CHAPTER 63 Experimental Meningioma Models
CELL LINES AND ANIMAL MODELS
Meningiomas arise from the meningothelial (arachnoidal cap) cells. They constitute approximately 20% of all intracranial tumors. About 85% of meningiomas are benign (grade I), while 10% are atypical (grade II), and 3% to 5% are malignant (anaplastic, grade III). Tumor models are critical to understand the function of genetic alterations in cancer, to evaluate their contribution to tumor initiation and progression, and to assess the toxicity and therapeutic efficacy of established and newly developed treatments. A major obstacle to research in meningioma biology and further therapeutic development has been the limited availability of long-term cell lines and animal models, which recapitulate the human clinical scenario. There has been and remains a continuing challenge to establish and maintain benign meningioma cell lines that retain the original phenotype both in vitro and in vivo similar to the original primary tumor specimen. An additional hindrance has been a lack of animal models with sufficiently high frequencies of spontaneous meningioma development and difficulties in implanting intracranial dural-based tumors to accurately model the human condition. These issues are further compounded by the lack of meningeal specific promoters. The LTAg2B was the first human leptomeningeal cell line available, although immortalization by transfection with an SV40 large T antigen gene construct under the control of the Rous sarcoma virus promoter. This cell line (LTAg2B) maintained the morphology of primary cultures and stained positive by immunohistochemistry for arachnoid-specific markers at early passages.1 The loss of intermediate filament staining in later passages may reflect a change in the phenotype of the cells owing to their high proliferative rate.
There are a few human and rat meningioma cell lines that have been established from malignant meningiomas.2–4 These cell lines have been derived from aggressive variants of meningioma, which represent only a small portion of meningiomas. There is a critical need to have additional cell lines that span the entire spectrum of meningioma histologic grades.
Most animal models have been developed by implanting human meningioma cells derived from a primary tumor subcutaneously into athymic mice. Grade I meningiomas grow very slowly with a large amount of variability in this environment.5 Recently, a novel genetic model was devised utilizing Cre recombinase technology to specifically inactivate NF2 in arachnoidal cells, resulting in the formation of intracranial meningothelial hyperplasia and meningiomas in roughly 30% of the mice.6 This powerful new technology significantly improves on prior models and may open avenues of investigation never before possible in meningioma research. This model is discussed in the latter part of this chapter.
CELL CULTURE
Cell culture provides a tool that is reliable, convenient, and, most important, reproducible for investigations into the biology of human cancers. A valid meningioma culture needs to fulfill the following requirements: (1) It must be composed of meningioma cells; (2) the growth rate of the cells should be predictable, reliable, and reproducible; and (3) the therapeutic response of the cells must be similar to that of human meningiomas. Studies clearly show that early passage cell lines (3–5) derived from benign human meningiomas undergo cellular senescence after only a few passages.7–12 Therefore any type of biological studies must be done quickly with the limited number of available cells. Usually the studies conducted on these cells are not reproducible because the cells cannot be frozen and then thawed; thus not allowing studies to be repeated in different laboratories under varied conditions. To overcome this problem, three separate studies have used hTERT to immortalize cells.
