CHAPTER 114 Frame and Frameless Stereotactic Brain Biopsy
Historical Landmarks
Pioneering neurosurgeons in the end of the 19th century and at the dawn of the 20th century, working without the benefit of imaging modalities, relied exclusively on anatomic knowledge and clinical findings to strategize their operative approaches. The first person to use a device for localizing brain structures was D. N. Zernov in 1889. A professor of anatomy in Moscow, Russia, he invented an apparatus that could be fastened to the head and adjusted to localize points that could then be identified on a map that showed the average positions of cranial structures.1 Zernov first used this device to identify and drain an abscess in the interparietal sulcus. Although his work was continued by others in Russia, his method did not become well known worldwide.2 Without being aware of Zernov’s method, Victor Horsley and Robert Clarke described a “steretotactic” method of localizing brain structures in animals with a frame based on a three-dimensional coordinate system in 1906 (Greek stereo meaning three-dimensional; taxis meaning to move toward).3 However, because subcortical structures could not be reliably localized, stereotaxy in humans was not widely adopted until intracranial imaging was available.2
It was not until 1947 that the first human stereotactic system, termed the encephalotome, was demonstrated by Spiegel and Wycis.4 By this time, x-ray technology combined with the development of ventriculography, a method whereby air or contrast material is introduced into the ventricles to facilitate clearer imaging on x-ray plates, made it practical to take films in the operating room and develop them in a timely manner.5 The underlying principle is to target an instrument toward a particular neural structure based on information regarding its location obtained on the preoperative images. Successful completion of this task required that the instrument involved be guided stably toward the target in a coordinate system common to both the preoperative images and the in vivo structures. Before image acquisition, a frame was firmly attached to the patient’s skull to produce images with markers that are referenced to a frame-based coordinate system.2 By using the same coordinate system during the operation, surgical instruments could be aimed toward the target structure shown on the images.
The pioneering development of the encephalotome led to development of other stereotactic frames, and with the introduction of CT in 19736,7 and MRI in 1980,8 frame-based stereotactic guidance took a firm place in the conduct of neurosurgical operations.2,5 These frame-based systems were initially used to generate lesions in different neural structures for treating movement disorders, pain, and epilepsy and then evolved for performing drainage of abscesses and biopsy of tumors.2,9 Although frame-based systems have proved to be reliable and accurate targeting devices,10,11 they have several disadvantages that limit their utility2: (1) attachment of the frame to the patient’s skull often needs to be done on the same day as the operation, especially when frame application requires general anesthesia, (2) posterior fossa lesions are difficult to target, (3) the frame can limit access to the surgical field, (4) guidance is limited to a single target or only a few targets, and (5) maneuvering the apparatus during surgery can be unwieldy.2 For these reasons, frameless stereotaxy has been developed to improve the efficacy and accuracy of targeting systems.
Background
Frame-Based Stereotaxy
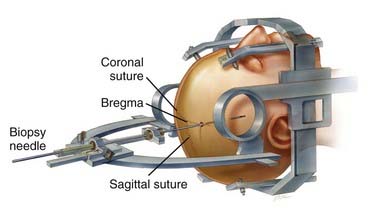
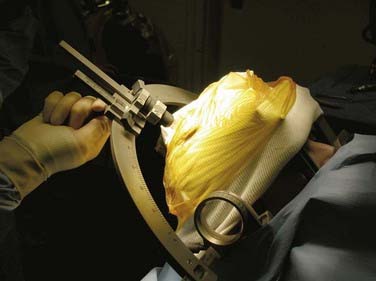
The Leksell frame (Fig. 114-1), introduced in 1949, is based on the arc-radius principle and is one of the more commonly used frame-based systems in modern neurosurgery. A frame is rigidly attached to the patient’s head to which the semicircular arc is mounted by pivoting shafts that in turn are connected to the graduated bars of the frame. Ultimately, the arc can be manipulated for vertical and anterior-posterior adjustments to permit differential targeting by moving the center of the arc.
The frame consists of four graduated bars that define the corners of a cube; on the left/right sides of the frame (in relation to the patient) there is a diagonal bar on each side connecting the corner of these posts to create an “N.” Once the patient is scanned (CT or MRI), the geometric center in the anterior-posterior plane is defined by looking at an axial image and drawing an “X” by connecting the corners of the frame. The center in the vertical plane is assessed by a line connecting the midpoint of the corner posts going through the “N.” After the center is defined, the location of the target in relation to the geometric center can be calculated and the semicircular arc manipulated (Fig. 114-2) accordingly to place that lesion at the center of the arc.
