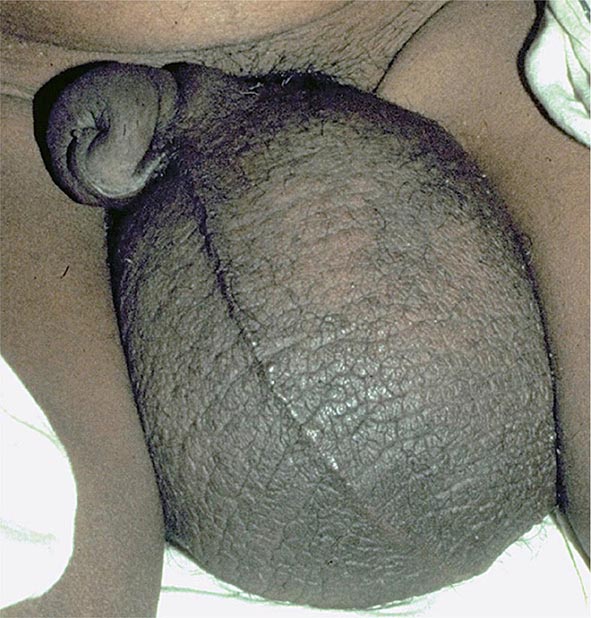
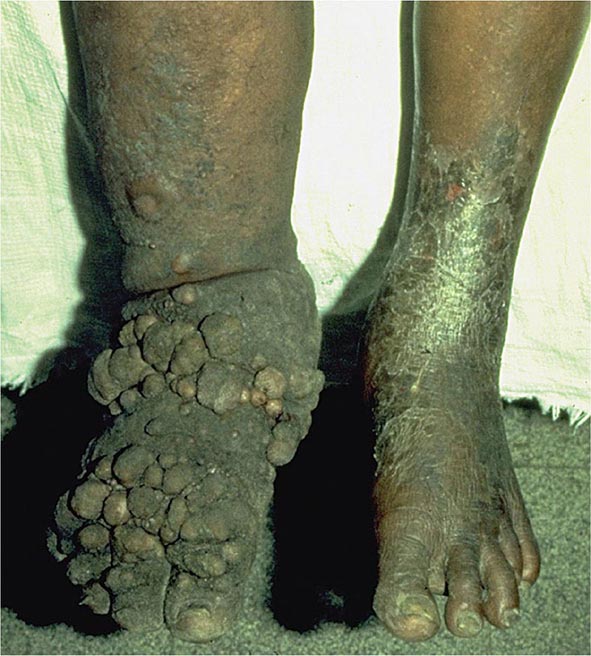
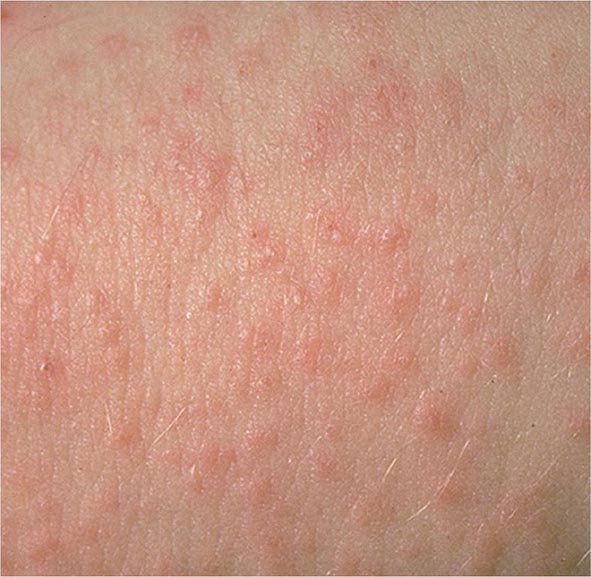
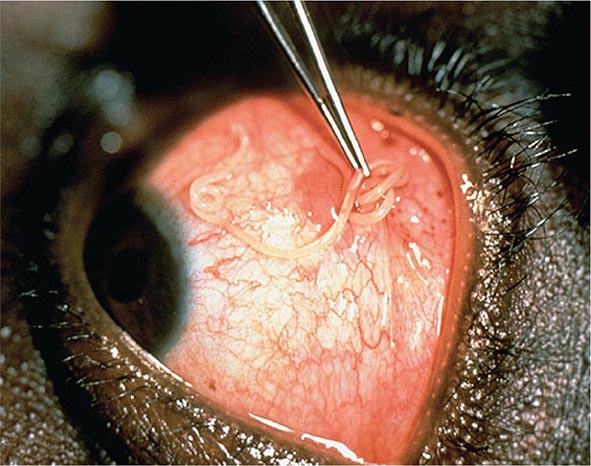
It is now appreciated that genotypes of T. gondii prevalent in South America may be more virulent than those typically seen in North America or Europe. These genotypes may be associated with acute or recurrent ocular disease in immunocompetent individuals and have also been associated with pneumonitis and a fulminant sepsis picture in immunologically normal individuals. Thus a detailed history is critical for establishing a diagnosis.
The results of routine laboratory studies are usually unremarkable except for minimal lymphocytosis, an elevated erythrocyte sedimentation rate, and a nominal increase in serum aminotransferase levels. Evaluation of cerebrospinal fluid (CSF) in cases with evidence of encephalopathy or meningoencephalitis shows an elevation of intracranial pressure, mononuclear pleocytosis (10–50 cells/mL), a slight increase in protein concentration, and (occasionally) an increase in the gamma globulin level. PCR amplification of the Toxoplasma DNA target sequence in CSF may be beneficial. The CSF of chronically infected individuals is normal.
Infection of Immunocompromised Patients Patients with AIDS and those receiving immunosuppressive therapy for lymphoproliferative disorders are at greatest risk for developing acute toxoplasmosis. Toxoplasmosis has also been reported after treatment with antibodies to tumor necrosis factor. The infection may be due either to reactivation of latent infection or to acquisition of parasites from exogenous sources such as blood or transplanted organs. In individuals with AIDS, >95% of cases of Toxoplasma encephalitis (TE) are believed to be due to recrudescent infection. In most of these cases, encephalitis develops when the CD4+ T cell count falls below 100/µL. In immunocompromised hosts, the disease may be rapidly fatal if untreated. Thus, accurate diagnosis and initiation of appropriate therapy are necessary to prevent fulminant infection.
Toxoplasmosis is a principal opportunistic infection of the CNS in persons with AIDS. Although geographic origin may be related to frequency of infection, it has no correlation with the severity of disease in immunocompromised hosts. Individuals with AIDS who are seropositive for T. gondii are at high risk for encephalitis. Before the advent of current cART, about one-third of the 15–40% of adult AIDS patients in the United States who were latently infected with T. gondii developed TE. TE may still be a presenting infection in individuals who are unaware of their positive HIV status.
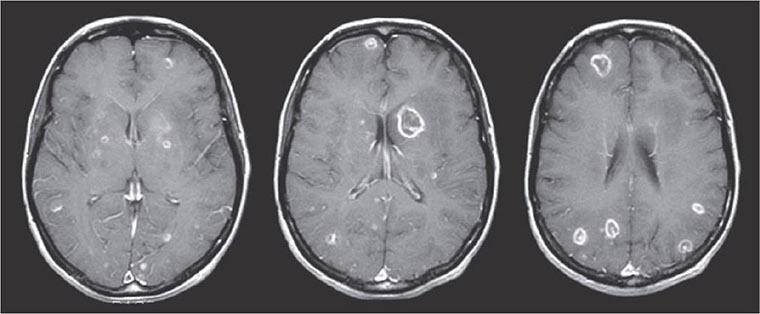
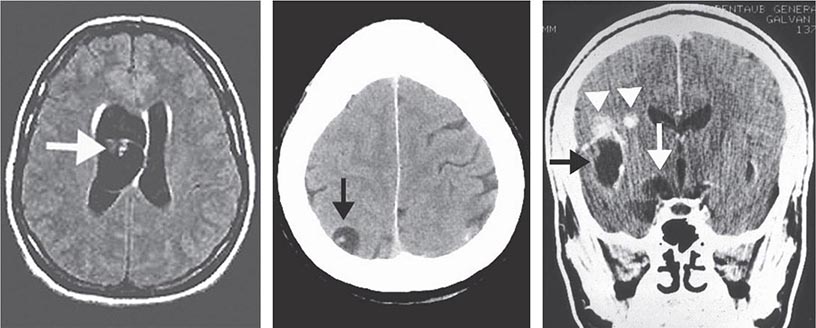
The signs and symptoms of acute toxoplasmosis in immunocompromised patients principally involve the CNS (Fig. 253-2). More than 50% of patients with clinical manifestations have intracerebral involvement. Clinical findings at presentation range from nonfocal to focal dysfunction. CNS findings include encephalopathy, meningoencephalitis, and mass lesions. Patients may present with altered mental status (75%), fever (10–72%), seizures (33%), headaches (56%), and focal neurologic findings (60%), including motor deficits, cranial nerve palsies, movement disorders, dysmetria, visual-field loss, and aphasia. Patients who present with evidence of diffuse cortical dysfunction develop evidence of focal neurologic disease as infection progresses. This altered condition is due not only to the necrotizing encephalitis caused by direct invasion by the parasite but also to secondary effects, including vasculitis, edema, and hemorrhage. The onset of infection can range from an insidious process over several weeks to an acute presentation with fulminant focal deficits, including hemiparesis, hemiplegia, visual-field defects, localized headache, and focal seizures.
FIGURE 253-2 Toxoplasmic encephalitis in a 36-year-old patient with AIDS. The multiple lesions are demonstrated by MRI scanning (T1-weighted with gadolinium enhancement). (Courtesy of Clifford Eskey, Dartmouth Hitchcock Medical Center, Hanover, NH; with permission.)
Although lesions can occur anywhere in the CNS, the areas most often involved appear to be the brainstem, basal ganglia, pituitary gland, and corticomedullary junction. Brainstem involvement gives rise to a variety of neurologic dysfunctions, including cranial nerve palsy, dysmetria, and ataxia. With basal ganglionic infection, patients may develop hydrocephalus, choreiform movements, and choreoathetosis. Toxoplasma usually causes encephalitis, and meningeal involvement is uncommon. CSF findings may be unremarkable or may include a modest increase in cell count and in protein—but not glucose—concentration. Nonetheless, the parasite may be detected by PCR in CSF from many patients with TE.
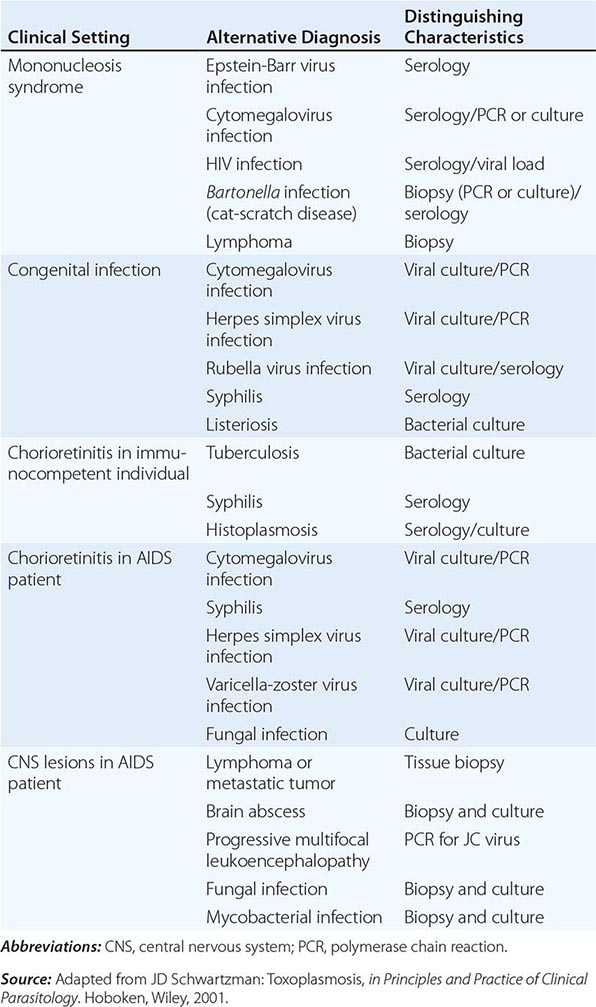
Cerebral toxoplasmosis must be differentiated from other opportunistic infections or tumors in the CNS of AIDS patients. The differential diagnosis includes herpes simplex encephalitis, cryptococcal meningitis, progressive multifocal leukoencephalopathy, and primary CNS lymphoma. Involvement of the pituitary gland can give rise to panhypopituitarism and hyponatremia from inappropriate secretion of vasopressin (antidiuretic hormone). HIV-associated neurocognitive disorder (HAND) may present as cognitive impairment, attention loss, and altered memory. Brain biopsy in patients who have been treated for TE but who continue to exhibit neurologic dysfunction often fails to identify organisms.
Autopsies of Toxoplasma-infected patients have demonstrated the involvement of multiple organs, including the lungs, gastrointestinal tract, pancreas, skin, eyes, heart, and liver. Toxoplasma pneumonia can be confused with Pneumocystis pneumonia (PcP). Respiratory involvement usually presents as dyspnea, fever, and a nonproductive cough and may rapidly progress to acute respiratory failure with hemoptysis, metabolic acidosis, hypotension, and (occasionally) disseminated intravascular coagulation. Histopathologic studies demonstrate necrosis and a mixed cellular infiltrate. The presence of organisms is a helpful diagnostic indicator, but organisms can also be found in healthy tissue. Infection of the heart is usually asymptomatic but can be associated with cardiac tamponade or biventricular failure. Infections of the gastrointestinal tract and the liver have been documented.
Congenital Toxoplasmosis Between 400 and 4000 infants born each year in the United States are affected by congenital toxoplasmosis. Acute infection in mothers acquiring T. gondii during pregnancy is usually asymptomatic; most such women are diagnosed via prenatal serologic screening. Infection of the placenta leads to hematogenous infection of the fetus. As gestation proceeds, the proportion of fetuses that become infected increases, but the clinical severity of the infection declines. Although infected children may initially be asymptomatic, the persistence of T. gondii can result in reactivation and clinical disease—most frequently chorioretinitis—decades later. Factors associated with relatively severe disabilities include delays in diagnosis and in initiation of therapy, neonatal hypoxia and hypoglycemia, profound visual impairment (see “Ocular Infection,” below), uncorrected hydrocephalus, and increased intracranial pressure. If treated appropriately, upwards of 70% of children have normal developmental, neurologic, and ophthalmologic findings at follow-up evaluations. Treatment for 1 year with pyrimethamine, a sulfonamide, and folinic acid is tolerated with minimal toxicity (see “Treatment,” below).
![]() Ocular Infection Infection with T. gondii is estimated to cause 35% of all cases of chorioretinitis in the United States and Europe. It was formerly thought that the majority of cases of ocular disease were due to congenital infection. New ocular toxoplasmosis in immunocompetent individuals occurs more commonly than was previously appreciated and has been associated with outbreaks in Victoria (British Columbia) and in South America. A variety of ocular manifestations are documented, including blurred vision, scotoma, photophobia, and eye pain. Macular involvement occurs, with loss of central vision, and nystagmus is secondary to poor fixation. Involvement of the extraocular muscles may lead to disorders of convergence and to strabismus. Ophthalmologic examination should be undertaken in newborns with suspected congenital infection. As the inflammation resolves, vision improves, but episodic flare-ups of chorioretinitis, which progressively destroy retinal tissue and lead to glaucoma, are common. The ophthalmologic examination reveals yellow-white, cotton-like patches with indistinct margins of hyperemia. As the lesions age, white plaques with distinct borders and black spots within the retinal pigment become more apparent. Lesions usually are located near the posterior pole of the retina; they may be single but are more commonly multiple. Congenital lesions may be unilateral or bilateral and show evidence of massive chorioretinal degeneration with extensive fibrosis. Surrounding these areas of involvement are a normal retina and vasculature. In patients with AIDS, retinal lesions are often large, with diffuse retinal necrosis, and include both free tachyzoites and cysts containing bradyzoites. Toxoplasmic chorioretinitis may be a prodrome to the development of encephalitis.
Ocular Infection Infection with T. gondii is estimated to cause 35% of all cases of chorioretinitis in the United States and Europe. It was formerly thought that the majority of cases of ocular disease were due to congenital infection. New ocular toxoplasmosis in immunocompetent individuals occurs more commonly than was previously appreciated and has been associated with outbreaks in Victoria (British Columbia) and in South America. A variety of ocular manifestations are documented, including blurred vision, scotoma, photophobia, and eye pain. Macular involvement occurs, with loss of central vision, and nystagmus is secondary to poor fixation. Involvement of the extraocular muscles may lead to disorders of convergence and to strabismus. Ophthalmologic examination should be undertaken in newborns with suspected congenital infection. As the inflammation resolves, vision improves, but episodic flare-ups of chorioretinitis, which progressively destroy retinal tissue and lead to glaucoma, are common. The ophthalmologic examination reveals yellow-white, cotton-like patches with indistinct margins of hyperemia. As the lesions age, white plaques with distinct borders and black spots within the retinal pigment become more apparent. Lesions usually are located near the posterior pole of the retina; they may be single but are more commonly multiple. Congenital lesions may be unilateral or bilateral and show evidence of massive chorioretinal degeneration with extensive fibrosis. Surrounding these areas of involvement are a normal retina and vasculature. In patients with AIDS, retinal lesions are often large, with diffuse retinal necrosis, and include both free tachyzoites and cysts containing bradyzoites. Toxoplasmic chorioretinitis may be a prodrome to the development of encephalitis.
DIAGNOSIS
Tissue and Body Fluids The differential diagnosis of acute toxoplasmosis can be made by appropriate culture, serologic testing, and PCR (Table 253-1). Although available only at specialized laboratories, the isolation of T. gondii from blood or other body fluids can be accomplished after subinoculation of the sample into the peritoneal cavity of mice. If no parasites are found in the mouse’s peritoneal fluid 6–10 days after inoculation, its anti-Toxoplasma serum titer can be evaluated 4–6 weeks after inoculation. Isolation of T. gondii from the patient’s body fluids reflects acute infection, whereas isolation from biopsied tissue is an indication only of the presence of tissue cysts and should not be misinterpreted as evidence of acute toxoplasmosis. Persistent parasitemia in patients with latent, asymptomatic infection is rare. Histologic examination of lymph nodes may suggest the characteristic changes described above. Demonstration of tachyzoites in lymph nodes establishes the diagnosis of acute toxoplasmosis. Like subinoculation into mice, histologic demonstration of cysts containing bradyzoites confirms prior infection with T. gondii but is nondiagnostic for acute infection.
|
DIFFERENTIAL LABORATORY DIAGNOSIS OF TOXOPLASMOSIS |
Serology The procedures mentioned above have great diagnostic value but are limited by difficulties encountered either in the growth of parasites in vivo or in the identification of tachyzoites by histochemical methods. Serologic testing has become the routine method of diagnosis.
Diagnosis of acute infection with T. gondii can be established by detection of the simultaneous presence of IgG and IgM antibodies to Toxoplasma in serum. The presence of circulating IgA favors the diagnosis of an acute infection. The Sabin-Feldman dye test, the indirect fluorescent antibody test, and the enzyme-linked immunosorbent assay (ELISA) all satisfactorily measure circulating IgG antibody to Toxoplasma. Positive IgG titers (>1:10) can be detected as early as 2–3 weeks after infection. These titers usually peak at 6–8 weeks and decline slowly to a new baseline level that persists for life. Antibody avidity increases with time and can be useful in difficult cases during pregnancy for establishing when infection may have occurred. The serum IgM titer should be measured in concert with the IgG titer to better establish the time of infection; either the double-sandwich IgM-ELISA or the IgM-immunosorbent assay (IgM-ISAGA) should be used. Both assays are specific and sensitive, with fewer false-positive results than other commercial tests. The double-sandwich IgA-ELISA is more sensitive than the IgM-ELISA for detecting congenital infection in the fetus and newborn. Although a negative IgM result with a positive IgG titer indicates distant infection, IgM can persist for >1 year and should not necessarily be considered a reflection of acute disease. If acute toxoplasmosis is suspected, a more extensive panel of serologic tests can be performed. In the United States, testing is available at the Toxoplasma Serology Laboratory at Palo Alto Medical Foundation (http://www.pamf.org/serology/clinicianguide.html).
Molecular Diagnostics Molecular approaches can directly detect T. gondii in biologic samples independent of the serologic response. Results obtained with PCR have suggested high sensitivity, specificity, and clinical utility in the diagnosis of TE, and PCR technology may be becoming more readily available in resource-poor settings. Real-time PCR is a promising technique that can provide quantitative results. Isolates can be genotyped and polymorphic sequences can be obtained, with consequent identification of the precise strain. Molecular epidemiologic studies with polymorphic markers have been useful in correlating clinical signs and symptoms of disease with different T. gondii genotypes.
The Immunocompetent Adult or Child For the patient who presents with lymphadenopathy only, a positive IgM titer is an indication of acute infection—and an indication for therapy, if clinically warranted (see “Treatment,” below). The serum IgM titer should be determined again in 3 weeks. An elevation in the IgG titer without an increase in the IgM titer suggests that infection is present but is not acute. If there is a borderline increase in either IgG or IgM, the titers should be reassessed in 3–4 weeks.
The Immunocompromised Host A presumptive clinical diagnosis of TE in patients with AIDS is based on clinical presentation, history of exposure (as evidenced by positive serology), and radiologic evaluation. To detect latent infection with T. gondii, HIV-infected persons should be tested for IgG antibody to Toxoplasma soon after HIV infection is diagnosed. When these criteria are used, the predictive value is as high as 80%. More than 97% of patients with AIDS and toxoplasmosis have IgG antibody to T. gondii in serum. IgM serum antibody usually is not detectable. Although IgG titers do not correlate with active infection, serologic evidence of infection virtually always precedes the development of TE. It is therefore important to determine the Toxoplasma antibody status of all patients infected with HIV. Antibody titers may range from negative to 1:1024 in patients with AIDS and TE. Fewer than 3% of patients have no demonstrable antibody to Toxoplasma at diagnosis of TE.
Patients with TE have focal or multifocal abnormalities demonstrable by CT or MRI. Neuroradiologic evaluation should include double-dose contrast CT of the head. By this test, single and frequently multiple contrast-enhancing lesions (<2 cm) may be identified. MRI usually demonstrates multiple lesions located in both hemispheres, with the basal ganglia and corticomedullary junction most commonly involved; MRI provides a more sensitive evaluation of the efficacy of therapy than does CT (Fig. 253-2). These findings are not pathognomonic of Toxoplasma infection, because 40% of CNS lymphomas are multifocal and 50% are ring-enhancing. For both MRI and CT scans, the rate of false-negative results is ~10%. The finding of a single lesion on an MRI scan increases the likelihood of primary CNS lymphoma (in which solitary lesions are four times more likely than in TE) and strengthens the argument for the performance of a brain biopsy. A therapeutic trial of anti-Toxoplasma medications is frequently used to assess the diagnosis. Treatment of presumptive TE with pyrimethamine plus sulfadiazine or clindamycin results in quantifiable clinical improvement in >50% of patients by day 3. Leucovorin is administered to prevent bone marrow toxicity. By day 7, >90% of treated patients show evidence of improvement. In contrast, if patients fail to respond or have lymphoma, clinical signs and symptoms worsen by day 7. Patients in this category require brain biopsy with or without a change in therapy. This procedure can now be performed by a stereotactic CT-guided method that reduces the potential for complications. Brain biopsy for T. gondii identifies organisms in 50–75% of cases. PCR amplification of CSF may also confirm toxoplasmosis or suggest alternative diagnoses (Table 253-1), such as progressive multifocal leukoencephalopathy (JC virus positive) or primary CNS lymphoma (Epstein-Barr virus positive).
CT and MRI with contrast are currently the standard diagnostic imaging tests for TE. As in other conditions, the radiologic response may lag behind the clinical response. Resolution of lesions may take from 3 weeks to 6 months. Some patients show clinical improvement despite worsening radiographic findings.
Congenital Infection The issue of concern when a pregnant woman has evidence of recent T. gondii infection is whether the fetus is infected. PCR analysis of the amniotic fluid for the B1 gene of T. gondii has replaced fetal blood sampling. Serologic diagnosis is based on the persistence of IgG antibody or a positive IgM titer after the first week of life (a time frame that excludes placental leak). The IgG determination should be repeated every 2 months. An increase in IgM beyond the first week of life is indicative of acute infection. Up to 25% of infected newborns may be seronegative and have normal routine physical examinations. Thus assessment of the eye and the brain, with ophthalmologic testing, CSF evaluation, and radiologic studies, is important in establishing the diagnosis.
Ocular Toxoplasmosis The serum antibody titer may not correlate with the presence of active lesions in the fundus, particularly in cases of congenital toxoplasmosis. In general, a positive IgG titer (measured in undiluted serum if necessary) in conjunction with typical lesions establishes the diagnosis. Antibody production in ocular fluids, expressed in terms of the Goldmann-Witmer coefficient, has been described for diagnosis of ocular disease but does not always correlate with PCR results. If lesions are atypical and the serum antibody titer is in the low-positive range, the diagnosis is presumptive. The parasitic antigen–specific polyclonal IgG assay as well as parasite-specific PCR may facilitate the diagnosis. Accordingly, the clinical diagnosis of ocular toxoplasmosis can be supported in 60–90% of cases by laboratory tests, depending on the time of anterior chamber puncture and the panel of antibody analyses used. In the remaining cases, the possibility of a falsely negative laboratory diagnosis or of an incorrect clinical diagnosis cannot be clarified further.
PREVENTION
All HIV-infected persons should be counseled regarding sources of Toxoplasma infection. The chances of primary infection with Toxoplasma can be reduced by not eating undercooked meat and by avoiding oocyst-contaminated material (i.e., a cat’s litter box). Specifically, lamb, beef, and pork should be cooked to an internal temperature of 165°–170°F; from a more practical perspective, meat cooked until it is no longer pink inside usually satisfies this requirement. Hands should be washed thoroughly after work in the garden, and all fruits and vegetables should be washed. Ingestion of raw shellfish is a risk factor for toxoplasmosis, given that the filter-feeding mechanism of clams and mussels concentrates oocysts.
If the patient owns a cat, the litter box should be cleaned or changed daily, preferably by an HIV-negative, nonpregnant person; alternatively, patients should wash their hands thoroughly after changing the litter box. Litter boxes should be changed daily if possible, as freshly excreted oocysts will not have sporulated and will not be infectious. Patients should be encouraged to keep their cats inside and not to adopt or handle stray cats. Cats should be fed only canned or dried commercial food or well-cooked table food, not raw or undercooked meats. Patients need not be advised to part with their cats or to have their cats tested for toxoplasmosis. Blood intended for transfusion into Toxoplasma-seronegative immunocompromised individuals should be screened for antibody to T. gondii. Although such serologic screening is not routinely performed, seronegative women should be screened for evidence of infection several times during pregnancy if they are exposed to environmental conditions that put them at risk for infection with T. gondii. HIV-positive individuals should adhere closely to these preventive measures.
254 |
Protozoal Intestinal Infections and Trichomoniasis |
PROTOZOAL INFECTIONS
GIARDIASIS
![]() Giardia intestinalis (also known as G. lamblia or G. duodenalis) is a cosmopolitan protozoal parasite that inhabits the small intestines of humans and other mammals. Giardiasis is one of the most common parasitic diseases in both developed and developing countries worldwide, causing both endemic and epidemic intestinal disease and diarrhea.
