19 Eyes
History
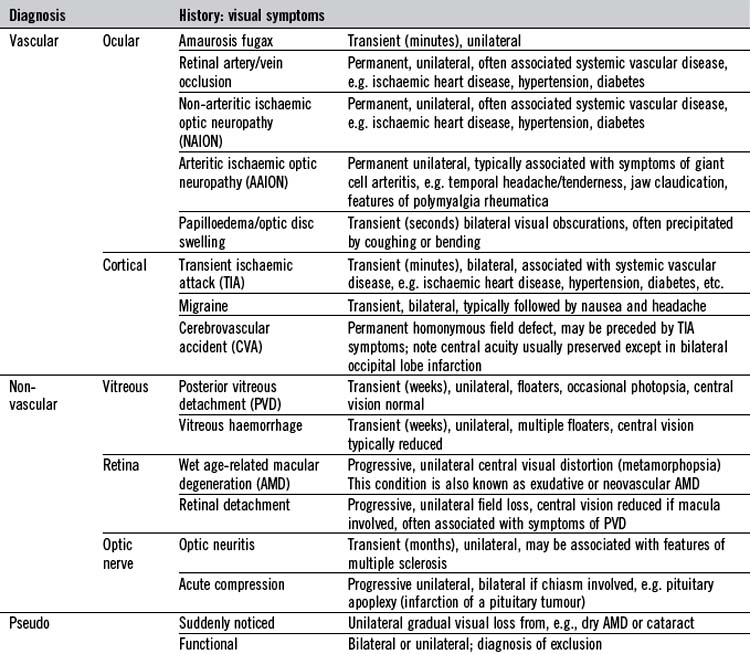
Disturbance of vision, the most important ocular symptom, may be sudden or gradual, unilateral or bilateral, and lead to loss of central vision or partial field loss. Simultaneous, bilateral visual symptoms are usually due to disease in optic pathways at or posterior to the optic chiasm. Sudden visual disturbance should be assessed urgently. Visual halluci-nations may be formed or unformed. Some visual symptoms have particular significance (Table 19.1). For example, haloes around lights occur in acute angle-closure glaucoma due to corneal oedema. ‘Floaters’ and flashes (photopsia) are indicative of vitreous or retinal disorders, respectively. The latter may also cause objects to appear smaller (micro-psia), larger (macropsia) or distorted (metamorph-opsia). Disorders of ocular movement may cause double vision (diplopia) or visual blurring. Are the visual symptoms binocular or monocular? Are they related to eye movements?
Other common presentations include a red eye, abnormal lid position, protrusion of the globe, pupillary or eyelid abnormality. Ocular pain is often associated with a red eye (Table 19.2). Ocular pain due to a foreign body may be described as ‘a gritty sensation’ in the eye, often worsened by blinking. It may be associated with sensitivity to light (photophobia) but this, particularly in conjunction with ocular aching, usually indicates serious corneal or intraocular disease. Severe ocular pain with vomiting may indicate acute glaucoma. Migraine often presents with bilateral visual symptoms and headache. Raised intracranial pressure and giant cell arteritis should also be considered when headache is associated with visual symptoms. Pain may be referred to the eye because of neighbouring disease, for example sinusitus. Excessive tear production (lacrimation) associated with discomfort may indicate ocular surface disease. There may be abnormal secretions from the eye, such as mucus or pus. With insufficient tears, the eye typically feels dry, whereas a painless overflow of tears (epiphora) typically indicates blockage of the lacrimal drainage system.
| Diagnosis | History | Examination |
|---|---|---|
| Subconjunctival haemorrhage | Typically asymptomatic, spontaneous May be associated with trauma |
Unilateral (except some trauma), contiguous red area |
| Viral conjunctivitis | FB sensation, watering, no visual loss or photophobia Recent contact with person with red eye or URTI? |
Bilateral, prominent inflamed conjunctival vessels, follicles, enlarged tender preauricular lymph node |
| Bacterial conjunctivitis | FB sensation, discharge, no visual loss or photophobia | Bilateral, prominent inflamed conjunctival vessels, mucopus |
| Allergic conjunctivitis | Itch, watering, no visual loss or photophobia, history of atopy | Bilateral, prominent inflamed conjunctival vessels, follicles |
| Iritis (anterior uveitis) | Reduced vision, aching sensation, photophobia PMH or systemic enquiry may elicit underlying disease |
Unilateral, prominent pericorneal vessels, small pupil, aqueous cells and protein (at slit lamp), hypopyon |
| Acute angle-closure glaucoma | Severe pain, haloes/rainbows around lights, reduced vision, hypermetropic, elderly | Unilateral, pericorneal prominent vessels, semidilated (oval) pupil, corneal oedema, shallow anterior chamber (slit lamp) |
| Episcleritis | Mild discomfort, tenderness, no visual loss or photophobia, young adult, otherwise fit and well | Unilateral, typically sectorial prominent inflamed subconjunctival vessels (may also be nodular or diffuse) |
| Scleritis | Significant aching pain, tender, photophobia, occasionally reduced vision, systemic enquiry may elicit underlying disease | Unilateral, typically sectorial prominent inflamed deep scleral vessels (may also be nodular or diffuse) |
| Bacterial keratitis | FB sensation, watering/discharge, visual loss, photophobia Pre-existing ocular surface disease, recent trauma or contact lens wear? |
Unilateral, opacity in cornea (slit lamp) stains with fluorescein |
| Herpetic viral keratitis | FB sensation, watering/discharge, visual loss, photophobia Cold sores or ophthalmic shingles? |
Unilateral, branching linear dendrite(s) on cornea (slit lamp) stains with fluorescein |
FB, foreign body; PMH, past medical history; URTI, upper respiratory tract infection.
