Chapter 170 Fever without a Focus
Fever without Localizing Signs
Fever of acute onset, with duration of <1 wk and without localizing signs, is a common diagnostic dilemma in children <36 mo of age. The etiology and evaluation of fever without localizing signs depends on the age of the child. Traditionally, 3 age groups are considered: neonates or infants to 1 mo of age, infants >1 mo to 3 mo of age, and children >3 mo to 3 yr of age. In 1993, practice guidelines were published to aid the clinician in evaluating the otherwise healthy 0 to 36 mo old with fever without a source. However, with the advent and extensive use of the conjugate Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae vaccines, the rates of infections with these 2 pathogens have decreased substantially. As a consequence, modifications to the 1993 guidelines have been advocated as described later. Children in high-risk groups (Table 170-1) require a more-aggressive approach and consideration of a broader differential diagnosis.
Table 170-1 FEBRILE PATIENTS AT INCREASED RISK FOR SERIOUS BACTERIAL INFECTIONS
| RISK GROUP | DIAGNOSTIC CONSIDERATIONS |
|---|---|
| IMMUNOCOMPETENT PATIENTS | |
| Neonates (<28 days) | Sepsis and meningitis caused by group B streptococcus, Escherichia coli, Listeria monocytogenes; neonatal herpes simplex virus infection, enteroviruses |
| Infants 1-3 mo | Serious bacterial disease in 10-15%, including bacteremia in 5%; urinary tract infection |
| Infants and children 3-36 mo | Occult bacteremia in <0.5% of children immunized with both Haemophilus influenzae type b and pneumococcal conjugate vaccines; urinary tract infections |
| Hyperpyrexia (>40°C) | Meningitis, bacteremia, pneumonia, heatstroke, hemorrhagic shock-encephalopathy syndrome |
| Fever with petechiae | Bacteremia and meningitis caused by Neisseria meningitidis, H. influenzae type b, and Streptococcus pneumoniae |
| IMMUNOCOMPROMISED PATIENTS | |
| Sickle cell disease | Sepsis, pneumonia, and meningitis caused by S. pneumoniae, osteomyelitis caused by Salmonella and Staphylococcus aureus |
| Asplenia | Bacteremia and meningitis caused by N. meningitidis, H. influenzae type b, and S. pneumoniae |
| Complement or properdin deficiency | Sepsis caused by N. meningitidis |
| Agammaglobulinemia | Bacteremia, sinopulmonary infections |
| AIDS | S. pneumoniae, H. influenzae type b, and Salmonella infections |
| Congenital heart disease | Infective endocarditis; brain abscess with right-to-left shunting |
| Central venous line | Staphylococcus aureus, coagulase-negative staphylococci, Candida |
| Malignancy | Bacteremia with gram-negative enteric bacteria, S. aureus, and coagulase-negative staphylococci; fungemia with Candida and Aspergillus |
1 Month to 3 Months
Many academic institutions have investigated the optimal management of low-risk patients in this age group with fever without a focus (Table 170-2). The use of viral diagnostic studies (enteroviruses, respiratory viruses, rotavirus, and herpesvirus) in combination with the Rochester Criteria or similar criteria can enhance the ability to determine which infants are at high risk for serious bacterial infections (see Table 170-2). Febrile infants in whom a virus has been detected are at low or no risk of a serious bacterial infection. Well-appearing infants 1-3 mo of age can be managed safely using low-risk laboratory and clinical criteria as indicated in Table 170-2 if reliable parents are involved and close follow-up is assured.
Table 170-2 LOW RISK CRITERIA IN 1-3 MONTHS OLD WITH FEVER
BOSTON CRITERIA
Infants are at low risk if they appear well, have normal physical examination, have a caretaker reachable by telephone, and laboratory tests are as follows:
PHILADELPHIA PROTOCOL
Infants are at low risk if they appear well, have a normal physical examination, and laboratory tests are as follows:
PITTSBURGH GUIDELINES
Infants are at low risk if they appear well, have a normal physical examination, and laboratory tests are as follows:
ROCHESTER CRITERIA
Infants are at low risk if they appear well, have a normal physical examination, and laboratory findings are as follows:
CBC, complete blood count; CSF, cerebrospinal fluid; HPF, high-powered field; RBC, red blood cell; WBC: white blood cell.