Frameless Stereotaxy
Principles of Neuronavigation
For both frame-based and frameless systems (Fig. 114-3), the relationship between the coordinate space for the preoperative images and that for the surgical field must be established. In frame-based systems, by using the same frame for both preoperative image acquisition and performing the surgery, the relationship between the two coordinate systems is known and no further transformation is required. For frameless systems, point-pair registration or surface contour registration can be used to establish the relationship between the preoperative images and that for the surgical field.
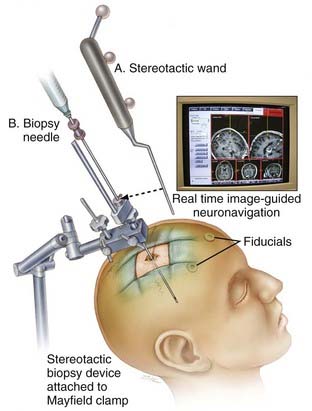
FIGURE 114-3 Image depicting a frameless stereotactic biopsy system that relies on neuronavigation systems for lesion targeting.
(From Woodworth GF, McGirt MJ, Samdani A, et al. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J Neurosurg. 2006;104:233-237.)
Point-Pair Registration
In frameless systems, the relationship between the two different coordinate systems, one related to the image and the other related to the patient during surgery, requires a patient-to-image registration procedure to create a mutual relationship.2 Point-pair matching was the first and has been the most common method to achieve transformation between the two coordinate systems.12,13 This method requires a set of at least three non-colinear points to be defined in the coordinates of the images. These points are known as fiducial points and can consist of either natural anatomic landmarks (i.e., nasion, lateral canthus, or tragus), skin-applied markers, or bone-implanted markers.2 These same fiducial points are defined again within the coordinate system of the surgical field in the operating room. Software is then used to establish the relationship between the coordinates of the fiducial points in the image space and their counterpart in the surgical space.2
Surface Contour Registration
In a comparison of natural landmarks and skin-applied fiducial markers, navigation based on registration of natural landmarks alone has generally been slightly less accurate, in large part because they consist of less discretely defined points. Golfinos and coauthors reported that anatomic landmark and surface-fit algorithms have an accuracy of half that achieved with fiducial-based registration.14 To this day, the sophistication of surface registration strategies continues to evolve. Although surface matching can be used as an alternative to fiducial mapping, its use in combination with fiducial point-pair matching may be an optimal strategy to further improve reliability in stereotactic localization for biopsy.
Once the spatial relationship is established with either point-pair registration or surface contour registration, it is used throughout the remainder of the operation to map anatomic targets of the patient to that of the preoperative images. This thus requires that the patient’s head be immobilized with a head clamp and the operating table not be moved during surgery. To overcome the error that may be induced with patient or operating table movement, tracking units attached to the patient’s head15 or a surgical head clamp16 can be used. These sets of reference markers, termed dynamic reference frames, can be used to correct for any unwanted movement. Markers attached to a pointing device or, alternatively, a microscope can be tracked to locate position and orient the axis given these fixed dynamic reference frames.
