CHAPTER 19 Fertilization to implantation
Prelude to Fertilization
Sexual dimorphism in gametogenesis
Spermatogenesis and oogenesis produce male and female gametes, respectively, and are characterized by a unique nuclear division, known as ‘meiosis’, which halves chromosome numbers. Notably however, the tempo of gametogenesis differs markedly between males and females. Spermatogenesis progresses uninterruptedly over approximately 60–70 days, whereas oogenesis often spans decades and is a discontinuous process punctuated by two arrest stages (Homer 2007).
With the exception of the relatively small amount of DNA associated with mitochondria which is exclusively oocyte-derived, spermatozoa and eggs have comparable stakes in the embryo’s genetic blueprint. In stark contrast, male and female gametes make very different cytoplasmic contributions (Figure 19.1). Virtually all of the embryo’s biosynthetic raw materials and organelles are derived from the egg, with the centriole being the only male cytoplasmic contribution. The fate of an embryo is therefore critically dependent upon stores built up in the oocyte during an extended growth phase lasting 2–3 months, resulting in a 100-fold increase in oocyte volume (Telfer and McLaughlin 2007). Consequently, any compromise to egg quality, such as is seen with advancing female age, has devastating consequences for embryonic and reproductive potential (Homer 2007). Accordingly, older women experience lower in-vitro fertilization (IVF) success rates, along with increased risks for miscarriage and chromosomally abnormal offspring.
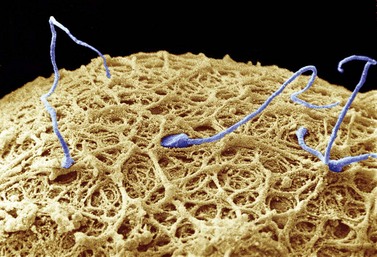
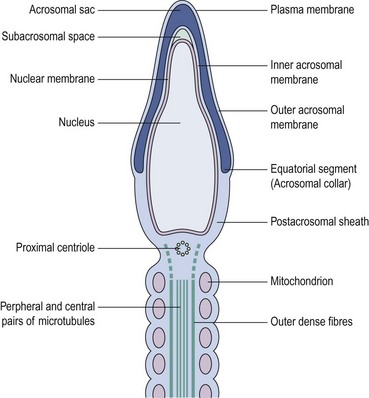
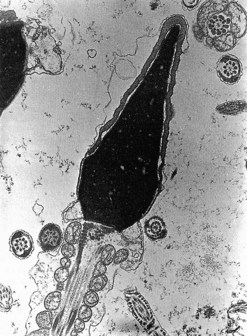
In stark contrast to oogenesis which has embryonic sustenance as an over-riding concern, spermatogenesis aims to produce a lightweight and motile gamete capable of penetrating the oocyte. Spermatogenesis fulfils this objective by incorporating a remodelling phase known as ‘spermiogenesis’, the most remarkable cellular metamorphosis in the human body. Spermiogenesis strips the spermatid of almost all of its cytoplasm, makes the nucleus highly compact by integrating unique DNA-binding proteins called ‘protamines’ (Sassone-Corsi 2002), and fashions a propulsive tail with an adjacent fuel source in the mitochondria-laden mid-piece (Figures 19.2 and 19.3). A specialized lysosomal-like secretory granule known as the acrosome is also forged, forming a cap-like structure over the nuclear surface (Figures 19.2 and 19.3). The acrosome contains enzymes essential for digesting a path through the egg’s outer covering known as the ‘zona pellucida’.
Maturation and capacitation
Maturation occurs in the epididymis endowing spermatozoa with the potential for movement and fertilization. The molecular basis for maturation remains unresolved, but is known to depend upon androgens and exposure to the dynamic microenvironment created by the secretory and absorptive functions of the epididymal epithelium (Nixon et al 2007).
Capacitation enables spermatozoa to recognize their full fertilization potential and occurs within the female genital tract. It is characterized by cyclic adenosine monophosphate (cAMP)-dependent tyrosine phosphorylation of intracellular proteins, most of which are components of the sperm exocytotic and motility machinery (Nixon et al 2007). The capacitated spermatozoon is now distinguished by hyperactivated motility and a change in surface membrane properties, rendering it sensitive to signals encountered in the immediate vicinity of the ovulated egg.
Transport of gametes in the female genital tract
At the time of ovulation, the egg and its tunic of cumulus cells are directed by tubal fimbria and cilia into the tubal ostium and then towards the tubal ampulla (Figure 19.4). The cumulus–egg complex attracts sperm by releasing chemoattractants that stimulate sperm odorant receptors, thereby inducing cAMP-mediated sperm chemotaxis.