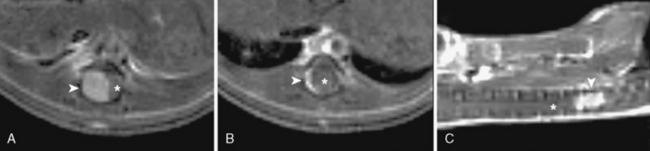
hTERT and Meningioma Cell Immortalization
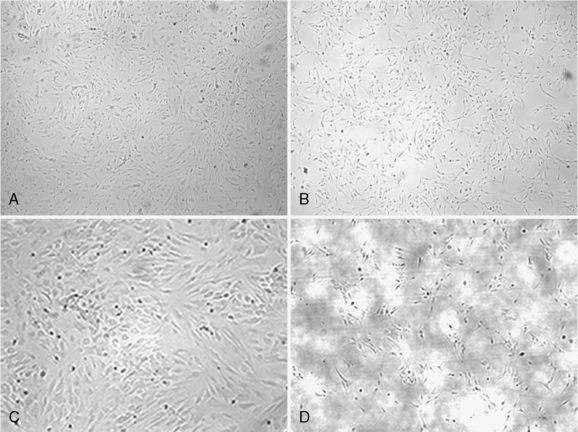
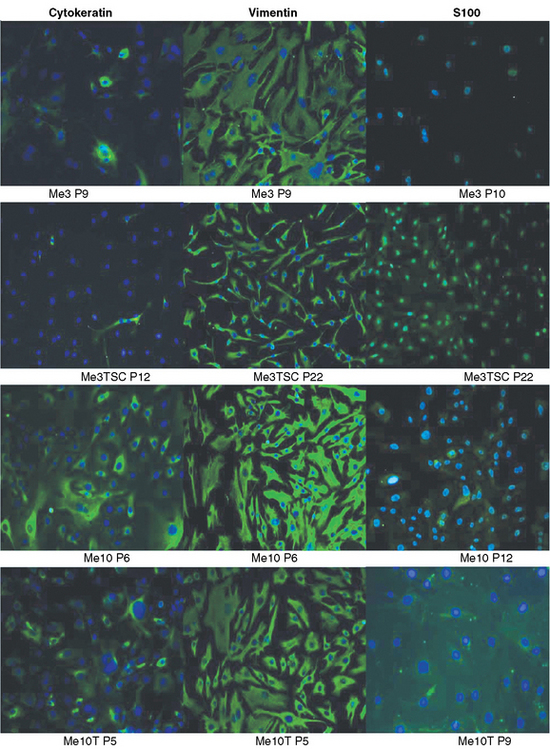
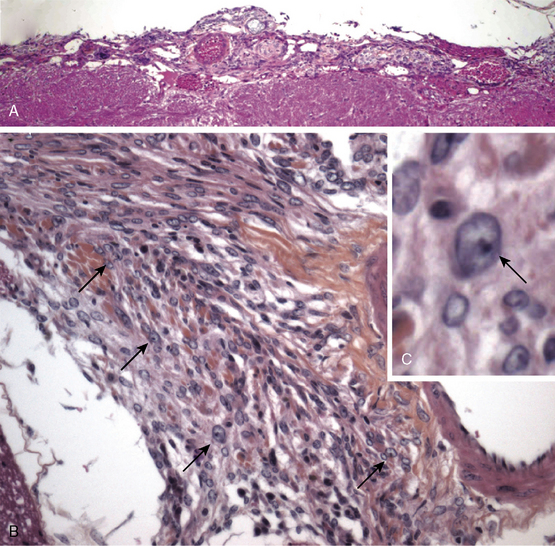
The major reason for the difficulty in generating established cell lines from benign meningiomas is when the cells from these tumors are put into culture they senesce. Many pathways are involved in cellular senescence and disruption of these pathways is required for continued cell growth and immortalization.9 Low or absent telomerase activity is in part one of the reasons for senescence; progressive shortening of telomerase occurs with each subsequent cell cycle. Telomeres are repetitive DNA sequences at the ends of chromosomal DNA. Telomerase is a ribonucleoprotein polymerase enzyme, composed of a integral RNA component (hTR) and several other proteins, including a reverse transcriptase subunit (hTERT). Telomerase activity is thought to be essential for maintaining chromosome ends by adding repetitive TTAGGG sequences to the telomeres; it is linked to cellular immortality.13 Cell cycle checkpoint pathways also control cellular senescence, and successful cell immortalization may also involve disruption of the p53 and/or Rb pathways.14 In meningiomas studies have shown that telomerase activity is directly related to tumor malignancy.7,8,15 Benign tumors have low or absent telomerase activity; whereas, the majority of malignant tumors are positive. In addition, hTERT-positive meningiomas have a higher incidence of recurrence, suggesting it use a potential predictor for recurrence.16 There are three reports, including one from our group, establishing immortalized meningioma cell lines derived from grade I tumors by transduction with hTERT, the catalytic subunit of telomerase.17–19 After immortalization, the morphologic features of the cell lines remained the same as the corresponding nontransduced cells (Fig. 63-1). On seeding of the primary cells, large nuclei and tight cell junctions were observed. Cells remained positive for cytokeratin, vimentin, and S-100 by immunohistochemistry (Fig. 63-2). There is some controversy as to whether transduction with hTERT alone is sufficient for immortalization of benign meningiomas. Baia and colleagues17report that immortalization of two benign meningiomas cell lines required disruption of the p53 and pRb pathways in addition to the expression of hTERT. In contrast, Puttmann and colleagues and Cargioli and colleagues18,19 reported that transduction with hTERT alone is sufficient for immortalization of benign meningioma. This most likely reflects the genetic alterations in the original