Chapter 90C Extended hepatic resections for biliary tumors
An alternative approach
Preoperative Diagnosis and Management of Hilar Cholangiocarcinoma
Accurate preoperative staging using extensive investigation is indispensable prior to radical resection for hilar cholangiocarcinoma (Nagino et al, 1998; Nimura, 1994, 1997; Nimura et al, 1990a, 1995b, 2000). Most cases of hilar cholangiocarcinoma are associated with jaundice as a result of obstruction of the proximal bile ducts, either within or outside of the liver. Obstruction usually involves multiple ducts as a result of proximal spread of carcinoma along the intrahepatic segmental bile ducts. Multiple applications of PTBD (Nagino et al 1992; Nimura et al 1995a; Takada et al 1976) to relieve the cholestasis and restore the functional reserve of the future remnant liver, we believe, is essential prior to operation involving extensive hepatic resection.
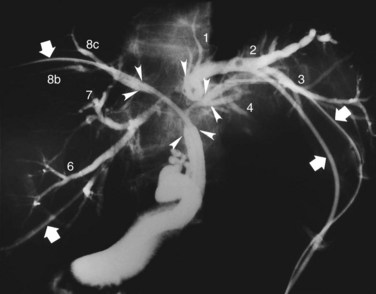
In the third edition of this text, we explained that the first step in the preoperative management of hilar cholangiocarcinoma was PTBD; however, MDCT is taken before biliary drainage to define which side of the liver should be resected, and PTBD applied by the direct anterior approach under fluoroscopic guidance (Takada et al, 1976) or ENBD for the future liver remnant (FLR) are selected to relieve cholestasis of the FLR. This offers both diagnostic and therapeutic advantage (Nagino et al, 1995a), and it minimizes PTBD sessions and the number of PTBD catheters. On the other hand, magnetic resonance cholangiopancreatography (MRCP) is occasionally insufficient to diagnose the difficult local anatomy of the separated intrahepatic segmental and/or sectoral ducts and to design an appropriate operative procedure, especially in patients with Bismuth type III or IV tumors (Bismuth & Corlette, 1975). In such cases, high-quality cholangiograms through the PTBD catheters, taken in various positions—supine, right and left anterior oblique, right and left lateral, and cephalad-anterior-oblique positions (Kamiya et al, 1994)—often clearly demonstrate the tumor location and provide the most reliable information on the proximal extension of hilar cholangiocarcinoma along the intrahepatic segmental bile ducts (Ebata et al, 2002; Sakamoto et al, 1998; Fig. 90C.1).
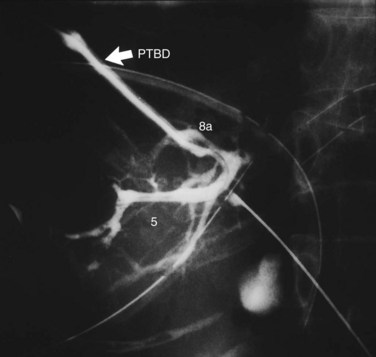
When cholangiography provides insufficient information on the tumor extent, additional PTBD is performed to the undrained bile ducts. After dilating the sinus tract of PTBD, percutaneous transhepatic cholangioscopy (PTCS) can also provide reliable information on anatomic variations of the biliary tree and mucosal extension of the cancer (Kamiya et al, 1994; Nimura, 1993; Nimura et al, 1989). Superficial mucosal extension of the carcinoma is often associated with polypoid and/or nodular tumors of a macroscopic type (Igami et al, 2009). Thus PTCS or per oral cholangioscopy with biopsy may be valuable in diagnosis of the extent of cancer (Itoi et al, 2000, 2007; Nimura et al, 1988; Sakamoto et al, 1997). On the other hand, coexistence of biliary tract infection negatively affects the functional reserve of the liver. When intrahepatic segmental cholangitis develops, even in patients with PTBD catheters during preoperative period, urgent additional selective PTBD to the affected segmental duct should be performed (Kanai et al, 1996; Fig. 90C.2).