Fertilization
Cumulus penetration
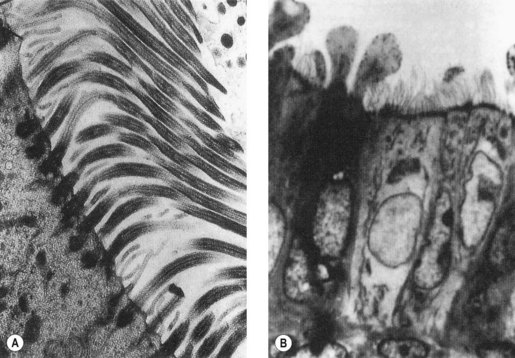
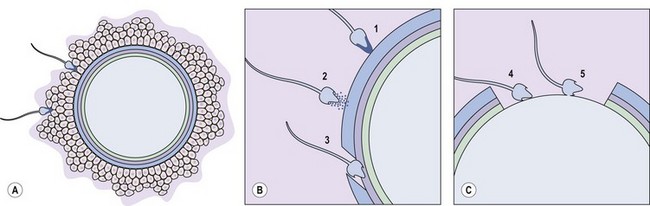
The first obstacle encountered by any would-be suitor of the ovulated egg is a covering of cumulus cells embedded in an extracellular matrix comprised primarily of the glycosaminoglycan, hyaluronan (Figure 19.5A). Spermatozoa digest a path through the extracellular matrix via hyperactivated motility and a glycophosphatidyl inositol (GPI)-anchored surface hyaluronidase termed ‘PH-20’ (Primakoff and Myles 2002). Notably, eggs influence sperm penetration, albeit indirectly, by secreting factors such as growth differentiation factor-9 and bone morphogenic protein-15 which regulate cumulus integrity (Swain and Pool 2008).
Sperm–zona interaction
After cumulus penetration, sperm must engage and penetrate the zona pellucida (Figure 19.5B and C). The zona is secreted by the growing oocyte and, in humans, comprises four glycoproteins: ZP1, ZP2, ZP3 and ZP4 (Wassarman 2008). ZP3 in concert with O-linked oligosaccharides functions as the primary sperm receptor that preferentially binds the plasma membrane overlying the intact acrosome. ZP2 is postulated to serve as a secondary receptor that attaches to the inner acrosomal membrane of acrosome-reacted sperm. The zona is also important for mediating species specificity in gamete interaction, and for preventing polyspermy following the cortical reaction (discussed later).
The complementary spermatozoal ligand for ZP3 defies unequivocal identification at present. It appears that the coordinated action of several proteins such as GalTI-1 (β-1,4-galactosyltransferase) and SED1 that are recruited and constrained by lipid rafts on the sperm surface may mediate docking of sperm on to the zona (Nixon et al 2007).
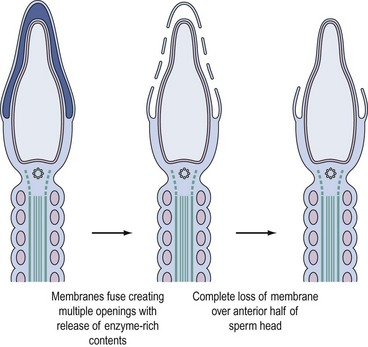
Binding of sperm ligands with ZP3 induces a rise in intracellular calcium, triggering multiple sites of fusion between the sperm membrane and the outer acrosomal membrane (Figure 19.6). This culminates in exocytosis of sperm acrosome contents, the acrosome reaction (Figures 19.5B and 19.6). The proteases and glycosidases thus released, in concert with hyperactivated motility, enable sperm to digest a path through the zona (Figure 19.5B).
Sperm–egg binding and fusion
Having penetrated the zona, spermatozoa enter the perivitelline space between the egg membrane (oolemma) and the zona (Figure 19.5B). Here, further receptor–ligand interactions enable binding of equatorial sperm membrane and oolemma, followed by insertion of the sperm membrane into the opposing oocyte bilayer at fusion (Figure 19.5C).
Initial interest in interplay between oolemmal integrins (e.g. α6β1) and sperm disintegrins (e.g. fertilin and cyritestin) as mediators of binding-fusion have dissipated due to lack of corroborative evidence from integrin/disintegrin knockout mouse models. Instead, focus is currently on alternative candidates such as sperm lysozyme-like protein and Izumo on sperm, and GPI-anchored proteins and the CD9 tetraspanin on eggs (Kaji et al 2000, Nixon et al 2007).
Egg activation
Sperm–egg fusion induces calcium oscillations in the egg emanating from the point of sperm entry. These calcium transients result from inositol triphosphate production brought about by a soluble sperm-specific factor known as ‘phospholipase-Cζ’ (Swann et al 2006). Calcium signals induce the two principal events associated with egg activation: breaking the second meiotic arrest and the cortical reaction.
At the time of fusion, the egg is arrested in meiosis II. This state of suspended animation is dependent upon maturation-promoting factor that is itself sustained by an ooplasmic activity known as ‘cytostatic factor’ (Tunquist and Maller 2003). The recent characterization of two proteins, early mitotic inhibitor 2 and the calcineurin phosphatase, has elucidated the link between calcium and meiosis II resumption. Following fusion, the ensuing calcium surge induces calcium/calmodulin-dependent-kinase-II-dependent early mitotic inhibitor 2 destruction and transient calcineurin activation, together resulting in cytostatic factor and maturation-promoting factor inactivation and relief from meiosis II arrest (Mochida and Hunt 2007, Wu and Kornbluth 2008).
