Chapter 59 Fetal Surgery for Open Neural Tube Defects
The goal of fetal surgery is to prevent or reduce the adverse consequences of a congenital disorder without increasing the risks for the fetus and mother. A number of diseases, such as congenital diaphragmatic hernia and sacrococcygeal teratoma, can be treated by surgical procedures performed directly on the fetus prior to the anticipated delivery date.1 An open neural tube defect, or myelomeningocele, is different in that it is not a fatal disease and fetuses with this condition will usually complete a normal gestation.2 This is an ethical challenge for the treating physicians because the potential benefits must be balanced by a well-defined risk to the fetus, and also to the mother who is a “bystander” with respect to the perceived benefits.
The development of fetal surgery for a specific condition has usually moved in a series of steps starting from the development of an animal model, definition of the natural history, refinement of techniques in test cases, and finally, evaluation of efficacy in prospective clinical trial. The reported benefits of fetal surgery for myelomeningocele as determined from retrospective case series include a reduction in shunt insertion rates and improvement in the hindbrain abnormality.3–5 To obtain more conclusive data, the potential benefit of fetal surgery for myelomeningoceles is being examined in a clinical trial directly comparing patients randomized into prenatal and postnatal treatment groups.
Rationale for Fetal Repair
Although there is large spectrum of abnormalities observed in children with myelomeningoceles, it is useful to separate the neurologic deficits into two groups: primary and secondary. The primary neurologic deficits are those directly caused by the arrested development of the neural placode, which usually occurs in the lumbosacral region.6 Because neural tube closure occurs during the third and fourth weeks of gestation, the spinal cord in this region is very immature at the stage when a myelomeningocele develops. Although the structure of the spinal cord is severely disrupted at the involved level, it is unknown whether the placode is capable of further development.7 The functional neurologic level is either at the same level as the vertebral anomaly, or actually higher than the vertebral level, resulting in worse neurologic function, in more than 80% of patients with open forms of spina bifida.8
The theoretical advantage of fetal repair for myelomeningoceles is that the neural tube is covered and protected many months before the expected delivery date. The basis for expecting improved neurologic function is that restoration of the dysplastic neural placode within the spinal canal isolates it from the amniotic fluid and prevents ongoing injury.9,10 Mueli and others surgically created a spinal-cord lesion in fetal sheep at 75 days of gestation that simulated a spina bifida lesion.11 After delivery at term, the gross and microscopic appearance of the exposed spinal cord resembled a human spina bifida lesion and the animals were incontinent and had loss of sensation and motor function below the lesion level. One group of animals with surgically created spina bifida lesions were then treated using a myocutaneous flap at 100 days of gestation. These animals were then carried to full-term gestation and had near-normal motor function and normal bowel and bladder control. The results of these experiments suggested that early repair of an exposed spinal cord may preserve neurologic function and may allow improvement through plasticity.12 Although provocative, these large animal experiments clearly rely on a model system that has distinct differences with the human disease.
Timing for Fetal Surgery
If closure of an open neural tube defect reduces secondary injury occurring to the placode, then surgical intervention should be performed as early as possible. In practice, surgical timing is determined by diagnosis and technical limitations of the actual procedure. Most myelomeningoceles are detected during the second trimester, either during an investigation of a positive maternal screening test, or during a routine ultrasound study. The quality of current ultrasonography allows detection of most fetuses with myelomeningoceles by the mid-portion of the second trimester.13 From a practical viewpoint, this means that a diagnosis is made between 18 and 22 weeks of gestation. Taking into consideration current obstetrical practice, it is unlikely that detection of fetuses with spina bifida will occur any earlier unless new, more sensitive screening tests are discovered.