Examination
Visual acuity
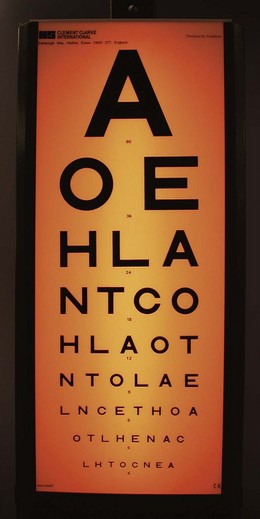
Visual acuity is most reliably tested at 6 m (20 ft) using a standard chart such as the Snellen chart (Fig. 19.1). Tests of acuity in near vision are portable but limited by age-related loss of accommodation (presbyopia), which necessitates refractive correction in older patients. These use test types of varying sizes, based on the point system of the printers (Fig. 19.2), and the smallest type that can be read comfortably at a distance of 33 cm is recorded (normally N4.5 or N5 type).
Snellen distance vision
Technique
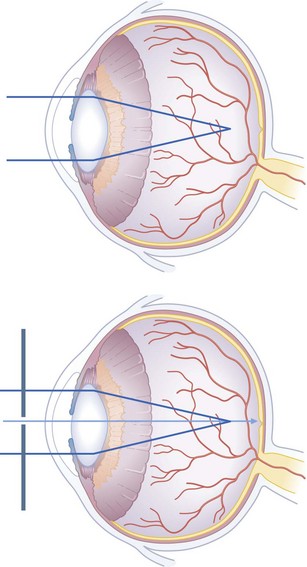
When testing low vision, ensure that the eye not being tested is completely covered. If a patient cannot read 6/6 or better in either eye, check the vision again using a pinhole occluder (Fig. 19.3). This test distinguishes patients with poor vision due to refractive error from those who have ocular or neurological conditions. In a myopic (short-sighted) eye, the rays of light are focused in front of the retina. In hypermetropia (long-sightedness), light is focused behind the eye, because the eye is abnormally short. In astigmatism, the cornea is not uniformly curved and light is not focused evenly on the retina. When using a pinhole, only the central rays of light pass through to the retina and the above refractive errors are significally reduced.
Recording visual acuity
Record a mistake of one letter in a line as ‘ −1’ and a single letter read from the next smaller line as ‘+1’. Thus, if all except one of the letters on the line designated 6 are read correctly, the visual acuity is recorded 6/6 −1. If only one letter on that line is read correctly, the visual acuity is recorded 6/9 +1. Like all ophthalmic findings except visual fields, right eye visual acuity is traditionally written on the left side of the page (as the patient’s eye appears to you), and vice versa. Whether the patient was using glasses, pinhole (ph) or was unaided (ua) is recorded in the middle (Fig. 19.4).
Colour vision
Tests of colour vision are important because colour perception, especially for red, is affected in optic nerve disease before changes in visual acuity can be detected. Show the patient a red target one eye at a time (any bright red target can be used) and ask if there is a difference between the eyes. In the affected eye, red appears ‘washed out’ (desaturated). Acquired defects of colour vision may also occur in macular disease. The Ishihara test (Fig. 19.5) was devised to test for congenital colour anomalies (colour blindness), but is often used to assess acquired visual disorders. Most inherited colour blindness occurs in males (sex-linked recessive inheritance). It ranges from total colour blindness (monochromatopsia) to subtle confusion between colours, typically between red and green. About 8% of men and 0.5% of women in the UK have congenital colour perception defects. Blue/yellow deficiencies and total colour blindness are uncommon.
Visual field testing
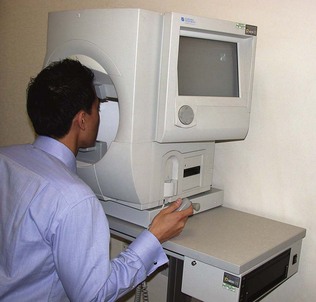
Visual field testing is described in Chapter 14. Field defects may affect one or both eyes. Symmetric bilateral (homonymous) field defects are characteristic of lesions posterior to the optic chiasm, and asymmetric field defects are usually due to lesions anterior to the chiasm (i.e. in the optic nerves or retinae). Characteristic field defects (scotoma) occur in glaucoma, when damage to nerve fibres occurs at the optic disc, typically at the inferior or superior aspect of the optic cup. Fundoscopy shows an increase in vertical length of the optic cup (see later in the chapter for details about how examine the fundi) and field loss is arc-shaped (arcuate scotoma). If both the inferior and superior fields are involved, a ring-shaped scotoma develops. Untreated glaucoma results in loss of the peripheral field so that only a small central island of vision remains (tunnel vision). Computerized perimetry is useful in identifying early visual field loss. The Humphrey field test analyser, for example, provides statistical information indicating the reliability of the test in comparison with a group of age-matched controls (Fig. 19.6).
Pupils
Examination of the pupils in neurology is discussed in Chapter 14. In ophthalmic practice, there are three key aspects to pupil examination: size, shape and reactions.
Pupil size: anisocoria
In 12% of normal individuals, the pupils are slightly unequal, particularly in bright light (anisocoria), but in these subjects they react normally. Abnormal pupils dilate and constrict abnormally, and the degree of anisocoria varies with the ambient illumination. However, it is often difficult to decide which pupil is abnormal. In the absence of local eye disease, a small pupil may be due to paralysis of the dilator pupillae muscle (sympathetic innervated), part of Horner’s syndrome (Fig. 19.7), in which anisocoria is more pronounced in low ambient light. An enlarged pupil suggests a parasympathetic lesion, which may be preganglionic in oculomotor lesions or postganglionic as in the tonic pupil of Adie’s syndrome. Adie’s tonic pupil tends to be dilated in bright light and is very slow to react. A feature of both parasympathetic and sympathetic lesions is denervation hypersensitivity caused by upregulation of receptors at the neuromuscular junction (adrenergic in sympathetic and cholinergic in parasympathetic). This is the basis of pharmacological pupil testing. In both pre- and postganglionic parasympathetic blockade, the pupil is supersensitive to weak cholinergic drops (e.g. pilocarpine 0.1%). In sympathetic block, dilute adrenergic agonists such as phenylephrine 1% are unreliable, so the uptake blocker, cocaine 4%, is used. This dilates normal pupils but has no effect in pre- or postganglionic lesions. Hydroxyamfetamine 1% causes noradrenaline (nor-epinephrine) release from normal or intact postganglionic neurones and allows pre- and postganglionic lesions to be distinguished (the synapse is located in the superior cervical ganglion). In complex pupil abnormalities, for example bilateral Horner’s syndrome, infrared pupil imaging can be valuable. Causes of anisocoria are highlighted in Box 19.1. In congenital Horner’s syndrome, the affected iris is depigmented and appears blue.
