Chapter 52 Extratemporal Procedures and Hemispherectomy for Epilepsy
• Intractable disabling epilepsy, a localizable epileptogenic zone, and a low risk of postoperative deficits are considered the three basic tenets that must be met before a patient can be considered a candidate for epilepsy surgery.
• One should apply surgical technique for extratemporal resections to match the underlying cause.
• Patients with hemimegalencephaly and intractable seizure disorder benefit from more tissue resection, and anatomical hemispherectomy is an excellent choice.
• Patients with encephalomalacia and large ventricles benefit from less tissue removal, and hemispherotomy is an excellent choice.
• Frontal lobe resections convey the lowest chance for successful postoperative results. Patients with a circumscribed lesion on preoperative magnetic resonance imaging (MRI) fared significantly better. Posterior cortex epilepsy resections convey a slightly higher chance of favorable outcome.
Wilder Penfield performed the first temporal lobe resection for epilepsy in 1936. Mesial temporal sclerosis has since become the most common indication for epilepsy surgery in adult patients. As a result, Penfield’s temporal lobectomy, or a variation thereof, has become the flagship procedure of epilepsy surgery in adults and is the only procedure for which class I medical evidence demonstrates a favorable outcome after surgery compared with medical management.1 However, a significant number of epilepsy patients do not have underlying temporal disease, but rather an epileptogenic zone extending into the regions outside the temporal lobe. These so-called extratemporal lobe epilepsy (ETLE) patients present unique management challenges. As the characteristics of ETLE have become better understood, the distinctive management challenges have become better defined. The result has been the development of new concepts of epileptogenesis, new diagnostic technologies, and from a neurosurgical perspective, the development and refinement of surgical techniques.
Unique Challenges in Extratemporal Lobe Epilepsy
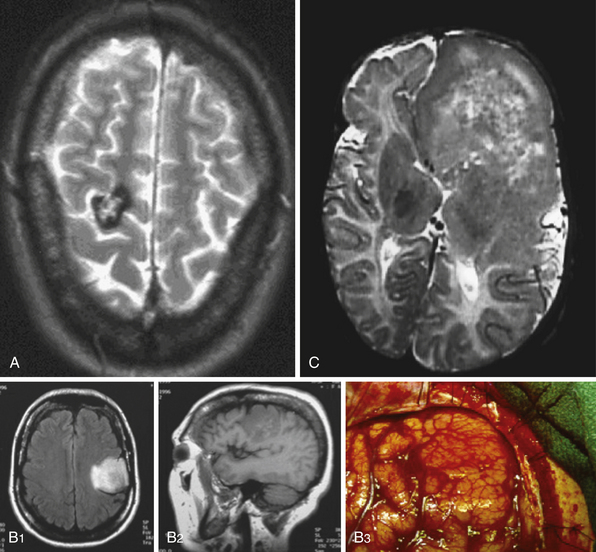
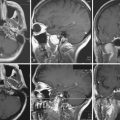
ETLE refers to epilepsy arising outside the temporal lobe and may result from a variety of causes including neoplasms, vascular malformations, malformations of cortical development, and encephalomalacias related to prior ischemic or traumatic events (Fig. 52.1). In addition to a variety of causes, the epilepsy may arise in the frontal, parietal, or occipital lobes and rapidly spread to involve large regions of cortex. These unique factors result in a broad spectrum of imaging findings, complex seizure semiology, and potentially diffuse electroencephalography (EEG) data.
Seizure semiology in ETLE may be complex depending on the site of seizure origin. The nature of ETLE lends itself to areas of ictal onset that may not necessarily result in clinical manifestations until the symptomatogenic zone becomes activated. With extratemporal areas of ictal onset, the location that is suggested by semiology has a greater propensity to be a result of secondary spread, resulting in potential diagnostic confusion. Similarly, EEG data may be particularly difficult to interpret because of rapid spread and artifact from muscle movement during the seizure. Deep cortical areas such as the interhemispheric and insular regions are particularly difficult to record epileptic activity from on-scalp EEG. Patients whose seizures start in these areas can present a particular challenge, as electrical seizure activity may be absent on standard EEG arrays, or it may falsely localize as originating in another area entirely.