Preimplantation
Timelines and key morphologic changes of embryonic development
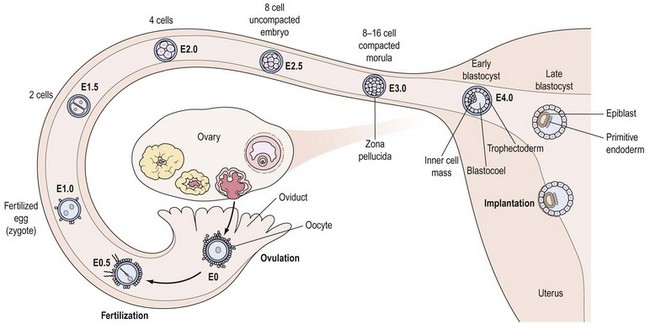
During early embryonic divisions, increasing cell number is not accompanied by any appreciable increase in embryonic size, reflected by the continued presence of the zona (Figure 19.7). Instead, during this time, the cytoplasm inherited from the egg is merely redistributed to the accumulating blastomeres which get progressively smaller, again vividly illustrating the central role played by the egg in embryogenesis.
Around the eight-cell stage (3–4 days post ovulation), the embryo undergoes compaction whereby membrane surfaces between neighbouring embryonic cells (or blastomeres) become indistinct, leading to the formation of a morula by the 16-cell stage. Around this time, the embryo enters the uterine cavity to begin its rapport with the endometrium (Figure 19.7). Shortly thereafter, at the 32–64-cell stage (4–5 days post ovulation), the blastocyst is formed, characterized by the presence of a cavity or blastocoel and the presence of two cell types: an outer trophoectoderm (TE) or trophoblast which is the placental precursor, and an eccentrically placed inner cell mass (ICM) which is the progenitor of the embryo proper (Figure 19.7). As discussed in greater detail later, isolation and culture of cells from the inner cell mass at this stage results in the derivation of an embryonic stem (ES) cell line (see Figure 19.10C).
Oviductal transport and nutritional support
Tubal embryo transport is dependent upon coordinated oviductal smooth muscle activity mediated by the sympathetic nervous system. The emerging picture is that sympathetic tone requires fine-tuning by lipid mediators, known as ‘endocannabinoids’ (e.g. anandamide), acting via CB1 receptors. Human fallopian tubes were recently shown to express CB1, the levels of which peak during the luteal phase, likely reflecting control by progesterone (Horne et al 2008). The importance of endocannabinoid signalling is underlined by high rates of oviductal embryo retention in CB1-deficient mice (Wang et al 2004), and lowered tubal CB1 expression in women with ectopic pregnancies (Horne et al 2008).
The embryo’s nutritional requirements change during early development. Thus, the early-cleavage-stage embryo is relatively metabolically quiescent and utilizes pyruvate as an energy source, whereas blastocysts demonstrate high metabolic activity and use glucose as their sole energy supply. In response to this, the oviduct is rich in pyruvate and non-essential amino acids, and low in glucose, whereas the uterine cavity is rich in glucose and essential amino acids (Watkins et al 2008).
Genetic aspects: embryonic reprogramming to embryonic genome activation
Underlying the overt structural changes described above are dramatic alterations in the regulation of gene expression. Under the influence of ooplasmic factors, the specialized gene expression programmes of both male and female gametes are largely erased to provide a tabula rasa on which to establish a well-orchestrated embryonic gene expression pattern required for preimplantation development (Niemann et al 2008).
This reprogramming is imposed by epigenetic modifications consisting of changes to DNA methylation status and to the histones on which the DNA is wound (Egli et al 2008). By altering the accessibility of DNA to the transcription machinery, epigenetic changes alter gene expression without altering the core genetic code. Epigenetic reprogramming is ultimately responsible for the acquisition of totipotency by early embryonic cells, conferring on them the capacity to form any cell type in the body, including extraembryonic trophoblast.
One group of genes must, however, remain shielded from this global reprogramming effort. These are the imprinted genes for which it is critically important that a parent-of-origin-specific pattern of expression is retained (Swales and Spears 2005). Male and female imprints are laid down during gametogenesis, and must be sequestered away from the reprogramming route if proper embryonic development is to follow.
Until its own genome is fully activated, the early embryo is heavily reliant on maternal transcripts present in the egg for directing new protein synthesis integral to early development. As epigenetic marks are laid down, the embryonic genome becomes progressively activated. Synchronous with this embryonic genome activation (EGA) is the degradation of oocyte-derived transcripts, thereby marking a maternal-to-embryonic baton change in genetic control. The major EGA burst occurs at the four-cell stage in humans and at the two-cell stage in mice. Recent evidence from mice suggests that EGA occurs in three successive and overlapping waves comprising minor and major EGA and mid-preimplantation EGA (Wang and Dey 2006).
