Chapter 84B External-beam radiotherapy for liver tumors
Technical Innovations
Intensity-Modulated Radiotherapy
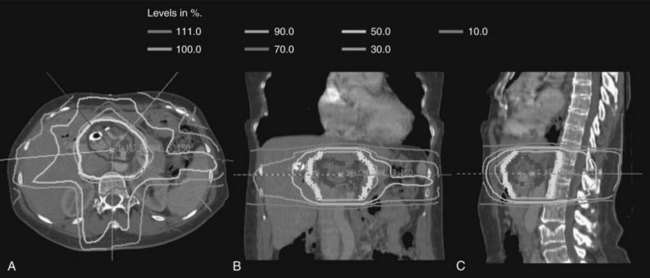
Over the last decade, more sophisticated imaging and treatment-planning techniques have allowed for more focal delivery of RT. Now routine is computed tomography (CT)-assisted three-dimensional conformal radiation therapy (3D-CRT), which uses diagnostic CT or magnetic resonance imaging (MRI) to define normal and target structures. IMRT is a further technical innovation, now frequently used in hepatobiliary radiation. As opposed to conventional radiation that uses large, fixed radiation portals and field borders based on correlations between the bony anatomy and the soft-tissue target, IMRT uses CT-based planning, and the target volumes and normal structures are delineated individually on axial CT imaging. With conventional RT, normal tissues adjacent to target structures cannot be spared and receive the prescribed radiotherapy dose, limiting the radiation dose that can be delivered safely. When planning treatment using IMRT, the radiation dose is prescribed to the target volume, and strict dose constraints are placed on normal tissues. The IMRT plan is then developed using a computer-optimized algorithm to deliver radiation of varying intensity to the target volume via multiple beams to meet the requirements for the target volume coverage and normal tissue constraints. The overall result is a highly conformal dose distribution customized to the shape of the tumor (Fig. 84B.1). This minimizes exposure of surrounding normal tissues and organs to high-dose radiation.
Image-Guided Radiotherapy and Motion Management
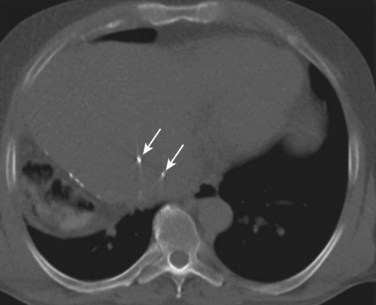
The development of 3D-CRT and IMRT, which allow for delivery of more conformal radiotherapy to the tumor, has made more accurate localization of the tumor at the time of treatment necessary to avoid missing the target. The need to improve targeting in radiation treatment has led to the development of image-guided radiation therapy to reduce the uncertainties of tumor positioning. On-board 3D CT imaging, known as cone-beam CT (CBCT), has been developed to allow real-time assessment of tumor positioning on the linear accelerator, while the patient is on the table prior to treatment delivery; however, CBCT scans may not show the target lesion in the liver because of limitations in resolution and contrast (Fig. 84B.2). Because liver tumors are not well visualized on standard megavoltage portal images or even on CBCT, surrogates for tumor location have been used to help verify tumor positioning prior to treatment. Fiducial markers (see Fig. 84B.2) have been introduced to help identify the tumor and allow for better visualization and improved setup for both conventionally fractionated treatments and, more importantly, for focal RT. Gold fiducial markers can be percutaneously inserted in or around the liver tumor, or postoperative clips can be used to help evaluate the postoperative bed on kilovoltage images. These fiducial markers are essential to allow for both tumor localization and assessment of tumor motion during treatment, and they are used in conjunction with many of the motion-management techniques described below.
Although 3D-CRT and IMRT offer the ability to tighten the margin around the target volume, the motion of the liver is complex because of organ deformation and rotation with respiration. Some series have found that the liver moves from 1 to 8 cm in the superior-inferior direction with respiration, and smaller shifts are noted in the anterior-posterior and left-right directions with breathing (Shirato et al, 2004). Because of this significant displacement over time, standard treatment techniques require large margins on the gross tumor volume to ensure a full dose to the tumor throughout the respiratory cycle.