3 Months to 36 Months of Age
Treatment of toxic-appearing febrile children 3-36 mo of age who do not have focal signs of infection includes hospitalization and prompt institution of antimicrobial therapy after specimens of blood, urine, and CSF are obtained for culture. Consensus practice guidelines published in 1993 recommended that children 3-36 mo of age who have a temperature of <39°C and do not appear toxic be observed as outpatients without performing diagnostic tests or administering antimicrobial agents. For nontoxic-appearing infants with a rectal temperature of ≥39°C, options include obtaining a blood culture and administering empirical antibiotic therapy (ceftriaxone, a single dose of 50 mg/kg, not to exceed 1 g); if the WBC count is >15,000/µL, obtaining a blood culture and beginning empirical antibiotic therapy; or obtaining a blood culture and observing as outpatients without empirical antibiotic therapy, with return for re-evaluation within 24 hr. Guidelines for managing febrile children 3-36 mo of age who have received both Hib and S. pneumoniae conjugate vaccines have not been established, but careful observation without empirical administration of antibiotic therapy is generally prudent. Because fully vaccinated young children are at a much lower risk of occult bacteremia and meningitis as the cause of acute fever without localizing signs, some advocate that the only laboratory tests needed in this age group when temperature is >39°C are a urinalysis and urine culture for circumcised boys <6 mo of age and uncircumcised boys and all girls <24 mo of age. Regardless of the management option (Table 170-3), the family should be instructed to return immediately if the child’s condition deteriorates or new symptoms develop.
If did not receive conjugate pneumococcal and H. influenzae type b vaccines, manage according to the 1993 Guidelines (see Baraff et al. Pediatrics 1993;92:1-12)
CSF, cerebrospinal fluid; HPF, high-powered field; RBC, red blood cell; WBC, white blood cell.
* Other tests may include chest radiograph, stool studies, herpes simplex polymerase chain reaction.
Fever of Unknown Origin
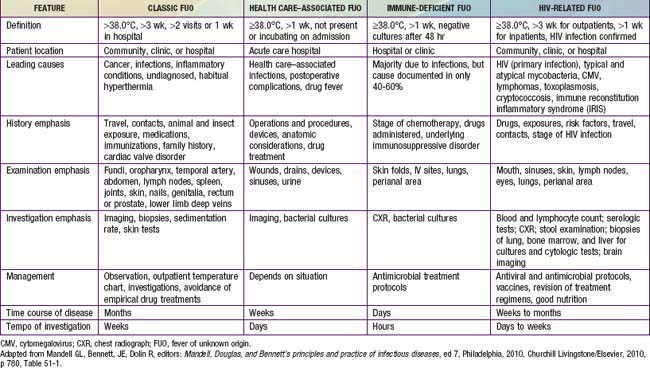
The classification of FUO is best reserved for children with fever documented by a health care provider and for which the cause could not be identified after 3 wk of evaluation as an outpatient or after 1 wk of evaluation in the hospital (Table 170-4).
Table 170-4 SUMMARY OF DEFINITIONS AND MAJOR FEATURES OF THE FOUR SUBTYPES OF FEVER OF UNKNOWN ORIGIN
Etiology
The many causes of FUO in children are infections and rheumatologic (connective tissue or autoimmune) diseases (Table 170-5). Neoplastic disorders should also be seriously considered, although most children with malignancies do not have fever alone. The possibility of drug fever should be considered if the patient is receiving any drug. Drug fever is usually sustained and not associated with other symptoms. Discontinuation of the drug is associated with resolution of the fever, generally within 72 hr, although certain drugs, such as iodides, are excreted for a prolonged period with fever that can persist for as long as 1 mo after drug withdrawal.