Expression of specific genes is required for segregation of the TE and ICM lineages at the morula-to-blastocyst transition. The current consensus is that Nanog and Oct4 are required for ICM specification and for suppressing the formation of extraembryonic lineages. On the other hand, Cdx2 is required for TE specification by ensuring the repression of Oct4 and Nanog in the TE (Wang and Dey 2006).
Implantation
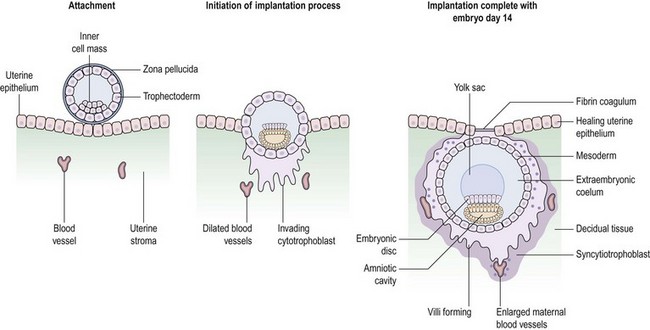
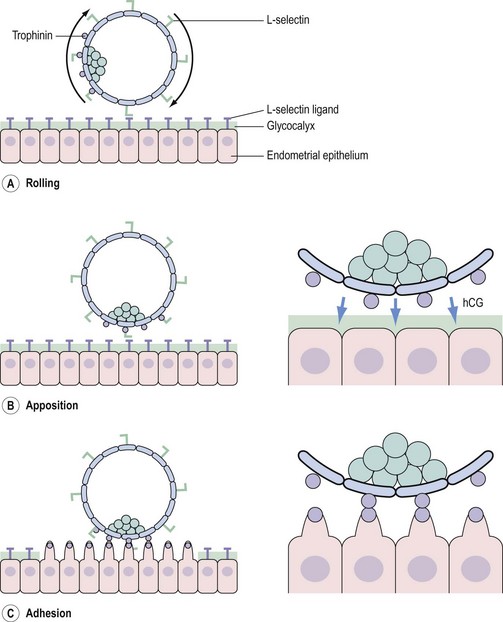
Having entered the uterine cavity, the trophoectoderm of the free-living blastocyst engages in foreplay with the endometrial luminal epithelium in the hope of securing a reliable nutrient supply. Successful implantation is the culmination of a highly intricate molecular dialogue between an implantation-competent blastocyst and a receptive endometrium. The process of implantation is schematically shown in Figure 19.8, but involves a series of complex biological interactions between the embryo and endometrium to be successful.
Morphologic changes and the window of implantation
Implantation requires the simultaneous presence of an activated embryo and a receptive endometrium. The endometrium is only receptive during a restricted timeframe referred to as the ‘implantation window’; prior to this window, the endometrium is prereceptive, whilst afterwards it is refractory and hostile to blastocyst survival. In humans, the receptive phase occurs approximately 5–9 days following ovulation, corresponding to days 20–24 of an idealized 28-day cycle (Diedrich et al 2007).
The principal regulators of uterine receptivity are oestrogen and progesterone acting via nuclear oestrogen receptors (ERα and ERβ) and progesterone receptors (PRα and PRβ). Endometrial expression of the homeobox gene, HOXA-10, rises dramatically at implantation and conveys progesterone responsiveness within the uterine stroma (Achache and Revel 2006). Oestrogen induces epithelial and stromal proliferation as well as PR expression. Progesterone, on the other hand, promotes stromal transformation or decidualization, glandular secretion and vascular remodelling. Bleb-like protrusions called ‘pinopods’ that extend beyond the epithelial microvilli tips are also under the influence of progesterone. Pinopods are preferential sites of embryo–endometrial interactions (see Figure 19.9C) and may serve as morphological markers of uterine receptivity (Achache and Revel 2006).
Within a few hours of attachment, penetration follows as trophoblastic processes erode the basal lamina. Implantation is described as interstitial in humans by virtue of the fact that the embryo invades the stroma deeply enough for the luminal epithelium to be reconstituted over it (Figure 19.8). Some trophoblastic cells fuse, forming the syncytiotrophoblast, whilst others retain their cellularity and serve as a trophoblastic reservoir known as the ‘cytotrophoblast’ (Figure 19.8). Maternal blood vessels are eroded so that the syncytiotrophoblast is bathed in maternal blood. These initial steps form the basis for the establishment of the elaborate placental circulation, the details of which can be found in textbooks devoted to obstetrics.
