Chapter 21 Femoral Tunnel Placement to Restore Normal Knee Laxity After Anterior Cruciate Ligament Reconstruction
Introduction
In order to perform a successful anterior cruciate ligament (ACL) reconstruction, the surgeon must make a number of steps that require correct judgment and execution, but there is evidence that the most frequent cause of failure is malpositioning of the graft tunnel in the femur.1 This is not surprising because of the anatomy of the interior of the knee joint and the difficulty of seeing the femoral attachment of the ACL. Because the surgeon views the interior of the intercondylar notch when the knee is flexed, the ACL attachment is carried back into the furthest recess of the knee. This means that there is plenty of scope to err with the tunnel placement if the ACL attachment is not visualized clearly. In particular, the undulating surface of the femoral intercondylar notch includes a transverse ridge or bulge that should come between the observer and the proximal part of the ACL attachment; the inexperienced surgeon may believe that this ridge is the posterior outlet of the notch and then place the graft tunnel shallow to that ridge in a nonanatomical position. The frequency of this error has led to common usage of the term “resident’s ridge” to describe this anatomical feature.
The aim of this chapter is to describe the evolution of knowledge regarding ACL graft placement on the femur, which relates closely to our understanding of the function of the ACL itself. There has been a recent move toward “anatomical” reconstructions, with two grafts in parallel, attempting to reproduce two functional fiber bundles of the ACL. This has prompted a better appreciation of the natural ACL attachment anatomy when performing a conventional single-bundle reconstruction. The tibial attachment is not considered here because changes of the femoral attachment have a much larger effect on ACL graft tension and length changes.2
In this chapter, two distinct sets of terminology will be used to describe femoral graft tunnel positions: (1) anatomical nomenclature for describing positions when the knee is in extension (anterior-posterior, proximal-distal) and (2) surgical nomenclature for describing what the surgeon views when the knee is flexed approximately 90 degrees (high-low, deep-shallow, respectively).3
Functional Anatomy of the Anterior Cruciate Ligament Related to Graft Tunnels
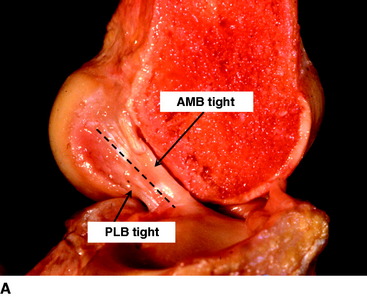
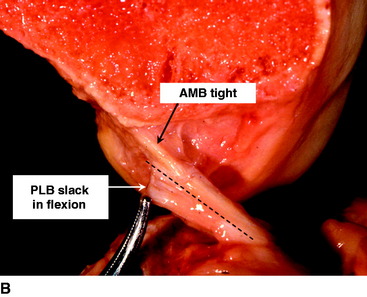
The ACL has a complex fiber structure composed of many fascicles bound together within a synovial covering layer. The fibers are not arranged simply in parallel, and this gives rise to the cross-sectional area being less at the mid-length than at the bony attachments: the fibers must splay out toward the bones.4 The functional significance of this architecture is not understood. However, at a gross level, the fibers of the ACL are arranged as a flat band, and all are tensed when the knee is extended (Fig. 21-1, A). This fiber band is oriented in a sagittal plane so that the ACL fits into and fills the narrow slot between the posterior cruciate ligament (PCL), which occupies most of the width of the intercondylar notch, and the lateral femoral condyle. The sagittal plane of the ACL orientation means that it attaches to the tibia over an area that is oriented anteroposterior (AP). The ACL attaches to the femur over an area that is oriented from anteroproximal to posterodistal.5 This femoral attachment is close to and bounded posteriorly by the condylar articular cartilage and has an overall alignment approximately 35 degrees posterior-distal to the axial.6
When the knee flexes, the axis of rotation moves within the distal femur and the kinematics are affected by the loads imposed on the knee, but the overall effect in the intact knee is that the most anterior fibers of the ACL remain close to a constant length and thus are often described as being “isometric.” Meanwhile, the more posterior the fibers, the more they slacken as the knee flexes, up to 90 degrees flexion2,7–10 (Fig. 21-1, B). These length change patterns have been measured in a number of studies,2,7,11 and it is generally accepted that an “isometry map” can be derived from such measurements.2,9,10 A modern surgical navigation system can produce such maps in response to the surgeon moving the knee during ACL reconstruction procedures, giving a patient-specific feedback on the likely length changes associated with choices of graft tunnel positions around the intercondylar notch12 (Fig. 21-2).