Indications for Stereotactic Biopsy
The significant development of intracranial imaging over the past few decades has allowed much earlier diagnosis of brain tumors. Although some tumors have a characteristic appearance on imaging, no imaging modality is yet able to provide sufficient diagnostic information to direct subsequent therapy. As a result, tissue biopsy remains indispensible in many cases. The goal of biopsy is to provide a representative sample for pathologic diagnosis to guide subsequent treatment, which can include cytoreductive surgery, radiotherapy, or chemotherapy.17
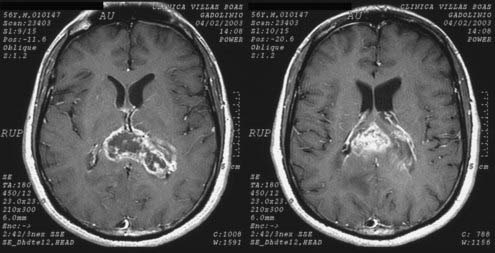
The main stimulus for the adoption of stereotactic biopsy over an open operative procedure is to achieve a higher rate of diagnostic accuracy while minimizing potential adverse effects. Diagnostic accuracy is important for dictating appropriate adjuvant therapy. Particular characteristics of the tumor that favor the use of stereotactic biopsy over open biopsy include (1) lesions that do not exert a symptomatic mass effect or that are not curable by surgical excision, such as metastases or malignant intrinsic brain tumors (Fig. 114-4); (2) deep-seated lesions or those occupying space in eloquent cortex or deep nuclei (i.e., basal ganglia, thalamus), where open resection would lead to unacceptable morbidity/mortality; and (3) infiltrative lesions (i.e., gliomatosis cerebri) that do not have a clear brain-tumor margin and are unlikely to be excised completely without significant loss of normal brain parenchyma.17 Moreover, if the lesion’s appearance on imaging or the course of the disease suggests an alternative cause such as an infectious or demyelinating process rather than a neoplastic one, stereotactic biopsy is a more appropriate first step than a large open procedure.17
Nevertheless, although stereotactic biopsy is a relatively safe procedure, there are relative contraindications. For example, vascular tumors such as metastatic renal cell carcinoma, choriocarcinoma, or metastatic melanoma should not be approached stereotactically, if possible, because of their inherently higher risk for hemorrhage.17 In such cases of metastatic tumor, a stronger effort to biopsy and diagnose the primary neoplasm instead is generally recommended. Furthermore, location of the tumor close to a major blood vessel, the vessel-rich sylvian fissure, the cavernous sinus, or the brain-pial border should also alert the neurosurgeon to the more elevated risk for hemorrhage.
Technical Considerations
Minimization of Brain Shift and Local Tissue Deformation
Intraoperative MRI (iMRI) in such a situation is ideal in that it allows intraoperative reregistration, as well as confirmation of biopsy needle placement within a target lesion. Comparison of iMRI-guided biopsy with stereotactic brain biopsy has demonstrated comparable if not higher diagnostic yield with iMRI-guided biopsy than with stereotaxis alone.18 An additional benefit of iMRI is that it provides an imaging modality to check for any hemorrhage that might occur on passing the biopsy needle or from the biopsy itself.19 Although the availability of iMRI is limited, a new generation of compact iMRI devices is emerging.
Complications of Stereotactic Biopsy
The most common complication of stereotactic biopsy is intracranial hemorrhage, which is frequently due to damage to blood vessels in the trajectory of the biopsy needle. In addition, damage to pathologically friable vessels within the targeted neoplastic lesion can account for a number of hemorrhagic complications. Reported rates of hemorrhage during stereotactic biopsy procedures range from 0% to 11.5%.9,20,21 Although most cases of blood vessel damage are minor, catastrophic consequences can take place if a lesion such as a vascular malformation is accidentally subjected to biopsy.17 Some metastatic tumors such as melanoma, choriocarcinoma, and renal cell carcinoma are more likely to lead to hemorrhage and should be approached more cautiously. Preoperative MRI studies should be studied closely before surgery to identify the most optimal approach. In the case of a hemorrhagic complication with mass effect, craniotomy may be necessary for evacuation of the hematoma.
A second, less frequent complication of stereotactic biopsy is a new neurological deficit secondary to trauma to the surrounding brain parenchyma as a result of direct damage by the needle biopsy or the resulting edema.17 Brain penumbral damage can be limited by reducing the number of needle passes, and edema in some cases can be prevented by avoiding bridging veins, which in rare instances can be spotted on preoperative MRI. In some cases, preoperative corticosteroids can prevent exacerbation of edema in lesions already associated with significant edema.17
A review of 3866 stereotactic biopsies performed in the 1980s placed the overall mortality rate at 0.6% and morbidity rate at 3.2%, divided as follows: hemorrhage, 1.9%; neurological deficit, 0.8%; seizure, 1%; and infection. 0.5%.17
Comparison of Frame-Based and Frameless Methods
With the increasing popularity of frameless biopsy, several studies have sought to compare frame-based and frameless biopsy techniques.22–25 Studies assessing the technical accuracy of frame-based versus frameless stereotactic localization have found acceptable ranges of accuracy when summing localization vector errors in models.26 Clinical studies have shown no difference in the diagnostic yield or diagnostic accuracy (concordance between initial frozen section diagnosis and final open resection diagnosis) of either technique.24,27,28 The most recent estimate of 391 procedures done over a 10-year span (227 frame based and 164 frameless) reported a diagnostic yield of 89.4% with no significant differences between frame-based and frameless methods.22 The reported diagnostic accuracy for either technique ranges between 51% and 78%.19,24,28,29 Series reporting lower diagnostic accuracy generally involved patient populations with larger and more complicated tumor morphologies.28 We have seen that increasing volumetric tumor size correlates with decreased concordance between biopsy and resection specimens. In one series performed at our institution, for a third of gliomas larger than 50 cm3, biopsy specimens were insufficient for accurate grading altogether.25 An increased volume of central necrosis and the higher degree of tissue heterogeneity seen in more aggressive gliomas may be responsible for such outcomes.
