Chapter 15 External Beam Radiotherapy for Tumors of the Spine
EXTERNAL BEAM RADIOTHERAPY TECHNIQUES
CONVENTIONAL 2-DIMENSIONAL RADIOTHERAPY
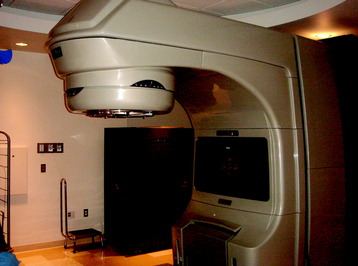
High-energy megavoltage radiation is produced by a linear accelerator (Fig. 15-1). The generated beam of radiation diverges from the head of the linear accelerator gantry and may be collimated or shaped using simple “jaws” that create square or rectangular fields. A secondary level of collimation can be achieved by placing lead-based alloy blocks in front of the beam to create a unique shape. Conventional radiotherapy using 2D techniques relies on simple 2D x-ray imaging and bony anatomical landmarks to determine where the beams of radiation should be focused. Historically, radiation therapists relied on a detailed knowledge of the relative relationship of internal organs with respect to the bony anatomy to define the radiation fields. These techniques are most commonly used for urgent radiation delivery or when treating simple bony lesions of the axial skeleton.
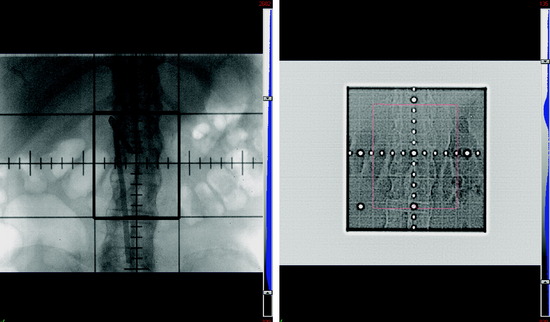
Figure 15-2 depicts a simulated radiation field created to treat a patient who presented with painful bony spinal metastasis at L2. In this x-ray image, the center of the beam is shown by the intersection of the perpendicular crosshairs. The thick continuous lines represent the “jaws,” which create a rectangular central area around the center of the beam that defines the target radiation field. It is customary to include at least one vertebral body above and below the target level when treating spinal metastases. The lateral borders of the field are set to encompass the paraspinal extent of the tumor with additional margin to account for uncertainties of internal organ motion, patient motion, and day-to-day variation in radiation field set-up. Parallel-opposed fields from anterior and posterior projections are used to treat the target within the rectangular field. Opposed lateral fields may be used for cervical spinal lesions. Thus, all the intervening organs and tissues contained in this field also will be irradiated. In this case, the amount of radiation that can be safely delivered is limited by the radiation sensitivity of the normal tissues, such as the spinal cord and small bowel.
THREE-DIMENSIONAL CONFORMAL RADIOTHERAPY AND INTENSITY MODULATED RADIOTHERAPY
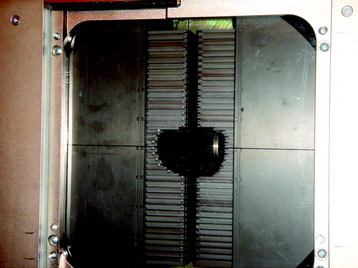
With the advent of computed tomography (CT), creation of complex radiation doses that conform to the shape of the clinical target is feasible. After contouring the target and normal structures on individual CT slices, a 3D representation of these structures is created by digital reconstruction. Unlike simple 2D treatment planning, 3D conformal radiotherapy (3D-CRT) and intensity modulated radiotherapy (IMRT) use an array of radiation beam angles. Custom field blocking of each field is based on the relative relationship between the target and normal structures from the beam’s eye view. The blocking is achieved by either lead-based alloy blocks or by a multi-leaf collimator (MLC). With the MLC, 3- to 10-mm rectangular tungsten leaves are independently positioned a certain distance from the center of the field to create a custom shape of the field (Fig. 15-3). In most cases of 3D-CRT, the radiation dose delivery through each field is both homogeneous (i.e., radiation dose does not vary in intensity) and static (i.e., there is no change in the beam angle or field blocking). The resultant radiation dose distribution will conform to the shape of the target relatively homogeneously. This technique is widely used.
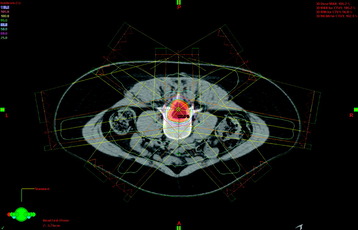
Figure 15-4 shows the radiation dose distribution of a seven-field IMRT plan for a spinal tumor. By converging beams from multiple angles and modulating the dose for each field, the prescription radiation dose, depicted by the yellow curve, tightly conforms to the shape of the target, depicted by the light red shaded contour. At the same time, the radiation dose to the bowel is about 25% of the prescribed dose.
