26 Eye Emergencies
• Eye emergencies can be classified into three major types: the red eye, the painful eye, and visual loss.
• Nausea and vomiting may be the only symptoms of acute angle-closure glaucoma, especially in elderly patients.
• Topical anesthetics should not be prescribed for a painful eye disorder because their use may lead to corneal ulcers.
• Close follow-up with an ophthalmologist should be recommended for most eye emergencies.
Epidemiology
Approximately 2% of emergency department (ED) visits involve complaints associated with the eye or vision.1 Eye injuries account for 3.5% of all occupational injuries in the United States, and about 2000 U.S. workers injure their eyes each day.2 Eye emergencies can be categorized as the red eye, the painful eye, and visual loss. This chapter discusses the various disorders that fall into each category. Table 26.1 summarizes the differential diagnosis and priority actions to be taken for any patient arriving at the ED with an eye complaint.
Table 26.1 Differential Diagnosis and Priority Actions for Eye Complaints in the Emergency Department
| Eye Pain? | |
Physiology
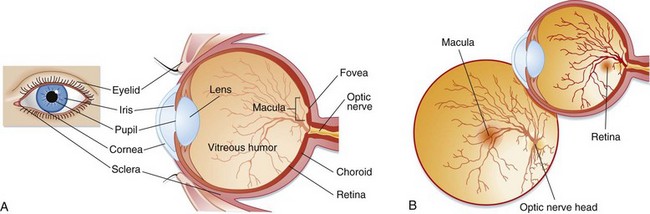
Light passes through the cornea and then through an opening in the iris, the pupil. The iris is responsible for controlling the amount of light that enters the eye by dilating and constricting the pupil. This light then reaches the lens, which refracts the light rays onto the retina. The anterior chamber is located between the lens and the cornea and contains aqueous humor, which is produced by the ciliary body. This fluid maintains pressure and provides nutrients to the lens and cornea. It is reabsorbed from the anterior chamber into the venous system through the canal of Schlemm. The vitreous chamber, located between the retina and the lens, contains a gelatinous fluid called vitreous humor. Light rays pass through the vitreous humor before reaching the retina. The retina lines the back of the eye and contains photoreceptor cells called rods and cones. Rods help vision in dim light, whereas cones aid light and color vision. The cones are located in the center of the retina in an area called the macula. The fovea is a small depression in the center of the macula that contains the highest concentration of cones. The optic nerve is located behind the retina and is responsible for transmitting signals from the photoreceptor cells to the brain (Fig. 26.1).
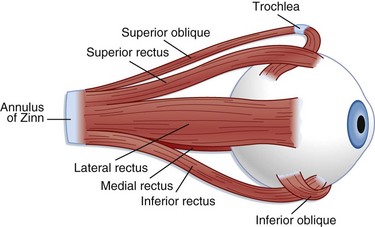
The extraocular muscles (Fig. 26.2) help in stabilization of the eye. Six extraocular muscles assist in horizontal, vertical, and rotational movement. These muscles are controlled by impulses from cranial nerves III, IV, and VI, which tell the muscles to relax or contract.
Glaucoma
Epidemiology
More than 3 million Americans suffer from glaucoma, the leading cause of preventable blindness in the United States.3 The term glaucoma refers to a group of disorders that damage the optic nerve and thereby lead to loss of vision. The two main classifications of glaucoma are open angle and angle closure. Acute angle-closure glaucoma is more common in white persons and women. Its peak incidence occurs between the ages of 55 and 70.4 African Americans, patients older than 65 years, and people with diabetes and ocular trauma are at increased risk for open-angle glaucoma. Differentiation between the two types of glaucoma lies in the mechanism of obstruction of outflow, as described later. Intraocular pressure (IOP) is determined by the rate of aqueous humor production relative to its outflow and removal. Normal IOP is between 10 and 20 mm Hg. This discussion focuses mainly on acute angle-closure glaucoma.