Hepatocellular Carcinoma
Early on, researchers determined that low doses of large-field hepatic irradiation were ineffective in controlling gross hepatocellular carcinoma (HCC) and that higher doses of whole-liver irradiation resulted in high rates of radiation hepatitis. Stevens (1994) states that 75% of patients treated with 40 Gy or greater radiation to the whole liver will develop liver dysfunction, and an early study of adjuvant liver irradiation for HCC (Fugitt et al, 1973) demonstrated that 4 of 52 patients treated with more than 55 Gy had fatal hepatitis. These widely quoted early results have resulted in RT being placed on the list of potential palliative therapies for HCC.
With the development of 3D-CRT, high-dose, partial liver irradiation was also attempted for patients with unresectable liver tumors ( Dawson et al, 2000; Lawrence et al, 1993; Robertson et al, 1997). Investigators at the University of Michigan have reported a Phase II study of 3D-CRT and concurrent intraarterial floxuridine chemotherapy for unresectable hepatobiliary disease. They treated 128 patients with unresectable HCC (n = 35), intrahepatic cholangiocarcinoma (n = 46), or colorectal hepatic metastases (n = 47). Doses ranged from 40 to 90 Gy (median, 60.75 Gy) and were based on the amount of normal liver tissue, evaluated by dose-volume histograms, that could be effectively excluded from the fields to a maximum risk of radiation-induced liver disease (RILD) of 10% to 15%. At a median follow-up time of 16 months (26 months in patients who were alive) the median survival was 15.8 months, and the 3-year survival was 17%; in addition, 38 patients (30%) developed grade 3 or greater toxicity, and 5 patients developed RILD, one of whom died.
More recently, studies of external-beam radiotherapy (EBRT) using IMRT have been reported. A dose-escalation trial of conformal radiotherapy using 3D-CRT (n = 24) or IMRT (n = 16) was performed at Fudan University in Shanghai (Ren et al, 2010). The median tumor dose was 51 Gy (range, 40 to 62 Gy), and the median dose to normal liver was 17 Gy (range, 9 to 22 Gy). None of the patients experienced grade 3 or greater toxicity. The acute hepatic toxicities were mainly elevated liver enzymes; in-field progression-free rates at 1 and 2 years were 100% and 93%, respectively; overall survival at 1 and 2 years was 72% and 62%, respectively.
Investigators from the University of Virginia reported their experience treating 20 patients with HCC using IMRT to deliver 50 Gy in 20 fractions with concurrent capecitabine (McIntosh et al, 2009). The median tumor size was 9 cm, and 45% of patients had Child-Turcotte-Pugh (CTP) class B cirrhosis. The average volume of liver that received greater than 30 Gy was 27%. None of the patients experienced grade 3 or greater acute or late toxicity, and no cases of RILD were reported. The median survival for patients who had class A and B disease was 22.5 months and 8 months, respectively. These numbers exceed those quoted for radiation or regional chemotherapy alone and match most surgical series, they should therefore serve as the basis for future studies using dose escalation and radiation sensitizers.
Alternative fractionation schemes have also been introduced in the treatment of HCC. Both hyperfractionation (lower dose per fraction, twice-daily treatments) and hypofractionation (high dose per fraction in fewer fractions) have been applied to improve response rates in liver malignancies. The rationale for hyperfractionation is the rapid doubling time of HCC tumors (estimated at 41 days) and the shortened treatment times. Hyperfractionation was used in the University of Michigan series (Ben-Josef et al, 2005; Dawson et al, 2000; Lawrence et al, 1993; Robertson et al, 1997). In a nonrandomized study by the Radiation Therapy Oncology Group (RTOG) (Stillwagon et al, 1989), 194 patients with advanced HCC—70% to 80% of whom had metastases or had failed prior treatment—received treatment to the whole liver. Conventional fractionation (3 Gy four times per week to 21 Gy) was used in 135 patients, whereas 59 received 24 Gy in 1.2 Gy fractions twice a day. Concurrent doxorubicin and fluorouracil were given intravenously every other day to both groups. The response rate for both was dismally low (approximately 20%) and unaffected by fractionation scheme. Both acute esophagitis and thrombocytopenia occurred significantly more often in the group that received twice-daily radiation.