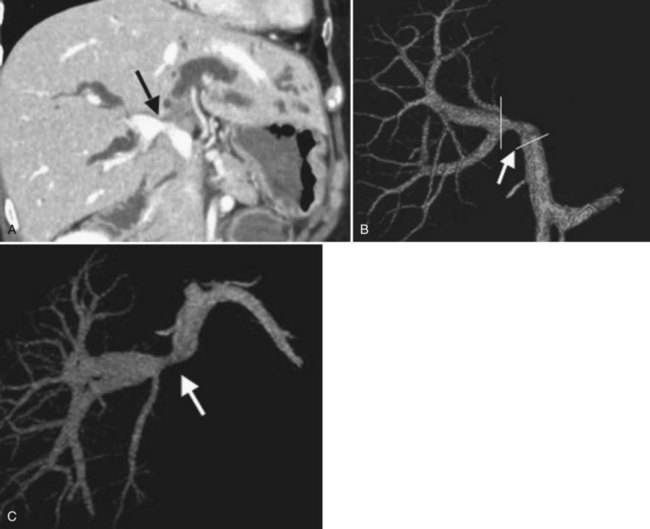
Concomitant vascular resection and reconstruction is indicated in some advanced cases of hilar cholangiocarcinoma (Nimura et al, 1991). We previously performed visceral angiography, percutaneous transhepatic portography, and selective retrograde hepatic venography to assess the vascular invasion of the tumor (Nishio et al, 1999, 2003). Recently, three-dimensional images of MDCT using techniques of volume rendering, multiplanar reformation, and maximum intensity projection have taken over because of the high-quality images and reduced invasiveness (see Fig. 90C.13). We usually estimate the relationship between a tumor and the vascular system in the liver and hepatoduodenal ligament and diagnose the vascular involvement of the tumor on the multiplanar reformatted images; we plan the methods of resection and reconstruction—wedge or segmental resection, direct end-to-end anastomosis, or segmental auto vein grafting—by volume-rendered images and maximum-intensity projection images (Senda et al, 2009; Sugiura et al, 2008).
Postoperative morbidity and mortality rates remain considerable, particularly if extensive resection is necessary (Nagino et al, 2001). Postoperative liver failure sometimes occurs and results in death. For this reason, liver resection is usually scheduled only after the liver has been decompressed and the serum total bilirubin concentration falls below 2 mg/dL. In addition, since 1989, we have routinely performed percutaneous transhepatic portal vein embolization (PVE) (Nagino et al, 1993, 1996, 2000, 2006a) as preoperative preparation for extensive liver resection. This is done to minimize the incidence of postoperative liver failure. After estimation of the resultant compensatory hypertrophy by volumetric CT scan (Nagino et al, 1995b, 1995c) and of the functional reserve of the liver as assessed by the indocyanine green clearance rate (Uesaka et al, 1996), hepatectomy is performed, usually 2 to 3 weeks after PVE. A Japanese series implemented a management strategy for patients with hilar cholangiocarcinoma that consisted of preoperative biliary drainage, PVE, and major hepatobiliary resection; it reported a 0% mortality rate in more than 100 consecutive cases (Sano et al, 2006).
Impaired intestinal barrier function does not recover with PTBD without bile replacement, which can restore the intestinal barrier function during external biliary drainage in patients with biliary obstruction, primarily as a result of repair of physical damage to the intestinal mucosa. Thus, in patients with hilar cholangiocarcinoma and external biliary drainage, we routinely replace bile prior to operation (Kamiya et al, 2004; Kanazawa et al, 2005). Also, perioperative administration of synbiotics can enhance immune responses, attenuate systemic postoperative inflammatory responses, and improve the intestinal microbial environment (Sugawara et al, 2006). These procedures may reduce postoperative infectious complications after major hepatobiliary resection in difficult patients with hilar cholangiocarcinoma.
