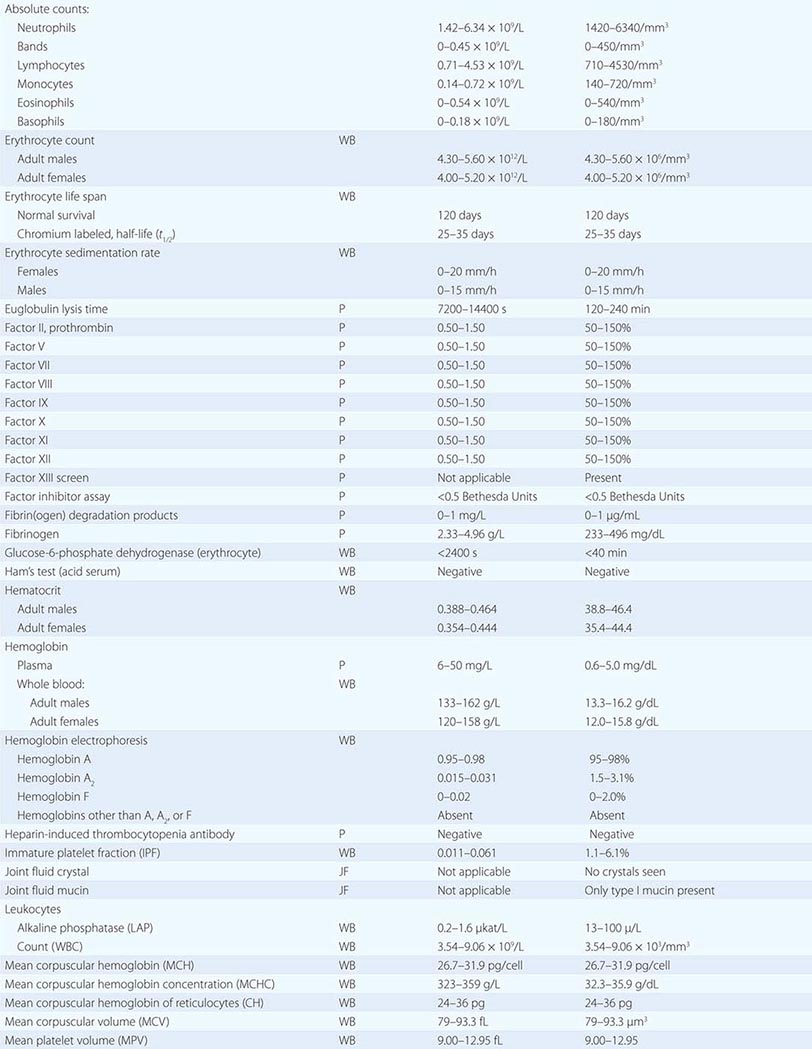
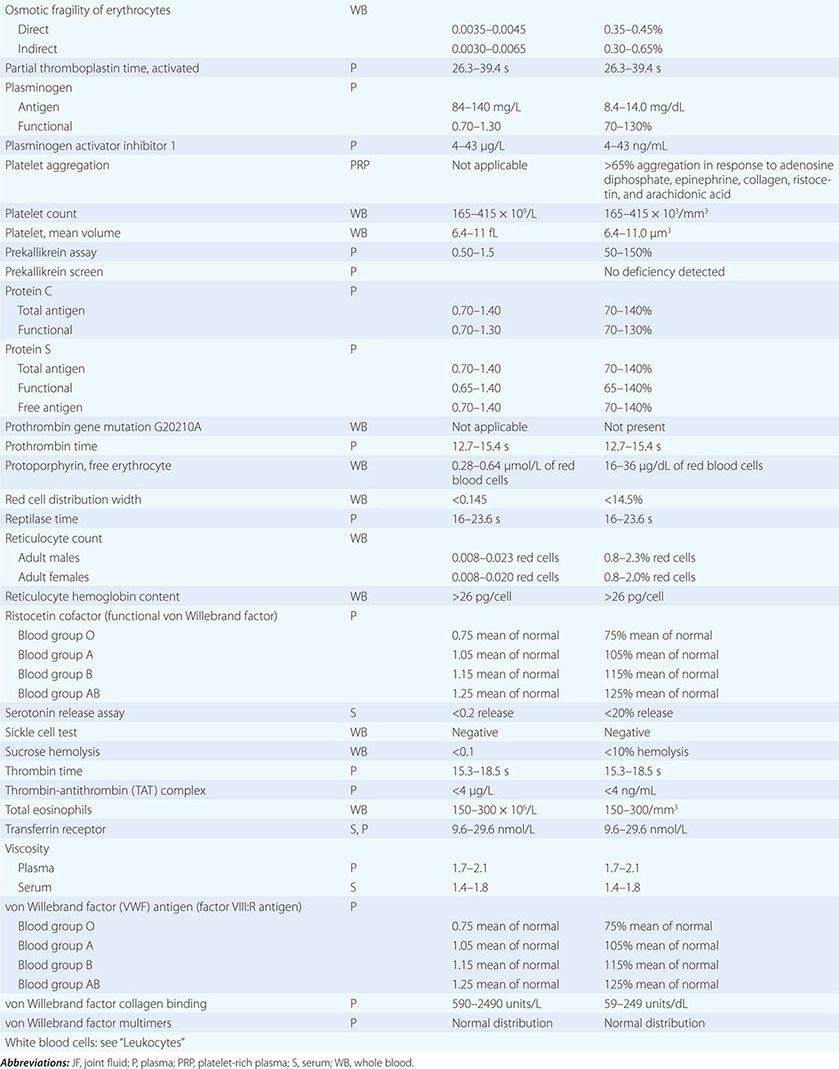
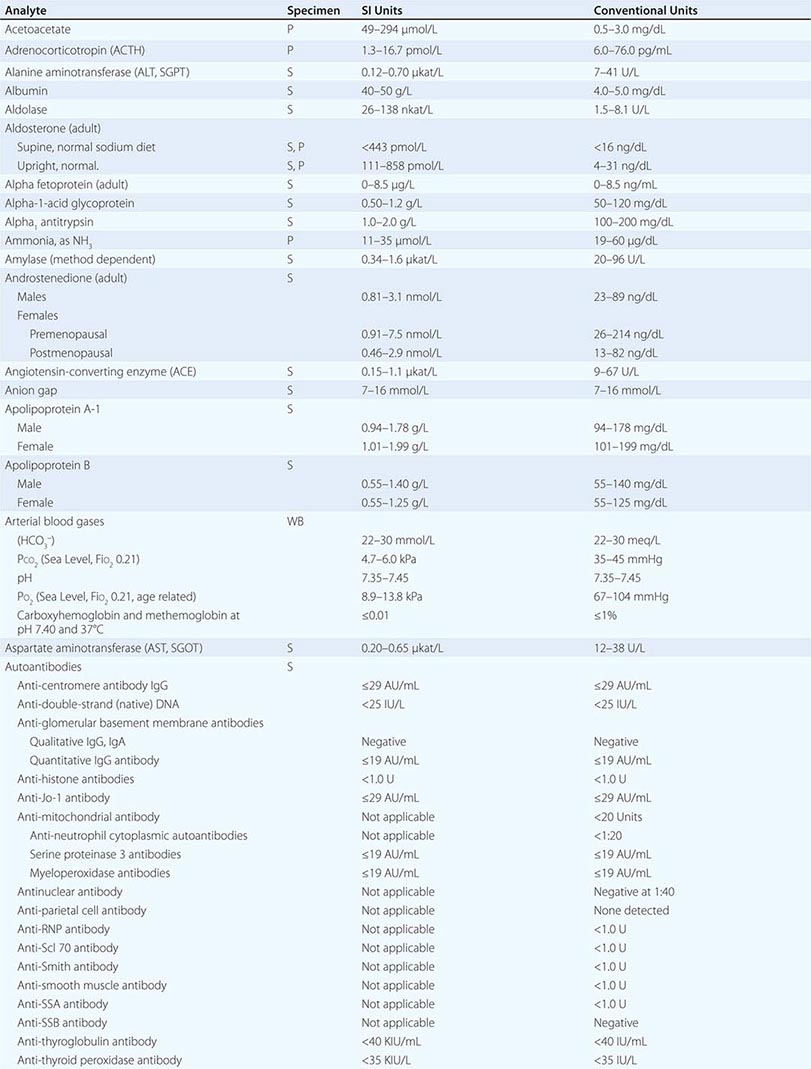
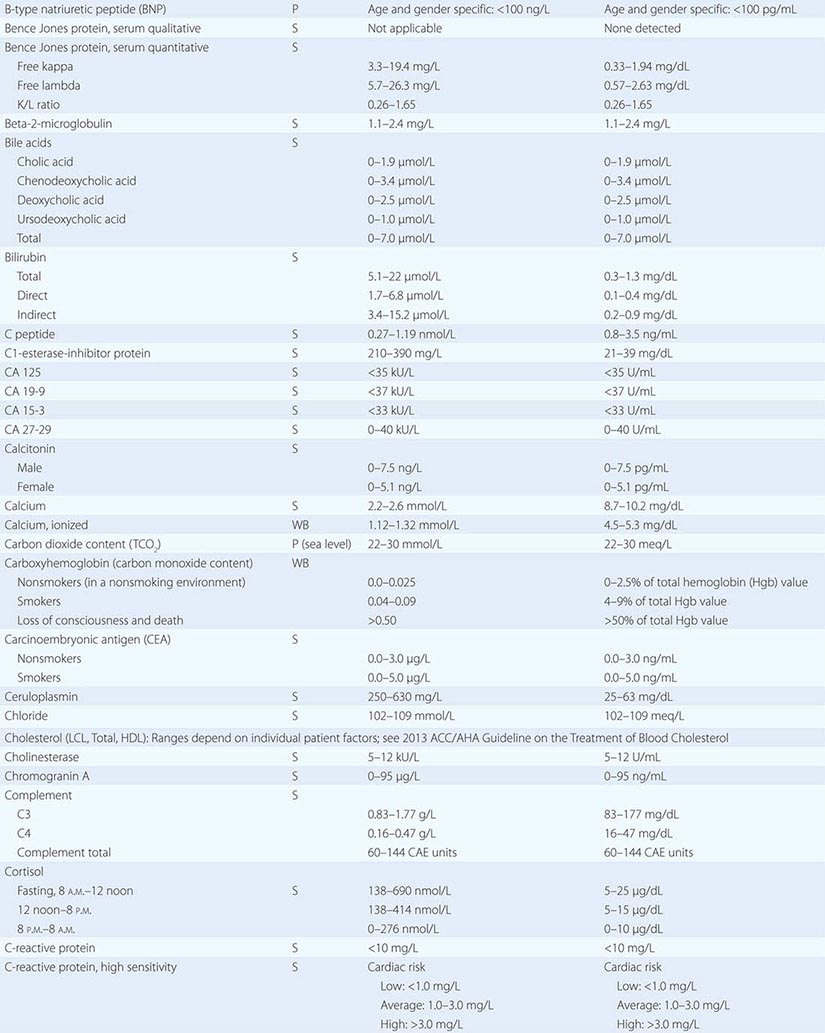
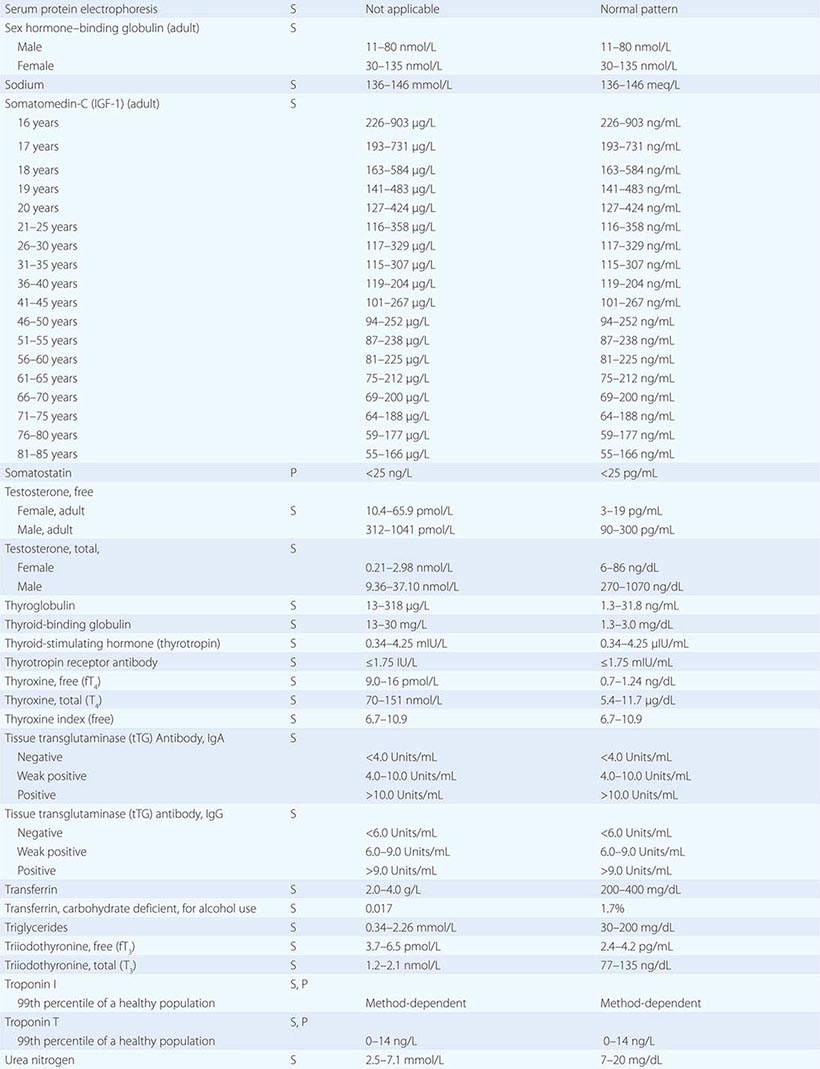
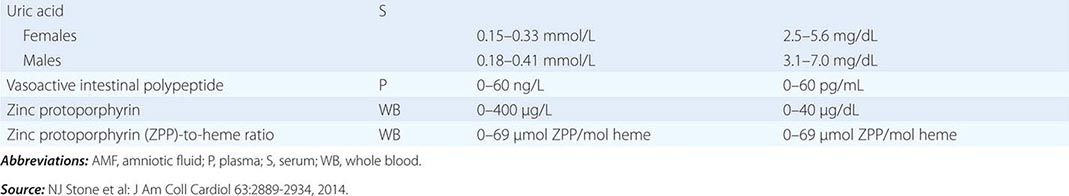
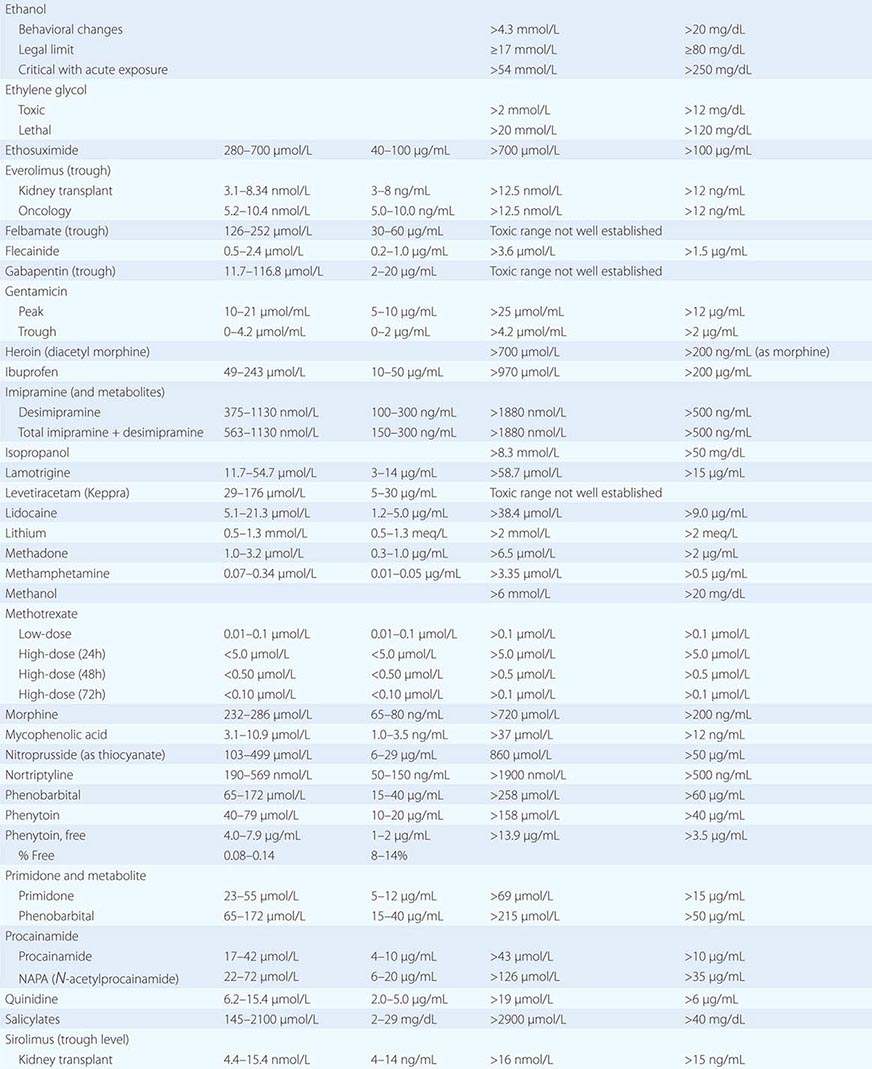
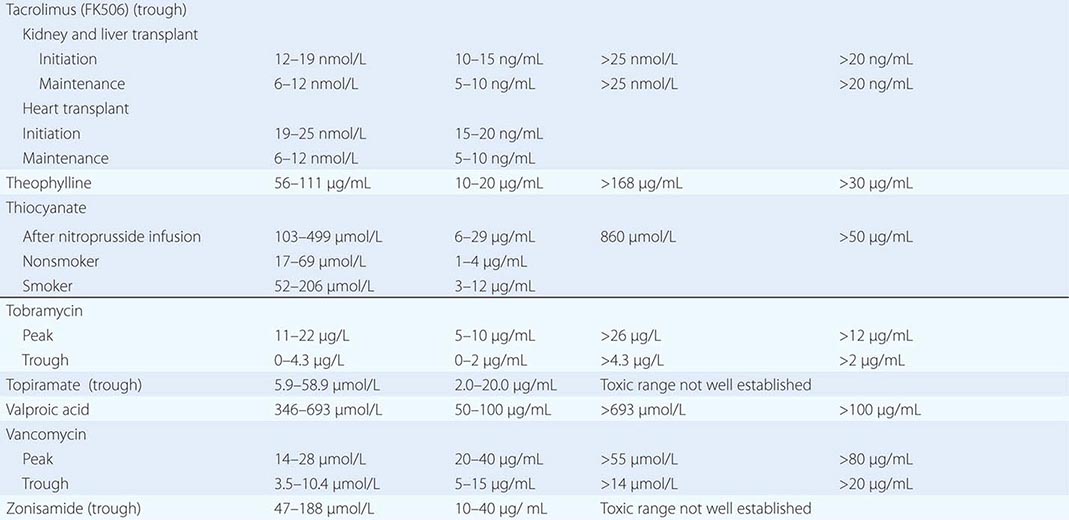
PART 19: Disorders Associated with Environmental Exposures
476e |
Altitude Illness |
EPIDEMIOLOGY
Mountains cover one-fifth of the earth’s surface; 38 million people live permanently at altitudes ≥2400 m, and 100 million people travel to high-altitude locations each year. Skiers in the Alps or Aspen; religious pilgrims to Lhasa or Kailash; trekkers and climbers to Kilimanjaro, Aconcagua, or Everest; and military personnel deployed to high-altitude locations are all at risk of developing acute mountain sickness (AMS), high-altitude cerebral edema (HACE), high-altitude pulmonary edema (HAPE), and other altitude-related problems. AMS is the benign form of altitude illness, whereas HACE and HAPE are life-threatening. Altitude illness is likely to occur above 2500 m but has been documented even at 1500–2500 m. In the Mount Everest region of Nepal, ~50% of trekkers who walk to altitudes >4000 m over ≥5 days develop AMS, as do 84% of people who fly directly to 3860 m. The incidences of HACE and HAPE are much lower than that of AMS, with estimates in the range of 0.1–4%.
PHYSIOLOGY
Ascent to a high altitude subjects the body to a decrease in barometric pressure that results in a decreased partial pressure of oxygen in the inspired gas in the lungs. This change leads in turn to less pressure driving oxygen diffusion from the alveoli and throughout the oxygen cascade. A normal initial “struggle response” to such an ascent includes increased ventilation—the cornerstone of acclimation—mediated by the carotid bodies. Hyperventilation may cause respiratory alkalosis and dehydration. Alkalosis may depress the ventilatory drive during sleep, with consequent periodic breathing and hypoxemia. During early acclimation, renal suppression of carbonic anhydrase and excretion of dilute alkaline urine combat alkalosis and tend to bring the pH of the blood to normal. Other physiologic changes during normal acclimation include increased sympathetic tone; increased erythropoietin levels, leading to increased hemoglobin levels and red blood cell mass; increased tissue capillary density and mitochondrial numbers; and higher levels of 2,3-bisphosphoglycerate, enhancing oxygen utilization. Even with normal acclimation, however, ascent to a high altitude decreases maximal exercise capacity (by ~1% for every 100 m gained above 1500 m) and increases susceptibility to cold injury due to peripheral vasoconstriction. Finally, if the ascent is made faster than the body can adapt to the stress of hypobaric hypoxemia, altitude-related disease states can result.
GENETICS
![]() Hypoxia-inducible factor, which is important in high-altitude adaptation, controls transcriptional responses to hypoxia throughout the body and is involved in the release of vascular endothelial growth factor (VEGF) in the brain, erythropoiesis, and other pulmonary and cardiac functions at high altitudes. In particular, the gene EPAS1, which codes for transcriptional regulator hypoxia-inducible factor 2α, appears to play an important role in the adaptation of Tibetans living at high altitude, resulting in lower hemoglobin concentrations than are found in the Han Chinese. For acute altitude illness, a single gene variant is unlikely to be found, but the differences in the susceptibility of individuals and populations, familial clustering of cases, and a positive association of some genetic variants all clearly support a role for genetics. Approximately 58 candidate genes have been tested, and at least one variant from 17 of these genes is associated with altitude illness.
Hypoxia-inducible factor, which is important in high-altitude adaptation, controls transcriptional responses to hypoxia throughout the body and is involved in the release of vascular endothelial growth factor (VEGF) in the brain, erythropoiesis, and other pulmonary and cardiac functions at high altitudes. In particular, the gene EPAS1, which codes for transcriptional regulator hypoxia-inducible factor 2α, appears to play an important role in the adaptation of Tibetans living at high altitude, resulting in lower hemoglobin concentrations than are found in the Han Chinese. For acute altitude illness, a single gene variant is unlikely to be found, but the differences in the susceptibility of individuals and populations, familial clustering of cases, and a positive association of some genetic variants all clearly support a role for genetics. Approximately 58 candidate genes have been tested, and at least one variant from 17 of these genes is associated with altitude illness.