With improved tumor localization techniques and motion-management modalities, hypofractionation using IGRT has been evaluated in the treatment of primary liver tumors. Focal, high-dose stereotactic body RT (SBRT) was evaluated in a Phase I study of 41 patients with primary liver tumors, the majority of which were HCC. Dawson and colleagues (2000) prescribed a variable dose of 24 to 54 Gy given over six fractions; the dose was dependent on the volume of liver irradiated and the estimated risk of liver toxicity using a normal tissue complication model. Grade 3 elevation of liver enzymes was seen in five patients (12%). No RILD or treatment-related grade 4 or 5 toxicity was seen within 3 months of SBRT, and overall clinical outcomes were better than expected for this high-risk population. This study was limited to patients with class A cirrhosis or better; however, the majority of patients with unresectable HCC have more advanced cirrhosis, and the underlying liver dysfunction must be considered when delivering any type of liver irradiation.
Emerging data show that dose-volume constraints may be used in cirrhotic patients receiving conformal RT for HCC (McGinn et al, 1998; Mornex et al, 2006). Such guidelines have been shown to allow safe delivery of relatively high doses of conformal RT for HCC (McGinn et al, 1998). These guidelines were used in a Phase II study that confirmed 3D-CRT could be used safely and effectively for patients with tumors less than 5 cm and class A or B cirrhosis (Mornex et al, 2006). SBRT appears to be a promising alternative for patients with unresectable primary liver tumors. This modality may prove to be an option not only for definitive treatment of primary liver tumors but also as a bridge to transplant for HCC patients.
Hypofractionated, focal RT using charged particles has also been studied, particularly in Asia. In small, nonrandomized studies, high-dose RT delivered with protons has been shown to result in acceptable local control and survival rates for unresectable primary liver tumors (Chiba et al, 2005; Fukumitsu et al, 2009; Matsuzaki et al, 1994). A recent prospective study evaluated 51 patients treated with proton RT to deliver 66 Gy equivalents in 10 fractions to those with one to three HCCs 10 cm or smaller (Fukumitsu et al, 2009); of these, 20% were CTP class B. The 5-year local control and survival were 88% and 39%, respectively, and 5-year survival was 46% among patients with solitary tumors and class A liver function. These results are similar to those obtained after surgery; liver function remained stable or improved in 84% of patients, and no RILD was observed. Investigators at Loma Linda Hospital reported the results of their Phase II study in which 34 HCC patients were treated with cobalt-60 Gy equivalents (CoGyEq) in 15 fractions using protons, resulting in a 2-year local control rate of 75% (Bush et al, 2004). Six patients underwent liver transplantation between 6 and 16 months after completion of RT, two of whom had no residual tumor. These newer modalities may be able to minimize normal tissue effects via precise dose localization and should be investigated further.
Selective internal RT (SIRT) or radioembolization using yttrium-90–labeled microspheres for the treatment of HCC has gained interest in recent years (see Chapter 84A). The 90Y microspheres have several advantages as a potential therapy for liver lesions, including the selective delivery of radiolabeled microspheres via the hepatic artery and the short-depth dose afforded by 90Y, a β-emitter. A meta-analysis of SIRT using either resin or glass microspheres demonstrated that for HCC, a treatment response—complete response, partial response, and/or stable disease—was seen in 89% for resin microspheres and 78% for glass microspheres (Vente et al, 2009). In a large, multiinstitutional, retrospective review of 515 patients who received 680 treatments of SIRT, stable disease was seen in 76.8%, a partial response in 9.5%, a complete response in 4.5%, and progressive liver disease in 9% (Salem et al, 2010). The treatment was well tolerated, with an incidence of RILD of only 4% and nonliver grade 3 or greater toxicity in 6% (gastritis or gastric ulceration) (Kennedy et al, 2009). Salem and colleagues (2010) recently reported long-term outcomes for 291 HCC patients treated with intraarterial 90Y microspheres. The median dose was 103 Gy per treatment, and the mean number of treatments per patient was 1.8. The overall time to progression was 7.9 months, and overall median survival was significantly longer among patients with class A disease (17.2 months) versus those with class B disease (7.7 months).