Pupil reactions: afferent and central defects
The swinging light test is used to detect a relative afferent pupillary defect (RAPD) resulting from retinal or optic nerve disease. The test loses sensiti-vity in symmetrical bilateral optic nerve disease, but this is rare, and in most bilateral cases the defect will be detected on the more abnormal side. RAPD is often associated with reduced vision, but if central vision is retained, the acuity will be normal, although severe peripheral field damage may cause RAPD. In neurosyphilis, the pupils are small and irregular and show the Argyll Robertson phenomenon (normal pupil constriction to a near target but reduced reaction to light) owing to a midbrain defect. In Parinaud’s syndrome, the pupils are typically large and poorly reactive to light, with abnormal vertical gaze, convergence retraction nystagmus and lid retraction on attempted upgaze (Collier’s sign). The causes of light-near dissociation are given in Box 19.2.
Pupil shape
Slit-lamp examination is the best technique to assess intrinsic ocular disease that may affect the shape and position of the pupil (Box 19.3).
Box 19.3 Causes of abnormal pupil shape
 Congenital: Rieger’s anomaly (anterior segment cleavage anomalies)
Congenital: Rieger’s anomaly (anterior segment cleavage anomalies)
 Iatrogenic (sphincterotomies, iridectomy, cataract surgery)
Iatrogenic (sphincterotomies, iridectomy, cataract surgery)
 Inflammatory (posterior synechiae in iritis)
Inflammatory (posterior synechiae in iritis)
 Tumour (ectropion uveae in ciliary body malignancies)
Tumour (ectropion uveae in ciliary body malignancies)
 Ischaemic: herpes zoster, angle-closure glaucoma
Ischaemic: herpes zoster, angle-closure glaucoma
 Idiopathic: iridocorneal endotheliopathy (ICE) syndrome (which also features multiple pupils (polycoria) and misplaced pupils (correctopia))
Idiopathic: iridocorneal endotheliopathy (ICE) syndrome (which also features multiple pupils (polycoria) and misplaced pupils (correctopia))
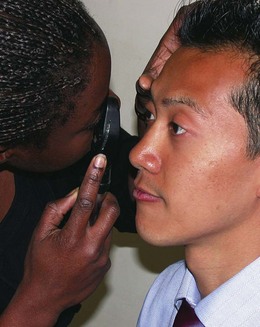
Direct ophthalmoscopy
Preparation
1 Decide the objective of the examination.
2 Consider dilating the pupils with 1% cyclopentolate (Mydrilate) or 1% tropicamide (Mydriacyl). This blurs the vision for at least 2 hours, so patients should be forewarned and instructed not to drive. Some patients have a predisposition to closed-angle glaucoma (Box 19.4) and dilatation may precipitate an attack. At-risk individuals must be warned to return if symptoms of acute angle closure occur.
3 Briefly explain the examination so that the patient can cooperate fully. Ask the patient to fixate on a distant target – he should continue to look in this direction even if the examiner’s head obscures the target.
5 Set the ophthalmoscope lens wheel to zero dioptres (D), unless correcting for your own short- or long-sightedness.
6 Check the ophthalmoscope light is bright. Unless the pupil is small, select a large light spot size (Box 19.5, Fig. 19.8).
Box 19.5 Technique of ophthalmoscopy
 Use your LEFT eye for the patient’s LEFT eye, holding the ophthalmoscope with your LEFT hand and using your RIGHT hand with thumb over the LEFT brow to steady the patient’s head (the opposite approach is used for the right eye) (Fig. 19.8).
Use your LEFT eye for the patient’s LEFT eye, holding the ophthalmoscope with your LEFT hand and using your RIGHT hand with thumb over the LEFT brow to steady the patient’s head (the opposite approach is used for the right eye) (Fig. 19.8).
 First, at arm’s length, look for and examine the red reflex (like that in flash photographs). The red reflex is dimmed in elderly people with cataracts, and these may obscure the retina. In a child, congenital cataract and retinoblastoma may abolish the red reflex.
First, at arm’s length, look for and examine the red reflex (like that in flash photographs). The red reflex is dimmed in elderly people with cataracts, and these may obscure the retina. In a child, congenital cataract and retinoblastoma may abolish the red reflex.
 Looking at the red reflex through the ophthalmoscope, gradually close on the eye to examine the anterior ocular structures. Use the +20 D lens to view the cornea and the +15 D lens for the iris. Some ophthalmoscopes have a lever to switch to these lenses.
Looking at the red reflex through the ophthalmoscope, gradually close on the eye to examine the anterior ocular structures. Use the +20 D lens to view the cornea and the +15 D lens for the iris. Some ophthalmoscopes have a lever to switch to these lenses.
 Then, with a zero lens setting, locate the optic disc. If it is difficult to find, locate any blood vessel and follow it towards the optic disc. Fine-tune the focus with 1-2 D lens steps; if the focus worsens, reverse the other way. If your eye is emmetropic and your accommodation is relaxed, the strength of the lens necessary to bring the fundus into focus gives an indication of the refractive error of the patient’s eye (plus or red lenses indicate hypermetropia and minus or black lenses myopia).
Then, with a zero lens setting, locate the optic disc. If it is difficult to find, locate any blood vessel and follow it towards the optic disc. Fine-tune the focus with 1-2 D lens steps; if the focus worsens, reverse the other way. If your eye is emmetropic and your accommodation is relaxed, the strength of the lens necessary to bring the fundus into focus gives an indication of the refractive error of the patient’s eye (plus or red lenses indicate hypermetropia and minus or black lenses myopia).