Anterior Cruciate Ligament Isometry and Reconstruction
The observation that the anterior fibers of the ACL remained tight across the range of knee flexion, whereas the more posterior parts slackened, led to the belief that the anterior fibers were the most important. This was reinforced by the finding that the more anterior fibers had a greater material failure strength,13 which suggests that they have adapted to a more mechanically demanding role. A similar finding has been made for the PCL.14 These findings have been correlated with a higher collagen density in the anterior fiber bundles of both the ACL and PCL.15 A more practical reason to place a graft isometrically is that this implies the graft will not be subjected to cyclical length changes when the knee is moving, thus helping to protect it from fatigue or loosening effects. For example, O’Meara et al16 reported that isometric grafts survived cyclical motion in a continuous passive motion machine, whereas nonisometric grafts did not.
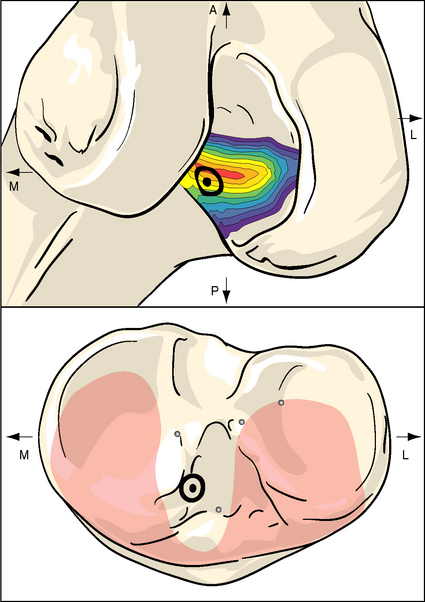
The problem with this line of reasoning is that isometry measurements depend on the ACL being intact; otherwise the kinematics may be abnormal. Even when the ACL is intact, the isometric area on the femoral condyle is influenced sensitively by the loads imposed on the knee while it is being moved. This was shown by Zavras et al,17 who published a map showing a range of different recommended isometric graft locations from the previous literature (Fig. 21-3). Their reproduction of the published works confirmed that isometric behavior could be found reliably for attachment points only at the extreme anteroproximal corner of the natural ACL attachment area.2,9 This means that “isometric” ACL reconstructions are nonanatomical, with the femoral graft tunnel centered higher and deeper in the notch (with the knee flexed) than the natural attachment area. Despite this, the mainstream of opinion through the 1990s favored femoral graft tunnels placed isometrically. Although many clinical papers were published to report a high percentage of good and excellent results, there remained a high level of interest in ACL research and development, reflecting an underlying dissatisfaction with clinical outcome and a desire to find ways of improvement.
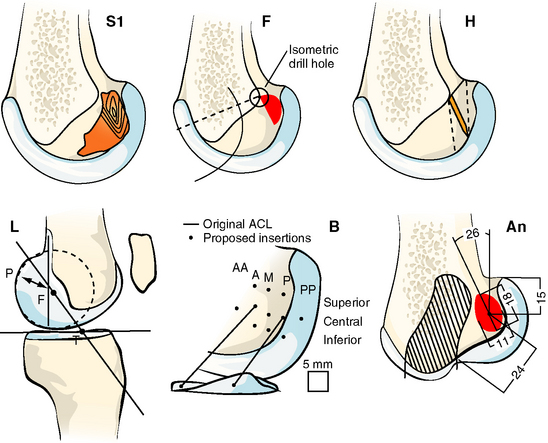
Fig. 21-3 Published isometric graft attachment sites: S1, Sidles et al10; F, Friederich and O’Brien9; H, Hefzy et al2; L, Cazenave and Laboureau31; B, Blankevoort et al32; An (anatomic), Odensten and Gillquist.33
(Reproduced from Zavras TD, Race A, Bull AMJ, et al. A comparative study of isometric points for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2001;9:28–33, with kind permission of Springer Science and Business Media.)
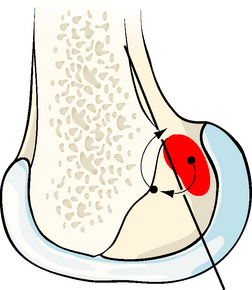
One of the underlying principles that emerged from the isometry research studies was that there is a transition line between attachments that causes graft tightening or slackening with knee flexion.2 The transition line passes through the isometric point at the anteroproximal edge of the ACL attachment and from there runs distal and slightly posterior.2,8,9 Attachments anterior to the transition line lead to graft tightening with knee flexion, whereas grafts posterior to the transition line slacken (Fig. 21-4).