Surgical Indications
Intractable disabling epilepsy, a localizable epileptogenic zone (defined as the area of tissue that must be removed to prevent further seizures), and a low risk of postoperative deficits are considered the three basic tenets that must be met before a patient can be considered a candidate for epilepsy surgery.2 It is important to recognize that medical intractability makes a patient a candidate for further investigation regarding the appropriateness to undergo surgery for epilepsy, but it does not guarantee that surgical management should ultimately be offered. Although no clear definition of medical intractability exists, recent reports in the literature suggest that successful treatment with anticonvulsant therapy is unlikely once two different medicines fail to control seizures.3 The optimal surgical patient will be highly motivated with a social support system in place, and additional coexisting psychiatric illness will be well controlled. Neurocognitive risks must be assessed and discussed with each patient, and risks should be deemed acceptable from both the perspective of the patient and the treatment team. A high probability of improving a patient’s overall quality of life must be confirmed before undertaking the procedure.4
Preoperative Evaluation
The starting point for any patient evaluation should include a detailed history and physical examination, with special focus on seizure semiology. Lateralizing and localizing signs, such as ictal eye movements, motor or sensory features, presence or absence of ictal speech, and the presence or absence of aura, should be noted. Video-electroencephalography (VEEG) data allow correlation of a patient’s behavior during a seizure with the changes recorded on scalp EEG. It allows for confirmation of the epilepsy and may provide helpful lateralization/localization to aid in the surgical hypothesis.
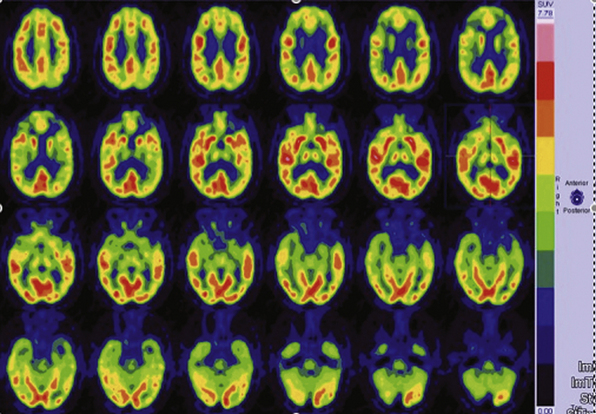
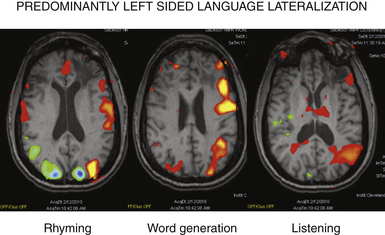
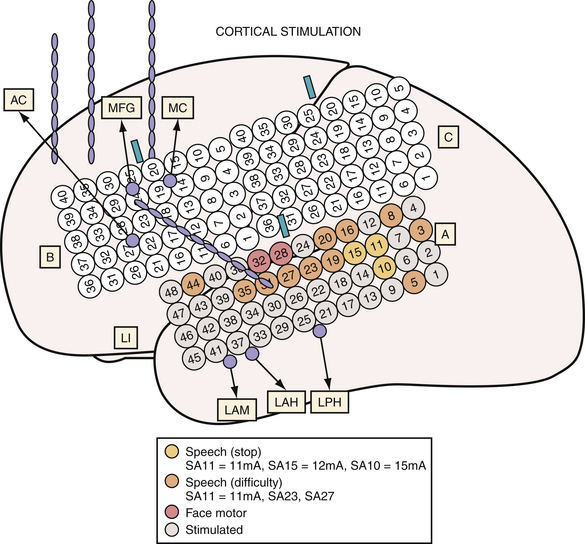
MRI studies for patients with suspected extratemporal lesions should include axial T2 sequences, axial and coronal fluid-attenuated inversion recovery (FLAIR) sequences, a sagittal T1 sequence, and a high-resolution (thin-cut) coronal magnetization prepared rapid gradient echo (MPRAGE) sequence.5 Although 1.5-tesla magnet strength is considered acceptable, 3-tesla or higher machines and the use of surface coils over the region of interest may add to the diagnostic yield. Additional studies such as subtraction single-photon emission computed tomography (SPECT), [18F]-fluorodeoxyglucose (FDG), positron emission tomography (PET), or magnetoencephalography (MEG) provide complementary information and may help to formulate the surgical hypothesis (Fig. 52.2). These studies may also be used as an adjunct in patients with multilobar lesions or discordant data.6 Functional MRI (fMRI), MEG, or intracarotid sodium amytal testing may be performed for localization of language and sensorimotor cortex (Fig. 52.3). After review of all available data, a likely cause should be determined for the patient’s seizures; an underlying cause has been correlated with the probability of postoperative seizure freedom across a number of surgical case series.7,8
