CHAPTER 189 Experimental Hydrocephalus
In the past 20 years our understanding of the pathophysiology of hydrocephalus has advanced significantly, yet critical gaps remain. A recent position paper identified 10 major areas that require immediate attention.1 Questions that are particularly amenable to experimental study include the following: How is cerebrospinal fluid (CSF) absorbed normally, and what are the causes of CSF malabsorption in hydrocephalus? Why do the ventricles dilate in communicating hydrocephalus? What happens to the structure and function of the brain when it is compressed and stretched by the expanding ventricles? What is the role of cerebrovenous pressure in hydrocephalus? What causes normal-pressure hydrocephalus? What causes low-pressure hydrocephalus? What is the pathophysiology of slit ventricle syndrome? What is the pathophysiologic basis for neurological impairment in hydrocephalus, and to what extent is it reversible? How is the brain of a child with hydrocephalus different from that of a young or elderly adult? It is difficult to address any of these important questions exclusively with clinical research, especially because of the wide variations in cause, onset, duration, and treatment complications that accompany hydrocephalus. Fortunately, animal models are available that approximate clinical conditions, and experimental hydrocephalus research is fundamental to promoting better treatments for this disorder. The recent white paper from a workshop sponsored by the National Institutes of Health bears witness to the importance of basic and clinical research in hydrocephalus.2
Unlike some neurological diseases or disorders, many mechanisms contribute to the pathophysiology of hydrocephalus. In fact, it is difficult to investigate these mechanisms individually because hydrocephalus is such a multifactorial disorder. Nevertheless, a clear distinction can be made between primary or causative mechanisms and secondary responses to ventriculomegaly. Primary mechanisms consist largely of developmental disorders that cause congenital hydrocephalus or pathologies such as intraventricular, subarachnoid, and intraparenchymal hemorrhage, meningitis and other infections, and tumors. Secondary mechanisms are far-reaching and include axonal damage, demyelination, cell death, gliosis and inflammation, biomechanical compression and stretch, edema, metabolic impairment, cerebrovascular effects and hypoxia-ischemia, synaptic and dendritic deterioration, neurotrophic changes, alterations of neurotransmitters and neuromodulators, and impaired clearance of toxins and metabolites. Often these secondary mechanisms overlap, making it difficult if not impossible to define the precise role of each. In spite of these obstacles, an emerging body of evidence is beginning to demonstrate that hydrocephalus (1) manifests, at least initially, as a disorder of periventricular white matter; (2) has a major impact on the structure and function of the cerebral vasculature; (3) involves metabolic and molecular changes that may not produce clinical symptoms but can have protracted effects on neurological function; (4) injures neurons in many ways but does not cause significant neuronal cell death unless ventriculomegaly is severe; (5) often follows a slowly progressive pathophysiologic pattern, which may allow considerable plasticity; and (6) is more effectively (but not completely) treated when intervention begins relatively soon after onset. A review by Del Bigio3 and several other summaries4–9 provide excellent introductions to the pathophysiology of hydrocephalus, and the reader is encouraged to consult these publications for details.
Experimental Models
Practically all the key questions listed earlier are best investigated, at least initially, in animals, and many in vivo models of hydrocephalus have been developed over the past 50 years.4,10,11 In addition, there is renewed interest in the use of mathematical models that can be tested in animals. It is appropriate to review these models here because they all have advantages and disadvantages as well as specific relevance to different clinical applications.