Giardia intestinalis (also known as G. lamblia or G. duodenalis) is a cosmopolitan protozoal parasite that inhabits the small intestines of humans and other mammals. Giardiasis is one of the most common parasitic diseases in both developed and developing countries worldwide, causing both endemic and epidemic intestinal disease and diarrhea.
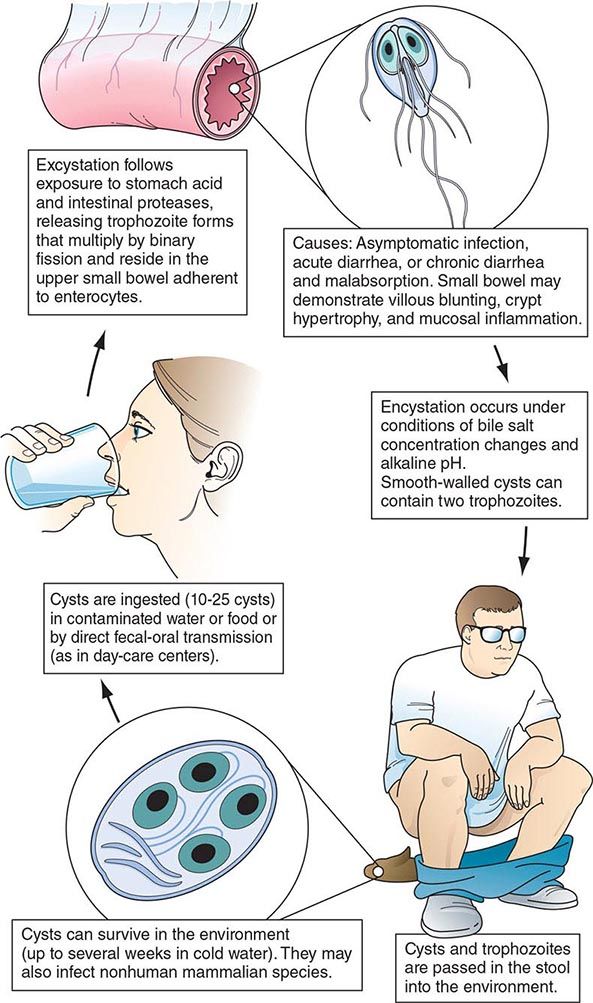
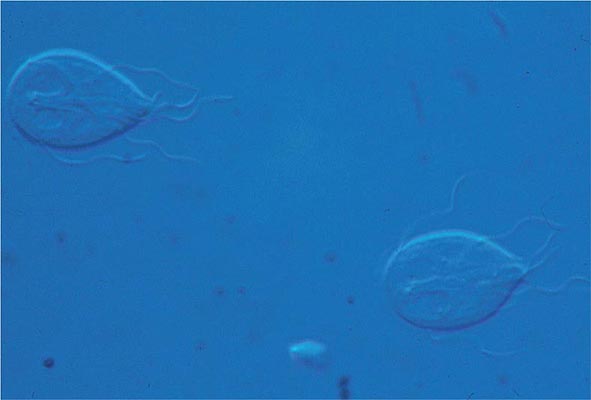
Life Cycle and Epidemiology (Fig. 254-1) Infection follows the ingestion of environmentally hardy cysts, which excyst in the small intestine, releasing flagellated trophozoites (Fig. 254-2) that multiply by binary fission. Giardia remains a pathogen of the proximal small bowel and does not disseminate hematogenously. Trophozoites remain free in the lumen or attach to the mucosal epithelium by means of a ventral sucking disk. As a trophozoite encounters altered conditions, it forms a morphologically distinct cyst, which is the stage of the parasite usually found in the feces. Trophozoites may be present and even predominate in loose or watery stools, but it is the resistant cyst that survives outside the body and is responsible for transmission. Cysts do not tolerate heating or desiccation, but they do remain viable for months in cold fresh water. The number of cysts excreted varies widely but can approach 107 per gram of stool.
FIGURE 254-1 Life cycle of Giardia. (Reprinted with permission from RL Guerrant et al [eds]: Tropical Infectious Diseases: Principles, Pathogens and Practice, 2nd ed, p 987. © 2006, with permission from Elsevier Science.)
FIGURE 254-2 Flagellated, binucleate Giardia trophozoites.
Ingestion of as few as 10 cysts is sufficient to cause infection in humans. Because cysts are infectious when excreted, person-to-person transmission occurs where fecal hygiene is poor. Giardiasis is especially prevalent in day-care centers; person-to-person spread also takes place in other institutional settings with poor fecal hygiene and during anal-oral contact. If food is contaminated with Giardia cysts after cooking or preparation, food-borne transmission can occur. Waterborne transmission accounts for episodic infections (e.g., in campers and travelers) and for major epidemics in metropolitan areas. Surface water, ranging from mountain streams to large municipal reservoirs, can become contaminated with fecally derived Giardia cysts. The efficacy of water as a means of transmission is enhanced by the small infectious inoculum of Giardia, the prolonged survival of cysts in cold water, and the resistance of cysts to killing by routine chlorination methods that are adequate for controlling bacteria. Viable cysts can be eradicated from water by either boiling or filtration.
![]() In the United States, Giardia (like Cryptosporidium; see below) is a common cause of waterborne epidemics of gastroenteritis. Giardia is common in developing countries, and infections may be acquired by travelers.
In the United States, Giardia (like Cryptosporidium; see below) is a common cause of waterborne epidemics of gastroenteritis. Giardia is common in developing countries, and infections may be acquired by travelers.
There are several recognized genotypes or assemblages of G. intestinalis. Human infections are due to assemblages A and B, whereas other assemblages are more common in other animals, including cats and dogs. Like beavers from reservoirs implicated in epidemics, dogs and cats have been found to be infected with assemblages A and B, an observation suggesting that these animals might be sources of human infection.
Giardiasis, like cryptosporidiosis, creates a significant economic burden because of the costs incurred in the installation of water filtration systems required to prevent waterborne epidemics, in the management of epidemics that involve large communities, and in the evaluation and treatment of endemic infections.
Pathophysiology The reasons that some, but not all, infected patients develop clinical manifestations and the mechanisms by which Giardia causes alterations in small-bowel function are largely unknown. Although trophozoites adhere to the epithelium, they are not invasive but may elicit apoptosis of enterocytes, epithelial barrier dysfunction, and epithelial cell malabsorption and secretion. Consequent lactose intolerance and, in a minority of infected adults and children, significant malabsorption are clinical signs of the loss of brush-border enzyme activities. In most infections, the morphology of the bowel is unaltered; however, in chronically infected, symptomatic patients, the histopathologic findings (including flattened villi) and the clinical manifestations at times resemble those of tropical sprue and gluten-sensitive enteropathy. The pathogenesis of diarrhea in giardiasis is not known.
The natural history of Giardia infection varies markedly. Infections may be aborted, transient, recurrent, or chronic. G. intestinalis parasites vary genotypically, and such variations might contribute to different courses of infection. Parasite as well as host factors may be important in determining the course of infection and disease. Both cellular and humoral responses develop in human infections, but their precise roles in disease pathogenesis and/or control of infection are unknown. Because patients with hypogammaglobulinemia suffer from prolonged, severe infections that are poorly responsive to treatment, humoral immune responses appear to be important. The greater susceptibilities of the young than of the old and of newly exposed persons than of chronically exposed populations suggest that at least partial protective immunity may develop.
Clinical Manifestations Disease manifestations of giardiasis range from asymptomatic carriage to fulminant diarrhea and malabsorption. Most infected persons are asymptomatic, but in epidemics the proportion of symptomatic cases may be higher. Symptoms may develop suddenly or gradually. In persons with acute giardiasis, symptoms develop after an incubation period that lasts at least 5–6 days and usually 1–3 weeks. Prominent early symptoms include diarrhea, abdominal pain, bloating, belching, flatus, nausea, and vomiting. Although diarrhea is common, upper intestinal manifestations such as nausea, vomiting, bloating, and abdominal pain may predominate. The duration of acute giardiasis is usually >1 week, although diarrhea often subsides. Individuals with chronic giardiasis may present with or without having experienced an antecedent acute symptomatic episode. Diarrhea is not necessarily prominent, but increased flatus, loose stools, sulfurous belching, and (in some instances) weight loss occur. Symptoms may be continual or episodic and may persist for years. Some persons who have relatively mild symptoms for long periods recognize the extent of their discomfort only in retrospect. Fever, the presence of blood and/or mucus in the stools, and other signs and symptoms of colitis are uncommon and suggest a different diagnosis or a concomitant illness. Symptoms tend to be intermittent yet recurring and gradually debilitating, in contrast with the acute disabling symptoms associated with many enteric bacterial infections. Because of the less severe illness and the propensity for chronic infections, patients may seek medical advice late in the course of the illness; however, disease can be severe, resulting in malabsorption, weight loss, growth retardation, and dehydration. A number of extraintestinal manifestations have been described, such as urticaria, anterior uveitis, and arthritis; whether these are caused by giardiasis or concomitant processes is unclear.
Giardiasis can be severe in patients with hypogammaglobulinemia and can complicate other preexisting intestinal diseases, such as that occurring in cystic fibrosis. In patients with AIDS, Giardia can cause enteric illness that is refractory to treatment.
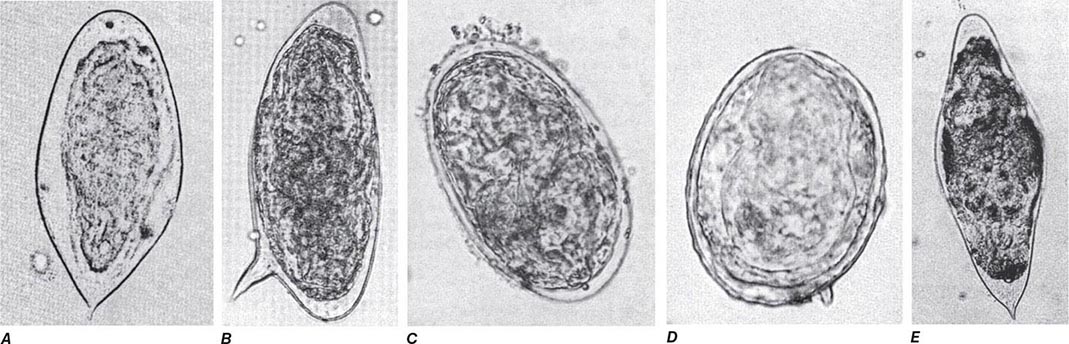
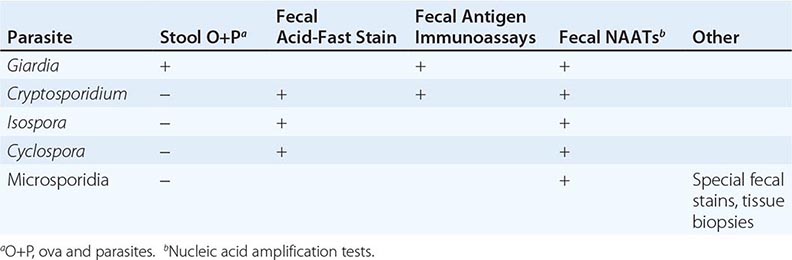
Diagnosis (Table 254-1) Giardiasis is diagnosed by detection of parasite antigens in the feces, by identification of cysts in the feces or of trophozoites in the feces or small intestines, or by nucleic acid amplification tests (NAATs). Cysts are oval, measure 8–12 μm × 7–10 μm, and characteristically contain four nuclei. Trophozoites are pear-shaped, dorsally convex, flattened parasites with two nuclei and four pairs of flagella (Fig. 254-2). The diagnosis is sometimes difficult to establish. Direct examination of fresh or properly preserved stools as well as concentration methods should be used. Because cyst excretion is variable and may be undetectable at times, repeated examination of stool, sampling of duodenal fluid, and biopsy of the small intestine may be required to detect the parasite. Tests for parasitic antigens in stool are at least as sensitive and specific as good microscopic examinations and are easier to perform. Newer NAATs are highly sensitive but are not always available for clinical use at present.
|
DIAGNOSIS OF INTESTINAL PROTOZOAL INFECTIONS |
Prevention Giardiasis can be prevented by consumption of uncontaminated food and water and by personal hygiene during the provision of care for infected children. Boiling or filtering potentially contaminated water prevents infection.
CRYPTOSPORIDIOSIS
![]() The coccidian parasite Cryptosporidium causes diarrheal disease that is self-limited in immunocompetent human hosts but can be severe in persons with AIDS or other forms of immunodeficiency. Two species of Cryptosporidium, C. hominis (especially in the United States, sub-Saharan Africa, and Asia) and C. parvum (in Europe), cause most human infections.
The coccidian parasite Cryptosporidium causes diarrheal disease that is self-limited in immunocompetent human hosts but can be severe in persons with AIDS or other forms of immunodeficiency. Two species of Cryptosporidium, C. hominis (especially in the United States, sub-Saharan Africa, and Asia) and C. parvum (in Europe), cause most human infections.
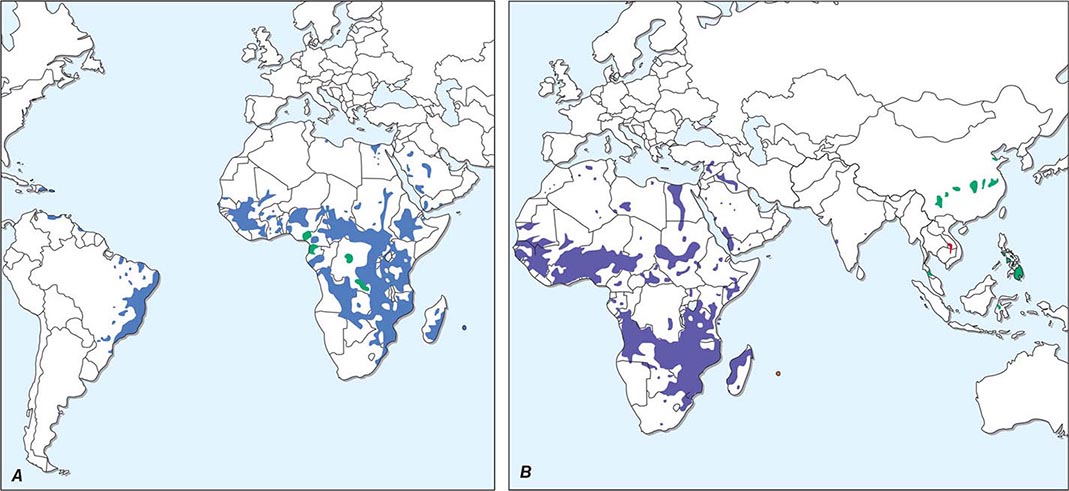
Life Cycle and Epidemiology Cryptosporidium species are widely distributed in the world. Cryptosporidiosis is acquired by the consumption of oocysts (50% infectious dose: ~132 C. parvum oocysts in nonimmune individuals), which excyst to liberate sporozoites that in turn enter and infect intestinal epithelial cells. The parasite’s further development involves both asexual and sexual cycles, which produce forms capable of infecting other epithelial cells and of generating oocysts that are passed in the feces. Cryptosporidium species infect a number of animals, and C. parvum can spread from infected animals to humans. Since oocysts are immediately infectious when passed in feces, person-to-person transmission takes place in day-care centers and among household contacts and medical providers. Waterborne transmission (especially that of C. hominis) accounts for infections in travelers and for common-source epidemics. Oocysts are quite hardy and resist killing by routine chlorination. Both drinking water and recreational water (e.g., pools, waterslides) have been increasingly recognized as sources of infection.
Pathophysiology Although intestinal epithelial cells harbor cryptosporidia in an intracellular vacuole, the means by which secretory diarrhea is elicited remain uncertain. No characteristic pathologic changes are found by biopsy. The distribution of infection can be spotty within the principal site of infection, the small bowel. Cryptosporidia are found in the pharynx, stomach, and large bowel of some patients and at times in the respiratory tract. Especially in patients with AIDS, involvement of the biliary tract can cause papillary stenosis, sclerosing cholangitis, or cholecystitis.
Clinical Manifestations Asymptomatic infections can occur in both immunocompetent and immunocompromised hosts. In immunocompetent persons, symptoms develop after an incubation period of ~1 week and consist principally of watery nonbloody diarrhea, sometimes in conjunction with abdominal pain, nausea, anorexia, fever, and/or weight loss. In these hosts, the illness usually subsides after 1–2 weeks. In contrast, in immunocompromised hosts (especially those with AIDS and CD4+ T cell counts <100/μL), diarrhea can be chronic, persistent, and remarkably profuse, causing clinically significant fluid and electrolyte depletion. Stool volumes may range from 1 to 25 L/d. Weight loss, wasting, and abdominal pain may be severe. Biliary tract involvement can manifest as mid-epigastric or right-upper-quadrant pain.
Diagnosis (Table 254-1) Evaluation starts with fecal examination for small oocysts, which are smaller (4–5 μm in diameter) than the fecal stages of most other parasites. Because conventional stool examination for ova and parasites (O+P) does not detect Cryptosporidium, specific testing must be requested. Detection is enhanced by evaluation of stools (obtained on multiple days) by several techniques, including modified acid-fast and direct immunofluorescent stains and enzyme immunoassays. Newer NAATs are being employed. Cryptosporidia can also be identified by light and electron microscopy at the apical surfaces of intestinal epithelium from biopsy specimens of the small bowel and, less frequently, the large bowel.
CYSTOISOSPORIASIS
The coccidian parasite Cystoisospora belli causes human intestinal disease. Infection is acquired by the consumption of oocysts, after which the parasite invades intestinal epithelial cells and undergoes both sexual and asexual cycles of development. Oocysts excreted in stool are not immediately infectious but must undergo further maturation.
Although C. belli infects many animals, little is known about the epidemiology or prevalence of this parasite in humans. It is most common in tropical and subtropical countries. Acute infections can begin abruptly with fever, abdominal pain, and watery nonbloody diarrhea and can last for weeks or months. In patients who have AIDS or are immunocompromised for other reasons, infections often are not self-limited but rather resemble cryptosporidiosis, with chronic, profuse watery diarrhea. Eosinophilia, which is not found in other enteric protozoan infections, may be detectable. The diagnosis (Table 254-1) is usually made by detection of the large (~25-μm) oocysts in stool by modified acid-fast staining. Oocyst excretion may be low-level and intermittent; if repeated stool examinations are unrevealing, sampling of duodenal contents by aspiration or small-bowel biopsy (often with electron microscopic examination) may be necessary. NAATs are promising newer diagnostic tools.
CYCLOSPORIASIS
![]() Cyclospora cayetanensis, a cause of diarrheal illness, is globally distributed: illness due to C. cayetanensis has been reported in the United States, Asia, Africa, Latin America, and Europe. The epidemiology of this parasite has not yet been fully defined, but waterborne transmission and food-borne transmission (e.g., by basil, sweet peas, and imported raspberries) have been recognized. The full spectrum of illness attributable to Cyclospora has not been delineated. Some infected patients may be without symptoms, but many have diarrhea, flulike symptoms, and flatulence and belching. The illness can be self-limited, can wax and wane, or, in many cases, can involve prolonged diarrhea, anorexia, and upper gastrointestinal symptoms, with sustained fatigue and weight loss in some instances. Diarrheal illness may persist for >1 month. Cyclospora can cause enteric illness in patients infected with HIV.
Cyclospora cayetanensis, a cause of diarrheal illness, is globally distributed: illness due to C. cayetanensis has been reported in the United States, Asia, Africa, Latin America, and Europe. The epidemiology of this parasite has not yet been fully defined, but waterborne transmission and food-borne transmission (e.g., by basil, sweet peas, and imported raspberries) have been recognized. The full spectrum of illness attributable to Cyclospora has not been delineated. Some infected patients may be without symptoms, but many have diarrhea, flulike symptoms, and flatulence and belching. The illness can be self-limited, can wax and wane, or, in many cases, can involve prolonged diarrhea, anorexia, and upper gastrointestinal symptoms, with sustained fatigue and weight loss in some instances. Diarrheal illness may persist for >1 month. Cyclospora can cause enteric illness in patients infected with HIV.
The parasite is detectable in epithelial cells of small-bowel biopsy samples and elicits secretory diarrhea by unknown means. The absence of fecal blood and leukocytes indicates that disease due to Cyclospora is not caused by destruction of the small-bowel mucosa. The diagnosis (Table 254-1) can be made by detection of spherical 8- to 10-μm oocysts in the stool, although routine stool O+P examinations are not sufficient. Specific fecal examinations must be requested to detect the oocysts, which are variably acid-fast and are fluorescent when viewed with ultraviolet light microscopy. Newer NAATs are proving to be sensitive. Cyclosporiasis should be considered in the differential diagnosis of prolonged diarrhea, with or without a history of travel by the patient to other countries.
MICROSPORIDIOSIS
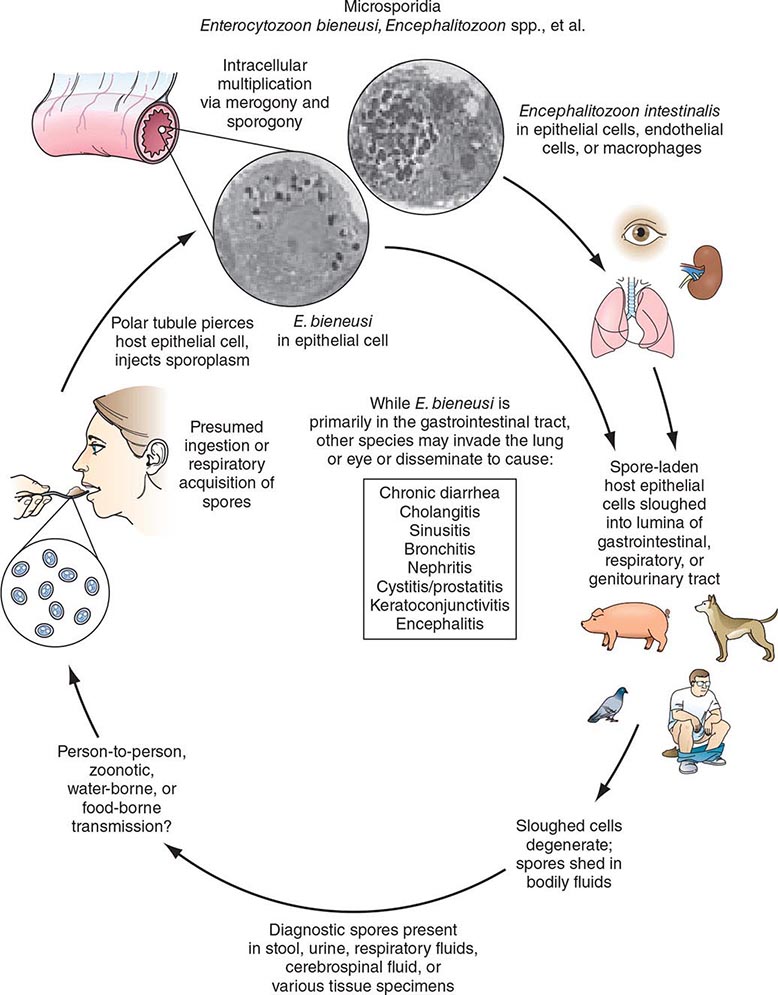
Microsporidia are obligate intracellular spore-forming protozoa that infect many animals and cause disease in humans, especially as opportunistic pathogens in AIDS. Microsporidia are members of a distinct phylum, Microspora, which contains dozens of genera and hundreds of species. The various microsporidia are differentiated by their developmental life cycles, ultrastructural features, and molecular taxonomy based on ribosomal RNA. The complex life cycles of the organisms result in the production of infectious spores (Fig. 254-3). Currently, eight genera of microsporidia—Encephalitozoon, Pleistophora, Nosema, Vittaforma, Trachipleistophora, Anncalia, Microsporidium, and Enterocytozoon—are recognized as causes of human disease. Although some microsporidia are probably prevalent causes of self-limited or asymptomatic infections in immunocompetent patients, little is known about how microsporidiosis is acquired.
FIGURE 254-3 Life cycle of microsporidia. (Reprinted with permission from RL Guerrant et al [eds]: Tropical Infectious Diseases: Principles, Pathogens and Practice, 2nd ed, p 1128. © 2006, with permission from Elsevier Science.)
Microsporidiosis is most common among patients with AIDS, less common among patients with other types of immunocompromise, and rare among immunocompetent hosts. In patients with AIDS, intestinal infections with Enterocytozoon bieneusi and Encephalitozoon (formerly Septata) intestinalis are recognized to contribute to chronic diarrhea and wasting; these infections had been found in 10–40% of patients with chronic diarrhea. Both organisms have been found in the biliary tracts of patients with cholecystitis. E. intestinalis may also disseminate to cause fever, diarrhea, sinusitis, cholangitis, and bronchiolitis. In patients with AIDS, Encephalitozoon hellem has caused superficial keratoconjunctivitis as well as sinusitis, respiratory tract disease, and disseminated infection. Myositis due to Pleistophora has been documented. Nosema, Vittaforma, and Microsporidium have caused stromal keratitis associated with trauma in immunocompetent patients.
Microsporidia are small gram-positive organisms with mature spores measuring 0.5–2 μm × 1–4 μm. Diagnosis of microsporidial infections in tissue often requires electron microscopy, although intracellular spores can be visualized by light microscopy with hematoxylin and eosin, Giemsa, or tissue Gram’s stain. For the diagnosis of intestinal microsporidiosis, modified trichrome or chromotrope 2R-based staining and Uvitex 2B or calcofluor fluorescent staining reveal spores in smears of feces or duodenal aspirates. Definitive therapies for microsporidial infections remain to be established. For superficial keratoconjunctivitis due to E. hellem, topical therapy with fumagillin suspension has shown promise (Chap. 246e). For enteric infections with E. bieneusi and E. intestinalis in HIV-infected patients, therapy with albendazole may be efficacious (Chap. 246e).
OTHER INTESTINAL PROTOZOA
![]() Balantidiasis Balantidium coli is a large ciliated protozoal parasite that can produce a spectrum of large-intestinal disease analogous to amebiasis. The parasite is widely distributed in the world. Since it infects pigs, cases in humans are more common where pigs are raised. Infective cysts can be transmitted from person to person and through water, but many cases are due to the ingestion of cysts derived from porcine feces in association with slaughtering, with use of pig feces for fertilizer, or with contamination of water supplies by pig feces.
Balantidiasis Balantidium coli is a large ciliated protozoal parasite that can produce a spectrum of large-intestinal disease analogous to amebiasis. The parasite is widely distributed in the world. Since it infects pigs, cases in humans are more common where pigs are raised. Infective cysts can be transmitted from person to person and through water, but many cases are due to the ingestion of cysts derived from porcine feces in association with slaughtering, with use of pig feces for fertilizer, or with contamination of water supplies by pig feces.
Ingested cysts liberate trophozoites, which reside and replicate in the large bowel. Many patients remain asymptomatic, but some have persisting intermittent diarrhea, and a few develop more fulminant dysentery. In symptomatic individuals, the pathology in the bowel—both gross and microscopic—is similar to that seen in amebiasis, with varying degrees of mucosal invasion, focal necrosis, and ulceration. Balantidiasis, unlike amebiasis, only rarely spreads hematogenously to other organs. The diagnosis is made by detection of the trophozoite stage in stool or sampled colonic tissue. Tetracycline (500 mg four times daily for 10 days) is an effective therapeutic agent.