 Compare the two eyes. There is a broad range of normal appearances, so if an unusual appearance is symmetrical, it could be a variant of normal.
Compare the two eyes. There is a broad range of normal appearances, so if an unusual appearance is symmetrical, it could be a variant of normal.
Examining the fundi
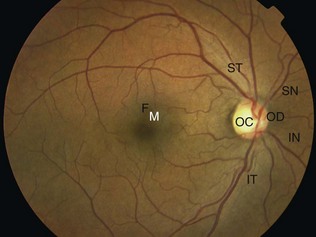
Look carefully at the optic disc/cup, the retinal vessels and the retina and macula (Fig. 19.9).
Optic disc
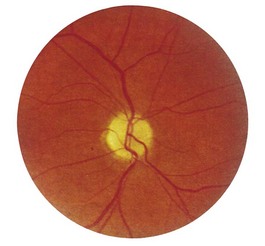
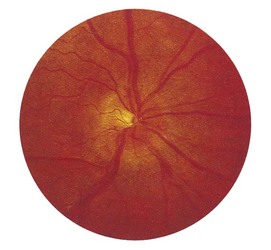
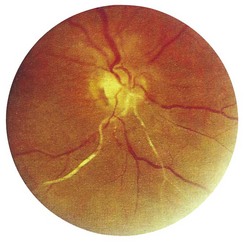
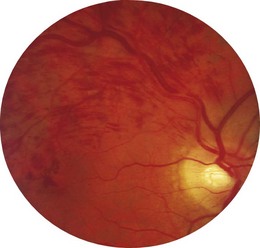
1 Is the colour normal? The normal disc is yellow-pink and its temporal side is paler. An abnormally pale disc is a sign of optic atrophy (Fig. 19.10). In addition, in optic atrophy there is a reduction in the number of capillaries crossing the edge of the disc, from the normal 10 to 7 or fewer (Kestenbaum’s sign). The causes of optic atrophy are listed in Box 19.6. In primary optic atrophy due to optic nerve lesions, the disc is flat and white, with clear-cut edges. Secondary optic atrophy follows swelling of the optic disc due to papilloedema: the disc is greyish-white, with indistinct edges.
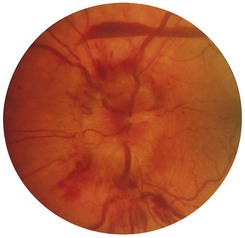
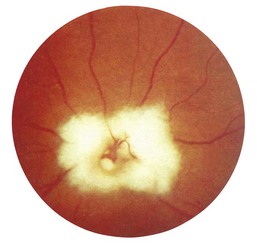
2 Are the margins distinct? The disc margin should be sharply defined. In optic disc swelling, this clear edge is obscured (Fig. 19.11) and venous pulsations are abolished. However, venous pulsations may be difficult to see in some normal subjects. Some important causes of disc swelling are listed in Box 19.7. In papilloedema, caused by raised intracranial pressure, the disc is abnormally red and its margins are blurred, especially at the upper and lower margins, and particularly in the upper nasal quadrant. The physiological cup becomes obliterated and the retinal veins are slightly distended. As the condition progresses, the disc becomes more definitely swollen (Fig. 19.12). In order to measure the degree of swelling, start with a high plus lens in the ophthalmoscope and reduce the power until the centre of the disc is just in focus. The retina, a short distance from the disc, is then brought into focus by further reduction of the lens power. This further reduction indicates the degree of swelling of the disc (3 D is equivalent to 1 mm of swelling). If papilloedema develops rapidly, there will be marked engorgement of the retinal veins with haemorrhages and exudates on and around the disc, but with papilloedema of slow onset there may be little or no vascular change, even though the disc may become very swollen. The retinal vessels will, however, bend sharply as they dip down from the swollen disc to the surrounding retina. The oedema may extend to the adjacent retina, producing greyish-white striations near the disc (Paton’s lines), and a macular fan of hard exudates temporal to the fovea may develop in some cases.
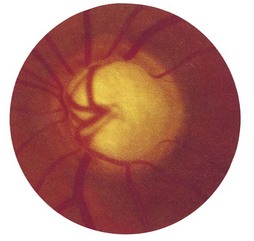
3 Is the central cup enlarged? The cup is a physiological central depression formed at the optic disc as nerve fibres leave the retina to form the optic nerve. It marks the point where the retinal vessels enter and leave the eye. It is paler than the surrounding rim of the disc. The optic cup : disc ratio is estimated by comparing their ratios vertically. In chronic open-angle glaucoma, the ratio is increased (>0.3) – optic disc cupping. When the cup is deep, in advanced glaucoma, retinal vessels disappear as they climb from the floor to the rim, and reappear as they bend sharply over the edge of the rim (bayonetting); in less advanced cases, the cup appears as a vertical oval extending to the edge of the disc (Fig. 19.13). In myopic individuals, the disc and cup appear large, and mimic glaucoma. Myopes often have a partial ring of pigmentation or white sclera surrounding the disc, which is easily mistaken for the edge of the cup. In severe myopia, degenerative chorioretinal changes may occur in the fundus, which can involve the macula and impair central vision.
4 Are there any other abnormal features? In proliferative diabetic retinopathy, new blood vessels (neovascularization) develop at the optic disc. Myelinated nerve fibres have a dramatic white appearance, but they are a unilateral, harmless and non-progressive congenital anomaly (Fig. 19.14). They have a characteristic feathered edge that may obscure the retinal vessels.
Blood vessels
There are four pairs of arterioles and venules, which form the main retinal vascular arcades that emerge from the optic disc: superotemporal (above the macula); inferotemporal (below the macula); superonasal; and inferonasal. Study each in turn. Arterioles are thin, bright red in colour and with a longitudinal streak of light reflection. In branch arteriolar occlusion, a bright yellow (cholesterol) embolus may occasionally be seen (Fig. 19.15). In diabetes or venous occlusion, the venules are larger, darker and often dilated or tortuous. Look carefully at arteriolar/venous crossings: compression and localized dilatation of venules (arteriovenous (AV) nipping) with arteriolar narrowing (attenuation) is a sign of hypertension (Box 19.8, Fig. 19.16). Spontaneous arteriolar pulsation is an abnormal finding that may occur if the IOP is very high or the central retinal artery pressure very low. Spontaneous venous pulsation is frequently seen in normal eyes, but is reduced in papilloedema.
