub-divisions of the corpus striatum. The ‘input’ region (striatum) consists of the caudate nucleus and putamen, which is shown in dark grey. The ‘output’ region (pallidum) consists of the internal segment of the globus pallidus, shown in light grey.
Internal capsule
The internal capsule is a thick sheet of white matter consisting of projection fibres (see Ch. 1, Fig. 1.17) passing to and from the cerebral cortex. It makes a sharp ‘knee-bend’ or genu around the apex of the lentiform nucleus (Latin: genu, knee). This gives it a V-shape when viewed in axial (horizontal) section (Fig. 3.22). There is therefore an anterior limb (between the lentiform nucleus and the head of the caudate nucleus) and a posterior limb (between the lentiform nucleus and the thalamus).
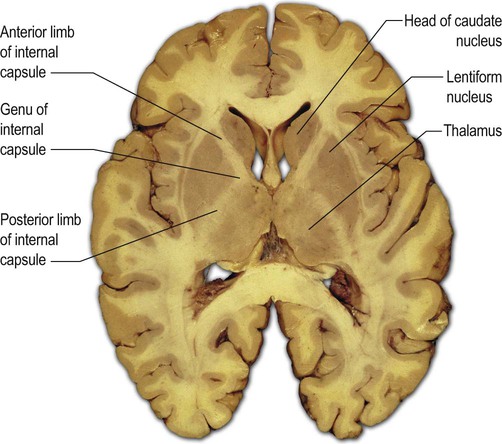
The section passes through the cone-shaped lentiform nucleus, with its base directed laterally and its apex pointing towards the midline. The V-shaped internal capsule can be seen wrapping around the apex of the cone as the genu (‘knee bend’), forming the anterior and posterior limbs. The anterior limb lies between the head of the caudate nucleus and the lentiform nucleus. The posterior limb is between the thalamus and the lentiform nucleus. From Standring: Gray’s Anatomy 40th edn (2008: Churchill Livingstone) with permission.
Functional divisions
Division of the corpus striatum into the caudate and lentiform nuclei reflects their physical separation by the white matter of the internal capsule (Fig. 3.21, left). However, it is also possible to identify two functional zones based upon afferent and efferent connections (Fig. 3.21, right):
 The striatum (caudate nucleus and putamen) is the ‘input’ portion of the basal ganglia which receives projections from the overlying cerebral cortex.
The striatum (caudate nucleus and putamen) is the ‘input’ portion of the basal ganglia which receives projections from the overlying cerebral cortex.
 The pallidum (internal segment of the globus pallidus) is the ‘output’ portion of the basal ganglia which projects to the thalamus.
The pallidum (internal segment of the globus pallidus) is the ‘output’ portion of the basal ganglia which projects to the thalamus.
The various terms used to describe parts of the corpus striatum are illustrated in Figure 3.23; note that the structural term ‘corpus striatum’ (caudate + lentiform nuclei) is not the same as the functional term ‘striatum’ (caudate nucleus + putamen; which is the afferent or ‘input’ region).
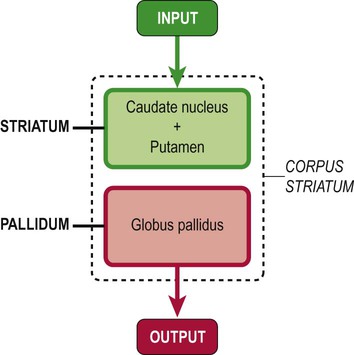
The striatum (caudate nucleus and putamen) is the ‘input’ portion of the basal ganglia which receives projections from the cerebral cortex. The pallidum (internal segment of the globus pallidus) is the ‘output’ portion of the basal ganglia and projects to the thalamus. The entire nuclear mass (caudate nucleus plus lentiform nucleus) is referred to as the corpus striatum.
Basal ganglia loops
The voluntary motor loop
The motor loop is the best understood component of the basal ganglia and is disturbed in movement disorders such as Parkinson’s disease. Projections arise from the supplementary motor area (SMA) in the medial frontal lobe and project into the putamen. For this reason the putamen is regarded as the ‘motor part’ of the striatum. The putamen gives rise to direct and indirect connections that converge on the internal pallidum. This projects in turn to the thalamus, which completes the loop via a thalamocortical projection back to the SMA. Dopamine deficiency in Parkinson’s disease leads to underactivity of the motor loop and SMA, interfering with the initiation of voluntary actions. The control of the motor loop (by the direct and indirect pathways) is discussed further in Chapter 13, in the context of Parkinson’s disease.
