CHAPTER 59 EXSANGUINATION: RELIABLE MODELS TO INDICATE DAMAGE CONTROL
Exsanguination has been defined as an extreme form of hemorrhage with ongoing bleeding that, if not surgically controlled, will lead to death. Therefore, the speed by which the exsanguinating trauma patient moves from the prehospital, emergency department, operating room, and intensive care unit is important to survival. Certain conditions and complexes of injuries require damage control to prevent exsanguination. This chapter will describe validated indicators that can be used both preoperatively and intraoperatively to improve outcomes. This chapter will also outline current guidelines for the institution of damage control in trauma patients. Emphasis is placed on the current indications for damage control as defined by key studies. Awareness of these guidelines can improve outcomes after major intra-abdominal injuries and hemorrhage and also assist in the management of one of the well-known sequelae of damage control, the post-traumatic open abdomen.
HISTORY
In 1983, Stone was first to describe the “bailout” approach of staged surgical procedures for severely injured patients. This approach emerged after his observation that early death following trauma was associated with severe metabolic and physiologic derangements following severe exsanguinating injuries. Following massive transfusion exceeding two blood volumes in trauma and emergency surgery, severe physiologic derangement ensued and mortality was found to be greater than 60%. Profound shock along with major blood loss initiates the cycle of hypothermia, acidosis, and coagulopathy. It was at this time that hypothermia, acidosis, and coagulopathy were described as the “trauma triangle of death” or the “bloody vicious cycle.” A fourth component, dysrhythmia, which usually heralded the patient’s death, was later added by Asensio. Coagulopathy, acidosis, and hypothermia make the prolonged and definitive operative management of trauma patients dangerous. This approach, now called “damage control,” describes it as multiphasic, where reoperation occurs after correcting physiologic abnormalities.
MODELS FOR DAMAGE CONTROL
To define the patient at greatest risk for exsanguination and death, one must determine the threshold levels of pH, temperature, and highest estimated level of blood loss. Therefore, in an attempt to institute the development of intraoperative guidelines for “damage control/bailout,” Asensio et al. first retrospectively evaluated 548 patients over 6 years who were admitted to a large urban trauma center with the diagnosis of exsanguination. Inclusion criteria were intraoperative blood loss of 2000 ml or more, minimum transfusion requirement of 1500 ml pRBCs or greater during the initial resuscitation, and diagnosis of exsanguination. Data collected included demographics, prehospital and admission vital signs and physiologic predictors of outcome, Revised Trauma Score (RTS), Glasgow Coma Scale (GCS), Injury Severity Score (ISS), volume of resuscitative fluids, need for thoracotomy in the emergency department (EDT), volume of fluids in the operating room, need for thoracotomy in the operating room (ORT), and intraoperative complications. In this patient population, the Revised Trauma Score was 4.38 and the mean ISS was 32, denoting a physiologically compromised and severely injured patient population. There were 180 patients that underwent EDT with aortic cross-clamping, open CPR, 99 (55%) succumbed in the emergency department. In addition to the 81 patients that survived EDT, 117 required ORT for a total of 198 EDT and ORT, of which 56 (28%) survived to leave the operating room and the hospital. In this series, mean admission pH was 7.15, mean temperature was 34.3° C in the operating room, and these patients received an average of 14,165 ml of crystalloid, blood, and blood products. Overall, 449 patients survived to arrive in the OR with some signs of life and 281 patients died; 37% of these patients survived damage control. Table 1 shows the objective intraoperative parameters developed to predict outcome and provide guidelines on when to institute damage control based on these findings. This series also provided independent risk factors for survival, which included an injury severity score less than 20, spontaneous ventilation in the emergency room, no EDT or ORT, and the absence of abdominal vascular injury.
Table 1 Physiologic Guidelines That Predict Need for Damage Control
One of the natural sequelae in patients surviving damage control is an open abdomen. These guidelines were prospectively validated in a series of 139 patients who underwent damage control and had post-traumatic open abdomen. This study consisted of two groups of patients: 86 patients studied retrospectively prior to instituting the guidelines, and 53 patients studied prospectively after instituting the guidelines. The groups were comparable in all relevant parameters. Although there was no difference in the mortality rate between the two groups (24% for each), there were statistically significant differences in the number of intraoperative transfusions, less hypothermia and bowel edema, less postoperative infections and gastrointestinal complications, and shorter intensive care unit and hospital length of stay for the prospective group. Another significant finding in this study was that 93% of patients were able to undergo definitive abdominal closure in their hospital stay as compared with the historic 22%.
PATIENT SELECTION
Not all trauma patients require damage control measures. In addition to the physiologic guidelines for the institution of damage control (see Table 1), certain conditions and complexes of injuries assessed both preoperatively and intraoperatively require damage control (Table 2). Multiple mass casualties and the need for EDT predict the need for damage control. In the multiply injured trauma patient sustaining major abdominal injury, the need to evaluate for other extra-abdominal injuries in a timely fashion may also indicate damage control. The preoperative duration of hypotension (systolic blood pressure <90 mm Hg) was significantly different in those patients that exsanguinated as compared with survivors (45 minutes vs. 85 minutes). Therefore, in addition to other factors such as the preoperative assessment of hypothermia and coagulopathy, a period of sustained hypotension greater than 60 minutes would predict the need for damage control. Intraoperatively, certain complexes of injuries also predict the need for this technique. These injuries include major abdominal vascular, complex hepatic, major thoracic vascular injuries, and the need for intraoperative thoracotomy.