The nonconcordance noted in studies comparing biopsy and open-resection pathologic diagnosis was most often secondary to misinterpretation of radiation-induced necrosis from recurrent glioma specimens.24 In such series, undergrading of high-grade astrocytic tumors was the most common occurrence. It is suspected that targeting stereotactic biopsy away from hypointense areas of necrotic tissue yields a higher chance of encountering tissue of lower grade appearance. Conversely, nonviable (and therefore nondiagnostic) tissue is often encountered if the biopsy is limited to highly necrotic areas of the neoplasm. Diagnostic yield and accuracy may increase with the number of tissue samples.24 However, the increased sampling has to be balanced with the potential for brain shift and increased risk for hemorrhagic complications.
On comparison of complication rates, transient or permanent morbidity, or mortality after either type of procedure, negligible significant differences have been reported between frame-based and frameless techniques.25,30 One series examining 76 cases of frameless and 79 cases of frame-based biopsies found that operating time and time under anesthesia were significantly shorter in frameless series.23 The frameless series also had a significantly lower rate of complications (14%) than did the frame-based series (22%).23 However, some of these findings might be related to the fact that frame-based techniques were used earlier when image-guidance support was not as accurate as in recent years and the surgical technique not as refined. Another series of 110 frame-based and 52 frameless procedures showed equal efficacy and similar complication rates.31 Those who advocate frameless stereotaxy cite the reduced operating time needed and the tendency for earlier discharge.22 Overall, these studies demonstrate that both frame-based and frameless techniques are efficacious in obtaining a tissue biopsy specimen, with the resulting complications probably secondary to the experience of the neurosurgeon and the instrumentation at that particular institution.
Stereotactic Biopsy of Brainstem Lesions
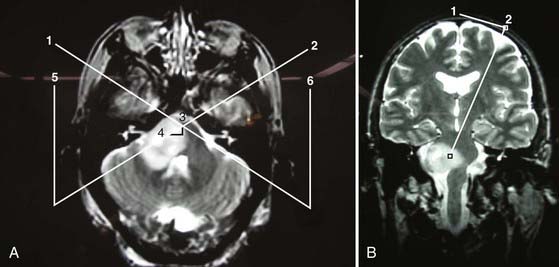
Lesions within the brainstem (Fig. 114-5A and B) have long been considered challenging to diagnose and treat. Although brainstem tumors represent only about 2% of all intracranial tumors in adults as compared with about 10% to 15% in the pediatric population,32 radiologic diagnosis of brainstem lesions in adults is inaccurate 10% to 20% of the time. Most brainstem tumors in children, in contrast, are gliomas, which arguably can be diagnosed with MRI alone.32 Anatomically, brainstem lesions are often classified as subsiding within either the midbrain or pons and occasionally reaching into the medulla. In general, because most adult brainstem tumors are not amenable to surgical excision, stereotactic biopsy is important for obtaining a pathologic diagnosis. Histologic diagnosis often enables replacement of empirical treatment modalities with more specific therapies, as well as determination of a more accurate prognosis.
Image-guided frame-based stereotactic biopsy of brainstem lesions has been performed since 1975,33 and the high diagnostic and low complication rates are comparable to those seen with supratentorial lesions.34 As with supratentorial lesions, there have been several reports demonstrating variable concordance between the actual findings on biopsy and preoperative assessments from imaging.34,35 For example, in the case of nonenhancing diffuse astrocytoma, half of those presumed to be low-grade glioma based on their characteristics on MRI were found to be anaplastic gliomas.34,36 In another study of 26 patients, MRI combined with positron emission tomography was concordant with biopsy findings in only 63% of cases, thus suggesting that stereotactic biopsy is necessary to obtain the correct diagnosis.34,37
A recent literature review of 469 cases of stereotactic brainstem biopsy in adults performed between 1975 and 2004 showed a diagnostic rate of 94.8%.34 In terms of complications, 6.6% have transient or mild symptoms and 1.8% have permanent deficits.34 Although the only noted mortality from this brainstem biopsy review occurred in a patient with capillary telangiectasia,38 it is notable that biopsies of many other vascular malformations, with most of them being cavernomas, have been performed without hemorrhage or complication.34 When hemorrhagic complications in the biopsy bed occur, prevention of postoperative swelling and close neurological observation are indicated.