tumors from which these cell lines were derived. In addition, the doubling time of the cells transduced with both SV40 large T antigen and hTERT was shorter than the cells transduced with hTERT only. Transplantation of the hTERT-immortalized cells subdurally into nude mice resulted in tumors with typical histologic features of meningioma. Both macroscopically and microscopically, the tumors showed features of meningioma (Fig. 63-3A, B). Grossly, the tumors were well-demarcated, firm, nodular masses of tan to white tissue adherent to the dura and leptomeninges on the brain surface. Microscopically, the tumor tissue was densely cellular and was composed of spindled to epithelioid cells with monomorphic round to oval nuclei and moderate amounts of cytoplasm. The tumors showed growth patterns that varied from small nodular meningothelial-like clusters to more fascicular spindled growth. Focal whorls and microcalcifications were present in some tumors. Although nuclei displayed some pleomorphism and had visible nucleoli, other atypical features such as small cells with a high nuclear-to-cytoplasmic ratio, brisk mitotic activity, and sheet-like growth were not observed. One tumor showed focal areas of nuclear condensation and fragmentation consistent with necrosis. Immunohistochemically, the tumors showed weak positive staining for EMA and focal weak staining for S-100 (Fig. 64-3C, D). It should be noted that the histopathologic examination revealed that the cell line transduced with both SV40 large T antigen and hTERT had some atypical changes when compared with the cells transduced with hTERT only. In conclusion, these studies show that genetic manipulation can be used to overcome the senescence of primary benign meningiomas and the cell lines generated can serve as useful model systems for biological studies and evaluation of novel therapies on meningioma.
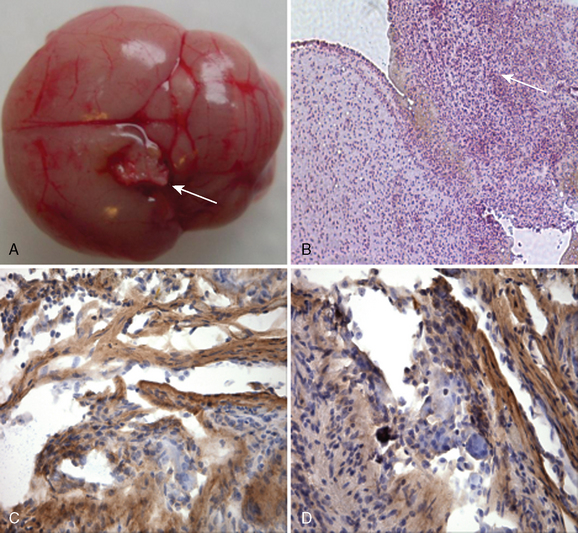
FIGURE 63-3 A, Photograph showing a grossly visible tumor attached to the cortex in the posterior parietal region of the left hemisphere. The tumor is well demarcated, firm, and white, and conforms macroscopically to meningioma characteristics observed in humans. B, Photomicrograph showing tumor tissue that is densely cellular and composed of spindled to epithelioid cells with monomorphic round to oval nuclei and moderate amounts of cytoplasm. The tumor volume was 2.03 mm3 (hematoxylin and eosin; original magnification, ×200). C, Photomicrograph showing that the tumor is weakly positive for EMA; original magnification, ×400. D, Photomicrograph showing that the tumor is weakly positive for S-100; original magnification, ×400.