Adhesion molecules and mucins
Members of the cell adhesion molecule (CAM) family, such as selectins, integrins and cadherins, positively regulate embryo–endometrial interaction (Achache and Revel 2006, Guzeloglu-Kayisli et al 2007). Embryonic L-selectin interacts with luminal epithelial oligosaccharides (e.g. MECA-79), constituting an initial step in the attachment process (Genbacev et al 2003) (Figure 19.9A). The trophoectoderm expresses another adhesion molecule termed ‘trophinin’, which is also expressed on endometrial pinopods under the influence of human chorionic gonadotrophin (hCG) secreted by the blastocyst (Fukuda and Sugihara 2008) (Figure 19.9B and C). The ensuing trophinin–trophinin interaction is proposed to be a more potent bond than that mediated by L-selectin (Fukuda and Sugihara 2008) (Figure 19.9C). Although the L-selectin/trophonin system is crucial for human implantation, it appears to be dispensable in mice.
Attachment is further facilitated by integrins such as trophoblastic α1β1 and endometrial αVβ3 which bind to ligands such as osteopontin and oncofetal fibronectin on the luminal epithelium and trophoblast, respectively (Achache and Revel 2006). During the proliferative phase, high oestrogen levels acting via ERα inhibit αVβ3 expression, whilst secretory-phase progesterone has the opposite effect possibly via increased epidermal growth factor (EGF) in combination with downregulation of ERα levels. Interestingly, the embryo may upregulate β3 integrins via its interleukin-1 (IL-1) system, thereby actively participating in establishing endometrial receptivity. Calcium-regulated expression of E-cadherin mediated by progesterone and endometrial calcitonin is also thought to contribute to implantation.
In contrast to CAMs, glycoproteins known as ‘mucins’ (e.g. MUC1) extend beyond the glycocalyx and sterically inhibit adhesion (Achache and Revel 2006) (see Figure 19.9). Although data are contradictory, it is felt that under the influence of progesterone, MUC1 levels are locally reduced on pinopods, thereby unmasking CAMs to facilitate embryo–endometrial interaction. Local downregulation of MUC1 at the implantation site, possibly via blastocyst tumour necrosis factor-α-mediated increases in sheddase enzymes, lends further support for an active embryonic input.
Cytokines and growth factors
Leukaemia inhibitory factor (LIF) is a member of the cytokine family whose endometrial expression peaks during the mid- to late-secretory phase. Implantation fails in mutant mice lacking LIF (Stewart et al 1992), and patients suffering from infertility and recurrent implantation failure display suboptimal endometrial LIF expression (Hambartsoumian 1998), underscoring a pivotal role for LIF in implantation. Embryonic signals such as hCG and insulin-like growth factor (IGF) may regulate LIF expression. Conversely, LIF may interact with embryonic LIF receptors to mediate trophoblast growth and differentiation.
Growth factors constitute another class of proimplantation mediators and include IGF, transforming growth factor-β and the EGF family, the latter embracing EGF itself, heparin-binding EGF-like growth factor (HB-EGF) and amphiregulin, amongst others (Achache and Revel 2006, Guzeloglu-Kayisli et al 2007). In mice, HB-EGF is the earliest molecular marker of implantation, found exclusively at the sites of active blastocysts (Wang and Dey 2006). HB-EGF derived from blastocysts induces endometrial HB-EGF expression in a paracrine manner, which in turn interacts with blastocyst receptors (ErbB1 and ErbB4). This HB-EGF autoinduction loop induces blastocyst activation, shedding of the zona pellucida and initiation of attachment. HB-EGF function is conserved as its expression in humans is maximal in the late secretory phase, and HB-EGF-expressing cells adhere to blastocyst ErbB4 (Chobotova et al 2002).
Matrix metalloproteinases and prostaglandins
Implantation is characterized by extensive tissue remodelling and vascular changes. Matrix metalloproteinases (e.g. MMP-9), in concert with tissue inhibitors of matrix metalloproteinases (e.g. TIMP-1), mediate controlled matrix degradation during invasion (Guzeloglu-Kayisli et al 2007).
Prostaglandins are members of the eicosanoid family that direct vascular events required for fully executing the implantation process (Achache and Revel 2006, Wang and Dey 2006). Prostaglandins are produced from membrane phospholipids under the consecutive action of phospholipase A2 (PLA2), cyclo-oxygenase (COX) and prostaglandin synthase enzymes. COX2-derived prostacyclin (PGI2) is the primary prostaglandin produced at the implantation site, where it acts via the peroxisome-proliferator-activated receptor-δ receptor. Prostaglandin-deficient mice, due to lack of either PLA2 or COX2, suffer higher rates of pregnancy failure resulting from delayed implantation. Furthermore, COX2-derived prostaglandins are also implicated in angiogenesis by differentially regulating vascular endothelial growth factor and angiopoietin signalling cascades. Evidence from mice intimates that prostaglandins are a focal downstream signalling target as LIF induces HB-EGF, which is in turn required for COX2 expression. In humans, COX expression is maximal during the menstrual and proliferative phases, and may be important for fine-tuning uterine receptivity during the window of implantation.