A study of alternative graft attachments investigated the effect of moving to different attachment points either at or around the isometric area on the femur.18 The in vitro study used artificial grafts secured into a barrel that was centered at the femoral isometric point (which had been ascertained by isometry measurements while the ACL was intact). Five attachment points were investigated: isometric, then anteroproximal, anterodistal, posteroproximal, or posterodistal to the isometric point, as shown in Fig. 21-5. It was found to be possible to restore tibiofemoral anterior laxity close to normal across the range of knee flexion investigated, with attachments that were either on that transition line or just posterior to it.18 The tendency of anterior femoral attachments to move away from the matching tibial attachment, and therefore cause the graft to tighten with knee flexion, led to overconstraint of the flexed knee; this was accompanied by elevated graft tension as the knee flexed. Grafts placed distal and posterior to the isometric point, which meant that they were in the anatomical ACL attachment, restored anterior laxity to that of the intact knee across the range of knee flexion investigated.
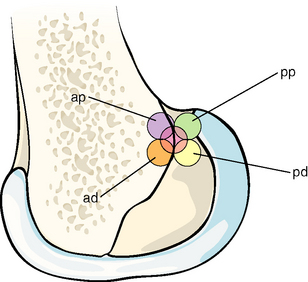
Fig. 21-5 The five anterior cruciate ligament (ACL) graft attachment points investigated by Zavras et al.17 The central isometric point and the more posterior points lead to restoration of normal anterior laxity across the range of knee flexion; the posterodistal point is close to the center of the anatomical ACL attachment area. ad, Anterodistal; ap, anteroproximal; pd, posterodistal; pp, posteroproximal.
(Reproduced from Zavras TD, Race A, Bull AMJ, et al. A comparative study of isometric points for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2001;9:28-33, with kind permission of Springer Science and Business Media.)
Anatomical Single-Bundle Anterior Cruciate Ligament Reconstruction
The trend from isometric toward anatomical graft placement was encouraged by growing evidence of limitations with isometric grafts. In particular, their placement high in the notch meant that they were close to the center of the knee, which is not efficient if they are supposed to limit tibial rotational laxity. There has been a growing awareness that restoration of physiological anterior laxity, as measured routinely by a KT-1000 or similar device, is not sufficient to define a return to the knee working normally and that tibial rotational laxity is also important. The drawback of grafts placed high in the notch has been demonstrated in vivo after ACL reconstruction: one study found that the majority of knees with a patellar tendon ACL reconstruction had traces of residual rotational laxities during pivot-shift testing (a mini-pivot remained).19,20 Other studies have found that the limb with a reconstructed ACL had a persistence of abnormal tibial rotation during gait analysis.21,22 In addition, Amis and Dawkins7 cut the fiber bundles sequentially and measured the reduction in force needed to induce a given tibial anterior translation. The reduction in force needed to displace the tibia indicated the contribution that the cut fiber bundle had made to resisting tibial anterior drawer. It was found that the anteromedial fiber bundle was dominant in the flexed knee, as expected, knowing that the rest of the ACL was then slackened (see Fig. 21-1, B). Conversely, the posterolateral fiber bundle was dominant when the knee was near extension. This, of course, is the posture in which knee stability is most important, when standing.
Such observations have led to a trend toward more anatomical graft placement. In single-bundle ACL reconstruction, that means that the tunnel should be placed at the center of the ACL attachment, which is distal and posterior to the isometric point. During surgery, with the knee flexed, this translates into a tunnel that is lower on the lateral side wall of the notch and also more shallow toward the surgeon compared with the isometric point. In practice, this translates into continuing to use a fixed offset from the posterior outlet but bringing the guide around from approximately the 11-o’clock or 11:30 position to approximately the 10-o’clock position in a right knee. If there is any doubt about the accuracy of finding this point, in a chronic case in which the ACL remnants have disappeared, a guidewire may be placed and checked radiographically using the quadrant method of Bernard et al,23 who documented the center of the femoral ACL attachment. A method to navigate to this point3 is shown in Fig. 21-6. Studies on cadaveric knees24,25 have found that the anatomical tunnel placement (at the 10 o’clock position) led to better control of tibial rotation than did a tunnel placed higher in the notch (at the 11 o’clock position).