(From Cargioli TG, Ugur HC, Ramakrishna N, et al. Establishment of an in vivo meningioma model with hTERT. Neurosurgery 2007;60:750–60.)
MALIGNANT MENINGIOMA CELL LINES
There have been several long-term cell lines, which have been established from malignant meningiomas.20 The most commonly used line was initiated from a tumor of a 61–year-old man with repeated recurrent primary intraosseous malignant meningioma of the skull.2 The doubling time of cultured cells was reported to be 62 hours. The mesenchymal tumor marker vimentin was identified by immunohistochemistry. Tumorigenicity in athymic nude mice was observed; with a tumor doubling time of 5 days. The ki-67 labeling index of cultured cells and xeonografted tumors was approximately 36% and 30%, respectively. The tumor retained its ultrastructural features including whorl formation patterns in vivo. This line has been useful to test the therapeutic efficacy of antitumor agents. An additional cell line, KT21-MG, has been established from a human malignant meningioma with amplified c-myc oncogene.3 Ultrastructurally, interdigitation of adjacent cell membranes with desmosomes was seen in the cultured cells that were observed in the original tumor. Immunohistochemical analysis showed the cells were positive for vimentin. The cell line was able to produce tumors in nude mice, proliferate under low serum conditions, anchorage independent growth in soft agar and many chromosomal abnormalities including monosomy of chromosome 22. Further, c-myc was found to be amplified in the original tumor and xenografts.
GENE EXPRESSION PROFILING: TISSUE VERSUS CELL CULTURE
Well established cell lines are commonly used for cell biology studies of cancer. As mentioned earlier, there are very few established cell lines for meningiomas. Most in vitro studies have been performed on primary meningioma cultures of the tumors. Studies in malignant tumors suggest the gene expression profiles of cultured tumor cells are distinct from the corresponding tumor.21 In addition, they are affected by culture conditions and number of passages. Sasaki and colleagues22 performed gene expression profiling using GeneChip on original frozen tumor specimens and primary cultures established from the corresponding tumors at two time points from 3 meningiomas (one meningioma of each World Health Organization Grade [WHO] Grades I, II, and III). Among the 12,000 genes examined, 51 genes were up regulated by fivefold or more and 19 genes were down-regulated by twofold or more in primary cultures compared with corresponding frozen tissue. The expression levels of a subset of these genes were confirmed by quantitative real-time transcription polymerase chain reaction. Increased expression of genes for extracellular matrix proteins, cytoskeletal proteins, and cell surface markers were noted in the primary cultures. This study does have several limitations including the small sample size; the original tumor tissue contains fibroblasts, endothelial cell, leukocytes and other cell types, which may confound the results. This article does serve as a warning that cell culture genetic studies may not accurately reflect the genetic milieu of a tumor in vivo. Therefore treatment studies in vitro may not directly correlate with treatment effect in vivo.