Central Retinal Artery Occlusion
Epidemiology
Retinal artery occlusion affects less than 1 per 100,000 persons annually.5,6 It is most commonly caused by an embolus from the carotid artery that lodges in a distal branch of the ophthalmic artery. Central retinal artery occlusion most commonly affects elderly patients and men. Although most emboli are formed from cholesterol, they may also be calcific, fat, or bacterial from cardiac valve vegetations.
Presenting Signs and Symptoms
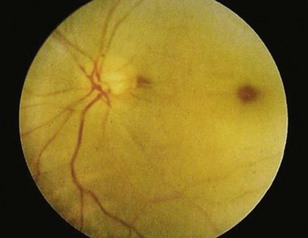
Sudden, painless visual loss is the classic manifestation of central retinal artery occlusion. Sometimes patients report transient visual loss before complete compromise. The visual loss is usually profound. Examination can often elicit an afferent pupillary defect (when light is shined into the abnormal eye, the pupil of the affected eye paradoxically dilates instead of constricting). Funduscopic examination typically demonstrates a pale retina with a cherry-red spot at the fovea (Fig. 26.3). Complete evaluation involves auscultation of the carotid arteries for bruits, palpation of the temporal artery for tenderness, and cardiac auscultation and palpation of the pulse to detect atrial fibrillation.
Treatment
Other treatment options are intraarterial thrombolysis and hyperbaric oxygen; however, studies have shown limited improvement in visual outcome with early administration of both these treatment modalities.7–10 One retrospective study found that even with thrombolysis, vision did not improve to better than 20/300 in the affected eye.7 Another study investigated the outcomes of 32 patients with central retinal artery occlusion, 17 of whom underwent fibrinolysis.6 This study found that all but six of the treated patients reported improvement in their visual compromise but that only five of the untreated patients had any improvement. In this study, patients with a duration of symptoms of up to 24 hours were treated.
Central Retinal Vein Occlusion
Epidemiology
Patients older than 50 years who have cardiovascular disease, hypertension, glaucoma, venous stasis, hypercoagulable conditions, collagen vascular diseases, or diabetes are at risk for central retinal vein occlusion.1
Presenting Signs and Symptoms
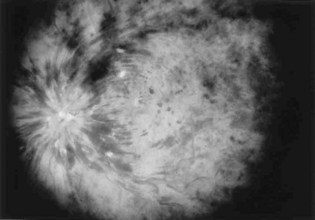
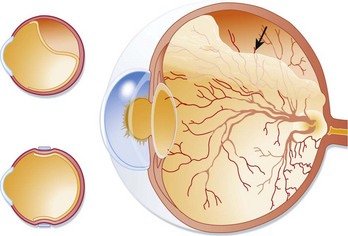
Typically, patients with ischemic retinal vein occlusion report an acute and relatively profound decrease in visual acuity. Those with the nonischemic type have progressively blurry vision that is worse in the morning. An afferent pupillary defect is found in the ischemic type. Funduscopic examination shows an edematous optic disk and macular, dilated retinal veins, retinal hemorrhage, and cotton-wool spots. Sometimes these findings are called the “blood and thunder” appearance of the fundus (Fig. 26.4).
Treatment
Although no specific treatment is available, a number of interventions have been proposed and practiced.11,12 However, these interventions have not been based on evidence of efficacy. Laser photocoagulation, for example, cauterizes leaking vessels with the aim of halting further visual loss. This procedure can be especially helpful for branch retinal vein occlusion. With nonischemic vein occlusion, attempts to reduce macular edema can be helpful. The reduction is accomplished with the administration of topical corticosteroids. Studies have been conducted to determine the benefit of steroids in treating both forms of retinal vein occlusion. Jonas et al.11 conducted a prospective, comparative, nonrandomized clinical interventional study to evaluate the visual outcomes in 32 patients with central retinal vein occlusion after intravitreal administration of triamcinolone acetate. The study included patients with both the ischemic and nonischemic forms of retinal vein occlusion. These researchers found that the medication resulted in temporary (up to 3 months) improvement in visual outcome but also raised IOP. Anticoagulants are not recommended because they may propagate hemorrhage.