Blastocystosis Blastocystis hominis remains an organism of uncertain pathogenicity. Some patients who pass B. hominis in their stools are asymptomatic, whereas others have diarrhea and associated intestinal symptoms. Diligent evaluation reveals other potential bacterial, viral, or protozoal causes of diarrhea in some but not all patients with symptoms. Because the pathogenicity of B. hominis is uncertain and because therapy for Blastocystis infection is neither specific nor uniformly effective, patients with prominent intestinal symptoms should be fully evaluated for other infectious causes of diarrhea. If diarrheal symptoms associated with Blastocystis are prominent, either metronidazole (750 mg thrice daily for 10 days) or TMP-SMX (160 mg/800 mg twice daily for 7 days) can be used.
Dientamoebiasis Dientamoeba fragilis is unique among intestinal protozoa in that it has a trophozoite stage but not a cyst stage. How trophozoites survive to transmit infection is not known. When symptoms develop in patients with D. fragilis infection, they are generally mild and include intermittent diarrhea, abdominal pain, and anorexia. The diagnosis is made by the detection of trophozoites in stool; the lability of these forms accounts for the greater yield when fecal samples are preserved immediately after collection. Since fecal excretion rates vary, examination of several samples obtained on alternate days increases the rate of detection. Iodoquinol (650 mg three times daily for 20 days) or paromomycin (25–35 mg/kg per day in three doses for 7 days) is appropriate for treatment.
TRICHOMONIASIS
Various species of trichomonads can be found in the mouth (in association with periodontitis) and occasionally in the gastrointestinal tract. Trichomonas vaginalis—one of the most prevalent protozoal parasites in the United States—is a pathogen of the genitourinary tract and a major cause of symptomatic vaginitis (Chap. 163).
Life Cycle and Epidemiology T. vaginalis is a pear-shaped, actively motile organism that measures about 10 × 7 μm, replicates by binary fission, and inhabits the lower genital tract of females and the urethra and prostate of males. In the United States, it accounts for ~3 million infections per year in women. While the organism can survive for a few hours in moist environments and could be acquired by direct contact, person-to-person venereal transmission accounts for virtually all cases of trichomoniasis. Its prevalence is greatest among persons with multiple sexual partners and among those with other sexually transmitted diseases (Chap. 163).
Clinical Manifestations Many men infected with T. vaginalis are asymptomatic, although some develop urethritis and a few have epididymitis or prostatitis. In contrast, infection in women, which has an incubation period of 5–28 days, is usually symptomatic and manifests with malodorous vaginal discharge (often yellow), vulvar erythema and itching, dysuria or urinary frequency (in 30–50% of patients), and dyspareunia. These manifestations, however, do not clearly distinguish trichomoniasis from other types of infectious vaginitis.
Diagnosis Detection of motile trichomonads by microscopic examination of wet mounts of vaginal or prostatic secretions has been the conventional means of diagnosis. Although this approach provides an immediate diagnosis, its sensitivity for the detection of T. vaginalis is only ~50–60% in routine evaluations of vaginal secretions. Direct immunofluorescent antibody staining is more sensitive (70–90%) than wet-mount examinations. T. vaginalis can be recovered from the urethra of both males and females and is detectable in males after prostatic massage. A new NAAT, APTIMA, is FDA approved and is highly sensitive and specific for urine and for endocervical and vaginal swabs from women.
SECTION 19 |
HELMINTHIC INFECTIONS |
255e |
Introduction to Helminthic Infections |
The word helminth is derived from the Greek helmins (“parasitic worm”). Helminthic worms are highly prevalent and, depending on the species, may exist as free-living organisms or as parasites of plant or animal hosts. The parasitic helminths have co-evolved with specific mammalian and other host species. Accordingly, most helminthic infections are restricted to nonhuman hosts, and only rarely do these zoonotic helminths accidentally cause human infections.
Helminthic parasites of humans belong to two phyla: Nemathelminthes, which includes nematodes (roundworms), and Platyhelminthes, which includes cestodes (tapeworms) and trematodes (flukes). Helminthic parasites of humans reside within the human body and hence are the cause of true infections. In contrast, parasites of other genera that reside only on mucocutaneous surfaces of humans (e.g., the parasites causing myiasis and scabies) are considered to represent infestations rather than infections.
Helminthic parasites differ substantially from protozoan parasites in several respects. First, protozoan parasites are unicellular organisms, whereas helminthic parasites are multicellular worms that possess differentiated organ systems. Second, helminthic parasites have complex life cycles that require sequential stages of development outside the human host. Thus, most helminths do not complete their replication within the human host; rather, they develop to a certain stage within the mammalian host and, as part of their obligatory life cycle, must mature further outside that host. During the “extra-human” stages of their life cycle, helminths exist either as free-living organisms or as parasites within another host species and thereafter mature into new developmental stages capable of infecting humans. Thus, with only two exceptions (Strongyloides stercoralis and Capillaria philippinensis, which are capable of internal reinfection), increases in the number of adult helminths (i.e., the “worm burden”) within the human host require repeated exogenous reinfections. In the case of protozoan parasites, a brief, even singular exposure (e.g., a single mosquito bite transmitting malaria) may lead rapidly to intense parasite loads and overwhelming infections; in contrast, for all but the two helminths noted above, increases in worm burden require multiple and usually ongoing exposures to infectious forms, such as ingestion of eggs of intestinal helminths or waterborne exposures to infectious cercariae of Schistosoma mansoni. This requirement is germane both to the consideration of helminthic infections in individuals and to ongoing global efforts to interrupt and/or minimize the acquisition of helminthic infections by humans.
Third, helminthic infections have a predilection toward stimulation of host immune responses that elicit eosinophilia within human tissues and blood. The many protozoan infections characteristically do not elicit eosinophilia in infected humans, with only three exceptions (two intestinal protozoan parasites, Cystoisospora belli and Dientamoeba fragilis, and tissue-borne Sarcocystis species). The magnitude of helminth-elicited eosinophilia tends to correlate with the extent of tissue invasion by larvae or adult helminths. For example, in several helminthic infections, including acute schistosomiasis (Katayama syndrome), paragonimiasis, and hookworm and Ascaris infections, eosinophilia is most pronounced during the early phases of infection, when migrations of infecting larvae and progression of subsequent developmental stages through the tissues are greatest. In established infections, local eosinophilia is often present around helminths in tissues, but blood eosinophilia may be intermittent, mild, or absent. In helminthic infections in which parasites are well contained within tissues (e.g., echinococcal cysts) or confined within the lumen of the intestinal tract (e.g., adult Ascaris or tapeworms), eosinophilia is usually absent.
NEMATODES
Nematodes are nonsegmented roundworms. Species of nematodes are remarkably diverse and abundant in nature. Among the many thousands of nematode species, few are parasites of humans. Most nematodes are free-living, and these species have variably evolved to survive in diverse ecologic niches, including saltwater, freshwater, or soil. The well-studied organism Caenorhabditis elegans is a free-living nematode. Nematodes can be either beneficial or deleterious parasites of plants. Parasitic nematodes have co-evolved with specific mammalian hosts and have no capacity to live their full life cycles in other hosts. Uncommonly, humans are exposed to infectious stages of nonhuman nematode parasites, and the resultant zoonotic nematode infections can elicit inflammatory and immune responses as larval forms migrate and die in the unsuitable human host. Examples include pulmonary coin lesions due to mosquito-transmitted infections with the dog heartworm Dirofilaria immitis; eosinophilic meningoencephalitis due to ingested eggs of the raccoon ascarid Baylisascaris procyonis; and eosinophilic meningitis due to ingestion of larvae of the rat lungworm Angiostrongylus cantonensis.
Nematode parasites of humans include worms that reside in the intestinal tract or localize in extraintestinal vascular or tissue sites. Roundworms are bisexual, with separate male and female forms (except for S. stercoralis, whose adult females are hermaphroditic in the human intestinal tract). Depending on the species, fertilized females release either larvae or eggs containing larvae. Nematodes have five developmental stages: an adult stage and four sequential larval stages. These parasites characteristically are surrounded by a durable outer cuticular layer. Nematodes have a nervous system; a muscular system, including muscle cells under the cuticle; and a developed intestinal tract, including an oral cavity and an elongated gut that ends in an anal pore. Adults may range in size from minute to >1 meter in length (with Dracunculus medinensis, for example, at the long end of this spectrum).
Humans acquire infections with nematode parasites by various routes, depending on the parasitic species. Ingestion of eggs passed in human feces is a major global health problem with many of the intestinal helminths (e.g., Ascaris lumbricoides). In other species, infecting larvae penetrate skin exposed to fecally contaminated soil (e.g., S. stercoralis) or traverse the skin after the bite of infected insect vectors (e.g., filariae). Some nematode infections are acquired by consumption of specific animal-derived foods (e.g., trichinellosis from raw or undercooked pork or wild carnivorous mammals). As noted above, only two nematodes, S. stercoralis and C. philippinensis, can internally reinfect humans.; thus, for all other nematodes, any increases in worm burden must be due to continued exogenous reinfections.
CESTODES
Tapeworms are the cestode parasites of humans. Adult tapeworms are elongated, segmented, hermaphroditic flatworms that reside in the intestinal lumen or, in their larval forms, may live in extraintestinal tissues. Tapeworms include a head (scolex) and a number of attached segments (proglottids). The worms attach to the intestinal tract via their scolices, which may possess suckers, hooks, or grooves. The scolex is the site of formation of new proglottids. Tapeworms do not have a functional gut tract; rather, each tapeworm segment passively and actively obtains nutrients through its specialized surface tegument. Mature proglottids possess both male and female sex organs, but insemination usually occurs between adjacent proglottids. Fertilized proglottids release eggs that are passed in the feces. When ingested by an intermediate host, an egg releases an oncosphere that penetrates the gut and develops further in tissues as a cysticercus. Humans acquire infection by ingesting animal tissues that contain cysticerci, and the resultant tapeworms develop and reside in the proximal small bowel (e.g., Taenia solium, T. saginata). Alternatively, if humans ingest eggs of these cestodes that have been passed in human or animal feces, oncospheres develop and can cause space-occupying extraintestinal cystic lesions in tissues; examples include cysticercosis due to T. solium and hydatid disease due to species of Echinococcus.
TREMATODES
Trematodes of medical importance include blood flukes, intestinal flukes, and tissue flukes. Adult flukes are often leaf-shaped flatworms. Oral and/or ventral suckers help adult flukes maintain their positions in situ. Flukes have an oral cavity but no distal anal pore. Nutrients are obtained both through their integument and by ingestion into the blind intestinal tract. Flukes are hermaphroditic except for blood flukes (schistosomes), which are bisexual. Eggs are passed in human feces (Fasciola, Fasciolopsis, Clonorchis, Schistosoma japonicum, S. mansoni), urine (S. haematobium), or sputum and feces (Paragonimus). Expelled eggs release miracidia—usually in water—that infect specific snail species. Within snails, parasites multiply and cercariae are released. Depending on the species, cercariae can penetrate the skin (schistosomes) or can develop into metacercariae that can be ingested with plants (e.g., watercress for Fasciola) or with fish (Clonorchis) or crabs (Paragonimus).
CONCLUSION
![]() Many of the so-called neglected tropical diseases are due to helminthic infections. The health impacts of many helminthic infections are varied and are based on the frequent need for repeated exposures to increase the worm burdens in infected humans. In global regions where exposures to specific helminths occur even in childhood (e.g., fecally derived intestinal nematodes, mosquito-transmitted filariae, or waterborne snail-transmitted schistosome infections), the morbidities in infected individuals can include nutritional, developmental, cognitive, and functional impairments. Ongoing global mass-treatment programs are currently aimed at diminishing the local prevalences of specific helminths and their consequent impacts on the health of local populations.
Many of the so-called neglected tropical diseases are due to helminthic infections. The health impacts of many helminthic infections are varied and are based on the frequent need for repeated exposures to increase the worm burdens in infected humans. In global regions where exposures to specific helminths occur even in childhood (e.g., fecally derived intestinal nematodes, mosquito-transmitted filariae, or waterborne snail-transmitted schistosome infections), the morbidities in infected individuals can include nutritional, developmental, cognitive, and functional impairments. Ongoing global mass-treatment programs are currently aimed at diminishing the local prevalences of specific helminths and their consequent impacts on the health of local populations.
256 |
Trichinellosis and Other Tissue Nematode Infections |
Nematodes are elongated, symmetric roundworms. Parasitic nematodes of medical significance may be broadly classified as either predominantly intestinal or tissue nematodes. This chapter covers the tissue nematodes that cause trichinellosis, visceral and ocular larva migrans, cutaneous larva migrans, cerebral angiostrongyliasis, and gnathostomiasis. All of these zoonotic infections result from incidental exposure to infectious nematodes. The clinical symptoms of these infections are due largely to invasive larval stages that (except in the case of Trichinella) do not reach maturity in humans.
TRICHINELLOSIS
Trichinellosis develops after the ingestion of meat containing cysts of Trichinella (e.g., pork or other meat from a carnivore). Although most infections are mild and asymptomatic, heavy infections can cause severe enteritis, periorbital edema, myositis, and (infrequently) death.
![]() Life Cycle and Epidemiology Eight species of Trichinella are recognized as causes of infection in humans. Two species are distributed worldwide: T. spiralis, which is found in a great variety of carnivorous and omnivorous animals, and T. pseudospiralis, which is found in mammals and birds. T. nativa is present in Arctic regions and infects bears; T. nelsoni is found in equatorial eastern Africa, where it is common among felid predators and scavengers such as hyenas and bush pigs; and T. britovi is found in Europe, western Africa, and western Asia among carnivores but not among domestic swine. T. murrelli is present in North American game animals.
Life Cycle and Epidemiology Eight species of Trichinella are recognized as causes of infection in humans. Two species are distributed worldwide: T. spiralis, which is found in a great variety of carnivorous and omnivorous animals, and T. pseudospiralis, which is found in mammals and birds. T. nativa is present in Arctic regions and infects bears; T. nelsoni is found in equatorial eastern Africa, where it is common among felid predators and scavengers such as hyenas and bush pigs; and T. britovi is found in Europe, western Africa, and western Asia among carnivores but not among domestic swine. T. murrelli is present in North American game animals.
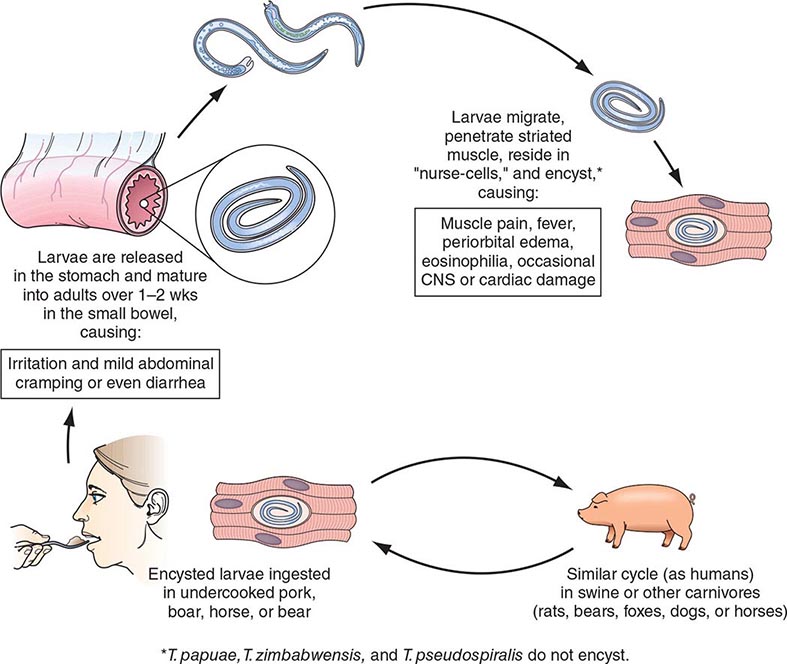
After human consumption of trichinous meat, encysted larvae are liberated by digestive acid and proteases (Fig. 256-1). The larvae invade the small-bowel mucosa and mature into adult worms. After ~1 week, female worms release newborn larvae that migrate via the circulation to striated muscle. The larvae of all species except T. pseudospiralis, T. papuae, and T. zimbabwensis then encyst by inducing a radical transformation in the muscle cell architecture. Although host immune responses may help to expel intestinal adult worms, they have few deleterious effects on muscle-dwelling larvae.
FIGURE 256-1 Life cycle of Trichinella spiralis (cosmopolitan); nelsoni (equatorial Africa); britovi (Europe, western Africa, western Asia); nativa (Arctic); murrelli (North America); papuae (Papua New Guinea); zimbabwensis (Tanzania); and pseudospiralis (cosmopolitan). CNS, central nervous system. (Reprinted from RL Guerrant et al [eds]: Tropical Infectious Diseases: Principles, Pathogens and Practice, 2nd ed, p 1218. © 2006, with permission from Elsevier Science.)
Human trichinellosis is often caused by the ingestion of infected pork products and thus can occur in almost any location where the meat of domestic or wild swine is eaten. Human trichinellosis may also be acquired from the meat of other animals, including dogs (in parts of Asia and Africa), horses (in Italy and France), and bears and walruses (in northern regions). Although cattle (being herbivores) are not natural hosts of Trichinella, beef has been implicated in outbreaks when contaminated or adulterated with trichinous pork. Laws that prohibit the feeding of uncooked garbage to pigs have greatly reduced the transmission of trichinellosis in the United States. About 12 cases of trichinellosis are reported annually in this country, but most mild cases probably remain undiagnosed. Recent U.S. and Canadian outbreaks have been attributable to consumption of wild game (especially bear meat) and, less frequently, of pork.
Pathogenesis and Clinical Features Clinical symptoms of trichinellosis arise from the successive phases of parasite enteric invasion, larval migration, and muscle encystment (Fig. 256-1). Most light infections (those with <10 larvae per gram of muscle) are asymptomatic, whereas heavy infections (which can involve >50 larvae per gram of muscle) can be life-threatening. Invasion of the gut by large numbers of parasites occasionally provokes diarrhea during the first week after infection. Abdominal pain, constipation, nausea, or vomiting also may be prominent.
Symptoms due to larval migration and muscle invasion begin to appear in the second week after infection. The migrating Trichinella larvae provoke a marked local and systemic hypersensitivity reaction, with fever and hypereosinophilia. Periorbital and facial edema is common, as are hemorrhages in the subconjunctivae, retina, and nail beds (“splinter” hemorrhages). A maculopapular rash, headache, cough, dyspnea, or dysphagia sometimes develops. Myocarditis with tachyarrhythmias or heart failure—and, less commonly, encephalitis or pneumonitis—may develop and accounts for most deaths of patients with trichinellosis.
Upon onset of larval encystment in muscle 2–3 weeks after infection, symptoms of myositis with myalgias, muscle edema, and weakness develop, usually overlapping with the inflammatory reactions to migrating larvae. The most commonly involved muscle groups include the extraocular muscles; the biceps; and the muscles of the jaw, neck, lower back, and diaphragm. Peaking ~3 weeks after infection, symptoms subside only gradually during a prolonged convalescence. Uncommon infections with T. pseudospiralis, whose larvae do not encapsulate in muscles, elicit prolonged polymyositis-like illness.
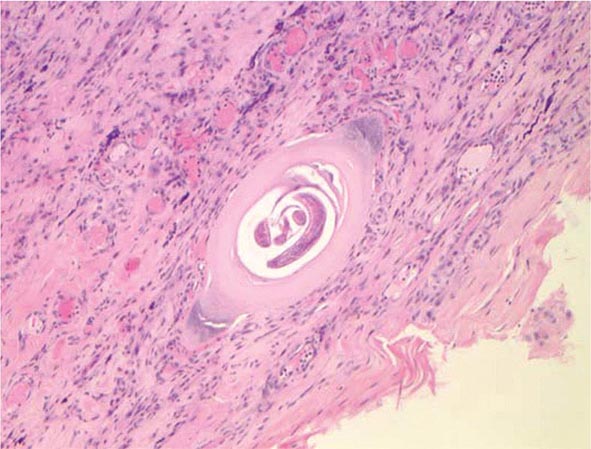
Laboratory Findings and Diagnosis Blood eosinophilia develops in >90% of patients with symptomatic trichinellosis and may peak at a level of >50% 2–4 weeks after infection. Serum levels of muscle enzymes, including creatine phosphokinase, are elevated in most symptomatic patients. Patients should be questioned thoroughly about their consumption of pork or wild animal meat and about illness in other individuals who ate the same meat. A presumptive clinical diagnosis can be based on fevers, eosinophilia, periorbital edema, and myalgias after a suspect meal. A rise in the titer of parasite-specific antibody, which usually does not occur until after the third week of infection, confirms the diagnosis. Alternatively, a definitive diagnosis requires surgical biopsy of at least 1 g of involved muscle; the yields are highest near tendon insertions. The fresh muscle tissue should be compressed between glass slides and examined microscopically (Fig. 256-2), because larvae may be missed by examination of routine histopathologic sections alone.
FIGURE 256-2 Trichinella larva encysted in a characteristic hyalinized capsule in striated muscle tissue. (Photo/Wadsworth Center, New York State Department of Health. Reprinted from MMWR 53:606, 2004; public domain.)
|
THERAPY FOR TISSUE NEMATODE INFECTIONS |
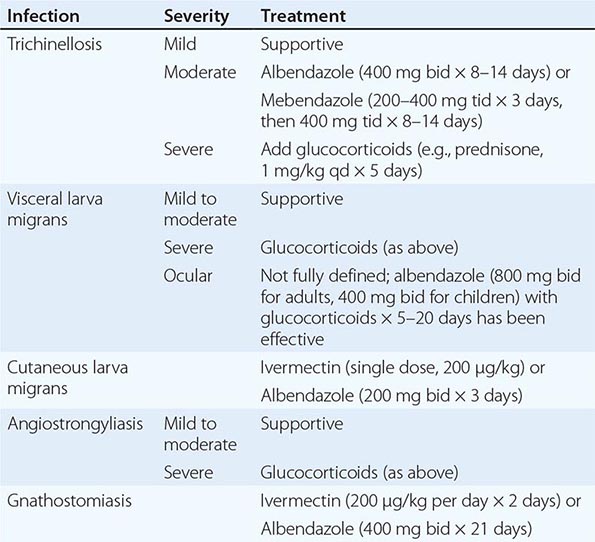
Prevention Larvae may be killed by cooking pork until it is no longer pink or by freezing it at –15°C for 3 weeks. However, Arctic T. nativa larvae in walrus or bear meat are relatively resistant and may remain viable despite freezing.
VISCERAL AND OCULAR LARVA MIGRANS
Visceral larva migrans is a syndrome caused by nematodes that are normally parasitic for nonhuman host species. In humans, these nematode larvae do not develop into adult worms but instead migrate through host tissues and elicit eosinophilic inflammation. The most common form of visceral larva migrans is toxocariasis due to larvae of the canine ascarid Toxocara canis; the syndrome is due less commonly to the feline ascarid T. cati and even less commonly to the pig ascarid Ascaris suum. Rare cases with eosinophilic meningoencephalitis have been caused by the raccoon ascarid Baylisascaris procyonis.
![]() Life Cycle and Epidemiology The canine roundworm T. canis is distributed among dogs worldwide. Ingestion of infective eggs by dogs is followed by liberation of Toxocara larvae, which penetrate the gut wall and migrate intravascularly into canine tissues, where most remain in a developmentally arrested state. During pregnancy, some larvae resume migration in bitches and infect puppies prenatally (through transplacental transmission) or after birth (through suckling). Thus, in lactating bitches and puppies, larvae return to the intestinal tract and develop into adult worms, which produce eggs that are released in the feces. Eggs must undergo embryonation over several weeks to become infectious. Humans acquire toxocariasis mainly by eating soil contaminated by puppy feces that contains infective T. canis eggs. Visceral larva migrans is most common among children who habitually eat dirt.
Life Cycle and Epidemiology The canine roundworm T. canis is distributed among dogs worldwide. Ingestion of infective eggs by dogs is followed by liberation of Toxocara larvae, which penetrate the gut wall and migrate intravascularly into canine tissues, where most remain in a developmentally arrested state. During pregnancy, some larvae resume migration in bitches and infect puppies prenatally (through transplacental transmission) or after birth (through suckling). Thus, in lactating bitches and puppies, larvae return to the intestinal tract and develop into adult worms, which produce eggs that are released in the feces. Eggs must undergo embryonation over several weeks to become infectious. Humans acquire toxocariasis mainly by eating soil contaminated by puppy feces that contains infective T. canis eggs. Visceral larva migrans is most common among children who habitually eat dirt.
Pathogenesis and Clinical Features Clinical disease most commonly afflicts preschool children. After humans ingest Toxocara eggs, the larvae hatch and penetrate the intestinal mucosa, from which they are carried by the circulation to a wide variety of organs and tissues. The larvae invade the liver, lungs, central nervous system (CNS), and other sites, provoking intense local eosinophilic granulomatous responses. The degree of clinical illness depends on larval number and tissue distribution, reinfection, and host immune responses. Most light infections are asymptomatic and may be manifest only by blood eosinophilia. Characteristic symptoms of visceral larva migrans include fever, malaise, anorexia and weight loss, cough, wheezing, and rashes. Hepatosplenomegaly is common. These features may be accompanied by extraordinary peripheral eosinophilia, which may approach 90%. Uncommonly, seizures or behavioral disorders develop. Rare deaths are due to severe neurologic, pneumonic, or myocardial involvement.
The ocular form of the larva migrans syndrome occurs when Toxocara larvae invade the eye. An eosinophilic granulomatous mass, most commonly in the posterior pole of the retina, develops around the entrapped larva. The retinal lesion can mimic retinoblastoma in appearance, and mistaken diagnosis of the latter condition can lead to unnecessary enucleation. The spectrum of eye involvement also includes endophthalmitis, uveitis, and chorioretinitis. Unilateral visual disturbances, strabismus, and eye pain are the most common presenting symptoms. In contrast to visceral larva migrans, ocular toxocariasis usually develops in older children or young adults with no history of pica; these patients seldom have eosinophilia or visceral manifestations.
Diagnosis In addition to eosinophilia, leukocytosis and hypergammaglobulinemia may be evident. Transient pulmonary infiltrates are apparent on chest x-rays of about one-half of patients with symptoms of pneumonitis. The clinical diagnosis can be confirmed by an enzyme-linked immunosorbent assay for toxocaral antibodies. Stool examination for parasite eggs is worthless in toxocariasis, since the larvae do not develop into egg-producing adults in humans.
CUTANEOUS LARVA MIGRANS
Cutaneous larva migrans (“creeping eruption”) is a serpiginous skin eruption caused by burrowing larvae of animal hookworms, usually the dog and cat hookworm Ancylostoma braziliense. The larvae hatch from eggs passed in dog and cat feces and mature in the soil. Humans become infected after skin contact with soil in areas frequented by dogs and cats, such as areas underneath house porches. Cutaneous larva migrans is prevalent among children and travelers in regions with warm humid climates, including the southeastern United States.
After larvae penetrate the skin, erythematous lesions form along the tortuous tracks of their migration through the dermal-epidermal junction; the larvae advance several centimeters in a day. The intensely pruritic lesions may occur anywhere on the body and can be numerous if the patient has lain on the ground. Vesicles and bullae may form later. The animal hookworm larvae do not mature in humans and, without treatment, will die after an interval ranging from weeks to a couple of months, with resolution of skin lesions. The diagnosis is made on clinical grounds. Skin biopsies only rarely detect diagnostic larvae. Symptoms can be alleviated by ivermectin or albendazole (Table 256-1).
