Chapter 13 Extracorporeal Membrane Oxygenation
3 How is ECMO different from cardiopulmonary bypass?
Cardiopulmonary bypass was developed as a means to provide short-term support to patients undergoing cardiac surgery. The setup necessary for cardiopulmonary bypass is also designed to accomplish several other tasks including suction and venting of the field and cardiac chambers and administration of cardioplegia (Table 13-1).
Table 13-1 Differences and Similarities Between ECMO and Cardiopulmonary Bypass
| Parameter | ECMO | Cardiopulmonary Bypass |
|---|---|---|
| Oxygenation | Yes | Yes |
| Ventilation | Yes | Yes |
| Circulatory support | Yes | Yes |
| Venous reservoir | No | Yes |
| Ability to deliver cardioplegia | No | Yes |
| Ability to administer medications into circuit | No | Yes |
| Supplemental pumps (e.g., suction, vent) | No | Yes |
| Heating and cooling | Yes | Yes |
| Ability to adjust oxygenation | Yes | Yes |
| Ability to add fluids directly to circuit | No | Yes |
| Ability to administer anesthetics in line | No | Yes |
4 What are clinical situations where ECMO may be beneficial?
ECMO has been used for the short-term hemodynamic and respiratory support for numerous conditions (Box 13-1).
Box 13-1 Potential Clinical Uses of ECMO
 Adult respiratory distress syndrome
Adult respiratory distress syndrome
 Acute respiratory failure associated with viral infection
Acute respiratory failure associated with viral infection
 Bridge to heart transplantation
Bridge to heart transplantation
 Bridge to lung transplantation
Bridge to lung transplantation
 Acute massive pulmonary emboli
Acute massive pulmonary emboli
 Primary graft failure after heart transplantation
Primary graft failure after heart transplantation
 Right ventricular failure after heart transplantation
Right ventricular failure after heart transplantation
 Severe refractory status asthmaticus
Severe refractory status asthmaticus
 Low cardiac output syndrome after cardiopulmonary bypass
Low cardiac output syndrome after cardiopulmonary bypass
 Support during liver transplantation
Support during liver transplantation
 Hemodynamics and respiratory support during surgery for mediastinal mass excision
Hemodynamics and respiratory support during surgery for mediastinal mass excision
 Support during carinal resection
Support during carinal resection
 Support during high-risk percutaneous coronary interventions
Support during high-risk percutaneous coronary interventions
 Support during high-risk electrophysiology procedures
Support during high-risk electrophysiology procedures
 Burn-associated respiratory failure
Burn-associated respiratory failure
 Respiratory failure due to near drowning
Respiratory failure due to near drowning
 Respiratory support for congenital diaphragmatic hernia
Respiratory support for congenital diaphragmatic hernia
 Rewarming after accidental hypothermia
Rewarming after accidental hypothermia
8 What are the components of an ECMO circuit?
Most ECMO circuits have a membrane gas exchange unit. This gas exchange unit consists of a series of hollow fibers through which the blood passes. Gas is passed in a countercurrent manner allowing exchange across the membranes. The pump system may be either a roller system or a centrifugal system. A roller pump system consists of flexible tubing in a track. The roller compresses the tubing and forces the blood forward with each turn. Such flow is independent of systemic vascular resistance, and high pressures can develop. Centrifugal pumps have been popular in Europe and are becoming more popular in the United States. Centrifugal pumps contain a magnetically driven impeller, which cycles at several thousand rotations per minute generating a pressure gradient across the pump head and propelling blood forward. It is preload and afterload dependent. If flow into the pump is decreased, then flow and pressure will be decreased. The CentriMag blood pump is a magnetically levitated centrifugal device that produces unidirectional flow and may generate flows up to 10 L/min (Figs. 13-1 and 13-2).
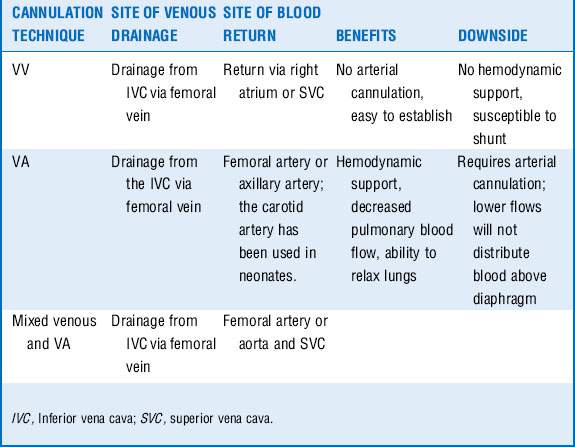
9 How is vascular access established for ECMO?
Several potential sites exist for both venous and arterial cannulation for patients requiring ECMO. The decision as to which cannulation strategy is necessary depends on the goals (Table 13-2). The venous or drainage cannula for adult patients should be 23F to 25F and the return cannula 17F to 21F.