Optic Neuritis
Treatment and Disposition
Ophthalmologic and neurologic consultation should be obtained if optic neuritis is suspected. Approximately 31% of patients with optic neuritis have a recurrence within 10 years of the initial episode.13 The goals of treatment are to restore visual acuity and prevent propagation of the underlying disease process. The Optic Neuritis Treatment Trial was a randomized, 15-center clinical trial involving 457 patients that was performed to evaluate both the benefit of corticosteroid treatment of optic neuritis and the relationship of this entity to multiple sclerosis. Use of intravenous steroids in conjunction with oral steroids reduced the short-term risk for the development of multiple sclerosis as determined by MRI evaluation. No long-term immunity from or benefit for multiple sclerosis was reported, however. The study concluded that although intravenous steroids have only minimal, if any effect on the patient’s ultimate visual acuity, they do expedite recovery from optic neuritis. Use of oral steroids alone is associated with a higher recurrence rate of optic neuritis. The dosage regimen recommended on the basis of the study results was methylprednisolone, 250 mg intravenously every 6 hours for 3 days, followed by prednisone, 1 mg/kg/day orally for 11 days.14
Retinal Detachment
Presenting Signs and Symptoms
Retinal detachment can occasionally be asymptomatic. More commonly, patients complain of flashes of light, floaters, or fine dots or cobwebs in their visual fields. Generally, a new onset of floaters associated with flashing lights is indicative of retinal detachment unless proved otherwise. Visual acuity correlates with the extent of macular involvement and can be minimally changed to severely decreased. The loss of vision is usually sudden in onset and starts peripherally, with propagation to the central visual field. The visual loss is commonly described as a filmy, cloudy, or curtainlike appearance. Visual field cuts relate to the location of the retinal detachment, and an afferent pupillary defect occurs if the detachment is large enough. Retinal detachment is painless. On examination, the detached retina may appear gray or translucent or may seem out of focus (Fig. 26.5). Retinal folds may be seen. The visual field defects are variable, depending on the involvement of the retina and macula. Bedside ED ultrasonography has been shown to be helpful in confirming the presence of retinal detachment.15 Left untreated, all cases of retinal detachment progress to involve the macula and result in complete loss of vision in the affected eye.
Treatment
The sooner that treatment of retinal detachment is initiated, the greater the chance of visual preservation and recovery. After detachment, retinal ischemia ensues because of loss of the choroidal blood supply. Patients with suspected or confirmed retinal breaks or detachments require emergency ophthalmologic consultation. Contrary to traditional belief, not all detachments are managed surgically. Laser photocoagulation or cryotherapy is frequently used to create small burns around the area of detachment to prevent further leakage of fluid between the retinal layers. Intraocular gas is sometimes used to tamponade the tear. These procedures are approximately 95% effective in halting the disease process.16 Surgical intervention may be needed to repair the tear and simultaneously remove the traction forces on the retina. The modality of treatment is at the discretion of the consulting ophthalmologist.
Temporal Arteritis
Presenting Signs and Symptoms
Unilateral headache, jaw or tongue claudication, constitutional symptoms, including anorexia and malaise, and visual impairment are common initial symptoms of temporal arteritis. Occasionally, the visual loss is preceded by amaurosis fugax. In one series of patients, visual loss was unilateral in 46%, sequential in 37%, and simultaneously bilateral in 17%.17 Patients with polymyalgia rheumatica may also complain of pain and stiffness in the shoulders or hips.