Table 170-5 DIAGNOSTIC CONSIDERATIONS OF FEVER OF UNKNOWN ORIGIN IN CHILDREN
ABSCESSES
BACTERIAL DISEASES
LOCALIZED INFECTIONS
FUNGAL DISEASES
VIRUSES
PARASITIC DISEASES
RHEUMATOLOGIC DISEASES
HYPERSENSITIVITY DISEASES
NEOPLASMS
GRANULOMATOUS DISEASES
FAMILIAL AND HEREDITARY DISEASES
MISCELLANEOUS
In the USA, the systemic infectious diseases most commonly implicated in children with FUO are salmonellosis, tuberculosis, rickettsial diseases, syphilis, Lyme disease, cat-scratch disease, atypical prolonged presentations of common viral diseases, infectious mononucleosis, cytomegalovirus (CMV) infection, viral hepatitis, coccidioidomycosis, histoplasmosis, malaria, and toxoplasmosis. Less common infectious causes of FUO include tularemia, brucellosis, leptospirosis, and rat-bite fever. AIDS alone is not usually responsible for FUO, although febrile illnesses often occur in patients with AIDS as a result of opportunistic infections (see Table 170-4).
Diagnosis
The evaluation of FUO requires a thorough history and physical examination supplemented by a few screening laboratory tests and additional laboratory and radiographic tests as indicated by the history or abnormalities on examination or initial screening (see Table 170-5).
Physical Examination
A complete physical examination is essential to find any physical clues to the underlying diagnosis (Table 170-6). The child’s general appearance, including sweating during fever, should be noted. The continuing absence of sweat in the presence of an elevated or changing body temperature suggests dehydration due to vomiting, diarrhea, or central or nephrogenic diabetes insipidus. It also should suggest anhidrotic ectodermal dysplasia, familial dysautonomia, or exposure to atropine.
Table 170-6 EXAMPLES OF SUBTLE PHYSICAL FINDINGS HAVING SPECIAL SIGNIFICANCE IN PATIENTS WITH FEVER OF UNKNOWN ORIGIN
| BODY SITE | PHYSICAL FINDING | DIAGNOSIS |
|---|---|---|
| Head | Sinus tenderness | Sinusitis |
| Temporal artery | Nodules, reduced pulsations | Temporal arteritis |
| Oropharynx | Ulceration | Disseminated histoplasmosis |
| Tender tooth | Periapical abscess | |
| Fundi or conjunctivae | Choroid tubercle | Disseminated granulomatosis* |
| Petechiae, Roth’s spot | Endocarditis | |
| Thyroid | Enlargement, tenderness | Thyroiditis |
| Heart | Murmur | Infective or marantic endocarditis |
| Abdomen | Enlarged iliac crest lymph nodes, splenomegaly | Lymphoma, endocarditis, disseminated granulomatosis* |
| Rectum | Perirectal fluctuance, tenderness | Abscess |
| Prostatic tenderness, fluctuance | Abscess | |
| Genitalia | Testicular nodule | Periarteritis nodosa |
| Epididymal nodule | Disseminated granulomatosis | |
| Lower extremities | Deep venous tenderness | Thrombosis or thrombophlebitis |
| Skin and nails | Petechiae, splinter hemorrhages, subcutaneous nodules, clubbing | Vasculitis, endocarditis |
* Includes tuberculosis, histoplasmosis, coccidioidomycosis, sarcoidosis, and syphilis.
From Mandell GL, Bennett, JE, Dolin R, editors: Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, ed 7, Philadelphia, 2010, Churchill Livingstone/Elsevier, 2010, p 785, Table 51-8.
American Academy of Pediatrics. Fever and antipyretic use in children. Pediatrics. 2011;127:580-587.
Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437-1441.
Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics. 1999;103:627-631.
Baraff LJ. Management of infants and young children with fever without source. Pediatr Ann. 2008;37:673-679.
Baraff LJ, Schriger DL, Bass JW, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Pediatrics. 1993;92:1-12.
Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days with intramuscular administration of ceftriaxone. J Pediatr. 1992;120:22-27.
Bateman SL, Seed PC. Procession to pediatric bacteremia and sepsis: covert operations and failures in diplomacy. Pediatrics. 2010;126(1):137-150.
Bleeker-Rovers CP, Vos FJ, de Kleijn MHA, et al. A prospective multicenter study on fever of unknown origin. Medicine. 2007;86:26-28.
