CHAPTER 50 Fractionated Radiation for Meningiomas
PRINCIPLES OF EXTERNAL BEAM RADIOTHERAPY
Molecular Mechanism and Cellular Endpoints
Radiation principally acts by ionizing molecules in irradiated tissues to cause DNA damage. Although radiation damage can be directly ionizing, in clinical therapy damage is most commonly caused indirectly, via the induction of highly reactive free radicals. The principal radiation-induced DNA damage is in the form of single- and double-strand breaks, the latter of which are generally considered the lethal event, leading to cell death.1 The majority of radiation-induced DNA lesions, particularly single-strand breaks, are effectively repaired via a number of complex repair mechanisms.2,3 It is assumed that DNA damage is responsible for radiation-induced inhibition of tumor growth in benign as well as in malignant tumors, and although likely, in benign tumors it remains unproven.
The end result of radiation damage after therapeutic radiation of malignant tumors is cell death, which is measured experimentally as clonogenic cell survival. Repeated small doses of radiation are less damaging to cells than an equivalent total dose given in a single fraction. Different dose fractionation schemes can be compared via a mathematical model (the linear quadratic [LQ] model), which compares different dose fractionation schemes through the concept of a biologically equivalent dose. The LQ model, which takes into account the total dose and the dose per fraction (and hence the number of doses/fractions), contains constants α and β, and it is the α/β ratio that distinguishes the responses of different tissues to radiation.1,4
Acute reacting tissues such as epithelium have a high α/β ratio generally in the range of 8 to 10 Gy (ranging from 7 to 20 Gy), and late-reacting tissues, exemplified by the normal central nervous system (CNS), have a low α/β ratio, usually 1 to 3 Gy (ranging from 0.5 to 6 Gy). The slow growth and presumed limited dividing capacity of benign tumors such as meningiomas has led to the assumption they have a low α/β ratio, but this remains unproven. Late-responding tissues are more spared by fractionation than early-responding tissues, and this is the principle of fractionated radiotherapy, particularly as applied to the CNS which has an α/β ratio of 1 to 2 Gy where fractionation preferentially spares normal brain compared to a high α/β ratio tumor.1
Technical Aspects of Radiotherapy
MLC leaves can also be used to alter the intensity of radiation, and this is described as intensity-modulated radiotherapy (IMRT). IMRT allows for better conformation of radiation to complex shape targets, particularly with concave regions containing critical normal tissue. Planning studies of complex meningiomas have demonstrated that IMRT may improve sparing of normal brain tissue in selected5,6 but not all cases.7
Proton radiation has the appeal of more localized deposition of energy than can be achieved with photons (X-rays), therefore offering the potential for better sparing of normal tissue particularly beyond the principal target. The rationale for the use of protons in meningiomas is based solely on the theoretical benefit of more localized conformal delivery of radiation shown in some cases.8,9
CLINICAL PRACTICE AND OUTCOME OF CONVENTIONAL FRACTIONATED RADIOTHERAPY IN BENIGN MENINGIOMAS
Tumor Control
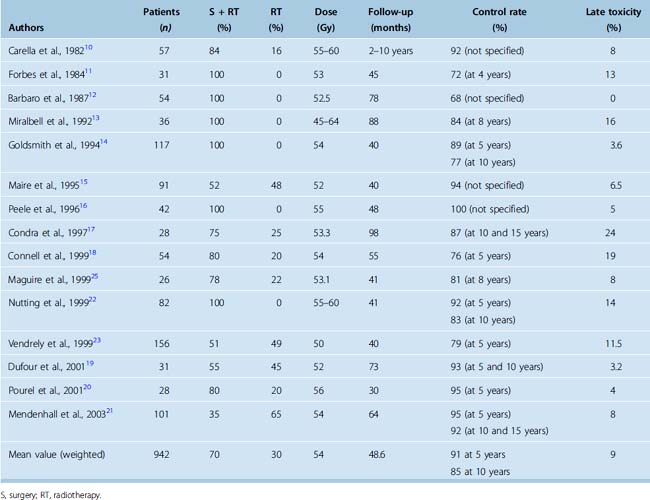
Retrospective studies of conventional fractionated radiotherapy in patients with residual and recurrent meningiomas have demonstrated apparent benefit in local tumor control after radiotherapy10–21 (Table 50-1). However, none of the studies are randomized or prospectively controlled and none have so far reported convincing survival gain with the early addition of irradiation. Nevertheless the reported progression-free survival (PFS) after radiotherapy of benign meningioma is in the region of 90% at 5 years and 80% to 90% at 10 years14,17,19,21,22 (see Table 50-1).