Cognitive-executive loops
Basal ganglia loops passing through the caudate nucleus arise and terminate in the prefrontal cortex. They influence cognition and behaviour, but also contribute to the control of visual attention and voluntary gaze via projections that arise and terminate in the frontal eye fields. Over-activity in caudate-prefrontal connections has been implicated in obsessive-compulsive disorder (Clinical Box 3.10).
Limbic-affective loops
Anteriorly, the caudate nucleus and putamen are fused underneath the anterior limb of the internal capsule (see Fig. 3.20) to form the ventral striatum. Due to its proximity to the septal area (Fig. 3.24) it is also known as the nucleus accumbens septi or nucleus accumbens (Latin: accumbens, leaning against). Projections taking part in the ‘limbic loops’ of the basal ganglia arise in the limbic lobe or amygdala and project to the ventral striatum. This region is rich in opiate receptors and has been implicated in motivation, reward-based learning and addictive behaviours.
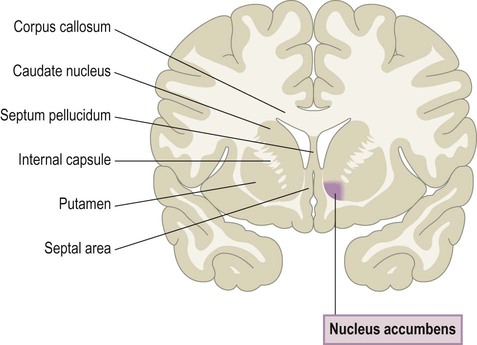
The most anterior parts of the caudate nucleus and putamen are fused underneath the anterior limb of the internal capsule (see Fig. 3.20) to form the ventral striatum (also known as the nucleus accumbens septi).
Thalamus and hypothalamus
The thalamus and hypothalamus are part of the diencephalon (Fig. 3.25). This is the portion of the cerebrum that is normally hidden from view between the cerebral hemispheres, surrounding the cavity of the third ventricle (Greek: dia, between; enkephalos, brain).
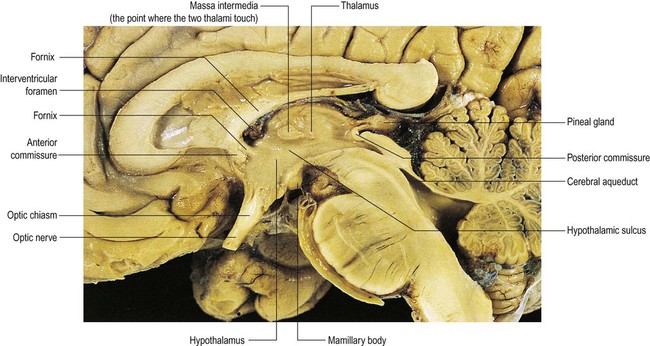
The fornix can be seen passing through the hypothalamus to reach the mamillary body in the floor of the third ventricle and forming the anterior boundary of the interventricular foramen. From Crossman: Neuroanatomy ICT 4e (Churchill Livingstone 2010) with permission.
Thalamus
Thalamic nuclei
Almost all ascending pathways synapse in a thalamic nucleus in order to reach the cerebral cortex. The thalamus is therefore described as the ‘gateway’ to the cortex. It is composed of more than a dozen nuclei, separated into anterior, medial and lateral groups by a Y-shaped internal medullary lamina. This thin sheet of white matter contains a few small intralaminar nuclei which are involved in arousal, wakefulness and pain (see Ch. 4). The lateral nuclear group of the thalamus has dorsal and ventral tiers. The ventral tier contains specific nuclei, which project to discrete cortical zones such as the primary sensory and motor areas. The non-specific nuclei form diffuse, reciprocal connections with large regions of the cortex.
Hypothalamus
The hypothalamus is a small structure which forms the lower side wall and floor of the third ventricle, just below and in front of the thalamus. It has numerous nuclei that are collectively responsible for maintaining a constant internal environment (homeostasis). It does this by regulating basic drives (e.g. hunger, thirst) and by co-ordinating the activity of the endocrine and autonomic nervous systems. It continuously monitors parameters such as core body temperature and blood glucose concentration and controls autonomic centres in the brain stem and spinal cord to keep them constant. It also influences hormonal release from the adjacent pituitary gland (Ch. 1).
Brain stem and cerebellum
The brain stem is composed of the midbrain, pons and medulla, which are closely related to the overlying cerebellum. Some important surface landmarks of the brain stem are illustrated in Figure 3.26.