Another factor favoring the decision to perform biopsy is the benefit of potential treatment. This benefit is significant given the great diversity of brainstem lesions. High-grade gliomas represent only 31% of cases. Low-grade gliomas and metastases account for 25% and 11% of cases, respectively.34 The remaining lesions consist of hematomas, lymphomas, abscesses, demyelination, radiation necrosis, infarcts, and other lesions.34 Patients most likely to benefit from stereotactic biopsy are those who are given a better prognosis based on biopsy results or who are spared a course of debilitating therapy.34 Such patients include those with radiation necrosis, chemotherapy-sensitive metastasis (breast, choriocarcinoma), a lymphoma, or an abscess rather than a malignant glioma.34
A number of approaches are available for stereotactic biopsy of an infratentorial brainstem lesion, including the transtentorial approach, the ipsilateral transfrontal trajectory, and the suboccipital transcerebellar route.39 Although all these approaches have been used successfully to reach lesions located within the midbrain, pons, and medulla, they all have limitations.
Approaches
The Transtentorial Approach
The transtentorial approach, which requires entry from the posterior cerebrum to reach the upper pons, has virtually been abandoned because it necessitates crossing the pia superior and inferior to the tentorium and places vital vasculature and cranial nerves at risk. Additionally, biopsy through this route may cause pain with tentorial puncture.10
The Suboccipital Transcerebellar Approach
The suboccipital transcerebellar approach has been described as a means for obtaining a biopsy sample of lesions of the lower midbrain, pons, and rostral medulla.40,41 Positioning often requires intubation and general anesthesia, although a method for using a local anesthetic has been described.42 The route is straightforward and projects through the ipsilateral middle cerebellar peduncle to the desired midbrain or brainstem lesion. One drawback to the suboccipital transcerebellar approach is the pain associated with muscle dissection before making the twist drill hole in the skull. A more decisive contraindication to this approach is the risk for posterior fossa subdural hematomas caused by blind puncture of the cerebellar cortex. Postoperative posterior fossa hematomas pose a higher risk for neurological sequelae than do similarly sized supratentorial subdural hematomas caused by a transfrontal approach.
The Ipsilateral Transfrontal Approach
The ipsilateral transfrontal approach is effective for biopsy of the midbrain, upper pons, and medulla.43–47 For midline lesions, an anterior coronal entry point is chosen such that the trajectory enters through the midpoint of a gyrus approximately 15 to 25 mm from the midline. For lower pons or medulla biopsies, an ipsilateral transfrontal approach necessitates a trajectory traversing the lateral ventricle before entering the anterior thalamus, cerebral peduncle, and then the brainstem. As a result, lesions of the brainstem below the lower pons are typically approached by the contralateral, extraventricular approach (described in the next section). Finally, to reach the lateral midbrain, a trajectory lateral to the ventricle suffices.46 This technique, however, has a few potential disadvantages. First, approaching the pons and medulla with this method often requires entering the frontal horn of the lateral ventricle, which violates two ependymal surfaces and places the patient at risk for intraventricular hemorrhage. Second, ventriculostomy may lead to intraoperative loss of cerebrospinal fluid, which may contribute to postoperative headache and shifting of intracranial contents, thereby possibly altering the location of the target. Third, this route is limited to midline regions of the pons and midbrain by the tentorial incisura.48
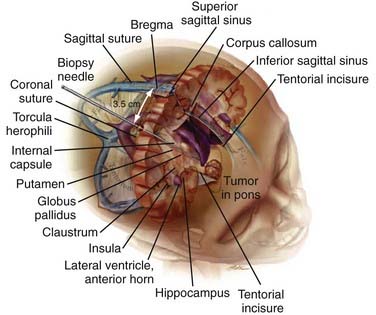
The Contralateral, Extraventricular Transfrontal Approach
We recently reported on a contralateral, transfrontal, extraventricular approach (Fig. 114-6) as a safe and straightforward alternative for biopsy of infratentorial brainstem targets.39 We have in particular found this approach advantageous for more lateral pontine lesions. After placement of the Leksell stereotactic frame and multiplanar MRI reconstruction, a point of entry through the midpoint of a gyrus approximately 3 cm lateral to the midline is chosen with special attention paid to avoiding large cortical vessels. An intraparenchymal trajectory is projected to avoid the lateral ventricle, the tentorium, major vessels, and the cerebral aqueduct. Biopsy specimens are sent for immediate intraoperative review by an attending neuropathologist. In cases requiring more than one sample to obtain adequate diagnostic material, we change the orientation of the needle and biopsy without performing additional passes. In our experience, a diagnosis was achieved in all cases, including metastatic adenocarcinoma, anaplastic astrocytoma (two cases), glioblastoma multiforme, and progressive multifocal leukoencephalopathy (two cases). Detailed intraoperative and postoperative neurological examinations showed no decline in function.