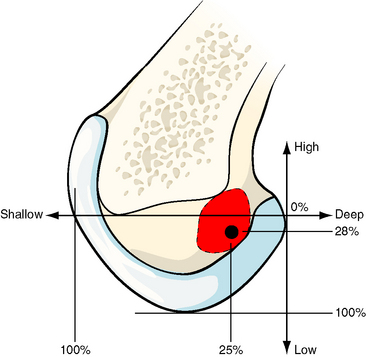
Fig. 21-6 The center of the femoral attachment of the anterior cruciate ligament (ACL) can be found by navigation in percentage terms from the over-the-top position in deep–shallow and high–low directions in the flexed knee.3 Bernard et al23 found the center of the ACL attachment to be 25% more shallow and 28% lower from the over-the-top position.
Anatomical Double-Bundle Anterior Cruciate Ligament Reconstruction
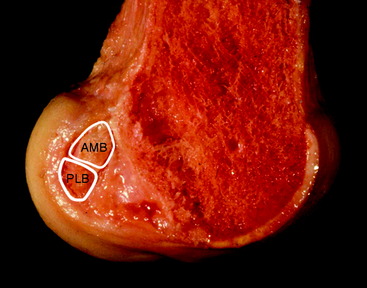
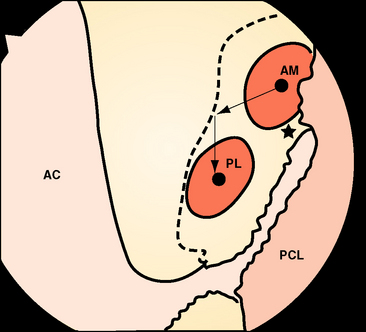
Recently there has been increasing interest in attempting to more closely achieve an anatomical reconstruction using a double-bundle graft. Although some studies have used double grafts passing to or from single tunnels in either the tibia or femur, it is usually accepted that an anatomical reconstruction has two grafts in parallel when the knee is extended, with two tunnels in each of the tibia and femur. The femoral ACL attachment has been split into the two bundle areas in Fig. 21-7.
If the knee is viewed arthroscopically, the anatomical ACL attachment area may be visualized via an anteromedial portal; the viewpoint across the notch gives a better appreciation of depth than can be gained when looking along the lateral side wall from an anterolateral portal.26 The differences in the double-bundle attachment sites, compared with the conventional tunnel high and deep in the notch, then become apparent. The tunnel for the anteromedial graft will still be close to the posterior outlet of the notch but will now be brought down to approximately the 10:30 position. Because of the sloping orientation of the posterior outlet of the notch, moving to the lateral wall also takes the graft tunnel toward the surgeon, which is more shallow (more distal). The tunnel for the posterolateral graft is farther distal and posterior anatomically, which means that it is lower on the side wall of the notch and much more shallow than the first tunnel (Fig. 21-8). Typical positions will be at the 9-o’clock orientation, with an offset sufficient to maintain a bone bridge between the tunnel mouths. With autogenous hamstring tendon grafts, the tunnels are typically 6 mm in diameter, and an offset of 8 mm between the tunnel centers maintains a bone bridge and matches the spacing of the natural fiber bundle attachments. This position will be much closer to the surgeon than with a conventional reconstruction and should also be low enough that the posterior edge of the tunnel is close to the articular cartilage margin at the place where it is closest to the tibia6 (see Fig. 21-8). Various instruments are being developed to allow the second (posterolateral) tunnel to be located relatively easily at a fixed offset distance from the first (anteromedial) tunnel,27 which can itself be located using a conventional offset drill guide hooked over the posterior rim of the intercondylar notch.
Discussion
This chapter has outlined some of the thinking and research behind the recent evolution of femoral ACL graft tunnel placement. At one period the predominant doctrine was that the tunnel placement should produce isometric graft behavior, but that resulted in the tunnel being placed high in the notch, which was not anatomical. The mainstream of opinion has more recently moved toward an acceptance of anatomical graft placement, a philosophy to which some surgeons have always adhered. However, until recently there has been little interest in making a comparison between these approaches. Biomechanical researchers have produced evidence in vitro to support a move toward placing the ACL graft more anatomically, which is onto the lateral side wall of the intercondylar notch, at approximately 10 o’clock, and more shallow compared with the conventional isometric placements. A more recent development is the anatomical double-bundle reconstruction,28,29 but at present there is no reliable clinical evidence to support a change from a single-bundle ACL reconstruction.30
1 Getelman MH, Friedman MJ. Revision anterior cruciate ligament surgery. J Am Acad Orthop Surg. 1999;7:189-198.
2 Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17:208-216.