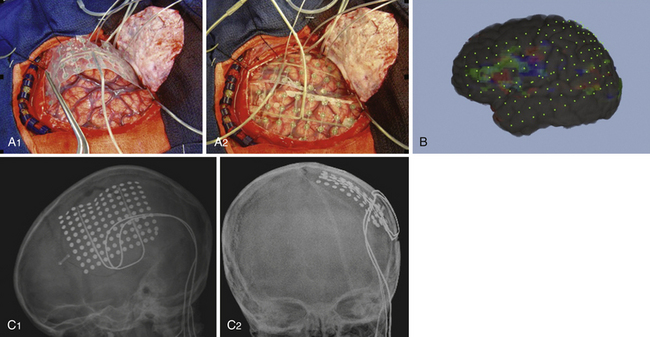
The strongest hypothesis is formed when the localization predicted by individual studies is concordant. Any discrepancies must be evaluated and resolved in relation to surgical planning. Discrepancies between semiology, EEG, or MRI are considered the most troubling. If localization differs based on these studies, or if the MRI is nonlesional, then potentially clarifying information should be sought with either FDG-PET or ictal SPECT. When discrepancies cannot be resolved, additional methods of strengthening the working surgical hypothesis should be considered. Chronic extraoperative intracranial recordings may provide critical information in such patients (Fig. 52.4). These may also be used when noninvasive evaluation leads to a hypothesis involving a large cortical area and further delineation of the epileptogenic zone is desired. A working hypothesis of the ictal onset zone based on noninvasive data helps guide the placement of the intracranial electrodes in an effort to confirm it prior to surgical resection.
Invasive intracranial electrodes also have a distinct advantage in mapping functional cortex. Consequently, their placement should be considered when epilepsy is thought to arise from within or near eloquent cortex. They are especially useful for localizing functional cortex in those patients unable to tolerate awake craniotomy (Fig. 52.5).
Surgical Procedures
There is a broad range of surgical techniques for the treatment of extratemporal epilepsy. These procedures can be loosely divided into resective techniques and palliative techniques.9 Resective techniques include lesionectomy, tailored focal resection (a “lesionectomy plus” approach), lobectomy, multilobar resections, and hemispherectomy and its variants. Palliative techniques include corpus callosotomy, multiple subpial transections, and vagal nerve stimulation. Other stimulation techniques currently under investigation include thalamic targeted deep brain stimulation, responsive cortical stimulation, and transcranial stimulation. Intraoperative techniques include stereotactic neuronavigation, ultrasound, intraoperative neuromonitoring with somatosensory evoked potentials (SSEPs) or electrocorticography (ECoG), and intraoperative MRI.
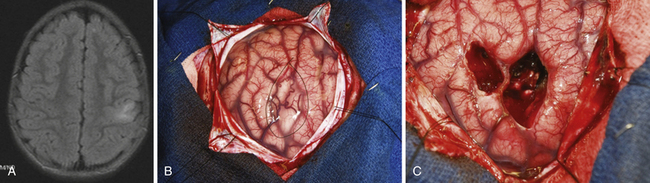
The surgical approach depends not only on surgical and functional anatomy, but also on age of the patient, medical comorbid conditions, and anticipated underlying disease. In particular, certain types of focal cortical dysplasias, neoplasms, and cavernomas can often be resected with pure lesionectomy, typically resulting in good surgical outcome (Fig. 52.6).10–12 The general tenet is focal resections for focal disease, and progressively larger areas of resection for more diffuse or for multifocal disease. The extension of a resection beyond the area of visible lesion may be performed with the assistance of intraoperative ECoG, SSEPs, or extraoperative recording with the use of implanted intracranial electrodes. The use of such techniques may be more important in cases of cortical dysplasia where the epileptogenic zone often extends beyond the margins of the radiographic lesion.