The contralateral, extraventricular transfrontal approach is a good option for biopsy of the lateral pons and medial middle cerebellar peduncle. One goal in choosing a trajectory is minimization of the number of pial and ependymal surfaces crossed to decrease the risk for hemorrhage. With an entry point approximately 4 cm off midline in the coronal plane, the needle’s trajectory remains completely intraparenchymal, thereby avoiding the ventricle and limiting the number of crossed pial surfaces to one. This approach thus limits the potential for intraventricular hemorrhage, shift associated with loss of cerebrospinal fluid, and subdural hematomas. Advancing the biopsy needle slowly also theoretically helps minimize trauma by allowing tracts, nuclei, and small vessels to be displaced rather than sheared.46 Intraoperative pathologic review by an attending neuropathologist may be helpful in minimizing the need to take multiple biopsy specimens. We believe that it also improves diagnostic yield and minimizes the potential morbidity and trauma associated with obtaining multiple samples, especially in a region as eloquent as the brainstem. This route also allows the use of a local anesthetic together with light intravenous sedation, which permits continuous patient participation in the neurological examination. Finally, although the length of the needle’s passage and therefore the number of structures displaced are greater when reaching the pons via the transfrontal route versus the transcerebellar route, we believe that the risk is negligible. In our previously published series of infratentorial brainstem biopsies, both ipsilateral and contralateral combined, there has been no significant differences in rates of complications, including symptomatic hemorrhage or hydrocephalus associated with aqueductal stenosis.48 This result is consistent with findings in other series featuring this approach—no significant correlation between the length of the needle tract and morbidity has been found.49
Amundson EW, McGirt MJ, Olivi A. A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. Technical note. J Neurosurg. 2005;102:565-570.
Blond S, Lejeune JP, Dupard T, et al. The stereotactic approach to brain stem lesions: a follow-up of 29 cases. Acta Neurochir Suppl. 1991;52:75-77.
Dorward NL, Paleologos TS, Alberti O, et al. The advantages of frameless stereotactic biopsy over frame-based biopsy. Br J Neurosurg. 2002;16:110-118.
Golfinos JG, Fitzpatrick BC, Smith LR, et al. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg. 1995;83:197-205.
Kondziolka D, Lunsford LD. Stereotactic biopsy for intrinsic lesions of the medulla through the long-axis of the brainstem: technical considerations. Acta Neurochir (Wien). 1994;129:89-91.
Kreth FW, Muacevic A, Medele R, et al. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours—a prospective study. Acta Neurochir (Wien). 2001;143:539-545.
Krieger MD, Chandrasoma PT, Zee CS, et al. Role of stereotactic biopsy in the diagnosis and management of brain tumors. Semin Surg Oncol. 1998;14:13-25.
McGirt MJ, Villavicencio AT, Bulsara KR, et al. MRI-guided stereotactic biopsy in the diagnosis of glioma: comparison of biopsy and surgical resection specimen. Surg Neurol. 2003;59:277-281.
McGirt MJ, Woodworth GF, Coon AL, et al. Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg. 2005;102:897-901.
Moriarty TM, Quinones-Hinojosa A, Larson PS, et al. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47:1138-1145.
Quinones-Hinojosa A, Ware ML, Sanai N, et al. Assessment of image guided accuracy in a skull model: comparison of frameless stereotaxy techniques vs. frame-based localization. J Neurooncol. 2006;76:65-70.
Samadani U, Stein S, Moonis G, et al. Stereotactic biopsy of brain stem masses: decision analysis and literature review. Surg Neurol. 2006;66:484-490.
Smith JS, Quinones-Hinojosa A, Barbaro NM, et al. Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. J Neurooncol. 2005;73:173-179.
Woodworth GF, McGirt MJ, Samdani A, et al. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J Neurosurg. 2006;104:233-237.
Woodworth G, McGirt MJ, Samdani A, et al. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol Res. 2005;27:358-362.