Recent data suggest that the use of combined RT and chemoembolization may give promising results. Seong and colleagues (2000) from South Korea evaluated 27 patients who had not responded to transarterial chemoembolization (TACE); failure was judged by incomplete tumor filling of Lipiodol adriamycin on angiogram/CT), and patients went on to receive RT (51.8 ± 7.9 Gy). They found objective responses in 67% of patients, a median survival of 14 months from the start of RT, and 1-year overall survival of 85% (Seong et al, 2000). In a separate review, Seong and colleagues (1999) evaluated 30 patients treated with TACE followed by planned RT and noted a 63% objective response rate and a median survival of 17 months. Cheng and colleagues (2000) reported similar results, with a median survival of 19.2 months in patients treated with combination radiation and TACE.
The role of radiotherapy for HCC tumor thrombosis has also been investigated. Vascular invasion is a relatively common and potentially lethal complication of advanced HCC, potentially leading to pulmonary metastases or infarction, Budd-Chiari syndrome, and cardiac and hepatic dysfunction. Portal vein or inferior vena cava (IVC) involvement by tumor thrombus is associated with an extremely poor prognosis and limits the ability to deliver therapy such as TACE. Radiotherapy has been used in many Asian centers to address portal vein or IVC thrombosis to reduce the thrombus size to minimize the risk of clot dissemination, alleviate portal hypertension, and allow for surgical resection or TACE (Koo et al, 2009; Zeng et al, 2005). Zeng and colleagues (2005) retrospectively reviewed the outcomes for 158 patients with HCC who had portal vein and/or IVC tumor thrombus diagnosed and treated at the Zhongshan hospital in Shanghai, China, from 1998 to 2003. Forty-four patients received EBRT (median dose of 50 Gy in 2-Gy fractions), and 144 did not receive EBRT. Approximately 80% in each group were also treated with TACE or resection. The 1-year survival was 0% for patients receiving no therapy, 10.7% for TACE alone, 27.8% for resection alone, and 34.8% for patients receiving any EBRT as part of their therapy. No deterioration of liver function was observed, and only seven patients developed additional elevations of alanine aminotransferase (ALT; less than twice upper limit of normal), and all returned to normal range within 1 month.
Biliary Tumors
Intrahepatic Cholangiocarcinoma (See Chapter 50A)
Complete resection remains the gold standard and best chance of cure for patients with intrahepatic cholangiocarcinoma (IHCC); however, even with a complete resection, the 5-year overall survival rates range from 21% to 63% (Carpizo & D’Angelica, 2009). Local recurrence is most common, thus adjuvant therapy has been investigated. A recent analysis of outcomes of patients with IHCC obtained from the Surveillance, Epidemiology, and End Results (SEER) database demonstrated an improvement in overall survival for patients undergoing adjuvant radiotherapy versus surgery alone (Shinohara et al, 2008). The median overall survival was 11 months for surgery and adjuvant RT versus 6 months for surgery alone.
Although patients with IHCC are somewhat less likely to have underlying liver disease than those with HCC, their tumors are often unresectable because of other limitations to curative surgical excision that include lymphadenopathy, vascular involvement, metastases, and multilobar or adjacent organ involvement (Chen et al, 1989).
Extrahepatic Cholangiocarcinoma (See Chapter 50B)
Historically, the use of EBRT for extrahepatic hepatocellular carcinoma (EHCC), including hilar and distal bile duct tumors, was largely limited to unresectable, recurrent, or gross residual disease treated in the pre-CT era, and this led to the conclusion that extrahepatic bile duct carcinomas were radioresistant; however, no evidence supports the idea that bile duct tumor cells have an inherent radioresistance, rather the doses of radiation generally administered have been limited by the surrounding structures. Indeed, some recent reports indicate that radiation limited to relatively small fields, but reaching higher dose levels, combined with more modern surgical approaches and techniques may have a positive effect on outcome (Cameron et al, 1990; Gonzalez Gonzalez et al, 1990; Schoenthaler et al, 1993). In general, this improvement has been found to be most marked in the setting of minimal residual disease (Schoenthaler et al, 1993), although occasionally an advantage is seen in unresectable patients treated with stents and radiation (Cameron et al, 1990).