Right Hepatic Resection with Total Caudate Lobectomy, Extrahepatic Bile Duct Resection, and Portal Vein Resection and Reconstruction
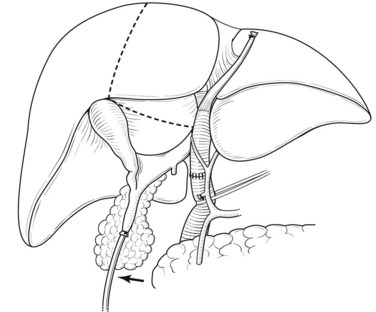
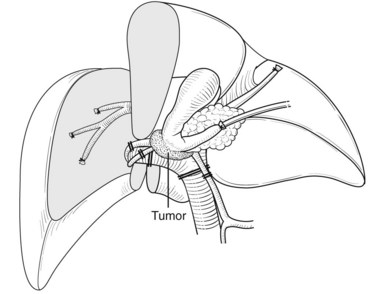
Right hepatectomy with total caudate lobectomy is carried out for tumors involving the right anterior and posterior sectoral bile ducts, with sparing of the left medial segmental bile duct to segment IV, or when the right hepatic artery is also involved (see Chapters 50B and 90B). If the cancer involves the right hepatic duct and the left medial segmental duct, anatomic right hepatic trisectionectomy with caudate lobectomy is indicated (Nagino et al, 2006b).
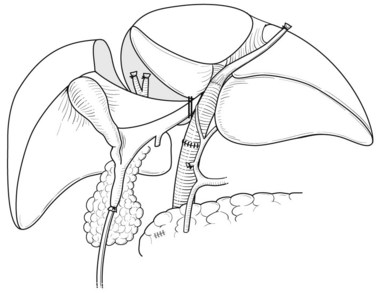
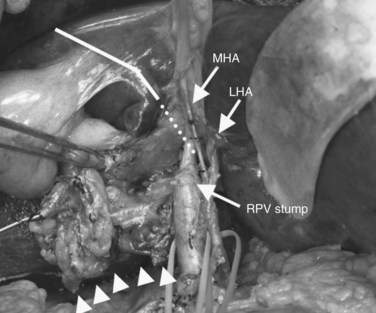
After retropancreatic lymph node dissection, the distal bile duct is divided above the pancreas with a histologic free margin; the hepatoduodenal ligament is dissected, and the vessels are skeletonized and removed (Bhuiya et al, 1992). The middle hepatic artery usually runs ventral to the left portal vein and enters the liver below the left medial segment branch of the portal vein; this artery is carefully preserved. The left hepatic artery enters the liver to the left of the umbilical portion of the portal vein (Figs. 90C.3 to 90C.5).
Following skeletonization of the hepatoduodenal ligament, preparation of the caudate lobe is carried out by isolation, ligation, and division of the arterial branches and portal branches of the caudate lobe. The details of the technique are described above (Figs. 90C.3 to 90C.7; see also Fig. 90C.12). Although the arterial branches directly arise from the left and right hepatic arteries, they cannot be accurately identified in some cases. Two to six branches of the portal vein arise from the left portal vein, two to three branches arise from the right portal vein, and one to two branches arise from the main trunk or the bifurcation of the portal vein.
The significance of total caudate lobectomy in cases of hilar cholangiocarcinoma is, of course, not resection of the parenchyma of the caudate lobe but clearance of the connective tissue of the hilar plate around the caudate bile duct branches, which are often involved by cancer (Nimura et al, 1990). After complete division of the caudate arteries and portal vein branches, the right hepatic lobe is mobilized, and short retrohepatic veins are ligated and divided. The caudate lobe is completely detached from the inferior vena cava (IVC) as described above, and the confluence of the right hepatic vein (RHV) with the IVC is carefully encircled, clamped, divided, and closed with a running suture or a vascular stapler.