1 ’Kandel EI, Shchavinskii YV. First stereotaxic apparatus created by Russian scientists in the 19th century. Biomed Eng (NY). 1973;7:121-124.
2 Willems PW, van der Sprenkel JW, Tulleken CA, et al. Neuronavigation and surgery of intracerebral tumours. J Neurol. 2006;253:1123-1136.
3 Clarke RH, Horsley V. The classic: on a method of investigating the deep ganglia and tracts of the central nervous system (cerebellum). Br Med J. 1906:1799-1800. Clin Orthop Relat Res. 2007;463:3-6
4 Spiegel EA, Wycis HT, Marks M, et al. Stereotaxic apparatus for operations on the human brain. Science. 1947;106:349-350.
5 Gildenberg PL. Spiegel and Wycis—the early years. Stereotact Funct Neurosurg. 2001;77:11-16.
6 Brown RA. A stereotactic head frame for use with CT body scanners. Invest Radiol. 1979;14:300-304.
7 Perry JH, Rosenbaum AE, Lunsford LD, et al. Computed tomography–guided stereotactic surgery: conception and development of a new stereotactic methodology. Neurosurgery. 1980;7:376-381.
8 Leksell L, Leksell D, Schwebel J. Stereotaxis and nuclear magnetic resonance. J Neurol Neurosurg Psychiatry. 1985;48:14-18.
9 Hall WA. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82:1749-1755.
10 Apuzzo ML, Chandrasoma PT, Cohen D, et al. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930-937.
11 Ostertag CB, Mennel HD, Kiessling M. Stereotactic biopsy of brain tumors. Surg Neurol. 1980;14:275-283.
12 Roberts DW, Strohbehn JW, Hatch JF, et al. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545-549.
13 Watanabe E, Watanabe T, Manaka S, et al. Three-dimensional digitizer (neuronavigator): new equipment for computed tomography–guided stereotaxic surgery. Surg Neurol. 1987;27:543-547.
14 Golfinos JG, Fitzpatrick BC, Smith LR, et al. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg. 1995;83:197-205.
15 Ryan MJ, Erickson RK, Levin DN, et al. Frameless stereotaxy with real-time tracking of patient head movement and retrospective patient-image registration. J Neurosurg. 1996;85:287-292.
16 Gumprecht HK, Widenka DC, Lumenta CB. BrainLab VectorVision neuronavigation system: technology and clinical experiences in 131 cases. Neurosurgery.. 1999;44:97-104.
17 Krieger MD, Chandrasoma PT, Zee CS, et al. Role of stereotactic biopsy in the diagnosis and management of brain tumors. Semin Surg Oncol. 1998;14:13-25.
18 Hall WA, Liu H, Martin AJ, et al. Comparison of stereotactic brain biopsy to interventional magnetic-resonance-imaging–guided brain biopsy. Stereotact Funct Neurosurg. 1999;73:148-153.
19 Moriarty TM, Quinones-Hinojosa A, Larson PS, et al. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47:1138-1145.
20 Kulkarni AV, Guha A, Lozano A, Bernstein M. Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. J Neurosurg. 1998;89:31-35.
21 Levy RM, Russell E, Yungbluth M, et al. The efficacy of image-guided stereotactic brain biopsy in neurologically symptomatic acquired immunodeficiency syndrome patients. Neurosurgery. 1992;30:186-189.
22 Dammers R, Haitsma IK, Schouten JW, et al. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien). 2008;150:23-29.
23 Dorward NL, Paleologos TS, Alberti O, et al. The advantages of frameless stereotactic biopsy over frame-based biopsy. Br J Neurosurg. 2002;16:110-118.
24 Woodworth G, McGirt MJ, Samdani A, et al. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol Res. 2005;27:358-362.
25 Woodworth GF, McGirt MJ, Samdani A, et al. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J Neurosurg. 2006;104:233-237.
26 Quinones-Hinojosa A, Ware ML, Sanai N, et al. Assessment of image guided accuracy in a skull model: comparison of frameless stereotaxy techniques vs. frame-based localization. J Neurooncol. 2006;76:65-70.
27 Chandrasoma PT, Smith MM, Apuzzo ML. Stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimen. Neurosurgery. 1989;24:160-165.
28 Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3:193-200.
29 McGirt MJ, Villavicencio AT, Bulsara KR, et al. MRI-guided stereotactic biopsy in the diagnosis of glioma: comparison of biopsy and surgical resection specimen. Surg Neurol. 2003;59:277-281.