ANGIOSTRONGYLIASIS
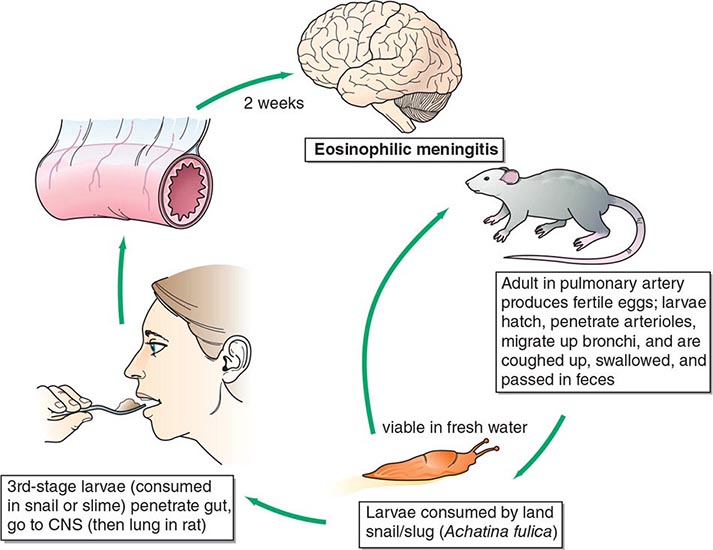
Angiostrongylus cantonensis, the rat lungworm, is the most common cause of human eosinophilic meningitis (Fig. 256-3).
FIGURE 256-3 Life cycle of Angiostrongylus cantonensis (rat lung worm), found in Southeast Asia, Pacific Islands, Cuba, Australia, Japan, China, Mauritius, and U.S. ports. CNS, central nervous system. (Reprinted from RL Guerrant et al [eds]: Tropical Infectious Diseases: Principles, Pathogens and Practice, 2nd ed, p 1225. © 2006, with permission from Elsevier Science.)
![]() Life Cycle and Epidemiology This infection occurs principally in Southeast Asia and the Pacific Basin but has spread to other areas of the world, including the Caribbean islands, countries in Central and South America, and the southern United States. A. cantonensis larvae produced by adult worms in the rat lung migrate to the gastrointestinal tract and are expelled with the feces. They develop into infective larvae in land snails and slugs. Humans acquire the infection by ingesting raw infected mollusks; vegetables contaminated by mollusk slime; or crabs, freshwater shrimp, and certain marine fish that have themselves eaten infected mollusks. The larvae then migrate to the brain.
Life Cycle and Epidemiology This infection occurs principally in Southeast Asia and the Pacific Basin but has spread to other areas of the world, including the Caribbean islands, countries in Central and South America, and the southern United States. A. cantonensis larvae produced by adult worms in the rat lung migrate to the gastrointestinal tract and are expelled with the feces. They develop into infective larvae in land snails and slugs. Humans acquire the infection by ingesting raw infected mollusks; vegetables contaminated by mollusk slime; or crabs, freshwater shrimp, and certain marine fish that have themselves eaten infected mollusks. The larvae then migrate to the brain.
Pathogenesis and Clinical Features The parasites eventually die in the CNS, but not before initiating pathologic consequences that, in heavy infections, can result in permanent neurologic sequelae or death. Migrating larvae cause marked local eosinophilic inflammation and hemorrhage, with subsequent necrosis and granuloma formation around dying worms. Clinical symptoms develop 2–35 days after the ingestion of larvae. Patients usually present with an insidious or abrupt excruciating frontal, occipital, or bitemporal headache. Neck stiffness, nausea and vomiting, and paresthesias are also common. Fever, cranial and extraocular nerve palsies, seizures, paralysis, and lethargy are uncommon.
Laboratory Findings Examination of cerebrospinal fluid (CSF) is mandatory in suspected cases and usually reveals an elevated opening pressure, a white blood cell count of 150–2000/μL, and an eosinophilic pleocytosis of >20%. The protein concentration is usually elevated and the glucose level normal. The larvae of A. cantonensis are only rarely seen in CSF. Peripheral-blood eosinophilia may be mild. The diagnosis is generally based on the clinical presentation of eosinophilic meningitis together with a compatible epidemiologic history.
GNATHOSTOMIASIS
Infection of human tissues with larvae of Gnathostoma spinigerum can cause eosinophilic meningoencephalitis, migratory cutaneous swellings, or invasive masses of the eye and visceral organs.
![]() Life Cycle and Epidemiology Human gnathostomiasis occurs in many countries and is notably endemic in Southeast Asia and parts of China and Japan. In nature, the mature adult worms parasitize the gastrointestinal tract of dogs and cats. First-stage larvae hatch from eggs passed into water and are ingested by Cyclops species (water fleas). Infective third-stage larvae develop in the flesh of many animal species (including fish, frogs, eels, snakes, chickens, and ducks) that have eaten either infected Cyclops or another infected second intermediate host. Humans typically acquire the infection by eating raw or undercooked fish or poultry. Raw fish dishes, such as som fak in Thailand and sashimi in Japan, account for many cases of human gnathostomiasis. Some cases in Thailand result from the local practice of applying frog or snake flesh as a poultice.
Life Cycle and Epidemiology Human gnathostomiasis occurs in many countries and is notably endemic in Southeast Asia and parts of China and Japan. In nature, the mature adult worms parasitize the gastrointestinal tract of dogs and cats. First-stage larvae hatch from eggs passed into water and are ingested by Cyclops species (water fleas). Infective third-stage larvae develop in the flesh of many animal species (including fish, frogs, eels, snakes, chickens, and ducks) that have eaten either infected Cyclops or another infected second intermediate host. Humans typically acquire the infection by eating raw or undercooked fish or poultry. Raw fish dishes, such as som fak in Thailand and sashimi in Japan, account for many cases of human gnathostomiasis. Some cases in Thailand result from the local practice of applying frog or snake flesh as a poultice.
Pathogenesis and Clinical Features Clinical symptoms are due to the aberrant migration of a single larva into cutaneous, visceral, neural, or ocular tissues. After invasion, larval migration may cause local inflammation, with pain, cough, or hematuria accompanied by fever and eosinophilia. Painful, itchy, migratory swellings may develop in the skin, particularly in the distal extremities or periorbital area. Cutaneous swellings usually last ~1 week but often recur intermittently over many years. Larval invasion of the eye can provoke a sight-threatening inflammatory response. Invasion of the CNS results in eosinophilic meningitis with myeloencephalitis, a serious complication due to ascending larval migration along a large nerve track. Patients characteristically present with agonizing radicular pain and paresthesias in the trunk or a limb, which are followed shortly by paraplegia. Cerebral involvement, with focal hemorrhages and tissue destruction, is often fatal.
Diagnosis and Treatment Cutaneous migratory swellings with marked peripheral eosinophilia, supported by an appropriate geographic and dietary history, generally constitute an adequate basis for a clinical diagnosis of gnathostomiasis. However, patients may present with ocular or cerebrospinal involvement without antecedent cutaneous swellings. In the latter case, eosinophilic pleocytosis is demonstrable (usually along with hemorrhagic or xanthochromic CSF), but worms are almost never recovered from CSF. Surgical removal of the parasite from subcutaneous or ocular tissue, though rarely feasible, is both diagnostic and therapeutic. Albendazole or ivermectin may be helpful (Table 256-1). At present, cerebrospinal involvement is managed with supportive measures and generally with a course of glucocorticoids. Gnathostomiasis can be prevented by adequate cooking of fish and poultry in endemic areas.
257 |
Intestinal Nematode Infections |
More than a billion persons worldwide are infected with one or more species of intestinal nematodes. Table 257-1 summarizes biologic and clinical features of infections due to the major intestinal parasitic nematodes. These parasites are most common in regions with poor fecal sanitation, particularly in resource-poor countries in the tropics and subtropics, but they have also been seen with increasing frequency among immigrants and refugees to resource-rich countries. Although nematode infections are not usually fatal, they contribute to malnutrition and diminished work capacity. It is interesting that these helminth infections may protect some individuals from allergic disease. Humans may on occasion be infected with nematode parasites that ordinarily infect animals; these zoonotic infections produce diseases such as trichostrongyliasis, anisakiasis, capillariasis, and abdominal angiostrongyliasis.
|
MAJOR HUMAN INTESTINAL PARASITIC NEMATODES |
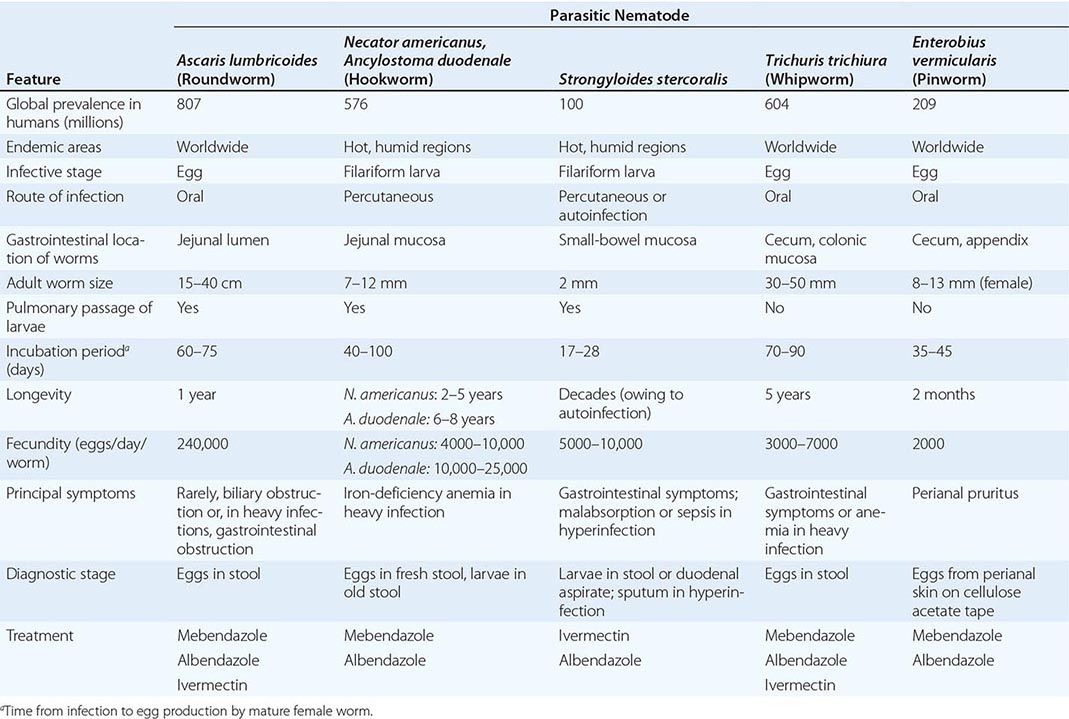
Intestinal nematodes are roundworms; they range in length from 1 mm to many centimeters when mature (Table 257-1). Their life cycles are complex and highly varied; some species, including Strongyloides stercoralis and Enterobius vermicularis, can be transmitted directly from person to person, while others, such as Ascaris lumbricoides, Necator americanus, and Ancylostoma duodenale, require a soil phase for development. Because most helminth parasites do not self-replicate, the acquisition of a heavy burden of adult worms requires repeated exposure to the parasite in its infectious stage, whether larva or egg. Hence, clinical disease, as opposed to asymptomatic infection, generally develops only with prolonged residence in an endemic area and is typically related to infection intensity. In persons with marginal nutrition, intestinal helminth infections may impair growth and development. Eosinophilia and elevated serum IgE levels are features of many helminth infections and, when unexplained, should always prompt a search for intestinal helminths. Significant protective immunity to intestinal nematodes appears not to develop in humans, although mechanisms of parasite immune evasion and host immune responses to these infections have not been elucidated in detail.
ASCARIASIS
A. lumbricoides is the largest intestinal nematode parasite of humans, reaching up to 40 cm in length. Most infected individuals have low worm burdens and are asymptomatic. Clinical disease arises from larval migration in the lungs or effects of the adult worms in the intestines.
Life Cycle Adult worms live in the lumen of the small intestine. Mature female Ascaris worms are extraordinarily fecund, each producing up to 240,000 eggs a day, which pass with the feces. Ascarid eggs, which are remarkably resistant to environmental stresses, become infective after several weeks of maturation in the soil and can remain infective for years. After infective eggs are swallowed, larvae hatched in the intestine invade the mucosa, migrate through the circulation to the lungs, break into the alveoli, ascend the bronchial tree, and return—through swallowing—to the small intestine, where they develop into adult worms. Between 2 and 3 months elapse between initial infection and egg production. Adult worms live for 1–2 years.
![]() Epidemiology Ascaris is widely distributed in tropical and subtropical regions as well as in other humid areas, including the rural southeastern United States. Transmission typically occurs through fecally contaminated soil and is due either to a lack of sanitary facilities or to the use of human feces as fertilizer. With their propensity for hand-to-mouth fecal carriage, younger children are most affected. Infection outside endemic areas, though uncommon, can occur when eggs on transported vegetables are ingested.
Epidemiology Ascaris is widely distributed in tropical and subtropical regions as well as in other humid areas, including the rural southeastern United States. Transmission typically occurs through fecally contaminated soil and is due either to a lack of sanitary facilities or to the use of human feces as fertilizer. With their propensity for hand-to-mouth fecal carriage, younger children are most affected. Infection outside endemic areas, though uncommon, can occur when eggs on transported vegetables are ingested.
Clinical Features During the lung phase of larval migration, ~9–12 days after egg ingestion, patients may develop an irritating nonproductive cough and burning substernal discomfort that is aggravated by coughing or deep inspiration. Dyspnea and blood-tinged sputum are less common. Fever is usually reported. Eosinophilia develops during this symptomatic phase and subsides slowly over weeks. Chest x-rays may reveal evidence of eosinophilic pneumonitis (Löffler’s syndrome), with rounded infiltrates a few millimeters to several centimeters in size. These infiltrates may be transient and intermittent, clearing after several weeks. Where there is seasonal transmission of the parasite, seasonal pneumonitis with eosinophilia may develop in previously infected and sensitized hosts.
In established infections, adult worms in the small intestine usually cause no symptoms. In heavy infections, particularly in children, a large bolus of entangled worms can cause pain and small-bowel obstruction, sometimes complicated by perforation, intussusception, or volvulus. Single worms may cause disease when they migrate into aberrant sites. A large worm can enter and occlude the biliary tree, causing biliary colic, cholecystitis, cholangitis, pancreatitis, or (rarely) intrahepatic abscesses. Migration of an adult worm up the esophagus can provoke coughing and oral expulsion of the worm. In highly endemic areas, intestinal and biliary ascariasis can rival acute appendicitis and gallstones as causes of surgical acute abdomen.
Laboratory Findings Most cases of ascariasis can be diagnosed by microscopic detection of characteristic Ascaris eggs (65 by 45 μm) in fecal samples. Occasionally, patients present after passing an adult worm—identifiable by its large size and smooth cream-colored surface—in the stool or, much less commonly, through the mouth or nose. During the early transpulmonary migratory phase, when eosinophilic pneumonitis occurs, larvae can be found in sputum or gastric aspirates before diagnostic eggs appear in the stool. The eosinophilia that is prominent during this early stage usually decreases to minimal levels in established infection. Adult worms may be visualized, occasionally serendipitously, on contrast studies of the gastrointestinal tract. A plain abdominal film may reveal masses of worms in gas-filled loops of bowel in patients with intestinal obstruction. Pancreaticobiliary worms can be detected by ultrasound and endoscopic retrograde cholangiopancreatography; the latter method also has been used to extract biliary Ascaris worms.
HOOKWORM
Two hookworm species (A. duodenale and N. americanus) are responsible for human infections. Most infected individuals are asymptomatic. Hookworm disease develops from a combination of factors—a heavy worm burden, a prolonged duration of infection, and an inadequate iron intake—and results in iron-deficiency anemia and, on occasion, hypoproteinemia.
Life Cycle Adult hookworms, which are ~1 cm long, use buccal teeth (Ancylostoma) or cutting plates (Necator) to attach to the small-bowel mucosa and suck blood (0.2 mL/d per Ancylostoma adult) and interstitial fluid. The adult hookworms produce thousands of eggs daily. The eggs are deposited with feces in soil, where rhabditiform larvae hatch and develop over a 1-week period into infectious filariform larvae. Infective larvae penetrate the skin and reach the lungs by way of the bloodstream. There they invade alveoli and ascend the airways before being swallowed and reaching the small intestine. The prepatent period from skin invasion to appearance of eggs in the feces is ~6–8 weeks, but it may be longer with A. duodenale. Larvae of A. duodenale, if swallowed, can survive and develop directly in the intestinal mucosa. Adult hookworms may survive over a decade but usually live ~6–8 years for A. duodenale and 2–5 years for N. americanus.
![]() Epidemiology A. duodenale is prevalent in southern Europe, North Africa, and northern Asia, and N. americanus is the predominant species in the Western Hemisphere and equatorial Africa. The two species overlap in many tropical regions, particularly Southeast Asia. In most areas, older children have the highest incidence and greatest intensity of hookworm infection. In rural areas where fields are fertilized with human feces, older working adults also may be heavily infected.
Epidemiology A. duodenale is prevalent in southern Europe, North Africa, and northern Asia, and N. americanus is the predominant species in the Western Hemisphere and equatorial Africa. The two species overlap in many tropical regions, particularly Southeast Asia. In most areas, older children have the highest incidence and greatest intensity of hookworm infection. In rural areas where fields are fertilized with human feces, older working adults also may be heavily infected.
Clinical Features Most hookworm infections are asymptomatic. Infective larvae may provoke pruritic maculopapular dermatitis (“ground itch”) at the site of skin penetration as well as serpiginous tracks of subcutaneous migration (similar to those of cutaneous larva migrans; Chap. 256) in previously sensitized hosts. Larvae migrating through the lungs occasionally cause mild transient pneumonitis, but this condition develops less frequently in hookworm infection than in ascariasis. In the early intestinal phase, infected persons may develop epigastric pain (often with postprandial accentuation), inflammatory diarrhea, or other abdominal symptoms accompanied by eosinophilia. The major consequence of chronic hookworm infection is iron deficiency. Symptoms are minimal if iron intake is adequate, but marginally nourished individuals develop symptoms of progressive iron-deficiency anemia and hypoproteinemia, including weakness and shortness of breath.
Laboratory Findings The diagnosis is established by the finding of characteristic 40- by 60-μm oval hookworm eggs in the feces. Stool-concentration procedures may be required to detect light infections. Eggs of the two species are indistinguishable by light microscopy. In a stool sample that is not fresh, the eggs may have hatched to release rhabditiform larvae, which need to be differentiated from those of S. stercoralis. Hypochromic microcytic anemia, occasionally with eosinophilia or hypoalbuminemia, is characteristic of hookworm disease.
![]() Ancylostoma caninum and Ancylostoma braziliense A. caninum, the canine hookworm, has been identified as a cause of human eosinophilic enteritis, especially in northeastern Australia. In this zoonotic infection, adult hookworms attach to the small intestine (where they may be visualized by endoscopy) and elicit abdominal pain and intense local eosinophilia. Treatment with mebendazole (100 mg twice daily for 3 days) or albendazole (400 mg once) or endoscopic removal is effective. Both of these animal hookworm species can cause cutaneous larva migrans (“creeping eruption”; Chap. 256).
Ancylostoma caninum and Ancylostoma braziliense A. caninum, the canine hookworm, has been identified as a cause of human eosinophilic enteritis, especially in northeastern Australia. In this zoonotic infection, adult hookworms attach to the small intestine (where they may be visualized by endoscopy) and elicit abdominal pain and intense local eosinophilia. Treatment with mebendazole (100 mg twice daily for 3 days) or albendazole (400 mg once) or endoscopic removal is effective. Both of these animal hookworm species can cause cutaneous larva migrans (“creeping eruption”; Chap. 256).
STRONGYLOIDIASIS
S. stercoralis is distinguished by its ability—unique among helminths (except for Capillaria; see below)—to replicate in the human host. This capacity permits ongoing cycles of autoinfection as infective larvae are internally produced. Strongyloidiasis can thus persist for decades without further exposure of the host to exogenous infective larvae. In immunocompromised hosts, large numbers of invasive Strongyloides larvae can disseminate widely and can be fatal.
Life Cycle In addition to a parasitic cycle of development, Strongyloides can undergo a free-living cycle of development in the soil (Fig. 257-1). This adaptability facilitates the parasite’s survival in the absence of mammalian hosts. Rhabditiform larvae passed in feces can transform into infectious filariform larvae either directly or after a free-living phase of development. Humans acquire strongyloidiasis when filariform larvae in fecally contaminated soil penetrate the skin or mucous membranes. The larvae then travel through the bloodstream to the lungs, where they break into the alveolar spaces, ascend the bronchial tree, are swallowed, and thereby reach the small intestine. There the larvae mature into adult worms that penetrate the mucosa of the proximal small bowel. The minute (2-mm-long) parasitic adult female worms reproduce by parthenogenesis; adult males do not exist. Eggs hatch in the intestinal mucosa, releasing rhabditiform larvae that migrate to the lumen and pass with the feces into soil. Alternatively, rhabditiform larvae in the bowel can develop directly into filariform larvae that penetrate the colonic wall or perianal skin and enter the circulation to repeat the migration that establishes ongoing internal reinfection. This autoinfection cycle allows strongyloidiasis to persist for decades.
FIGURE 257-1 Life cycle of Strongyloides stercoralis. (Adapted from Guerrant RL et al [eds]: Tropical Infectious Diseases: Principles, Pathogens and Practice, 2nd ed, p 1276. © 2006, with permission from Elsevier Science.)
![]() Epidemiology S. stercoralis is spottily distributed in tropical areas and other hot, humid regions and is particularly common in Southeast Asia, sub-Saharan Africa, and Brazil. In the United States, the parasite is endemic in parts of the Southeast and is found in immigrants, refugees, travelers, and military personnel who have lived in endemic areas.
Epidemiology S. stercoralis is spottily distributed in tropical areas and other hot, humid regions and is particularly common in Southeast Asia, sub-Saharan Africa, and Brazil. In the United States, the parasite is endemic in parts of the Southeast and is found in immigrants, refugees, travelers, and military personnel who have lived in endemic areas.
Clinical Features In uncomplicated strongyloidiasis, many patients are asymptomatic or have mild cutaneous and/or abdominal symptoms. Recurrent urticaria, often involving the buttocks and wrists, is the most common cutaneous manifestation. Migrating larvae can elicit a pathognomonic serpiginous eruption, larva currens (“running larva”). This pruritic, raised, erythematous lesion advances as rapidly as 10 cm/h along the course of larval migration. Adult parasites burrow into the duodenojejunal mucosa and can cause abdominal (usually midepigastric) pain, which resembles peptic ulcer pain except that it is aggravated by food ingestion. Nausea, diarrhea, gastrointestinal bleeding, mild chronic colitis, and weight loss can occur. Small-bowel obstruction may develop with early, heavy infection. Pulmonary symptoms are rare in uncomplicated strongyloidiasis. Eosinophilia is common, with levels fluctuating over time.
The ongoing autoinfection cycle of strongyloidiasis is normally constrained by unknown factors of the host’s immune system. Abrogation of host immunity, especially with glucocorticoid therapy and much less commonly with other immunosuppressive medications, leads to hyperinfection, with the generation of large numbers of filariform larvae. Colitis, enteritis, or malabsorption may develop. In disseminated strongyloidiasis, larvae may invade not only gastrointestinal tissues and the lungs but also the central nervous system, peritoneum, liver, and kidneys. Moreover, bacteremia may develop because of the passage of enteric flora through disrupted mucosal barriers. Gram-negative sepsis, pneumonia, or meningitis may complicate or dominate the clinical course. Eosinophilia is often absent in severely infected patients. Disseminated strongyloidiasis, particularly in patients with unsuspected infection who are given glucocorticoids, can be fatal. Strongyloidiasis is a frequent complication of infection with human T cell lymphotropic virus type 1, but disseminated strongyloidiasis is not common among patients infected with HIV-1.
Diagnosis In uncomplicated strongyloidiasis, the finding of rhabditiform larvae in feces is diagnostic. Rhabditiform larvae are ~250 μm long, with a short buccal cavity that distinguishes them from hookworm larvae. In uncomplicated infections, few larvae are passed and single stool examinations detect only about one-third of cases. Serial examinations and the use of the agar plate detection method improve the sensitivity of stool diagnosis. In uncomplicated strongyloidiasis (but not in hyperinfection), stool examinations may be repeatedly negative. Strongyloides larvae may also be found by sampling of the duodenojejunal contents by aspiration or biopsy. An enzyme-linked immunosorbent assay for serum antibodies to antigens of Strongyloides is a sensitive method for diagnosing uncomplicated infections. Such serologic testing should be performed for patients whose geographic histories indicate potential exposure, especially those who exhibit eosinophilia and/or are candidates for glucocorticoid treatment of other conditions. In disseminated strongyloidiasis, filariform larvae should be sought in stool as well as in samples obtained from sites of potential larval migration, including sputum, bronchoalveolar lavage fluid, or surgical drainage fluid.
TRICHURIASIS
![]() Most infections with Trichuris trichiura are asymptomatic, but heavy infections may cause gastrointestinal symptoms. Like the other soil-transmitted helminths, whipworm is distributed globally in the tropics and subtropics and is most common among poor children from resource-poor regions of the world.
Most infections with Trichuris trichiura are asymptomatic, but heavy infections may cause gastrointestinal symptoms. Like the other soil-transmitted helminths, whipworm is distributed globally in the tropics and subtropics and is most common among poor children from resource-poor regions of the world.
Life Cycle Adult Trichuris worms reside in the colon and cecum, the anterior portions threaded into the superficial mucosa. Thousands of eggs laid daily by adult female worms pass with the feces and mature in the soil. After ingestion, infective eggs hatch in the duodenum, releasing larvae that mature before migrating to the large bowel. The entire cycle takes ~3 months, and adult worms may live for several years.
Clinical Features Tissue reactions to Trichuris are mild. Most infected individuals have no symptoms or eosinophilia. Heavy infections may result in anemia, abdominal pain, anorexia, and bloody or mucoid diarrhea resembling inflammatory bowel disease. Rectal prolapse can result from massive infections in children, who often suffer from malnourishment and other diarrheal illnesses. Moderately heavy Trichuris burdens also contribute to growth retardation.
Diagnosis and Treatment The characteristic 50- by 20-μm lemon-shaped Trichuris eggs are readily detected on stool examination. Adult worms, which are 3–5 cm long, are occasionally seen on proctoscopy. Mebendazole (500 mg once) or albendazole (400 mg daily for 3 doses) is safe and moderately effective for treatment, with cure rates of 70–90%. Ivermectin (200 μg/kg daily for 3 doses) is also safe but is not quite as efficacious as the benzimidazoles.
ENTEROBIASIS (PINWORM)
![]() E. vermicularis is more common in temperate countries than in the tropics. In the United States, ~40 million persons are infected with pinworms, with a disproportionate number of cases among children.
E. vermicularis is more common in temperate countries than in the tropics. In the United States, ~40 million persons are infected with pinworms, with a disproportionate number of cases among children.
Life Cycle and Epidemiology Enterobius adult worms are ~1 cm long and dwell in the cecum. Gravid female worms migrate nocturnally into the perianal region and release up to 2000 immature eggs each. The eggs become infective within hours and are transmitted by hand-to-mouth passage. From ingested eggs, larvae hatch and mature into adults. This life cycle takes ~1 month, and adult worms survive for ~2 months. Self-infection results from perianal scratching and transport of infective eggs on the hands or under the nails to the mouth. Because of the ease of person-to-person spread, pinworm infections are common among family members.
Clinical Features Most pinworm infections are asymptomatic. Perianal pruritus is the cardinal symptom. The itching, which is often worse at night as a result of the nocturnal migration of the female worms, may lead to excoriation and bacterial superinfection. Heavy infections have been alleged to cause abdominal pain and weight loss. On rare occasions, pinworms invade the female genital tract, causing vulvovaginitis and pelvic or peritoneal granulomas. Eosinophilia is uncommon.