Retina and macula
As each main vascular arcade is followed and examined, the adjacent and peripheral retina can be systematically assessed. The macula is the central retinal area bounded by temporal vascular arcades. It mea-sures approximately five disc diameters across. The fovea at its centre is one disc diameter in size. The fovea, with its high density of cone photoreceptors, is responsible for fine discriminatory vision. To find the fovea, locate the optic disc and move the ophthalmoscope beam temporally (move yourself nasally). Alternatively, ask the patient to look directly into the light. However, if the pupil is undilated, it tends to constrict at this point, and the patient may recoil because of dazzle (you can dim the light beam to make it more comfortable). In young patients, the retina is very reflective and there is often a small yellow dot in the middle of the fovea (macula lutea or fovea centralis). Box 19.9 and Figures 19.17 and 19.18 identify common retinal abnormalities by their colour and appearance.
Box 19.9 Common retinal abnormalities
White
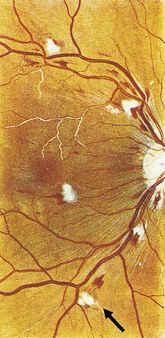
 Cotton wool spots: white, fluffy, indistinct areas indicative of retinal ischaemia. This is the accumulation of axonal proteins in the nerve fibre layer. Causes include severe hypertension, diabetes and retinal vein occlusion
Cotton wool spots: white, fluffy, indistinct areas indicative of retinal ischaemia. This is the accumulation of axonal proteins in the nerve fibre layer. Causes include severe hypertension, diabetes and retinal vein occlusion
 Chorioretinal atrophy: well-defined ‘punched-out’ lesions (the white is the sclera). May occur in conjunction with retinal pigment hypertrophy. Associated with previous retinal inflammation or injury (including retinal laser)
Chorioretinal atrophy: well-defined ‘punched-out’ lesions (the white is the sclera). May occur in conjunction with retinal pigment hypertrophy. Associated with previous retinal inflammation or injury (including retinal laser)
Yellow
 Hard exudates: bright yellow with well-demarcated edges consisting of lipid deposits that have leaked out of abnormal blood vessels. Most commonly associated with microaneurysms in diabetes (Fig. 19.17)
Hard exudates: bright yellow with well-demarcated edges consisting of lipid deposits that have leaked out of abnormal blood vessels. Most commonly associated with microaneurysms in diabetes (Fig. 19.17)
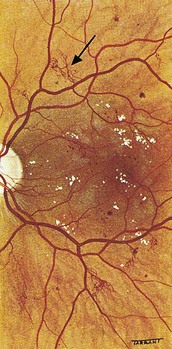
 Drusen: small multifocal round yellow features, usually located in the central macula. Generally smaller and less bright yellow than hard exudates. Typically bilateral and relatively symmetrical. Common in elderly people associated with ‘dry’ age-related macular degeneration
Drusen: small multifocal round yellow features, usually located in the central macula. Generally smaller and less bright yellow than hard exudates. Typically bilateral and relatively symmetrical. Common in elderly people associated with ‘dry’ age-related macular degeneration
Red
 Microaneurysms: the dots that typify diabetic retinopathy. They may leak to cause exudates or bleed to cause blot haemorrhages (Fig. 19.17)
Microaneurysms: the dots that typify diabetic retinopathy. They may leak to cause exudates or bleed to cause blot haemorrhages (Fig. 19.17)
 Blot haemorrhages: rounded localized intraretinal blood, typically due to diabetic retinopathy, but other causes include severe hypertension and retinal vein occlusion
Blot haemorrhages: rounded localized intraretinal blood, typically due to diabetic retinopathy, but other causes include severe hypertension and retinal vein occlusion
 Deep large haemorrhages: associated with retinal ischaemia when numerous
Deep large haemorrhages: associated with retinal ischaemia when numerous
 Flame haemorrhages: have a characteristic feathery shape as the blood is in the nerve fibre layer; may be present in retinal vein occlusion (Fig. 19.18). Not typically associated with ischaemia
Flame haemorrhages: have a characteristic feathery shape as the blood is in the nerve fibre layer; may be present in retinal vein occlusion (Fig. 19.18). Not typically associated with ischaemia
Slit lamp and intraocular pressure
The slit lamp (Fig. 19.19) provides a stereoscopic, magnified view of the eye and is the key examination tool for ophthalmologists. Many accident and emergency departments have a slit lamp, and it can be invaluable for assessing suspected foreign bodies and corneal abrasions. Some direct ophthalmoscopes are equipped with a slit-lamp beam, which can be useful. Alternatively, the anterior orbital structures and globe can be examined with a bright torch and basic magnification, and the same principles of systematic examination apply. The slit lamp comprises a table-mounted binocular microscope column with an adjustable illumination source that produces a narrow, slit beam of light.
The following structures can be examined:
 Lid margins, meibomian gland orifices and lashes. Inflammation of the lid margins (blepharitis) is one of the commonest ophthalmic conditions. It is related to chalazia, blocked meibomian glands and infected lash follicles (styes). The puncta, on the medial aspect of the lids, drain tears into the canalicular tear drainage pathway. Misdirected lashes (trichiasis) causing foreign body sensations occur with chronic lid disease.
Lid margins, meibomian gland orifices and lashes. Inflammation of the lid margins (blepharitis) is one of the commonest ophthalmic conditions. It is related to chalazia, blocked meibomian glands and infected lash follicles (styes). The puncta, on the medial aspect of the lids, drain tears into the canalicular tear drainage pathway. Misdirected lashes (trichiasis) causing foreign body sensations occur with chronic lid disease.