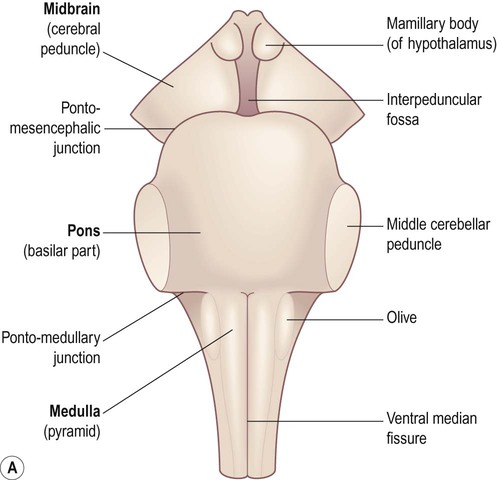
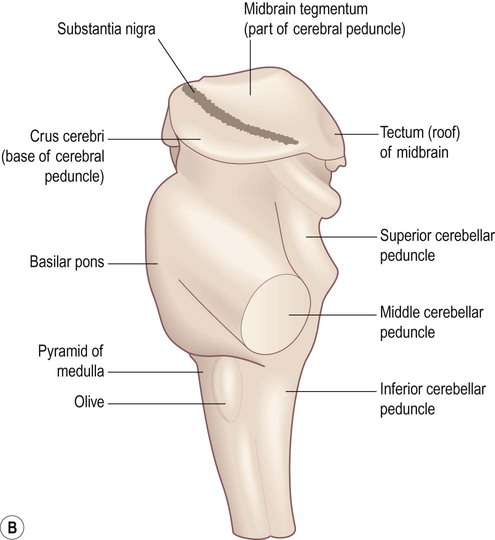
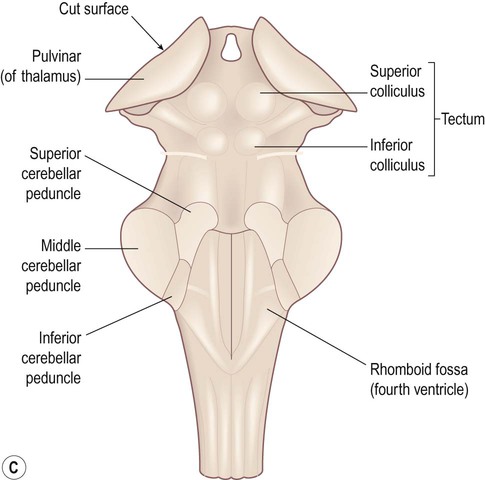
(A) Anterior aspect; (B) Lateral aspect; (C) Posterior aspect. The cerebellum and cerebral hemispheres have been removed.
Midbrain
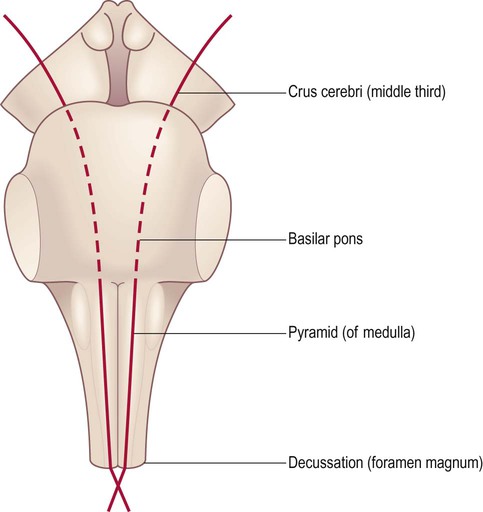
The midbrain is the most rostral part of the brain stem (Figs 3.26 and 3.27). It contains the cerebral aqueduct which runs between the third ventricle (above) and the fourth ventricle (below). The small part of the midbrain dorsal to the aqueduct is the tectum or ‘roof’ of the midbrain (Latin: tectum, roof). The large portion in front of the aqueduct (making up half of the midbrain on each side, excluding the tectum) consists of the left and right cerebral peduncles.
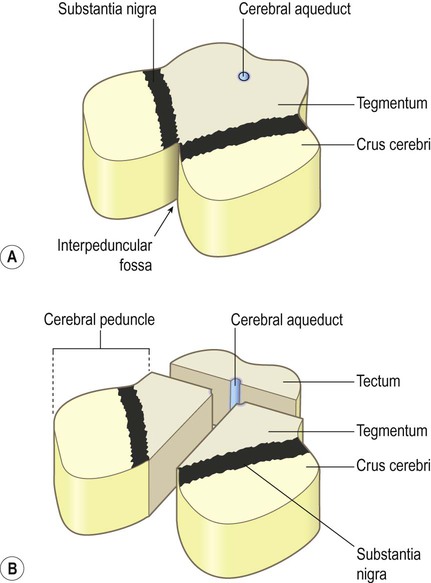
(A) Anterolateral view of the intact midbrain separated from the rest of the brain stem; (B) Exploded schematic view of the midbrain illustrating the tectum and the paired cerebral peduncles. Note that the ‘cerebral peduncle’ makes up one half of the midbrain on each side (excluding the tectum), but the term is frequently misused to describe only the anterior part of the midbrain in which the corticospinal tract is found (the correct term for this region is the crus cerebri).