Immune Factors in Implantation
Immune tolerance
Although half of its genes are paternally derived, the implanting embryo is able to evade the maternal immune system during normal pregnancy (Trowsdale and Betz 2006). One aspect of immune tolerance is related to the unique expression of human leukocyte antigen (HLA) antigens on extravillous trophoblast cells which display HLA-C, HLA-E and HLA-G molecules, but not HLA-A or HLA-B which mediate allograft rejection. Other likely factors include trophoblastic expression of Fas ligand (CD95L) that promotes apoptosis of activated lymphocytes expressing Fas (CD95), as well as suppression of T-cell proliferation secondary to reduced levels of uterine tryptophan, and the systemic and decidual expansion of regulatory T-cells (CD4+CD25+Foxp3+ T-cells).
Uterine natural killer cells
A hallmark of decidualization is the presence of uterine natural killer cells (uNK cells; CD56+bright/CD16−) which represent 70% of the uterine lymphocyte population at the implantation site (Moffett-King 2002). The term ‘killer’ is misleading as there is no compelling evidence that uNK cells kill trophoblast cells; unlike their highly cytotoxic blood counterparts (CD56+dim/CD16+), uNK cells are of low cytotoxicity. On the contrary, it is currently felt that uNK cells may exert a beneficial effect in controlling trophoblast invasion, possibly mediated via interaction between uNK cell killer immunoglobulin receptor and trophoblastic HLA-C (Moffett and Loke 2006). Given the uncertain role of the uNK cell in human pregnancy and the equally uncertain relationship between blood and uNK cells, tenuous links between blood NK cell levels and risk of pregnancy failure remain of uncertain significance (Moffett et al 2004).
Clinical and Scientific Correlates
Embryo–endometrial synchronization
A greater appreciation of embryo development has translated into improved IVF success rates by altering parameters to mimic the in-vivo situation more closely. During natural conception, the cleavage-stage embryo (2–3 days post fertilization) is first geared towards interacting with the oviductal milieu, whilst the blastocyst-stage embryo (5–6 days post fertilization) is more temporally synchronized with the endometrium. In keeping with this, significantly better IVF implantation rates accrue after transferring blastocyst-stage embryos into the uterine cavity compared with transferring cleavage-stage embryos (Papanikolaou et al 2008). Culture of embryos to the blastocyst stage in the laboratory setting has become increasingly more achievable with the development of improved sequential embryo culture media. These media formulations have, in turn, arisen from a greater understanding of the evolving needs of the preimplantation embryo during its journey from the oviduct to the uterine cavity.
Embryonic aneuploidy and preimplantation genetic screening
Studies involving PGS have produced conflicting results. The only two completed randomized controlled trials to date did not provide any compelling evidence that PGS improves pregnancy outcomes (Anderson and Pickering 2008). The results of these studies present something of an enigma in so far as there is no a priori reason why a euploid embryo should not result in a better outcome than one that is aneuploid. It is only when better diagnostic techniques emerge that are less detrimental to the embryo and which give a more comprehensive readout of the embryo’s entire chromosomal constitution (not just a nine-chromosome sample) that the role of PGS in assisted reproductive technology (ART) will be better informed.
IVF/ICSI: proceed with caution
Since its introduction over a decade and a half ago, intracytoplasmic sperm injection (ICSI) has become an established tool for overcoming male factor subfertility (Varghese et al 2007). However, ICSI bypasses many of the stringent natural selection mechanisms detailed previously that would normally preclude successful fertilization by defective spermatozoa. Thus, ICSI will ensure that infertility-related genetic traits such as Y-chromosome microdeletions in the azoospermia factor region are handed down to male offspring. Apart from propagating male infertility traits, it is unknown at present whether such genetic defects harbour any additional, more sinister, consequences. It is notable in this respect that there have been reports of increased rates (albeit small) of congenital abnormalities, especially of the genitourinary system, amongst ICSI-derived offspring. However, because the oldest child conceived through ICSI is still only a teenager, long-term studies are lacking and clarification on any such links, if indeed they do exist, remain outstanding.
Another area of concern is the potential effect of the artificial embryonic environment during IVF on the epigenome, in particular on imprinted genes (Watkins et al 2008).
Stem Cell Technology
The remarkable capacity of the oocyte to reprogramme gametic nuclei following fertilization has contributed to the birth of, arguably, the most exciting and promising scientific endeavour of our generation: somatic-cell nuclear transfer (SCNT) and the generation of embryonic stem cells (ES cells) (Hochedlinger and Jaenisch 2003).