There is limited information on the appropriate dose of irradiation. The reported tumor control is similar for doses ranging from 50 to 60 Gy at less than or equal to 2 Gy per fraction. Doses less than 50 Gy were reported to be associated with worse disease control,13,21,23 although this is based on retrospective studies. The recommended dose for benign meningiomas is generally 50 to 60 Gy (usually 54–56 Gy) at less than 2 Gy per fraction. Our practice is to limit the dose to 50 Gy for meningiomas involving the optic pathways.
Tumor site and size have been reported as predictors of tumor control.14,15 In skull-base tumors, the generally larger sphenoid ridge tumors fare worse than small cavernous sinus lesions.22 There is little evidence that timing of radiotherapy is important, as local control and survival rates are similar whether radiotherapy is given as part of primary treatment or at the time of recurrence.14,21,22 Age and gender have little influence on long-term disease control.
Neurologic Function
An important endpoint of treatment of benign meningiomas is the preservation or improvement of neurologic function. More than 70% of patients with skull-base meningiomas have a neurologic deficit that may be a consequence of tumor growth or previous surgery. An improvement or more often stabilization of neurologic deficits has been reported in up to two thirds of patients after conventional radiotherapy,15,19,23 although this is generally in the absence of objective prospective recording of function. Our experience suggests that the majority of patients have stabilization of neurologic deficit for the duration of disease control.
Toxicity of Fractionated Radiotherapy
The risk of late normal CNS toxicity of external beam radiotherapy to doses less than 60 Gy at less than 2 Gy per fraction is low. Radiation injury to the optic apparatus (radiation optic neuropathy), manifest as decreased visual acuity or visual field defect, is reported in 0 to 2% of patients irradiated for meningiomas.10,12–25 No injuries were noted in 106 optic nerves treated to doses less than 59 Gy, while the 15-year actuarial risk of radiation optic neuropathy was up to 47% in patients receiving a dose greater than or equal to 60 Gy.26 Other cranial deficits have been reported in 1% to 3% of patients. Radiation-induced necrosis of brain parenchyma is rare, and remains an exceptional event (<1% risk) after modern conformal radiotherapy to doses less than or equal to 60 Gy.
Neurocognitive dysfunction is a recognized consequence of large-volume cranial irradiation, particularly using hypofractionated schedules.27,28 There are no large prospective cognitive function studies in patients with benign meningiomas treated with radiation. Although impairment of short-term memory has been recorded, the cause is likely to be multifactorial, especially in the elderly patient population. There is little evidence that small-volume fractionated irradiation for meningioma is responsible for cognitive dysfunction beyond the deleterious effect of surgery and the tumor itself.29
Hypopituitarism is reported in less than 4% of irradiated patients with meningiomas,19,21–23 although endocrine function has not been systematically evaluated. Patients with large parasellar meningiomas involving the sella and suprasellar region are at risk of hypopituitarism due to the disease itself and as a consequence of irradiation of the hypothalamic–pituitary axis. Regular endocrine assessment should be standard practice in patients with parasellar meningiomas and any tumors where the hypothalamic–pituitary axis received significant doses of irradiation.
Although there is an increased incidence of cerebrovascular accidents and excess cerebrovascular mortality in patients with benign tumors treated with radiotherapy,30,31 the risk in patients with meningioma treated with radiation has not been defined and the influence of radiation on its frequency is also not clear.
High-dose radiation may be associated with the development of a second brain tumor, usually a meningioma or an astrocytoma, as reported after irradiation of a pituitary adenoma.32 The estimated risk after radiotherapy of pituitary adenoma is in the region of 2% at 20 years; the risk after localized radiotherapy of meningioma is not defined.