Treatment
Treatment of temporal arteritis should never be delayed to await the results of biopsy because outcome is contingent on early medical treatment. Initial treatment should consist of prednisone, 60 to 80 mg, or high-dose intravenous methylprednisolone. The exact duration and regimen of steroid therapy are determined on a case-by-case basis. Generally, treatment is continued until the symptoms improve and the ESR begins to normalize. It has also been suggested that methotrexate, infliximab, and aspirin may also halt progression of the visual symptoms, but further studies are necessary to establish practice patterns.18
Orbital Cellulitis and Periorbital Cellulitis
Presenting Signs and Symptoms
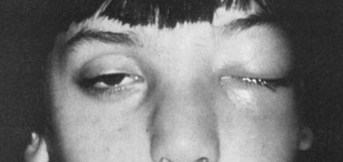
Patients with infection limited to the periorbital tissues typically have erythema, edema, and warmth of the external eye tissues. Though generally unilateral, the infection can be bilateral. Constitutional symptoms, including fever and malaise, may be present. The presence of ocular pain, ophthalmoplegia, and pain with extraocular movement suggests orbital involvement. Patients with orbital infection may also have decreased visual acuity and pupillary paralysis. Elevated IOP, preseptal (periorbital) cellulitis, conjunctival injection, and proptosis may additionally be present (Fig. 26.6).
The Red Eye
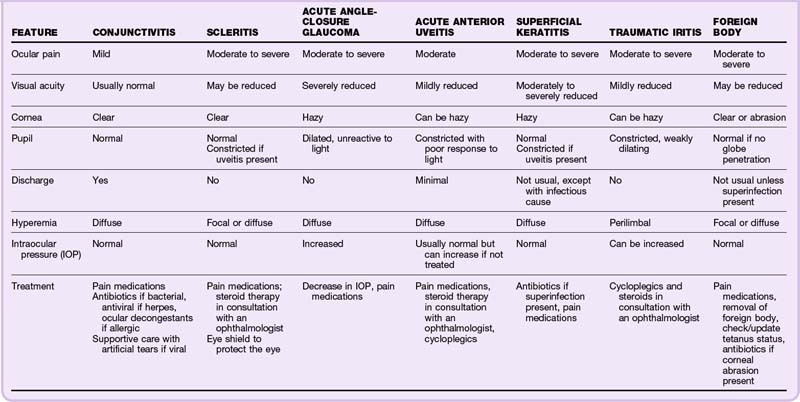
An injected, red eye signals an inflammatory reaction. Fortunately, most inflammation is self-limited and can be treated on an outpatient basis. The specific cause, treatment, course, and prognosis, as well as the impact on vision, depend on the underlying cause (Table 26.2).
This discussion focuses on conjunctivitis, the diagnosis in 30% of patients seen in the ED with ocular complaints and the most common cause of red eye.19 When the cornea is involved as well, the process is called keratoconjunctivitis.
![]() Patient Teaching Tips
Patient Teaching Tips
A patient with a red eye should seek evaluation by a primary care physician because this disorder has several benign and serious causes.
If the pain worsens, visual acuity decreases, a discharge appears, or a fever develops, the patient should immediately return to the emergency department for reevaluation.
Patients at high risk for eye problems (e.g., those with diabetes) should be encouraged to obtain yearly eye examinations. More information for patients with diabetes is available from the American Diabetes Association (www.diabetes.org).
Presenting Signs and Symptoms
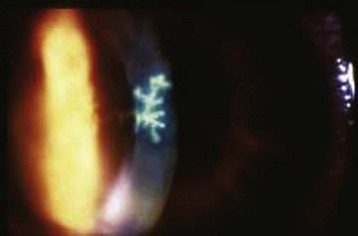
Herpes zoster ophthalmicus is caused by activation of the virus along the ophthalmic branch of the trigeminal nerve. A vesicular rash is present along the involved dermatome and results in forehead and upper eyelid lesions. Lesions on the tip of the nose, called the Hutchinson sign, signify involvement of the nasociliary branch of the fifth cranial nerve. The presence of the Hutchinson sign indicates a much higher likelihood of ocular involvement (76% risk versus a 34% risk in the absence of such lesions). Fluorescein examination may show punctate, ulcerated, or dendritic corneal lesions (Fig. 26.7).