ACUTE MOUNTAIN SICKNESS AND HIGH-ALTITUDE CEREBRAL EDEMA
AMS is a neurologic syndrome characterized by nonspecific symptoms (headache, nausea, fatigue, and dizziness), with a paucity of physical findings, developing 6–12 h after ascent to a high altitude. AMS is a clinical diagnosis. For uniformity in research studies, the Lake Louise Scoring System, created at the 1991 International Hypoxia Symposium, is generally used. AMS must be distinguished from exhaustion, dehydration, hypothermia, alcoholic hangover, and hyponatremia. AMS and HACE are thought to represent opposite ends of a continuum of altitude-related neurologic disorders. HACE (but not AMS) is an encephalopathy whose hallmarks are ataxia and altered consciousness with diffuse cerebral involvement but generally without focal neurologic deficits. Progression to these signal manifestations can be rapid. Papilledema and, more commonly, retinal hemorrhages may also develop. In fact, retinal hemorrhages occur frequently at ≥5000 m, even in individuals without clinical symptoms of AMS or HACE. It is unclear whether retinal hemorrhage and cerebral hemorrhage at high altitude are caused by the same mechanism.
Risk Factors The most important risk factors for the development of altitude illness are the rate of ascent and a prior history of high-altitude illness. Exertion is a risk factor, but lack of physical fitness is not. An attractive but still speculative hypothesis proposes that AMS develops in people who have inadequate cerebrospinal capacity to buffer the brain swelling that occurs at high altitude. Children and adults seem to be equally affected, but people >50 years of age may be less likely to develop AMS than younger people. Aging appears to be associated with blunting of cardiac chronotropic function and an increase in ventilatory response leading to maintenance of arterial oxygen saturation in hypoxia. Most studies reveal no gender difference in AMS incidence. A recent study showed that, in women, adaptive responses to hypoxia with aging are blunted by menopause but can be maintained with endurance training. Sleep desaturation—a common phenomenon at high altitude—is associated with AMS. Debilitating fatigue consistent with severe AMS on descent from a summit is also an important risk factor for death in mountaineers. A recently published prospective study involving trekkers and climbers who ascended to altitudes between 4000 m and 8848 m showed that high oxygen desaturation and low ventilatory response to hypoxia during exercise are independent predictors of severe altitude illness. However, because there may be overlap between groups of susceptible and nonsusceptible individuals, accurate cutoff values are hard to define. Prediction is made more difficult because the pretest probabilities of HAPE and HACE are low. Neck irradiation or surgery damaging the carotid bodies, respiratory tract infections, and dehydration appear to be other potential risk factors for altitude illness.
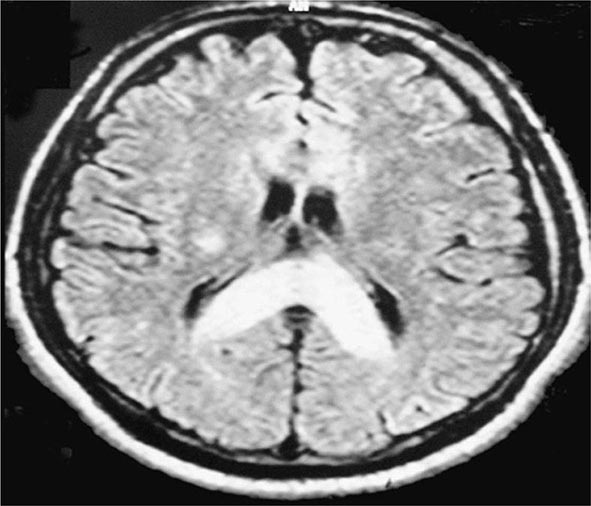
Pathophysiology The exact mechanisms causing AMS and HACE are unknown. Evidence points to a central nervous system process. MRI studies have suggested that vasogenic (interstitial) cerebral edema is a component of the pathophysiology of HACE. In the setting of high-altitude illness, the MRI findings shown in Fig. 476e-1 are confirmatory of HACE, with increased signal in the white matter and particularly in the splenium of the corpus callosum. Quantitative analysis in a 3-tesla MRI study revealed that hypoxia is associated with mild vasogenic cerebral edema irrespective of AMS. This finding is in keeping with case reports of suddenly symptomatic brain tumors and of cranial nerve palsies without AMS at high altitudes. Vasogenic edema may become cytotoxic (intracellular) in severe HACE.
FIGURE 476e-1 T2 magnetic resonance image of the brain of a patient with high-altitude cerebral edema (HACE) shows marked swelling and a hyperintense posterior body and splenium of the corpus callosum (area with dense opacity). The patient, a climber, went on to climb Mount Everest about 9 months after this episode of HACE. (With permission from Wilderness Environ Med 15:53–55, 2004.)
Impaired cerebral autoregulation in the presence of hypoxic cerebral vasodilation and altered permeability of the blood-brain barrier due to hypoxia-induced chemical mediators like histamine, arachidonic acid, and VEGF may all contribute to brain edema. In 1995, VEGF was first proposed as a potent promoter of capillary leakage in the brain at high altitude, and studies in mice have borne out this role. Although preliminary studies of VEGF in climbers have yielded inconsistent results regarding its association with altitude illness, indirect evidence of a role for this growth factor in AMS and HACE comes from the observation that dexamethasone, when used in the prevention and treatment of these conditions, blocks hypoxic upregulation of VEGF. Other factors in the development of cerebral edema may be the release of calcium-mediated nitric oxide and neuronally mediated adenosine, which may promote cerebral vasodilation.
Increased sympathetic activity triggered by hypoxia may also contribute to blood-brain barrier leakage. Enhanced optic-nerve sheath diameter with increasing severity of AMS has been noted and suggests an important role for increased intracranial pressures in the pathophysiology of AMS. Microhemorrhage formation caused by cytokines or damage through increased hydrostatic pressure is an important feature of HACE. Lesions in the globus pallidum (which is sensitive to hypoxia) leading to Parkinson’s disease have also been reported to be complications of HACE. Finally, the effect of hypoxia on reactive oxygen species and the role of these species in clinical AMS are unclear.
The pathophysiology of the most common and prominent symptom of AMS—headache—remains unclear because the brain itself is an insensate organ; only the meninges contain trigeminal sensory nerve fibers. The cause of high-altitude headache is multifactorial. Various chemicals and mechanical factors activate a final common pathway, the trigeminovascular system. In the genesis of high-altitude headache, the response to nonsteroidal anti-inflammatory drugs and glucocorticoids provides indirect evidence for involvement of the arachidonic acid pathway and inflammation. Although the International Headache Society acknowledges that high altitude may be a trigger for migraine, it is unclear whether high-altitude headache and migraine share the same pathophysiology.
Prevention and Treatment (Table 476e-1) Gradual ascent, with adequate time for acclimation, is the best method for the prevention of altitude illness. Even though there may be individual variation in the rate of acclimation, a graded ascent of ≤400 m from the previous day’s sleeping altitude is recommended above 3000 m, and taking every third day of gain in sleeping altitude as an extra day for acclimation is helpful. Spending one night at an intermediate altitude before proceeding to a higher altitude may enhance acclimation and attenuate the risk of AMS. Another protective factor in AMS is high-altitude exposure during the preceding 2 months; for example, the incidence and severity of AMS at 4300 m are reduced by 50% with an ascent after 1 week at an altitude of ≥2000 m rather than with an ascent from sea level. Studies have examined whether exposure to a normobaric hypoxic environment (in a room or a tent) before an ascent can provide protection against AMS. In double-blind placebo-controlled trials, repeated intermittent exposure (60–90 min) to normobaric hypoxia (up to 4500 m) or continuous exposure to 3000 m during 8 h of sleep for 7 consecutive days failed to reduce the incidence of AMS at altitudes of 4300–4559 m.
|
MANAGEMENT OF ALTITUDE ILLNESS |
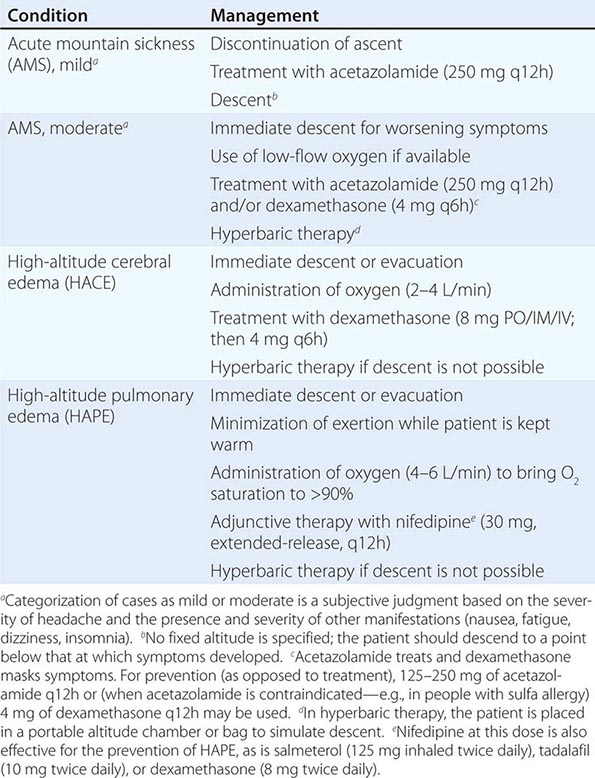
Clearly, a flexible itinerary that permits additional rest days will be helpful. Sojourners to high-altitude locations must be aware of the symptoms of altitude illness and should be encouraged not to ascend further if these symptoms develop. Any hint of HAPE (see below) or HACE mandates descent. Finally, proper hydration (but not overhydration) in high-altitude trekking and climbing, aimed at countering fluid loss due to hyperventilation and sweating, may also play a role in avoiding AMS. Pharmacologic prophylaxis at the time of travel to high altitudes is warranted for people with a history of AMS or when a graded ascent and acclimation are not possible—e.g., when rapid ascent is necessary for rescue purposes or when flight to a high-altitude location is required. Acetazolamide is the drug of choice for AMS prevention. It inhibits renal carbonic anhydrase, causing a prompt bicarbonate diuresis that leads to metabolic acidosis and hyperventilation. Acetazolamide (125–250 mg twice a day), administered for 1 day before ascent and continued for 2 or 3 days, is effective. Higher doses are not required. A meta-analysis limited to randomized controlled trials revealed that 125 mg of acetazolamide twice daily was effective in the prevention of AMS, with a relative-risk reduction of ~48% from values obtained with placebo. Paresthesia and a tingling sensation are common side effects of acetazolamide. This drug is a nonantibiotic sulfonamide that has low-level cross-reactivity with sulfa antibiotics; as a result, severe reactions are rare. Dexamethasone (8 mg/d in divided doses) is also effective. A large-scale, randomized, double-blind, placebo-controlled trial in partially acclimated trekkers has clearly shown that Ginkgo biloba is ineffective in the prevention of AMS. Recently, ibuprofen (600 mg three times daily) was shown to be beneficial in the prevention of AMS, but more definitive studies and proper gastrointestinal-bleeding risk assessment need to be conducted before ibuprofen can be routinely recommended for AMS prevention. Many drugs, including spironolactone, medroxyprogesterone, magnesium, calcium channel blockers, and antacids, confer no benefit in the prevention of AMS. Similarly, no efficacy studies are available for coca leaves (a weak form of cocaine), which are offered to high-altitude travelers in the Andes, or for soroche pills, which contain aspirin, caffeine, and acetaminophen and are sold over the counter in Bolivia and Peru. Finally, a word of caution applies in the pharmacologic prevention of altitude illness. A fast-growing population of climbers in pursuit of a summit are using prophylactic drugs such as glucocorticoids in an attempt to improve their performance; the outcome can be tragic because of potentially severe side effects of these drugs.
For the treatment of mild AMS, rest alone with analgesic use may be adequate. Descent and the use of acetazolamide and (if available) oxygen are sufficient to treat most cases of moderate AMS. Even a minor descent (400–500 m) may be adequate for symptom relief. For moderate AMS or early HACE, dexamethasone (4 mg orally or parenterally) is highly effective. For HACE, immediate descent is mandatory. When descent is not possible because of poor weather conditions or darkness, a simulation of descent in a portable hyperbaric chamber is effective and, like dexamethasone administration, “buys time.” Thus, in certain high-altitude locations (e.g., remote pilgrimage sites), the decision to bring along the light-weight hyperbaric chamber may prove life-saving. Like nifedipine, phosphodiesterase-5 inhibitors have no role in the treatment of AMS or HACE.
HIGH-ALTITUDE PULMONARY EDEMA
Risk Factors and Manifestations Unlike HACE (a neurologic disorder), HAPE is primarily a pulmonary problem and therefore is not necessarily preceded by AMS. HAPE develops within 2–4 days after arrival at high altitude; it rarely occurs after more than 4 or 5 days at the same altitude, probably because of remodeling and adaptation that render the pulmonary vasculature less susceptible to the effects of hypoxia. A rapid rate of ascent, a history of HAPE, respiratory tract infections, and cold environmental temperatures are risk factors. Men are more susceptible than women. People with abnormalities of the cardiopulmonary circulation leading to pulmonary hypertension—e.g., large patent foramen ovale, mitral stenosis, primary pulmonary hypertension, and unilateral absence of the pulmonary artery—are at increased risk of HAPE, even at moderate altitudes. For example, patent foramen ovale is four times more common among HAPE-susceptible individuals than in the general population. Echocardiography is recommended when HAPE develops at relatively low altitudes (<3000 m) and whenever cardiopulmonary abnormalities predisposing to HAPE are suspected.
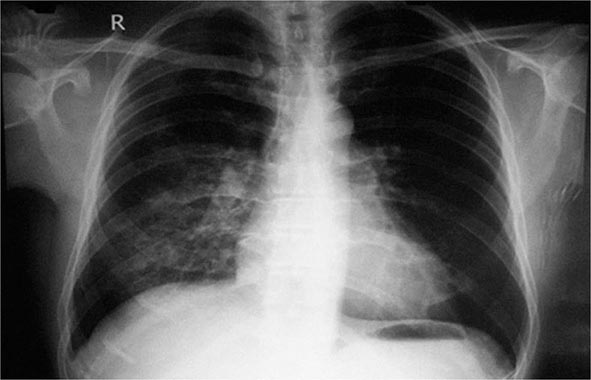
The initial manifestation of HAPE may be a reduction in exercise tolerance greater than that expected at the given altitude. Although a dry, persistent cough may presage HAPE and may be followed by the production of blood-tinged sputum, cough in the mountains is almost universal and the mechanism is poorly understood. Tachypnea and tachycardia, even at rest, are important markers as illness progresses. Crackles may be heard on auscultation but are not diagnostic. HAPE may be accompanied by signs of HACE. Patchy or localized opacities (Fig. 476e-2) or streaky interstitial edema may be noted on chest radiography. In the past, HAPE was mistaken for pneumonia due to the cold or for heart failure due to hypoxia and exertion. Kerley B lines or a bat-wing appearance are not seen on radiography. Electrocardiography may reveal right ventricular strain or even hypertrophy. Hypoxemia and respiratory alkalosis are consistently present unless the patient is taking acetazolamide, in which case metabolic acidosis may supervene. Assessment of arterial blood gases is not necessary in the evaluation of HAPE; an oxygen saturation reading with a pulse oximeter is generally adequate. The existence of a subclinical form of HAPE has been suggested by an increased alveolar-arterial oxygen gradient in Everest climbers near the summit, but hard evidence correlating this abnormality with the development of clinically relevant HAPE is lacking.
FIGURE 476e-2 Chest radiograph of a patient with high-altitude pulmonary edema shows opacity in the right middle and lower zones simulating pneumonic consolidation. The opacity cleared almost completely in 2 days with descent and supplemental oxygen.
Pathophysiology HAPE is a noncardiogenic pulmonary edema characterized by patchy pulmonary vasoconstriction that leads to overperfusion in some areas. This abnormality leads in turn to increased pulmonary capillary pressure (>18 mmHg) and capillary “stress” failure. The exact mechanism for the vasoconstriction is unknown. Endothelial dysfunction due to hypoxia may play a role by impairing the release of nitric oxide, an endothelium-derived vasodilator. At high altitude, HAPE-prone persons have reduced levels of exhaled nitric oxide. The effectiveness of phosphodiesterase-5 inhibitors in alleviating altitude-induced pulmonary hypertension, decreased exercise tolerance, and hypoxemia supports the role of nitric oxide in the pathogenesis of HAPE. One study demonstrated that prophylactic use of tadalafil, a phosphodiesterase-5 inhibitor, decreases the risk of HAPE by 65%. In contrast, the endothelium also synthesizes endothelin-1, a potent vasoconstrictor whose concentrations are higher than average in HAPE-prone mountaineers. Bosentan, an endothelin receptor antagonist, attenuates hypoxia-induced pulmonary hypertension, but further field studies with this drug are necessary.
Exercise and cold lead to increased pulmonary intravascular pressure and may predispose to HAPE. In addition, hypoxia-triggered increases in sympathetic drive may lead to pulmonary venoconstriction and extravasation into the alveoli from the pulmonary capillaries. Consistent with this concept, phentolamine, which elicits α-adrenergic blockade, improves hemodynamics and oxygenation in HAPE more than do other vasodilators. The study of tadalafil cited above also investigated dexamethasone in the prevention of HAPE. Surprisingly, dexamethasone reduced the incidence of HAPE by 78%—a greater decrease than with tadalafil. Besides possibly increasing the availability of endothelial nitric oxide, dexamethasone may have altered the excessive sympathetic activity associated with HAPE: the heart rate of participants in the dexamethasone arm of the study was significantly lowered. Finally, people susceptible to HAPE also display enhanced sympathetic activity during short-term hypoxic breathing at low altitudes.
Because many patients with HAPE have fever, peripheral leukocytosis, and an increased erythrocyte sedimentation rate, inflammation has been considered an etiologic factor in HAPE. However, strong evidence suggests that inflammation in HAPE is an epiphenomenon rather than the primary cause. Nevertheless, inflammatory processes (e.g., those elicited by viral respiratory tract infections) do predispose persons to HAPE—even those who are constitutionally resistant to its development.
Another proposed mechanism for HAPE is impaired transepithelial clearance of sodium and water from the alveoli. β-Adrenergic agonists upregulate the clearance of alveolar fluid in animal models. In a double-blind, randomized, placebo-controlled study of HAPE-susceptible mountaineers, prophylactic inhalation of the β-adrenergic agonist salmeterol reduced the incidence of HAPE by 50%. Other effects of β agonists may also contribute to the prevention of HAPE, but these findings are in keeping with the concept that alveolar fluid clearance may play a pathogenic role in this illness.
Prevention and Treatment (Table 476e-1) Allowing sufficient time for acclimation by ascending gradually (as discussed above for AMS and HACE) is the best way to prevent HAPE. Sustained-release nifedipine (30 mg), given once or twice daily, prevents HAPE in people who must ascend rapidly or who have a history of HAPE. Other drugs for the prevention of HAPE are listed in Table 476e-1 (footnote e). Although dexamethasone is listed for prevention, its adverse-effect profile requires close monitoring. Acetazolamide has been shown to blunt hypoxic pulmonary vasoconstriction in animal models, and this observation warrants further study in HAPE prevention. However, one large study failed to show a decrease in pulmonary vasoconstriction in partially acclimated individuals given acetazolamide.
Early recognition is paramount in the treatment of HAPE, especially when it is not preceded by the AMS symptoms of headache and nausea. Fatigue and dyspnea at rest may be the only initial manifestations. Descent and the use of supplementary oxygen (aimed at bringing oxygen saturation to >90%) are the most effective therapeutic interventions. Exertion should be kept to a minimum, and the patient should be kept warm. Hyperbaric therapy in a portable altitude chamber may be used if descent is not possible and oxygen is not available. Oral sustained-release nifedipine (30 mg once or twice daily) can be used as adjunctive therapy. Inhaled β agonists, which are safe and convenient to carry, are useful in the prevention of HAPE and may be effective in its treatment, although no trials have yet been carried out. Inhaled nitric oxide and expiratory positive airway pressure may also be useful therapeutic measures but may not be available in high-altitude settings. No studies have investigated phosphodiesterase-5 inhibitors in the treatment of HAPE, but reports have described their use in clinical practice. The mainstays of treatment remain descent and (if available) oxygen.
In AMS, if symptoms abate (with or without acetazolamide), the patient may reascend gradually to a higher altitude. Unlike that in acute respiratory distress syndrome (another noncardiogenic pulmonary edema), the architecture of the lung in HAPE is usually well preserved, with rapid reversibility of abnormalities (Fig. 476e-2). This fact has allowed some people with HAPE to reascend slowly after a few days of descent and rest. In HACE, reascent after a few days may not be advisable during the same trip.
OTHER HIGH-ALTITUDE PROBLEMS
Sleep Impairment The mechanisms underlying sleep problems, which are among the most common adverse reactions to high altitude, include increased periodic breathing; changes in sleep architecture, with increased time in lighter sleep stages; and changes in rapid eye movement sleep. Sojourners should be reassured that sleep quality improves with acclimation. In cases where drugs do need to be used, acetazolamide (125 mg before bedtime) is especially useful because this agent decreases hypoxemic episodes and alleviates sleeping disruptions caused by excessive periodic breathing. Whether combining acetazolamide with temazepam or zolpidem is more effective than administering acetazolamide alone is unknown. In combinations, the doses of temazepam and zolpidem should not be increased by >10 mg at high altitudes. Limited evidence suggests that diazepam causes hypoventilation at high altitudes and therefore is contraindicated. For trekkers with obstructive sleep apnea who are using a continuous positive airway pressure (CPAP) machine, the addition of acetazolamide, which will decrease centrally mediated sleep apnea, may be helpful. There is evidence to show that obstructive sleep apnea at high altitude may decrease and “convert” to central sleep apnea.
Gastrointestinal Issues High-altitude exposure may be associated with increased gastric and duodenal bleeding, but further studies are required to determine whether there is a causal effect. Because of decreased atmospheric pressure and consequent intestinal gas expansion at high altitudes, many sojourners experience abdominal bloating and distension as well as excessive flatus expulsion. In the absence of diarrhea, these phenomena are normal, if sometimes uncomfortable. Accompanying diarrhea, however, may indicate the involvement of bacteria or Giardia parasites, which are common at many high-altitude locations in the developing world. Prompt treatment with fluids and empirical antibiotics may be required to combat dehydration in the mountains. Finally, hemorrhoids are common on high-altitude treks; treatment includes hot soaks, application of hydrocortisone ointment, and measures to avoid constipation.
High-Altitude Cough High-altitude cough can be debilitating and is sometimes severe enough to cause rib fracture, especially at >5000 m. The etiology is probably multifactorial. Although high-altitude cough has been attributed to inspiration of cold dry air, this explanation appears not to be sufficient by itself; in long-duration studies in hypobaric chambers, cough has occurred despite controlled temperature and humidity. The implication is that hypoxia also plays a role. Exercise can precipitate cough at high altitudes, possibly because of water loss from the respiratory tract. Long-acting β agonists and glucocorticoids prevent bronchoconstriction that otherwise may be brought on by cold and exercise. In general, infection does not seem to be a common etiology. Anecdotal reports have described the efficacy of an inhaled combination of fluticasone and salmeterol in the treatment of high-altitude cough; however, a placebo-controlled, randomized trial failed to support this beneficial effect. Furthermore, anecdotal evidence supports the utility of the proton pump inhibitor omeprazole in preventing gastroesophageal reflux in some trekkers and climbers. In most situations, cough resolves upon descent.
High-Altitude Neurologic Events Unrelated to “Altitude Illness” Transient ischemic attacks (TIAs) and strokes have been well described in high-altitude sojourners outside the setting of altitude sickness. However, these descriptions are not based on cause (hypoxia) and effect. In general, symptoms of AMS present gradually, whereas many of these neurologic events happen suddenly. The population that suffers strokes and TIAs at sea level is generally an older age group with other risk factors, whereas those so afflicted at high altitudes are generally younger and probably have fewer risk factors for atherosclerotic vascular disease. Other mechanisms (e.g., migraine, vasospasm, focal edema, hypocapneic vasoconstriction, hypoxia in the watershed zones of minimal cerebral blood flow, or cardiac right-to-left shunt) may be operative in TIAs and strokes at high altitude.