RADIOTHERAPY FOR SPINAL METASTASES AND MALIGNANT SPINAL CORD COMPRESSION
The spine is the most common site of osseous tumor spread of cancer, comprising 40% of skeletal metastases.1–4 Adjuvant radiotherapy after surgery or radiotherapy alone remains a mainstay of palliation of spinal metastases.5–8 Most spinal metastases are either asymptomatic or cause pain only. A smaller percentage evolve to cause epidural compression of the spinal cord or cauda equine.9
Radiotherapy is very effective in palliating pain caused by spinal metastases. A recent review by Falkmer et al10 showed that more than 80% overall pain relief is achievable by radiotherapy for skeletal metastases. Currently, radiotherapy alone is recommended for patients with painful spinal metastases without epidural spinal compression, patients with metastatic epidural spinal cord compression (MESCC) caused by radioresponsive tumors (e.g., lymphoma, leukemia, germ cell tumors), medically inoperable patients, and patients with multiple levels of compression.5,9
Conventional radiotherapy for painful bony metastases is generally delivered either as a single fraction of approximately 8 Gy or as a fractionated course of 20–40 Gy over 5–20 daily fractions. Multiple randomized trials and retrospective reviews have explored the relative palliative benefit of short duration radiation schedules (e.g., 8 Gy in a single fraction) vs. longer schedules (e.g., 30 Gy in 10 fractions).11–16 Even though short and longer schedules of radiotherapy generally have similar effectiveness in early pain relief, re-treatment is required more frequently after the shorter courses.10,12 Therefore, when death does not appear to be imminent, 30 Gy in 10 fractions is generally the preferred palliative course of treatment.
In recent years the important role of surgical resection in addition to radiotherapy for MESCC has been better defined. Patchell et al7 reported a randomized control trial of direct decompressive surgical resection plus adjuvant radiotherapy vs. radiotherapy alone; the results strongly support the use of surgery plus adjuvant radiotherapy for MESCC. In this study, 101 patients with MESCC were randomized. After initiating high-dose steroids, patients were treated as randomized. Ninety-four percent of surgical patients who were ambulatory on study entry retained their ambulatory status compared with only 74% of radiation alone patients. Sixty-two percent of surgical patients who were non-ambulatory at study entry regained walking ability after treatment compared with only 19% of radiotherapy alone patients. These results translate into an overall post-treatment ambulatory rate of 84% in surgery plus radiotherapy patients and 57% in radiotherapy alone patients. The radiation alone results in this study are similar to the results shown by randomized radiation trials of MESCC exploring different radiation schedules. Maranzano et al17–19 reported on several prospective trials including a randomized, multi-institutional trial comparing two different hypofractionation schedules and showed that approximately three-quarters of patients are ambulatory post-treatment and 54–59% show complete relief of back pain. Figure 15-5 shows an example of MESCC at the left posterior aspect of T4.
RADIOTHERAPY FOR PRIMARY SPINAL TUMORS
PRIMARY EPIDURAL SPINAL TUMORS
Multiple myeloma and solitary plasmacytoma (SP) are quite radiosensitive tumors. When multiple myeloma or SP involves the spine, it often presents with spinal cord compression. Whereas radiotherapy is most often used palliatively for multiple myeloma, radiotherapy may be curative for SP. Therefore, local field radiotherapy is the treatment of choice for SP of bone involving the spine. Although a definite dose response for radiation doses of more than 30 Gy has not been proven, 40–50 Gy or more delivered in conventional fractions of 1.8–2 Gy is most commonly recommended, particularly for bulky tumors.20–24
Ewing’s sarcoma (ES) is the second most common malignant pediatric bone tumor, typically arising in the pelvis and the diaphyseal portion of long bones of the lower extremities. Primary vertebral Ewing’s sarcoma occurs in approximately 8–10% of patients.25–27 Vertebral body distribution was cervical (3%), thoracic (11%), lumbar (25%), sacral (53%), and multilevel (8%) in a review of 125 patients.25 Current treatment approaches consist of induction chemotherapy, followed by local treatment with surgery or radiotherapy and adjuvant chemotherapy. Given its unfavorable location and lack of local control benefit to subtotal resection of tumor, primary radiotherapy is generally preferred for primary vertebral body ES.