Gonococcal infection generally results in unilateral conjunctival injection, copious purulent discharge, and edema and erythema of the lids. The infection is sudden in onset and progresses rapidly.19 Patient populations usually affected are infants, health care workers, and sexually active young adults. The amount of discharge helps distinguish gonococcal infection from other bacterial pathogens. Patients may have associated urethral discharge or arthritis.
Diagnosis
The diagnosis of conjunctivitis is based on the history, physical examination, and appropriate exclusion of other causes of red eye. It is important, in the correct setting, to perform a thorough examination to rule out both other causes of red eye and potential complications of conjunctivitis such as corneal ulceration. When particularly virulent pathogens are a consideration, Gram stain and cultures may be necessary. Because the diagnosis of viral conjunctivitis can be made clinically, viral culture and laboratory investigation are not necessary.20
Treatment and Disposition
Herpes Simplex Conjunctivitis
Ophthalmologic consultation is indicated immediately for patients with herpes simplex conjunctivitis. The ophthalmologist may consider mechanical débridement. Topical antivirals such as 3% vidarabine and 1% trifluridine are prescribed in consultation with an ophthalmologist. Up to 97% of patients have been shown to heal within 2 weeks following trifluridine therapy.4 Topical prophylactic antibiotics and cycloplegia are also used.
Herpes Zoster Ophthalmicus
The EP should consult an ophthalmologist about a patient with herpes zoster ophthalmicus. Systemic antiviral agents are indicated early in the disorder. Early treatment with antiviral therapy within 72 hours of onset of the rash has been shown to reduce acute pain and ocular complications.21 Corticosteroid therapy may be considered in consultation with the ophthalmologist.
Corneal Abrasions
Presenting Signs and Symptoms
Common complaints of patients with corneal abrasions include photophobia, foreign body sensation, pain, and tearing. Conjunctival injection and blepharospasm may or may not be seen. Depending on the location and size of the abrasion, patients may also complain of decreased visual acuity. Fluorescein examination demonstrates the abrasion as a staining defect (Fig. 26.8). If a linear abrasion is noted, the EP should search carefully for a retained foreign body on the inner side of the upper eyelid. Corneal ulceration should be excluded with a slit-lamp examination, especially in contact lens wearers. A topical anesthetic should be administered to facilitate the examination.
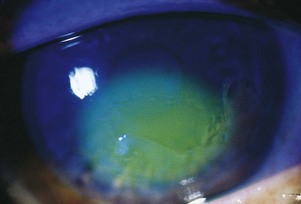
Fig. 26.8 Corneal abrasions.
(From Goldman L, Ausiello D, editors. Cecil medicine. 23rd ed. Philadelphia: Saunders; 2007.)
Tips and Tricks
Perform a slit-lamp examination before fluorescein examination to prevent false-positive results of assessment for corneal abrasions.
Apply local anesthetic (e.g., tetracaine) to facilitate a slit-lamp examination.
A cotton-tipped applicator can be used to evert the upper eyelid.
Never send a patient home with topical anesthetic eyedrops, which can raise the risk for further injury.
Treatment
Tetanus prophylaxis has been a long-standing component of the treatment of corneal abrasions, but evidence suggests that this practice is not routinely indicated. In the absence of infection, corneal perforation, or devitalized tissue, no benefit is seen with the routine administration of a tetanus booster. However, current Centers for Disease Control and Prevention guidelines recommend a tetanus booster within 5 years if the event causing the corneal abrasion involved a dirty vector such as vegetable matter and within 10 years if the corneal injury was caused by a clean, uncontaminated vector.22,23 Corneal abrasions heal within 3 to 5 days, and patients can be discharged with arrangements for close outpatient follow-up.