Subarachnoid hemorrhage, transient global amnesia, delirium, and cranial nerve palsies (e.g., lateral rectus palsy) occurring at high altitudes but outside the setting of altitude sickness have also been well described. Syncope is common at moderately high altitudes, generally occurs shortly after ascent, usually resolves without descent, and appears to be a vasovagal event related to hypoxemia. Seizures occur rarely with HACE, but hypoxemia and hypocapnia, which are prevalent at high altitudes, are well-known triggers that may contribute to new or breakthrough seizures in predisposed individuals. Nevertheless, the consensus among experts is that sojourners with well-controlled seizure disorders can ascend to high altitudes. Ophthalmologic problems, such as cortical blindness, amaurosis fugax, and retinal hemorrhage with macular involvement and compromised vision, are well recognized. Visual problems from previous refractive surgery and blurred monocular vision—due either to the use of a transdermal scopolamine patch (touching the eye after touching the patch) or to dry-eye syndrome—may also occur in the field at high altitudes and may be confused with neurologic conditions. Finally, persons with hypercoagulable conditions (e.g., antiphospholipid syndrome, protein C deficiency) who are asymptomatic at sea level may experience cerebral venous thrombosis (possibly due to the enhanced blood viscosity triggered by polycythemia and dehydration) at high altitudes. Proper history taking, examination, and prompt investigations where possible will help define these conditions as entities separate from altitude sickness. Administration of oxygen (where available) and prompt descent are the cornerstones of treatment of most of these neurologic conditions.
Psychological/Psychiatric Problems Delirium characterized by a sudden change in mental status, a short attention span, disorganized thinking, and an agitated state during the period of confusion has been well described in mountain climbers and trekkers without a prior history. In addition, anxiety attacks, often triggered at night by excessive periodic breathing, are well documented. The contribution of hypoxia to these conditions is unknown. Expedition medical kits need to include antipsychotic injectable drugs to control psychosis in patients in remote high-altitude locations.
PREEXISTING MEDICAL ISSUES
Because travel to high altitudes is increasingly popular, common conditions such as hypertension, coronary artery disease, and diabetes are more frequently encountered among high-altitude sojourners. This situation is of particular concern for the thousands of elderly pilgrims with medical problems who visit high-altitude sacred areas (e.g., in the Himalayas) each year. In recent years, high-altitude travel has attracted intrepid trekkers who are taking immunosuppressive medications (e.g., kidney transplant recipients or patients undergoing chemotherapy). Recommended vaccinations and other precautions (e.g., hand washing) may be especially important for this group. Although most of these medical conditions do not appear to influence susceptibility to altitude illness, they may be exacerbated by ascent to altitude, exertion in cold conditions, and hypoxemia. Advice regarding the advisability of high-altitude travel and the impact of high-altitude hypoxia on these preexisting conditions is becoming increasingly relevant, but there are no evidence-based guidelines. In addition, recommendations made for relatively low altitudes (~3000 m) may not hold true for higher altitudes (>4000 m), where hypoxic stress is greater. Personal risks and benefits must be clearly thought through before ascent.
Hypertension At high altitudes, enhanced sympathetic activity may lead to a transient rise in blood pressure. Occasionally, nonhypertensive, healthy, asymptomatic trekkers have pathologically high blood pressure at high altitude that rapidly normalizes without medicines on descent. Sojourners should continue to take their antihypertensive medications at high altitudes. Hypertensive patients are not more likely than others to develop altitude illness. Because the probable mechanism of high-altitude hypertension is α-adrenergic activity, anti-α-adrenergic drugs like prazosin have been suggested for symptomatic patients and those with labile hypertension. It is best to start taking the drug several weeks before the trip and to carry a sphygmomanometer if a trekker has labile hypertension. Sustained-release nifedipine may also be useful. For a common problem like hypertension, there is clearly inadequate knowledge on which to base appropriate recommendations.
Coronary Artery Disease Myocardial oxygen demand and maximal heart rate are reduced at high altitudes because the VO2 max (maximal oxygen consumption) decreases with increasing altitude. This effect may explain why signs of cardiac ischemia or dysfunction usually are not seen in healthy persons at high altitudes. Asymptomatic, fit individuals with no risk factors need not undergo any tests for coronary artery disease before ascent. For persons with ischemic heart disease, previous myocardial infarction, angioplasty, and/or bypass surgery, an exercise treadmill test is indicated. A strongly positive treadmill test is a contraindication for high-altitude trips. Patients with poorly controlled arrhythmias should avoid high-altitude travel, but patients with arrhythmias that are well controlled with antiarrhythmic medications do not seem to be at increased risk. Sudden cardiac deaths are not noted with a greater frequency in the Alps than at lower altitudes; although sudden cardiac deaths are encountered every trekking season in the higher Himalayan range, accurate documentation is lacking.
Asthma Although cold air and exercise may provoke acute bronchoconstriction, asthmatic patients usually have fewer problems at high than at low altitudes, possibly because of decreased allergen levels and increased circulating catecholamine levels. Nevertheless, asthmatic individuals should carry all their medications, including oral glucocorticoids, with proper instructions for use in case of an exacerbation. Severely asthmatic persons should be cautioned against ascending to high altitudes.
Pregnancy In general, low-risk pregnant women ascending to 3000 m are not at special risk except for the relative unavailability of medical care in many high-altitude locations, especially in developing countries. Despite the lack of firm data on this point, venturing higher than 3000 m to altitudes at which oxygen saturation drops steeply seems unadvisable for pregnant women.
Obesity Although living at a high altitude has been suggested as a means of controlling obesity, obesity has also been reported to be a risk factor for AMS, probably because nocturnal hypoxemia is more pronounced in obese individuals. Hypoxemia may also lead to greater pulmonary hypertension, thus possibly predisposing the trekker to HAPE.
Sickle Cell Disease High altitude is one of the rare environmental exposures that occasionally provokes a crisis in persons with the sickle cell trait. Even when traversing mountain passes as low as 2500 m, people with sickle cell disease have been known to have a vasoocclusive crisis. Sickle cell disease needs to be considered when persons traveling to high altitudes become unwell and develop left-upper-quadrant pain. Patients with known sickle cell disease who need to travel to high altitudes should use supplemental oxygen and travel with caution.
Diabetes Mellitus Trekking at high altitudes may enhance sugar uptake. Thus, high-altitude travel may not pose problems for persons with diabetes that is well controlled with oral hypoglycemic agents. An eye examination before travel may be useful. Patients taking insulin may require lower doses on trekking/climbing days than on rest days. Because of these variations, diabetic patients need to carry a reliable glucometer and use fast-acting insulin. Ready access to sweets is also essential. It is important for companions of diabetic trekkers to be fully aware of potential problems like hypoglycemia.
Chronic Lung Disease Depending on disease severity and access to medical care, preexisting lung disease may not always preclude high-altitude travel. A proper pretravel evaluation must be conducted. Supplemental oxygen may be required if the predicted PaO2 for the altitude is <50–55 mmHg. Preexisting pulmonary hypertension may also need to be assessed in these patients. If the result is positive, patients should be discouraged from ascending to high altitudes; if such travel is necessary, treatment with sustained-release nifedipine (20 mg twice a day) should be considered. Small-scale studies have revealed that when patients with bullous disease reach ~5000 m, bullous expansion and pneumothorax are not noted. Compared with information on chronic obstructive pulmonary disease, fewer data exist about the safety of travel to high altitude for people with pulmonary fibrosis, but acute exacerbation of pulmonary fibrosis has been seen at high altitude. A handheld pulse oximeter can be useful to check for oxygen saturation.
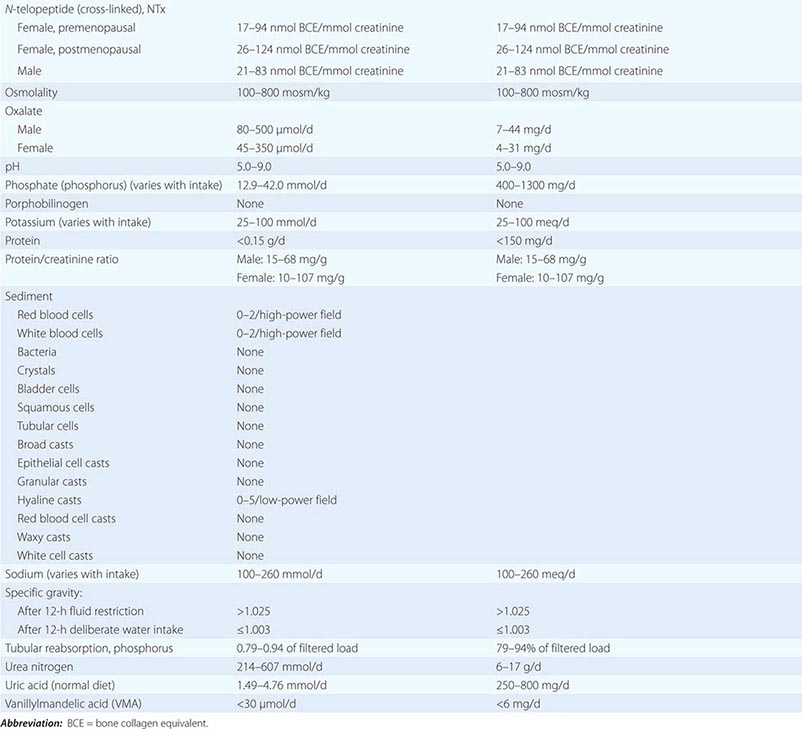
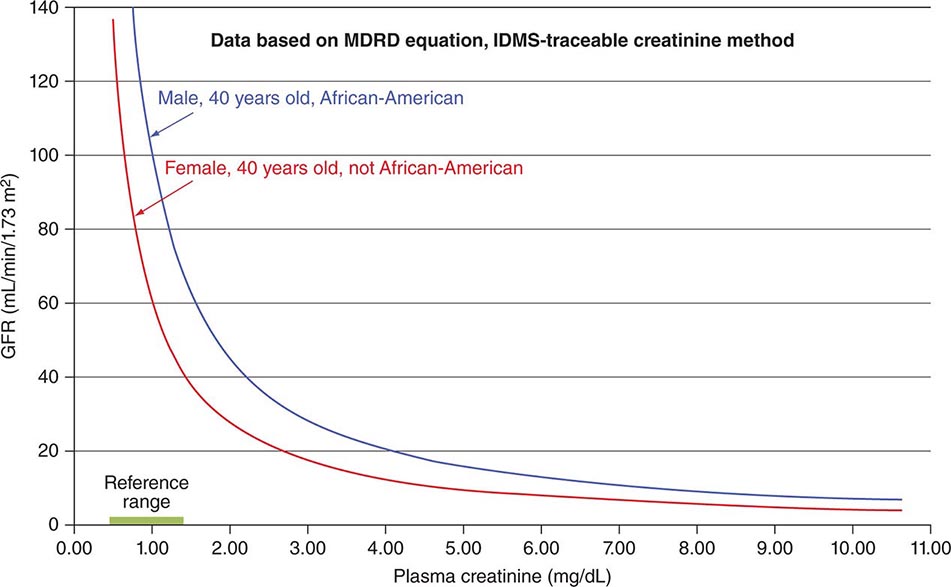
Chronic Kidney Disease Patients with chronic kidney disease can tolerate short-term stays at high altitudes, but theoretical concern persists about progression to end-stage renal disease. Acetazolamide, the drug most commonly used for altitude sickness, should be avoided by anyone with preexisting metabolic acidosis, which can be exacerbated by this drug. In addition, the acetazolamide dosage should be adjusted when the glomerular filtration rate falls below 50 mL/min, and the drug should not be used at all if this value falls below 10 mL/min.
CHRONIC MOUNTAIN SICKNESS AND HIGH-ALTITUDE PULMONARY HYPERTENSION
Chronic mountain sickness (Monge’s disease) is a disease of long-term residents of altitudes above 2500 m that is characterized by excessive erythrocytosis with moderate to severe pulmonary hypertension leading to cor pulmonale. This condition was originally described in South America and has also been documented in Colorado and in the Han Chinese population in Tibet. Migration to a low altitude results in the resolution of chronic mountain illness. Venesection and acetazolamide are helpful.
High-altitude pulmonary hypertension is also a subacute disease of long-term high-altitude residents. Unlike Monge’s disease, this syndrome is characterized primarily by pulmonary hypertension (not erythrocytosis) leading to heart failure. Indian soldiers living at extreme altitudes for prolonged periods and Han Chinese infants born in Tibet have presented with the adult and infantile forms, respectively. High-altitude pulmonary hypertension bears a striking pathophysiologic resemblance to brisket disease in cattle. Descent to a lower altitude is curative.
477e |
Hyperbaric and Diving Medicine |
WHAT IS HYPERBARIC AND DIVING MEDICINE?
Hyperbaric medicine is the treatment of health disorders using whole-body exposure to pressures greater than 101.3 kPa (1 atmosphere or 760 mmHg). In practice, this almost always means the administration of hyperbaric oxygen therapy (HBO2T). The Undersea and Hyperbaric Medical Society (UHMS) defines HBO2T as: “a treatment in which a patient breathes 100% oxygen … while inside a treatment chamber at a pressure higher than sea level pressure (i.e., >1 atmosphere absolute or ATA).” The treatment chamber is an airtight vessel variously called a hyperbaric chamber, recompression chamber, or decompression chamber, depending on the clinical and historical context. Such chambers may be capable of compressing a single patient (a monoplace chamber) or multiple patients and attendants as required (a multiplace chamber) (Figs. 477e-1 and 477e-2). Historically, these compression chambers were first used for the treatment of divers and compressed air workers suffering decompression sickness (DCS; “the bends”). Although the prevention and treatment of disorders arising after decompression in diving, aviation, and space flight has developed into a specialized field of its own, it remains closely linked to the broader practice of hyperbaric medicine.
FIGURE 477e-1 A monoplace chamber. (Prince of Wales Hospital, Sydney.)
FIGURE 477e-2 A chamber designed to treat multiple patients. (Karolinska University Hospital.)
Despite an increased understanding of mechanisms and an improving evidence basis, hyperbaric medicine has struggled to achieve widespread recognition as a “legitimate” therapeutic measure. There are several contributing factors, but high among them are a poor grounding in general oxygen physiology and oxygen therapy at medical schools and a continuing tradition of charlatans advocating hyperbaric therapy (often using air) as a panacea. Funding for both basic and clinical research has been difficult in an environment where the pharmacologic agent under study is abundant, cheap, and unpatentable. Recently, however, there are signs of an improved appreciation of the potential importance of HBO2T with significant National Institutes of Health (NIH) funding for mechanisms research and from the U.S. military for clinical investigation.
MECHANISMS OF HYPERBARIC OXYGEN
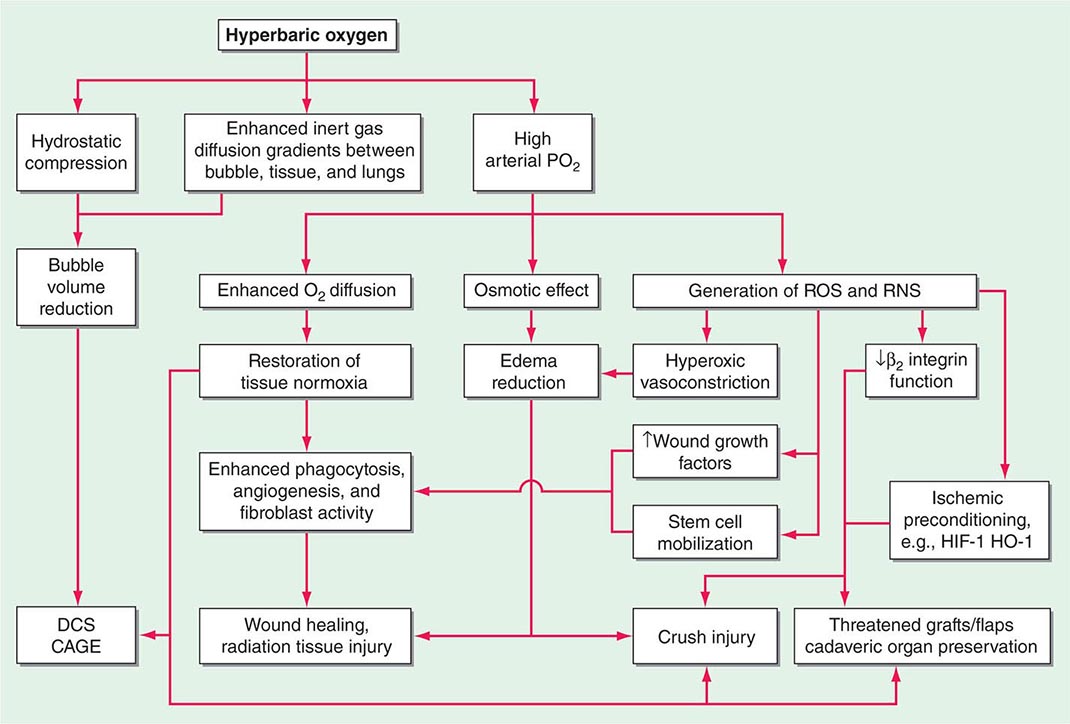
Increased hydrostatic pressure will reduce the volume of any bubbles present within the body (see “Diving Medicine”), and this is partly responsible for the success of prompt recompression in DCS and arterial gas embolism. Supplemental oxygen breathing has a dose-dependent effect on oxygen transport, ranging from improvement in hemoglobin oxygen saturation when a few liters per minute are delivered by simple mask at 101.3 kPa (1 ATA) to raising the dissolved plasma oxygen sufficiently to sustain life without the need for hemoglobin at all when 100% oxygen is breathed at 303.9 kPa (3 ATA). Most HBO2T regimens involve oxygen breathing at between 202.6 kPa and 283.6 kPa (2 and 2.8 ATA), and the resultant increase in arterial oxygen tensions to >133.3 kPa (1000 mmHg) has widespread physiologic and pharmacologic consequences (Fig. 477e-3).
FIGURE 477e-3 Mechanisms of action of hyperbaric oxygen. There are many consequences of compression and oxygen breathing. The cell-signaling effects of HBO2T are the least understood but potentially most important. Examples of indications for use are shown in the shaded boxes. CAGE, cerebral arterial gas embolism; DCS, decompression sickness; HIF-1, hypoxia inducible factor-1; HO-1, hemoxygenase 1; RNS, reactive nitrogen species; ROS, reactive oxygen species.
One direct consequence of such high intravascular tension is to increase greatly the effective capillary-tissue diffusion distance for oxygen such that oxygen-dependent cellular processes can resume in hypoxic tissues. Important as this may be, the mechanism of action is not limited to this restoration of oxygenation in hypoxic tissue. Indeed, there are pharmacologic effects that are profound and long-lasting. Although removal from the hyperbaric chamber results in a rapid return of poorly vascularized tissues to their hypoxic state, even a single dose of HBO2T produces changes in fibroblast, leukocyte, and angiogenic functions and antioxidant defenses that persist many hours after oxygen tensions are returned to pretreatment levels.
It is widely accepted that oxygen in high doses produces adverse effects due to the production of reactive oxygen species (ROS) such as superoxide (O2–) and hydrogen peroxide (H2O2). It has become increasingly clear over the last decade that both ROS and reactive nitrogen species (RNS) such as nitric oxide (NO) participate in a wide range of intracellular signaling pathways involved in the production of a range of cytokines, growth factors, and other inflammatory and repair modulators. Such mechanisms are complex and at times apparently paradoxical. For example, when used to treat chronic hypoxic wounds, HBO2T has been shown to enhance the clearance of cellular debris and bacteria by providing the substrate for macrophage phagocytosis; stimulate growth factor synthesis by increased production and stabilization of hypoxia-inducible factor 1 (HIF-1); inhibit leukocyte activation and adherence to damaged endothelium; and mobilize CD34+ pluripotent vasculogenic progenitor cells from the bone marrow. The interactions between these mechanisms remain a very active field of investigation. One exciting development is the concept of hyperoxic preconditioning in which a short exposure to HBO2 can induce tissue protection against future hypoxic/ischemic insult, most likely through an inhibition of mitochondrial permeability transition pore (MPTP) opening and the release of cytochrome c. By targeting these mechanisms of cell death during reperfusion events, HBO2 has potential applications in a variety of settings including organ transplantation. One randomized clinical trial suggested that HBO2T prior to coronary artery bypass grafting reduces biochemical markers of ischemic stress and improves neurocognitive outcomes.
ADVERSE EFFECTS OF THERAPY
HBO2T is generally well tolerated and safe in clinical practice. Adverse effects are associated with both alterations in pressure (barotrauma) and the administration of oxygen.
BAROTRAUMA
Barotrauma occurs when any noncompliant gas-filled space within the body does not equalize with environmental pressure during compression or decompression. About 10% of patients complain of some difficulty equalizing middle-ear pressure early in compression, and although most of these problems are minor and can be overcome with training, 2–5% of conscious patients require middle-ear ventilation tubes or formal grommets across the tympanic membrane. Unconscious patients cannot equalize and should have middle-ear ventilation tubes placed prior to compression if possible. Other less common sites for barotrauma of compression include the respiratory sinuses and dental caries. The lungs are potentially vulnerable to barotrauma of decompression as described below in the section on diving medicine, but the decompression following HBO2T is so slow that pulmonary gas trapping is extremely rare in the absence of an undrained pneumothorax or lesions such as bullae.
OXYGEN TOXICITY
The practical limit to the dose of oxygen, either in a single treatment session or in a series of daily sessions, is oxygen toxicity. The most common acute manifestation is a seizure, often preceded by anxiety and agitation, during which time a switch from oxygen to air breathing may avoid the convulsion. Hyperoxic seizures are typically generalized tonic-clonic seizures followed by a variable postictal period. The cause is an overwhelming of the antioxidant defense systems within the brain. Although clearly dose-dependent, onset is very variable both between individuals and within the same individual on different days. In routine clinical hyperbaric practice, the incidence is about 1:1500 to 1:2000 compressions.
Chronic oxygen poisoning most commonly manifests as myopic shift. This is due to alterations in the refractive index of the lens following oxidative damage that reduces the solubility of lenticular proteins in a process similar to that associated with senescent cataract formation. Up to 75% of patients show deterioration in visual acuity after a course of 30 treatments at 202.6 kPa (2 ATA). Although most return to pretreatment values 6–12 weeks after cessation of treatment, a small proportion do not recover. A more rapid maturation of preexisting cataracts has occasionally been associated with HBO2T. Although a theoretical problem, the development of pulmonary oxygen toxicity over time does not seem to be problematic in practice—probably due to the intermittent nature of the exposure.
CONTRAINDICATIONS TO HYPERBARIC OXYGEN
There are few absolute contraindications to HBO2T. The most commonly encountered is an untreated pneumothorax. A pneumothorax may expand rapidly on decompression and come under tension. Prior to any compression, patients with a pneumothorax should have a patent chest drain in place. The presence of other obvious risk factors for pulmonary gas trapping such as bullae should trigger a very cautious analysis of the risks of treatment versus benefit. Prior bleomycin treatment deserves special mention because of its association with a partially dose-dependent pneumonitis in about 20% of people. These individuals appear to be at particular risk for rapid deterioration of ventilatory function following exposure to high oxygen tensions. The relationship between distant bleomycin exposure and subsequent risk of pulmonary oxygen toxicity is uncertain, however late pulmonary fibrosis is a potential complication of bleomycin, and any patient with a history of receiving this drug should be carefully counseled prior to exposure to HBO2T. For those recently exposed to doses above 300,000 IU (200 mg) and whose course was complicated by a respiratory reaction to bleomycin, compression should be avoided except in a life-threatening situation.