Diagnosis Since pinworm eggs are not released in feces, the diagnosis cannot be made by conventional fecal ova and parasite tests. Instead, eggs are detected by the application of clear cellulose acetate tape to the perianal region in the morning. After the tape is transferred to a slide, microscopic examination will detect pinworm eggs, which are oval, measure 55 by 25 μm, and are flattened along one side.
TRICHOSTRONGYLIASIS
![]() Trichostrongylus species, which are normally parasites of herbivorous animals, occasionally infect humans, particularly in Asia and Africa. Humans acquire the infection by accidentally ingesting Trichostrongylus larvae on contaminated leafy vegetables. The larvae do not migrate in humans but mature directly into adult worms in the small bowel. These worms ingest far less blood than hookworms; most infected persons are asymptomatic, but heavy infections may give rise to mild anemia and eosinophilia. In stool examinations, Trichostrongylus eggs resemble hookworm eggs but are larger (85 by 115 μm). Treatment consists of mebendazole or albendazole (Chap. 246e).
Trichostrongylus species, which are normally parasites of herbivorous animals, occasionally infect humans, particularly in Asia and Africa. Humans acquire the infection by accidentally ingesting Trichostrongylus larvae on contaminated leafy vegetables. The larvae do not migrate in humans but mature directly into adult worms in the small bowel. These worms ingest far less blood than hookworms; most infected persons are asymptomatic, but heavy infections may give rise to mild anemia and eosinophilia. In stool examinations, Trichostrongylus eggs resemble hookworm eggs but are larger (85 by 115 μm). Treatment consists of mebendazole or albendazole (Chap. 246e).
ANISAKIASIS
![]() Anisakiasis is a gastrointestinal infection caused by the accidental ingestion in uncooked saltwater fish of nematode larvae belonging to the family Anisakidae. The incidence of anisakiasis in the United States has increased as a result of the growing popularity of raw fish dishes. Most cases occur in Japan, the Netherlands, and Chile, where raw fish—sashimi, pickled green herring, and ceviche, respectively—are national culinary staples. Anisakid nematodes parasitize large sea mammals such as whales, dolphins, and seals. As part of a complex parasitic life cycle involving marine food chains, infectious larvae migrate to the musculature of a variety of fish. Both Anisakis simplex and Pseudoterranova decipiens have been implicated in human anisakiasis, but an identical gastric syndrome may be caused by the red larvae of eustrongylid parasites of fish-eating birds.
Anisakiasis is a gastrointestinal infection caused by the accidental ingestion in uncooked saltwater fish of nematode larvae belonging to the family Anisakidae. The incidence of anisakiasis in the United States has increased as a result of the growing popularity of raw fish dishes. Most cases occur in Japan, the Netherlands, and Chile, where raw fish—sashimi, pickled green herring, and ceviche, respectively—are national culinary staples. Anisakid nematodes parasitize large sea mammals such as whales, dolphins, and seals. As part of a complex parasitic life cycle involving marine food chains, infectious larvae migrate to the musculature of a variety of fish. Both Anisakis simplex and Pseudoterranova decipiens have been implicated in human anisakiasis, but an identical gastric syndrome may be caused by the red larvae of eustrongylid parasites of fish-eating birds.
When humans consume infected raw fish, live larvae may be coughed up within 48 h. Alternatively, larvae may immediately penetrate the mucosa of the stomach. Within hours, violent upper abdominal pain accompanied by nausea and occasionally vomiting ensues, mimicking an acute abdomen. The diagnosis can be established by direct visualization on upper endoscopy, outlining of the worm by contrast radiographic studies, or histopathologic examination of extracted tissue. Extraction of the burrowing larvae during endoscopy is curative. In addition, larvae may pass to the small bowel, where they penetrate the mucosa and provoke a vigorous eosinophilic granulomatous response. Symptoms may appear 1–2 weeks after the infective meal, with intermittent abdominal pain, diarrhea, nausea, and fever resembling the manifestations of Crohn’s disease. The diagnosis may be suggested by barium studies and confirmed by curative surgical resection of a granuloma in which the worm is embedded. Anisakid eggs are not found in the stool, since the larvae do not mature in humans. Serologic tests have been developed but are not widely available.
Anisakid larvae in saltwater fish are killed by cooking to 60°C, freezing at -20°C for 3 days, or commercial blast freezing, but usually not by salting, marinating, or cold smoking. No medical treatment is available; surgical or endoscopic removal should be undertaken.
CAPILLARIASIS
![]() Intestinal capillariasis is caused by ingestion of raw fish infected with Capillaria philippinensis. Subsequent autoinfection can lead to a severe wasting syndrome. The disease occurs in the Philippines and Thailand and, on occasion, elsewhere in Asia. The natural cycle of C. philippinensis involves fish from fresh and brackish water. When humans eat infected raw fish, the larvae mature in the intestine into adult worms, which produce invasive larvae that cause intestinal inflammation and villus loss. Capillariasis has an insidious onset with nonspecific abdominal pain and watery diarrhea. If untreated, progressive autoinfection can lead to protein-losing enteropathy, severe malabsorption, and ultimately death from cachexia, cardiac failure, or superinfection. The diagnosis is established by identification of the characteristic peanut-shaped (20- by 40-μm) eggs on stool examination. Severely ill patients require hospitalization and supportive therapy in addition to prolonged anthelmintic treatment with albendazole (200 mg twice daily for 10 days; Chap. 246e).
Intestinal capillariasis is caused by ingestion of raw fish infected with Capillaria philippinensis. Subsequent autoinfection can lead to a severe wasting syndrome. The disease occurs in the Philippines and Thailand and, on occasion, elsewhere in Asia. The natural cycle of C. philippinensis involves fish from fresh and brackish water. When humans eat infected raw fish, the larvae mature in the intestine into adult worms, which produce invasive larvae that cause intestinal inflammation and villus loss. Capillariasis has an insidious onset with nonspecific abdominal pain and watery diarrhea. If untreated, progressive autoinfection can lead to protein-losing enteropathy, severe malabsorption, and ultimately death from cachexia, cardiac failure, or superinfection. The diagnosis is established by identification of the characteristic peanut-shaped (20- by 40-μm) eggs on stool examination. Severely ill patients require hospitalization and supportive therapy in addition to prolonged anthelmintic treatment with albendazole (200 mg twice daily for 10 days; Chap. 246e).
ABDOMINAL ANGIOSTRONGYLIASIS
Abdominal angiostrongyliasis is found in Latin America and Africa. The zoonotic parasite Angiostrongylus costaricensis causes eosinophilic ileocolitis after the ingestion of contaminated vegetation. A. costaricensis normally parasitizes the cotton rat and other rodents, with slugs and snails serving as intermediate hosts. Humans become infected by accidentally ingesting infective larvae in mollusk slime deposited on fruits and vegetables; children are at highest risk. The larvae penetrate the gut wall and migrate to the mesenteric artery, where they develop into adult worms. Eggs deposited in the gut wall provoke an intense eosinophilic granulomatous reaction, and adult worms may cause mesenteric arteritis, thrombosis, or frank bowel infarction. Symptoms may mimic those of appendicitis, including abdominal pain and tenderness, fever, vomiting, and a palpable mass in the right iliac fossa. Leukocytosis and eosinophilia are prominent. CT with contrast medium typically shows inflamed bowel, often with concomitant obstruction, but a definitive diagnosis is usually made surgically with partial bowel resection. Pathologic study reveals a thickened bowel wall with eosinophilic granulomas surrounding the Angiostrongylus eggs. In nonsurgical cases, the diagnosis rests solely on clinical grounds because larvae and eggs cannot be detected in the stool. Medical therapy for abdominal angiostrongyliasis is of uncertain efficacy. Careful observation and surgical resection for severe symptoms are the mainstays of treatment.
258 |
Filarial and Related Infections |
Filarial worms are nematodes that dwell in the subcutaneous tissues and the lymphatics. Eight filarial species infect humans (Table 258-1); of these, four—Wuchereria bancrofti, Brugia malayi, Onchocerca volvulus, and Loa loa—are responsible for most serious filarial infections. Filarial parasites, which infect an estimated 170 million persons worldwide, are transmitted by specific species of mosquitoes or other arthropods and have a complex life cycle, including infective larval stages carried by insects and adult worms that reside in either lymphatic or subcutaneous tissues of humans. The offspring of adults are microfilariae, which, depending on their species, are 200–250 μm long and 5–7 μm wide, may or may not be enveloped in a loose sheath, and either circulate in the blood or migrate through the skin (Table 258-1). To complete the life cycle, microfilariae are ingested by the arthropod vector and develop over 1–2 weeks into new infective larvae. Adult worms live for many years, whereas microfilariae survive for 3–36 months. The bacterial endosymbiont Wolbachia has been found intracellularly in all stages of Brugia, Wuchereria, Mansonella, and Onchocerca species and has become a target for antifilarial chemotherapy.
|
CHARACTERISTICS OF THE FILARIAE |
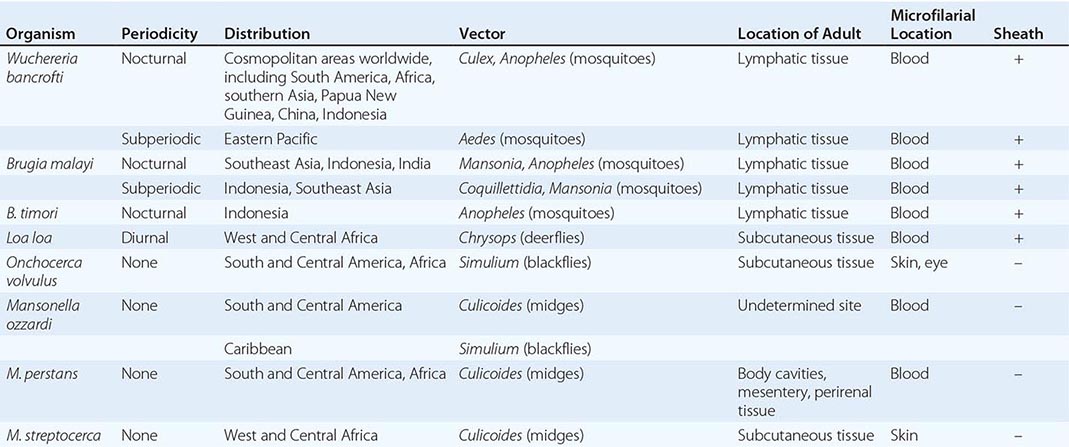
Usually, infection is established only with repeated, prolonged exposures to infective larvae. Since the clinical manifestations of filarial diseases develop relatively slowly, these infections should be considered to induce chronic infections with possible long-term debilitating effects. In terms of the nature, severity, and timing of clinical manifestations, patients with filarial infections who are native to endemic areas and have lifelong exposure may differ significantly from those who are travelers or who have recently moved to these areas. Characteristically, filarial disease is more acute and intense in newly exposed individuals than in natives of endemic areas.
LYMPHATIC FILARIASIS
Lymphatic filariasis is caused by W. bancrofti, B. malayi, or B. timori. The threadlike adult parasites reside in lymphatic channels or lymph nodes, where they may remain viable for more than two decades.
EPIDEMIOLOGY
![]() W. bancrofti, the most widely distributed filarial parasite of humans, affects an estimated 110 million people and is found throughout the tropics and subtropics, including Asia and the Pacific Islands, Africa, areas of South America, and the Caribbean basin. Humans are the only definitive host for the parasite. Generally, the subperiodic form is found only in the Pacific Islands; elsewhere, W. bancrofti is nocturnally periodic. Nocturnally periodic forms of microfilariae are scarce in peripheral blood by day and increase at night, whereas subperiodic forms are present in peripheral blood at all times and reach maximal levels in the afternoon. Natural vectors for W. bancrofti are Culex fatigans mosquitoes in urban settings and Anopheles or Aedes mosquitoes in rural areas.
W. bancrofti, the most widely distributed filarial parasite of humans, affects an estimated 110 million people and is found throughout the tropics and subtropics, including Asia and the Pacific Islands, Africa, areas of South America, and the Caribbean basin. Humans are the only definitive host for the parasite. Generally, the subperiodic form is found only in the Pacific Islands; elsewhere, W. bancrofti is nocturnally periodic. Nocturnally periodic forms of microfilariae are scarce in peripheral blood by day and increase at night, whereas subperiodic forms are present in peripheral blood at all times and reach maximal levels in the afternoon. Natural vectors for W. bancrofti are Culex fatigans mosquitoes in urban settings and Anopheles or Aedes mosquitoes in rural areas.
Brugian filariasis due to B. malayi occurs primarily in eastern India, Indonesia, Malaysia, and the Philippines. B. malayi also has two forms distinguished by the periodicity of microfilaremia. The more common nocturnal form is transmitted in areas of coastal rice fields, while the subperiodic form is found in forests. B. malayi naturally infects cats as well as humans. The distribution of B. timori is limited to the islands of southeastern Indonesia.
PATHOLOGY
The principal pathologic changes result from inflammatory damage to the lymphatics, which is typically caused by adult worms and not by microfilariae. Adult worms live in afferent lymphatics or sinuses of lymph nodes and cause lymphatic dilation and thickening of the vessel walls. The infiltration of plasma cells, eosinophils, and macrophages in and around the infected vessels, along with endothelial and connective tissue proliferation, leads to tortuosity of the lymphatics and damaged or incompetent lymph valves. Lymphedema and chronic stasis changes with hard or brawny edema develop in the overlying skin. These consequences of filarial infection are due both to the direct effects of the worms and to the host’s inflammatory response to the parasite. Inflammatory responses are believed to cause the granulomatous and proliferative processes that precede total lymphatic obstruction. It is thought that the lymphatic vessel remains patent as long as the worm remains viable and that the death of the worm leads to enhanced granulomatous reactions and fibrosis. Lymphatic obstruction results, and, despite collateralization, lymphatic function is compromised.
CLINICAL FEATURES
The most common presentations of the lymphatic filariases are asymptomatic (or subclinical) microfilaremia, hydrocele (Fig. 258-1), acute adenolymphangitis (ADL), and chronic lymphatic disease. In areas where W. bancrofti or B. malayi is endemic, the overwhelming majority of infected individuals have few overt clinical manifestations of filarial infection despite large numbers of circulating microfilariae in the peripheral blood. Although they may be clinically asymptomatic, virtually all persons with W. bancrofti or B. malayi microfilaremia have some degree of subclinical disease that includes microscopic hematuria and/or proteinuria, dilated (and tortuous) lymphatics (visualized by imaging), and—in men with W. bancrofti infection—scrotal lymphangiectasia (detectable by ultrasound). Despite these findings, the majority of individuals appear to remain clinically asymptomatic for years; in relatively few does the infection progress to either acute or chronic disease.
FIGURE 258-1 Hydrocele associated with Wuchereria bancrofti infection.
ADL is characterized by high fever, lymphatic inflammation (lymphangitis and lymphadenitis), and transient local edema. The lymphangitis is retrograde, extending peripherally from the lymph node draining the area where the adult parasites reside. Regional lymph nodes are often enlarged, and the entire lymphatic channel can become indurated and inflamed. Concomitant local thrombophlebitis can occur as well. In brugian filariasis, a single local abscess may form along the involved lymphatic tract and subsequently rupture to the surface. The lymphadenitis and lymphangitis can involve both the upper and lower extremities in both bancroftian and brugian filariasis, but involvement of the genital lymphatics occurs almost exclusively with W. bancrofti infection. This genital involvement can be manifested by funiculitis, epididymitis, and scrotal pain and tenderness. In endemic areas, another type of acute disease—dermatolymphangioadenitis (DLA)—is recognized as a syndrome that includes high fever, chills, myalgias, and headache. Edematous inflammatory plaques clearly demarcated from normal skin are seen. Vesicles, ulcers, and hyperpigmentation may also be noted. There is often a history of trauma, burns, irradiation, insect bites, punctiform lesions, or chemical injury. Entry lesions, especially in the interdigital area, are common. DLA is often diagnosed as cellulitis.
If lymphatic damage progresses, transient lymphedema can develop into lymphatic obstruction and the permanent changes associated with elephantiasis (Fig. 258-2). Brawny edema follows early pitting edema, the subcutaneous tissues thicken, and hyperkeratosis occurs. Fissuring of the skin develops, as do hyperplastic changes. Superinfection of these poorly vascularized tissues becomes a problem. In bancroftian filariasis, in which genital involvement is common, hydroceles may develop (Fig. 258-1); in advanced stages, this condition may evolve into scrotal lymphedema and scrotal elephantiasis. Furthermore, if there is obstruction of the retroperitoneal lymphatics, increased renal lymphatic pressure leads to rupture of the renal lymphatics and the development of chyluria, which is usually intermittent and most prominent in the morning.
FIGURE 258-2 Elephantiasis of the lower extremity associated with Wuchereria bancrofti infection.
The clinical manifestations of filarial infections in travelers or transmigrants who have recently entered an endemic region are distinctive. Given a sufficient number of bites by infected vectors, usually over a 3- to 6-month period, recently exposed patients can develop acute lymphatic or scrotal inflammation with or without urticaria and localized angioedema. Lymphadenitis of epitrochlear, axillary, femoral, or inguinal lymph nodes is often followed by retrogradely evolving lymphangitis. Acute attacks are short-lived and are not usually accompanied by fever. With prolonged exposure to infected mosquitoes, these attacks, if untreated, become more severe and lead to permanent lymphatic inflammation and obstruction.
DIAGNOSIS
A definitive diagnosis can be made only by detection of the parasites and hence can be difficult. Adult worms localized in lymphatic vessels or nodes are largely inaccessible. Microfilariae can be found in blood, in hydrocele fluid, or (occasionally) in other body fluids. Such fluids can be examined microscopically, either directly or—for greater sensitivity—after concentration of the parasites by the passage of fluid through a polycarbonate cylindrical-pore filter (pore size, 3 μm) or by the centrifugation of fluid fixed in 2% formalin (Knott’s concentration technique). The timing of blood collection is critical and should be based on the periodicity of the microfilariae in the endemic region involved. Many infected individuals do not have microfilaremia, and definitive diagnosis in such cases can be difficult. Assays for circulating antigens of W. bancrofti permit the diagnosis of microfilaremic and cryptic (amicrofilaremic) infection. Two tests are commercially available: an enzyme-linked immunosorbent assay (ELISA) and a rapid-format immunochromatographic card test. Both assays have sensitivities of 93–100% and specificities approaching 100%. There are currently no tests for circulating antigens in brugian filariasis.
Polymerase chain reaction (PCR)–based assays for DNA of W. bancrofti and B. malayi in blood have been developed. A number of studies indicate that the sensitivity of this diagnostic method is equivalent to or greater than that of parasitologic methods.
In cases of suspected lymphatic filariasis, examination of the scrotum, the lymph nodes, or (in female patients) the breast by means of high-frequency ultrasound in conjunction with Doppler techniques may result in the identification of motile adult worms within dilated lymphatics. Worms may be visualized in the lymphatics of the spermatic cord in up to 80% of men infected with W. bancrofti. Live adult worms have a distinctive pattern of movement within the lymphatic vessels (termed the filarial dance sign). Radionuclide lymphoscintigraphic imaging of the limbs reliably demonstrates widespread lymphatic abnormalities in both subclinical microfilaremic persons and those with clinical manifestations of lymphatic pathology. Although of potential utility in the delineation of anatomic changes associated with infection, lymphoscintigraphy is unlikely to assume primacy in the diagnostic evaluation of individuals with suspected infection; it is principally a research tool, although it has been used more widely for assessment of lymphedema of any cause. Eosinophilia and elevated serum concentrations of IgE and antifilarial antibody support the diagnosis of lymphatic filariasis. There is, however, extensive cross-reactivity between filarial antigens and antigens of other helminths, including the common intestinal roundworms; thus, interpretations of serologic findings can be difficult. In addition, residents of endemic areas can become sensitized to filarial antigens (and thus be serologically positive) through exposure to infected mosquitoes without having patent filarial infections.
The ADL associated with lymphatic filariasis must be distinguished from thrombophlebitis, infection, and trauma. Retrograde evolution is a characteristic feature that helps distinguish filarial lymphangitis from ascending bacterial lymphangitis. Chronic filarial lymphedema must also be distinguished from the lymphedema of malignancy, postoperative scarring, trauma, chronic edematous states, and congenital lymphatic system abnormalities.
PREVENTION AND CONTROL
To protect themselves against filarial infection, individuals must avoid contact with infected mosquitoes by using personal protective measures, including bed nets, particularly those impregnated with insecticides such as permethrin. Community-based intervention is the current approach to elimination of lymphatic filariasis as a public health problem. The underlying tenet of this approach is that mass annual distribution of antimicrofilarial chemotherapy—albendazole with either DEC (for all areas except those where onchocerciasis is coendemic; see section on onchocerciasis treatment, below) or ivermectin—will profoundly suppress microfilaremia. If the suppression is sustained, then transmission can be interrupted.
![]() Created by the World Health Organization in 1997, the Global Programme to Eliminate Lymphatic Filariasis is based on mass administration of single annual doses of DEC plus albendazole in non-African regions and of albendazole plus ivermectin in Africa. Available information from late 2013 indicated that more than 792 million persons in 53 countries had thus far participated. Not only has lymphatic filariasis been eliminated in some defined areas, but collateral benefits—avoidance of disability and treatment of intestinal helminths and other conditions (e.g., scabies and louse infestation)—have also been noted. The strategy of the global program is being refined, and attempts are being made to integrate this effort with other mass-treatment strategies (e.g., deworming programs, malaria control, and trachoma control) in an integrated control strategy.
Created by the World Health Organization in 1997, the Global Programme to Eliminate Lymphatic Filariasis is based on mass administration of single annual doses of DEC plus albendazole in non-African regions and of albendazole plus ivermectin in Africa. Available information from late 2013 indicated that more than 792 million persons in 53 countries had thus far participated. Not only has lymphatic filariasis been eliminated in some defined areas, but collateral benefits—avoidance of disability and treatment of intestinal helminths and other conditions (e.g., scabies and louse infestation)—have also been noted. The strategy of the global program is being refined, and attempts are being made to integrate this effort with other mass-treatment strategies (e.g., deworming programs, malaria control, and trachoma control) in an integrated control strategy.
TROPICAL PULMONARY EOSINOPHILIA
![]() Tropical pulmonary eosinophilia (TPE) is a distinct syndrome that develops in some individuals infected with the lymphatic-dwelling filarial species. This syndrome affects males and females in a ratio of 4:1, often during the third decade of life. The majority of cases have been reported from India, Pakistan, Sri Lanka, Brazil, Guyana, and Southeast Asia.
Tropical pulmonary eosinophilia (TPE) is a distinct syndrome that develops in some individuals infected with the lymphatic-dwelling filarial species. This syndrome affects males and females in a ratio of 4:1, often during the third decade of life. The majority of cases have been reported from India, Pakistan, Sri Lanka, Brazil, Guyana, and Southeast Asia.
Clinical Features The main features include a history of residence in filarial-endemic regions, paroxysmal cough and wheezing (usually nocturnal and probably related to the nocturnal periodicity of microfilariae), weight loss, low-grade fever, lymphadenopathy, and pronounced blood eosinophilia (>3000 eosinophils/μL). Chest x-rays or CT scans may be normal but generally show increased bronchovascular markings. Diffuse miliary lesions or mottled opacities may be present in the middle and lower lung fields. Tests of pulmonary function show restrictive abnormalities in most cases and obstructive defects in half. Characteristically, total serum IgE levels (4–40 KIU/mL) and antifilarial antibody titers are markedly elevated.
Pathology In TPE, microfilariae and parasite antigens are rapidly cleared from the bloodstream by the lungs. The clinical symptoms result from allergic and inflammatory reactions elicited by the cleared parasites. In some patients, trapping of microfilariae in other reticuloendothelial organs can cause hepatomegaly, splenomegaly, or lymphadenopathy. A prominent, eosinophil-enriched, intraalveolar infiltrate is often reported, and with it comes the release of cytotoxic proinflammatory eosinophil granule proteins that may mediate some of the pathology seen in TPE. In the absence of successful treatment, interstitial fibrosis can lead to progressive pulmonary damage.
Differential Diagnosis TPE must be distinguished from asthma, Löffler’s syndrome, allergic bronchopulmonary aspergillosis, allergic granulomatosis with angiitis (Churg-Strauss syndrome), the systemic vasculitides (most notably, periarteritis nodosa and granulomatosis with polyangiitis), chronic eosinophilic pneumonia, and the idiopathic hypereosinophilic syndrome.
ONCHOCERCIASIS
EPIDEMIOLOGY
![]() Onchocerciasis (“river blindness”) is caused by the filarial nematode O. volvulus, which infects an estimated 37 million individuals in 35 countries worldwide. The majority of individuals infected with O. volvulus live in the equatorial region of Africa extending from the Atlantic coast to the Red Sea. In the Americas, isolated foci were identified in Mexico, Guatemala, Colombia, Ecuador, Venezuela, and Brazil. The infection is also found in Yemen.
Onchocerciasis (“river blindness”) is caused by the filarial nematode O. volvulus, which infects an estimated 37 million individuals in 35 countries worldwide. The majority of individuals infected with O. volvulus live in the equatorial region of Africa extending from the Atlantic coast to the Red Sea. In the Americas, isolated foci were identified in Mexico, Guatemala, Colombia, Ecuador, Venezuela, and Brazil. The infection is also found in Yemen.
ETIOLOGY
Infection in humans begins with the deposition of infective larvae on the skin by the bite of an infected blackfly. The larvae develop into adults, which are typically found in subcutaneous nodules. About 7 months to 3 years after infection, the gravid female releases microfilariae that migrate out of the nodule and throughout the tissues, concentrating in the dermis. Infection is transmitted to other persons when a female fly ingests microfilariae from the host’s skin and these microfilariae then develop into infective larvae. Adult O. volvulus females and males are ~40–60 cm and ~3–6 cm in length, respectively. The life span of adults can be as long as 18 years, with an average of ~9 years. Because the blackfly vector breeds along free-flowing rivers and streams (particularly in rapids) and generally restricts its flight to an area within several kilometers of these breeding sites, both biting and disease transmission are most intense in these locations.
PATHOLOGY
Onchocerciasis primarily affects the skin, eyes, and lymph nodes. In contrast to the pathology in lymphatic filariasis, the damage in onchocerciasis is elicited by microfilariae and not by adult parasites. In the skin, there are mild but chronic inflammatory changes that can result in loss of elastic fibers, atrophy, and fibrosis. The subcutaneous nodules (onchocercomata) consist primarily of fibrous tissues surrounding the adult worm, often with a peripheral ring of inflammatory cells (characterized as lymphatic in origin) surrounded by an endothelial layer. In the eye, neovascularization and corneal scarring lead to corneal opacities and blindness. Inflammation in the anterior and posterior chambers frequently results in anterior uveitis, chorioretinitis, and optic atrophy. Although punctate opacities are due to an inflammatory reaction surrounding dead or dying microfilariae, the pathogenesis of most manifestations of onchocerciasis is still unclear.
CLINICAL FEATURES
Skin Pruritus and rash are the most common manifestations of onchocerciasis. The pruritus can be incapacitating; the rash is typically a papular eruption (Fig. 258-3) that is generalized rather than localized to a particular region of the body. Long-term infection results in exaggerated and premature wrinkling of the skin, loss of elastic fibers, and epidermal atrophy that can lead to loose, redundant skin and hypo- or hyperpigmentation. Localized eczematoid dermatitis can cause hyperkeratosis, scaling, and pigmentary changes. In an immunologically hyperreactive form of onchodermatitis (commonly termed sowdah or localized onchodermatitis), the affected skin darkens as a consequence of the profound inflammation that occurs as microfilariae in the skin are cleared.