Corneal Ulcers
Presenting Signs and Symptoms
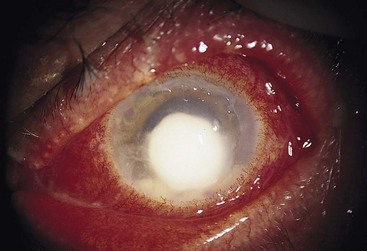
Corneal ulcers cause significant eye pain, ciliary injection, tearing, foreign body sensation, blurry vision, and photophobia. Eyelid swelling and purulent drainage may be present. Depending on the location and extent of the lesion, visual acuity may be decreased. Inspection may show eyelid swelling and erythema. Large ulcers can be seen as round or oval white spots on the cornea with the naked eye. Fluorescein and slit-lamp examinations demonstrate a corneal defect, usually with sharply demarcated borders and a gray appearance of the infiltrated ulcer base. On examination of the anterior chamber, hypopyon or a flare consistent with iritis is often seen. With the exception of the classic dendritic lesions that occur with herpes simplex virus infection, no pathognomonic signs or symptoms can be used to diagnose the cause of the ulcer seen on examination (Fig. 26.9).
Ocular Foreign Bodies
Presenting Signs and Symptoms
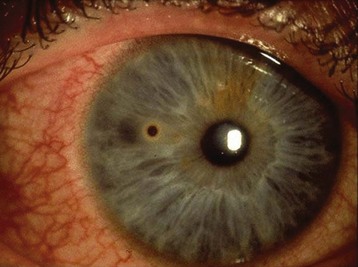
Typical symptoms of an ocular foreign body include pain, photophobia, tearing, conjunctival injection, and a foreign body sensation. Examination may show conjunctival injection, a visible foreign body, a corneal epithelial defect, and corneal edema. White blood cell mobilization may occur and can be detected as anterior chamber flare and the presence of cells. Visual acuity can be decreased. Metallic foreign bodies can cause visible rust rings (Fig. 26.10).
Ocular Burns
Retrobulbar Hematoma
Presenting Signs and Symptoms
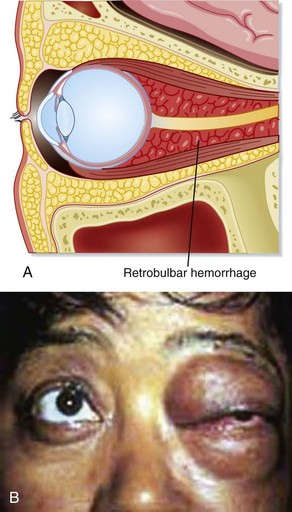
Severe eye pain, nausea, vomiting, diplopia, and decreases in both visual acuity and eye movement are common complaints at initial evaluation of a patient with retrobulbar hematoma. Physical findings include proptosis, decreased ocular motility, visual loss, elevated IOP, and hemorrhagic chemosis. An afferent pupillary defect is common (Fig. 26.11).
Treatment
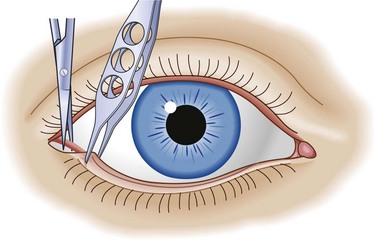
The rate of development of retrobulbar hematoma dictates the treatment. If the condition develops over minutes, the eye must be decompressed immediately via lateral canthotomy (Fig. 26.12). Orbital CT demonstrates the hematoma; however, treatment should not be delayed while waiting for imaging to be performed. If the process is slower and develops over a period of hours, conservative management can be effective and consists of head elevation, ice packs to reduce swelling, intravenous acetazolamide and mannitol, and topical beta-blockers. Progress is monitored through serial measurements of IOP and pupillary reactivity. An ophthalmologist should be notified for consultation as soon as the diagnosis is suspected. Patients with retrobulbar hematoma are admitted to the hospital to monitor progress.