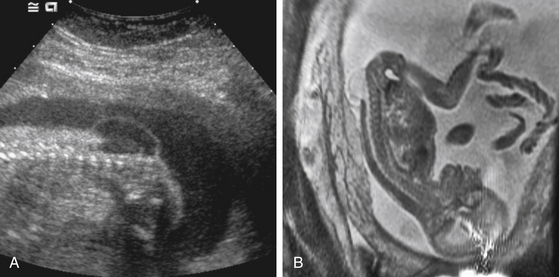
Preoperative fetal imaging studies usually begin with a detailed ultrasonogram (Fig. 59-1A). This study is able to determine some anatomic features with great precision. These include size of the overlying sac, the level of the defect, the position of the cerebellar tonsils, and the presence of lower-extremity deformities. Limitations include difficulty determining some associated brain anomalies, other intraspinal anomalies, and at times, the exact dysraphic level. Most patients being considered for fetal surgery will undergo a fetal MRI study (Fig. 59-1B). Because of motion, images in sagittal, axial, and coronal planes are obtained randomly by repeatedly imaging the fetus over time. The preferred MRI technique is a single-shot, fast spin-echo T2-weighted sequence. There is some evidence that MRI may improve the ability to detect coexisting spinal and brain anomalies that may not be apparent on ultrasound studies.14,15
Hysterotomy and Exposure
Several technical hurdles needed to be overcome before fetal surgery could be performed safely. These include: (1) the ability to open the uterus and prevent separation of the chorioamniotic membranes, (2) achieve watertight uterine closure, and (3) prevent the onsent of preterm labor in the post-operative period.1 The ability of the mother to carry and deliver subsequent pregnancies does not appear to be jeopardized by fetal surgery.
Surgical Repair of the Defect
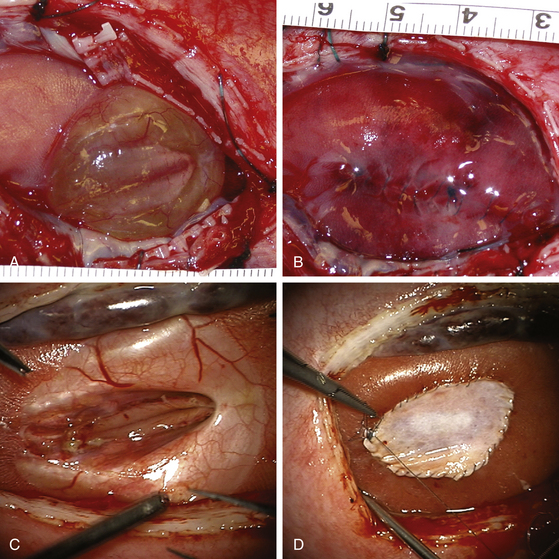
The neural placode is usually more visible in the fetus than in the term infant. The arachnoid is extremely thin and translucent and the junction between it and the placode is readily apparent. If the myelomeningocele sac is intact, the placode is usually lifted upward away from the surface of the back (Fig. 59-2A). In other situations, the placode is flat and at the same level as the surrounding skin (Fig. 59-2C). The epithelium of the skin does not usually reach the edge of the placode. The clear identification of the intervening arachnoid usually allows the placode to be divided from its attachments with sharp dissection. Depending on the consistency of the placode, the neural tube can be retubularized; however, if the placode is particularly fragile, this step may not be possible.
The dura is loosely attached to the underlying subcutaneous tissues just lateral to the spinal canal. After incising the dura at its lateral junction with the dermis, gentle instillation of saline into the epidural plane with a small angiocatheter lifts the dura away from the underlying tissues, which minimizes trauma. Between 18 to 20 weeks of gestation, the dura can be very thin and difficult to handle. After 22 weeks of gestation, the dura becomes more substantial and can be handled more easily. Once the dura is circumferentially detached from the dermis and separated from the underlying lumbar fascia, it can be closed using a running suture. If the amount of dura is insufficient, then a patch is used to close the opening. The use of acellular human dermis to repair the dura may contribute to the formation of intracellular dermoid cysts.16 For this reason, a synthetic collagen matrix (DuraGen, Integra Life Sciences, Plainsboro, NJ) can be used to create a dural barrier.