MATRIGEL AND XENOGRAFT AUGMENTATION
An in vivo model of meningioma cell growth is essential for a better understanding of meningioma biology and as a model for testing the efficacy of novel therapeutics before initiating clinical trials. The first transplantation of human meningiomas was described in 1945.23 In these studies, tumors were injected into the eyes of guinea pigs. Successful subcutaneous implantation of human malignant meningioma was first reported in 1977.24 This was followed by a study that reported the successful serial passage of transplanted meningioma that remained histologically unchanged. Implantation of meningioma cells in the subrenal capsule of nude mice has the most successful tumor take.25 The subrenal capsule is a very different microenvironment than the brain and does not allow for serial tumor measurements. Matrigel has enhanced the growth of a number of cell lines in vivo; it is a substance derived from a mouse sarcoma and is a mixture of basement membrane proteins and growth factors. A high tumor take has been demonstrated by subcutaneous meningioma cells mixed with Matrigel at the time of injection. The histology of the xenografted tumor closely resembled that of the original human tumor.26 By immunohistochemistry, the tumors were positive for human fibronectin and collagen IV and negative for human or mouse laminin. About half the tumors were immunohistochemically positive for epithelial membrane antigen. The reason for the enhancement of the growth of the cells in the presence of Matrigel may be the enhancement of growth of the tumor cells and the concentration of cellular and circulating growth factors. Thus far, the majority of preclinical trials have been conducted in xenograft mouse meningioma model.27 Recently, a mouse model of intracranial meningioma that use the novel nonivasive bioluminescent imaging system has been developed.28,29 However, whereas xenograft models are straightforward and relatively easy to use, their accuracy in predicting the efficacy of an anticancer agent in patients is questionable. This may be secondary to cell line selection, the environment or location of the xenograft transplant, or the lack of de novo tumor development.
MOUSE MODELING IN MENINGIOMA PRECLINICAL AND TRANSLATIONAL RESEARCH
Individuals with neurofibromatosis type 2 (NF2) are at significantly elevated risk for developing meningiomas,30 suggesting that the NF2 gene might play a central role in regulating arachnoidal cell proliferation as a tumor suppressor gene. Biallelic inactivation of the NF2 gene has been identified in 30% to 70% of sporadic meningiomas, leading to loss of expression of the NF2 gene product, merlin.31 In addition, NF2 inactivation is likely an early event in sporadic meningioma pathogenesis and is observed as frequently in grade I meningiomas as it is in high-grade tumors.32
Although cancer prone, heterozygous traditional Nf2 knock-out mice (Nf2+/-) do not develop meningiomas, but rather die with osteosarcomas and other tumor types not found in humans with NF2. Nf2-/- mice die early during embryonic development of a failure to initiate gastrulation.33,34
To examine the consequences of Nf2 loss in selected cell types of adult mice, a conditional knock-out allele (Nf2flox2) has been generated. It was clearly demonstrated that conditional biallelic Nf2 inactivation is a rate-limiting step in murine Schwann cell tumorigenesis by using the P0 promoter to specifically express the Cre recombinase in Schwann cells.34 Remarkably, meningioma was not observed in these mice, suggesting that Cre recombinase expressed from a Schwann cell-specific promoter does not affect meningioma progenitor cells.
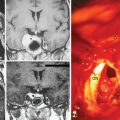
Because of the lack of arachnoidal cell specific promoter, an alternative approach was used for delivery of Cre recombinase by direct injection of a recombinant Cre adenovirus.6 This approach also had the advantage of targeting the initiating genetic lesion (in this case, homozygous inactivation of Nf2) to a small population of susceptible arachnoidal cells, which is likely to model human cancer more accurately than when all of the cells in a targeted tissue are mutated. Direct injection of recombinant Cre adenovirus into the cerebrospinal fluid (CSF) circulation in Nf2flox2 pups allowed targeting of leptomeninges. Beginning at four months of age, 30% of mice with arachnoidal cell Cre-mediated excision of Nf2 exon 2 developed a range of benign meningiomas subtypes histologically similar to the human tumors: transitional, meningothelial and fibroblastic6 (Fig. 63-4). In addition, similar to human patients in whom histologically benign meningiomas may invade brain, mouse meningiomas showed a similar pattern with tumors cells infiltrating the adjacent cerebral parenchyma causing reactive astrogliosis. Because of adenovirus-Cre diffusion through the CSF circulation, spinal meningiomas with spinal cord compression were also observed.