FIGURE 258-3 Papular eruption as a consequence of onchocerciasis.
Onchocercomata These subcutaneous nodules, which can be palpable and/or visible, contain the adult worm. In African patients, they are common over the coccyx and sacrum, the trochanter of the femur, the lateral anterior crest, and other bony prominences; in patients from South and Central America, nodules tend to develop preferentially in the upper part of the body, particularly on the head, neck, and shoulders. Nodules vary in size and characteristically are firm and not tender. It has been estimated that, for every palpable nodule, there are four deeper nonpalpable ones.
Ocular Tissue Visual impairment is the most serious complication of onchocerciasis and usually affects only those persons with moderate or heavy infections. Lesions may develop in all parts of the eye. The most common early finding is conjunctivitis with photophobia. Punctate keratitis—acute inflammatory reactions surrounding dying microfilariae and manifested as “snowflake” opacities—is common among younger patients and resolves without apparent complications.
![]() Sclerosing keratitis occurs in 1–5% of infected persons and is the leading cause of onchocercal blindness in Africa. Anterior uveitis and iridocyclitis develop in ~5% of infected persons in Africa. In Latin America, complications of the anterior uveal tract (pupillary deformity) may cause secondary glaucoma. Characteristic chorioretinal lesions develop as a result of atrophy and hyperpigmentation of the retinal pigment epithelium. Constriction of the visual fields and overt optic atrophy may occur.
Sclerosing keratitis occurs in 1–5% of infected persons and is the leading cause of onchocercal blindness in Africa. Anterior uveitis and iridocyclitis develop in ~5% of infected persons in Africa. In Latin America, complications of the anterior uveal tract (pupillary deformity) may cause secondary glaucoma. Characteristic chorioretinal lesions develop as a result of atrophy and hyperpigmentation of the retinal pigment epithelium. Constriction of the visual fields and overt optic atrophy may occur.
Lymph Nodes Mild to moderate lymphadenopathy is common, particularly in the inguinal and femoral areas, where the enlarged nodes may hang down in response to gravity (“hanging groin”), sometimes predisposing to inguinal and femoral hernias.
Systemic Manifestations Some heavily infected individuals develop cachexia with loss of adipose tissue and muscle mass. Among adults who become blind, there is a three- to fourfold increase in the mortality rate.
DIAGNOSIS
Definitive diagnosis depends on the detection of an adult worm in an excised nodule or, more commonly, of microfilariae in a skin snip. Skin snips are obtained with a corneal-scleral punch, which collects a blood-free skin biopsy sample extending to just below the epidermis, or by lifting of the skin with the tip of a needle and excision of a small (1- to 3-mm) piece with a sterile scalpel blade. The biopsy tissue is incubated in tissue culture medium or in saline on a glass slide or flat-bottomed microtiter plate. After incubation for 2–4 h (or occasionally overnight in light infections), microfilariae emergent from the skin can be seen by low-power microscopy.
Eosinophilia and elevated serum IgE levels are common but, because they occur in many parasitic infections, are not diagnostic in themselves. Assays to detect specific antibodies to Onchocerca and PCR to detect onchocercal DNA in skin snips are used in specialized laboratories and are highly sensitive and specific.
PREVENTION
Vector control has been beneficial in highly endemic areas in which breeding sites are vulnerable to insecticide spraying, but most areas endemic for onchocerciasis are not suited to this type of control. Community-based administration of ivermectin every 6–12 months is being used to interrupt transmission in endemic areas. This measure, in conjunction with vector control, has already helped eliminate the infection in most of Latin America and has reduced the prevalence of disease in many endemic foci in Africa. No drug has proved useful for prophylaxis of O. volvulus infection.
LOIASIS
ETIOLOGY AND EPIDEMIOLOGY
Loiasis is caused by L. loa (the African eye worm), which is present in the rainforests of West and Central Africa. Adult parasites (females, 50–70 mm long and 0.5 mm wide; males, 25–35 mm long and 0.25 mm wide) live in subcutaneous tissues. Microfilariae circulate in the blood with a diurnal periodicity that peaks between 12:00 noon and 2:00 P.M.
CLINICAL FEATURES
Manifestations of loiasis in natives of endemic areas may differ from those in temporary residents or visitors. Among the indigenous population, loiasis is often an asymptomatic infection with microfilaremia. Infection may be recognized only after subconjunctival migration of an adult worm (Fig. 258-4) or may be manifested by episodic Calabar swellings—evanescent localized areas of angioedema and erythema developing on the extremities and less frequently at other sites. Nephropathy, encephalopathy, and cardiomyopathy can occur but are rare. In patients who are not residents of endemic areas, allergic symptoms predominate, episodes of Calabar swelling tend to be more frequent and debilitating, microfilaremia is less common, and eosinophilia and increased levels of antifilarial antibodies are characteristic.
FIGURE 258-4 Adult Loa loa worm being surgically removed after its subconjunctival migration.
PATHOLOGY
The pathogenesis of the manifestations of loiasis is poorly understood. Calabar swellings are thought to result from a hypersensitivity reaction to adult worm antigens.
DIAGNOSIS
Definitive diagnosis of loiasis requires the detection of microfilariae in the peripheral blood or the isolation of the adult worm from the eye (Fig. 258-4) or from a subcutaneous biopsy specimen collected from a site of swelling developing after treatment. PCR-based assays for the detection of L. loa DNA in blood are available in specialized laboratories and are highly sensitive and specific, as are some newer recombinant antigen–based serologic techniques. In practice, the diagnosis must often be based on a characteristic history and clinical presentation, blood eosinophilia, and elevated levels of antifilarial antibodies, particularly in travelers to an endemic region, who are usually amicrofilaremic. Other clinical findings in travelers include hypergammaglobulinemia, elevated levels of serum IgE, and elevated leukocyte and eosinophil counts.
STREPTOCERCIASIS
![]() Mansonella streptocerca, found mainly in the tropical forest belt of Africa from Ghana to the Democratic Republic of the Congo, is transmitted by biting midges. The major clinical manifestations involve the skin and include pruritus, papular rashes, and pigmentation changes. Many infected individuals have inguinal adenopathy, although most are asymptomatic. The diagnosis is made by detection of the characteristic microfilariae in skin snips. Ivermectin at a single dose of 150 μg/kg leads to sustained suppression of microfilariae in the skin and is probably the treatment of choice for streptocerciasis.
Mansonella streptocerca, found mainly in the tropical forest belt of Africa from Ghana to the Democratic Republic of the Congo, is transmitted by biting midges. The major clinical manifestations involve the skin and include pruritus, papular rashes, and pigmentation changes. Many infected individuals have inguinal adenopathy, although most are asymptomatic. The diagnosis is made by detection of the characteristic microfilariae in skin snips. Ivermectin at a single dose of 150 μg/kg leads to sustained suppression of microfilariae in the skin and is probably the treatment of choice for streptocerciasis.
MANSONELLA PERSTANS INFECTION
![]() M. perstans, distributed across the center of Africa and in northeastern South America, is transmitted by midges. Adult worms reside in serous cavities—pericardial, pleural, and peritoneal—as well as in the mesentery and the perirenal and retroperitoneal tissues. Microfilariae circulate in the blood without periodicity. The clinical and pathologic features of the infection are poorly defined. Most patients appear to be asymptomatic, but manifestations may include transient angioedema and pruritus of the arms, face, or other parts of the body (analogous to the Calabar swellings of loiasis); fever; headache; arthralgias; and right-upper-quadrant pain. Occasionally, pericarditis and hepatitis occur. The diagnosis is based on the demonstration of microfilariae in blood or serosal effusions. Perstans filariasis is often associated with peripheral-blood eosinophilia and antifilarial antibody elevations.
M. perstans, distributed across the center of Africa and in northeastern South America, is transmitted by midges. Adult worms reside in serous cavities—pericardial, pleural, and peritoneal—as well as in the mesentery and the perirenal and retroperitoneal tissues. Microfilariae circulate in the blood without periodicity. The clinical and pathologic features of the infection are poorly defined. Most patients appear to be asymptomatic, but manifestations may include transient angioedema and pruritus of the arms, face, or other parts of the body (analogous to the Calabar swellings of loiasis); fever; headache; arthralgias; and right-upper-quadrant pain. Occasionally, pericarditis and hepatitis occur. The diagnosis is based on the demonstration of microfilariae in blood or serosal effusions. Perstans filariasis is often associated with peripheral-blood eosinophilia and antifilarial antibody elevations.
With the identification of a Wolbachia endosymbiont in M. perstans, doxycycline (200 mg twice a day) for 6 weeks has been established as the first effective treatment for this infection.
MANSONELLA OZZARDI INFECTION
![]() The distribution of M. ozzardi is restricted to Central and South America and certain Caribbean islands. Adult worms are rarely recovered from humans. Microfilariae circulate in the blood without periodicity. Although this organism has often been considered nonpathogenic, headache, articular pain, fever, pulmonary symptoms, adenopathy, hepatomegaly, pruritus, and eosinophilia have been ascribed to M. ozzardi infection. The diagnosis is made by detection of microfilariae in peripheral blood. Ivermectin is effective in treating this infection.
The distribution of M. ozzardi is restricted to Central and South America and certain Caribbean islands. Adult worms are rarely recovered from humans. Microfilariae circulate in the blood without periodicity. Although this organism has often been considered nonpathogenic, headache, articular pain, fever, pulmonary symptoms, adenopathy, hepatomegaly, pruritus, and eosinophilia have been ascribed to M. ozzardi infection. The diagnosis is made by detection of microfilariae in peripheral blood. Ivermectin is effective in treating this infection.
DRACUNCULIASIS (GUINEA WORM INFECTION)
ETIOLOGY AND EPIDEMIOLOGY
![]() The incidence of dracunculiasis, caused by Dracunculus medinensis, has declined dramatically because of global eradication efforts. In 2012, only 542 cases worldwide had been identified. The infection is currently endemic only in Chad, Ethiopia, Mali, and South Sudan.
The incidence of dracunculiasis, caused by Dracunculus medinensis, has declined dramatically because of global eradication efforts. In 2012, only 542 cases worldwide had been identified. The infection is currently endemic only in Chad, Ethiopia, Mali, and South Sudan.
Humans acquire D. medinensis when they ingest water containing infective larvae derived from Cyclops, a crustacean that is the intermediate host. Larvae penetrate the stomach or intestinal wall, mate, and mature. The adult male probably dies; the female worm develops over a year and migrates to subcutaneous tissues, usually in the lower extremity. As the thin female worm, ranging in length from 30 cm to 1 m, approaches the skin, a blister forms that, over days, breaks down and forms an ulcer. When the blister opens, large numbers of motile, rhabditiform larvae can be released into stagnant water; ingestion by Cyclops completes the life cycle.
CLINICAL FEATURES
Few or no clinical manifestations of dracunculiasis are evident until just before the blister forms, when there is an onset of fever and generalized allergic symptoms, including periorbital edema, wheezing, and urticaria. The emergence of the worm is associated with local pain and swelling. When the blister ruptures (usually as a result of immersion in water) and the adult worm releases larva-rich fluid, symptoms are relieved. The shallow ulcer surrounding the emerging adult worm heals over weeks to months. Such ulcers, however, can become secondarily infected, the result being cellulitis, local inflammation, abscess formation, or (uncommonly) tetanus. Occasionally, the adult worm does not emerge but becomes encapsulated and calcified.
DIAGNOSIS
The diagnosis is based on the findings developing with the emergence of the adult worm, as described above.
PREVENTION
Prevention, which remains the only real control measure, depends on the provision of safe drinking water.
ZOONOTIC FILARIAL INFECTIONS
Dirofilariae that affect primarily dogs, cats, and raccoons occasionally infect humans incidentally, as do Brugia and Onchocerca parasites that affect small mammals. Because humans are an abnormal host, the parasites never develop fully. Pulmonary dirofilarial infection caused by the canine heartworm Dirofilaria immitis generally presents in humans as a solitary pulmonary nodule. Chest pain, hemoptysis, and cough are uncommon. Infections with D. repens (from dogs) or D. tenuis (from raccoons) can cause local subcutaneous nodules in humans. Zoonotic Brugia infection can produce isolated lymph node enlargement, whereas zoonotic Onchocerca can cause subconjunctival masses. Eosinophilia levels and antifilarial antibody titers are not commonly elevated. Excisional biopsy is both diagnostic and curative. These infections usually do not respond to chemotherapy.
259 |
Schistosomiasis and Other Trematode Infections |
Trematodes, or flatworms, are a group of morphologically and biologically heterogeneous organisms that belong to the phylum Platyhelminthes. Human infection with trematodes occurs in many geographic areas and can cause considerable morbidity and mortality. The dependence on one drug—praziquantel—for treatment of most infections caused by trematodes raises the specter of developing resistance in these worms; several instances of reduced drug efficacy have already been reported. The widespread use of oxamniquine in the 1970s to reduce the impact of schistosomiasis resulted in the development of significant resistance. Recently, a single quantitative trait locus on schistosomal chromosome 6 was identified as the genetic basis for resistance.
ETIOLOGIC AGENTS AND THEIR LIFE CYCLES
For clinical purposes, significant trematode infections of humans may be divided according to the tissues invaded by the adult stage of the fluke, whether bloodstream, biliary tree, intestines, or lungs (Table 259-1). Trematodes share some common morphologic features, including macroscopic size (from one to several centimeters); dorsoventral, flattened, bilaterally symmetric bodies (adult worms); and the prominence of two suckers. Except for schistosomes, all human parasitic trematodes are hermaphroditic. Their life cycles involve a definitive host (mammalian/human), in which adult worms initiate sexual reproduction, and an intermediate host (snail), in which asexual multiplication of larvae occurs. More than one intermediate host may be necessary for some species of trematodes. Human infection is initiated either by direct penetration of intact skin or by ingestion. Upon maturation within humans, adult flukes initiate sexual reproduction and egg production. Helminth ova leave the definitive host in excreta or sputum and, upon reaching suitable environmental conditions, they hatch, releasing free-living miracidia that seek specific snail intermediate hosts. After asexual reproduction, cercariae are released from infected snails. In certain species, these organisms infect humans; in others, they find a second intermediate host to allow encystment into metacercariae—the infective stage for humans.
|
MAJOR HUMAN TREMATODE INFECTIONS |
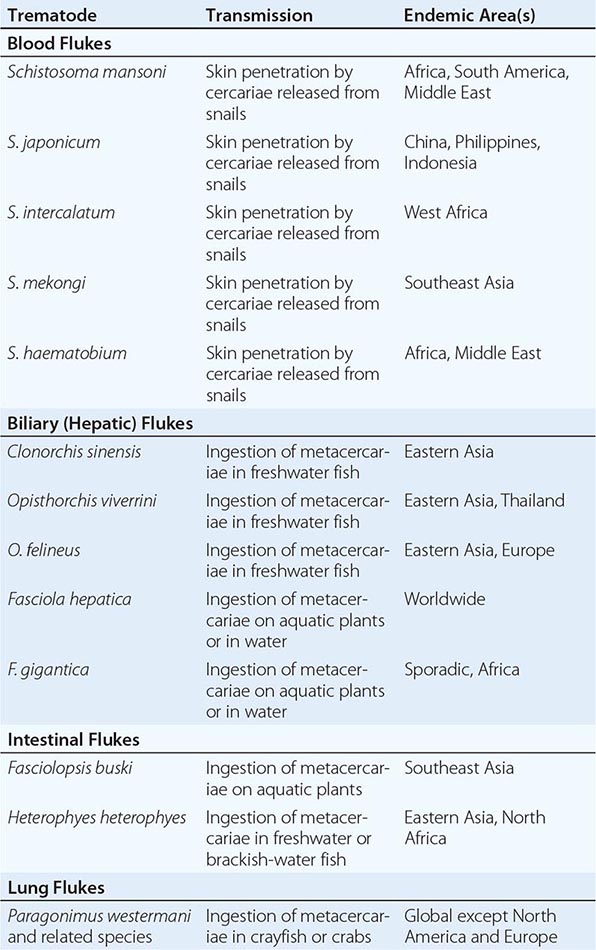
The host-parasite relationship in trematode infections is a product of certain biologic features of these organisms: they are multicellular, undergo several developmental changes within the host, and usually result in chronic infections. In general, the distribution of worm infections in human populations is overdispersed; i.e., it follows a negative binomial statistical distribution in which most infected individuals harbor low worm burdens while a small percentage are heavily infected. It is the heavily infected minority who are particularly prone to disease sequelae and who constitute an epidemiologically significant reservoir of infection in endemic areas. Recent evidence indicates that the prevalence of morbidity in infected populations is greater than was previously thought. Morbidity and death due to trematode infections reflect a multifactorial process that results from the tipping of a delicate balance between intensity of infection and host reactions, which initiate and modulate immunologic and pathologic outcome. Furthermore, the genetics of the parasite and of the human host contribute to the outcome of infection and disease. Infections with trematodes that migrate through or reside in host tissues are associated with a moderate to high degree of peripheral-blood eosinophilia; this association is of significance in protective and immunopathologic sequelae and is a useful clinical indicator of infection.
GLOBAL CONSIDERATIONS: EPIDEMIOLOGY OF TREMATODE INFECTIONS
![]() Except among international travelers, trematode infections are quite rare in high-income countries because good sanitation and hygiene block trematode transmission and because transmission is tied to the distribution of the specific snail species that serve as intermediate hosts during the parasites’ life cycle. In contrast, parasitic fluke infections are quite common in underdeveloped areas of Africa, Asia, and South America, with an estimated 440 million people affected by past or present Schistosoma infection and another 60 million people affected by the other foodborne trematodes. These infections are not benign; they result in multiyear chronic inflammatory disorders that significantly affect performance status and health-related quality of life. Global disease burden estimates indicate that at least 5 million years of healthy life are lost each year in the more than 90 endemic countries around the world.
Except among international travelers, trematode infections are quite rare in high-income countries because good sanitation and hygiene block trematode transmission and because transmission is tied to the distribution of the specific snail species that serve as intermediate hosts during the parasites’ life cycle. In contrast, parasitic fluke infections are quite common in underdeveloped areas of Africa, Asia, and South America, with an estimated 440 million people affected by past or present Schistosoma infection and another 60 million people affected by the other foodborne trematodes. These infections are not benign; they result in multiyear chronic inflammatory disorders that significantly affect performance status and health-related quality of life. Global disease burden estimates indicate that at least 5 million years of healthy life are lost each year in the more than 90 endemic countries around the world.
BLOOD FLUKES: SCHISTOSOMIASIS
Human schistosomiasis is caused by five species of the parasitic trematode genus Schistosoma: S. mansoni, S. japonicum, S. mekongi, and S. intercalatum cause intestinal and hepatic schistosomiasis, and S. haematobium causes urogenital schistosomiasis. Infection may cause considerable morbidity in the intestines, liver, or urinary tract, and a small proportion of affected individuals die. Other schistosomes (e.g., avian species) may invade human skin but then die in subcutaneous tissue, producing only self-limiting cutaneous manifestations.
ETIOLOGY
Human infection is initiated by penetration of intact skin with infective cercariae. These organisms, which are released from infected snails in freshwater bodies, measure ~2 mm in length and possess an anterior and a ventral sucker that attach to the skin and facilitate penetration. Once in subcutaneous tissue, cercariae transform into schistosomula, with morphologic, membrane, and immunologic changes. The cercarial outer membrane changes from a trilaminar to a heptalaminar structure that is then maintained throughout the organism’s life span in humans. This transformation is thought to be the schistosome’s main adaptive mechanism for survival in humans. Schistosomula begin their migration within 2–4 days via venous or lymphatic vessels, reaching the lungs and finally the liver parenchyma. Sexually mature worms descend into the venous system at specific anatomic locations: intestinal veins (S. mansoni, S. japonicum, S. mekongi, and S. intercalatum) and vesical and other pelvic veins (S. haematobium). After mating, adult gravid females travel against venous blood flow to small tributaries, where they deposit their ova intravascularly. Schistosome ova (Fig. 259-1) have specific morphologic features that vary with the species. Aided by enzymatic secretions through minipores in eggshells, ova move through the venous wall, traversing host tissues to reach the lumen of the intestinal or urinary tract, and are voided with stools or urine. Approximately 50% of ova are retained in host tissues locally (intestines or urinary tract) or are carried by venous blood flow to the liver and other organs. Schistosome ova that reach freshwater bodies hatch, releasing free-living miracidia that seek the snail intermediate host and undergo several cycles of asexual multiplication. Finally, infective cercariae are shed from snails to complete the transmission cycle.
FIGURE 259-1 Morphology of schistosome eggs, the diagnostic stage of the parasite’s life cycle. A. Schistosoma haematobium egg (in a urine sample) is large (~140 mm long), with a terminal spine. B. S. mansoni egg (in a fecal sample) is large (~150 mm long), with a thin shell and lateral spine. C. S. japonicum egg (fecal) is smaller than that of S. mansoni (~90 mm long), with a small spine or hooklike structure. D. S. mekongi egg (fecal) is similar to that of S. japonicum but smaller (~65 mm long). E. S. intercalatum egg (fecal) is larger than that of S. haematobium (~190 mm long), with a longer, sharply pointed spine. (From LR Ash, TC Orihel: Atlas of Human Parasitology, 3rd ed. Chicago, ASCP Press, 1990; with permission.)
Adult schistosomes are ~1–2 cm long. Males are slightly shorter than females, with flattened bodies and anteriorly curved edges forming the gynecophoral canal, in which mature adult females are usually held. Females are longer, slender, and rounded in cross-section. The precise nature of biochemical and reproductive exchanges between the two sexes is unknown, as are the regulatory mechanisms for pairing. Adult schistosomes parasitize specific sites in the host venous system. What guides adult intestinal schistosomes to branches of the superior or inferior mesenteric veins or adult S. haematobium worms to the vesical plexus is unknown. In addition, adult worms inhibit the coagulation cascade and evade the effector arms of the host immune responses by still-undetermined mechanisms. The genome of schistosomes is relatively large (~270 Mb) and is arrayed on seven pairs of autosomes and one pair of sex chromosomes. Sequencing of the S. japonicum, S. mansoni, and S. haematobium genomes has provided insight into the worms’ genomic and proteomic features, offering an opportunity to discover new drug targets and to understand the molecular basis of pathogenesis.
EPIDEMIOLOGY
![]() The global distribution of schistosome infection in human populations (Fig. 259-2) is dependent on both parasite and host factors. Information on prevalence and global distribution is inexact. At present, the five Schistosoma species are estimated to infect 200–300 million individuals (mostly children and young adults) in South America, the Caribbean, Africa, the Middle East, and Southeast Asia. Notably, parasite-related disease persists after active infection resolves, leaving a substantial health burden among adult populations. Thus, the overall number of humans likely to be affected by Schistosoma-related disease is now ~440 million. The total population living under conditions favoring transmission risk numbers ~700 million—a fact reflecting the global public health significance of schistosomiasis.
The global distribution of schistosome infection in human populations (Fig. 259-2) is dependent on both parasite and host factors. Information on prevalence and global distribution is inexact. At present, the five Schistosoma species are estimated to infect 200–300 million individuals (mostly children and young adults) in South America, the Caribbean, Africa, the Middle East, and Southeast Asia. Notably, parasite-related disease persists after active infection resolves, leaving a substantial health burden among adult populations. Thus, the overall number of humans likely to be affected by Schistosoma-related disease is now ~440 million. The total population living under conditions favoring transmission risk numbers ~700 million—a fact reflecting the global public health significance of schistosomiasis.
FIGURE 259-2 Global distribution of schistosomiasis. A. Schistosoma mansoni infection (dark blue) is endemic in Africa, the Middle East, South America, and a few Caribbean countries. S. intercalatum infection (green) is endemic in sporadic foci in West and Central Africa. B. S. haematobium infection (purple) is endemic in Africa and the Middle East. The major endemic countries for S. japonicum infection (green) are China, the Philippines, and Indonesia. S. mekongi infection (red) is endemic in sporadic foci in Southeast Asia.
In endemic areas, the rate of yearly onset of new infection (incidence) is generally low. Prevalence, on the other hand, starts to be appreciable by the age of 3–4 years and builds to a maximum that varies by endemic region (up to 100%) in the 12- to 20-year age group. Prevalence then stabilizes or decreases slightly in older age groups (>40 years). Intensity of infection (as measured by fecal or urinary egg counts, which correlate with adult worm burdens in most circumstances) follows the increase in prevalence up to the age of 12–20 years and then declines markedly in older age groups. This decline may reflect acquisition of resistance or may be due to changes in water contact patterns, since older people have less exposure. Infection with schistosomes in human populations has a peculiar pattern. Most infected individuals harbor low worm burdens, and only a small proportion suffer from high-intensity infection. This pattern may be due to differences in worm infectivity or to a spectrum of genetic susceptibilities in human populations.
Disease due to schistosome infection is the consequence of parasitologic, host, and associated viral infections and of nutritional and environmental factors. Most disease syndromes relate to the presence of one or more of the parasite stages in humans. Disease manifestations in the populations of endemic areas correlate, in general, with intensity and duration of infection as well as with age and genetic susceptibility of the host. Overall, severe Schistosoma-specific disease manifestations are relatively rare among persons infected with any of the intestinal schistosomes. In contrast, symptoms of urogenital schistosomiasis manifest clinically in most S. haematobium–infected individuals. In addition, all forms of Schistosoma infection are associated with subclinical systemic morbidities that can significantly affect physical and cognitive performance, causing, for example, growth stunting, undernutrition, and anemia of chronic inflammation. New estimates of total morbidity due to chronic schistosomiasis indicate a significantly greater burden than was previously appreciated.
Schistosomiasis appears to be a cofactor in the spread and progression of HIV/AIDS in areas where both diseases are endemic. Increased emphasis should be placed on the treatment of schistosome infections in persons at risk of HIV/AIDS.
PATHOGENESIS AND IMMUNITY
Cercarial invasion is associated with dermatitis arising from dermal and subdermal inflammatory responses, both humoral and cell-mediated. As the parasites approach sexual maturity in the liver of infected individuals and as oviposition commences, acute schistosomiasis or Katayama syndrome (a serum sickness–like illness; see “Clinical Features,” below) may occur. The associated antigen excess results in formation of soluble immune complexes, which may be deposited in several tissues, initiating multiple pathologic events. In chronic schistosomiasis, most disease manifestations are due to eggs retained in host tissues. The granulomatous response around these ova is cell-mediated and is regulated both positively and negatively by a cascade of cytokine, cellular, and humoral responses. Granuloma formation begins with recruitment of a host of inflammatory cells in response to antigens secreted by the living organism within the ova. Cells recruited initially include phagocytes, antigen-specific T cells, and eosinophils. Fibroblasts, giant cells, and B lymphocytes predominate later. Over time, these cumulative lesions reach a size many times that of parasite eggs, thus inducing organomegaly and obstruction. Immunomodulation or downregulation of host responses to schistosome eggs plays a significant role in limiting the extent of the granulomatous lesions—and consequently disease—in chronically infected experimental animals or humans. The underlying mechanisms involve another cascade of regulatory cytokines and idiotypic antibodies. Subsequent to the granulomatous response, fibrosis sets in, resulting in more permanent disease sequelae. Because schistosomiasis is also a chronic infection, the accumulation of antigen–antibody complexes results in deposits in renal glomeruli and may cause significant kidney disease.