Somatic-cell nuclear transfer
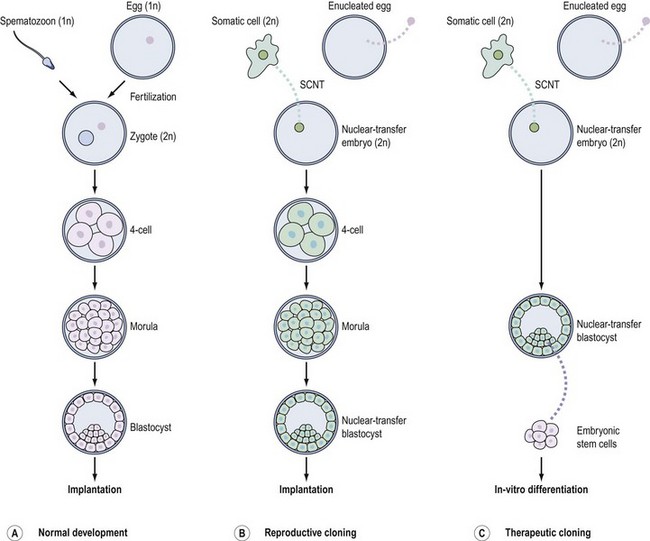
SCNT denotes the introduction of a donor somatic cell nucleus into an enucleated egg (Hochedlinger and Jaenisch 2003) (Figure 19.10B). The transcriptional programme of the donor nucleus is erased by ooplasmic factors and reprogrammed towards an embryonic schedule of development. Once activated using an artificial stimulus, the egg containing the donor nucleus can then be coaxed into developing into a so-called ‘cloned’ blastocyst in the same way that an egg fertilized by a spermatozoon can develop into a blastocyst during IVF. Notably, unlike the IVF embryo which is a genetic composite of two gametic genomes, the SCNT embryo is genetically identical to that of (or a clone of) the donor somatic cell (Figure 19.10, compare A and B). The first cloned human blastocysts derived after SCNT with human ES cells (Stojkovic et al 2005) and adult fibroblasts (French et al 2008) have been reported recently.
‘Reproductive cloning’ and ‘therapeutic cloning’
The blastocyst resulting from SCNT could be transferred into a recipient uterus with the aim of producing cloned offspring with the identical genetic composition of the donor nucleus (Figure 19.10B). Although highly inefficient, such ‘reproductive cloning’ has resulted in the birth of animals from 12 species, the first mammalian clone being ‘Dolly the sheep’ (Wilmut et al 1997). Importantly, however, human reproductive cloning is unanimously considered morally and ethically repugnant, and is legally prohibited in the UK by the Human Reproductive Cloning Act (2001) (Lovell-Badge 2008).
The overwhelming interest in this field does not surround ‘reproductive cloning’ but instead centres largely on ‘therapeutic cloning’. For therapeutic cloning, cells from the inner cell mass of an SCNT blastocyst are explanted in culture to yield a line of pluripotent ES cells potentially capable of differentiating into any adult cell type (Hochedlinger and Jaenisch 2003) (Figure 19.10C). ES cell lines have recently been derived from primates following SCNT (Byrne et al 2007).
Alternative methods of nuclear reprogramming
One of the major obstacles to stem cell research is the dearth of human oocytes available for SCNT (Hall and Stojkovic 2006). One means for sidestepping this problem is the startling revelation that nuclear reprogramming could be effected by a cocktail of just four transcription factors (OCT4, SOX2, KLF4 and MYC) to produce so-called ‘induced pluripotent stem’ (iPS) cells (Okita et al 2007). This is a field that is progressing with unprecedented rapidity, driven by the goal of regenerative medicine. There are clear links between the nuclear reprogramming events that underpin the earliest events of embryo development and the molecular processes that are needed to reprogramme an adult nucleus. It is anticipated that our understanding of the latter may lead to explanations for many of the problems that occur during human fertilization and preimplantation development.
Concluding Remarks
Relentless scientific toil, largely undertaken in animal models like the mouse, has made us privy to many of the molecular forces driving critical stages in mammalian reproduction. However, it is becoming increasingly evident that humans exhibit unique signalling details that are not replicated in animal models (Diedrich et al 2007, Childs et al 2008, Fukuda and Sugihara 2008). It will therefore be important that greater inroads be made into the workings of the human system if clinical practice is to be better served. In the future, techniques such as gene expression profiling will lead to a better appreciation of the molecular signature defining a receptive human endometrium (Diedrich et al 2007). The growing emphasis on single embryo transfer during IVF as a means for reducing unacceptably high multiple pregnancy rates places greater burden on selecting the best quality embryos (Cutting et al 2008). In this regard, the application of metabolomics, proteomics and comparative genomic hybridization to embryo selection holds promise for ‘sifting the wheat from the chaff’.
KEY POINTS
Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Human Reproduction Update. 2006;12:731-746.
Anderson RA, Pickering S. The current status of preimplantation genetic screening: British Fertility Society policy and practice guidelines. Human Fertility (Cambridge, England). 2008;11:71-75.
Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497-502.
Childs AJ, Saunders PT, Anderson RA. Modelling germ cell development in vitro. Molecular Human Reproduction. 2008;14:501-511.
Chobotova K, Spyropoulou I, Carver J, et al. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mechanisms of Development. 2002;119:137-144.