30 McGirt MJ, Woodworth GF, Coon AL, et al. Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg. 2005;102:897-901.
31 Smith JS, Quinones-Hinojosa A, Barbaro NM, et al. Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. J Neurooncol. 2005;73:173-179.
32 Samadani U, Stein S, Moonis G, et al. Stereotactic biopsy of brain stem masses: decision analysis and literature review. Surg Neurol. 2006;66:484-490.
33 Mathisen JR, Giunta F, Marini G, et al. Transcerebellar biopsy in the posterior fossa: 12 years experience. Surg Neurol. 1987;28:100-104.
34 Samadani U, Stein S, Moonis G, et al. Stereotactic biopsy of brain stem masses: decision analysis and literature review. Surg Neurol. 2006;66:484-490.
35 Friedman WA, Sceats DJJr, Nestok BR, et al. The incidence of unexpected pathological findings in an image-guided biopsy series: a review of 100 consecutive cases. Neurosurgery. 1989;25:180-184.
36 Guillamo JS, Monjour A, Taillandier L, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528-2539.
37 Massager N, David P, Goldman S, et al. Combined magnetic resonance imaging– and positron emission tomography–guided stereotactic biopsy in brainstem mass lesions: diagnostic yield in a series of 30 patients. J Neurosurg. 2000;93:951-957.
38 Frank F, Fabrizi AP, Frank-Ricci R, et al. Stereotactic biopsy and treatment of brain stem lesions: combined study of 33 cases (Bologna-Marseille). Acta Neurochir Suppl. 1988;42:177-181.
39 Amundson EW, McGirt MJ, Olivi A. A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. Technical note. J Neurosurg. 2005;102:565-570.
40 Abernathey CD, Camacho A, Kelly PJ. Stereotaxic suboccipital transcerebellar biopsy of pontine mass lesions. J Neurosurg. 1989;70:195-200.
41 Goncalves-Ferreira AJ, Herculano-Carvalho M, Pimentel J. Stereotactic biopsies of focal brainstem lesions. Surg Neurol. 2003;60:311-320.
42 Spiegelmann R, Friedman WA. Stereotactic suboccipital transcerebellar biopsy under local anesthesia using the Cosman-Roberts-Wells frame. Technical note. J Neurosurg. 1991;75:486-488.
43 Blond S, Lejeune JP, Dupard T, et al. The stereotactic approach to brain stem lesions: a follow-up of 29 cases. Acta Neurochir Suppl. 1991;52:75-77.
44 Coffey RJ, Lunsford LD. Stereotactic surgery for mass lesions of the midbrain and pons. Neurosurgery. 1985;17:12-18.
45 Favre J, Taha JM, Burchiel KJ. An analysis of the respective risks of hematoma formation in 361 consecutive morphological and functional stereotactic procedures. Neurosurgery. 2002;50:48-56.
46 Kondziolka D, Lunsford LD. Stereotactic biopsy for intrinsic lesions of the medulla through the long-axis of the brainstem: technical considerations. Acta Neurochir (Wien). 1994;129:89-91.
47 Steck J, Friedman WA. Stereotactic biopsy of brainstem mass lesions. Surg Neurol. 1995;43:563-567.
48 Hood TW, Gebarski SS, McKeever PE, et al. Stereotaxic biopsy of intrinsic lesions of the brain stem. J Neurosurg. 1986;65:172-176.
49 Kreth FW, Muacevic A, Medele R, et al. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours—a prospective study. Acta Neurochir (Wien). 2001;143:539-545.

 -inch hole in the skull and the dura is perforated with a sharp needle The biopsy needle is then passed along the path of the trajectory, and at least two specimens approximately 8 mm long × 1 mm in diameter are obtained and sent for pathologic evaluation. Further biopsy samples are taken if the initial specimens are nondiagnostic.
-inch hole in the skull and the dura is perforated with a sharp needle The biopsy needle is then passed along the path of the trajectory, and at least two specimens approximately 8 mm long × 1 mm in diameter are obtained and sent for pathologic evaluation. Further biopsy samples are taken if the initial specimens are nondiagnostic. -inch opening in the skull. Tissue specimens 8 mm long and 1 mm in diameter are obtained, with additional biopsy specimens being taken if the initial ones are nondiagnostic.
-inch opening in the skull. Tissue specimens 8 mm long and 1 mm in diameter are obtained, with additional biopsy specimens being taken if the initial ones are nondiagnostic.