3 Amis AA, Beynnon B, Blankevoort L, et al. Proceedings of the ESSKA scientific workshop on reconstruction of the anterior and posterior cruciate ligaments. Knee Surg Sports Traumatol Arthrosc. 1994;2:124-132.
4 Harner CD, Baek GH, Vogrin TM, et al. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15:741-749.
5 Giron F, Cuomo P, Aglietti P, et al. Femoral attachment of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14:250-256.
6 Yasuda K, Kondo E, Ichiyama H, et al. Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon. Arthroscopy. 2004;20:1015-1025.
7 Amis AA, Dawkins GPC. Functional anatomy of the anterior cruciate ligament—fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg. 1991;73B:260-267.
8 Amis AA, Zavras TD. Review article: isometricity and graft placement during anterior cruciate ligament reconstruction. Knee. 1995;2:5-17.
9 Friederich NF, O’Brien WR. Functional anatomy of the cruciate ligaments. In: Jakob RP, Staubli HU, editors. The knee and the cruciate ligaments. Berlin: Springer Verlag; 1992:78-91.
10 Sidles JA, Larson RV, Garbini JL, et al. Ligament length relationships in the moving knee. J Orthop Res. 1988;6:583-610.
11 Sapega AA, Moyer RA, Schneck C, et al. Testing for isometry during reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 1990;72A:259-267.
12 Colombet P. Personal communication. December, 2005.
13 Butler DL, Guan Y, Kay MD, et al. Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech. 1992;25:511-518.
14 Race A, Amis AA. The mechanical properties of the two bundles of the human posterior cruciate ligament. J Biomech. 1994;27:13-24.
15 Mommersteeg TJ, Blankevoort L, Kooloos JG, et al. Nonuniform distribution of collagen density in human knee ligaments. J Orthop Res. 1994;12:238-245.
16 O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. Clin Orthop. 1992;277:201-209.
17 Zavras TD, Race A, Bull AMJ, et al. A comparative study of isometric points for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2001;9:28-33.
18 Zavras TD, Race A, Amis AA. The effect of femoral attachment location on anterior cruciate ligament reconstruction: graft tension patterns and restoration of normal anterior-posterior laxity patterns. Knee Surg Sports Traumatol Arthrosc. 2005;13:92-100.
19 Amis AA, Bull AMJ, Lie DTT. Biomechanics of rotational instability and anatomic anterior cruciate ligament reconstruction. Op Tech Orthop. 2005;15:29-35.
20 Bull AMJ, Earnshaw PH, Smith A, et al. Intraoperative measurement of knee kinematics in reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 2002;84B:1075-1081.
21 Ristanis S, Giakas G, Papageorgiou CD, et al. The effects of anterior cruciate ligament reconstruction on tibial rotation during pivoting after descending stairs. Knee Surg Sports Traumatol Arthrosc. 2003;11:360-365.
22 Tashman S, Collon D, Anderson K, et al. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975-983.
23 Bernard M, Hertel P, Hornung H, et al. Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg. 1997;10:14-21.
24 Loh JC, Fukuda Y, Tsuda E, et al. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. Arthroscopy. 2003;19:297-304.
25 Scopp JM, Jasper JE, Belkoff SM, et al. The effect of oblique femoral tunnel placement on rotational contraint of the knee reconstructed using patellar tendon autografts. Arthroscopy. 2004;20:294-299.
26 Fu F. Personal communication. August, 2005.
27 Christel P, et al. Personal communication. April, 2005.
28 Radford WJP, Amis AA. Biomechanics of a double prosthetic ligament in the anterior cruciate deficient knee. J Bone Joint Surg. 1990;73B:1038-1043.
29 Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660-666.
30 Adachi N, Ochi M, Uchio Y, et al. Reconstruction of the anterior cruciate ligament: single versus double-bundle multistranded hamstring tendons. J Bone Joint Surg. 2004;86B:515-520.
31 Cazenave A, Laboureau JP. Isometric reconstruction of the anterior cruciate ligament. Pre- and peri-operative determination of the femoral isometric point. French J Orthop Surg. 1990;4:255-259.
32 Blankevoort L, Huiskes R, van Kampen A. ACL reconstruction: simply a matter of isometry?. Passive motion characteristics of the human knee joint—experiments and computer simulations. 1991;151-162. PhD thesis, University of Nijmegen
33 Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for its reconstruction. J Bone Joint Surg. 1985;67A:257-262.