Following dural closure, the skin is closed as a single layer incorporating the superficial and deeper tissues (Fig. 59-2B). In general, dissection of the underlying muscle and fascia is not attempted because excessive fetal blood loss must be avoided and the duration of the procedure minimized. Elevation of the skin and separation from the underlying subcutaneous tissues is relatively easy, although increased tension on the skin inevitably leads to tearing. Small openings in the skin caused by handling with forceps or tension from suture points generally close rapidly. If the skin can be brought together, the final postnatal appearance is often excellent. For situations where insufficient skin is available to close the lesion, either skin flaps, relaxing incisions, or acellular dermis can be used as a patch (Fig. 59–2D). In most cases, this patch becomes incorporated into the healing scar tissue.
Results
Experimental evidence suggested that early closure of myelomeningoceles should improve neurologic function by preventing the secondary injury to the exposed nervous tissue.9,11 Early clinical results, however, from fetal repair of human myelomeningoceles have been disappointing. Tubbs and colleagues examined a cohort of patients (n = 37) who had undergone fetal repair between 20 and 28 weeks of gestation and compared their neurologic function to a cohort (n = 40) of patients who underwent postnatal procedures.17 No statistical difference was observed in lower extremity function between the two groups. This study, along with others, has limitations inherent with any retrospective analysis, such as an unmatched control group, lack of standardization of surgical technique, and lack of randomization to treatment arms. Nevertheless, the lack of clear improvement in neurologic function with fetal surgery suggests that the animal models used to study this disorder do not recapitulate the human disease.
The incidence of delayed signs and symptoms such as lower-extremity weakness, worsening of bladder and bowel control, and/or pain in patients who have had fetal surgery is unknown. Based on a few cases, re-exploration in patients who have had previous fetal repair appears to be more difficult because tissue planes in the area of the placode are poorly defined. Urodynamics performed on a small group of children who had undergone fetal surgery showed clear abnormalities such as vesicoureteral reflux and a significant postvoid residual urine volume. These results were indistinguishable from those of patients who had undergone postnatal repair.18 This is not unexpected since urologic function should be strongly related to sacral spinal cord function.
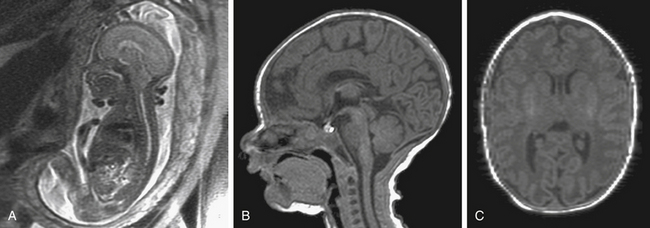
Data from centers performing fetal surgery for myelomeningocele have indicated that the benefits of surgery are a reduction in the rate of CSF shunt insertion, and improvement in the appearance of the Chiari II malformation on imaging studies (Fig. 59-3).5,19 The shunt rate in a cohort of 116 children treated with fetal surgery and followed in the postnatal period for at least 12 months was 54%.20 The strongest predictor for postnatal shunt placement was the upper level of the spinal lesion, with those above L3 showing the highest rates of shunt insertion. This trend is similar to a historical series where lesion level affected shunt rates.8 The overall percentage of patients requiring shunt placement, based on retrospective series, is usually in the range of 80% to 95%. By this measure, the reduction in shunt insertion rates reported in the fetal surgery group is encouraging. However, it is possible that selection bias alone may account for this benefit. In order to measure this presumed reduction in shunt insertion rates and to accurately assess maternal and fetal risks, a randomized, prospective clinical trial sponsored by the National Institutes of Health is underway (see Addendum).
Bruner J.P., Tulipan N., Paschall R.L., et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282(19):1819-1825.
Bruner J.P., Tulipan N., Reed G., et al. Intrauterine repair of spina bifida: preoperative predictors of shunt-dependent hydrocephalus. Am J Obstet Gynecol. 2004;190(5):1305-1312.