INDICATIONS FOR HYPERBARIC OXYGEN
The appropriate indications for HBO2T are controversial and evolving. Practitioners in this area are in an unusual position. Unlike most branches of medicine, hyperbaric physicians do not deal with a range of disorders within a defined organ system (e.g., cardiology), nor are they masters of a therapy specifically designed for a single category of disorders. Inevitably, the encroachment of hyperbaric physicians into other medical fields generates suspicion from specialist practitioners in those fields. At the same time this relatively benign therapy, the prescription and delivery of which requires no medical license in most jurisdictions (including the United States), attracts both charlatans and well-motivated proselytizers who tout the benefits of oxygen for a plethora of chronic incurable diseases. This battle on two fronts has meant that mainstream hyperbaric physicians have been particularly careful to claim effectiveness only for those conditions where there is a reasonable body of supporting evidence.
In 1977, the UHMS systematically examined claims for the use of HBO2T in more than 100 disorders and found sufficient evidence to support routine use in only 12. The Hyperbaric Oxygen Therapy Committee of that organization has continued to update this list periodically with an increasingly formalized system of appraisal for new indications and emerging evidence (Table 477e-1). Around the world, other relevant medical organizations have generally taken a similar approach, although indications vary considerably—particularly those recommended by hyperbaric medical societies in Russia and China where HBO2T has gained much wider support than in the United States, Europe, and Australasia. Recently, several Cochrane reviews have examined the randomized trial evidence for many putative indications, including attempts to examine the cost-effectiveness of HBO2T. Table 477e-2 is a synthesis of these two approaches and lists the estimated cost of attaining health outcomes with the use of HBO2T. Any savings associated with alternative treatment strategies avoided as a result of HBO2T are not accounted for in these estimates (e.g., the avoidance of lower leg amputation in diabetic foot ulcers). Following are short reviews of three important indications currently accepted by the UHMS.
|
CURRENT LIST OF INDICATIONS FOR HYPERBARIC OXYGEN THERAPY |
Source: The Undersea and Hyperbaric Medical Society (2013).
|
SELECTED INDICATIONS FOR WHICH THERE IS PROMISING EFFICACY FOR THE APPLICATION OF HYPERBARIC OXYGEN THERAPY |
LATE RADIATION TISSUE INJURY
Radiotherapy is a well-established treatment for suitable malignancies. In the United States alone, approximately 300,000 individuals annually will become long-term survivors of cancer treated by irradiation. Serious radiation-related complications developing months or years after treatment (late radiation tissue injury [LRTI]) will significantly affect between 5 and 15% of those long-term survivors, although incidence varies widely with dose, age, and site. LRTI is most common in the head and neck, chest wall, breast, and pelvis.
Pathology and Clinical Course With time, tissues undergo a progressive deterioration characterized by a reduction in the density of small blood vessels (reduced vascularity) and the replacement of normal tissue with dense fibrous tissue (fibrosis). An alternative model of pathogenesis suggests that rather than a primary hypoxia, the principle trigger is an overexpression of inflammatory cytokines that promote fibrosis, probably through oxidative stress and mitochondrial dysfunction, and a secondary tissue hypoxia. Ultimately, and often triggered by a further physical insult such as surgery or infection, there may be insufficient oxygen to sustain normal function, and the tissue becomes necrotic (radiation necrosis). LRTI may be life-threatening and significantly reduce quality of life. Historically, the management of these injuries has been unsatisfactory. Conservative treatment is usually restricted to symptom management, whereas definitive treatment traditionally entails surgery to remove the affected part and extensive repair. Surgical intervention in an irradiated field is often disfiguring and associated with an increased incidence of delayed healing, breakdown of a surgical wound, or infection. HBO2T may act by several mechanisms to improve this situation, including edema reduction, vasculogenesis, and enhancement of macrophage activity (Fig. 477e-3). The intermittent application of HBO2 is the only intervention shown to increase the microvascular density in irradiated tissue.
Clinical Evidence The typical course of HBO2T consists of 30 once-daily compressions to 202.6–243.1 kPa (2–2.4 ATA) for 1.5–2 h each session, often bracketed around surgical intervention if required. Although HBO2T has been used for LRTI since at least 1975, most clinical studies have been limited to small case series or individual case reports. In a review, Feldmeier and Hampson located 71 such reports involving a total of 1193 patients across eight different tissues. There were clinically significant improvements in the majority of patients, and only 7 of 71 reports indicated a generally poor response to HBO2T. A Cochrane systematic review with meta-analysis included six randomized trials published since 1985 and drew the following conclusions (see Table 477e-2 for numbers needed to treat): HBO2T improves healing in radiation proctitis (relative risk [RR] of healing with HBO2T 2.7; 95% confidence interval [CI] 1.2–6) and after hemimandibulectomy and reconstruction of the mandible (RR 1.4; 95% CI 1.1–1.8); HBO2T improves the probability of achieving mucosal coverage (RR 1.4; 95% CI 1.2–1.6) and the restoration of bony continuity with osteoradionecrosis (ORN) (RR 1.4; 95% CI 1.1–1.8); HBO2T prevents the development of ORN following tooth extraction from a radiation field (RR 1.4; 95% CI 1.08–1.7) and reduces the risk of wound dehiscence following grafts and flaps in the head and neck (RR 4.2; 95% CI 1.1–16.8). Conversely, there was no evidence of benefit in established radiation brachial plexus lesions or brain injury.
SELECTED PROBLEM WOUNDS
A problem wound is any cutaneous ulceration that requires a prolonged time to heal, does not heal, or recurs. In general, wounds referred to hyperbaric facilities are those where sustained attempts to heal by other means have failed. Problem wounds are common and constitute a significant health problem. It has been estimated that 1% of the population of industrialized countries will experience a leg ulcer at some time. The global cost of chronic wound care may be as high as U.S. $25 billion per year.
Pathology and Clinical Course By definition, chronic wounds are indolent or progressive and resistant to the wide array of treatments applied. Although there are many contributing factors, most commonly these wounds arise in association with one or more comorbidities such as diabetes, peripheral venous or arterial disease, or prolonged pressure (decubitus ulcers). First-line treatments are aimed at correction of the underlying pathology (e.g., vascular reconstruction, compression bandaging, or normalization of blood glucose level), and HBO2T is an adjunctive therapy to good general wound care practice to maximize the chance of healing.
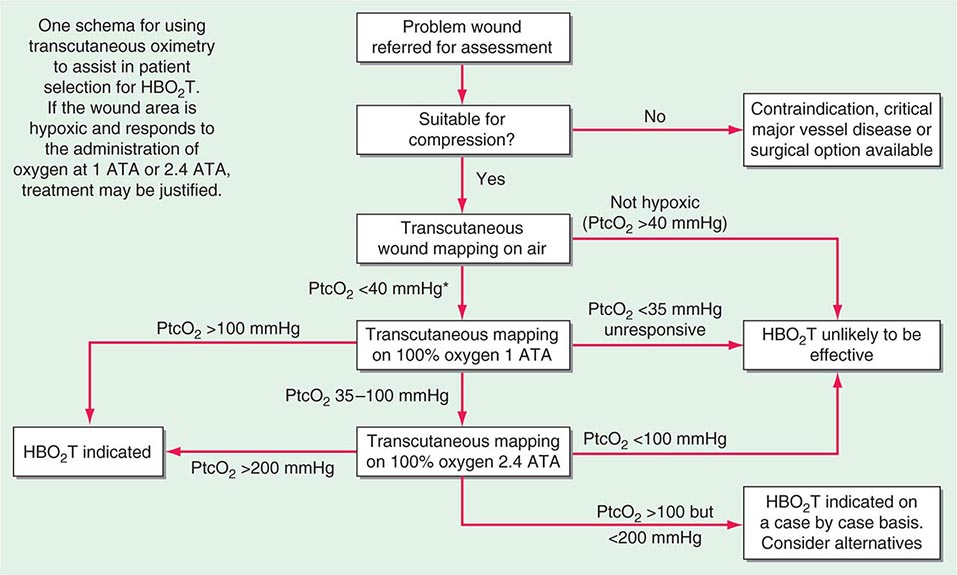
For most indolent wounds, hypoxia is a major contributor to failure to heal. Many guidelines to patient selection for HBO2T include the interpretation of transcutaneous oxygen tensions around the wound while breathing air and oxygen at pressure (Fig. 477e-4). Wound healing is a complex and incompletely understood process. While it appears that in acute wounds healing is stimulated by the initial hypoxia, low pH, and high lactate concentrations found in freshly injured tissue, some elements of tissue repair are extremely oxygen dependent, for example, collagen elaboration and deposition by fibroblasts, and bacterial killing by macrophages. In this complicated interaction between wound hypoxia and peri-wound oxygenation, successful healing relies on adequate tissue oxygenation in the area surrounding the fresh wound. Certainly, wounds that lie in hypoxic tissue beds are those that most often display poor or absent healing. Some causes of tissue hypoxia will be reversible with HBO2T, whereas some will not (e.g., in the presence of severe large vessel disease). When tissue hypoxia can be overcome by a high driving pressure of oxygen in the arterial blood, this can be demonstrated by measuring the tissue partial pressure of oxygen using an implantable oxygen electrode or, more commonly, a modified transcutaneous Clarke electrode.
FIGURE 477e-4 Determining suitability for hyperbaric oxygen therapy (HBO2T) guided by transcutaneous oximetry around the wound bed. *In diabetic patients, <50 mmHg may be more appropriate. PtcO2, transcutaneous oxygen pressure.
The intermittent presentation of oxygen to those hypoxic tissues facilitates a resumption of healing. These short exposures to high oxygen tensions have long-lasting effects (at least 24 h) on a wide range of healing processes (Fig. 477e-3). The result is a gradual improvement in oxygen tension around the wound that reaches a plateau in experimental studies at about 20 treatments over 4 weeks. Improvements in oxygenation are associated with an eight- to ninefold increase in vascular density over both normobaric oxygen and air-breathing controls.
Clinical Evidence The typical course of HBO2T consists of 20–30 once-daily compressions to 2–2.4 ATA for 1.5–2 h each session, but is highly dependent on the clinical response. There are many case series in the literature supporting the use of HBO2T for a wide range of problem wounds. Both retrospective and prospective cohort studies suggest that 6 months after a course of therapy, about 70% of indolent ulcers will be substantially improved or healed. Often these ulcers have been present for many months or years, suggesting that the application of HBO2T has a profound effect, either primarily or as a facilitator of other strategies. A recent Cochrane review included nine randomized controlled trials (RCTs) and concluded that the chance of ulcer healing improved about fivefold with HBO2T (RR 5.20; 95% CI 1.25–21.66; p = .02). Although there was a trend to benefit with HBO2T, there was no statistically significant difference in the rate of major amputations (RR 0.36; 95% CI 0.11–1.18).
CARBON MONOXIDE POISONING
Carbon monoxide (CO) is a colorless, odorless gas formed during incomplete hydrocarbon combustion. Although CO is an essential endogenous neurotransmitter linked to NO metabolism and activity, it is also a leading cause of poisoning death, and in the United States alone results in more than 50,000 emergency department visits per year and about 2000 deaths. Although there are large variations from country to country, about half of nonlethal exposures are due to self-harm. Accidental poisoning is commonly associated with defective or improperly installed heaters, house fires, and industrial exposures. The motor vehicle is by far the most common source of intentional poisoning.
Pathology and Clinical Course The pathophysiology of carbon monoxide exposure is incompletely understood. CO binds to hemoglobin with an affinity more than 200 times that of oxygen, directly reducing the oxygen-carrying capacity of blood, and further promoting tissue hypoxia by shifting the oxyhemoglobin dissociation curve to the left. CO is also an anesthetic agent that inhibits evoked responses and narcotizes experimental animals in a dose-dependent manner. The associated loss of airway patency together with reduced oxygen carriage in blood may cause death from acute arterial hypoxia in severe poisoning. CO may also cause harm by other mechanisms including direct disruption of cellular oxidative processes, binding to myoglobin and hepatic cytochromes, and peroxidation of brain lipids.
The brain and heart are the most sensitive target organs due to their high blood flow, poor tolerance of hypoxia, and high oxygen requirements. Minor exposure may be asymptomatic or present with vague constitutional symptoms such as headache, lethargy, and nausea, whereas higher doses may present with poor concentration and cognition, short-term memory loss, confusion, seizures, and loss of consciousness. While carboxyhemoglobin (COHb) levels on admission do not necessarily reflect the severity or the prognosis of CO poisoning, cardiorespiratory arrest carries a very poor prognosis. Over the longer term, surviving patients commonly have neuropsychological sequelae. Motor disturbances, peripheral neuropathy, hearing loss, vestibular abnormalities, dementia, and psychosis have all been reported. Risk factors for poor outcome are age >35 years, exposure for >24 h, acidosis, and loss of consciousness.
Clinical Evidence The typical course of HBO2T consists of two to three compressions to 2–2.8 ATA for 1.5–2 h each session. It is common for the first two compressions to be delivered within 24 h of the exposure. CO poisoning is one of the longest-standing indications for HBO2T—based largely on the obvious connection between exposure, tissue hypoxia, and the ability of HBO2T rapidly to overcome this hypoxia. CO is eliminated rapidly via the lungs on application of HBO2T, with a half-life of about 21 min at 2.0 ATA versus 5.5 h breathing air and 71 min breathing oxygen at sea level. In practice, however, it seems unlikely that HBO2T can be delivered in time to prevent either acute hypoxic death or irreversible global cerebral hypoxic injury. If HBO2T is beneficial in CO poisoning, it must reduce the likelihood of persisting and/or delayed neurocognitive deficit through a mechanism other than the simple reversal of arterial hypoxia due to high levels of COHb. The difficulty in accurately assessing neurocognitive deficit has been one of the primary sources of controversy surrounding the clinical evidence in this area. To date there have been six randomized controlled trials of HBO2T for CO poisoning, although only four have been reported in full. While a Cochrane review suggested that overall there is insufficient evidence to confirm a beneficial effect of HBO2T on the chance of persisting neurocognitive deficit following poisoning (34% of patients treated with oxygen at 1 atmosphere vs 29%, of those treated with HBO2T; odds ratio [OR] 0.78; 95% CI 0.54–1.1), this may have more to do with poor reporting and inadequate follow-up than with evidence that HBO2T is not effective. The interpretation of the literature has much to do with how one defines neurocognitive deficit. In the most methodologically rigorous of these studies (Weaver et al.), a professionally administered battery of validated neuropsychological tests and a definition based on the deviation of individual subtest scores from the age-adjusted normal values was used; if the patient complained of memory, attention, or concentration difficulties, the required decrement was decreased. Using this approach, 6 weeks after poisoning, 46% of patients treated with normobaric oxygen alone had cognitive sequelae compared to 25% of those who received HBO2T (p = .007; number needed to treat [NNT] = 5; 95% CI 3–16). At 12 months, the difference remained significant (32% vs 18%; p = .04; NNT = 7; 95% CI 4–124) despite considerable loss to follow-up.
On this basis, HBO2T remains widely advocated for the routine treatment of patients with moderate to severe poisoning—in particular in those older than 35 years, presenting with a metabolic acidosis on arterial blood-gas analysis, exposed for lengthy periods, or with a history of unconsciousness. Conversely, many toxicologists remain unconvinced about the place of HBO2T in this situation and call for further well-designed studies.
DIVING MEDICINE
INTRODUCTION
Underwater diving is both a popular recreational activity and a means of employment in a range of tasks from underwater construction to military operations. It is a complex activity with unique hazards and medical complications arising mainly as a consequence of the dramatic changes in pressure associated with both descent and ascent through the water column. For every 10.13 m increase in depth of seawater, the ambient pressure (Pamb) increases by 101.3 kPa (1 atmosphere) so that a diver at 20 m depth is exposed to a Pamb of approximately 303.9 kPa (3 ATA), made up of 1 ATA due to atmospheric pressure and 2 ATA generated by the water column.
BREATHING EQUIPMENT
Most diving is undertaken using a self-contained underwater breathing apparatus (scuba) consisting of one or more cylinders of compressed gas connected to a pressure-reducing regulator and a demand valve activated by inspiratory effort. Some divers use “rebreathers,” which are scuba devices that are closed or semiclosed circle systems with a carbon dioxide scrubber and a system designed to maintain a safe inspired PO2. Exhaled gas is recycled, and gas consumption is limited to little more than the oxygen metabolized by the diver. Rebreathers are therefore popular for deep dives where expensive helium is included in the respired mix (see below). Occupational divers frequently use “surface supply” equipment where gas, along with other utilities such as communications and power, is supplied via an umbilical from the surface.
All these systems must supply gas to the diver at the Pamb of the surrounding water or inspiration would be impossible against the surrounding water pressure. For most recreational diving, the respired gas is air. Pure oxygen is rarely used because oxygen may provoke seizures above an inspired PO2 of 162 kPa (1.6 ATA) in aquatic environments, limiting the practical safe depth to 6 m. This is a conspicuously lower PO2 than routinely used for hyperbaric therapy, reflecting a higher risk of both seizures and pulmonary toxicity during immersion. For the same reason, very deep diving requires the use of oxygen fractions lower than in air (FO2 0.21). This is because breathing air at 66 m means inspiring 1.6 ATA of oxygen, the maximum allowable pressure. To dive any deeper, breathing gases must contain less oxygen than air. Deep-diving gases often include helium instead of nitrogen to reduce both the narcotic effect and high gas density that result from breathing nitrogen at high pressures.
SUITABILITY FOR DIVING
The most common reason for physician consultation in relation to diving is for the evaluation of suitability for diver training or after a health event. Occupational diver candidates are usually compelled to see doctors with specialist training in the field, both at entry to the industry and periodically thereafter, and their medical evaluations are usually conducted according to legally mandated standards. In contrast, in most jurisdictions prospective recreational diver candidates simply complete a self-assessment medical questionnaire prior to diver training. If there are no positive responses, the candidate proceeds directly to training, but positive responses mandate the candidate see a doctor for evaluation of the identified medical issue. Prospective divers will often present to their family medicine practitioner for this purpose. In the modern era, such consultations have evolved from a simple proscriptive exercise of excluding those with potential contraindications to a more “risk analysis” approach in which each case is evaluated on its own merits. Such analyses require integration of diving physiology, the impact of associated medical problems, and a detailed knowledge of the specific medical condition of the candidate. A detailed discussion of the subject is beyond the scope of this chapter, but a few important principles are outlined below.
There are three primary questions that should be answered: (1) Could the underlying condition be exacerbated by diving? (2) Could the condition make a diving medical problem more likely? (3) Could the condition prevent the diver from meeting the functional requirements of diving? As examples, epilepsy is usually considered a contraindication because there are epileptogenic stimuli encountered in diving that could make a seizure more likely (such as thermal stress and exercise). Active asthma is a relative contraindication because it could predispose to pulmonary barotrauma (see below), and untreated ischemic heart disease is a contraindication because it could prevent a diver from exercising sufficiently to get out of a difficult situation such as being caught in a current. It can be a complex matter to recognize the relevant interactions between diving and medical conditions and to determine the impact on suitability for diving. Physicians interested in regularly conducting such evaluations should obtain relevant training. Short courses providing relevant training are offered by specialist groups in most countries.
BAROTRAUMA
The problem of middle-ear barotrauma (MEBT) with diving is similar to the problem that may occur during descent from altitude in an airplane, but difficulties with equalizing pressure in the middle ear are exaggerated underwater by both the rapidity and magnitude of pressure change as a diver descends or ascends. Failure to periodically insufflate the middle-ear spaces via the eustachian tubes during descent results in increasing pain. As the Pamb increases, the tympanic membrane (TM) may be bruised or even ruptured as it is pushed inward. Negative pressure in the middle ear results in engorgement of blood vessels in the mucous membranes and leads to effusion or bleeding, which can be associated with a conductive hearing loss. MEBT is much less common during ascent because expanding gas in the middle-ear space tends to open the eustachian tube easily and “automatically.” Barotrauma may also affect the respiratory sinuses, although the sinus ostia are usually widely patent and allow automatic pressure equalization without the need for specific maneuvers. If equalization fails, pain usually results in termination of the dive. Difficulty with equalizing ears or sinuses may respond to oral or nasal decongestants.
Much less commonly the inner ear may suffer barotrauma (IEBT). Several explanations have been proposed, of which the most favored holds that forceful attempts to insufflate the middle-ear space by Valsalva maneuvers during descent cause sudden transmission of pressure to the perilymph via the cochlear aqueduct and outward rupture of the round window already under tension because of negative middle ear pressure. The clinician should be alerted to possible IEBT after diving by a sensorineural hearing loss or true vertigo (which is often accompanied by nausea, vomiting, nystagmus, and ataxia). These manifestations can also occur in vestibulocochlear DCS (see below) but should never be attributed to MEBT. Immediate review by an expert diving physician is recommended, and urgent referral to an otologist will often follow.
The lungs are also vulnerable to barotrauma but are at most risk during ascent. If expanding gas becomes trapped in the lungs as Pamb falls, this may rupture alveoli and associated vascular tissue. Gas trapping may occur if divers intentionally or involuntarily hold their breath during ascent or if there are bullae. The extent to which asthma predisposes to pulmonary barotrauma is debated, but the presence of active bronchoconstriction must increase risk. For this reason, asthmatics who regularly require bronchodilator medications or whose airways are sensitive to exercise or cold air are usually discouraged from diving. While possible consequences of pulmonary barotrauma include pneumothorax and mediastinal emphysema, the most feared is the introduction of gas into the pulmonary veins leading to cerebral arterial gas embolism (CAGE). Manifestations of CAGE include loss of consciousness, confusion, hemiplegia, visual disturbances, and speech difficulties, appearing immediately or within minutes after surfacing. The management is the same as for DCS described below. It is notable that the natural history of CAGE often includes substantial or complete resolution of symptoms early after the event. This is probably the clinical correlate of bubble involution and redistribution with consequent restoration of flow. Patients exhibiting such remissions should still be reviewed at specialist diving medical centers because secondary deterioration or reembolization can occur. Unsurprisingly, these events can be misdiagnosed as typical strokes or transient ischemic attacks (TIAs) (Chap. 455) when patients are seen by those unfamiliar with diving medicine. All patients presenting with neurologic symptoms after diving should have their symptoms discussed with a specialist in diving medicine and be considered for recompression therapy.
DECOMPRESSION SICKNESS
DCS is caused by the formation of bubbles from dissolved inert gas (usually nitrogen) during or after ascent (decompression) from a compressed gas dive. Bubble formation is also possible following decompression for extravehicular activity during space flight and with ascent to altitude in unpressurized aircraft. DCS in the latter scenarios is probably rare in comparison with diving, where the incidence is approximately 1:10,000 recreational dives.
Breathing at elevated Pamb results in increased uptake of inert gas into blood and then into tissues. The rate at which tissue inert gas equilibrates with the inspired inert gas pressure is proportional to tissue blood flow and the blood-tissue partition coefficient for the gas. Similar factors dictate the kinetics of inert gas washout during ascent. If the rate of gas washout from tissues does not match the rate of decline in Pamb, then the sum of dissolved gas pressures in the tissue will exceed Pamb, a condition referred to as “supersaturation.” This is the prerequisite for bubbles to form during decompression, although other less well-understood factors are also involved. Deeper and longer dives result in greater inert gas absorption and greater likelihood of tissue supersaturation during ascent. Divers control their ascent for a given depth and time exposure using algorithms that often include periods where ascent is halted for a prescribed period at different depths to allow time for gas washout (“decompression stops”). Although a breach of these protocols increases the risk of DCS, adherence does not guarantee against it. DCS should be considered in any diver manifesting symptoms not readily explained by an alternative mechanism.