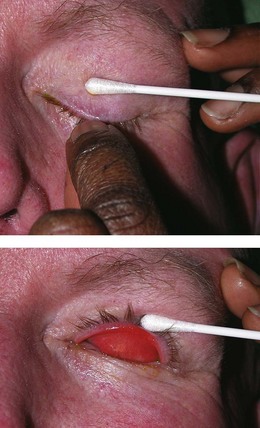
 Conjunctival surfaces (tarsal, forniceal and bulbar). This mucous membrane lines the eyeball (bulbar conjunctiva) and the inner surface of the eyelids (tarsal conjunctiva). The conjunctiva may be pale in anaemia, yellow in jaundice or red (injected) in conjunctivitis and other inflammatory eye disorders. Directing the patient’s gaze up, down, left and right ensures that all the bulbar conjuctiva is viewed. To examine the inferior tarsal conjunctiva of the lower lid, the lower lid should be gently everted and the patient asked to look upwards. To examine the superior tarsal conjunctiva – for example if a foreign body is suspected – ask the patient to look downwards (Fig. 19.20). Grasp the lashes between the forefinger and thumb, gently pull down on them and rotate the eyelid upwards over either the other thumb or a cotton bud.
Conjunctival surfaces (tarsal, forniceal and bulbar). This mucous membrane lines the eyeball (bulbar conjunctiva) and the inner surface of the eyelids (tarsal conjunctiva). The conjunctiva may be pale in anaemia, yellow in jaundice or red (injected) in conjunctivitis and other inflammatory eye disorders. Directing the patient’s gaze up, down, left and right ensures that all the bulbar conjuctiva is viewed. To examine the inferior tarsal conjunctiva of the lower lid, the lower lid should be gently everted and the patient asked to look upwards. To examine the superior tarsal conjunctiva – for example if a foreign body is suspected – ask the patient to look downwards (Fig. 19.20). Grasp the lashes between the forefinger and thumb, gently pull down on them and rotate the eyelid upwards over either the other thumb or a cotton bud.
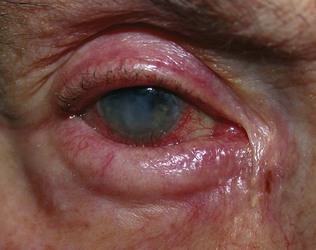
 Cornea and tear film. The transparent cornea can be viewed in cross-section. The addition of a drop of 2% fluorescein reveals defects or foreign bodies in the corneal epithelium and the tear film can be assessed (Fig. 19.21). The tear meniscus on the lower eyelid should be symmetrical and less than 1 mm thick, and the tear break-up time should be more than 10 seconds. Fluorescein also aids the identification of aqueous leakage in a penetrating corneal injury (Seidel’s test). Arcus senilis is a common crescentic opacity near the periphery of the cornea. It usually starts at the lower part of the cornea, extending to form a complete circle. It is common in old people, but may occur in the young (arcus juvenilis) in association with type IV hyperlipoproteinaemia. Corneal sensation should be tested.
Cornea and tear film. The transparent cornea can be viewed in cross-section. The addition of a drop of 2% fluorescein reveals defects or foreign bodies in the corneal epithelium and the tear film can be assessed (Fig. 19.21). The tear meniscus on the lower eyelid should be symmetrical and less than 1 mm thick, and the tear break-up time should be more than 10 seconds. Fluorescein also aids the identification of aqueous leakage in a penetrating corneal injury (Seidel’s test). Arcus senilis is a common crescentic opacity near the periphery of the cornea. It usually starts at the lower part of the cornea, extending to form a complete circle. It is common in old people, but may occur in the young (arcus juvenilis) in association with type IV hyperlipoproteinaemia. Corneal sensation should be tested.
 Anterior chamber (filled with aqueous). In iritis, a cause of red eye, there is inflammation in the aqueous, with flare (protein) and cells (typically leukocytes). In severe iritis, the inflammatory exudate settles inferiorly to create a white fluid level in the anterior chamber (hypopyon). Hyphaema has a similar but red appearance caused by bleeding into the anterior chamber, usually due to trauma.
Anterior chamber (filled with aqueous). In iritis, a cause of red eye, there is inflammation in the aqueous, with flare (protein) and cells (typically leukocytes). In severe iritis, the inflammatory exudate settles inferiorly to create a white fluid level in the anterior chamber (hypopyon). Hyphaema has a similar but red appearance caused by bleeding into the anterior chamber, usually due to trauma.
 Iris. Note any difference in the colour of the two eyes (heterochromia), abnormality in the shape or size of the pupils or signs of iritis. In iritis, the pupil may be constricted (miosis) or irregular owing to the formation of adhesions (posterior synechiae) between the edge of the pupil and the anterior surface of the lens. Blunt trauma can cause a dilated (mydriasis) unreactive pupil with radial ruptures in the iris. An irregular or teardrop-shaped pupil with a history of a high-velocity foreign body is highly suspicious of a penetrating eye injury where the iris has plugged the leaking wound. Other abnormalities of the pupils are described in Chapter 14.
Iris. Note any difference in the colour of the two eyes (heterochromia), abnormality in the shape or size of the pupils or signs of iritis. In iritis, the pupil may be constricted (miosis) or irregular owing to the formation of adhesions (posterior synechiae) between the edge of the pupil and the anterior surface of the lens. Blunt trauma can cause a dilated (mydriasis) unreactive pupil with radial ruptures in the iris. An irregular or teardrop-shaped pupil with a history of a high-velocity foreign body is highly suspicious of a penetrating eye injury where the iris has plugged the leaking wound. Other abnormalities of the pupils are described in Chapter 14.
 Lens. Cataracts are usually due to ageing (central nuclear sclerosis), but also occur in diabetes mellitus, after injury and in certain hereditary diseases, for example myotonic dystrophy. Posterior subcapsular cataract is a common side-effect of corticosteroid therapy. Blunt eye injury may cause partial dislocation of the lens (subluxation) or complete dislocation into the vitreous cavity.