Meuli M., Meuli-Simmen C., Hutchins G.M., et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med. 1995;1(4):342-347.
Tulipan N., Hernanz-Schulman M., Lowe L.H., et al. Intrauterine myelomeningocele repair reverses preexisting hindbrain herniation. Pediatr Neurosurg. 1999;31(3):137-142.
Adzick N.S., Thom E.A., Spong C.Y. A randomized trial of prenatal versus postnatal repair of myelomeningocele. NEJM. 2011;364:993-1004.
1. Harrison M.R. The Unborn Patient: The Art and Science of Fetal Therapy, 3rd ed. Philadelphia: Saunders; 2001.
2. Mitchell L.E., Adzick N.S., Melchionne J., et al. Spina bifida. Lancet. 2004;364(9448):1885-1895.
3. Bruner J.P., Tulipan N., Paschall R.L., et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282(19):1819-1825.
4. Tulipan N., Hernanz-Schulman M., Lowe L.H., et al. Intrauterine myelomeningocele repair reverses preexisting hindbrain herniation. Pediatr Neurosurg. 1999;31(3):137-142.
5. Johnson M.P., Sutton L.N., Rintoul N., et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol. 2003;189(2):482-487.
6. Copp A.J., Greene N.D., Murdoch J.N. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4(10):784-793.
7. Hutchins G.M., Meuli M., Mueli-Simmon C., et al. Acquired spinal cord injury in human fetuses with myelomeningocele. Pediatr Pathol Lab Med. 1996;16(5):701-712.
8. Rintoul N.E., Sutton L.N., Hubbard A.M., et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics. 2002;109(3):409-413.
9. Heffez D.S., Aryanpur J., Hutchins G.M., et al. The paralysis associated with myelomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery. 1990;26(6):987-992.
10. Heffez D.S., Aryanpur J., Rotellini N.A., et al. Intrauterine repair of experimental surgically created dysraphism. Neurosurgery. 1993;32(6):1005-1010.
11. Meuli M., Meuli-Simmen C., Hutchins G.M., et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med. 1995;1(4):342-347.
12. Meuli M., Meuli-Simmen C., Yingling C.D., et al. In utero repair of experimental myelomeningocele saves neurological function at birth. J Pediatr Surg. 1996;31(3):397-402.
13. Patel T.R., Bannister C.M., Thorne J., et al. A study of prenatal ultrasound and postnatal magnetic imaging in the diagnosis of central nervous system abnormalities. Eur J Pediatr Surg. 2003;13(Suppl 1):S18-S22.
14. Glenn O.A., Goldstein R.B., et al. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med. 2005;24(6):791-804.
15. von Koch C.S., Glenn O.A., Li K.C., et al. Fetal magnetic resonance imaging enhances detection of spinal cord anomalies in patients with sonographically detected bony anomalies of the spine. J Ultrasound Med. 2005;24(6):781-789.
16. Mazzola C.A., Albright A.L., Sutton L.N., et al. Dermoid inclusion cysts and early spinal cord tethering after fetal surgery for myelomeningocele. N Engl J Med. 2002;347(4):256-259.
17. Tubbs R.S., Chambers M.R., Smyth M.D., et al. Late gestational intrauterine myelomeningocele repair does not improve lower extremity function. Pediatr Neurosurg. 2003;38(3):128-132.
18. Holmes N.M., Nguyen H.T., Harrison M.R., et al. Fetal intervention for myelomeningocele: effect on postnatal bladder function. J Urol. 2001;166(6):2383-2386.
19. Tulipan N., Hernanz-Schulman M., Bruner J.P. Reduced hindbrain herniation after intrauterine myelomeningocele repair: a report of four cases. Pediatr Neurosurg. 1998;29(5):274-278.
20. Bruner J.P., Tulipan N., Reed G., et al. Intrauterine repair of spina bifida: preoperative predictors of shunt-dependent hydrocephalus. Am J Obstet Gynecol. 2004;190(5):1305-1312.