So far, only few of the individual genes affected by the chromosomal alterations found in atypical and malignant meningiomas are known. Deletion of the p16INK4a/p14ARF locus or monosomy of chromosome 9 was found in the majority of higher-grade meningiomas.32,35
In the mouse Nf2 meningioma model additional nullizygosity for p16Ink4a increased the frequency of meningioma and meningothelial proliferation without modifying the tumor grade,36 suggesting that loss of the p14Arf rather than p16Ink4a component of the locus is critical for malignant transformation of meningiomas.
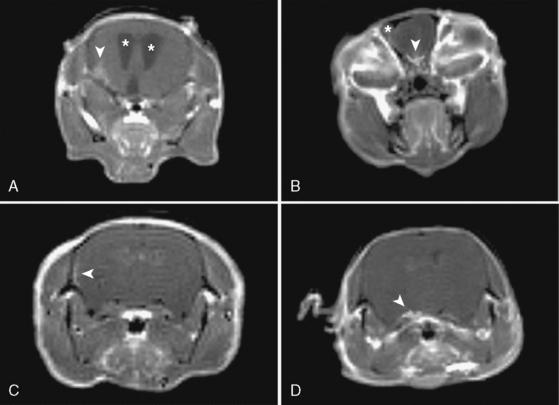
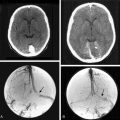
In addition, magnetic resonance imaging (MRI) can be used to screen for meningothelial proliferation and meningioma development in mice, a crucial step in the translation in this mouse meningiomas model with low tumor penetrance and relatively slow growth rate into a preclinical model (Figs. 63-5 and 63-6). Monitoring meningioma growth by MRI opens the way to future studies in which therapeutic interventions can be tested as preclinical assessment of the potential for clinical application.
[1] Murphy M., Chen J.N., George D.L. Establishment and characterization of a human leptomeningeal cell line. J Neurosci Res. 1991;30:475-483.
[2] Lee W.H. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery. 1990;27:389-395. discussion 396
[3] Tanaka K., Sato C., Maeda Y., et al. Establishment of a human malignant meningioma cell line with amplified c-myc oncogene. Cancer. 1989;64:2243-2249.
[4] Tsujino K., Yamate J., Tsukamoto Y., et al. Establishment and characterization of cell lines derived from a transplantable rat malignant meningioma: morphological heterogeneity and production of nerve growth factor. Acta Neuropathol (Berl). 1997;93:461-470.
[5] McCutcheon I.E., Friend K.E., Gerdes T.M., et al. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg. 2000;92:306-314.
[6] Kalamarides M., Niwa-Kawakita M., Leblois H., et al. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev. 2002;16:1060-1065.
[7] Langford L.A., Piatyszek M.A., Xu R., et al. Telomerase activity in ordinary meningiomas predicts poor outcome. Hum Pathol. 1997;28:416-420.
[8] Leuraud P., Dezamis E., Aguirre-Cruz L., et al. Prognostic value of allelic losses and telomerase activity in meningiomas. J Neurosurg. 2004;100:303-309.
[9] Sasaki M., Honda T., Yamada H., et al. Evidence for multiple pathways to cellular senescence. Cancer Res. 1994;54:6090-6093.
[10] Dirven C.M., Grill J., Lamfers M.L., et al. Gene therapy for meningioma: improved gene delivery with targeted adenoviruses. J Neurosurg. 2002;97:441-449.
[11] Ikeda K., Saeki Y., Gonzalez-Agosti C., et al. Inhibition of NF2–negative and NF2–positive primary human meningioma cell proliferation by overexpression of merlin due to vector-mediated gene transfer. J Neurosurg. 1999;91:85-92.
[12] Shu J., Lee J.H., Harwalkar J.A., et al. Adenovirus-mediated gene transfer of dominant negative Ha-Ras inhibits proliferation of primary meningioma cells. Neurosurgery. 1999;44:579-587. discussion 587–8
[13] Weinrich S.L., Pruzan R., Ma L., et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498-502.