Hemispherectomy, and related procedures, tend to be limited primarily to the pediatric population. Anatomical hemispherectomy and disconnective hemispherectomy procedures are the available techniques.7,13 To be considered for hemispherectomy, the ideal patient should have epilepsy arising from a single affected hemisphere. The underlying disease should be unilateral and most patients considered for these procedures will have preexisting neurological deficits, including homonymous hemianopia and contralateral hemiparesis with absence of functional fine motor movements of the hand. If they do not, counseling regarding these anticipated deficits should be extensive, including the likelihood that the patient will not drive due to the visual field defect.
Anatomical hemispherectomy remains in use for selected pathological processes such as hemimegalencephaly and diffuse malformations of cortical development. It is also commonly utilized when patients have failed prior disconnective hemispherectomy techniques.14 Disconnective hemispherectomy techniques are usually considered in those patients with perinatal stroke, Sturge-Weber syndrome, or Rasmussen’s encephalitis.7 The goal of these less extensive procedures is isolation of the hemispheric cortex while reducing the perioperative risks that are more prevalent with the anatomical technique (blood loss and hydrocephalus). From a practical standpoint, this translates to disconnection of the horizontal frontal fibers, mesial temporal structures, corpus callosum, and the internal capsule and corona radiata (see video).15 The completeness of disconnection, in addition to seizure etiology, relates directly to surgical outcome.16
Functional hemispherectomy as described by Rasmussen implies removal of the temporal lobe (including mesial structures) followed by a limited central resection of frontoparietal tissue.17 This limited resection allows access to the lateral ventricle, facilitating the disconnection of the remaining hemisphere through the ventricular space. The majority of the disconnected hemisphere is then left in situ in an attempt to reduce perioperative morbidity (blood loss, hydrocephalus) and the long-term risk of superficial cerebral hemosiderosis.
The Rasmussen technique, initially reported in 1973, has since undergone a number of modifications. The resulting evolution has yielded both the hemispherotomy and hemispherical deafferentation techniques.18,19 The main variation among these procedures is the amount of tissue resected as the surgeon enters the ventricular space, with more recent refinements of technique focusing on reducing the amount of tissue resected. The smaller resection window of hemispherotomy and hemispherical deafferentation is ideally suited for those patients with anatomical variations that make the process of disconnection straightforward, such as large ventricles or encephalomalacia.
Surgical Technique: Anatomical Hemispherectomy
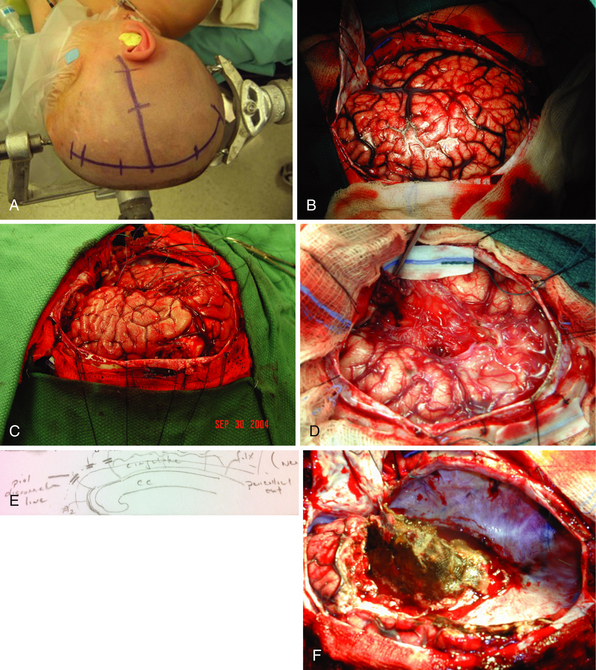
The patient is positioned either on a rigid fixation device or resting on a head support. The head is turned 90 degrees and elevated above the level of the heart to assist with venous return. An ipsilateral shoulder support is placed. A T incision is then marked such that it consists of one line at least 0.5 cm from midline and another perpendicular line extending from the zygomatic root just anterior to the tragus. The midline incision should extend from hairline to 4 to 5 cm above the inion (Fig. 52.7). The incision is infiltrated with a local anesthetic and the skin is incised, taking care not to injure the sagittal sinus in the area of an open anterior fontanelle. Careful attention is paid to hemostasis throughout the procedure, with all bleeding points controlled with bipolar coagulation and hemostatic agents. The temporalis muscle is incised and reflected inferiorly to allow adequate exposure of the temporal lobe. Bur holes are then placed just above the zygomatic arch, the keyhole, and along the parasagittal area to allow an exposure of the entire hemisphere. High-speed air craniotome is then used to remove the craniotomy flap. Dural tack-up sutures are then placed and the dura is opened in an H-fashion. The surface of the cortex is examined, the sylvian fissure identified, and venous drainage patterns noted (see Fig. 52.7). Major draining veins to the sagittal sinus are carefully protected to avoid potentially devastating hemorrhage. Initial dissection is performed to open the sylvian fissure (see Fig. 52.7). The middle cerebral artery (MCA) is exposed at a point just distal to the lenticulostriate branches. The inferior and superior circular sulci of the insula are then exposed (see Fig. 52.7) and the MCA is ligated with bipolar cautery and surgical clips, if necessary.