Animal Models of Congenital and Transgenic Hydrocephalus
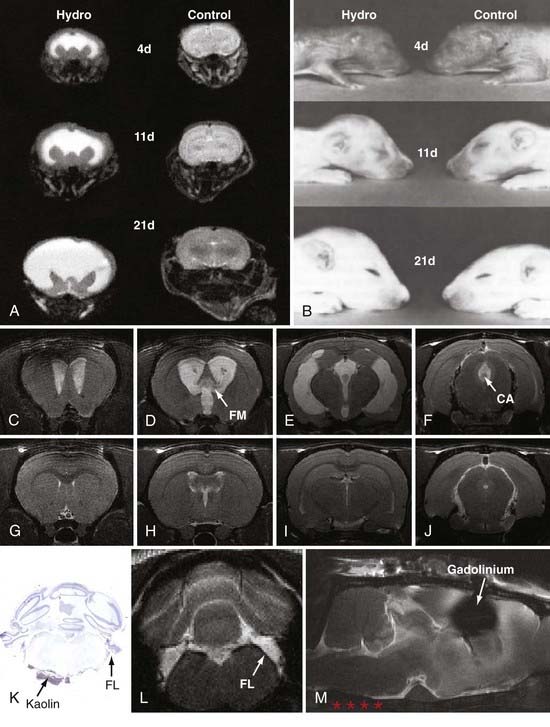
The rat congenital model most often used in experimental research is the H-Tx strain, which develops obstructive hydrocephalus from aqueductal stenosis in the perinatal period.11–14 From four chromosomes within a heterozygous background13,15,16 and incomplete penetrance,17 these animals develop hydrocephalus within several days of birth, which in rats corresponds to the third trimester of human brain maturation. In this model, ventriculomegaly becomes severe by the second postnatal week (Fig. 189-1), and the animals usually expire by 20 to 25 days of age if intracranial pressure (which does not rise until postnatal day 12) is not reduced by shunting. Drainage of CSF can be accomplished in H-Tx rats with either ventriculoperitoneal or ventriculosubcutaneous shunts, which are more effective when placed early (3 to 5 days of age) rather than late (12 to 14 days of age). Inbred strains of Wistar-Lewis (LEW/Jms) rats also develop aqueductal stenosis through nonmendelian mechanisms as early as day 17 in a 21-day gestational period.18–21 The frequency of hydrocephalus and the ratio of affected males to females are significantly higher when the LEW/Jms parent is male. These rat models are excellent for studies of neonatal and juvenile hydrocephalus, especially that caused by aqueductal stenosis, because ventriculomegaly occurs naturally, the brain is large enough for customized shunting, the rats are amenable to behavioral testing, the cost is relatively low, and a wealth of data is available for correlation. Nevertheless, they are not ideal for long-term experiments unless shunting is performed, and their size restricts the use of clinical shunt systems and pressure probes.
Several interesting mouse models of hydrocephalus have provided valuable insights into the causes of ventriculomegaly.11,22–33 The most widely used models include the SUMS/NP,28,32 hy3,31,34–40 transforming growth factor (TGF)-β1 overexpression,26,30,41–46 hyh with point mutation in α-SNAP and ependymal denudation that precedes aqueductal stenosis,47–51 fibroblast growth factor (FGF)-2,52 L1-cell adhesion molecule deficient,27,53–55 aquaporin deficient,56–59 hpy,60 members of the conserved forkhead–winged helix transcription factor gene (previously Mf1),29,61–63 heparin-binding epidermal growth factor,64 and collagen deficienct.65 Most recently, Sweger and colleagues66 developed a double transgenic mouse model that allows expression of the G1-coupled Ro1 receptor exclusive to astrocytes. By controlling Ro1 expression with a tetracycline-on promoter in drinking water, these mice develop enlarged ventricles, partial ependymal denudation, morphologic changes in the subcommissural organ, and obliteration of the cerebral aqueduct at designated times. This represents a powerful experimental model because the pathogenesis of hydrocephalus can be studied with neonatal, juvenile, and adult onset. The main disadvantage of these mouse models is that their size limits the use of CSF shunts and invasive physiologic sensors.
Animal Models of Acquired Hydrocephalus
Communicating extraventricular hydrocephalus has been more difficult to achieve with acquired approaches. Attempts to produce communicating hydrocephalus with kaolin injections into the cortical subarachnoid space of adult rats67,68 and dogs69–74 or with silicone injections into the subarachnoid space75,76 have consistently produced only moderate ventriculomegaly and usually require a long time (several months) to develop. Likewise, Silastic has been infused into the basal cisterns of monkeys to produce mild forms of communicating hydrocephalus.77 Recently, Li and colleagues78 developed a novel model of communicating hydrocephalus by injecting kaolin into the basal cisterns of adult rats (see Fig. 189-1); these animals develop moderate ventriculomegaly within a week of induction and exhibit impairments in CSF absorption and pulsatility (see later).
Nonmechanical induction methods, such as viral79 and bacterial inoculations,80 have also been used to produce communicating hydrocephalus, but these procedures involve the additional influence of the induction substances on pathophysiology and thus are not true representations of the effects of hydrocephalus alone on the brain. Growth factors such as TGF-β and FGF-1 and -2,30,41,52 as well as neurotoxins,3,81 have all been successful to varying degrees.
An important advance in experimental studies has been the application of CSF shunting techniques.82–89 Initially, larger models such as feline,83,88 lagomorph,90 and canine91–97 were employed, and these still have the advantage of accommodating commercially available shunt systems. With improved neurosurgical techniques, smaller neonatal, infantile, and juvenile rats have been shunted successfully,98–104 but the catheters are usually custom made, and valves are seldom used. It is also important to realize that shunted animals, especially the youngest and smallest ones, develop shunt malfunctions at approximately the same rate as human patients. Nevertheless, it is surprising that no studies have attempted to evaluate the effects of repetitive shunt malfunction.