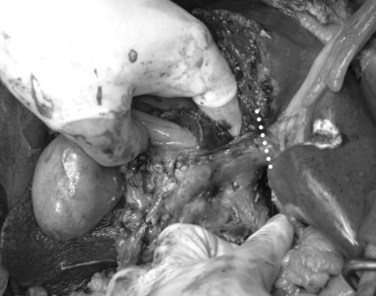
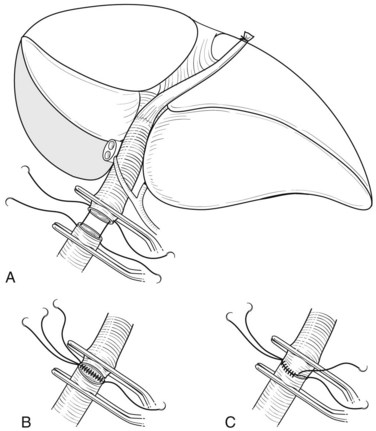
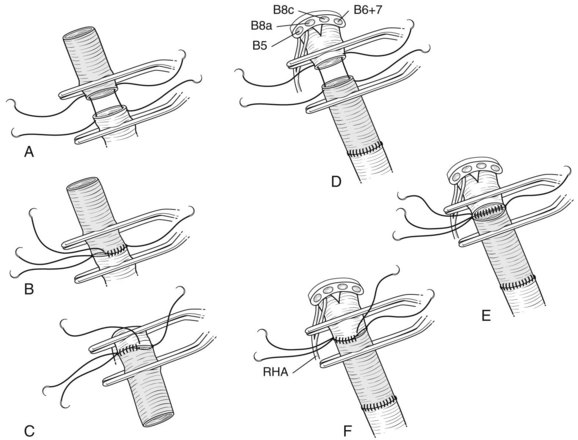
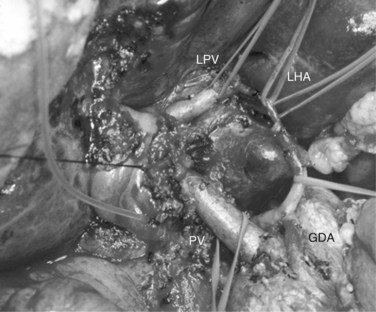
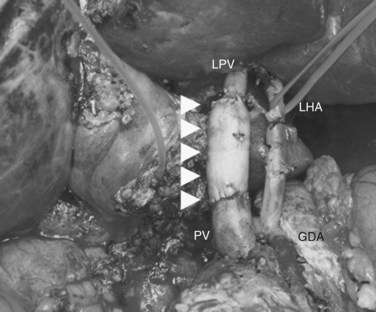
In case of direct cancer invasion of the portal bifurcation, portal vein resection and reconstruction is performed prior to liver resection (Figs. 90C.8 to 90C.12). Direct end-to-end anastomosis of the main trunk of the portal vein and the left branch is carried out (Ebata et al, 2003; Nimura et al, 1991; see Chapter 91B). Portal vein resection and reconstruction is carried out during the hilar dissection prior to hepatic parenchymal transection, because the portal flow to the FLR is easily impeded as a result of kinking during operative manipulations. Direct end-to-end anastomosis between the main portal trunk and the left portal vein is usually possible during right-sided resections (Kondo et al, 2003). On the other hand, interposition of an autogenous vein graft is often required during portal vein reconstruction, between the right portal vein and the main trunk during left-sided resections (Figs. 90C.9 to 90C.15). We usually harvest an external iliac vein for an autogenous vein graft by an extraperitoneal approach. The key to portal vein resection and reconstruction during right-sided hepatectomy is the feasibility of cross clamping the root of the umbilical portion of the left portal vein. In the case of left hepatectomy, isolation and clamping of the right posterior sectoral and/or the right anterior sectoral portal vein are key maneuvers.