The better-studied pathologic sequelae in schistosomiasis are those observed in liver disease. Ova that are carried by portal blood embolize to the liver. Because of their size (~150 × 60 μm in the case of S. mansoni), they lodge at presinusoidal sites, where granulomas are formed. These granulomas contribute to the hepatomegaly observed in infected individuals (Fig. 259-3). Schistosomal liver enlargement is also associated with certain class I and class II human leukocyte antigen (HLA) haplotypes and markers; its genetic basis appears to be polygenic. Presinusoidal portal blockage causes several hemodynamic changes, including portal hypertension and associated development of portosystemic collaterals at the esophagogastric junction and other sites. Esophageal varices are most likely to break and cause repeated episodes of hematemesis. Because changes in hepatic portal blood flow occur slowly, compensatory arterialization of the blood flow through the liver is established. Although this compensatory mechanism may be associated with certain metabolic side effects, retention of hepatocyte perfusion permits maintenance of normal liver function for several years.
FIGURE 259-3 Chronic hepatosplenomegaly caused by schistosomiasis mansoni. Liver and spleen enlargement, ascites, and wasting are characteristically seen in patients with chronic Schistosoma mansoni infection.
The second most significant pathologic change in the liver relates to fibrosis. It is characteristically periportal (Symmers’ clay pipe–stem fibrosis) but may be diffuse. Fibrosis, when diffuse, may be seen in areas of egg deposition and granuloma formation but is also seen in distant locations such as portal tracts. Schistosomiasis results in pure fibrotic lesions in the liver; cirrhosis occurs only when other toxic factors or infectious agents (e.g., hepatitis B or C virus) are involved. Deposition of fibrotic tissue in the extracellular matrix results from the interaction of T lymphocytes with cells of the fibroblast series; several cytokines, such as interleukin (IL) 2, IL-4, IL-1, and transforming growth factor β, are known to stimulate fibrogenesis. The process may be dependent on the genetic constitution of the host. Furthermore, regulatory cytokines that can suppress T cell responses and fibrogenesis, such as IL-10, interferon γ, or IL-12, may play a role in modulating the response.
Although the above description focuses on granuloma formation and fibrosis of the liver, similar processes occur in urogenital schistosomiasis. Granuloma formation at the lower end of the ureters obstructs urinary flow, with subsequent development of hydroureter and hydronephrosis. Similar lesions in the urinary bladder cause the protrusion of papillomatous structures into its cavity; these may ulcerate and/or bleed. The chronic stage of infection is associated with scarring and deposition of calcium in the bladder wall. Among women, involvement of the birth canal can cause cervical or vaginal wall polyps and friability leading to contact bleeding, with an apparently increased risk of HIV transmission. Secondary infertility or subfecundity can also result from female genital schistosomiasis involving the uterus, fallopian tubes, or ovaries. Among men, S. haematobium infection can result in prostatic and testicular lesions with hematospermia. Superficial cutaneous lesions of the perineum can occur in both sexes.
Studies on immunity to schistosomiasis, whether innate or adaptive, have expanded our knowledge of the components of these responses and target antigens. The critical question, however, is whether humans acquire immunity to schistosomes. Epidemiologic data suggest the onset of acquired immunity during the course of infection in young adults. Curative treatment of infected populations in endemic areas is followed by differentiation in the pattern of reinfection. Some (susceptible) individuals acquire reinfection rapidly, whereas other (resistant) individuals are reinfected slowly. This difference may be explained by differences in transmission, immunologic response, or genetic susceptibility. The mechanism of acquired immunity involves antibodies, complement, and several effector cells, particularly eosinophils. Furthermore, the intensity of schistosome infection has been correlated with a region in chromosome 5. In several studies, a few protective schistosome antigens have been identified as vaccine candidates, but none has been fully evaluated in human populations to date.
CLINICAL FEATURES
In general, disease manifestations of schistosomiasis occur in three stages, which vary not only by species but also by intensity of infection and other host factors, such as age and genetics of the human host. During the phase of cercarial invasion, a form of dermatitis may be observed. This so-called swimmers’ itch occurs most often with S. mansoni and S. japonicum infections, manifesting 2 or 3 days after invasion as an itchy maculopapular rash on the affected areas of the skin. The condition is particularly severe when humans are exposed to avian schistosomes. This form of cercarial dermatitis is also seen around freshwater lakes in the northern United States, particularly in the spring and summer months. Cercarial dermatitis is a self-limiting clinical entity. During worm maturation and at the beginning of oviposition (i.e., 4–8 weeks after skin invasion), acute schistosomiasis or Katayama syndrome—a serum sickness–like illness with fever, generalized lymphadenopathy, and hepatosplenomegaly—may develop. Individuals with acute schistosomiasis have a high degree of peripheral-blood eosinophilia. Parasite-specific antibodies may be detected before schistosome eggs are identified in excreta.
![]() Acute schistosomiasis has become an important clinical entity worldwide because of increased travel to endemic areas. Travelers are exposed to parasites while swimming or wading in freshwater bodies and upon their return present with acute manifestations. The course of acute schistosomiasis is generally benign, but central nervous system (CNS) schistosomiasis and even deaths are occasionally reported in association with heavy exposure to schistosomes among travelers and migrants.
Acute schistosomiasis has become an important clinical entity worldwide because of increased travel to endemic areas. Travelers are exposed to parasites while swimming or wading in freshwater bodies and upon their return present with acute manifestations. The course of acute schistosomiasis is generally benign, but central nervous system (CNS) schistosomiasis and even deaths are occasionally reported in association with heavy exposure to schistosomes among travelers and migrants.
The main clinical manifestations of chronic schistosomiasis are species-dependent. Intestinal species (S. mansoni, S. japonicum, S. mekongi, and S. intercalatum) cause intestinal and hepatosplenic disease as well as several manifestations associated with portal hypertension. During the intestinal phase, which may begin a few months after infection and may last for years, symptomatic patients characteristically have colicky abdominal pain, bloody diarrhea, and anemia. Patients may also report fatigue and an inability to perform daily routine functions and may show evidence of growth retardation and anemia. This more subtle form of schistosomiasis morbidity is generally underappreciated. The severity of intestinal schistosomiasis is often related to the intensity of the worm burden. The disease runs a chronic course and may result in colonic polyposis, which has been reported from some endemic areas, such as Egypt and Uganda.
The hepatosplenic phase of disease manifests early (during the first year of infection, particularly in children) with liver enlargement due to parasite-induced granulomatous lesions. Hepatomegaly is seen in ~15–20% of infected individuals; it correlates roughly with intensity of infection, occurs more often in children, and may be related to specific HLA haplotypes. In subsequent phases of infection, presinusoidal blockage of blood flow leads to portal hypertension and splenomegaly (Fig. 259-3). Moreover, portal hypertension may lead to varices at the lower end of the esophagus and at other sites. Patients with schistosomal liver disease may have right-upper-quadrant “dragging” pain during the hepatomegaly phase, and this pain may move to the left upper quadrant as splenomegaly progresses. Bleeding from esophageal varices may, however, be the first clinical manifestation of this phase. Patients may experience repeated bleeding but seem to tolerate its impact, because an adequate total hepatic blood flow permits normal liver function for a considerable period. In late-stage disease, typical fibrotic changes occur along with liver function deterioration and the onset of ascites, hypoalbuminemia, and defects in coagulation. Intercurrent viral infections of the liver (especially hepatitis B and C), toxic insults (excessive ethanol ingestion or exposure to organic poisons or aflatoxin), or nutritional deficiencies may well accelerate or exacerbate the deterioration of hepatic function.
The extent and severity of intestinal and hepatic disease in schistosomiasis mansoni and japonica have been well described. Although it was originally thought that S. japonicum might induce more severe disease manifestations because the adult worms can produce 10 times more eggs than S. mansoni, subsequent field studies have not supported this claim. Clinical observations of individuals infected with S. mekongi or S. intercalatum have been less detailed, partly because of the limited geographic distribution of these organisms.
The clinical manifestations of S. haematobium infection occur relatively early and involve a high percentage of infected individuals. Up to 80% of children infected with S. haematobium have dysuria, frequency, and hematuria. Hematuria may sometimes occur only at the end of voiding. Urine examination reveals blood and albumin as well as an unusually high frequency of bacterial urinary tract infections and urinary sediment cellular metaplasia. These manifestations correlate with the intensity of infection, the presence of urinary bladder granulomas, and subsequent ulceration. Along with local effects of granuloma formation in the urinary bladder, obstruction of the lower end of the ureters results in hydroureter and hydronephrosis, which may be seen in 25–50% of infected children. As infection progresses, bladder granulomas undergo fibrosis, which results in typical sandy patches visible on cystoscopy. In many endemic areas, an association between squamous cell carcinoma of the bladder and S. haematobium infection has been observed. Such malignancy is detected in a younger age group than is transitional cell carcinoma. In fact, S. haematobium has now been classified as a human carcinogen. Genital schistosomiasis (described in the previous section) is a common presenting symptom among adults of both sexes.
Significant disease may occur in other organs during chronic schistosomiasis. Lung and CNS disease have been documented; other sites, such as the skin and the genital organs, are less frequently affected. In pulmonary schistosomiasis, embolized eggs lodge in small arterioles, producing acute necrotizing arteriolitis and granuloma formation. During S. mansoni and S. japonicum infection, schistosome eggs reach the lungs after the development of portosystemic collateral circulation; in S. haematobium infection, ova may reach the lungs directly via connections between the vesical and systemic circulation. Subsequent fibrous tissue deposition leads to endarteritis obliterans, pulmonary hypertension, and cor pulmonale. The most common symptoms are cough, fever, and dyspnea. Cor pulmonale may be diagnosed radiologically on the basis of prominence of the right side of the heart and dilation of the pulmonary artery. Frank evidence of right-sided heart failure may be seen in late cases.
Although less common than pulmonary manifestations, CNS schistosomiasis is important, characteristically occurring in association with S. japonicum infection. Migratory worms deposit eggs in the brain and induce a granulomatous response. The frequency of this manifestation among infected individuals in some endemic areas (e.g., the Philippines) is calculated at 2–4%. Jacksonian epilepsy due to S. japonicum infection is the second most common cause of epilepsy in these areas. S. mansoni and S. haematobium infections have been associated with transverse myelitis. This syndrome is thought to be due to eggs traveling to the venous plexus around the spinal cord. In schistosomiasis mansoni, transverse myelitis is usually seen in the chronic stage after the development of portal hypertension and portosystemic shunts, which allow ova to travel to the spinal cord veins. This proposed sequence of events has been challenged because of a few reports of transverse myelitis occurring early in the course of S. mansoni infection. More information is needed to confirm these observations. During schistosomiasis caused by Schistosoma haematobium, ova may travel through communication between vesical and systemic veins, resulting in spinal cord disease that may be detected at any stage of infection. Pathologic study of lesions in schistosomal transverse myelitis may reveal eggs along with necrotic or granulomatous lesions. Patients usually present with acute or rapidly progressing lower-leg weakness accompanied by sphincter dysfunction.
DIAGNOSIS
Physicians in areas not endemic for schistosomiasis face considerable diagnostic challenges. In the most common clinical presentation, a traveler returns with symptoms and signs of acute syndromes of schistosomiasis—namely, cercarial dermatitis or Katayama syndrome. Central to a correct diagnosis is a thorough inquiry into the patient’s history of travel and exposure to freshwater bodies—whether slow- or fast-running—in an endemic area. Differential diagnosis of fever in returned travelers includes a spectrum of infections whose etiologies are viral (e.g., dengue fever), bacterial (e.g., enteric fever, leptospirosis), rickettsial, or protozoal (e.g., malaria). In cases of Katayama syndrome, prompt diagnosis is essential and is based on clinical presentation, high-level peripheral-blood eosinophilia, and a positive serologic assay for schistosomal antibodies. Two tests are available at the CDC: the Falcon assay screening test/enzyme-linked immunosorbent assay (FAST-ELISA) and the confirmatory enzyme-linked immunoelectrotransfer blot (EITB). Both tests are highly sensitive and ~96% specific. In some instances, examination of stool or urine for ova may yield positive results.
Individuals with established infection are diagnosed by a combination of geographic history, characteristic clinical presentation, and presence of schistosome ova in excreta. The diagnosis may also be established with the serologic assays mentioned above or with those that detect circulating schistosome antigens. These assays can be applied to blood, urine, or other body fluids (e.g., cerebrospinal fluid). For suspected schistosome infection, stool examination by the Kato thick smear or any other concentration method generally identifies most patients with heavy infection but does not identify all lightly infected individuals. For the latter patients, a point-of-care test to detect parasite circulating cathodic antigen in urine may prove very useful in establishing the presence of active S. mansoni infection and in monitoring the clearance of infection after treatment. For S. haematobium, urine may be examined by microscopy of sediment or by filtration of a known volume through Nuclepore filters. Sensitivity can be further improved by testing for parasite DNA in urine sediment. The Kato thick smear and Nuclepore filtration provide quantitative data on the intensity of infection, which is of value in assessing the degree of tissue damage and in monitoring the effect of chemotherapy. Schistosome infection may also be diagnosed by examination of tissue specimens, typically rectal biopsy samples; except in rare circumstances, other biopsy procedures (e.g., liver biopsy) are not needed.
The differential diagnosis of schistosomal hepatomegaly must include viral hepatitis of all etiologies, miliary tuberculosis, malaria, visceral leishmaniasis, ethanol abuse, and causes of hepatic and portal vein obstruction. The differential diagnosis of hematuria in S. haematobium infection includes bacterial cystitis, tuberculosis, urinary stones, and malignancy.
|
DRUG THERAPY FOR HUMAN TREMATODE INFECTIONS |
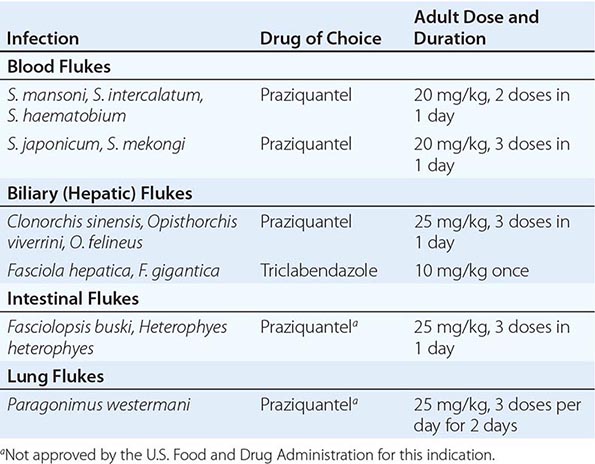
PREVENTION AND CONTROL
![]() Transmission of schistosomiasis is dependent on human behavior. Because the geographic distribution of infections in endemic regions of the world is not clearly demarcated, it is prudent for travelers to endemic areas to avoid contact with all freshwater bodies, irrespective of the speed of water flow or unsubstantiated claims of safety. Some topical agents, when applied to the skin, may inhibit cercarial penetration, but none is currently available. If exposure occurs, a follow-up visit with a health care provider is strongly recommended. Prevention of infection in inhabitants of endemic areas is a significant challenge. Residents of these regions use freshwater bodies for sanitary, domestic, recreational, and agricultural purposes. Several control measures have been used, including application of molluscicides, provision of sanitary water and sewage disposal, chemotherapy, and health education to effect behavioral change in terms of water-contact activities. Current recommendations to countries endemic for schistosomiasis emphasize the use of multiple approaches. With the advent of an oral, safe, and effective broad-spectrum antischistosomal agent (praziquantel), chemotherapy has been most successful in reducing the intensity of infection and reversing disease. The duration of this positive impact depends on the transmission dynamics of the parasite in any specific endemic region. The ultimate goal of research on prevention and control is the development of a vaccine. Although there are a few promising leads, this goal probably is not within reach during the next decade.
Transmission of schistosomiasis is dependent on human behavior. Because the geographic distribution of infections in endemic regions of the world is not clearly demarcated, it is prudent for travelers to endemic areas to avoid contact with all freshwater bodies, irrespective of the speed of water flow or unsubstantiated claims of safety. Some topical agents, when applied to the skin, may inhibit cercarial penetration, but none is currently available. If exposure occurs, a follow-up visit with a health care provider is strongly recommended. Prevention of infection in inhabitants of endemic areas is a significant challenge. Residents of these regions use freshwater bodies for sanitary, domestic, recreational, and agricultural purposes. Several control measures have been used, including application of molluscicides, provision of sanitary water and sewage disposal, chemotherapy, and health education to effect behavioral change in terms of water-contact activities. Current recommendations to countries endemic for schistosomiasis emphasize the use of multiple approaches. With the advent of an oral, safe, and effective broad-spectrum antischistosomal agent (praziquantel), chemotherapy has been most successful in reducing the intensity of infection and reversing disease. The duration of this positive impact depends on the transmission dynamics of the parasite in any specific endemic region. The ultimate goal of research on prevention and control is the development of a vaccine. Although there are a few promising leads, this goal probably is not within reach during the next decade.
LIVER (BILIARY) FLUKES
![]() Several species of biliary fluke infecting humans are particularly common in Southeast Asia and Russia. Other species are transmitted in Europe, Africa, and the Americas. On the basis of their migratory pathway in humans, these infections may be divided into the Clonorchis and Fasciola groups (Table 259-1).
Several species of biliary fluke infecting humans are particularly common in Southeast Asia and Russia. Other species are transmitted in Europe, Africa, and the Americas. On the basis of their migratory pathway in humans, these infections may be divided into the Clonorchis and Fasciola groups (Table 259-1).
CLONORCHIASIS AND OPISTHORCHIASIS
![]() Infection with Clonorchis sinensis, the Chinese or oriental fluke, is endemic among fish-eating mammals in Southeast Asia. Humans are an incidental host; the prevalence of human infection is highest in China, Vietnam, and Korea. Infection with Opisthorchis viverrini and O. felineus is zoonotic in cats and dogs. Transmission to humans occurs occasionally, particularly in Thailand (O. viverrini) and in Southeast Asia and eastern Europe (O. felineus). Data on the exact geographic distribution of these infectious agents in human populations are rudimentary.
Infection with Clonorchis sinensis, the Chinese or oriental fluke, is endemic among fish-eating mammals in Southeast Asia. Humans are an incidental host; the prevalence of human infection is highest in China, Vietnam, and Korea. Infection with Opisthorchis viverrini and O. felineus is zoonotic in cats and dogs. Transmission to humans occurs occasionally, particularly in Thailand (O. viverrini) and in Southeast Asia and eastern Europe (O. felineus). Data on the exact geographic distribution of these infectious agents in human populations are rudimentary.
Infection with any of these three species is established by ingestion of raw or inadequately cooked freshwater fish harboring metacercariae. These organisms excyst in the duodenum, releasing larvae that travel through the ampulla of Vater and mature into adult worms in bile canaliculi. Mature flukes are flat and elongated, measuring 1–2 cm in length. The hermaphroditic worms reproduce by releasing small operculated eggs, which pass with bile into the intestines and are voided with stools. The life cycle is completed in the environment in specific freshwater snails (the first intermediate host) along with later encystment of snail-derived cercariae as infectious metacercariae in freshwater fish.
Except for late sequelae, the exact clinical syndromes caused by clonorchiasis and opisthorchiasis are not well defined. Because most infected individuals harbor a low worm burden, many are minimally symptomatic. Moderate to heavy infection may be associated with vague right-upper-quadrant pain. In contrast, chronic or repeated infection is associated with manifestations such as cholangitis, cholangiohepatitis, and biliary obstruction. Cholangiocarcinoma is epidemiologically related to C. sinensis infection in China and to O. viverrini infection in northeastern Thailand. This association has resulted in classification of these infectious agents as human carcinogens.
FASCIOLIASIS
![]() Infections with Fasciola hepatica and F. gigantica are worldwide zoonoses that are particularly endemic in sheep-raising countries. Human cases have been reported in South America, Europe, Africa, and Australia. Recent estimates indicate a worldwide prevalence of 17 million cases. High endemicity has been reported in certain areas of Peru and Bolivia. In most endemic areas the predominant species is F. hepatica, but in Asia and Africa a varying degree of overlap with F. gigantica has been observed.
Infections with Fasciola hepatica and F. gigantica are worldwide zoonoses that are particularly endemic in sheep-raising countries. Human cases have been reported in South America, Europe, Africa, and Australia. Recent estimates indicate a worldwide prevalence of 17 million cases. High endemicity has been reported in certain areas of Peru and Bolivia. In most endemic areas the predominant species is F. hepatica, but in Asia and Africa a varying degree of overlap with F. gigantica has been observed.
Humans acquire fascioliasis by ingestion of metacercariae attached to certain aquatic plants, such as watercress, water caltrop, and water chestnuts. Infection may also be acquired by consumption of contaminated water or ingestion of food items washed with such water. Acquisition of human infection through consumption of freshly prepared raw liver containing immature flukes has been reported. Infection is initiated when metacercariae excyst, penetrate the gut wall, and travel through the peritoneal cavity to invade the liver capsule. Adult worms migrate through the liver parenchyma and finally reach bile ducts, where they produce large operculated eggs that are voided in bile through the gastrointestinal tract to the outside environment. The flukes’ life cycle is completed in specific snails (the first intermediate host) followed by encystment on aquatic plants.
Clinical features of fascioliasis relate to the stage and intensity of infection. Acute disease develops during parasite migration (1–2 weeks after infection) and includes fever, right-upper-quadrant pain, hepatomegaly, and eosinophilia. Computed tomography (CT) of the liver may show multiple parenchymal holes/or migratory tracks. Symptoms and signs usually subside as the parasites reach their final habitat. In individuals with chronic infection, bile duct obstruction and biliary cirrhosis are infrequently demonstrated. No relation to hepatic malignancy has been ascribed to fascioliasis.
DIAGNOSIS
Diagnosis of infection with any of the biliary flukes depends on a high degree of suspicion, elicitation of an appropriate geographic history, and stool examination for characteristically shaped parasite ova. Additional evidence may be obtained by documenting peripheral-blood eosinophilia or imaging the liver. Serologic testing is helpful, particularly in lightly infected individuals.
INTESTINAL FLUKES
![]() Two species of intestinal flukes cause human infection in defined geographic areas worldwide (Table 259-1). The large Fasciolopsis buski (adults measure 2 × 7 cm) is endemic in Southeast Asia, whereas the smaller Heterophyes heterophyes is found in the Nile Delta of Egypt. Infection is initiated by ingestion of metacercariae attached to aquatic plants (F. buski) or encysted in freshwater or brackish-water fish (H. heterophyes). Flukes mature in human intestines, and eggs are passed with stools. Most individuals infected with intestinal flukes are asymptomatic. In heavy F. buski infection, diarrhea, abdominal pain, and malabsorption may be encountered. Heavy infection with H. heterophyes may be associated with abdominal pain and mucous diarrhea. The diagnosis is established by detection of characteristically shaped ova in stool samples. The drug of choice for treatment is praziquantel (Table 259-2).
Two species of intestinal flukes cause human infection in defined geographic areas worldwide (Table 259-1). The large Fasciolopsis buski (adults measure 2 × 7 cm) is endemic in Southeast Asia, whereas the smaller Heterophyes heterophyes is found in the Nile Delta of Egypt. Infection is initiated by ingestion of metacercariae attached to aquatic plants (F. buski) or encysted in freshwater or brackish-water fish (H. heterophyes). Flukes mature in human intestines, and eggs are passed with stools. Most individuals infected with intestinal flukes are asymptomatic. In heavy F. buski infection, diarrhea, abdominal pain, and malabsorption may be encountered. Heavy infection with H. heterophyes may be associated with abdominal pain and mucous diarrhea. The diagnosis is established by detection of characteristically shaped ova in stool samples. The drug of choice for treatment is praziquantel (Table 259-2).
LUNG FLUKES
![]() Infection with the lung fluke Paragonimus westermani (Table 259-1) and related species (e.g., P. africanus) is endemic in many parts of the world, excluding North America and Europe. Endemicity is particularly noticeable in West Africa, Central and South America, and Asia. In nature, the reservoir hosts of P. westermani are wild and domestic felines. In Africa, P. africanus has been found in other species, such as dogs. Adult lung flukes, which are 7–12 mm in length, are found encapsulated in the lungs of infected persons. In rare circumstances, flukes are found encysted in the CNS (cerebral paragonimiasis) or the abdominal cavity. Humans acquire lung fluke infection by ingesting infective metacercariae encysted in the muscles and viscera of crayfish and freshwater crabs. In endemic areas, these crustaceans are consumed raw, marinated, or pickled. Once the organisms reach the duodenum, they excyst, penetrate the gut wall, and travel through the peritoneal cavity, diaphragm, and pleural space to reach the lungs. Mature flukes are found in the bronchioles surrounded by cystic lesions. Parasite eggs are either expectorated with sputum or swallowed and passed to the outside environment with feces. The life cycle is completed in snails and freshwater crustaceans.
Infection with the lung fluke Paragonimus westermani (Table 259-1) and related species (e.g., P. africanus) is endemic in many parts of the world, excluding North America and Europe. Endemicity is particularly noticeable in West Africa, Central and South America, and Asia. In nature, the reservoir hosts of P. westermani are wild and domestic felines. In Africa, P. africanus has been found in other species, such as dogs. Adult lung flukes, which are 7–12 mm in length, are found encapsulated in the lungs of infected persons. In rare circumstances, flukes are found encysted in the CNS (cerebral paragonimiasis) or the abdominal cavity. Humans acquire lung fluke infection by ingesting infective metacercariae encysted in the muscles and viscera of crayfish and freshwater crabs. In endemic areas, these crustaceans are consumed raw, marinated, or pickled. Once the organisms reach the duodenum, they excyst, penetrate the gut wall, and travel through the peritoneal cavity, diaphragm, and pleural space to reach the lungs. Mature flukes are found in the bronchioles surrounded by cystic lesions. Parasite eggs are either expectorated with sputum or swallowed and passed to the outside environment with feces. The life cycle is completed in snails and freshwater crustaceans.
When maturing flukes lodge in lung tissues, they cause hemorrhage and necrosis, resulting in cyst formation. The adjacent lung parenchyma shows evidence of inflammatory infiltration, predominantly by eosinophils. Cysts usually measure 1–2 cm in diameter and may contain one or two worms each. With the onset of oviposition, cysts usually rupture in adjacent bronchioles—an event allowing ova to exit the human host. Older cysts develop thickened walls, which may undergo calcification. During the active phase of paragonimiasis, lung tissues surrounding parasite cysts may show evidence of pneumonia, bronchitis, bronchiectasis, and fibrosis.
Pulmonary paragonimiasis is particularly symptomatic in persons with moderate to heavy infection. Productive cough with brownish sputum or frank hemoptysis associated with peripheral-blood eosinophilia is usually the presenting feature. Chest examination may reveal signs of pleurisy. In chronic cases, bronchitis or bronchiectasis may predominate, but these conditions rarely proceed to lung abscess. Imaging of the lungs demonstrates characteristic features, including patchy densities, cavities, pleural effusion, and ring shadows. Cerebral paragonimiasis presents as either space-occupying lesions or epilepsy.
DIAGNOSIS
Pulmonary paragonimiasis is diagnosed by detection of parasite ova in sputum and/or stools. Serology is of considerable help in egg-negative cases and in cerebral paragonimiasis. The differential diagnosis includes active tuberculosis, bacterial lung abscess, and lung carcinoma.
CONTROL AND PREVENTION OF TISSUE FLUKES
![]() For residents of nonendemic areas who are visiting an endemic region, the only effective preventive measure is to avoid ingestion of local plants, fish, or crustaceans; if their ingestion is necessary, these items should be washed and cooked thoroughly. Instruction on water and food preparation and consumption should be included in physicians’ advice to travelers (Chap. 149). Interruption of transmission among residents of endemic areas depends on avoiding ingestion of infective stages and disposing of feces and sputum appropriately to prevent hatching of eggs in the environment. These two approaches rely greatly on socioeconomic development, health education, and significant behavioral change. In countries where economic progress has resulted in financial and social improvements, transmission has decreased. The third approach to control in endemic communities entails selective use of chemotherapy for individuals posing the highest risk of transmission (i.e., those with heavy infections). The availability of praziquantel—a broad-spectrum, safe, and effective anthelmintic agent—provides a means for reducing the reservoirs of infection in human populations. However, the existence of most of these helminthic infections as zoonoses in several animal species complicates control efforts.