Brauner M, Goldman M, Kozer E. Extreme leucocytosis and the risk of serious bacterial infections in febrile children. Arch Dis Child. 2010;95:209-212.
Bressan S, Andreola B, Cattelan F, et al. Predicting severe bacterial infections in well-appearing febrile neonates. Pediatr Infect Dis J. 2010;29:227-232.
Centers for Disease Control and Prevention. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states. 1998-2005. MMWR. 2008;57:144-148.
Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15,781 febrile illnesses. BMJ. 2010;340:c1594.
Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86:363-367.
Dagan R, Powell KR, Hall CB, et al. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr. 1985;107:855-860.
Dawes M. Identifying sick children on primary care. Lancet. 2010;375:784-785.
Downs SM. Technical report: urinary tract infections in febrile infants and young children. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement. Pediatrics. 1999;103(4):e54.
Flynn PM. Diagnosis and management of central venous catheter-related bloodstream infections in pediatric patients. Pediatr Infect Dis J. 2009;28(11):1016-1017.
Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without focus. Arch Dis Child. 2010;95:968-973.
Ginsburg CM, McCracken GHJr. Urinary tract infections in young infants. Pediatrics. 1982;69:409-412.
Gómez B, Mintegi S, Benito J, et al. Blood culture and bacteremia predictors in infants less than three months of age with fever without source. Pediatr Infect Dis J. 2010;29:43-47.
Herr SM, Wald ER, Pitetti RD, et al. Enhanced urinalysis improves identification of febrile infants age 60 days and younger at low risk for serious bacterial illness. Pediatrics. 2001;108:866-871.
Herz AM, Greenhow TL, Alcantara J, et al. Changing epidemiology of outpatient bacteremia in 3 to 36 month-old children after the introduction to the heptavalent-conjugate pneumococcal vaccine. Pediatr Infect Dis J. 2006;25:293-299.
Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125:228-233.
Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester Criteria and implications for management. Pediatrics. 1994;94:390-396.
Kopterides P, Siempos II, Tsangaris I, et al. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229-2241.
Krief WI, Levine DA, Platt SL, et al. Influenza virus infection and the risk of serious bacterial infections in young febrile infants. Pediatrics. 2009;124:30-39.
Laupland KB, Gregson DB, Vanderkooi OG, et al. The changing burden of pediatric bloodstream infections in Calgary, Canada, 2000-2006. Pediatr Infect Dis J. 2009;28:114-117.
Lee GM, Harper MB. Risk of bacteremia for febrile young children in the post-Haemophilus influenzae type b era. Arch Pediatr Adolesc Med. 1998;152:624-628.
Naik JD, Sathiyaseelan SRK, Vasudev NS. Febrile neutropenia. BMJ. 2011;342:103-104.
O’Donnell DR. Recognizing severe infection: in hoc signo vinces? Arch Dis Child. 2010;95(12):957-958.
Olaciregui I, Hernández U, Muñoz JA, et al. Makers that predict serious bacterial infection in infants under 3 months of age presenting with fever of unknown origin. Arch Dis Child. 2009;94:501-505.
Rudinsky SL, Carstairs KL, Reardon JM, et al. Serious bacterial infections in febrile infants in the post-pneumococcal conjugate vaccine era. Acad Emerg Med. 2009;16:585-590.
Shah SS, Downes KJ, Elliott MR, et al. How long does it take to “rule out” bacteremia in children with central venous catheters? Pediatrics. 2008;121:135-141.
Thompson MJ, Van den Bruel A. Diagnosing serious bacterial infection in young febrile children. BMJ. 2010;340:986-987.
Van den Bruel A, Haj-Hassan T, Thompson M, et al. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet. 2010;375:834-844.
Vidwan G, Geis G. Evaluation, management, and outcome of focal bacterial infections (FBIs) in nontoxic infants under two months of age. J Hosp Med. 2010;5(2):76-82.
Waddle E, Jhaveri R. Outcomes of febrile children without localizing signs after pneumococcal conjugate vaccine. Arch Dis Child. 2009;94:144-147.