Lens. Cataracts are usually due to ageing (central nuclear sclerosis), but also occur in diabetes mellitus, after injury and in certain hereditary diseases, for example myotonic dystrophy. Posterior subcapsular cataract is a common side-effect of corticosteroid therapy. Blunt eye injury may cause partial dislocation of the lens (subluxation) or complete dislocation into the vitreous cavity.
 Anterior vitreous. This is best examined when the pupil is dilated. Opacities may be observed, most easily using a green light. Cells in the vitreous may be associated with ocular inflammation (vitritis), trauma (vitreous haemorrhage) or retinal holes/detachment (retinal pigment).
Anterior vitreous. This is best examined when the pupil is dilated. Opacities may be observed, most easily using a green light. Cells in the vitreous may be associated with ocular inflammation (vitritis), trauma (vitreous haemorrhage) or retinal holes/detachment (retinal pigment).
Measuring intraocular pressure: applanation tonometry
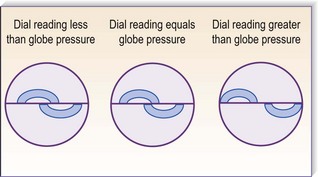
Intraocular pressures between 10 and 21 mmHg are considered normal. An increased IOP is a characteristic feature of glaucoma. A diminished IOP occurs in diabetic coma and in severe dehydration from any cause. The IOP may be assessed by palpating the eyeball, although only gross variations from normal can be appreciated. More accurate is applanation tonometry, in which the force required to flatten (applanate) an area of a sphere (the cornea) is proportional to the pressure within the sphere (Fig. 19.22). Topical anaesthestic and fluorescein are applied to the cornea and a bright cobalt blue filter is used to illuminate the sterile tonometer head. Contact between tonometer head and cornea creates a thin green circular outline of fluorescein, and a prism in the head splits this into two semicircles. The tonometer force is adjusted manually until the semicircles just overlap, and is read in millimeters of mercury (mmHg; Fig. 19.23).
Eyelid, lacrimal and orbital assessment
Eyelids
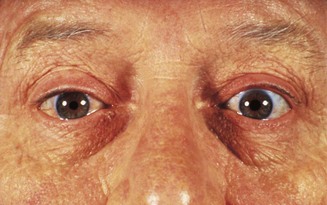
Normally no sclera is visible above the limbus (the corneoscleral junction). The commonest cause of scleral show is eyelid retraction (Fig. 19.24) due to dysthyroid eye disease, accompanied by other signs such as lid lag, in which movement of the upper lid seems to lag behind that of the eyeball when the patient looks downwards. In parkinsonism, there may be reduced blink frequency. Look for reduced eyelid closure (lagophthalmos) and levator muscle function. Ptosis (drooping of the upper lid) may be congenital or acquired (check old photographs). In age-related ptosis, owing to levator disinsertion, levator function is retained and there is a high upper eyelid skin crease. In ptosis due to myogenic or neurogenic lesions, there is reduced levator function (see Ch. 14). In entropion, there is inversion of the lid margin with associated malpositioning of the lashes, which may rub on the cornea (Fig. 19.25); and in ectropion, eversion of the eyelid is often associated with watering. The lower lid is prone to skin tumours, particularly basal cell and squamous cell carcinomas (see Fig. 15.25). Xanthelasmas are fatty deposits that develop in the upper and lower eyelids in patients with longstanding hypercholesterolaemia.
Lacrimal
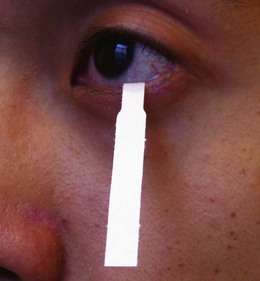
Assess the position and size of the puncta (see above). Painless watering is a feature of obstruction of the tear drainage pathway, but exclude reflex tearing and overflow from, for example, a dry eye. Schirmer’s test uses a standardized strip of filter paper to detect dry eyes by assessing the extent of wetting at 5 minutes (Fig. 19.26). Overt nasolacrimal duct blockage can be excluded if the patient reports fluid at the back of the throat on probing and syringing with normal saline (Fig. 19.27).
Orbit
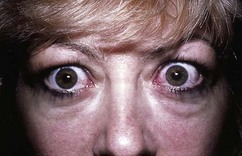
The most common cause of forward displacement of the eyeball – proptosis when unilateral, or exophthalmos when bilateral – is thyroid eye disease (TED) (see Fig. 16.13). This can cause corneal exposure and ulceration. Optic nerve damage may occur despite minimal proptosis. Axial proptosis, in the primary direction of the eye in forward gaze, is typical of TED and of tumours in the extraocular muscle cone behind the eye (intraconal mass lesions) (Fig. 19.28). Non-axial proptosis occurs in association with space-occupying orbital lesions outside the muscle cone, for example lacrimal gland tumours; these displace the globe forward and inferomedially. Apparent (‘pseudo’) proptosis causes diagnostic confusion: for example in ipsilateral eyelid retraction or myopia (where the eye is longer than normal) or when there is contralateral ptosis or enophthalmos.
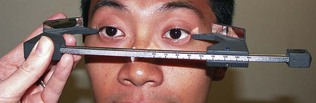
Proptosis and enophthalmos can be measured with the Hertel exophthalmometer (Fig. 19.29). A difference of >2 mm between sides is abnormal. A proptosis that increases while the patient performs a Valsalva manoeuvre is suggestive of a venous abnormality. Pulsatile proptosis with an orbital bruit is a feature of carotid cavernous fistula.
Examination of the eye in children
The advice given in Chapter 5 on the examination of children in general is also important when examining children’s eyes. Children may object strongly to lights and instruments, particularly when they are wielded by white-coated strangers. Allow the child to get used to the surroundings while taking a history from the parent, but do not ignore the child. Constantly observe the child, noting visual behaviour, the position and movements of the eyes and the general appearance of each eye.