Cutting R, Morroll D, Roberts SA, et al. Elective single embryo transfer: guidelines for practice. British Fertility Society and Association of Clinical Embryologists. Human Fertility (Cambridge, England). 2008;11:131-146.
Diedrich K, Fauser BC, Devroey P, Griesinger G, Evian Annual Reproduction (EVAR) Workshop Group. The role of the endometrium and embryo in human implantation. Human Reproduction Update. 2007;13:365-377.
Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nature Reviews. Molecular Cell Biology. 2008;9:505-516.
French AJ, Adams CA, Anderson LS, Kitchen JR, Hughes MR, Wood SH. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells. 2008;26:485-493.
Fukuda MN, Sugihara K. An integrated view of L-selectin and trophinin function in human embryo implantation. Journal of Obstetrics and Gynaecology Research. 2008;34:129-136.
Genbacev OD, Prakobphol A, Foulk RA, et al. Trophoblast L-selectin-mediated adhesion at the maternal–fetal interface. Science. 2003;299:405-408.
Guzeloglu-Kayisli O, Basar M, Arici A. Basic aspects of implantation. Reproductive Biomedicine Online. 2007;15:728-739.
Hall VJ, Stojkovic M. The status of human nuclear transfer. Stem Cell Reviews. 2006;2:301-308.
Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. American Journal of Reproductive Immunology. 1998;39:137-143.
Hochedlinger K, Jaenisch R. Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. New England Journal of Medicine. 2003;349:275-286.
Homer HA. Ageing, aneuploidy and meiosis: eggs in a race against time. In: Hillard T, editor. The Yearbook of Obstetrics and Gynaecology. RCOG Press London; 2007:139-158.
Horne AW, Phillips JA, Kane N, et al. CB1 expression is attenuated in fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE. 2008;3:e3969.
Kaji K, Oda S, Shikano T, et al. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nature Genetics. 2000;24:279-282.
Lovell-Badge R. The regulation of human embryo and stem-cell research in the United Kingdom. Nature Reviews. Molecular Cell Biology. 2008;9:998-1003.
Mastroianni L, Coutifaris C 1990 Reproductive Physiology, Vol. I. Parthenon, Carnforth.
Mochida S, Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336-340.
Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nature Reviews. Immunology. 2006;6:584-594.
Moffett A, Regan L, Braude P. Natural killer cells, miscarriage, and infertility. BMJ (Clinical Research Ed.). 2004;329:1283-1285.
Moffett-King A. Natural killer cells and pregnancy. Nature Reviews. Immunology. 2002;2:656-663.
Niemann H, Tian XC, King WA, Lee RS. Epigenetic reprogramming in embryonic and foetal development upon somatic cell nuclear transfer cloning. Reproduction. 2008;135:151-163.
Nixon B, Aitken RJ, McLaughlin EA. New insights into the molecular mechanisms of sperm–egg interaction. Cellular and Molecular Life Sciences. 2007;64:1805-1823.
Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-317.
Papanikolaou EG, Kolibianakis EM, Tournaye H, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Human Reproduction. 2008;23:91-99.
Primakoff P, Myles DG. Penetration, adhesion, and fusion in mammalian sperm–egg interaction. Science. 2002;296:2183-2185.
Sassone-Corsi P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science. 2002;296:2176-2178.
Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76-79.
Stojkovic M, Stojkovic P, Leary C, et al. Derivation of a human blastocyst after heterologous nuclear transfer to donated oocytes. Reproductive Biomedicine Online. 2005;11:226-231.
Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Human Reproduction Update. 2008;14:431-446.
Swales AK, Spears N. Genomic imprinting and reproduction. Reproduction. 2005;130:389-399.
Swann K, Saunders CM, Rogers NT, Lai FA. PLCzeta(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Seminars in Cell and Developmental Biology. 2006;17:264-273.
Telfer EE, McLaughlin M. Natural history of the mammalian oocyte. Reproductive Biomedicine Online. 2007;15:288-295.
Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal–fetal tolerance. Nature Immunology. 2006;7:241-246.
Tunquist BJ, Maller JL. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes and Development. 2003;17:683-710.
Varghese AC, Goldberg E, Agarwal A. Current and future perspectives on intracytoplasmic sperm injection: a critical commentary. Reproductive Biomedicine Online. 2007;15:719-727.
Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nature Reviews. Genetics. 2006;7:185-199.
Wang H, Guo Y, Wang D, et al. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nature Medicine. 2004;10:1074-1080.
Wassarman PM. Zona pellucida glycoproteins. Journal of Biological Chemistry. 2008;283:24285-24289.
Watkins AJ, Papenbrock T, Fleming TP. The preimplantation embryo: handle with care. Seminars in Reproductive Medicine. 2008;26:175-185.
Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810-813.
Wu JQ, Kornbluth S. Across the meiotic divide — CSF activity in the post-Emi2/XErp1 era. Journal of Cell Science. 2008;121:3509-3514.