The indications for postoperative RT are not yet clear. Patients with negative resection margins are rarely referred for consideration of RT, although the available data suggest isolated locoregional recurrence rates of up to 60% after curative resection. Investigators at the University of Michigan published their retrospective experience with adjuvant RT after a potentially curative resection in 81 patients with localized EHCC (Ben-David et al, 2006). With a median follow-up of 1.2 years, the median overall survival and progression-free survival were 14.7 and 11 months, respectively. Complete resection (R0) was the only predictive factor significantly associated with increases in both overall and progression-free survival, and no difference in outcomes was reported between R1 and R2 resections. The first site of failure was locoregional in over two thirds of patients. Clearly, local failure plays a significant role in many EHCC deaths, and this is a persistent problem after surgery with or without irradiation and after irradiation alone (Gonzalez Gonzalez et al, 1990; Schoenthaler et al, 1993).
Prognostic factors that may help determine which patients might benefit from adjuvant therapy include age younger than 65 years and small tumor size (Schoenthaler et al, 1994). The degree of differentiation, lymph node involvement, local invasiveness, and papillary characteristics may also serve as prognosticators. All of these factors must be weighed with the demands of upper quadrant irradiation, the patient’s overall medical status, and the significant quality of life issues raised by this disease. As always, by far the most important prognosticator for survival is the extent of resection.
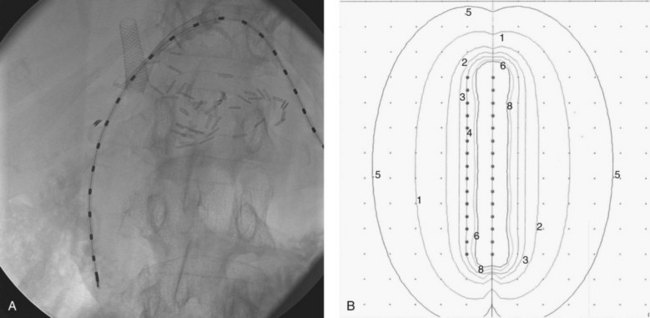
Brachytherapy has been used at numerous institutions. Overall, a slight trend has been observed toward improved survival with its use as a boost (Jarnagin, 2000). The potential advantages are clear: the area is reasonably accessible via stents placed during surgery or by the interventional radiologist; a high dose can be limited to a few surrounding centimeters; and the risk of serious short-term toxicity is low, although most series report rates of cholangitis of 30% to 50%. Dose distributions can be quite satisfactory with the use of iridium-192 catheters. Ideally, an external-beam dose designed to eradicate microscopic disease (i.e., 45 to 50 Gy) is given first, followed by seed placement designed to give an additional high dose to the site of gross or highly suspect disease. Doses ranging from 15 to 100 Gy have been administered using 192Ir, 131I, and gold-198. Dose is usually prescribed at 0.5 to 1.0 cm from the radiation sources (Fig. 84B.3).
Long-term complications secondary to the high doses and use of stents are common and include stricture, bowel obstruction, and bleeding. In addition, the rapid dose falloff means that some of the high-risk area may well lie outside the high-dose area, so brachytherapy should only be used in conjunction with EBRT. No study has shown that brachytherapy alone has anything but the briefest of palliative effects, although reports of high-dose-rate brachytherapy are just beginning to appear in the literature (Kurisu et al, 1991).
Intraoperative RT has been used for EHCCs at several institutions. After promising preliminary work in Japan, an RTOG protocol (85-06) gave 14 to 22 Gy as intraoperative RT before 45 to 50 Gy external beam to eight evaluable patients with unresectable, unresected, or recurrent tumors. Two patients were alive, one with no evidence of disease, at the time of publication, with a median follow-up of 10.5 months (Wolkov et al, 1992), In their study of 15 patients, Busse and colleagues (1989) had a median survival of 14 months with a 50% local control rate using 5 to 20 Gy. In a study by Buskirk and colleagues (1992), the only long-term survivors had either intraoperative RT or a brachytherapy boost. All of these reports involve the use of both intraoperative RT and EBRT, as the use of the intraoperative RT alone and brachytherapy alone have not been shown to have a durable impact. Intraoperative electron doses of 15 to 25 Gy have been given, again before external-beam treatments of approximately 45 to 50 Gy. Buskirk and colleagues (1992) leave transhepatic catheters in place after this procedure.