Visual maturation continues until the age of 8. Without a focused retinal image, the visual pathways fail to develop properly, a condition known as amblyopia. After this age, sight loss from amblyopia becomes irreversible. It is therefore important to assess visual acuity in preverbal and young children. Babies should rapidly fix a large object – for example the examiner’s face – and follow it. After 6 months, ‘continuous’ and ‘steady’ fixation that is ‘maintained’ (‘CSM’) during a blink should be demonstrable. If an infant strongly objects to your covering an eye for even a short time, consider whether the non-covered eye may not be seeing well. A more sophisticated assessment can be made using ‘preferential looking’. Cards are presented to the child with a grating drawn at one end and none at the other. The child will prefer to look at the image rather than nothing. Successively smaller spatial gratings are shown until the child does not see them. The grating seen can be converted to an approximate Snellen acuity (the vision of a 1-year-old equates to approximately 6/12), although testing each eye independently in this age group is difficult. From the age of 2 years, a more accurate estimate of acuity can be made using the Kay picture-matching test (Fig. 19.30), and from 3 years the Sheridan-Gardiner letter-matching test. In these tests, the child, or the child’s parent, holds a card with a number of pictures or numbers on it. The examiner holds up an image and asks the child to match this target to one on the card – the targets vary in size.
Imaging
Plain X-rays
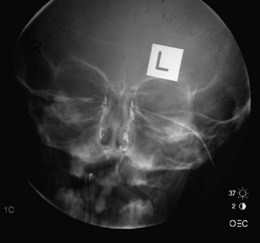
Plain X-rays have a limited role in the detection of foreign bodies, but have been largely superseded by computed tomography (CT) or magnetic resonance imaging (MRI). Ultrasound is used to assess the globe. A dacryocystogram uses a radiopaque dye introduced into the lacrimal drainage system to identify sites of lacrimal duct obstruction (Fig. 19.31). It is particularly useful in the watering eye, when carcinoma is suspected, when repeat surgery is planned or when trauma has occurred.
Computed tomography and magnetic resonance imaging
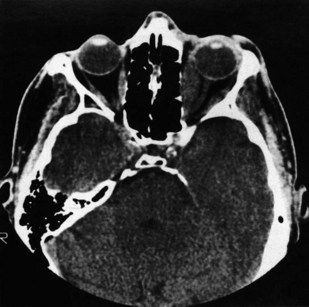
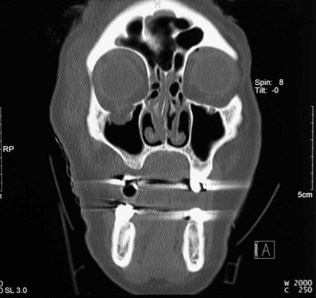
These are used extensively in the diagnosis of orbital disease. CT is often considered superior because it defines the bony orbit, but the X-ray dose to the eye and lens is not inconsiderable. CT is the investigation of choice in blunt orbital trauma and blowout fractures (see above), where fine-cut coronal spiral images are desirable (Fig. 19.32).
A- and B-mode ultrasound
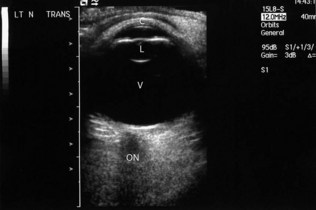
The A-mode scan is a one-dimensional time-amplitude study commonly used to assess axial length, which is an essential measurement for lens implant calculation prior to cataract surgery. The B-mode scan gives a two-dimensional cross-sectional view of the eye for the diagnosis of both intra-ocular and orbital tumours, retinal detachments and intraocular disorders when the fundal view is impaired (e.g. with vitreous haemorrhage; Fig. 19.33).
Retinal photography and fundus fluorescein angiography
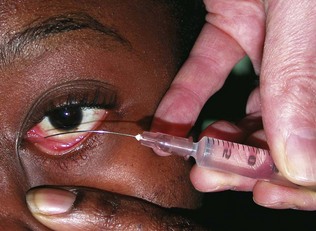
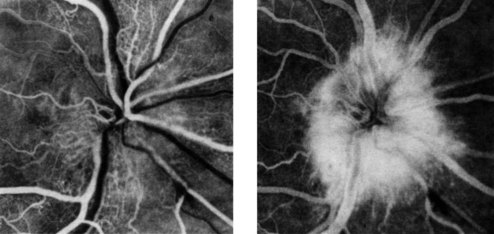
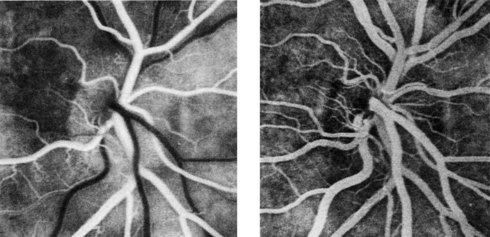
Retinal photography in conjunction with the intravenous injection of sodium fluorescein gives a detailed assessment of the retinal and choroidal vasculature (Figs 19.34 and 19.35). A blue filtered light excites fluorescence (530 nm) as the dye circulates. Fundus fluorescein angiography is useful in investigating diabetic retinopathy, age-related macular degeneration and retinal ischaemia. Minor side-effects, including transient nausea and yellow discoloration of the skin and urine, are common. Severe anaphylaxis is, fortunately, very rare.
Special examination techniques
Amsler grid
The Amsler grid is a sensitive test of macular function. It comprises a series of vertical and horizontal lines with a central spot for fixation (Fig. 19.36). The patient is asked to look at this spot and describe any distortions or missing areas in the grid.
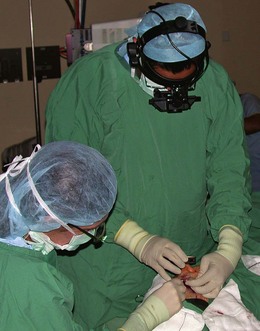
Indirect ophthalmoscopy
Binocular indirect ophthalmoscopy, using a light source supported on the examiner’s head and a hand-held lens in front of the patient’s eye, allows a much greater area of the fundus to be visualized (Fig. 19.37). The retinal periphery is more readily seen.