10 What does one monitor while using ECMO?
Monitoring patients subject to ECMO is a multidisciplinary task. Surgeons, intensivists, perfusionists, anesthesiologists, respiratory therapists, and nurses may all be involved in the care of these patients. Maintenance of normal physiologic parameters is the goal (Table 13-3). Flow rates, sweep speed, and FiO2 are adjusted to attain such measure.
| Parameter | Goal |
|---|---|
| Flow rates (mL/kg/min) | 50-80 |
| Mean arterial pressure (adult) (mm Hg) | 65-95 |
| Sweep speed (gas flow) (mL/kg/min) | 50-80 |
| FiO2 (%) | 100 |
| pH | 7.35-7.45 |
| PaCO2 (mm Hg) | 35-45 |
| SpO2 (arterial or return cannula) (%) | 100 |
| SpO2 (VA ECMO) (%) | > 95 |
| SpO2 (VV ECMO) (%) | 85-92 |
13 How does one wean a patient from ECMO?
The technique of weaning a patient from ECMO support depends on the type of ECMO (VV or VA) and the purpose of the support. In general the lungs and heart assume more responsibility for oxygenation, ventilation, and circulation. Sidebotham et al. describe their technique for weaning ECMO (Table 13-4).
| Measure | VV ECMO | VA ECMO |
|---|---|---|
| Purpose of support | Oxygenation-ventilation | Hemodynamic support |
| Measure of improvement | Increased SpO2 and PaO2 Decreased PaCO2 Improved CXR Increased lung compliance |
Return of pulsatile arterial waveform Improved cardiac function by echocardiography |
| Technique | Provide full ventilatory support Reduce gas “sweep” Reduce flows to 1-2 L/min |
Adjust inotropic support to provide acceptable hemodynamics Reduce flows to 1-2 L/min |
| Monitoring during weaning | Maintenance of SpO2/PaO2 | Echocardiography (cardiac function) Blood pressure Cardiac output Central venous pressure Pulmonary artery pressure |
| When to discontinue | Acceptable ABG 2-3 hr after weaning and tolerance of flow reduction to zero | Acceptable hemodynamics after 1-2 hr of minimal or no hemodynamics support. |
ABG, Arterial blood gases; CXR, chest radiograph.
Modified from Sidebotham D, McGeorge A, McGuinness S, et al: Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2—technical considerations. J Cardiothorac Vasc Anesth 24:164-172, 2010.
15 How does one transfer a patient receiving ECMO?
Key Points Extracorporeal Membrane Oxygenation
ECMO may provide temporary support to patients with failure of the cardiopulmonary system by:
1. Providing primary oxygenation and circulation for patients with primary cardiac failure (VA ECMO)
2. Providing extracorporal oxygenation and carbon dioxide removal for patients with respiratory failure (VV ECMO)
ECMO is different from cardiopulmonary bypass in several ways:
1 Augustin P., Lasocki S., Dufour G., et al. Abdominal compartment syndrome due to extracorporeal membrane oxygenation in adults. Ann Thorac Surg. 2010;90:e40–e41.
2 Aziz T.A., Singh G., Popjes E., et al. Initial experience with CentriMag extracorporeal membrane oxygenation for support of critically ill patients with refractory cardiogenic shock. J Heart Lung Transplant. 2010;29:66–71.
3 Brogan T.V., Thiagarajan R.R., Rycos P.T., et al. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35:2105–2114.
4 Foley D.S., Pranikoff T., Younger J.G., et al. A review of 100 patients transported on extracorporeal life support. ASAIO J. 2002;48:612–619.
5 Hill J.D., O’Brien T.G., Murray J.J., et al. Prolonged extracorporeal oxygenation in severe acute respiratory failure (shock lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–634.
6 Meilck F., Quintel M. Extracorporeal membrane oxygenation. Curr Opin Crit Care. 2005;11:87–93.
7 Peek G.J., Mugford M., Tiruvoipai R., et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilator support versus extracorporeal membrane oxygenation for severe adult respiratory failure [CESAR]; a multicenter randomized controlled trial. Lancet. 2009;374:1351–1363.
8 Saito S., Nakatani T., Kobayashi J., et al. Is extracorporeal life support contraindicated in elderly patients? Ann Thorac Surg. 2007;83:140–145.
9 Sidebotham D., McGeorge A., McGuinness S., et al. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2—technical considerations. J Cardiothorac Vasc Anesth. 2010;24:164–172.
10 Terragni P.P., Del Sorbo L., Mascia L., et al. Tidal volume lower than 6 ml/kg enhances lung protection. Role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–835.
11 Zapol W.M., Snider M.T., Hill J.D., et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196.
12 Zapol W.M., Snider M.T., Schneider R.C. Extracorporeal membrane oxygenation for acute respiratory failure. Anesthesiology. 1977;46:272–285.