The role of liver transplantation for unresectable hilar cholangiocarcinoma is under active investigation (see Chapter 97E). Results from the Mayo Clinic are encouraging; they have been using 4500 cGy neoadjuvant chemoradiation, concurrent fluorouracil, and a brachytherapy boost, followed by liver transplantation (Rea et al, 2005). Of 71 patients enrolled, 38 underwent liver transplantation. The 3- and 5-year survival rates for this group were 82% and 82%. These results compared favorably with another group of patients who underwent resection, whose 3- and 5-year survival rates were 48% and 21%, respectively. It must be emphasized that transplantation is reserved for highly selected patients, and selection criteria are quite stringent, favoring those at earlier stages of disease compared with patients undergoing resection.
Ben-David MA, et al. External-beam radiotherapy for localized extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66:772-779.
Ben-Josef E, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739-8747.
Bush DA, et al. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127:S189-S193.
Buskirk SJ, et al. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg. 1992;215:125-131.
Busse PM, et al. Intraoperative radiation therapy for biliary tract carcinoma: results of a 5-year experience. Surgery. 1989;105:724-733.
Cameron JL, et al. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159:91-97. discussion 97-98
Carpizo DR, D’Angelica M. Management and extent of resection for intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2009;18:289-305. viii-ix
Chen MF, et al. Hepatic resection in 120 patients with hepatocellular carcinoma. Arch Surg. 1989;124:1025-1028.
Cheng JC, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:435-442.
Chiba T, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799-3805.
Dawson LA, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210-2218.
Fugitt RB, Wu GS, Martinelli LC. An evaluation of postoperative radiotherapy in hypernephroma treatment: a clinical trial. Cancer. 1973;32:1332-1340.
Fukumitsu N, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831-836.
Gonzalez Gonzalez D, et al. Results of radiation therapy in carcinoma of the proximal bile duct (Klatskin tumor). Semin Liver Dis. 1990;10:131-141.
Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19:156-176.
Kennedy AS, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494-1500.
Koo JE, et al. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2009;78:180-187.
Kurisu K, et al. High-dose-rate intraluminal brachytherapy for bile duct carcinoma after surgery. Radiother Oncol. 1991;21:65-66.
Lawrence TS, Kessler ML, Robertson JM. Conformal high-dose radiation plus intraarterial floxuridine for hepatic cancer. Oncology (Williston Park). 1993;7:51-57. discussion 57-58, 63
Matsuzaki Y, et al. A new, effective, and safe therapeutic option using proton irradiation for hepatocellular carcinoma. Gastroenterology. 1994;106:1032-1041.
McGinn CJ, et al. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16:2246-2252.
McIntosh A, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. 2009;115:5117-5125.
Mornex F, et al. Feasibility and efficacy of high-dose three-dimensional conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies: mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152-1158.
Rea DJ, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-458. discussion 458-461
Ren ZG, et al. Three-dimensional conformal radiation therapy and intensity-modulated radiation therapy combined with transcatheter arterial chemoembolization for locally advanced hepatocellular carcinoma: an irradiation dose escalation study. Int J Radiat Oncol Biol Phys. 2010;79:496-502.
Robertson JM, et al. Long-term results of hepatic artery fluorodeoxyuridine and conformal radiation therapy for primary hepatobiliary cancers. Int J Radiat Oncol Biol Phys. 1997;37:325-330.
Salem R, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64.
Schoenthaler R, et al. Definitive postoperative irradiation of bile duct carcinoma with charged particles and/or photons. Int J Radiat Oncol Biol Phys. 1993;27:75-82.
Schoenthaler R, et al. Carcinoma of the extrahepatic bile ducts: the University of California at San Francisco experience. Ann Surg. 1994;219:267-274.
Seong J, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393-397.
Seong J, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331-1335.
Shinohara ET, et al. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1495-1501.
Shirato H, et al. Intrafractional tumor motion: lung and liver. Semin Radiat Oncol. 2004;14:10-18.
Stevens KJr. Moss’ Radiation Oncology: Rationale, Technique, Results. St Louis: Mosby-Year Book; 1994.
Stillwagon GB, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group study. Int J Radiat Oncol Biol Phys. 1989;17:1223-1229.
Vente MA, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19:951-959.
Wolkov HB, et al. Intraoperative radiation therapy of extrahepatic biliary carcinoma: a report of RTOG-8506. Am J Clin Oncol. 1992;15:323-327.
Zeng ZC, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432-443.