FRACTIONATED STEREOTACTIC, INTENSITY MODULATED, AND PROTON BEAM RADIOTHERAPY IN BENIGN MENINGIOMAS
Stereotactic Radiotherapy
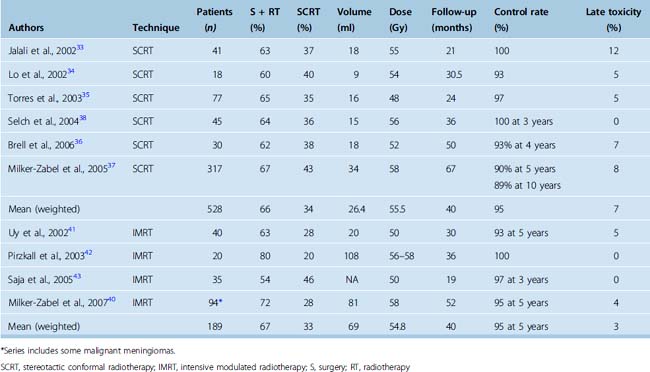
The reported control after SCRT is greater than 90% at 5 years (weighted mean 95% at a median follow-up of 40 months)33–38 (Table 50-2). The largest series of 189 patients with benign skull-base meningioma treated with fractionated stereotactic conformal radiotherapy reported a 5-year local PFS and survival of 94% and 97%, respectively.39 An update of 317 patients reported a 5-year and 10-year PFS of 91% and 89% and respective survival of 95% and 90% for Grade 1 meningiomas.37 Tumor volume greater than 60 cm3 was associated with 16% recurrence rate compared to 4% for smaller tumors less than or equal to 60 cm3. The 5-year local PFS of 110 patients with benign skull-base meningioma treated with SCRT between 1994 and 2006 at the Royal Marsden Hospital is 89% and the 5-year survival is 96% (unpublished update). Patients with small cavernous and parasellar meningiomas had a local PFS of 100%.
Improvement in neurologic function, usually in the form of improvement or recovery of cranial nerve deficit, has been reported in 14% to 44% patients.33,37 In the authors’ experience, pain due to meningiomas is rarely improved by fractionated irradiation. Late toxicity including cranial nerve deficit (e.g., visual problems), hypopituitarism, and impairment of neurocognitive function have been reported in fewer than 8% of patients treated with SCRT33–38 (see Table 50-2). However, the evaluation of complications is frequently retrospective without defined objective severity scales and long-term prospective studies are needed.
IMRT
Based on reports of improved normal tissue sparing in comparison to conventional radiotherapy and SCRT in selected meningiomas, IMRT has been employed particularly in patients with complex shaped tumors. The reported local tumor control in the largest cohort of patients with benign meningiomas treated with IMRT was 94%, with improvement in neurologic function in approximately 40%.40 Similar results have been reported in other smaller cohorts41–43 (see Table 50-2). Although IMRT is a feasible technique of radiation delivery for complex shaped meningiomas, tumor and morbidity control are similar to those achieved with other means of photon irradiation without clear evidence of benefit.
Protons
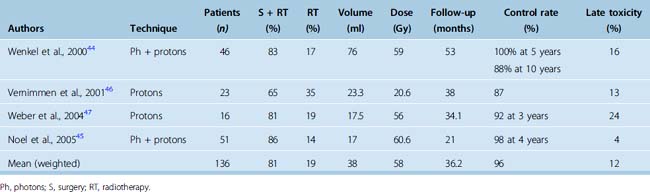
Relatively few patients with meningioma received proton irradiation. Reported tumor control rates after proton therapy is shown in Table 50-3. The 3- to 5-year local PFS is 91% to 100%44–47 and the only reported 10-year PFS is 88%.44 The treatment as reported was associated with a relatively high incidence of late morbidity, with 16% actuarial risk at 2 years in one study44 and a 13% to 24% risk in other smaller studies,46,47 usually associated with doses greater than 60 GyE. In summary, although proton irradiation appears equally effective as photon radiotherapy in achieving local control of benign meningiomas, the reported morbidity of treatment, particularly when employing doses beyond tolerance of normal CNS, appears higher.