Mesial frontoparietal disconnection and corpus callosotomy are then performed. Identification of the corpus callosum is facilitated by careful aspiration of the roof of the lateral ventricle just above its intersection with the septum pellucidum. The gray matter of the cingulate is easily identified. Aspiration of a portion of the cingulate allows clear visualization of the pericallosal arteries and corpus callosum. During this portion of the procedure the surgeon must be careful to avoid injuring either pericallosal artery, as it is difficult to ascertain which branch supplies the contralateral hemisphere. The cingulate and corpus callosum are then aspirated to achieve complete disconnection, being careful to avoid injury to the contralateral frontal lobe. The ipsilateral fornix is then interrupted by aspiration just anterior to the splenium in the posterior aspect of the lateral ventricle.
The anterior callosal dissection is continued, dividing the pia of the mesial frontal lobe and any branches from the anterior circulation, until the olfactory nerve is reached. The falx and pericallosal arteries provide an excellent anatomical reference to ensure complete frontal disconnection (see Fig. 52.7). The mesial frontoparietal pia and anterior circulation branches should be coagulated and divided above the inferior aspect of the falx to ensure that the contralateral frontal lobe is not injured. Posterior dissection is then performed along the falx until it transitions to the tentorium, effectively connecting to the infrasylvian basal temporal disconnection.
Once the entire mesial pia has been divided, the draining veins to the sinuses are coagulated and divided sequentially. During this portion of the procedure it is common to have bleeding from the dura around the sagittal sinus. Packing the bleeding areas with a combination of hemostatic agents and cotton balls works well to avoid excessive blood loss. Once the draining veins have been ligated, the entire hemisphere has been disconnected and can be carefully removed. Finally, removal of the temporal mesial structures and insula is performed and the cavity irrigated and hemostasis achieved (see Fig. 52.7). A ventricular drain is placed in the cavity and the dura is closed in a watertight fashion. The bone flap is replaced and the wound is closed in anatomical layers.
Complication Avoidance
Tanreverdi and associates, in 2009, published an analysis of postoperative epilepsy surgery morbidity rates in 1905 patients over the course of 2449 epilepsy surgery procedures. Of these 1905 patients, 517 underwent extratemporal procedures consisting either of lobectomy or multilobar resections. Morbidity was subdivided by frontal, central, parietal, occipital, or multilobar resection location, resulting in major neurological morbidity rates of 0.7%, 1.4%, 0%, 5.1%, and 2.6%, and minor morbidity rates of 5.2%, 5.9%, 0%, 2.5%, and 12%, respectively. When surgical morbidity was analyzed across the same subdivisions of frontal, central, parietal, occipital, or multilobar resection, rates of complication were 5.6%, 1.5%, 14%, 0%, and 4%, respectively. Whereas parietal resections had the lowest neurological morbidity rate, they had the highest surgical morbidity rate of any reported group. Infection was the most common surgical complication, occurring in 2.1% of extratemporal procedures, followed by hematoma at 1.5%. Only one patient developed hydrocephalus.20
With extratemporal resections, the most common neurological complications are those secondary to working in or near areas of eloquent cortex. Parieto-occipital resections, for example, are reported to have visual field deficits as their most common postoperative complication. This is followed by new or worsening hemiparesis, hemisensory loss, and aphasia.8 Resections in or near the dominant parietal lobe may result in a characteristic pattern of deficit. The most common complication of frontal lobe surgery is hemiparesis; however, aphasia is also a potential complication when operating in or near the dominant frontal operculum.21 The use of invasive monitoring to delineate eloquent cortex prior to resection aids in preventing and predicting postoperative neurological deficits. When surgical planning involves resection in or near functional cortex the relationship of the epileptogenic zone to eloquent cortex may limit the potential for seizure-free outcome.