[14] Campisi J., d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729-740.
[15] Boldrini L., Pistolesi S., Gisfredi S., et al. Telomerase in intracranial meningiomas. Int J Mol Med. 2003;12:943-947.
[16] Maes L., Lippens E., Kalala J.P., de Ridder L. The hTERT-protein and Ki-67 labelling index in recurrent and non-recurrent meningiomas. Cell Prolif. 2005;38:3-12.
[17] Baia G.S., Slocum A.L., Hyer J.D., et al. A genetic strategy to overcome the senescence of primary meningioma cell cultures. J Neurooncol. 2006;78:113-121.
[18] Cargioli T.G., Ugur H.C., Ramakrishna N., et al. Establishment of an in vivo meningioma model with human telomerase reverse transcriptase. Neurosurgery. 2007;60:750-759. discussion 759–60
[19] Puttmann S., Senner V., Braune S., et al. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest. 2005;85:1163-1171.
[20] Yazaki T., Manz H.J., Rabkin S.D., Martuza R.L. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55:4752-4756.
[21] Hess K.R., Fuller G.N., Rhee C.H., Zhang W. Statistical pattern analysis of gene expression profiles for glioblastoma tissues and cell lines. Int J Mol Med. 2001;8:183-188.
[22] Sasaki T., Hankins G.R., Helm G.A. Comparison of gene expression profiles between frozen original meningiomas and primary cultures of the meningiomas by GeneChip. Neurosurgery. 2003;52:892-898. discussion 898–9
[23] Greene H.S.N., Arnold H. The homologous and heterologous transplantation of brain and brain tumors. J Neurosurg. 1945;2:315-329.
[24] Rana M.W., Pinkerton H., Thornton H., Nagy D. Heterotransplantation of human glioblastoma multiforme and meningioma to nude mice. Proc Soc Exp Biol Med. 1977;155:85-88.
[25] Medhkour A., Van Roey M., Sobel R.A., et al. Implantation of human meningiomas into the subrenal capsule of the nude mouse. A model for studies of tumor growth. J Neurosurg. 1989;71:545-550.
[26] Jensen R.L., Leppla D., Rokosz N., Wurster R.D. Matrigel augments xenograft transplantation of meningioma cells into athymic mice. Neurosurgery. 1998;42:130-135. discussion 135–6
[27] Ragel B.T., Jensen R.L., Gillespie D.L., et al. A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Cancer. 2007;109:588-597.
[28] Baia G.S., Dinca E.B., Ozawa T., et al. An orthotopic skull base model of malignant meningioma. Brain Pathol. 2008;18:172-179.
[29] Ragel B.T., Elam I.L., Gillespie D.L., et al. Celecoxib inhibits meningioma tumor growth in a mouse xenograft model. J Neurosurg. 2008;108:304-310.
[30] Evans D.G., Huson S.M., Donnai D., et al. A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 1992;29:841-846.
[31] Gutmann D.H., Giordano M.J., Fishback A.S., Guha A. Loss of merlin expression in sporadic meningiomas, ependymomas and schwannomas. Neurology. 1997;49:267-270.
[32] Perry A., Cai D.X., Scheithauer B.W., et al. Merlin, DAL-1, and progesterone receptor expression in clinicopathologic subsets of meningioma: A correlative immunohistochemical study of 175 cases. J Neuropathol Exp Neurol. 2000;59:872-879.
[33] McClatchey A.I., Saotome I., Mercer K., et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12:1121-1133.
[34] Giovannini M., Robanus-Maandag E., van der Valk M., et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617-1630.
[35] Perry A., Banerjee R., Lohse C.M., et al. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol. 2002;12:183-190.
[36] Kalamarides M., Stemmer-Rachamimov A.O., Takahashi M., et al. Natural history of meningioma development in mice reveals a synergy of Nf2 and p16(Ink4a) mutations. Brain Pathol. 2008;18:62-70.