Bubbles may form within tissues themselves, where they cause symptoms by mechanical distraction of pain-sensitive or functionally important structures. They also appear in the venous circulation as blood passes through supersaturated tissues. Some venous bubbles are tolerated without symptoms and are filtered from the circulation in the pulmonary capillaries. However, in sufficiently large numbers, these bubbles are capable of inciting inflammatory and coagulation cascades, damaging endothelium, activating formed elements of blood such as platelets, and causing symptomatic pulmonary vascular obstruction. Moreover, if there is a right-to-left shunt such as through a patent foramen ovale (PFO) or an intrapulmonary shunt, then venous bubbles may enter the arterial circulation (25% of adults have a probe-patent PFO). The risk of cerebral, spinal cord, inner ear, and skin manifestations appears higher in the presence of significant shunts, suggesting that these “arterialized” venous bubbles can cause harm, perhaps by disrupting flow in the microcirculation of target organs. Circulating endothelial microparticles, which are elevated in number and size after diving, are currently under investigation as indicators of decompression stress or possibly as injurious agents in their own right. How they arise, and their role in DCS, remain unclear.
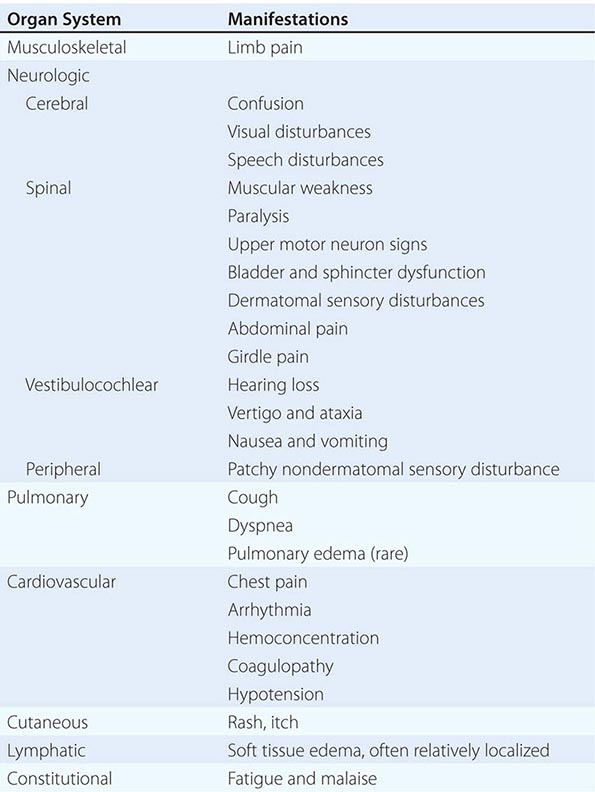
Table 477e-3 lists manifestations of DCS grouped according to organ system. The majority of cases present with mild symptoms, including musculoskeletal pain, fatigue, and minor neurologic manifestations such as patchy paresthesias. Serious presentations are much less common. Pulmonary and cardiovascular manifestations can be life-threatening, and spinal cord involvement frequently results in permanent disability. Latency is variable. Serious DCS usually manifests within minutes of surfacing, but mild symptoms may not appear for several hours. Symptoms arising more than 24 h after diving are very unlikely to be DCS. The presentation may be confusing and nonspecific, and there are as yet no useful diagnostic investigations. Diagnosis is based on integration of findings from examination of the dive profile, the nature and temporal relationship of symptoms, and the clinical examination. Some DCS presentations may be difficult to separate from CAGE following pulmonary barotrauma, but from a clinical perspective the distinction is unimportant because the first aid and definitive management of both conditions are the same.
|
MANIFESTATIONS OF DECOMPRESSION SICKNESS |
478e |
Hypothermia and Frostbite |
HYPOTHERMIA
Accidental hypothermia occurs when there is an unintentional drop in the body’s core temperature below 35°C (95°F). At this temperature, many of the compensatory physiologic mechanisms that conserve heat begin to fail. Primary accidental hypothermia is a result of the direct exposure of a previously healthy individual to the cold. The mortality rate is much higher for patients who develop secondary hypothermia as a complication of a serious systemic disorder.
CAUSES
Primary accidental hypothermia is geographically and seasonally pervasive. Although most cases occur in the winter months and in colder climates, this condition is surprisingly common in warmer regions as well. Multiple variables render individuals at the extremes of age—both the elderly and neonates—particularly vulnerable to hypothermia (Table 478e-1). The elderly have diminished thermal perception and are more susceptible to immobility, malnutrition, and systemic illnesses that interfere with heat generation or conservation. Dementia, psychiatric illness, and socioeconomic factors often compound these problems by impeding adequate measures to prevent hypothermia. Neonates have high rates of heat loss because of their increased surface-to-mass ratio and their lack of effective shivering and adaptive behavioral responses. At all ages, malnutrition can contribute to heat loss because of diminished subcutaneous fat and as a result of depleted energy stores used for thermogenesis.
|
RISK FACTORS FOR HYPOTHERMIA |
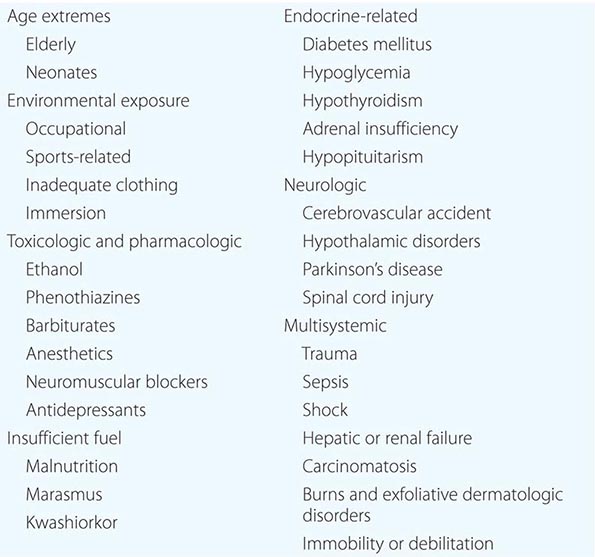
Individuals whose occupations or hobbies entail extensive exposure to cold weather are at increased risk for hypothermia. Military history is replete with hypothermic tragedies. Hunters, sailors, skiers, and climbers also are at great risk of exposure, whether it involves injury, changes in weather, or lack of preparedness.
Ethanol causes vasodilation (which increases heat loss), reduces thermogenesis and gluconeogenesis, and may impair judgment or lead to obtundation. Phenothiazines, barbiturates, benzodiazepines, heterocyclic antidepressants, and many other medications reduce centrally mediated vasoconstriction. Up to 25% of patients admitted to an intensive care unit because of drug overdose are hypothermic. Anesthetics can block shivering responses; their effects are compounded when patients are not insulated adequately in the operating or recovery units.
Several types of endocrine dysfunction can lead to hypothermia. Hypothyroidism—particularly when extreme, as in myxedema coma—reduces the metabolic rate and impairs thermogenesis and behavioral responses. Adrenal insufficiency and hypopituitarism also increase susceptibility to hypothermia. Hypoglycemia, most commonly caused by insulin or oral hypoglycemic drugs, is associated with hypothermia, in part because of neuroglycopenic effects on hypothalamic function. Increased osmolality and metabolic derangements associated with uremia, diabetic ketoacidosis, and lactic acidosis can lead to altered hypothalamic thermoregulation.
Neurologic injury from trauma, cerebrovascular accident, subarachnoid hemorrhage, and hypothalamic lesion increases susceptibility to hypothermia. Agenesis of the corpus callosum (Shapiro syndrome) is one cause of episodic hypothermia; in this syndrome, profuse perspiration is followed by a rapid fall in temperature. Acute spinal cord injury disrupts the autonomic pathways that lead to shivering and prevents cold-induced reflex vasoconstrictive responses.
Hypothermia associated with sepsis is a poor prognostic sign. Hepatic failure causes decreased glycogen storage and gluconeogenesis as well as a diminished shivering response. In acute myocardial infarction associated with low cardiac output, hypothermia may be reversed after adequate resuscitation. With extensive burns, psoriasis, erythrodermas, and other skin diseases, increased peripheral-blood flow leads to excessive heat loss.
THERMOREGULATION
Heat loss occurs through five mechanisms: radiation (55–65% of heat loss), conduction (10–15% of heat loss, much increased in cold water), convection (increased in the wind), respiration, and evaporation; both of the latter two mechanisms are affected by the ambient temperature and the relative humidity.
The preoptic anterior hypothalamus normally orchestrates thermoregulation (Chap. 23). The immediate defense of thermoneutrality is via the autonomic nervous system, whereas delayed control is mediated by the endocrine system. Autonomic nervous system responses include the release of norepinephrine, increased muscle tone, and shivering, leading to thermogenesis and an increase in the basal metabolic rate. Cutaneous cold thermoreception causes direct reflex vasoconstriction to conserve heat. Prolonged exposure to cold also stimulates the thyroid axis, leading to an increased metabolic rate.
CLINICAL PRESENTATION
In most cases of hypothermia, the history of exposure to environmental factors (e.g., prolonged exposure to the outdoors without adequate clothing) makes the diagnosis straightforward. In urban settings, however, the presentation is often more subtle, and other disease processes, toxin exposures, or psychiatric diagnoses should be considered.
After initial stimulation by hypothermia, there is progressive depression of all organ systems. The timing of the appearance of these clinical manifestations varies widely (Table 478e-2). Without knowing the core temperature, it can be difficult to interpret other vital signs. For example, tachycardia disproportionate to the core temperature suggests secondary hypothermia resulting from hypoglycemia, hypovolemia, or a toxin overdose. Because carbon dioxide production declines progressively, the respiratory rate should be low; persistent hyperventilation suggests a central nervous system (CNS) lesion or one of the organic acidoses. A markedly depressed level of consciousness in a patient with mild hypothermia raises suspicion of an overdose or CNS dysfunction due to infection or trauma.
|
PHYSIOLOGIC CHANGES ASSOCIATED WITH ACCIDENTAL HYPOTHERMIA |
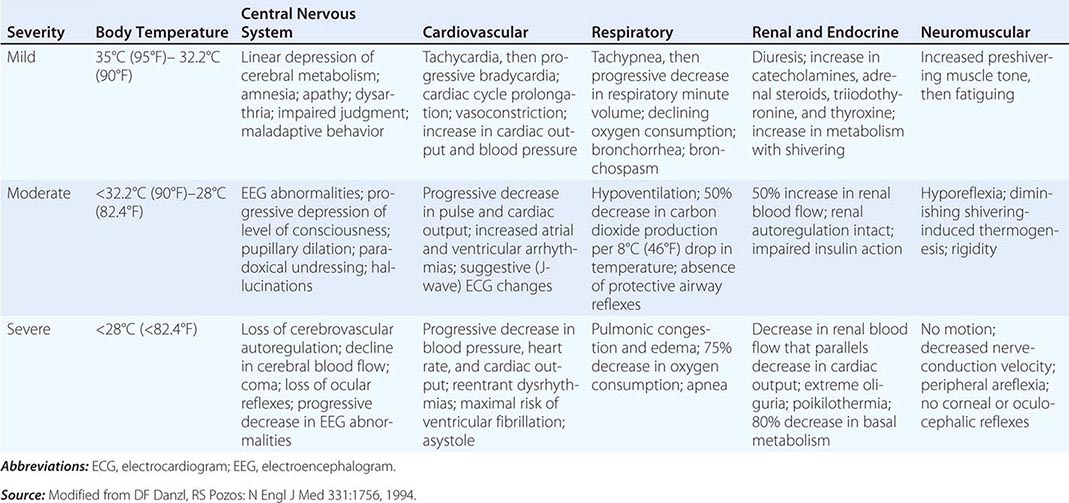
Physical examination findings can also be altered by hypothermia. For instance, the assumption that areflexia is solely attributable to hypothermia can obscure and delay the diagnosis of a spinal cord lesion. Patients with hypothermia may be confused or combative; these symptoms abate more rapidly with rewarming than with chemical or physical restraint. A classic example of maladaptive behavior in patients with hypothermia is paradoxical undressing, which involves the inappropriate removal of clothing in response to a cold stress. The cold-induced ileus and abdominal rectus spasm can mimic or mask the presentation of an acute abdomen (Chap. 20).
When a patient in hypothermic cardiac arrest is first discovered, cardiopulmonary resuscitation is indicated unless (1) a do-not-resuscitate status is verified, (2) obviously lethal injuries are identified, or (3) the depression of a frozen chest wall is not possible. As the resuscitation proceeds, the prognosis is grave if there is evidence of widespread cell lysis, as reflected by potassium levels >10–12 mmol/L (10–12meq/L). Other findings that may preclude continuing resuscitation include a core temperature <10–12°C (<50–54°F), a pH <6.5, and evidence of intravascular thrombosis with a fibrinogen value <0.5 g/L (<50 mg/dL). The decision to terminate resuscitation before rewarming the patient past 33°C (91°F) should be predicated on the type and severity of the precipitants of hypothermia. There are no validated prognostic indicators for recovery from hypothermia. A history of asphyxia with secondary cooling is the most important negative predictor of survival.
DIAGNOSIS AND STABILIZATION
Hypothermia is confirmed by measurement of the core temperature, preferably at two sites. Rectal probes should be placed to a depth of 15 cm and not adjacent to cold feces. A simultaneous esophageal probe should be placed 24 cm below the larynx; it may read falsely high during heated inhalation therapy. Relying solely on infrared tympanic thermography is not advisable.
After a diagnosis of hypothermia is established, cardiac monitoring should be instituted, along with attempts to limit further heat loss. If the patient is in ventricular fibrillation, it is unclear at what core temperature ventricular defibrillation (2 J/kg) should first be attempted. One attempt below 30°C is warranted. Further defibrillation attempts should be deferred until some rewarming (1°–2°C) is achieved and ventricular fibrillation is coarser. Although cardiac pacing for hypothermic bradydysrrhythmias is rarely indicated, the transthoracic technique is preferable.
Supplemental oxygenation is always warranted, since tissue oxygenation is affected adversely by the leftward shift of the oxyhemoglobin dissociation curve. Pulse oximetry may be unreliable in patients with vasoconstriction. If protective airway reflexes are absent, gentle endotracheal intubation should be performed. Adequate preoxygenation will prevent ventricular arrhythmias.
Insertion of a gastric tube prevents dilation secondary to decreased bowel motility. Indwelling bladder catheters facilitate monitoring of cold-induced diuresis. Dehydration is encountered commonly with chronic hypothermia, and most patients benefit from an intravenous or intraosseous bolus of crystalloid. Normal saline is preferable to lactated Ringer’s solution, as the liver in hypothermic patients inefficiently metabolizes lactate. The placement of a pulmonary artery catheter can cause perforation of the less compliant pulmonary artery. Insertion of a central venous catheter deeply into the cold right atrium should be avoided since this procedure can precipitate arrhythmias.
Arterial blood gases should not be corrected for temperature (Chap. 66). An uncorrected pH of 7.42 and a PCO2 of 40 mmHg reflect appropriate alveolar ventilation and acid-base balance at any core temperature. Acid-base imbalances should be corrected gradually, since the bicarbonate buffering system is inefficient. A common error is overzealous hyperventilation in the setting of depressed CO2 production. When the PCO2 decreases by 10 mmHg at 28°C (82°F), it doubles the pH increase of 0.08 that occurs at 37°C (99°F).
The severity of anemia may be underestimated because the hematocrit increases 2% for each 1°C drop in temperature. White blood cell sequestration and bone marrow suppression are common, potentially masking an infection. Although hypokalemia is more common in chronic hypothermia, hyperkalemia also occurs; the expected electrocardiographic changes can be obscured by hypothermia. Patients with renal insufficiency, metabolic acidoses, or rhabdomyolysis are at greatest risk for electrolyte disturbances.
Coagulopathies are common because cold inhibits the enzymatic reactions required for activation of the intrinsic cascade. In addition, thromboxane B2 production by platelets is temperature dependent, and platelet function is impaired. The administration of platelets and fresh-frozen plasma is therefore not effective. The prothrombin or partial thromboplastin times or the international normalized ratio can be deceptively normal and contrast with the observed in vivo coagulopathy. This contradiction occurs because all coagulation tests are routinely performed at 37°C (99°F), and the enzymes are thus rewarmed.
REWARMING STRATEGIES
The key initial decision is whether to rewarm the patient passively or actively. Passive external rewarming simply involves covering and insulating the patient in a warm environment. With the head also covered, the rate of rewarming is usually 0.5°–2°C (1.10°–4.4°F) per hour. This technique is ideal for previously healthy patients who develop acute, mild primary accidental hypothermia. The patient must have sufficient glycogen to support endogenous thermogenesis.
The application of heat directly to the extremities of patients with chronic severe hypothermia should be avoided because it can induce peripheral vasodilation and precipitate core temperature “afterdrop,” a response characterized by a continual decline in the core temperature after removal of the patient from the cold. Truncal heat application reduces the risk of afterdrop.
Active rewarming is necessary under the following circumstances: core temperature <32°C (<90°F) (poikilothermia), cardiovascular instability, age extremes, CNS dysfunction, hormone insufficiency, and suspicion of secondary hypothermia. Active external rewarming is best accomplished with forced-air heating blankets. Other options include devices that circulate water through external heat exchange pads, radiant heat sources, and hot packs. Monitoring a patient with hypothermia in a heated tub is extremely difficult. Electric blankets should be avoided because vasoconstricted skin is easily burned.
There are numerous widely available options for active core rewarming. Airway rewarming with heated humidified oxygen (40°–45°C [104°–113°F]) via mask or endotracheal tube is a convenient option. Although airway rewarming provides less heat than do some other forms of active core rewarming, it eliminates respiratory heat loss and adds 1°–2°C (1.1°–4.4°F) to the overall rewarming rate. Crystalloids should be heated to 40°–42°C (104°–108°F), but the quantity of heat provided is significant only during massive volume resuscitation. The most efficient method for heating and delivering fluid or blood is with a countercurrent in-line heat exchanger. Heated irrigation of the gastrointestinal tract or bladder transfers minimal heat because of the limited available surface area. These methods should be reserved for patients in cardiac arrest and then used in combination with all available active rewarming techniques.
Closed thoracic lavage is far more efficient in severely hypothermic patients with cardiac arrest. The hemithoraxes are irrigated through two inserted large-bore thoracostomy tubes. Thoracostomy tubes should not be placed in the left chest of a spontaneously perfusing patient for purposes of rewarming. Peritoneal lavage with the dialysate at 40°–45°C (104°–113°F) efficiently transfers heat when delivered through two catheters with outflow suction. Like peritoneal dialysis, standard hemodialysis is especially useful for patients with electrolyte abnormalities, rhabdomyolysis, or toxin ingestion. Another option involves the central venous insertion of a rapid endovascular warming device.
Extracorporeal blood rewarming options (Table 478e-3) should be considered in severely hypothermic patients, especially those with primary accidental hypothermia. Cardiopulmonary bypass should be considered in nonperfusing patients without documented contraindications to resuscitation. Circulatory support may be the only effective option in patients with completely frozen extremities or those with significant tissue destruction coupled with rhabdomyolysis. There is no evidence that extremely rapid rewarming improves survival in perfusing patients. The best strategy is usually a combination of passive, truncal active, and active core rewarming techniques.
|
OPTIONS FOR EXTRACORPOREAL BLOOD REWARMING |
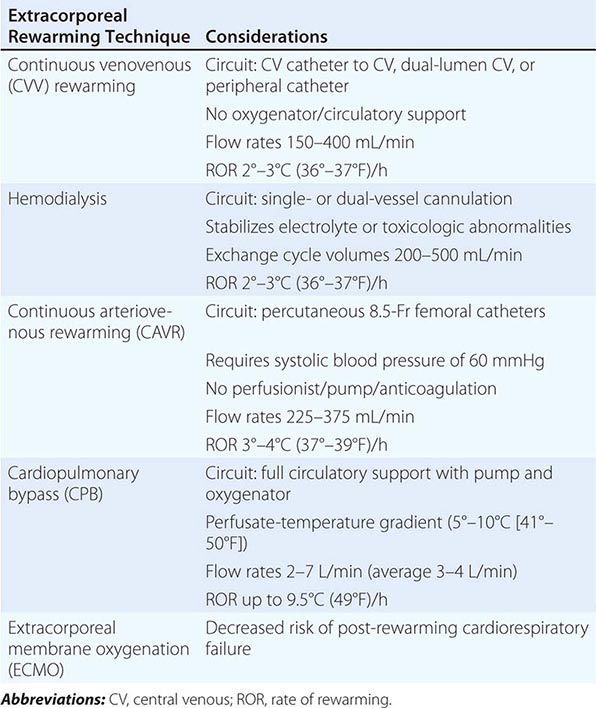
FROSTBITE
Peripheral cold injuries include both freezing and nonfreezing injuries to tissue. Tissue freezes quickly when in contact with thermal conductors such as metal and volatile solutions. Other predisposing factors include constrictive clothing or boots, immobility, and vasoconstrictive medications. Frostbite occurs when the tissue temperature drops below 0°C (32°F). Ice-crystal formation subsequently distorts and destroys the cellular architecture. Once the vascular endothelium is damaged, stasis progresses rapidly to microvascular thrombosis. After the tissue thaws, there is progressive dermal ischemia. The microvasculature begins to collapse, arteriovenous shunting increases tissue pressures, and edema forms. Finally, thrombosis, ischemia, and superficial necrosis appear. The development of mummification and demarcation may take weeks to months.
CLINICAL PRESENTATION
The initial presentation of frostbite can be deceptively benign. The symptoms always include a sensory deficiency affecting light touch, pain, and temperature perception. The acral areas and distal extremities are the most common insensate areas. Some patients describe a clumsy or “chunk of wood” sensation in the extremity.
Deep frostbitten tissue can appear waxy, mottled, yellow, or violaceous-white. Favorable presenting signs include some warmth or sensation with normal color. The injury is often superficial if the subcutaneous tissue is pliable or if the dermis can be rolled over bony prominences.
Frostnip may precede frostbite. Frostnip is a nonfreezing cold injury resulting from intense vasoconstriction of exposed acral skin.
Clinically, it is most practical to classify frostbite as superficial or deep. Superficial frostbite does not entail tissue loss but rather causes only anesthesia and erythema. The appearance of vesiculation surrounded by edema and erythema implies deeper involvement (Fig. 478e-1). Hemorrhagic vesicles reflect a serious injury to the microvasculature and indicate severe frostbite. Damages in subcuticular, muscular, or osseous tissues may result in amputation.
FIGURE 478e-1 Frostbite with vesiculation, surrounded by edema and erythema.
The two most common nonfreezing peripheral cold injuries are chilblain (pernio) and immersion (trench) foot. Chilblain results from neuronal and endothelial damage induced by repetitive exposure to dry cold. Young females, particularly those with a history of Raynaud’s phenomenon, are at greatest risk. Persistent vasospasticity and vasculitis can cause erythema, mild edema, and pruritus. Eventually plaques, blue nodules, and ulcerations develop. These lesions typically involve the dorsa of the hands and feet. In contrast, immersion foot results from repetitive exposure to wet cold above the freezing point. The feet initially appear cyanotic, cold, and edematous. The subsequent development of bullae is often indistinguishable from frostbite. This vesiculation rapidly progresses to ulceration and liquefaction gangrene. Patients with milder cases report hyperhidrosis, cold sensitivity, and painful ambulation for many years.
479e |
Heat-Related Illnesses |
Heat-related illnesses include a spectrum of disorders ranging from heat syncope, muscle cramps, and heat exhaustion to medical emergencies such as heatstroke. The core body temperature is normally maintained within a very narrow range. Although significant levels of hypothermia are tolerated (Chap. 478e), multiorgan dysfunction occurs rapidly at temperatures >41°–43°C. In contrast to severe hyperthermia, the far more common sign of fever reflects intact thermoregulation.