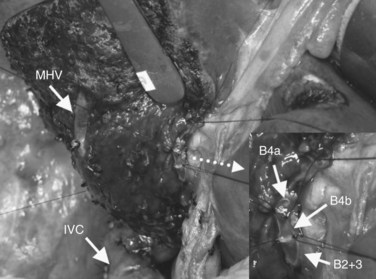
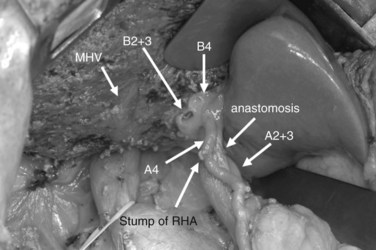
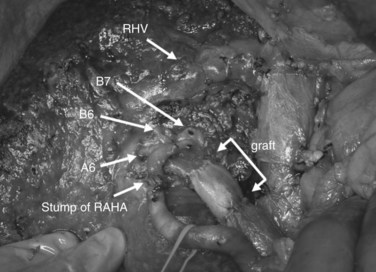
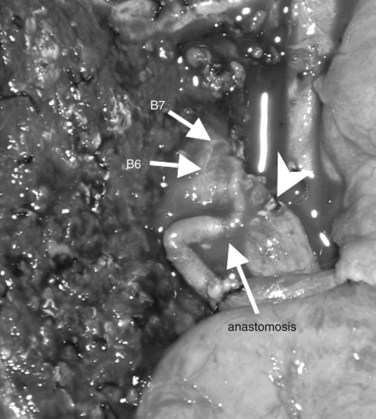
After confirmation of the course of the middle hepatic vein (MHV) using intraoperative ultrasonography, liver transection is performed along the demarcation line that appears after ligation of the right hepatic artery. On the caudal aspect of segment IV, the liver transection line is horizontally advanced approximately 1 cm ventral to the hilar plate to achieve a free surgical margin. When the MHV is exposed, liver transection is reduced toward the left, lateral to the distal portion of the ligamentum venosum. When the caudodorsal aspect of the MHV is completely exposed, the confluence of the MHV and IVC is seen. At this point, the caudate lobe can be turned and pulled ventrally from behind the left lateral segment. Finally, the bile duct is transected after placing a stay suture, and the right hepatic lobe is resected en bloc with the entire caudate lobe, portal bifurcation, and extrahepatic bile duct. The transected bile duct stumps, left medial sectional branch (segment IV), left lateral anterior sectional branch (segment III), and left lateral posterior sectional branch (segment II) are noted just to the right of the umbilical portion of the left portal vein (see Fig. 90C.7).
Bilioenteric continuity is reestablished using a Roux-en-Y jejunal loop. In our fashion, the jejunal limb is brought to the transected hepatic ducts via the retrocolic-antegastric or the retrocolic-retrogastric route (Nagino et al, 2002). We perform hepaticojejunostomy in one layer using a 5-0 absorbable monofilament suture; a biliary drainage tube is placed across all anastomoses either through the jejunal loop and/or transhepatically (Nagino et al, 2007).
Extended Left Hepatic Resection, Caudate Lobectomy, Extrahepatic Bile Duct Resection, and Portal Vein Resection and Reconstruction
Extended left hepatic resection with caudate lobectomy is used for hilar cholangiocarcinoma, which predominantly involves the left intrahepatic bile ducts in continuity with the duct to the right anterior section (see Chapters 50B and 90B). During the subhepatic dissection of the hepatoduodenal ligament, the left hepatic artery and middle hepatic artery, if present, are ligated and divided at their origins, and the right hepatic artery is carefully isolated up to its bifurcation into the anterior and posterior sectoral branches. Then the anterior sectoral branch and caudate lobe branches of the right hepatic artery are ligated and divided. Mobilization of the gallbladder provides a good operative view of the right posterior pedicle. The left portal vein is ligated and divided, followed by division of the right anterior branch of the right portal vein and caudate lobe branches arising from the main portal trunk and right portal vein. If cancer invasion of the portal bifurcation or the right portal vein is observed, combined portal vein resection and reconstruction is usually performed after liver transection for left hepatectomy or before liver transection in some cases of right hepatectomy. The caudate lobe and segment II and III are mobilized to the right, and the short retrohepatic caudate veins are ligated and divided, beginning on the left caudally and progressing cranially. When the confluence of the ligamentum venosum and the left hepatic vein (LHV) or IVC is displayed, the ligament is ligated and divided. The LHV and MHV are divided and closed with a running suture or a vascular stapler. The caudate lobe and the left lobe are then completely detached from the IVC.