The most commonly given dose for primary treatment of ES, 55.8 Gy, exceeds the tolerance of the spinal cord; therefore, doses of 45–50.4 Gy are given over cord, containing levels. Outcomes for primary vertebral ES are similar to that of extremity ES, with local control achieved in approximately three-quarters of patients.25–29
The effective treatment of chordomas is challenging because of their locally aggressive nature and propensity for local recurrence. Chordomas occur in the sacrococcygeal region (50%), base of skull (35%), and other levels of the vertebral column (15%).30,31 Characteristically, chordomas exhibit indolent growth. Generally speaking, chordomas rarely metastasize, although sacral chordomas metastasize more frequently than those with other sites of origin. Chordomas and chondrosarcomas are relatively radioresistant tumors and are prone to local recurrence despite treatment. Chondrosarcomas may occur in the vertebral column, but typically occur in long bones or the pelvis. Complete surgical resection is the treatment of choice, and radiotherapy is recommended after incomplete resection. In a retrospective review of 27 patients with sacral chordoma reported by York et al,32 the 2.27-year disease-free interval of patients undergoing radical resection was statistically different compared with the disease-free interval of 8 months observed in patients undergoing subtotal surgery. Although some series have failed to show a significant dose-response relationship with doses up to 60 Gy after subtotal resection, it is widely believed that even higher doses in a range more than 70 Gy are likely required to yield meaningful control for these tumors.33–35
PRIMARY INTRAMEDULLARY GLIAL TUMORS
Intramedullary spinal tumors account for 25% of all spinal cord tumors, with ependymomas and astrocytomas representing the majority of cases.36 Ependymoma is the most common intramedullary spinal tumor in adults. The long-term outcome and risk of recurrence of intramedullary ependymoma are largely dependent on the extent of surgical resection. The recurrence rate after gross total resection is approximately 10%, whereas after subtotal resection recurrence can be as high as 70%. Adjuvant radiotherapy after adjuvant subtotal resection reduces recurrences to 30%.37–39 Although some series report similar survival and local control of subtotal resection followed by radiotherapy as compared with gross total resection alone, the results are not as favorable as those for gross total resection.40
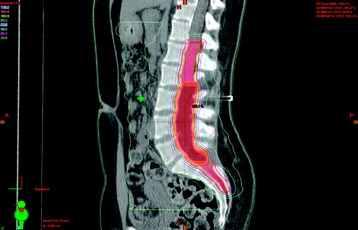
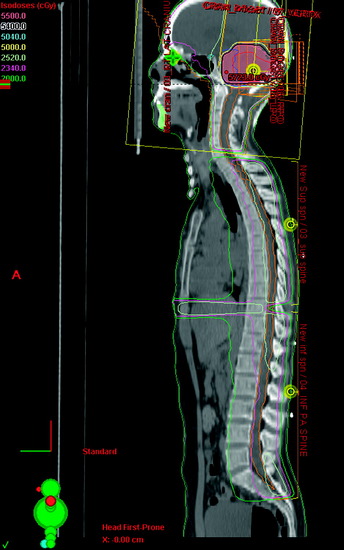
For subtotally resected ependymomas, radiation doses less than 40 Gy are associated with unacceptably low survival rates. No significant dose-response relationship has been noted for doses between 40–54 Gy, and there is no clear evidence for improved local control with doses greater than 54 Gy.41 Figure 15-6 shows a case of a patient with a lumbosacral Grade II spinal ependymoma initially extending from L2–S2. After subtotal resection, residual deposits of tumor persisted in the region of L3–S1. IMRT was used to treat this patient postoperatively. The target volumes are depicted by two separate volumes: the orange contour from L1–S3 represents the area containing potential microscopic tumor, whereas the central brown contour represents the region containing visible tumor deposits. By using IMRT, the central region was treated with a higher dose of 54 Gy in 30 fractions, whereas the area containing subclinical disease was treated with approximately 48 Gy. Craniospinal irradiation is rarely required, but is reserved for patients with disseminated or multifocal disease. Figure 15-7 shows a typical radiation dose distribution for craniospinal irradiation for a posterior fossa tumor. Because late recurrences have been documented up to 12 years after treatment, long-term follow-up is critical.
Astrocytomas are the second most common spinal cord tumors in adults. Unlike ependymomas, in which extent of resection is a major determinant of outcome, the most important prognostic factor for survival for patients with spinal astrocytomas is histological grade. The 5-year survival rate for patients with low-grade astrocytomas treated with postoperative radiation is 50–91%.39,42 Comparatively, the outcome of high-grade astrocytomas is extremely poor, with only a 6- to 8-month average survival after surgery with or without postoperative radiotherapy.42,43 Therefore, radiation therapy generally has an adjuvant role for incompletely resected low-grade astrocytomas and a palliative role for malignant astrocytomas. Radiotherapy after gross resection of low-grade astrocytomas remains controversial. The typical recommended dose for spinal astrocytoma is 45–55 Gy in conventional fractions.39,42,44