OPTIC NERVE SHEATH MENINGIOMA
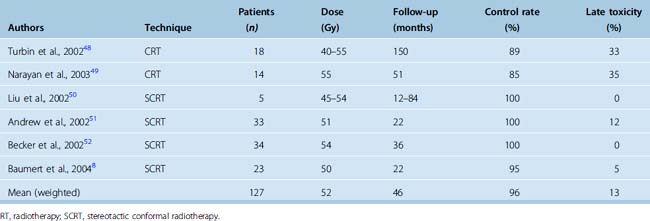
Early use of conventional and 3D conformal radiotherapy resulted in improvement and stabilization of vision in 61% to 82% patients48,49 (Table 50-4). High-dose irradiation to an already compromised optic nerve and to the retina may lead to late visual deterioration, and cases of radiation retinopathy have been reported. After stereotactic radiotherapy (SCRT), stabilization and improvement in vision has been reported in 92% to 100% patients8,50–52 (see Table 50-4). Few if any recurrences occur after irradiation, as would be expected in such an indolent tumor.
CLINICAL RESULTS OF EXTERNAL BEAM RADIOTHERAPY IN NON-BENIGN MENINGIOMAS
The majority of patients with non-benign (WHO grades II and III) meningiomas treated with surgery alone ultimately recur. The reported recurrence rate is in the region of 50% for subtotally and 90% for completely excised tumors and recurrent disease adversely affects survival.53–57 The 5-year survival rates of patients with non-benign meningiomas is 28% to 70%,4,58–63 with worse survival in patients after incomplete resections at first presentation.58,63
As in patients with benign meningioma, radiotherapy is usually administered after incomplete resection or after recurrence. There are no published prospective studies in patients with grade II and III meningiomas. A small retrospective study suggested that adjuvant radiotherapy for recurrent disease after reexcision improves the 2-year PFS (50% vs. 89%; P = 0.002) although not the 5-year PFS.60 This supports the belief that radiotherapy may reduce the need for further surgical procedures and this may be of particular value for patients with a high risk of tumor recurrence. Nevertheless, despite radiotherapy, 5-year survival in this study was only 28%. In larger cohorts including both atypical and malignant meningiomas treated with radiation after subtotal resection, the reported 5-year survival is 40% to 58%.14,63
The principal aim of modern radiotherapy for non-benign meningioma is to achieve better tumor control, ideally to allow both for safe dose escalation and effective normal tissue avoidance. Retrospective studies of conventional external beam radiotherapy suggest that higher doses may increase local tumor control and survival in WHO grade II and grade III meningiomas14,61,64,65 (Table 50-5). The cutoff level is in the region of 50 Gy; information on dose response beyond 60 Gy is not available.
TABLE 50-5 Outcome of grade WHO II and III meningioma patients after external beam radiotherapy to either low- or high-target dose
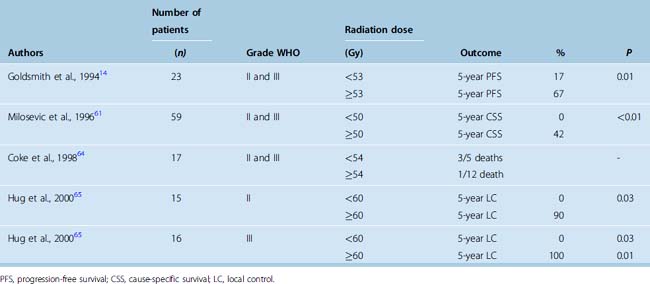
Patients with non-benign meningiomas have been treated with radiosurgery with varying results. The 5-year PFS in a small cohort of patients after single-fraction radiosurgery of 30 Gy was 83% for atypical and 72% for malignant meningiomas.66 Other centers reported worse tumor control, with 68% and 0% 5-year PFS for atypical and malignant meningiomas.67 Encouraging results have also been reported after proton therapy, where doses greater than 60 Gy were associated with improved outcome,65 although such apparent dose–response relationship has not been confirmed by others.68,69
In summary, limited retrospective data suggest that radiotherapy may improve disease control in patients with non-benign meningiomas with a potential for improved local tumor control with higher doses of radiation therapy, although this is based on retrospective studies of small numbers of patients with all the problems of selection and both patient and treatment heterogeneity (see Table 50-5). Currently, a phase 2 dose escalation study organised by the EORTC (22042-26042) is in progress wherein patients with grade II and III meningioma receive postoperative radiotherapy to 60 Gy and 70 Gy after Simpson55 grade 1 to 3 and greater than 3 resection. A similar study by Radiation Therapy Oncology Group (RTOG 0539) is in preparation.