Subsequently, the liver is transected along a demarcation line in the right portal scissura between the right anterior and right posterior sections. The course of the RHV is confirmed ultrasonographically. After parenchymal transection, the RHV should be exposed on the raw surface of the liver. The plane of the liver transection courses between the right posterior superior section (segment VII) and the right portion of the caudate lobe and begins from the sulcus on the right side of the caudate process and advances cranially along the right margin of the IVC. Finally, the right wall of the IVC is clearly exposed (Fig. 90C.16; see also Fig. 90C.15).
The right posterior sectoral bile duct is exposed cranially to the right portal vein and is divided so as to obtain histologically free margins. Finally, vascular clamps are placed distally and proximally to the involved portal bifurcation: the distal clamp is placed obliquely to adjust the caliber of the distal portal vein to the transversely transected main portal trunk, and the specimen is resected en bloc, including the entire caudate lobe, the involved segment of the portal vein, and the extrahepatic bile duct. The portal vein reconstruction between the main portal trunk and the right posterior sectoral portal branch is then performed (see Fig. 90C.16), and an interposition graft of autogenous vein is often necessary (see Fig. 90C.15).
In some locally advanced cases, combined resection and reconstruction of the right hepatic artery with an end-to-end anastomosis is a requisite to achieve a negative margin (Fig. 90C.17; see also Fig. 90C.16). Microsurgical techniques are used for arterial reconstruction with or without using right gastroepiploic artery or radial artery as an interposition graft (Sakamoto et al, 2006). When arterial reconstruction is impossible, one potential countermeasure is creation of an arterioportal shunt to prevent possible leakage of the hepaticojejunostomy, liver abscess, and fatal liver failure (Kondo et al, 2004). Oblique-to-side anastomosis is performed between the common hepatic artery and the main portal vein. Three weeks after surgery, transcatheter arterial embolization of the common hepatic artery is carried out to prevent further portal hypertension. It must be remembered, however, that the development of liver failure or an abscess in the remnant liver are not eliminated by arterioportal shunting.
Bhuiya MR, et al. Clinicopathological studies on perineural invasion of bile duct carcinoma. Ann Surg. 1992;215:344-349.
Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170-178.
Ebata T, et al. Pathological appraisal of lines of resection for bile duct carcinoma. Br J Surg. 2002;89:1260-1267.
Ebata T, et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720-727.
Igami T, et al. Clinicopathologic study of cholangiocarcinoma with superficial spread. Ann Surg. 2009;249:296-302.
Itoi T, et al. Detection of telomerase activity in biopsy specimens for diagnosis of biliary tract cancers. Gastrointest Endosc. 2000;52:380-386.
Itoi T, et al. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest Endosc. 2007;66:730-736.
Kamiya J, et al. Preoperative cholangiography of the caudate lobe: surgical anatomy and staging for biliary carcinoma. J Hepatobiliary Pancreat Surg. 1994;4:385-389.
Kamiya S, et al. The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity, and microflora. Ann Surg. 2004;239:510-517.
Kanai M, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498-504.
Kanazawa H, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104-113.
Kondo S, et al. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br J Surg. 2003;90:694-697.
Kondo S, et al. Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br J Surg. 2004;91:248-251.
Nagino M, Nimura Y, Hayakawa N. Percutaneous transhepatic portal embolization using newly devised catheters: preliminary report. World J Surg. 1993;17:520-524.