Hyphema
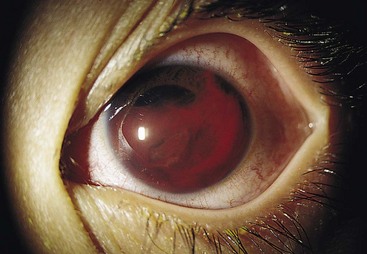
Presenting Signs and Symptoms
Patient symptoms and findings on examination correlate with the size of the hyphema. Typically, patients complain of eye pain, decreased visual acuity, and photophobia. If the patient is upright, the hyphema usually layers out in the inferior portion of the anterior chamber. Depending on the size of the hyphema, it can be seen with either the naked eye or a slit-lamp examination. If the hyphema is large, IOP can be elevated. IOP is elevated in approximately 27% of patients acutely but is usually mild and self-limited. Pressures greater than 35 mm Hg with durations greater than 5 to 14 days are more likely to be associated with optic atrophy.20 Generally, no afferent pupillary defect is present (Fig. 26.13).
Treatment and Disposition
Surgery may be necessary if the elevated IOP is refractory to medical therapy or to remove a large clot. Patients with hyphemas larger than 50%, decreased vision, increased IOP, and sickle cell disease should all be considered for admission.24 In consultation with an ophthalmologist it may be determined that a patient with an extremely small hyphema can receive outpatient management. The major complication of hyphema is rebleeding after 2 to 5 days when the initial clot loosens, which results in potentially severe elevations in IOP. The reported incidence of rebleeding is between 6% and 33%.20
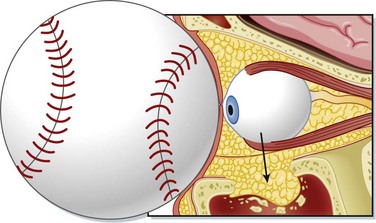
Orbital Wall or Blow-Out fractures
Blunt force to the orbital region can raise intraorbital pressure, relief of which is accomplished by fracture of the orbital walls. A fracture of the orbital wall should always be suspected when a patient has soft tissue swelling following trauma to the globe The inferior and medial walls are most frequently involved. The orbital contents slip into the corresponding sinus: the maxillary sinus for inferior wall fractures and the ethmoid sinus for medial wall fractures. Concomitant facial injuries should be sought in a patient with an orbital wall or blow-out fracture (Fig. 26.14).
Diagnosis
A Waters view radiograph can show indirect signs of fracture: a cloudy sinus, a bulge extending from the orbit into the maxillary sinus (the teardrop sign), or an air-fluid level in the maxillary sinus. CT is the diagnostic study of choice because it demonstrates the fracture, as well as other injuries. The use of bedside ultrasound has also shown to be helpful in the identification of orbital wall fractures.25
Treatment and Disposition
Indications for surgical repair include muscle entrapment and cosmetic deformity with significant enophthalmos. Immediate surgery is not necessary. Surgery is delayed to allow abatement of the swelling and a better examination. The preferred time frame for operative repair is 10 to 14 days, which optimizes the balance between reduced swelling and absence of scar tissue formation. Administration of prophylactic antibiotics for an orbital wall or blow-out fracture without evidence of sinus infection is controversial. Data are inadequate for a definitive recommendation, as review of 214 studies to examine this very question found.26,27
Ruptured Globe
Globe rupture involves a full-thickness defect in the cornea, sclera, or both. Penetrating mechanisms are almost always involved. Rarely, enough force is generated by a blunt injury that transmission of the force results in eventual rupture. Ruptures are most common at the insertions of the intraocular muscles or at the limbus, where the sclera is thinnest.28 This entity is a true ophthalmologic emergency and always requires surgical intervention.
Traumatic Iritis
Treatment and Disposition
![]() Priority Actions
Priority Actions
Determine whether the patient’s complaint is monocular or binocular.
Determine whether the complaint is related to trauma.
Ask whether the patient wears contact lenses or glasses.
Check the patient’s visual acuity.
Determine whether vision is monocular or binocular.
Involve an ophthalmologist in the treatment of a patient with an eye complaint as soon as possible if there is reasonable suspicion of a vision-compromising process, such as acute angle-closure glaucoma or central retinal artery occlusion.