[1] Steel G. Clonogenic cells and the concept of cell survival. In: Steel G., editor. Basic Clinical Radiobiology. London: Arnold; 2002:52-54.
[2] Pawlik T.M., Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928-942.
[3] Helleday T., et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193-204.
[4] Chadwick K.H., Leenhouts H.P. A molecular theory of cell survival. Phys Med Biol. 1973;18:78-87.
[5] Pirzkall A., et al. Comparison of intensity-modulated radiotherapy with conventional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys. 2000;48:1371-1380.
[6] Baumert B.G., Norton I.A., Davis J.B. Intensity-modulated stereotactic radiotherapy vs. stereotactic conformal radiotherapy for the treatment of meningioma located predominantly in the skull base. Int J Radiat Oncol Biol Phys. 2003;57:580-592.
[7] Khoo V.S., et al. Comparison of intensity-modulated tomotherapy with stereotactically guided conformal radiotherapy for brain tumors. Int J Radiat Oncol Biol Phys. 1999;45:415-425.
[8] Baumert B.G., et al. Dose conformation of intensity-modulated stereotactic photon beams, proton beams, and intensity-modulated proton beams for intracranial lesions. Int J Radiat Oncol Biol Phys. 2004;60:1314-1324.
[9] Cozzi L., et al. Comparative planning study for proton radiotherapy of benign brain tumors. Strahlenther Onkol. 2006;182:376-381.
[10] Carella R., Ransohoff J., Newall J. Role of radiation therapy in the management of meningioma. Neurosurgery. 1982;10:332-339.
[11] Forbes A.R., Goldberg I.D. Radiation therapy in the treatment of meningioma: the Joint Center for Radiation Therapy experience 1970 to 1982. J Clin Oncol. 1984;2:1139-1143.
[12] Barbaro N.M., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
[13] Miralbell R., et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13:157-164.
[14] Goldsmith B., et al. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
[15] Maire J.P., et al. Fractionated radiation therapy in the treatment of intracranial meningiomas: local control, functional efficacy, and tolerance in 91 patients. Int J Radiat Oncol Biol Phys. 1995;33:315-321.
[16] Peele K.A., et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996;103:1761-1766. discussion 1766–7
[17] Condra K.S., et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
[18] Connell P.P., et al. Tumor size predicts control of benign meningiomas treated with radiotherapy. Neurosurgery. 1999;44:1194-1199. discussion 1199–200
[19] Dufour H., et al. Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: is there an alternative to aggressive tumor removal? Neurosurgery. 2001;48:285-294. discussion 294–6
[20] Pourel N., et al. Efficacy of external fractionated radiation therapy in the treatment of meningiomas: a 20–year experience. Radiother Oncol. 2001;61:65-70.
[21] Mendenhall W.M., et al. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003;98:1473-1482.
[22] Nutting C., et al. Radiotherapy in the treatment of benign meningioma of the skull base. J Neurosurg. 1999;90:823-827.
[23] Vendrely V., et al. [Fractionated radiotherapy of intracranial meningiomas: 15 years’ experience at the Bordeaux University Hospital Center]. Cancer Radiother. 1999;3:311-317.
[24] Forbes A.R., Goldberg I.D. Radiation therapy in the treatment of meningioma: the Joint Center for Radiation Therapy experience 1970 to 1982. J Clin Oncol. 1984;2:1139-1143.
[25] Maguire P.D., et al. Fractionated external-beam radiation therapy for meningiomas of the cavernous sinus. Int J Radiat Oncol Biol Phys. 1999;44:75-79.
[26] Parsons J.T., et al. Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:755-763.
[27] Laack N.N., Brown P.D. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31:702-713.
[28] Taphoorn M.J., Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159-168.