Nagino M, et al. Percutaneous transhepatic biliary drainage in patients with malignant biliary obstruction of the hepatic confluence. Hepatogastroenterology. 1992;39:296-300.
Nagino M, et al. Preoperative management of hilar cholangiocarcinoma. J Hepatobiliary Pancreatic Surg. 1995;2:215-223.
Nagino M, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21:434-439.
Nagino M, et al. Right or left trisegment portal vein embolization before hepatic trisectionectomy for hilar bile duct carcinoma. Surgery. 1995;117:677-681.
Nagino M, et al. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology. 1996;200:559-563.
Nagino M, et al. Segmental liver resections for hilar cholangiocarcinoma. Hepatogastroenterology. 1998;45:7-13.
Nagino M, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155-160.
Nagino M, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25:1277-1283.
Nagino M, et al. Hepaticojejunostomy using Roux-en-Y jejunal limb via retrocolic-retrogastric route. Langenbecks Arch Surg. 2002;387:188-189.
Nagino M, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372.
Nagino M, et al. “Anatomic” right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243:28-32.
Nagino M, et al. Intrahepatic cholangiojejunostomy following hepatobiliary resection. Br J Surg. 2007;94:70-77.
Nimura Y. Staging of biliary carcinoma: cholangiography and cholangioscopy. Endoscopy. 1993;25:76-80.
Nimura Y. Hepatectomy for proximal bile duct cancer. In: Braasch, JW, Tompkins, RK. Surgical Disease of the Biliary Tract and Pancreas. St Louis: Mosby–Year Book; 1994:251-261.
Nimura Y. Surgical anatomy of the biliary ducts. In: Rossi, P, Bezzi, M. Medical Radiology Biliary Tract Radiology. Berlin: Springer-Verlag; 1997:21-30.
Nimura Y, et al. Value of percutaneous transhepatic cholangioscopy (PTCS). Surgical Endoscopy. 1988;2:213-219.
Nimura Y, et al. Cholangioscopic differentiation of biliary stricture and polyps. Endoscopy. 1989;21:351-356.
Nimura Y, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:533-544.
Nimura Y, et al. Combined portal vein and liver resection for carcinoma of the biliary tract. Br J Surg. 1991;78:727-731.
Nimura Y, et al. Technique of inserting multiple biliary drains and management. Hepatogastroenterology. 1995;42:323-331.
Nimura Y, et al. Hilar cholangiocarcinoma: surgical anatomy and curative resection. J Hepatobiliary Pancreat Surg. 1995;2:239-248.
Nimura Y, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155-162.
Nishio H, et al. Value of percutaneous transhepatic portography before hepatectomy for hilar cholangiocarcinoma. Br J Surg. 1999;86:1415-1421.
Nishio H, et al. Most informative projection for portography: quantitative analysis of 47 percutaneous transhepatic portograms. World J Surg. 2003;27:433-436.
Sakamoto E, et al. Clinicopathological studies of mucin-producing cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 1997;4:157-162.
Sakamoto E, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma. Ann Surg. 1998;227:405-411.
Sakamoto Y, et al. Clinical significance of reconstruction of the right hepatic artery for biliary malignancy. Langenbecks Arch Surg. 2006;391:203-208.
Sano T, et al. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240-247.
Senda Y, et al. Value of multidetector row CT in the assessment of longitudinal extension of cholangiocarcinoma: correlation between MDCT and microscopic findings. World J Surg. 2009;33:1459-1467.
Sugawara G, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706-714.
Sugiura T, et al. Value of multidetector-row computed tomography in diagnosis of portal vein invasion by perihilar cholangiocarcinoma. World J Surg. 2008;32:1478-1484.
Takada T, et al. Percutaneous transhepatic cholangial drainage: direct approach under fluoroscopic control. J Surg Oncol. 1976;8:83-97.
Uesaka K, Nimura Y, Nagino M. Changes in hepatic lobar function after right portal vein embolization. Ann Surg. 1996;223:77-83.