THERMOREGULATION
Humans are capable of significant heat generation. Strenuous exercise can increase heat generation twentyfold. The heat load from metabolic heat production and environmental heat absorption is balanced by a variety of heat dissipation mechanisms. These dissipation pathways are orchestrated by the central thermostat, which is located in the preoptic nucleus of the anterior hypothalamus. Efferent signals sent via the autonomic nervous system trigger cutaneous vasodilation and diaphoresis to facilitate heat loss.
Normally, the body dissipates heat into the environment via four mechanisms. The evaporation of skin moisture is the single most efficient mechanism of heat loss but becomes progressively ineffective as the relative humidity rises to >70%. The radiation of infrared electromagnetic energy directly into the surrounding environment occurs continuously. (Conversely, radiation is a major source of heat gain in hot climates.) Conduction—the direct transfer of heat to a cooler object—and convection—the loss of heat to air currents—become ineffective when the environmental temperature exceeds the skin temperature.
Factors that interfere with the evaporation of diaphoresis significantly increase the risk of heat illness. Examples include dripping of sweat off the skin, constrictive or occlusive clothing, dehydration, and excessive humidity. While air is an effective insulator, the thermal conductivity of water is 25 times greater than that of air at the same temperature. The wet-bulb globe temperature is a commonly used index to assess the environmental heat load. This calculation considers the ambient air temperature, the relative humidity, and the degree of radiant heat.
The regulation of this heat load is complex and involves the central nervous system (CNS), thermosensors, and thermoregulatory effectors. The central thermostat activates the effectors that produce peripheral vasodilation and sweating. The skin surface is in effect the radiator and the principal location of heat loss, since skin blood flow can increase 25–30 times over the basal rate. This dramatic increase in skin blood flow, coupled with the maintenance of peripheral vasodilation, efficiently radiates heat. At the same time, there is a compensatory vasoconstriction of the splanchnic and renal beds.
Acclimatization to heat reflects a constellation of physiologic adaptations that permit the body to lose heat more efficiently. This process often requires one to several weeks of exposure and work in a hot environment. During acclimatization, the thermoregulatory set point is altered, and this alteration affects the onset, volume, and content of diaphoresis. The threshold for the initiation of sweating is lowered, and the amount of sweat increases, with a lowered salt concentration. Sweating rates can be 1–2 L/h in acclimated individuals during heat stress. Plasma volume expansion also occurs and improves cutaneous vascular flow. The heart rate lowers, with a higher stroke volume. After the individual leaves the hot environment, improved tolerance to heat stress dissipates rapidly, the plasma volume decreases, and de-acclimatization occurs within weeks.
PREDISPOSING FACTORS AND DIFFERENTIAL DIAGNOSIS
When there is an excessive heat load, unacclimated individuals can develop a variety of heat-related illnesses. Heat waves exacerbate the mortality rate, particularly among the elderly and poor and among persons lacking adequate nutrition and access to air-conditioned environments. Secondary vascular events, including cerebrovascular accidents and myocardial infarctions, occur at least 10 times more often in conditions of extreme heat.
Exertional heat illness continues to occur when laborers, military personnel, or athletes exercise strenuously in the heat. A variety of common factors predispose to heat illness. In addition to the very young and very old, preadolescents and teenagers are at risk since they may use poor judgment when vigorously exercising in high humidity and heat. Other risk factors include obesity, poor conditioning and lack of acclimatization, and mild dehydration.
Cardiovascular inefficiency is a common feature of heat illness. Any physiologic or pharmacologic impediment to cutaneous perfusion will impair heat loss. Many patients are unaware of the heat risk associated with their medications. Anticholinergic agents impair sweating and blunt the normal cardiovascular response to heat. Phenothiazines also have anticholinergic properties that interfere with the function of the preoptic nucleus of the anterior hypothalamus due to central depletion of dopamine.
Calcium channel blockers, beta blockers, and various stimulants also inhibit sweating by reducing peripheral blood flow. To maintain the mean arterial blood pressure, increased cardiac output must be capable of compensating for progressive dehydration. A variety of stimulants and substances of abuse also increase muscle activity and heat production. Careful consideration of the differential diagnosis is important in the evaluation of a patient for a potential heat-related illness. The clinical setting may suggest other etiologies, such as malignant hyperthermia after general anesthesia or neuroleptic malignant syndrome in a patient taking certain antipsychotic medications. A variety of infectious and endocrine disorders as well as conditions with toxicologic or CNS etiologies may initially mimic heatstroke (Table 479e-1).
|
HEAT-RELATED ILLNESS: PREDISPOSING FACTORS AND DIFFERENTIAL DIAGNOSIS |
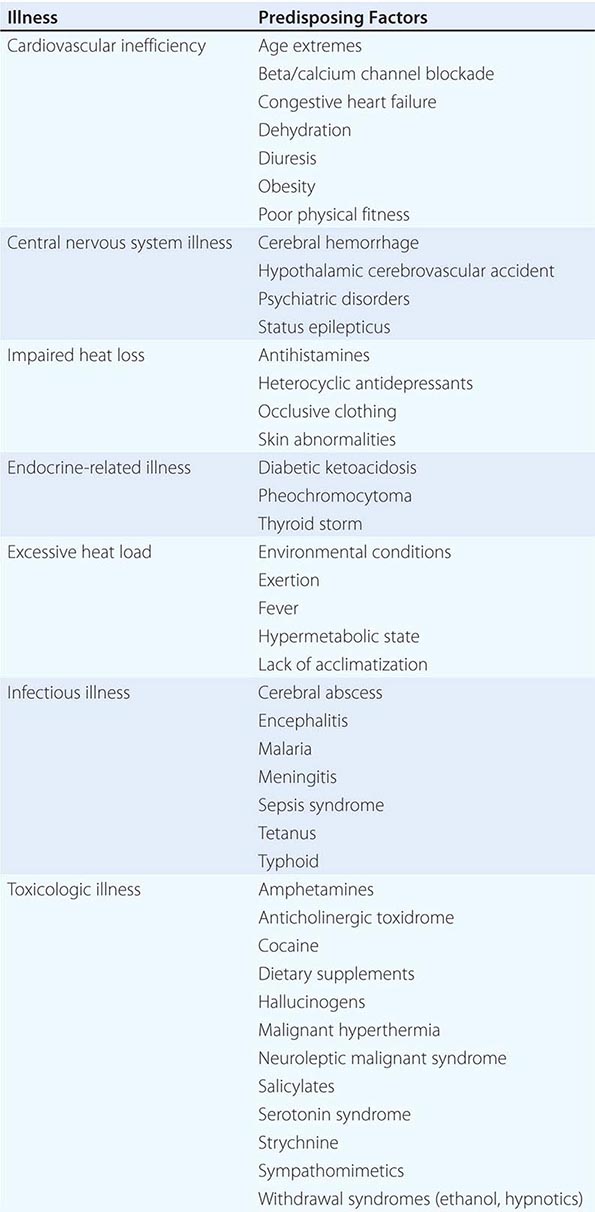
MINOR HEAT-EMERGENCY SYNDROMES
Heat edema is characterized by mild swelling of the hands, feet, and ankles during the first few days of significant heat exposure. The principal mechanism involves cutaneous vasodilation and pooling of interstitial fluid in response to heat stress. Heat also increases the secretion of antidiuretic hormone and aldosterone. Systemic causes of edema, including cirrhosis, nephrotic syndrome, and congestive heart failure, can usually be excluded by the history and physical examination. Heat edema generally resolves without treatment in several days. Diuretics are not effective and, in fact, predispose to volume depletion and the development of more serious heat-related illnesses.
Prickly heat (miliaria rubra, lichen tropicus) is a maculopapular, pruritic, erythematous rash that commonly occurs in clothed areas. Blockage of the sweat pores by debris from macerated stratum corneum causes inflammation in the sweat ducts. As the ducts dilate, they rupture and produce superficial vesicles. The predominant symptom is pruritus. In addition to antihistamines, chlorhexidine may provide some relief. Localized areas may benefit from 1% salicylic acid, with caution taken to avoid salicylate intoxication. Clothing should be clean and loose fitting, and activities or environments that induce diaphoresis should be avoided.
Heat syncope (exercise-associated collapse) can follow endurance exercise or can occur in the elderly. Other common clinical scenarios include prolonged standing while stationary in the heat and sudden standing after prolonged exposure to heat. Heat stress routinely causes relative volume depletion, decreased vasomotor tone, and peripheral vasodilation. The cumulative effect of this decrease in venous return is postural hypotension, especially in nonacclimated elderly individuals. Many of those affected also have comorbidities. Therefore, other cardiovascular, neurologic, and metabolic causes of syncope should be considered. After removal from the heat source, most patients will recover promptly with cooling and rehydration.
Hyperventilation tetany occurs in some individuals when exposure to heat stimulates hyperventilation, producing respiratory alkalosis, paresthesias, and carpopedal spasm. Unlike heat cramps, heat tetany causes very little muscle-compartment pain. Treatment includes providing reassurance, moving the patient out of the heat, and addressing the hyperventilation.
HEAT CRAMPS
Heat cramps (exercise-associated muscle cramps) are intermittent, painful, and involuntary spasmodic contractions of skeletal muscles. They typically occur in an unacclimated individual who is at rest after vigorous exertion in a humid, hot environment. In contrast, cramps that occur in athletes during exercise last longer, are relieved by stretching and massage, and resolve spontaneously.
Of note, not all muscle cramps are related to exercise, and the differential diagnosis includes many other disorders. A variety of medications, myopathies, endocrine disorders, and sickle cell trait are other possible causes.
The typical patient with heat cramps is usually profusely diaphoretic and has been replacing fluid losses with copious water or other hypotonic fluids. Roofers, firefighters, military personnel, athletes, steel workers, and field workers are commonly affected. Other predisposing factors include insufficient sodium intake before intense activity in the heat and lack of heat acclimatization, resulting in sweat with a high salt concentration.
The precise pathogenesis of heat cramps is unclear but appears to involve a relative deficiency of sodium, potassium, and fluid at the intracellular level. Coupled with copious hypotonic fluid ingestion, large amounts of sodium in the diaphoresis cause hyponatremia and hypochloremia, resulting in muscle cramps due to calcium-dependent muscle relaxation. Total-body depletion of potassium may be observed during the period of heat acclimatization. Rhadomyolysis is very rare with routine exercise-associated muscle cramps.
Heat cramps that are not accompanied by significant dehydration can be treated with commercially available electrolyte solutions. Although the flavored electrolyte solutions are far more palatable, two 650-mg salt tablets dissolved in 1 quart of water produces a 0.1% saline solution. Individuals should avoid the ingestion of undissolved salt tablets, which are a gastric irritant and may induce vomiting.
HEAT EXHAUSTION
The physiologic hallmarks of heat exhaustion—in contrast to heatstroke—are the maintenance of thermoregulatory control and CNS function. The core temperature is usually elevated but is generally <40.5°C (<105°F). The two physiologic precipitants are water depletion and sodium depletion, which often occur in combination. Laborers, athletes, and elderly individuals exerting themselves in hot environments, without adequate fluid intake, tend to develop water-depletion heat exhaustion. Persons working in the heat frequently consume only two-thirds of their net water loss and are voluntarily dehydrated. In contrast, salt-depletion heat exhaustion occurs more slowly in unacclimated persons who have been consuming large quantities of hypotonic solutions.
Heat exhaustion is usually a diagnosis of exclusion because of the multitude of nonspecific symptoms. If any signs of heatstroke are present, rapid cooling and crystalloid resuscitation should be initiated immediately during stabilization and evaluation. Mild neurologic and gastrointestinal influenza-like symptoms are common. These symptoms may include headache, vertigo, ataxia, impaired judgment, malaise, dizziness, nausea, and muscle cramps. Orthostatic hypotension and sinus tachycardia develop frequently. More significant CNS impairment suggests heatstroke or other infectious, neurologic, or toxicologic diagnoses.
Hemoconcentration does not always develop, and rapid infusion of isotonic IV fluids should be guided by frequent electrolyte determinations and perfusion requirements. Most cases of heat exhaustion reflect mixed sodium and water depletion. Sodium-depletion heat exhaustion is characterized by hyponatremia and hypochloremia. Hepatic aminotransferases are mildly elevated in both types of heat exhaustion. Urinary sodium and chloride concentrations are usually low.
Some patients with heat exhaustion develop heatstroke after removal from the heat-stress environment. Aggressive cooling of nonresponders is indicated until their core temperature is 39°C (102.2°F). Except in mild cases, free water deficits should be replaced slowly over 24–48 h to avoid a decrease of serum osmolality by >2 mOsm/h.
The disposition of younger, previously healthy heat-exhaustion patients who have no major laboratory abnormalities may include hospital observation and discharge after IV rehydration. Older patients with comorbidities (including cardiovascular disease) or predisposing factors often require inpatient fluid and electrolyte replacement, monitoring, and reassessment.
HEATSTROKE
The clinical manifestations of heatstroke reflect a total loss of thermoregulatory function. Typical vital-sign abnormalities include tachypnea, various tachycardias, hypotension, and a widened pulse pressure. Although there is no single specific diagnostic test, the historical and physical triad of exposure to a heat stress, CNS dysfunction, and a core temperature >40.5°C helps establish the preliminary diagnosis. the definitive diagnosis should be reserved until the other potential causes of hyperthermia are excluded. Many of the usual laboratory abnormalities seen with heatstroke overlap with other conditions. If the patient’s mental status does not improve with cooling, toxicologic screening may be indicated, and cranial CT and spinal fluid analysis can be considered.
The premonitory clinical characteristics may be nonspecific and include weakness, dizziness, disorientation, ataxia, and gastrointestinal or psychiatric symptoms. These prodromal symptoms often resemble heat exhaustion. The sudden onset of heatstroke occurs when the maintenance of adequate perfusion requires peripheral vasoconstriction to stabilize the mean arterial blood pressure. As a result, the cutaneous radiation of heat ceases. At this juncture, the core temperature rises dramatically. Since many patients with heatstroke also meet the criteria for systemic inflammatory response syndrome and have a broad differential diagnosis, rapid cooling is essential during the extensive diagnostic evaluation (Table 479e-1).
There are two forms of heatstroke with significantly different manifestations (Table 479e-2). Classic (epidemic) heatstroke (CHS) usually occurs during long periods of high ambient temperature and humidity, as during summer heat waves. Patients with CHS commonly have chronic diseases that predispose to heat-related illness, and they may have limited access to oral fluids. Heat dissipation mechanisms are overwhelmed by both endogenous heat production and exogenous heat stress. Patients with CHS are often compliant with prescribed medications that can impair tolerance to a heat stress. In many of these dehydrated CHS patients, sweating has ceased and the skin is hot and dry. If cooling is delayed, severe hepatic dysfunction, renal failure, disseminated intravascular coagulation, and fulminant multisystem organ failure may occur. Hepatocytes are very heat sensitive. On presentation, the serum level of aspartate aminotransferase (AST) is routinely elevated. Eventually, levels of both AST and alanine aminotransferase (ALT) often increase to >100 times the normal values. Coagulation studies commonly demonstrate decreased platelets, fibrinogen, and prothrombin. Most patients with CHS require cautious crystalloid resuscitation, electrolyte monitoring, and—in certain refractory cases—consideration of central venous pressure (CVP) measurements. Hypernatremia is secondary to dehydration in CHS. Many patients exhibit significant stress leukocytosis, even in the absence of infection.
|
TYPICAL MANIFESTATIONS OF HEATSTROKE |
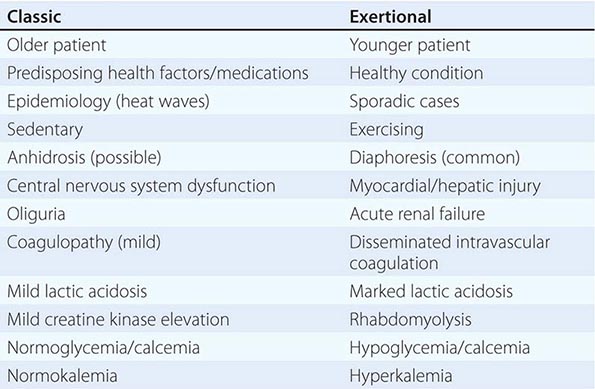
Patients with exertional heatstroke (EHS), in contrast to those with CHS, are often young and previously healthy, and their diagnosis is usually more obvious from the history. Athletes, laborers, and military recruits are common victims. Unlike those with CHS, many EHS patients present profusely diaphoretic despite significant dehydration. As a result of muscular exertion, rhabdomyolysis and acute renal failure are more common in EHS. Studies to detect rhabdomyolysis and its complications, including hypocalcemia and hyperphosphatemia, should be considered. Hyponatremia, hypoglycemia, and coagulopathies are frequent findings. Elevated creatine kinase and lactate dehydrogenase levels also suggest EHS. Oliguria is a common finding. Renal failure can result from direct thermal injury, untreated rhabdomyolysis, or volume depletion. Common urinalysis findings include microscopic hematuria, myoglobinuria, and granular or red cell casts.
With both CHS and EHS, heat-related reversible increases in cardiac biomarker levels are often present. Heatstroke often causes thermal cardiomyopathy. As a result, the CVP may be elevated despite significant dehydration. In addition, the patient often presents with potentially deceptive noncardiogenic pulmonary edema and basilar rales despite being significantly hypovolemic. The electrocardiogram commonly displays a variety of tachyarrhythmias, nonspecific ST-T wave changes, and heat-related ischemia or infarction. Rapid cooling—not the administration of antiarrhythmic medications—is essential.
Above 42°C (107.6° F), heat can rapidly produce direct cellular injury. Thermosensitive enzymes become nonfunctional, and eventually there is irreversible uncoupling of oxidative phosphorylation. The production of heat-shock proteins increases, and cytokines mediate a systemic inflammatory response. The vascular endothelium is also damaged, and this injury activates the coagulation cascade. Significant shunting away from the splanchnic circulation produces gastrointestinal ischemia. Endotoxins further impair normal thermoregulation. As a result, if cooling is delayed, severe hepatic dysfunction, permanent renal failure, disseminated intravascular coagulation, and fulminant multisystem organ failure may occur.
COOLING STRATEGIES
Before cooling is initiated, endotracheal intubation, CVP determination, and continuous core-temperature monitoring should be considered. Hypoglycemia is a frequent finding and can be addressed by glucose infusion. Since peripheral vasoconstriction delays heat dissipation, repeated administration of discrete boluses of isotonic crystalloid for hypotension is preferable to the administration of α-adrenergic agonists.
Evaporative cooling is usually the most practical and effective technique. Rapid cooling is essential in both CHS and EHS, and an immediate improvement in vital signs and mental status may prove valuable for diagnostic purposes. Cool water (15°C [60° F]) is sprayed on the exposed skin while fans direct continuous airflow over the moistened skin. Cold packs applied to the axillae and groin are a useful cooling adjunct. If cardiac electrodes will not adhere, they can be applied to the patient’s back. To avoid “overshoot hypothermia,” active cooling should be terminated at ~38°–39°C (100.4°–102.2°F).
Immersion cooling in cold water is an alternative option in EHS but induces peripheral vasoconstriction and intense shivering. This technique presents significant monitoring and resuscitation challenges in most clinical settings. The safety of immersion cooling is best established for young, previously healthy patients with EHS (but not for those with CHS).
Cooling with commercially available cooling blankets should not be the sole technique used, since the rate of cooling is far too slow. Other methods are less efficacious and rarely indicated, such as IV infusion of cold fluids and cold irrigation of the bladder or gastrointestinal tract. Cold thoracic and peritoneal lavage are efficient maneuvers but are invasive and rarely necessary. Cardiopulmonary bypass provides fast and effective cooling but is labor intensive and is rarely available on a stat basis.
RESUSCITATION
Aspiration and seizures commonly occur in heatstroke, and endotracheal intubation is usually necessary. The metabolic demands are high, and supplemental oxygenation is essential due to hypoxemia induced by thermal stress and pulmonary dysfunction. Pneumonitis, pulmonary infarction, hemorrhage, edema, and acute respiratory distress syndrome occur frequently in heatstroke patients.
The circulatory fluid requirements, particularly in CHS, may be deceptively modest. Aggressive cooling and modest volume repletion usually elevate the CVP to 12–14 mmHg. The reading, however, may be deceptive. Many patients present with a thermally induced hyperdynamic circulation accompanied by a high cardiac index, low peripheral vascular resistance, and an elevated CVP caused by right-sided heart failure. Rarely, wedge pressures measured via a pulmonary artery catheter may be necessary to guide resuscitation. In contrast, most patients with EHS require far more zealous isotonic crystalloid resuscitation.
The hypotension that is initially common among patients with heatstroke results from both dehydration and high-output cardiac failure caused by peripheral vasodilation. Inotropes causing α-adrenergic stimulation (e.g., norepinephrine) can impede cooling by causing significant vasoconstriction. Vasoactive catecholamines such as dopamine or dobutamine may be necessary if the cardiac output remains depressed despite an elevated CVP, particularly in patients with a hyperdynamic circulation.
A wide variety of tachyarrhythmias are routinely observed on presentation and usually resolve during cooling. The administration of atrial or ventricular antiarrhythmic medications is rarely indicated during cooling. Anticholinergic medications (including atropine) that inhibit sweating should not be given, and electrical cardioversion of the hyperthermic myocardium should be avoided except when there is ventricular fibrillation.
Significant shivering, discomfort, or extreme agitation is preferably mitigated with short-acting benzodiazepines, which are ideal due to their renal clearance. On the other hand, chlorpromazine may lower the seizure threshold, has anticholinergic properties, and can exacerbate the hypotension or cause neuroleptic malignant syndrome. Because of likely hepatic dysfunction, barbiturates should be avoided and seizures should be treated with benzodiazepines.
Coagulopathies more commonly occur after the first day of illness. After cooling, the patient should be monitored for laboratory signs of disseminated intravascular coagulation, and replacement therapy with fresh-frozen plasma and platelets should be considered.
There is no therapeutic role for antipyretics in the control of environmentally induced hyperthermia; these drugs block the actions of pyrogens at hypothalamic receptor sites. Salicylates can further uncouple oxidative phosphorylation in heatstroke and exacerbate coagulopathies. Acetaminophen may further stress hepatic function. The safety and efficacy of other medications, including dantrolene and aminocaproic acid, are not established.
DISPOSITION
Most patients with minor heat-emergency syndromes (including heat edema, heat syncope, and heat cramps) require only stabilization and treatment with outpatient follow-up. Although there are no decision rules to guide disposition choices in heat exhaustion, many of these patients have multiple predisposing factors and comorbidities that will require prolonged observation or hospital admission.
Essentially all patients with actual heatstroke require admission to a monitored setting, and most require intensive care. Many of these patients also require prolonged tracheal intubation, invasive hemodynamic monitoring, and support for various degrees of multiorgan dysfunction syndrome. The prognosis worsens if the initial core temperature exceeds 42°C (107.6°F) or if there was a prolonged period during which the core temperature exceeded this level. Other features of a negative prognosis include acute renal failure, massively elevated liver enzymes, and significant hyperkalemia. As expected, the number of dysfunctional organ systems also correlates directly with mortality risk.
APPENDIX: |
Laboratory Values of Clinical Importance |
This Appendix contains tables of reference values for common laboratory tests. A variety of factors can influence reference values. Such variables include the population studied, the duration and means of specimen transport, laboratory methods and instrumentation, and even the type of container used for the collection of the specimen. The reference or “normal” ranges given in this appendix may therefore not be appropriate for all laboratories, and these values should only be used as general guidelines. Whenever possible, reference values provided by the laboratory performing the testing should be used in the interpretation of laboratory data. Values supplied in this Appendix reflect typical reference ranges in nonpregnant adults. Pediatric reference ranges and values in pregnant patients may vary significantly from the data presented in the Appendix.