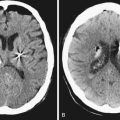
Hemispherectomy and multilobar resection patients are at increased risk of specific complications, including hemorrhage, infarction, hydrocephalus, coagulopathy, anemia, and aseptic meningitis, when compared to those patients who undergo lobar resections.14 These risks are elevated in both the acute and delayed postoperative setting. In addition, disconnective hemispherectomy can be complicated by postoperative hemispheric cerebral edema or infarct, with resultant intracranial hypertension and death.22
Conclusion
Patients with ETLE compose a significant portion of medically intractable epilepsy. Surgical management provides an effective therapeutic option for many of these patients. Success rates vary by location, pathology, and extent of resection. Despite the team-based approach for preoperative evaluation of the epilepsy patient, the primary responsibility for selection of surgical approach remains with the neurosurgeon. The importance of this decision is underscored by the fact that, in recent surgical series, 1% to 11% of epilepsy patients underwent reoperation due to surgical failure.23–25
Surgical failure has been noted to be more common in neocortical resections. Possible reasons for this include incomplete resection of the lesion and the epileptogenic zone. Jehi and colleagues remark in a review of outcomes in 57 patients who underwent posterior cortex epilepsy surgery, resection was felt to be complete in 67% of patients. Within this cohort, at 6-month routine EEG follow-up, 56% of patients with incomplete resection of their MRI lesion had spiking on their EEG versus 27% of those patients who had complete resections.8
Frontal lobe resections convey the lowest chance for successful postoperative results. Overall seizure-free outcome for a combined series of both lesional and nonlesional frontal lobe epilepsy patients was 56% at 1 year, 45% at 3 years, and 30% at 5 years. Patients with a circumscribed lesion on MRI preoperatively fared significantly better, having a 79% seizure-free rate at 5 years.21
Posterior cortex epilepsy resections convey a slightly higher chance of favorable outcome. In parietal, occipital, and parieto-occipital resections in a series of 57 patients, 5-year seizure-free rates were 52%, 89%, and 93%, respectively. The overall 5-year seizure-free rate for the posterior cortex cohort was 54%.8
Older single institution series of hemispherectomy patients report rates of seizure freedom from 53% to 67%, with malformative lesions having the worst prognosis. The more recent University of California at Los Angeles series of 141 pediatric patients reports a seizure-free rate of 83% for all hemispherectomies performed after 1997.26 Outcomes are certainly not perfect for patients undergoing surgery for management of their ETLE; however, for patients who are deemed surgical candidates, these percentages of seizure freedom far exceed those offered by any other treatment strategy.
Gonzalez-Martinez J., Srikijvilaikul T., Nair D., Bingaman W.E. Longterm seizure outcome in reoperation after failure of epilepsysurgery. Neurosurgery. 2007;60:873-880.
Marras C.E., Granata T., Franzini A., et al. Hemispherotomy and functional hemispherectomy: indications and outcome. Epilepsy Res. 2010;89(1):104-112.
Rasmussen T., Villemure J.G. Cerebral hemispherectomy for seizures with hemiplegia. Cleve Clin J Med. 1983;56:S62-S68.
Schramm J., Clusmann H. The surgery of epilepsy. Neurosurgery. 2008;62:463-481.
Wiebe S., Blume W.T., Girvin J.P., et al. A randomized, controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:311-318.
Please go to expertconsult.com to view the complete list of references.
1. Wiebe S., Blume W.T., Girvin J.P., et al. A randomized, controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:311-318.