1 Sharma R, Bruncette DD. Ophthalmology. Marx JA, Hockberger RS, Walls RM. Rosen’s emergency medicine, 7th ed, Philadelphia: Mosby, 2009.
2 Xiang H, Stallones L, Chen G, et al. Work-related eye injuries treated in hospital emergency departments in the US. Am J Ind Med. 2005;48:57–62.
3 Tham CC, Lai JS, Leung DY, et al. Acute angle-closure glaucoma. Ophthalmology. 2005;112:1479–1480.
4 Dargin JM, Lowenstein RA. The painful eye. Emerg Med Clin North Am. 2008;26:199–216.
5 Rumelt S, Brown GC. Update on treatment of retinal arterial occlusions. Curr Opin Ophthalmol. 2003;14:139–141.
6 Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995;102:2029–2035.
7 Pettersen JA, Hill MD, Demchuk AM, et al. Intra-arterial thrombolysis for retinal artery occlusion: the Calgary experience. Can J Neurol Sci. 2005;32:507–511.
8 Weber J, Remonda L, Mattle HP, et al. Selective intra-arterial fibrinolysis of acute central retinal artery occlusion. Stroke. 1998;29:2076–2079.
9 Beiran I, Goldenberg I, Adir Y, et al. Early hyperbaric oxygen therapy for retinal artery occlusion. Eur J Ophthalmol. 2002;11:345–350.
10 Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128:733–738.
11 Jonas JB, Akkoyun I, Kamppeter B, et al. Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide. Eye. 2005;19:65–71.
12 Horio N, Horiguchi M. Retinal blood flow and macular edema after radial optic neurotomy for central retinal vein occlusion. Am J Ophthalmol. 2006;141:145–146.
13 Vortmann M, Schneider JI. Acute monocular vision loss. Emerg Med Clin North Am. 2008;26:73–96.
14 Chan CK, Lam DS. Optic neuritis treatment trial: 10 year follow-up results. Am J Ophthalmol. 2004;138:695.
15 Lewin MR, Williams SR, Ahuja Y. Ultrasonographic diagnosis of retinal detachment in the emergency department. Ann Emerg Med. 2005;45:97–98.
16 Quintyn JC, Benouaich X, Pagot-Mathis V, et al. Retinal detachment, a condition little known to patients. Retina. 2006;26:1077–1078.
17 Liu GT, Glaser JS, Schatz NJ, et al. Visual morbidity in giant cell arteritis: clinical characteristics and prognosis for vision. Ophthalmology. 1994;101:1779–1785.
18 Brown SM, Raflo GT, Harper DK, et al. Temporal arteritis management. Am J Ophthalmol. 2006;113:1059–1060.
19 Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician. 2010;81:137–144.
20 Yanoff M, Duker JS. Ophthalmology, 3rd ed, Philadelphia: Mosby, 2008.
21 Liesegang TJ. Herpes zoster virus infection. Curr Opin Ophthalmol. 2004;15:531.
22 Mukherjee P, Sivakumar A. Tetanus prophylaxis in superficial corneal abrasions. Emerg Med J. 2003;20:62–64.
23 Benson WH, Snyder IS, Granus V, et al. Tetanus prophylaxis following ocular injuries. J Emerg Med. 1993;11:677–683.
24 Bord SP, Linden J. Trauma to the globe and orbit. Emerg Med Clin North Am. 2008;26:97–123.
25 Mcllrath ST, Blaivas M, Lyon M. Diagnosis of periorbital gas on ocular ultrasound after facial trauma. Am J Emerg Med. 2005;23:517–520.
26 Martin B, Ghosh A, Mackway-Jones K. Antibiotics in orbital floor fractures. Emerg Med J. 2003;20:66.
27 Westfall CT, Shore JW. Isolated fractures of the orbital floor: risk of infection and the role of antibiotic prophylaxis. Ophthalmic Surg. 1991;22:409–411.
28 Lubeck D. Penetrating ocular injuries. Emerg Med Clin North Am. 1988;6:127.