In preparing the Appendix, the authors have taken into account the fact that the system of international units (SI, système international d’unités) is used in most countries and in some medical journals. However, clinical laboratories may continue to report values in “traditional” or conventional units. Therefore, both systems are provided in the Appendix. The dual system is also used in the text except for those instances in which the numbers remain the same and only the terminology is changed (mmol/L for meq/L or IU/L for mIU/mL), when only the SI units are given.
|
HEMATOLOGY AND COAGULATION |
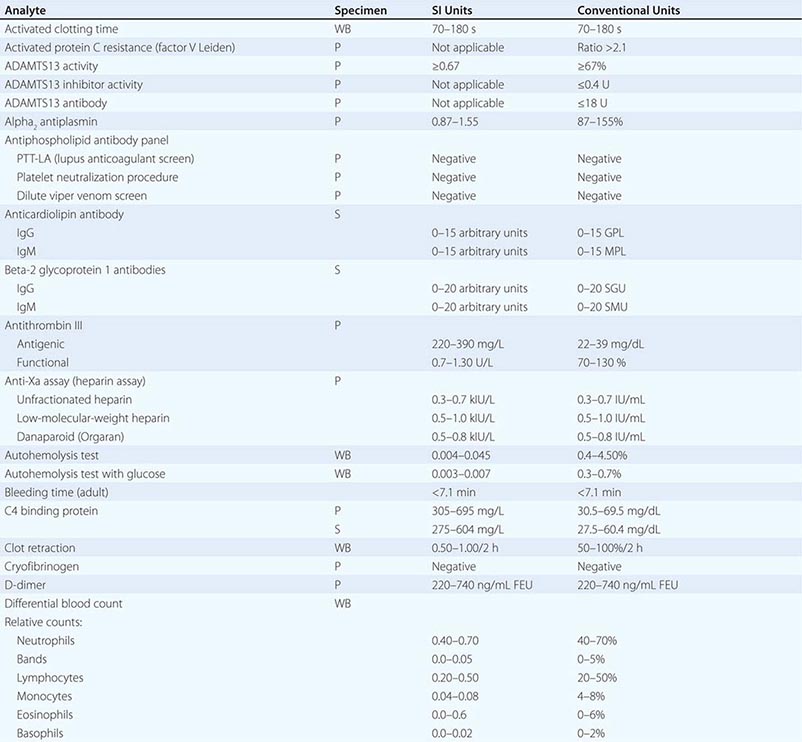
|
CLINICAL CHEMISTRY AND IMMUNOLOGY |
|
TOXICOLOGY AND THERAPEUTIC DRUG MONITORING |
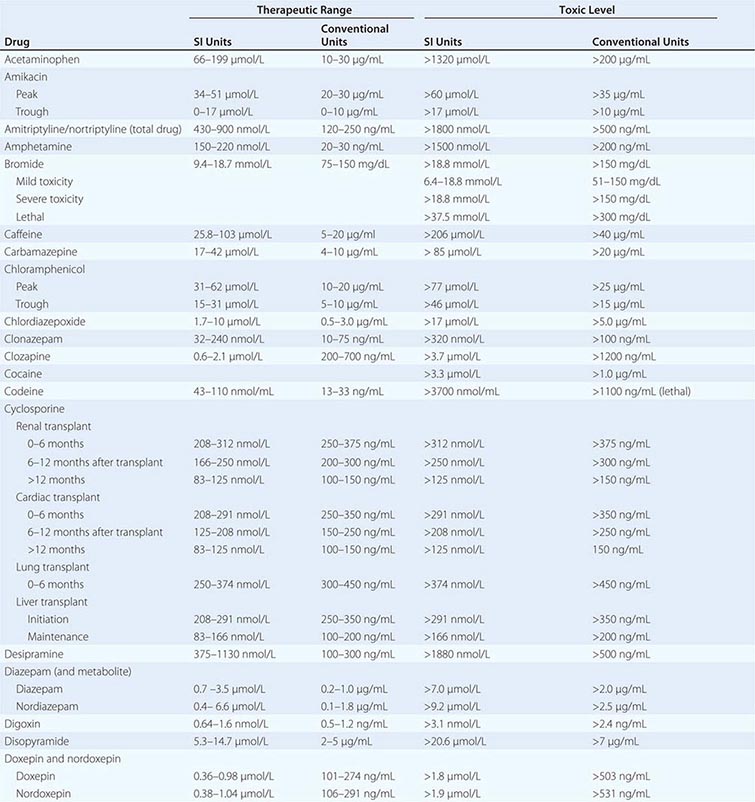
|
VITAMINS AND SELECTED TRACE MINERALS |
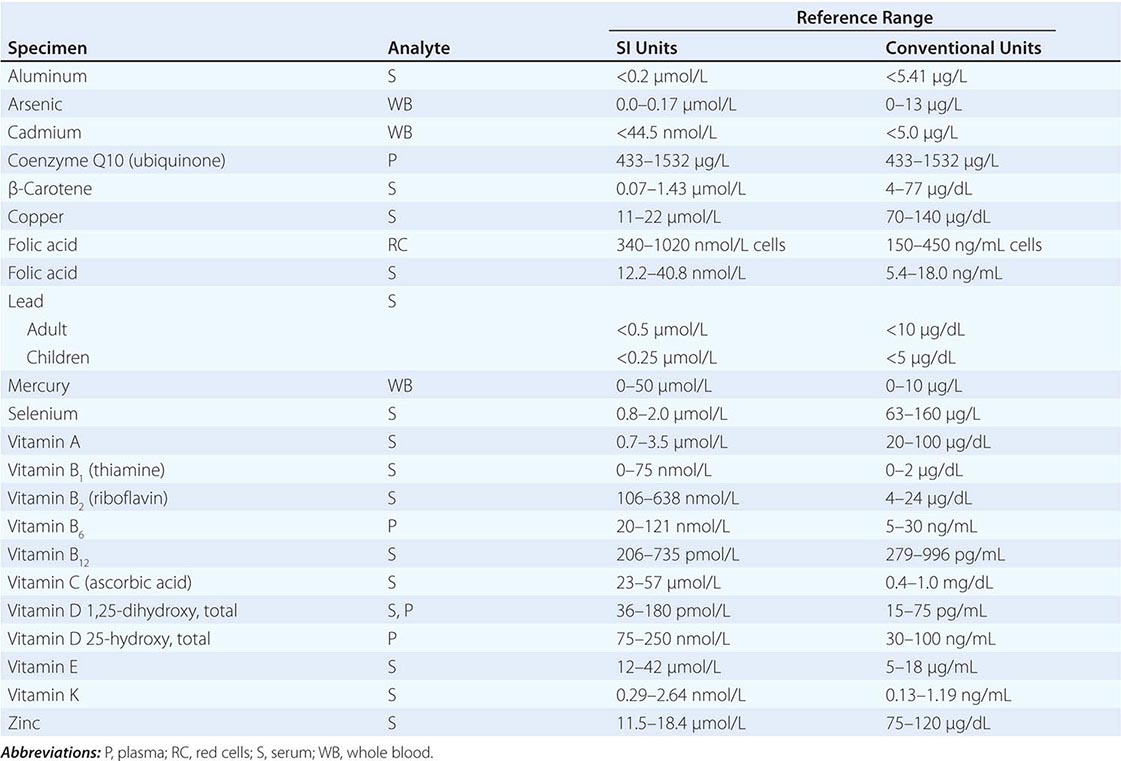
|
CEREBROSPINAL FLUIDa |
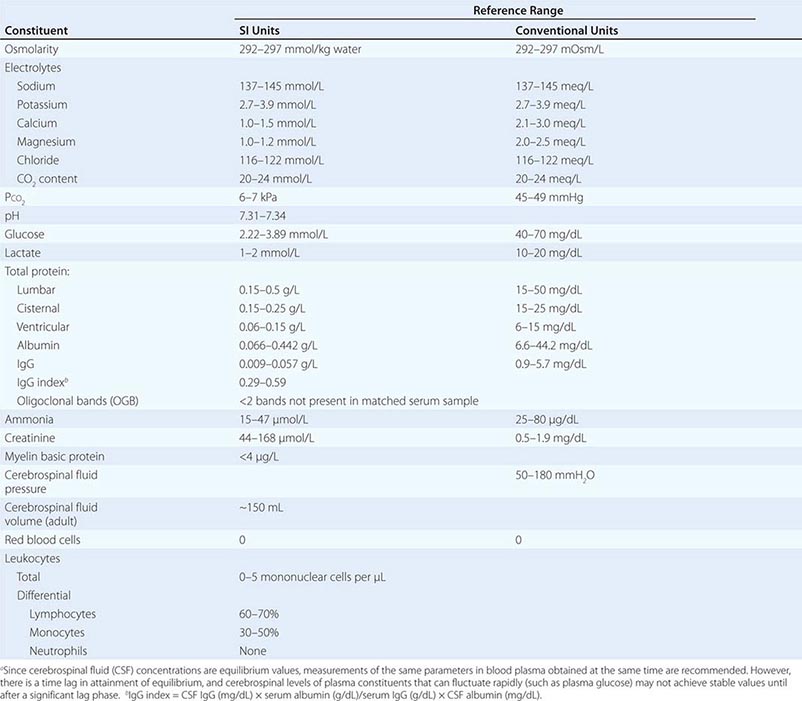
|
URINE ANALYSIS AND RENAL FUNCTION TESTS |
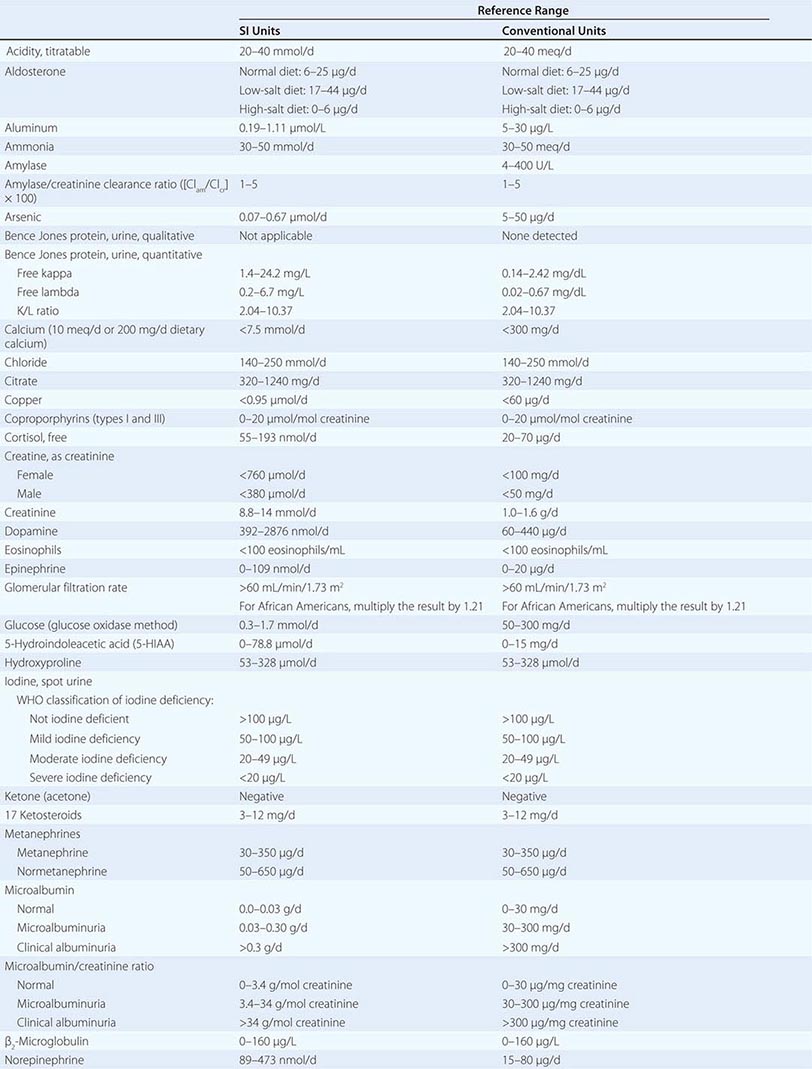
ACKNOWLEDGMENT
The contributions of Drs. Daniel J. Fink, Patrick M. Sluss, James L. Januzzi, Kent B. Lewandrowski, Amudha Palanisamy, and Scott Fink to this chapter in previous editions are gratefully acknowledged. We also express our gratitude to Drs. Alex Rai and Jeffrey Jhang for helpful suggestions.
480e |
The Clinical Laboratory in Modern Health Care |
Modern medicine relies extensively on the clinical laboratory as a key component of health care. It is estimated that, in current practice, at least 60–70% of all clinical decisions rely to some extent on a laboratory result. For many diseases, the clinical laboratory provides essential diagnostic information. As an example, histopathologic analysis provides basic information about histologic type and classification of tumors and their degree of invasion into adjacent tissues. Microbiologic testing is required to identify infectious organisms and determine antibiotic susceptibility. For many common diseases, expert groups have produced standard guidelines for diagnosis that rely on defined clinical laboratory values, e.g., blood glucose or hemoglobin A1C levels form the basis for diagnosis of diabetes mellitus; the presence of specific serum antibodies is required for diagnosis of many rheumatologic diseases; and serum levels of cardiac markers are a mainstay in diagnosis of acute coronary syndromes.
With their ever-increasing number and scope, clinical laboratory tests provide the clinician with a powerful set of tools but pose challenges in terms of appropriate selection and judicious, cost-effective use to deliver effective patient care.
RATIONALE FOR PERFORMING CLINICAL LABORATORY TESTS
DIAGNOSIS OF DISEASE
One of the most frequent reasons for performing clinical laboratory tests is to support, confirm, or refute a diagnosis of disease that is suspected on the basis of other information sources, such as history, physical examination findings, and imaging studies. The following questions need to be considered: Which clinical laboratory tests may be of value in supporting, confirming, or excluding the clinical impression? What is the most efficient test-ordering strategy? Will a positive test result confirm the clinical impression or even definitively establish the diagnosis? Will a negative result disprove the clinical suspicion, and, if so, what further testing or alternative approach will be needed? What are the known sources of false-positive and false-negative results, and how are these misleading results recognized?
SCREENING FOR DISEASE
Another reason for ordering clinical laboratory tests is to screen for disease in asymptomatic individuals (Chap. 4). Perhaps the most common examples of this application are the newborn screening programs now routinely used in most developed countries. Their purpose is to identify newborns with treatable conditions for which early intervention—even before clinical symptoms develop—is known to be beneficial. In adults, screening tests for diabetes mellitus, renal disease, prostate cancer (measurement of serum prostate-specific antigen [PSA] levels), and colorectal cancer (testing for occult blood in stool), for example, are widely applied to apparently healthy individuals on the grounds that early diagnosis and intervention lead to improved long-term outcomes.
Differences between Screening Tests and Confirmatory Tests It is important to distinguish between clinical laboratory tests that can be used to screen for disease and those that offer a confirmatory result. Screening tests are generally less expensive and more widely available than are confirmatory tests, which often require more specialized equipment or personnel. As a general principle, screening tests are designed to identify all individuals who have the disease of interest, even if, in the process, some healthy individuals are misidentified as possibly having the disease. In other words, maximization of the diagnostic sensitivity of screening tests inevitably comes at the expense of reduced diagnostic specificity. Confirmatory testing is intended to correctly separate those individuals who have a disease from those who do not.
These principles are exemplified by screening for hepatitis C virus (HCV) infection. A common approach is to test first for the presence of antibodies to HCV in serum. A positive result generally indicates either a current infection or a previous infection that the patient’s immune system has successfully cleared. In the latter situation, antibodies may persist at detectable levels for life. However, a small proportion of patients have false-positive results in the serologic screening test for HCV. To resolve uncertainty in these instances, a positive serologic screening test should be followed by confirmatory identification of HCV RNA with molecular techniques. This confirmatory testing can provide evidence of current viral infection and can identify patients who are not infected.
RISK ASSESSMENT OF FUTURE DISEASE
Another reason for clinical laboratory testing is to assess a patient’s risk of developing disease in the future. A number of diseases are associated with well-established clinical laboratory–defined risk factors that, if present, indicate the need for more frequent monitoring for the disease in question. The need for risk assessment is even clearer if there are useful interventions that decrease the risk of developing disease. For example, hypercholesterolemia is a well-established risk factor for coronary artery disease that may be modified by pharmacologic intervention (Chap. 291e). Many genetic mutations are known to be associated with increased risk of cancer. For example, hereditary mutations in the BRCA1 and BRCA2 genes predispose to breast and/or ovarian cancer. Individuals who are known to carry these mutations require more vigilant monitoring for early signs of cancer and may even opt for prophylactic surgery in an attempt to prevent cancer (Chap. 84). Individuals with factor V Leiden are at increased risk of developing deep venous thrombosis and may benefit from prophylactic anticoagulation in the perioperative period.
MONITORING DISEASE AND THERAPY
Many clinical laboratory tests offer useful information on the progress of disease and the response to therapy. One example is the measurement of viral load in HIV-1-infected patients who are taking antiretroviral agents. According to current guidelines from the Centers for Disease Control and Prevention, a successful antiretroviral response is defined by a fall in plasma HIV-1 levels of 0.5 log10 copies/mL, and a key goal of treatment is a reduction in the viral load to below the level of detection, which is typically in the range of 40–75 copies/mL. Other examples of the use of clinical laboratory testing for monitoring disease include measurement of tumor markers such as PSA, especially following surgical removal of tumors. In this situation, the expectation is that successful treatment of a tumor will cause a decrease in the level of the tumor marker. A later increase in the level of the tumor marker suggests a recurrence of the disease. Finally, the clinical laboratory offers direct monitoring of levels of some therapeutic agents, such as drugs. This monitoring is important if a drug has a defined therapeutic concentration range above which it is toxic and below which it is ineffective. Monitoring of drug levels in this situation facilitates optimal dosing and avoidance of toxicity.
CRITICAL VALUES
It is common practice for clinical laboratories to establish a list of “critical values.” These are values of test results that indicate immediate risk to the health or life of the patient and therefore require urgent communication with the patient’s physician so that appropriate medical intervention may be initiated. Critical values are reported regardless of whether the test was ordered as a “stat” or a routine test. The critical values themselves are generally created by the clinical laboratory medical director in conjunction with the medical staff. A representative list of critical values is shown in Table 480e-1.
|
SELECTED EXAMPLES OF LABORATORY CRITICAL VALUES |
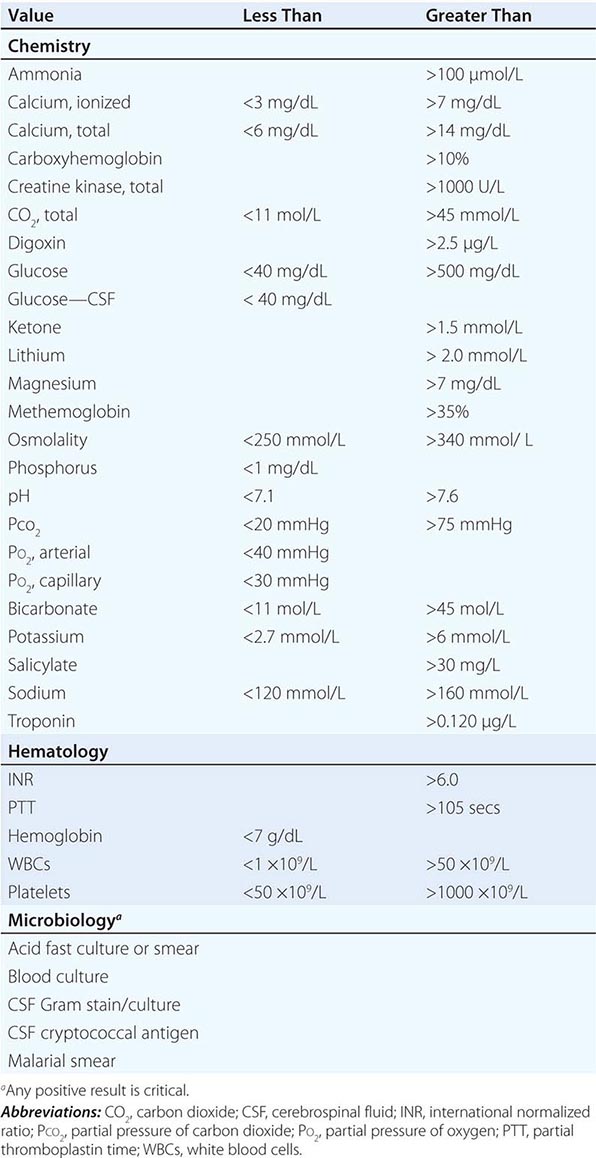
“STAT” ORDERS
Tests that are ordered on a “stat” basis receive priority in the clinical laboratory’s testing queue; testing of specimens from other patients may be delayed while a stat specimen is analyzed. Ordering a test stat should be reserved for situations in which a result is needed for urgent medical care—a judgment that must be made by the ordering physician. Stat testing should not be used merely for the convenience of either the patient or the health care provider.
SENSITIVITY AND SPECIFICITY IN THE CLINICAL LABORATORY
The commonly used metrics of a clinical laboratory test are the diagnostic sensitivity, specificity, and positive and negative predictive values. These concepts are discussed in Chap. 3. In the clinical laboratory, the terms sensitivity and specificity have alternative meanings that are applied to tests, and the different uses of these terms may cause confusion. Analytic sensitivity can refer to the lowest detectable concentration of analyte that can be measured with some defined certainty or to the rate of change of signal intensity as analyte concentration changes. For example, newer generations of laboratory assays frequently exhibit improved sensitivity over that of earlier generations, i.e., they can detect lower concentrations of the analyte—a feature that is often of value in disease diagnosis. Analytic specificity refers to the extent to which other substances in the test system interfere with measurement of the analyte of interest. This concept is frequently applied to immunoassays, in which a detection antibody may also bind with compounds that have a structure similar to the substance sought. For instance, immunoassays for drugs may show cross-reactivity with drug metabolites, and immunoassays for glucocorticoids may show cross-reactivity with other glucocorticoids of similar structure. Certain chemical assays are also subject to nonspecificity. For example, the Jaffe reaction, a chemical method commonly used to measure creatinine, is subject to positive interference from a number of other compounds, including glucose, certain ketones, and cephalosporin antibiotics. Elevated concentrations of bilirubin, free hemoglobin, or turbidity in plasma or serum specimens may also interfere with some assays. The clinical laboratory should be able to provide advice about the presence or magnitude of these effects in the assays it performs.
CLINICAL LABORATORY DIAGNOSTIC PRINCIPLES
Clinical laboratory diagnosis, like all diagnosis, is based on observation of disease-related changes from normality.
1. Tissue injury or necrosis allows leakage of intracellular components into the circulation, with consequent detectable rises in blood levels of these components. Many intracellular molecules are common across tissue types and are therefore not indicative of injury to a specific tissue. Other constituents are selectively expressed in relatively high concentrations—or are even uniquely present—in certain tissues. Therefore, their presence in the blood is evidence of injury to that tissue. This principle forms the rationale for measurement of blood levels of, for example, liver enzymes in evaluating liver disease (Chap. 358), cardiac troponins in acute coronary syndromes (Chap. 295), and myoglobulin in muscle injury. The extent of the rise in blood levels of these markers generally correlates with the extent of tissue damage, although there are exceptions; for example, liver enzyme levels may fall in end-stage liver disease.