Table 2 Preoperative and Intraoperative States That Suggest Need for Damage Control
| Preoperative | Intraoperative |
|---|---|
| Multiple mass casualties | Need for intraoperative thoracotomy |
| Multisystem trauma with major abdominal injury | Major abdominal vascular injuries |
| Open pelvic fracture with major abdominal injury | Major thoracic vascular injuries |
| Major abdominal injury with need to evaluate extra-abdominal injury | Severe complex hepatic injuries |
| Traumatic amputation with major abdominal injury | Presence of sustained hypotension |
| Need for emergency department thoracotomy | Presence of coagulopathy |
| Presence of sustained hypotension | Presence of hypothermia |
| Presence of coagulopathy | |
| Presence of hypothermia | |
| Need for adjunctive use of angio-embolization |
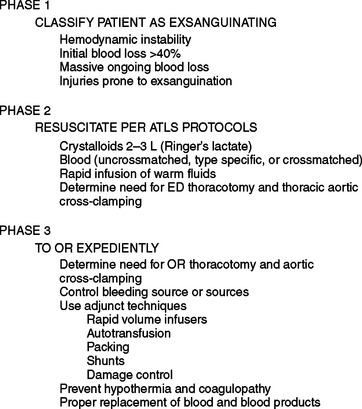
Patients with exsanguination are perhaps the best candidates to undergo damage control. Asensio has described an algorithm for the management of exsanguination that involves three phases (Figure 1). First, the patient is classified as exsanguinating; and second, resuscitation under the Advanced Trauma Life Support protocols is begun (see Figure 1). The third phase comprises rapid transport to the operating room (exsanguination from penetrating injuries is a dramatic ill-defined entity that requires leadership, fast thinking, aggressive surgical intervention, and a well-thought-out plan). Rapid damage control can lead to effective management of exsanguination and improve survival.
TECHNIQUE OF DAMAGE CONTROL
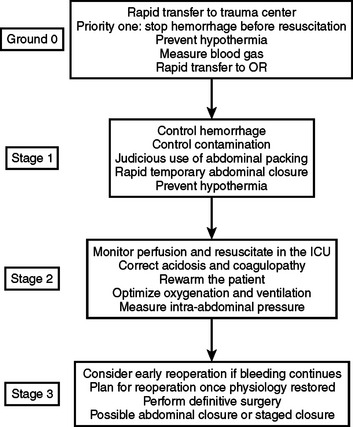
The most important goal of early institution of damage control is patient survival. A four-stage damage control approach has been recently defined by Johnson in a study of 24 patients who underwent damage control and were retrospectively compared with patients who underwent damage control a decade earlier (Figure 2). The “ground-zero” stage includes the prehospital phase as well as early resuscitation in the emergency room. This ground-zero phase includes short paramedic scene times and identification of injury patterns in the ED that require damage control, as well as rewarming maneuvers that begin in the trauma bay.
Aoki N, Wall M, Demsar J, et al. Predictive model for survival at the conclusion of damage control laparotomy. Am J Surg. 2001;180:540-545.
Asensio JA, Britt LD, Borzotta A, et al. Multiinstitutional experience with the management of superior mesenteric artery injuries. J Am Coll Surg. 2001;193:354-365.
Asensio JA, McDuffie L, Petrone P, et al. Reliable variables in the exsanguinated patient which indicate damage control and predict outcome. Am J Surg. 2001;182:743-751.
Asensio JA, Petrone P, O’Shanahan G, Kuncir EJ. Managing exsanguination: what we know about damage control/bailout is not enough. BUMC Proc. 2003;16:294-296.
Asensio JA, Petrone P, Roldan G, et al. Has evolution in awareness of guidelines for institution of damage control improved outcome in the management of the posttraumatic open abdomen? Arch Surg. 2004;139:209-214.
Burch JM, Ortiz VB, Richardson RJ, et al. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg. 1992;215:476-483.
Cosgriff N, Moore EE, Sauaia A, et al. Predicting life threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857-861.
Johnson JW, Gracias VH, Schwab CW, et al. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51:261-269.
Moore EE. Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am J Surg. 1996;172:405-410.
Morris JAJr, Eddy VA, Blinman TA, Rutherford EJ, Sharp KW. The staged celiotomy for trauma: issues in unpacking and reconstruction. Ann Surg. 1993;217:576-584.
Phillips TF, Soulier G, Wilson RF. Outcome of massive transfusion exceeding two blood volumes in trauma and emergency surgery. J Trauma. 1987;27:903-910.
Rotondo MF, Schwab CW, McGonigal MD, et al. “Damage control”: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375-382.
Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532-535.