For residents of nonendemic areas who are visiting an endemic region, the only effective preventive measure is to avoid ingestion of local plants, fish, or crustaceans; if their ingestion is necessary, these items should be washed and cooked thoroughly. Instruction on water and food preparation and consumption should be included in physicians’ advice to travelers (Chap. 149). Interruption of transmission among residents of endemic areas depends on avoiding ingestion of infective stages and disposing of feces and sputum appropriately to prevent hatching of eggs in the environment. These two approaches rely greatly on socioeconomic development, health education, and significant behavioral change. In countries where economic progress has resulted in financial and social improvements, transmission has decreased. The third approach to control in endemic communities entails selective use of chemotherapy for individuals posing the highest risk of transmission (i.e., those with heavy infections). The availability of praziquantel—a broad-spectrum, safe, and effective anthelmintic agent—provides a means for reducing the reservoirs of infection in human populations. However, the existence of most of these helminthic infections as zoonoses in several animal species complicates control efforts.
260 |
Cestode Infections |
Cestodes, or tapeworms, are segmented worms. The adults reside in the gastrointestinal tract, but the larvae can be found in almost any organ. Human tapeworm infections can be divided into two major clinical groups. In one group, humans are the definitive hosts, with the adult tapeworms living in the gastrointestinal tract (Taenia saginata, Diphyllobothrium, Hymenolepis, and Dipylidium caninum). In the other, humans are intermediate hosts, with larval-stage parasites present in the tissues; diseases in this category include echinococcosis, sparganosis, and coenurosis. Humans may be either the definitive or the intermediate hosts for Taenia solium. Both stages of Hymenolepis nana are found simultaneously in the human intestines.
The ribbon-shaped tapeworm attaches to the intestinal mucosa by means of sucking cups or hooks located on the scolex. Behind the scolex is a short, narrow neck from which proglottids (segments) form. As each proglottid matures, it is displaced further back from the neck by the formation of new, less mature segments. The progressively elongating chain of attached proglottids, called the strobila, constitutes the bulk of the tapeworm. The length varies among species. In some, the tapeworm may consist of more than 1000 proglottids and may be several meters long. The mature proglottids are hermaphroditic and produce eggs, which are subsequently released. Because eggs of the different Taenia species are morphologically identical, differences in the morphology of the scolex or proglottids provide the basis for diagnostic identification to the species level.
Most human tapeworms require at least one intermediate host for complete larval development. After ingestion of the eggs or proglottids by an intermediate host, the larval oncospheres are activated, escape the egg, and penetrate the intestinal mucosa. The oncosphere migrates to tissues and develops into an encysted form known as a cysticercus (single scolex), a coenurus (multiple scolices), or a hydatid (cyst with daughter cysts, each containing several protoscolices). The definitive host’s ingestion of tissues containing a cyst enables a scolex to develop into a tapeworm.
TAENIASIS SAGINATA AND TAENIASIS ASIATICA
![]() The beef tapeworm T. saginata occurs in all countries where raw or undercooked beef is eaten. It is most prevalent in sub-Saharan African and Middle Eastern countries. T. asiatica is closely related to T. saginata and is found in Asia, with pigs as intermediate hosts. The clinical manifestations and morphology of these two species are very similar and are therefore discussed together.
The beef tapeworm T. saginata occurs in all countries where raw or undercooked beef is eaten. It is most prevalent in sub-Saharan African and Middle Eastern countries. T. asiatica is closely related to T. saginata and is found in Asia, with pigs as intermediate hosts. The clinical manifestations and morphology of these two species are very similar and are therefore discussed together.
Etiology and Pathogenesis Humans are the only definitive host for the adult stage of T. saginata and T. asiatica. The tapeworms, which can reach 8 m in length with 1000–2000 proglottids, inhabit the upper jejunum. The scolex of T. saginata has four prominent suckers, whereas T. asiatica has an unarmed rostellum. Each gravid segment has 15–30 uterine branches (in contrast to 8–12 for T. solium). The eggs are indistinguishable from those of T. solium; they measure 30–40 μm, contain the oncosphere, and have a thick brown striated shell. Eggs deposited on vegetation can live for months or years until they are ingested by cattle or other herbivores (T. saginata) or pigs (T. asiatica). The embryo released after ingestion invades the intestinal wall and is carried to striated muscle or viscera, where it transforms into the cysticercus. When ingested in raw or undercooked meat, this form can infect humans. After the cysticercus is ingested, it takes ~2 months for the mature adult worm to develop.
Clinical Manifestations Patients become aware of the infection most commonly by noting passage of proglottids in their feces. The proglottids are often motile, and patients may experience perianal discomfort when proglottids are discharged. Mild abdominal pain or discomfort, nausea, change in appetite, weakness, and weight loss can occur.
Diagnosis The diagnosis is made by the detection of eggs or proglottids in the stool. Eggs may also be present in the perianal area; thus, if proglottids or eggs are not found in the stool, the perianal region should be examined with use of a cellophane-tape swab (as in pinworm infection; Chap. 257). Distinguishing T. saginata or T. asiatica from T. solium requires examination of mature proglottids. All three species can be distinguished by examining the scolex. Available serologic tests are not helpful diagnostically. Eosinophilia and elevated levels of serum IgE may be detected.
Prevention The major method of preventing infection is the adequate cooking of beef or pork viscera; exposure to temperatures as low as 56°C for 5 min will destroy cysticerci. Refrigeration or salting for long periods or freezing at –10°C for 9 days also kills cysticerci in beef. General preventive measures include inspection of beef and proper disposal of human feces.
TAENIASIS SOLIUM AND CYSTICERCOSIS
The pork tapeworm T. solium can cause two distinct forms of infection in humans: adult tapeworms in the intestine or larval forms in the tissues (cysticercosis). Humans are the only definitive hosts for T. solium; pigs are the usual intermediate hosts, although other animals may harbor the larval forms.
![]() T. solium is found worldwide in areas where pigs are raised and have access to human feces. However, it is most prevalent in Latin America, sub-Saharan Africa, China, India, and Southeast Asia. Cysticercosis occurs in industrialized nations largely as a result of the immigration of infected persons from endemic areas.
T. solium is found worldwide in areas where pigs are raised and have access to human feces. However, it is most prevalent in Latin America, sub-Saharan Africa, China, India, and Southeast Asia. Cysticercosis occurs in industrialized nations largely as a result of the immigration of infected persons from endemic areas.
Etiology and Pathogenesis The adult tapeworm generally resides in the upper jejunum. The scolex attaches by both sucking disks and two rows of hooklets. The adult worm usually lives for a few years. The tapeworm, usually ~3 m in length, may have as many as 1000 proglottids, each of which produces up to 50,000 eggs. Proglottids are released and excreted into the feces, and the eggs in these proglottids are infective for both humans and animals. The eggs may survive in the environment for several months. After ingestion of eggs by the pig intermediate host, the larvae are activated, escape the egg, penetrate the intestinal wall, and are carried to many tissues; they are most frequently identified in striated muscle of the neck, tongue, and trunk. Within 60–90 days, the encysted larval stage develops. These cysticerci can survive for months to years. By ingesting undercooked pork containing cysticerci, humans acquire infections that lead to intestinal tapeworms. Infections that cause human cysticercosis follow the ingestion of T. solium eggs, usually from close contact with a tapeworm carrier. Autoinfection may occur if an individual with an egg-producing tapeworm ingests eggs derived from his or her own feces.
Clinical Manifestations Intestinal infections with T. solium may be asymptomatic. Fecal passage of proglottids may be noted by patients. Other symptoms are infrequent.
In cysticercosis, the clinical manifestations are variable. Cysticerci can be found anywhere in the body but are most commonly detected in the brain, cerebrospinal fluid (CSF), skeletal muscle, subcutaneous tissue, or eye. The clinical presentation of cysticercosis depends on the number and location of cysticerci as well as on the extent of associated inflammatory responses or scarring. Neurologic manifestations are the most common (Fig. 260-1). Seizures are associated with inflammation surrounding cysticerci in the brain parenchyma. These seizures may be generalized, focal, or Jacksonian. Hydrocephalus results from CSF flow obstruction by cysticerci and accompanying inflammation or by CSF outflow obstruction from arachnoiditis. Symptoms of increased intracranial pressure, including headache, nausea, vomiting, changes in vision, dizziness, ataxia, or confusion, are often evident. Patients with hydrocephalus may develop papilledema or display altered mental status. When cysticerci develop at the base of the brain or in the subarachnoid space, they may cause chronic meningitis or arachnoiditis, communicating hydrocephalus, hemorrhages, or strokes.
FIGURE 260-1 Neurocysticercosis is caused by Taenia solium. Neurologic infection can be classified on the basis of the location and viability of the parasites. When the parasites are in the ventricles, they often cause obstructive hydrocephalus. Left: Magnetic resonance imaging showing a cysticercus in the lateral ventricle, with resultant hydrocephalus. The arrow points to the scolex within the cystic parasite. Center: CT showing a parenchymal cysticercus, with enhancement of the cyst wall and an internal scolex (arrow). Right: Multiple cysticerci, including calcified lesions from prior infection (arrowheads), viable cysticerci in the basilar cisterns (white arrow), and a large degenerating cysticercus in the Sylvian fissure (black arrow). (Modified with permission from JC Bandres et al: Clin Infect Dis 15:799, 1992. © The University of Chicago Press.)
Diagnosis The diagnosis of intestinal T. solium infection is made by the detection of eggs or proglottids, as described for T. saginata. More sensitive methods, including antigen-capture enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), and serology for tapeworm stage-specific antigens, are currently available only as research techniques. In cysticercosis, diagnosis can be difficult. A consensus conference has delineated absolute, major, minor, and epidemiologic criteria for diagnosis (Table 260-1). Diagnostic certainty is possible only with definite demonstration of the parasite (absolute criteria). This task can be accomplished by histologic observation of the parasite in excised tissue, by funduscopic visualization of the parasite in the eye (in the anterior chamber, vitreous, or subretinal spaces), or by neuroimaging studies demonstrating cystic lesions containing a characteristic scolex (Fig. 260-1). With improving resolution of neuroimaging studies, the scolex can now be identified in many cases. In other instances, a clinical diagnosis is based on a combination of clinical presentation, radiographic studies, serologic tests, and exposure history.
|
DIAGNOSTIC CRITERIA FOR HUMAN CYSTICERCOSISa |
aDiagnosis is confirmed by either one absolute criterion or a combination of two major criteria, one minor criterion, and one epidemiologic criterion. A probable diagnosis is supported by the fulfillment of (1) one major criterion plus two minor criteria; (2) one major criterion plus one minor criterion and one epidemiologic criterion; or (3) three minor criteria plus one epidemiologic criterion.
Source: Modified from OH Del Brutto et al: Neurology 57:177, 2001.
Neuroimaging findings suggestive of neurocysticercosis constitute the primary major diagnostic criterion (Fig. 260-1). These findings include cystic lesions with or without enhancement (e.g., ring enhancement), one or more nodular calcifications (which may also have associated enhancement), or focal enhancing lesions. Cysticerci in the brain parenchyma are usually 5–20 mm in diameter and rounded. Cystic lesions in the subarachnoid space or fissures may enlarge up to 6 cm in diameter and may be lobulated. For cysticerci within the subarachnoid space or ventricles, the walls may be very thin and the cyst fluid is often isodense with CSF. Thus, obstructive hydrocephalus or enhancement of the basilar meninges may be the only finding on CT in extraparenchymal neurocysticercosis. Cysticerci in the ventricles or subarachnoid space are usually visible to an experienced neuroradiologist on MRI or on CT with intraventricular contrast injection. CT is more sensitive than MRI in identifying calcified lesions, whereas MRI is better for identifying cystic lesions, scolices, and enhancement.
The second major diagnostic criterion is detection of specific antibodies to cysticerci. Although most tests using unfractionated antigen have high rates of false-positive and false-negative results, this problem can be overcome by using the more specific immunoblot assay. An immunoblot assay using lentil lectin purified glycoproteins is >99% specific and highly sensitive. However, patients with single intracranial lesions or with calcifications may be seronegative. With this assay, serum samples provide greater diagnostic sensitivity than CSF. All of the diagnostic antigens have been cloned, and assays using recombinant antigens are being developed. Antigen detection assays using monoclonal antibodies to detect parasite antigen in the blood or CSF may also facilitate diagnosis and patient follow-up. These assays are only now becoming available for patient care.
Studies have demonstrated that clinical criteria can aid in diagnosis in selected cases. In patients from endemic areas who had single enhancing lesions presenting with seizures, a normal physical examination, and no evidence of systemic disease (e.g., no fever, adenopathy, or chest radiographic abnormalities), the constellation of rounded CT lesions 5–20 mm in diameter with no midline shift was almost always caused by neurocysticercosis. Finally, spontaneous resolution or resolution after therapy with albendazole alone is consistent with neurocysticercosis.
Minor diagnostic criteria include neuroimaging findings consistent with but less characteristic of cysticercosis, clinical manifestations suggestive of neurocysticercosis (e.g., seizures, hydrocephalus, or altered mental status), evidence of cysticercosis outside the central nervous system (CNS) (e.g., cigar-shaped soft-tissue calcifications), or detection of antibody in CSF by ELISA. Epidemiologic criteria include exposure to a tapeworm carrier or household member infected with T. solium, current or prior residence in an endemic area, and frequent travel to an endemic area.
The diagnosis is confirmed in patients with either one absolute criterion or a combination of two major criteria, one minor criterion, and one epidemiologic criterion (Table 260-1). A probable diagnosis is supported by the fulfillment of (1) one major criterion plus two minor criteria; (2) one major criterion plus one minor criterion and one epidemiologic criterion; or (3) three minor criteria plus one epidemiologic criterion. Although the CSF is usually abnormal in neurocysticercosis, CSF abnormalities are not pathognomonic. Patients may have CSF pleocytosis with a predominance of lymphocytes, neutrophils, or eosinophils. The protein level in CSF may be elevated; the glucose concentration is usually normal but may be depressed.
Prevention Measures for the prevention of intestinal T. solium infection consist of the application to pork of precautions similar to those described above for beef with regard to T. saginata infection. The prevention of cysticercosis involves minimizing the opportunities for ingestion of fecally derived eggs by means of good personal hygiene, effective fecal disposal, and treatment and prevention of human intestinal infections. Mass chemotherapy has been administered to human and porcine populations in efforts at disease eradication. Finally, vaccines to prevent porcine cysticercosis have shown promise in studies and are under development.
ECHINOCOCCOSIS
![]() Echinococcosis is an infection caused in humans by the larval stage of the Echinococcus granulosus complex, E. multilocularis, or E. vogeli. E. granulosus complex parasites produce cystic hydatid disease, with unilocular cystic lesions. These infections are prevalent in most areas where livestock is raised in association with dogs. Molecular evidence suggests that E. granulosus strains may actually belong to more than one species; specifically, strains from sheep, cattle, pigs, horses, and camels probably represent separate species. These parasites are found on all continents, with areas of high prevalence in China, central Asia, the Middle East, the Mediterranean region, eastern Africa, and parts of South America. E. multilocularis, which causes multilocular alveolar lesions that are locally invasive, is found in Alpine, sub-Arctic, or Arctic regions, including Canada, the United States, and central and northern Europe; China; and central Asia. E. vogeli causes polycystic hydatid disease and is found only in Central and South America.
Echinococcosis is an infection caused in humans by the larval stage of the Echinococcus granulosus complex, E. multilocularis, or E. vogeli. E. granulosus complex parasites produce cystic hydatid disease, with unilocular cystic lesions. These infections are prevalent in most areas where livestock is raised in association with dogs. Molecular evidence suggests that E. granulosus strains may actually belong to more than one species; specifically, strains from sheep, cattle, pigs, horses, and camels probably represent separate species. These parasites are found on all continents, with areas of high prevalence in China, central Asia, the Middle East, the Mediterranean region, eastern Africa, and parts of South America. E. multilocularis, which causes multilocular alveolar lesions that are locally invasive, is found in Alpine, sub-Arctic, or Arctic regions, including Canada, the United States, and central and northern Europe; China; and central Asia. E. vogeli causes polycystic hydatid disease and is found only in Central and South America.
Like other cestodes, echinococcal species have both intermediate and definitive hosts. The definitive hosts are canines that pass eggs in their feces. After the ingestion of eggs, cysts develop in the intermediate hosts—sheep, cattle, humans, goats, camels, and horses for the E. granulosus complex and mice and other rodents for E. multilocularis. When a dog (E. granulosus) or fox (E. multilocularis) ingests infected meat containing cysts, the life cycle is completed.
Etiology The small (5-mm-long) adult E. granulosus complex worms, which live for 5–20 months in the jejunum of dogs, have only three proglottids: one immature, one mature, and one gravid. The gravid segment splits to release eggs that are morphologically similar to Taenia eggs and are extremely hardy. After humans ingest the eggs, embryos escape from the eggs, penetrate the intestinal mucosa, enter the portal circulation, and are carried to various organs, most commonly the liver and lungs. Larvae develop into fluid-filled unilocular hydatid cysts that consist of an external membrane and an inner germinal layer. Daughter cysts develop from the inner aspect of the germinal layer, as do germinating cystic structures called brood capsules. New larvae, called protoscolices, develop in large numbers within the brood capsule. The cysts expand slowly over a period of years.
The life cycle of E. multilocularis is similar except that wild canines, such as foxes, serve as the definitive hosts and small rodents serve as the intermediate hosts. The larval form of E. multilocularis, however, is quite different in that it remains in the proliferative phase, the parasite is always multilocular, and vesicles without brood capsule or protoscolices progressively invade the host tissue by peripheral extension of processes from the germinal layer.
Clinical Manifestations Slowly enlarging echinococcal cysts generally remain asymptomatic until their expanding size or their space-occupying effect in an involved organ elicits symptoms. The liver and the lungs are the most common sites of these cysts. The liver is involved in about two-thirds of E. granulosus infections and in nearly all E. multilocularis infections. Because a period of years elapses before cysts enlarge sufficiently to cause symptoms, they may be discovered incidentally on a routine x-ray or ultrasound study.
Patients with hepatic echinococcosis who are symptomatic most often present with abdominal pain or a palpable mass in the right upper quadrant. Compression of a bile duct or leakage of cyst fluid into the biliary tree may mimic recurrent cholelithiasis, and biliary obstruction can result in jaundice. Rupture of or episodic leakage from a hydatid cyst may produce fever, pruritus, urticaria, eosinophilia, or anaphylaxis. Pulmonary hydatid cysts may rupture into the bronchial tree or pleural cavity and produce cough, salty phlegm, dyspnea, chest pain, or hemoptysis. Rupture of hydatid cysts, which can occur spontaneously or at surgery, may lead to multifocal dissemination of protoscolices, which can form additional cysts. Other presentations are due to the involvement of bone (invasion of the medullary cavity with slow bone erosion producing pathologic fractures), the CNS (space-occupying lesions), the heart (conduction defects, pericarditis), and the pelvis (pelvic mass).
The larval forms of E. multilocularis characteristically present as a slowly growing hepatic tumor, with progressive destruction of the liver and extension into vital structures. Patients commonly report upper-quadrant and epigastric pain. Liver enlargement and obstructive jaundice may be apparent. The lesions may infiltrate adjoining organs (e.g., diaphragm, kidneys, or lungs) or may metastasize to the spleen, lungs, or brain.
Diagnosis Radiographic and related imaging studies are important in detecting and evaluating echinococcal cysts. Plain x-rays will define pulmonary cysts of E. granulosus—usually as rounded masses of uniform density—but may miss cysts in other organs unless there is cyst wall calcification (as occurs in the liver). MRI, CT, and ultrasound reveal well-defined cysts with thick or thin walls. When older cysts contain a layer of hydatid sand that is rich in accumulated protoscolices, these imaging methods may detect this fluid layer of different density. However, the most pathognomonic finding, if demonstrable, is that of daughter cysts within the larger cyst. This finding, like eggshell or mural calcification on CT, is indicative of E. granulosus infection and helps to distinguish the cyst from carcinomas, bacterial or amebic liver abscesses, or hemangiomas. In contrast, ultrasound or CT of alveolar hydatid cysts reveals indistinct solid masses with central necrosis and plaquelike calcifications.
A specific diagnosis of E. granulosus infection can be made by the examination of aspirated fluids for protoscolices or hooklets, but diagnostic aspiration is not usually recommended because of the risk of fluid leakage resulting in either dissemination of infection or anaphylactic reactions. Serodiagnostic assays can be useful, although a negative test does not exclude the diagnosis of echinococcosis. Cysts in the liver elicit positive antibody responses in ~90% of cases, whereas up to 50% of individuals with cysts in the lungs are seronegative. Detection of antibody to specific echinococcal antigens by immunoblotting has the highest degree of specificity.
![]() Prevention In endemic areas, echinococcosis can be prevented by administering praziquantel to infected dogs, by denying dogs access to infected animals, or by vaccinating sheep. Limitation of the number of stray dogs is helpful in reducing the prevalence of infection among humans. In Europe, E. multilocularis infection has been associated with gardening; gloves should be used when working with soil.
Prevention In endemic areas, echinococcosis can be prevented by administering praziquantel to infected dogs, by denying dogs access to infected animals, or by vaccinating sheep. Limitation of the number of stray dogs is helpful in reducing the prevalence of infection among humans. In Europe, E. multilocularis infection has been associated with gardening; gloves should be used when working with soil.
HYMENOLEPIASIS NANA
![]() Infection with Hymenolepis nana, the dwarf tapeworm, is the most common of all the cestode infections. H. nana is endemic in both temperate and tropical regions of the world. Infection is spread by fecal/oral contamination and is common among institutionalized children.
Infection with Hymenolepis nana, the dwarf tapeworm, is the most common of all the cestode infections. H. nana is endemic in both temperate and tropical regions of the world. Infection is spread by fecal/oral contamination and is common among institutionalized children.
Etiology and Pathogenesis H. nana is the only cestode of humans that does not require an intermediate host. Both the larval and adult phases of the life cycle take place in the human. The adult—the smallest tapeworm parasitizing humans—is ~2 cm long and dwells in the proximal ileum. Proglottids, which are small and rarely seen in the stool, release spherical eggs 30–44 μm in diameter, each of which contains an oncosphere with six hooklets. The eggs are immediately infective and are unable to survive for >10 days in the external environment. When the egg is ingested by a new host, the oncosphere is freed and penetrates the intestinal villi, becoming a cysticercoid larva. Larvae migrate back into the intestinal lumen, attach to the mucosa, and mature into adult worms over 10–12 days. Eggs may also hatch before passing into the stool, causing internal autoinfection with increasing numbers of intestinal worms. Although the life span of adult H. nana worms is only ~4–10 weeks, the autoinfection cycle perpetuates the infection.
Clinical Manifestations H. nana infection, even with many intestinal worms, is usually asymptomatic. When infection is intense, anorexia, abdominal pain, and diarrhea develop.
Diagnosis Infection is diagnosed by the finding of eggs in the stool.
Prevention Good personal hygiene and improved sanitation can eradicate the disease. Epidemics have been controlled by mass chemotherapy coupled with improved hygiene.
HYMENOLEPIASIS DIMINUTA
Hymenolepis diminuta, a cestode of rodents, occasionally infects small children, who ingest the larvae in uncooked cereal foods contaminated by fleas and other insects in which larvae develop. Infection is usually asymptomatic and is diagnosed by the detection of eggs in the stool. Treatment with praziquantel results in cure in most cases.
DIPHYLLOBOTHRIASIS
![]() Diphyllobothrium latum and other Diphyllobothrium species are found in the lakes, rivers, and deltas of the Northern Hemisphere, central Africa, and South America.
Diphyllobothrium latum and other Diphyllobothrium species are found in the lakes, rivers, and deltas of the Northern Hemisphere, central Africa, and South America.
Etiology and Pathogenesis The adult worm—the longest tapeworm (up to 25 m)—attaches to the ileal and occasionally to the jejunal mucosa by its suckers, which are located on its elongated scolex. The adult worm has 3000–4000 proglottids, which release ~1 million eggs daily into the feces. If an egg reaches water, it hatches and releases a free-swimming embryo that can be eaten by small freshwater crustaceans (Cyclops or Diaptomus species). After an infected crustacean containing a developed procercoid is swallowed by a fish, the larva migrates into the fish’s flesh and grows into a plerocercoid, or sparganum larva. Humans acquire the infection by ingesting infected raw or smoked fish. Within 3–5 weeks, the tapeworm matures into an adult in the human intestine.
Clinical Manifestations Most D. latum infections are asymptomatic, although manifestations may include transient abdominal discomfort, diarrhea, vomiting, weakness, and weight loss. Occasionally, infection can cause acute abdominal pain and intestinal obstruction; in rare cases, cholangitis or cholecystitis may be produced by migrating proglottids.
![]() Because the tapeworm absorbs large quantities of vitamin B12 and interferes with ileal B12 absorption, vitamin B12 deficiency can develop, but this effect has been noted only in Scandinavia, where up to 2% of infected patients, especially the elderly, have megaloblastic anemia resembling pernicious anemia and may exhibit neurologic sequelae of B12 deficiency.
Because the tapeworm absorbs large quantities of vitamin B12 and interferes with ileal B12 absorption, vitamin B12 deficiency can develop, but this effect has been noted only in Scandinavia, where up to 2% of infected patients, especially the elderly, have megaloblastic anemia resembling pernicious anemia and may exhibit neurologic sequelae of B12 deficiency.
Diagnosis The diagnosis is made readily by the detection of the characteristic eggs in the stool. The eggs possess a single shell with an operculum at one end and a knob at the other. Mild to moderate eosinophilia may be detected.
Prevention Infection can be prevented by heating fish to 54°C for 5 min or by freezing it at –18°C for 24 h. Placing fish in brine with a high salt concentration for long periods kills the eggs.
DIPYLIDIASIS
Dipylidium caninum, a common tapeworm of dogs and cats, may accidentally infect humans. Dogs, cats, and occasionally humans become infected by ingesting fleas harboring cysticercoids. Children are more likely to become infected than adults. Most infections are asymptomatic, but abdominal pain, diarrhea, anal pruritus, urticaria, eosinophilia, or passage of segments in the stool may occur. The diagnosis is made by the detection of proglottids or ova in the stool. As in D. latum infection, therapy consists of praziquantel. Prevention requires anthelmintic treatment and flea control for pet dogs or cats.
SPARGANOSIS
Humans can be infected by the sparganum, or plerocercoid larva, of a diphyllobothrid tapeworm of the genus Spirometra. Infection can be acquired by the consumption of water containing infected Cyclops; by the ingestion of infected snakes, birds, or mammals; or by the application of infected flesh as poultices. The worm migrates slowly in tissues, and infection commonly presents as a subcutaneous swelling. Periorbital tissues can be involved, and ocular sparganosis may destroy the eye. Surgical excision is used to treat localized sparganosis.
COENUROSIS
This rare infection of humans by the larval stage (coenurus) of the dog tapeworm Taenia multiceps or T. serialis results in a space-occupying cystic lesion. As in cysticercosis, involvement of the CNS and subcutaneous tissue is most common. Both definitive diagnosis and treatment require surgical excision of the lesion. Chemotherapeutic agents generally are not effective.