2. An increase in blood levels of some analytes indicates failure of normal excretory processes. This principle is illustrated by elevations in conjugated bilirubin that accompany obstruction of the biliary system, by elevations in ammonia in advanced or metabolic liver disease, by rises in creatinine and potassium levels in renal failure, and by increases in Pco2 in some pulmonary diseases.
3. Increases in the blood concentration of tissue-specific markers may result from expansion of the total volume of that tissue. This principle forms the basis for the measurement of levels of many tumor markers such as PSA (prostate cancer), CA-125 (ovarian cancer), CEA (colon cancer), and CA-19–9 (pancreatic cancer). In practice, the usefulness of these markers varies with the degree to which they are produced by a tumor and by the tumor’s size. Small colon cancers, for example, may not produce a significant rise in CEA levels, whereas small prostate cancers often produce detectable rises in PSA concentrations.
4. Disease processes often manifest characteristic patterns of coincident changes in levels of several analytes. These patterns of change can be understood by consideration of the underlying pathophysiology. For example, acute intravascular hemolysis is characterized by a fall in levels of hemoglobin and haptoglobin and by a rise in unconjugated bilirubin. In endocrine diseases, there are often changes in concentrations of several hormones because of disturbance of feedback loops. Primary hyperthyroidism, as an example, is characterized by increases in thyroxine and by suppression of thyroid-stimulating hormone. In diabetic ketoacidosis caused by insulin deficiency, there are concomitant elevations of plasma glucose, ketones, and (frequently) potassium. In response to metabolic acidosis, levels of bicarbonate are typically reduced.
5. Genetic changes underlie many diseases, both inherited and acquired. In the era of molecular medicine, there is increasing recognition of the contribution of hereditary factors to many common diseases. Often, the epidemiology of common diseases such as hypertension is characterized by a minority of families that have mutations in recognized genes, whereas the genetic basis of the same disease phenotype in the larger population is unclear. The search for the genetic factors that contribute to many common diseases remains a topic of intense research interest. It is now clear that essentially all tumors have genetic abnormalities. Although there is an inherited predisposition in some families, most of these genetic changes are acquired. Identification of the genetic abnormalities in cancer offers new tools for clinical laboratory diagnosis and classification of tumors in ways that surpass traditional histopathology and also provides insights into cellular processes that may be targets for treatment.
6. Clinical laboratory results should always be interpreted in the context of the patient’s history and physical examination as well as any other relevant information (e.g., imaging studies). The clinician should avoid treating laboratory results rather than the patient.
7. Recommended clinical laboratory tests change with time. As new markers of disease emerge, they may replace older markers. For example, measurement of serum creatine kinase (CK) levels was introduced for diagnosis of acute myocardial infarction in the 1980s. Use of the cardiac-specific isoenzyme CK-MB later became widespread in clinical practice. Today, cardiac troponins are replacing CK (or CK-MB) measurements in recommended guidelines. Many other assays have fallen out of use as better assays have become available. Measurement of urine 17-ketosteroids (arising from androgens) and of urine 17-hydroxycorticosteroids (arising from glucocorticoids) has been supplanted by immunoassays or mass spectroscopy determinations of specific steroid hormones. Today, many steroid hormones are measured by mass spectrometry, often with better analytic specificity than is provided by immunoassays. As new tests are introduced, it is essential that they be evaluated critically before adoption for clinical use. At a minimum, consideration needs to be given to questions of clinical validation, specimen stability, diagnostic sensitivity and specificity, positive and negative predictive values, analytic accuracy and precision, and relative costs.
REFERENCE RANGES
In the interpretation of clinical laboratory results, comparison is usually made to a reference range (sometimes called a normal range) that defines the values seen in health or considered to be desirable for health. Several common methods are used to describe reference ranges in the clinical laboratory.
1. For many quantitative clinical laboratory tests, the range of observed values in a healthy population shows an approximately Gaussian distribution. The factors that contribute to this range include the inter- and intraindividual variation in the concentration of the analyte and the analytic imprecision. When there is an approximately Gaussian distribution of values in the population, the reference range is commonly defined as being the central 95% of the range of distribution of those values. According to this method, 2.5% of the population will have a measured value that is below the reference range for the analyte, and 2.5% will have a value that is above the reference range. The fact that 5% of healthy individuals will have a test value that is outside the reference range has important implications when multiple tests are ordered. If N independent tests are performed on a specimen, then the probability that at least one result will be outside the reference range is (1–0.95N). The greater the number of tests ordered (even for a healthy individual), the greater is the likelihood of an abnormal result (Fig. 480e-1). If 20 independent tests are performed on a healthy subject, the probability of at least one abnormal result is almost two-thirds.
FIGURE 480e-1 Probability that at least one laboratory result will be abnormal in a healthy individual as an increasing number of independent tests are performed. The reference range is the central 95% of values measured in a healthy population.
In some settings, a narrower range of values is considered to be abnormal. For example, current American Heart Association guidelines recommend the use of a serum level of cardiac troponins that is greater than the 99th percentile of values found in a healthy population as evidence of acute myocardial infarction.
2. An alternative approach to using population means and standard deviations is to define a range of analyte values that is judged to be consistent with health on the basis of expert consensus opinion. These ranges are often referred to as decision limits. Examples of reference ranges established in this way include those for total, high-density, and low-density cholesterol (Table 480e-2). Such ranges may deviate considerably from those that would be established if the analyte concentrations of the population (mean ± 2 standard deviations) were used as a basis for establishing the reference range. For example, the “desirable” total cholesterol value according to the National Cholesterol Education Program is <200 mg/dL. This value is actually very close to the mean concentration among U.S. adults; in fact, almost one-half of U.S. adults have a total cholesterol concentration that is above the “desirable” range. If the central 95% of cholesterol concentrations in the population were taken as the reference range, the upper end of that range would be ~240 mg/dL, well beyond what is considered desirable.
|
NATIONAL CHOLESTEROL EDUCATION PROGRAM ADULT TREATMENT PANEL III GUIDELINES FOR CHOLESTEROL |
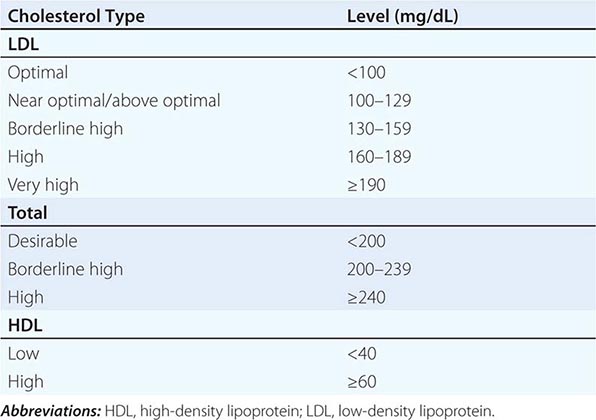
Reference ranges may vary with age, gender, ethnic background, and physiologic state (e.g., pregnancy, high-altitude adaptation). Some examples of these variations are shown in the Appendix. The existence of different reference ranges poses challenges for interpretation of results. In particular, creatinine stands out as an analyte for which conventional reference ranges are not always easy to apply in clinical practice. Plasma levels of creatinine vary with age, gender, and ethnic group. This fact makes it difficult in practice to use a simple reference range for this analyte when attempting to gauge a patient’s renal function. A large decrease in glomerular filtration rate (GFR) is associated with slight increases in the plasma creatinine concentration within the typical reference range provided by many laboratories (Fig. 480e-2). A 60-year-old white woman with a serum creatinine level of 1.00 mg/dL, which is well within the typical reference range, has an estimated GFR of only 57 mL/min per 1.73 m2, whereas the same creatinine concentration in a 20-year-old African-American male is consistent with normal renal function. To better estimate the GFR, which is widely considered to be the most useful index of overall renal function, it has become customary to use equations that incorporate plasma creatinine with other parameters. The most widely used of these equations in current practice is the 4-parameter Modification of Diet in Renal Disease (MDRD) equation that incorporates plasma creatinine, age, gender, and ethnic group (African American or not African American). The more recent CKD-EPI equation, which uses the same 4 parameters, is beginning to replace the MDRD equation. Recommended clinical laboratory practice is to report the estimated GFR with all creatinine measurements in adults. This addition provides more useful information than would a creatinine reference range alone.
FIGURE 480e-2 Relationship between plasma creatinine and estimated glomerular filtration rate (eGFR) using the 4-parameter Modification of Diet in Renal Disease (MDRD) equation. IDMS, isotope dilution mass spectrometry.
SOURCES OF ERROR IN CLINICAL LABORATORY TESTING
Errors can arise at all stages of the testing process, from specimen collection to result interpretation. An error arising at any stage may adversely affect patient care. In clinical laboratory practice, it is customary to divide the testing process into three phases: preanalytic, analytic, and postanalytic. Examples of each type of error are shown in Table 480e-3. The most common error in the testing process is specimen mislabeling, in which a specimen from one patient is placed in a container labeled with another patient’s name or identifiers. Specimen mislabeling errors may have very serious consequences for a patient. For example, if erroneous typing of a patient’s blood group results from specimen mislabeling and is followed by transfusion of a mismatched unit of blood, the outcome may be fatal. A mislabeled biopsy specimen can lead either to an erroneous diagnosis and inappropriate therapy or to a failure to make a diagnosis and institute appropriate therapy.
|
EXAMPLES OF PREANALYTIC, ANALYTIC, AND POSTANALYTIC ERRORS DURING THE LABORATORY TESTING PROCESS |
In addition to errors, many preanalytic factors can influence clinical laboratory results. Posture (i.e., recumbent versus upright), exercise, diet, recently ingested food, and use of prescribed or recreational drugs (including tobacco, alcohol, caffeine, and herbal supplements) can influence a variety of analyte concentrations. After blood has been collected, certain analytes undergo changes in their concentration during storage or transportation. Glucose levels fall as a result of red cell metabolism. Ammonia levels rise as a result of protein breakdown. Increasing permeability and breakdown of red cell membranes leads to increases in plasma potassium and free hemoglobin levels. Bacterial contamination can lead to overgrowth of specimens. To minimize these precollection alterations, specimens should be processed or transported to the clinical laboratory as soon as possible after collection. The list of known preanalytic variables and their effects is extensive, and the reader is referred to the compendium on this subject (see Young DS: Effects of Preanalytical Variables on Clinical Laboratory Tests, 3rd ed. Washington, DC, AACC Press, 2007).
POINT-OF-CARE TESTING
The great majority of tests continue to be performed in dedicated clinical laboratory facilities, but for several decades there has been a trend toward point-of-care testing. This change has been made possible by the development of portable analytic devices, including single-purpose instruments such as glucometers and oxygen saturation monitors, and multifunction instruments that can perform a wider variety of analyses, particularly in chemistry and hematology but also in some areas of microbiology. The use of these devices is driven largely by the convenience of faster result availability. In some settings (e.g., in rural areas and developing countries), there may be no easily accessible clinical laboratory and a point-of-care device may be the best or only option for testing. However, the per-specimen cost of point-of-care testing, in terms both of reagents and supplies and of personnel, is often greater than that of centralized testing. Other concerns relate to the adequacy of personnel training for point-of-care testing, the quality of the results, and the incorporation of results into the medical record.
HOME TESTING BY PATIENTS
One of the largest markets for point-of-care testing is home testing by patients, which has long been an important element in the management of persons with diabetes who monitor their own blood glucose levels. Over-the-counter kits for home pregnancy testing have been available for decades. More recently, kits have become available for home testing of the international normalized ratio or prothrombin time by patients taking oral anticoagulants. Kits are also available for cholesterol monitoring, fecal occult-blood detection, and hemoglobin measurement. In these areas, there is often little information on the quality of test performance, the accuracy of the results, or the correctness of result interpretation.
ISSUES SPECIFIC TO GENETIC TESTING
![]() The principles of genetic medicine in clinical practice are discussed in Chaps. 82–84. Here we will concentrate on issues related to clinical laboratory testing for genetic disease.
The principles of genetic medicine in clinical practice are discussed in Chaps. 82–84. Here we will concentrate on issues related to clinical laboratory testing for genetic disease.
The distinction between genetic testing for inherited disorders and that for acquired disorders affects the type of tissue that should be obtained for analysis. In inherited disorders, all nucleated cells are expected to carry the inherited mutation; thus white blood cells or buccal cells (obtained by scraping the inside of the cheek) are convenient sources of DNA for clinical laboratory testing. For prenatal testing of the fetus, chorionic villi or amniocytes are commonly used. In tests for acquired genetic disorders (e.g., in tumors), the tissue of interest that contains a suspected mutation must be sampled. It is often useful to compare tumor DNA with the patient’s normal DNA in order to identify acquired mutations (e.g., testing for microsatellite instability in colorectal cancer; Chap. 101e).
INFORMED CONSENT FOR GENETIC TESTING
Although it is assumed that all clinical laboratory testing is performed with the consent of the patient (or, in the case of minors, the parents), regulations may require formal written consent for genetic testing. Such regulations vary among jurisdictions, and the practicing clinician should be aware of local regulations. In some jurisdictions, there are regulations on the storage and use of genetic information and on the maximal period for which genetic specimens may be stored.
For some late-onset genetic diseases, such as Huntington’s disease (Chap. 449), genetic testing allows a prediction about the future development of the disease. The degree of certainty that is possible on the basis of this testing surpasses that associated with identification of more traditional disease risk factors (e.g., hyperlipidemia as a risk factor for future myocardial infarction). When deciding to undertake predictive genetic testing, it is important for the patient to consider the broad implications of a positive or negative test result, to be made aware of any support and counseling that is available, and to understand the implications of a result for other family members. In dealing with these issues, genetic counselors play an important role (Chap. 84). Their expertise includes the ability to explain genetic disorders at an understandable level to patients and their families, to arrange for support services, and to provide genetic risk assessments to members of families with genetic disorders.
When testing for genetic disorders, the clinical laboratory will use different analytic approaches according to the disease of interest. Some disorders, such as sickle cell anemia, are caused by single-point mutations. Testing for these disorders involves mere assessment for one or a few mutations in a single gene. Other disorders (e.g., hyperphenylalaninemias) may be caused by numerous mutations in a single gene. Still others (e.g., hereditary breast cancer) may be caused by mutations in many genes. The number of possible mutations and genes that underlie a clinical phenotype affects the cost of and time required for clinical laboratory testing as well as the likelihood of finding a disease-causing mutation.
If a disease phenotype can be caused by many mutations, a clinical laboratory result that is negative should be interpreted with care. For example, it is common to screen healthy pregnant women (and their partners) for mutations in the CFTR gene, which is mutated in patients with cystic fibrosis (CF). The goal of this screening is to identify women who are carriers of a CFTR mutation and therefore are at increased risk of having a baby with CF. Because CF is an autosomal recessive disorder, a fetus has a 1:4 chance of being affected if both parents are carriers of disease-causing CFTR mutations. The screening test approach that is commonly used to identify mutations in carriers detects 80–85% of all known disease-causing CFTR mutations in Caucasians and up to 97% of mutations among Ashkenazi Jews. A negative screening result therefore does not completely eliminate the possibility that a woman (or her partner) actually has a mutation. What can be inferred from a negative test result is that the risk of having a CF-affected baby has decreased significantly to an extent that depends on the woman’s ethnic group and the mutations that were examined. The clinical laboratory should calculate and report the woman’s new risk of being a carrier if the screening result is negative.
The increasing availability of large-scale (next-generation) sequencing of a patient’s whole genome or exome will greatly affect genetic testing over the next decade, with implications for the number of mutations that can be detected and the increased complexity of result interpretation.
LIMITATIONS TO MOLECULAR GENETIC TESTING
Genetic testing has limitations that are often unique to this field. Results may be inconclusive. For example, a search for mutations in a gene that is suspected of causing a disease may fail to reveal any known disease-causing mutations. A mutation may be discovered that is of unknown clinical significance. In this situation, consideration of the predicted change in the amino acid sequence of the encoded protein may suggest a biologic effect—e.g., replacement of a charged amino acid by one of the opposite charge or by a neutral amino acid; replacement of an amino acid by one of a different size; or replacement of an amino acid that is conserved across multiple species. Further information may be obtained by determining whether the mutation is found in healthy individuals. Even with all of these considerations, it is not uncommon that the biologic significance of an identified mutation remains uncertain, and further research may be needed to assess its significance.
It is also important to understand the limitations of the clinical laboratory approach used to detect mutations. At this time, next-generation sequencing remains an impractical undertaking for financial reasons, although extensive sequence analysis has become the standard of care for a few genes (e.g., analysis of BRCA1 and BRCA2 in assessing the risk of breast and ovarian cancer in individuals with a strong family history of these disorders). As sequencing technologies become less expensive, they can be expected to be more commonly used for both identifying mutations in patients with genetic disorders and screening asymptomatic individuals at risk of genetic disease.
Another unique aspect of genetic testing is the concern that genetic information about individuals may be used to discriminate against them by employers or by insurance companies. In the United States, the Genetic Information Nondiscrimination Act of 2008 (GINA) prohibits the use of genetic information by employers in making decisions related to employment and by health insurance companies in issuing insurance policies or setting premium rates based on knowledge of the applicant’s genetic status. GINA does not cover disability insurance, long-term care insurance, or life insurance policies.
Although public attention has been most closely focused on DNA testing, other clinical laboratory investigations that are not usually thought of as genetic may provide important genetic information about the person being tested. For example, serum protein electrophoresis may reveal α-1 antitrypsin deficiency. Depending on the clinical laboratory technology used, measurement of hemoglobin A1C, commonly used for monitoring diabetes control, may reveal a hemoglobin variant such as HbS (sickle cell). Measurement of cholesterol and triglyceride levels may reveal any of a number of hereditary disorders. All of these results are types of genetic information.
REGULATION OF THE CLINICAL LABORATORY
In the United States, all clinical laboratory testing performed for clinical purposes (but not for research purposes) is regulated by the federal Clinical Laboratory Improvement Amendments Act of 1988 (CLIA). Home monitoring by patients who are testing their own specimens is not covered by CLIA. The statute and the regulations, which are administered by the Centers for Medicare and Medicaid Services, apply to all laboratories, whether located in a physician’s office, a large hospital, or a reference laboratory; and all laboratories are required to hold a valid CLIA certificate that is appropriate for the highest complexity level of the tests they perform. The U.S. Food and Drug Administration is responsible for assigning the complexity level of commercial tests. The lowest category of complexity is the “waived” category, which is followed (in order of increasing complexity) by the categories of “provider-performed microscopy,” “moderate-complexity testing,” and “high-complexity testing.” The category of provider-performed microscopy is used to cover tests such as the use of potassium hydroxide preparations on skin scrapings to examine for fungi, fern tests, and sperm motility tests; it does not encompass histopathology that falls into the high-complexity category. Even if a clinical laboratory is performing only testing in the “waived” category, it must still hold a valid CLIA certificate. Laboratories that hold certificates for nonwaived tests are required to participate in proficiency testing and are regularly inspected to monitor their performance.
481e |
Clinical Procedure Tutorial: Central Venous Catheter Placement |
Clinical procedures are an important component of medical student and resident training, and some are required for board and hospital certification. In these Harrison’s Chaps. 481e–486e, video tutorials are presented for performing abdominal paracentesis, thoracentesis, endotracheal intubation, central venous catheter placement, percutaneous arterial blood gas sampling, and lumbar puncture. These videos have been created specifically for Harrison’s. Each includes the indications, contraindications, equipment, potential complications, and related patient safety considerations. Additional video tutorials covering clinical procedures such as breast biopsy, IV line insertion, phlebotomy, arterial line insertion, arthrocentesis, bone marrow biopsy, pelvic examination, thyroid aspiration, basic suturing, and urethral catheterization are available to subscribers of Harrison’s Online and AccessMedicine (available at www.accessmedicine.com).
482e |
Clinical Procedure Tutorial: Thoracentesis |
Clinical procedures are an important component of medical student and resident training, and some are required for board and hospital certification. In these new Harrison’s Chaps. 481e–486e, video tutorials are presented for performing abdominal paracentesis, thoracentesis, endotracheal intubation, central venous catheter placement, percutaneous arterial blood gas sampling, and lumbar puncture. These videos have been created specifically for Harrison’s. Each includes the indications, contraindications, equipment, potential complications, and related patient safety considerations. Additional video tutorials covering clinical procedures such as breast biopsy, IV line insertion, phlebotomy, arterial line insertion, arthrocentesis, bone marrow biopsy, pelvic examination, thyroid aspiration, basic suturing, and urethral catheterization are available to subscribers of Harrison’s Online and AccessMedicine (available at www.accessmedicine.com).
483e |
Clinical Procedure Tutorial: Abdominal Paracentesis |
Clinical procedures are an important component of medical student and resident training, and some are required for board and hospital certification. In these new Harrison’s Chaps. 481e–486e video tutorials are presented for performing abdominal paracentesis, thoracentesis, endotracheal intubation, central venous catheter placement, percutaneous arterial blood gas sampling, and lumbar puncture. These videos have been created specifically for Harrison’s. Each includes the indications, contraindications, equipment, potential complications, and related patient safety considerations. Additional video tutorials covering clinical procedures such as breast biopsy, IV line insertion, phlebotomy, arterial line insertion, arthrocentesis, bone marrow biopsy, pelvic examination, thyroid aspiration, basic suturing, and urethral catheterization are available to subscribers of Harrison’s Online and AccessMedicine (available at www.accessmedicine.com).
484e |
Clinical Procedure Tutorial: Endotracheal Intubation |
Clinical procedures are an important component of medical student and resident training, and some are required for board and hospital certification. In these new Harrison’s Chaps. 481e–486e, video tutorials are presented for performing abdominal paracentesis, thoracentesis, endotracheal intubation, central venous catheter placement, percutaneous arterial blood gas sampling, and lumbar puncture. These videos have been created specifically for Harrison’s. Each includes the indications, contraindications, equipment, potential complications, and related patient safety considerations. Additional video tutorials covering clinical procedures such as breast biopsy, IV line insertion, phlebotomy, arterial line insertion, arthrocentesis, bone marrow biopsy, pelvic examination, thyroid aspiration, basic suturing, and urethral catheterization are available to subscribers of Harrison’s Online and AccessMedicine (available at www.accessmedicine.com).
485e |
Clinical Procedures Tutorial: Percutaneous Arterial Blood Gas Sampling |
Clinical procedures are an important component of medical student and resident training, and some are required for board and hospital certification. In these Harrison’s Chaps. 481e–486e, video tutorials are presented for performing abdominal paracentesis, thoracentesis, endotracheal intubation, central venous catheter placement, percutaneous arterial blood gas sampling, and lumbar puncture. These videos have been created specifically for Harrison’s. Each includes the indications, contraindications, equipment, potential complications, and related patient safety considerations. Additional video tutorials covering clinical procedures such as breast biopsy, IV line insertion, phlebotomy, arterial line insertion, arthrocentesis, bone marrow biopsy, pelvic examination, thyroid aspiration, basic suturing, and urethral catheterization are available to subscribers of Harrison’s Online and AccessMedicine (available at www.accessmedicine.com).
486e |
Clinical Procedures Tutorial: Lumbar Puncture |
Clinical procedures are an important component of medical student and resident training, and some are required for board and hospital certification. In these Harrison’s Chaps. 481e–486e, video tutorials are presented for performing abdominal paracentesis, thoracentesis, endotracheal intubation, central venous catheter placement, percutaneous arterial blood gas sampling, and lumbar puncture. These videos have been created specifically for Harrison’s. Each includes the indications, contraindications, equipment, potential complications, and related patient safety considerations. Additional video tutorials covering clinical procedures such as breast biopsy, IV line insertion, phlebotomy, arterial line insertion, arthrocentesis, bone marrow biopsy, pelvic examination, thyroid aspiration, basic suturing, and urethral catheterization are available to subscribers of Harrison’s Online and AccessMedicine (available at www.accessmedicine.com).