2. Wyllie E. Surgical treatment of epilepsy in children. Pediatr Neurol. 1998;19:179-188.
3. Kwan P., Brodie M.J. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314-319.
4. Noachtar S., Winkler P.A., Luders H.O. Surgical therapy of epilepsy. In: Brandt T., Caplan L.R., Dichgans J., et al, editors. Neurological Disorders: Course and Treatment. 2nd ed. San Diego: Academic Press; 2003:235-244.
5. Chapman K., Wyllie E., Najm I., et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76:710-713.
6. Diaz R.J., Sherman E.M., Hader W.J. Surgical treatment of intractable epilepsy associated with focal cortical dysplasia. Neurosurg Focus. 2008;25(3):E6.
7. Marras C.E., Granata T., Franzini A., et al. Hemispherotomy and functional hemispherectomy: indications and outcome. Epilepsy Res. 2010;89(1):104-112.
8. Jehi L., O’Dwyer R., Najm I., et al. A longitudinal study of surgical outcome and its determinants following posterior cortex epilepsy surgery. Epilepsia. 2009;9:1-13.
9. Spencer S., Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525-537.
10. Palmini A., Chandler C., Andermann F., et al. Resection of the lesion in patients with hypothalamic hamartomas and catastrophic epilepsy. Neurology. 2002;58:1338-1347.
11. Urbach H., Scheffler B., Heinrichsmeier T., et al. Focal cortical dysplasia of Taylor’s balloon cell type: a clinicopathological entity with characteristic neuroimaging and histopathological features, and favorable postsurgical outcome. Epilepsia. 2002;43:33-40.
12. Schramm J., Clusmann H. The surgery of epilepsy. Neurosurgery. 2008;62:463-481.
13. De Almeida A.N., Marino R.Jr., Aguiar P.H., Jacobsen Teixeira M. Hemispherectomy: a schematic review of the current techniques. Neurosurg Rev. 2006;29:97-102.
14. Boongird A., Bingaman W.E. Anatomical hemispherectomy. In: Starr P.A., Barbaro N.M., Larson P.S., editors. Neurosurgical Operative Atlas. Functional Neurosurgery. 2nd ed. New York: Thieme; 2009:49-55.
15. De Ribaupierre S., Delalande O. Hemispherotomy and other disconnective techniques. Neurosurg Focus. 2008;25(3):E14.
16. Gonzalez-Martinez J., Srikijvilaikul T., Nair D., Bingaman W.E. Longterm seizure outcome in reoperation after failure of epilepsy surgery. Neurosurgery. 2007;60:873-880.
17. Rasmussen T., Villemure J.G. Cerebral hemispherectomy for seizures with hemiplegia. Cleve Clin J Med. 1983;56:S62-S68.
18. Villemure J.G., Mascott C.R. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37:975-981.
19. Schramm J., Behrens E., Entzian W. Hemispherical deafferentation: a modified functional hemispherectomy technique. Epilepsia. 1994;35:71. (abstract)
20. Tanriverdi T., Ajlan A., Poulin N., Olivier A. Morbidity in epilepsy surgery: an experience based on 2449 epilepsy surgery procedures from a single institution. J Neurosurg. 2009;110:1111-1123.
21. Jehi L.E., Najm I., Bingaman W., et al. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130(Pt 2):574-584.
22. Delalande O., Bulteau C., Dellatolas G., et al. 2007. Vertical parasagittal hemispherotomy: surgical procedures and clinical long term outcomes in a population of 83 children. Oper Neurosurg. 2007;60:19-32.
23. Aykut-Bingol C., Bronen R.A., Kim J.H., et al. Surgical outcome in occipital lobe epilepsy: implications for pathophysiology. Ann Neurol. 1998;44:60-69.
24. Siegel A.M., Cascino G.D., Meyer F.B., et al. Resective reoperation for failed epilepsy surgery: seizure outcome in 64 patients. Neurology. 2004;63:2298-2302.
25. Salanova V., Markland O., Worth R. Temporal lobe epilepsy: analysis of failures and the role of reoperation. Acta Neurol Scand. 2005;111:126-133.
26. Mathern G.W., Giza C.C., Yudovin S. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986-1997. Epilepsia. 1999;40(12):1740-1749.