Tectum (3.26C and 3.27B)
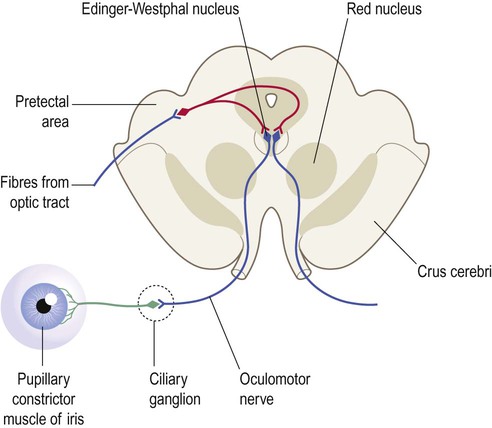
The tectum bears four smooth elevations or colliculi (Latin: colliculus, little hill). The superior colliculi (collectively: the optic tectum) give rise to the tectospinal tracts which co-ordinate head, neck and eye movements during orientation reflexes (e.g. involuntary turning towards a novel or unexpected stimulus). The inferior colliculi are part of a complex pathway between the cochlea and primary auditory cortex. The role of the midbrain in the control of pupil size is discussed in Clinical Box 3.11.
Pons
Pontine tegmentum
The dorsal third of the pons is the tegmentum, the posterior surface of which forms part of the floor of the fourth ventricle. The tegmentum contains the pontine reticular formation, together with several cranial nerve nuclei and ascending tracts. The loci coerulei (singular: locus coeruleus) are found in the rostral pons, just beneath the floor of the fourth ventricle on each side. These pigmented nuclei (Latin: locus, place; coeruleus, dark-blue) give rise to a diffuse projection for noradrenaline (see Ch. 1).
Medulla oblongata
Basilar region
The anterior medulla contains two tapering columns of white matter called the pyramids, which are equivalent to the ‘basilar region’ in the midbrain and pons. The pyramids transmit the primary motor pathway (the corticospinal tract) which is therefore also known as the pyramidal tract (Fig. 3.29). Just lateral to the pyramids in the upper medulla are the olives (see Fig. 3.26), which send an ascending projection to the opposite cerebellum. This olivo-cerebellar pathway contributes to motor learning by signalling unexpected events (e.g. dropping a ball whilst learning to juggle) and provides a ‘training signal’ to the cerebellum which alters synaptic strengths in such a way that the error is less likely to be repeated.
Cerebellum
The gross anatomy of the cerebellum has been outlined in Chapter 1. In this section its three functional divisions will be reviewed. It is important to remember that, in contrast to the cerebral hemispheres, cerebellar disease causes symptoms and signs on the same (ipsilateral) side of the body.
Functional divisions
The cerebellum has three functional divisions, corresponding to: (i) the flocculonodular lobe; (ii) the vermis/paravermal region; and (iii) the cerebellar hemispheres (Fig. 3.30). It is important to note that the functional regions do not coincide exactly with the gross anatomical (lobar) boundaries.
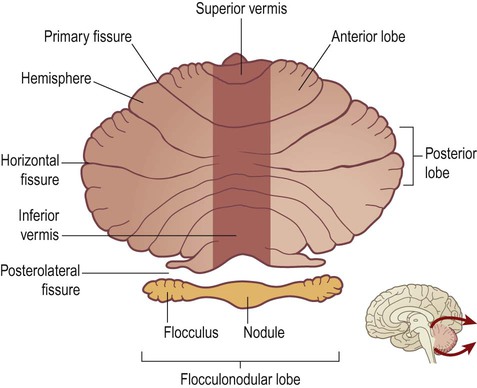
The cerebellar cortex has been ‘unfolded’ and laid flat to illustrate the approximate position of the three functional sub-divisions: flocculonodular lobe (orange), vermis/paravermis (dark brown) and cerebellar hemispheres (light brown). From Crossman: Neuroanatomy ICT 4e (Churchill Livingstone 2010) with permission.
Role in movement control
The cerebellum acts as a comparator. The motor and premotor areas of the frontal lobe provide information about intended movements whereas the spinocerebellar tracts (and other pathways) provide information about actual movements. Any discrepancy between the two creates an ‘error signal’ that is fed back to the motor cortex, permitting continuous modification and improvement of on-going movements. Cerebellar disease therefore leads to incoordination (Clinical Box 3.12).