[29] van Nieuwenhuizen D., et al. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84:271-278.
[30] Brada M., et al. Cerebrovascular mortality in patients with pituitary adenoma. Clin Endocrinol (Oxf). 2002;57:713-717.
[31] Brada M., et al. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 1999;45:693-698.
[32] Minniti G., et al. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab. 2005;90:800-804.
[33] Jalali R., et al. High precision focused irradiation in the form of fractionated stereotactic conformal radiotherapy (SCRT) for benign meningiomas predominantly in the skull base location. Clin Oncol (R Coll Radiol). 2002;14:103-109.
[34] Lo S.S., et al. Single dose versus fractionated stereotactic radiotherapy for meningiomas. Can J Neurol Sci. 2002;29:240-248.
[35] Torres R.C., et al. Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus. 2003;14:e5.
[36] Brell M., et al. Fractionated stereotactic radiotherapy in the treatment of exclusive cavernous sinus meningioma: functional outcome, local control, and tolerance. Surg Neurol. 2006;65:28-33.
[37] Milker-Zabel S., et al. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61:809-816.
[38] Selch M.T., et al. Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2004;59:101-111.
[39] Debus J., et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. 2001;19:3547-3553.
[40] Milker-Zabel S., et al. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858-863.
[41] Uy N.W., et al. Intensity-modulated radiation therapy (IMRT) for meningioma. Int J Radiat Oncol Biol Phys. 2002;53:1265-1270.
[42] Pirzkall A., et al. Intensity modulated radiotherapy (IMRT) for recurrent, residual, or untreated skull-base meningiomas: preliminary clinical experience. Int J Radiat Oncol Biol Phys. 2003;55:362-372.
[43] Sajja R., et al. Intensity-modulated radiation therapy (IMRT) for newly diagnosed and recurrent intracranial meningiomas: preliminary results. Technol Cancer Res Treat. 2005;4:675-682.
[44] Wenkel E., et al. Benign meningioma: Partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363-1370.
[45] Noel G., et al. Functional outcome of patients with benign meningioma treated by 3D conformal irradiation with a combination of photons and protons. Int J Radiat Oncol Biol Phys. 2005;62:1412-1422.
[46] Vernimmen F.J., et al. Stereotactic proton beam therapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2001;49:99-105.
[47] Weber D.C., et al. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol. 2004;71:251-258.
[48] Turbin R.E., et al. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890-899. discussion 899–900
[49] Narayan S., et al. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56:537-543.
[50] Liu J.K., et al. Optic nerve sheath meningiomas: visual improvement after stereotactic radiotherapy. Neurosurgery. 2002;50:950-955. discussion 955–7
[51] Andrews D., et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002;51:890-902.
[52] Becker G., et al. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2002;54:1422-1429.
[53] Salazar O.M. Ensuring local control in meningiomas. Int J Radiat Oncol Biol Phys. 1988;15:501-504.
[54] Chan R.C., Thompson G.B. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60:52-60.
[55] Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
[56] Taylor B.W.Jr, et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
[57] Yamashita J., et al. Recurrence of intracranial meningiomas, with special reference to radiotherapy. Surg Neurol. 1980;14:33-40.
[58] Palma L., et al. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. Neurosurg Focus. 1997;2:e3.
[59] Jaaskelainen J., Haltia M., Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233-242.
[60] Dziuk T., et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37:177-188.
[61] Milosevic M.F., et al. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys. 1996;34:817-822.
[62] Mahmood A., et al. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33:955-963.
[63] Younis G.A., et al. Aggressive meningeal tumors: review of a series. J Neurosurg. 1995;82:17-27.
[64] Coke C., et al. Atypical and malignant meningiomas: an outcome report of seventeen cases. J Neurooncol. 1998;39:65-70.
[65] Hug E., et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151-160.
[66] Harris A.E., et al. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60:298-305. discussion 305
[67] Stafford S.L., et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037. discussion 1037–8
[68] Katz T.S., et al. Pushing the limits of radiotherapy for atypical and malignant meningioma. Am J Clin Oncol. 2005;28:70-74.
[69] Pasquier D., Bijmolt S., Veninga T., et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71:1388-1393.