Mathematical Models of Hydrocephalus
Several mathematical models have revealed possible mechanisms associated with hydrocephalus.105–122 One of the prevailing hypotheses is that the mechanical properties of hydrocephalic brain tissue are fundamentally changed compared with healthy brain tissue. However, this change in material properties has not yet been quantified, so these models and others like them are not useful as predictive models. One particular mathematical model that has been reliable in matching patient outcomes describes the brain as a quasi-linear viscoelastic tissue,123 which essentially means that there is an initial elastic response to ventriculomegaly, followed by a different long-term viscoelastic response. The flaw that prevents this model and all others from being predictive is that the material properties of the brain are not known precisely enough to be useful. CSF infusion tests are available to measure compliance (stiffness) of the brain by monitoring intracranial pressure changes during the injection of artificial CSF into the ventricles or the lumbar subarachnoid space.124 These tests have shown that compliance decreases in the hydrocephalic brain.124–133 However, compliance is an extrinsic property of the entire contents of the cranial cavity and can be influenced by the amount of tissue, the amount and flow velocity of blood, the tension of the meninges, and the possible expansion of the skull. In contrast, the material properties needed to construct a predictive mathematical model, such as shear or elastic modulus, are intrinsic properties of brain tissue independent of the amount of tissue. Current tissue testing methods to acquire intrinsic properties typically involve the removal of brain tissue for mechanical tests, but this approach eliminates many structural and physiologic factors of the “living” brain, especially the mechanical support offered by blood volume and flow, that tend to hydraulically stiffen the tissue. Fortunately, a mechanical testing method has been developed that allows direct measurement of the initial elastic response as well as the long-term viscoelastic response of “living” brain tissue.134,135 This method uses a mechanical probe that displaces the surface of the cortical tissue inward slightly and holds the displacement for a period of time, which is an ideal way to investigate tissue properties without damaging the brain.
Magnetic resonance elastography136,137 measures the elastic properties of brain tissue in vivo, based on the propagation of waves through deformed tissue detected with magnetic resonance imaging (MRI). Tissues with different material properties conduct these waves differently. Although this method holds great potential for noninvasive tissue property measurement, the true material properties of the brain (healthy or hydrocephalic) have yet to be measured directly, so the accuracy of this method has not been verified. In addition, the device used to perform the mechanical perturbation of the tissue requires a flat surface,137 so more developed gyral hemispheres are problematic, and the device is too large for rodent brains.
Pathophysiologic Mechanisms and Treatment Possibilities
Several previous reviews have provided excellent summaries of the pathophysiology of hydrocephalus.2,4,5,138–140 In general, mechanisms of injury include morphologic and functional changes in the cerebral vasculature, hypoxia-ischemia, gliosis and neuroinflammation, edema, axonal degeneration and impaired intracellular transport, demyelination and oligodendrocyte death, dendritic and synaptic degeneration and plasticity, altered levels of neurotransmitters and neuromodulators, and impaired protein clearance141 and lymphatic absorption. Exciting new data have revealed several novel mechanisms, dramatically broadening the view of the pathogenesis and secondary pathophysiology of hydrocephalus. These new findings are presented here rather than providing an exhaustive review of experimental studies.
Gliosis and Inflammation
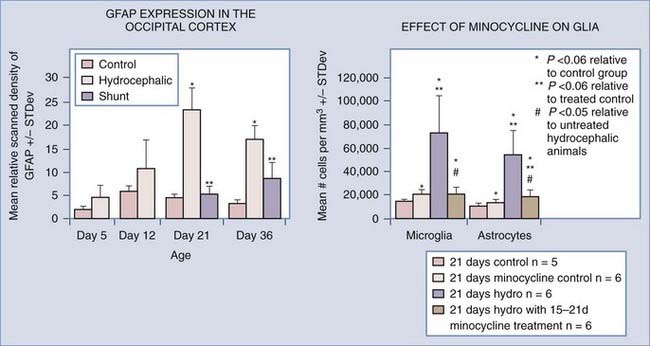
Gliosis is a consistent finding in hydrocephalus, and inflammation and glial scar formation could play a major role in creating the chronic problems that plague hydrocephalic patients, but the time course and permanence of the reaction are not completely known.28,138,142–153 It has been suggested by many investigators that scar formation is a permanent fixture in hydrocephalic brains,89,144,152 even those that have been shunted successfully. Previous studies in both congenital and acquired models of hydrocephalus have shown that glial fibrillary acidic protein (GFAP) RNA and protein levels increase with the progression of hydrocephalus. Additionally, Mangano and colleagues152 illustrated that microglial cell proliferation and activation increased in regions distant from the cortical “lesion,” suggesting that neuroinflammation is related to damage throughout the cortical pathways. Furthermore, GFAP-labeled reactive astrocytes surround cystic lesions in severely hydrocephalic H-Tx animals but are not present in the white matter surrounding the ventricles.154,155 Experimental models of hydrocephalus have demonstrated that shunting can reduce the amount of GFAP protein and RNA present in the cerebral cortex, but these levels begin to rise over time,89 suggesting that reactive astrocytosis is highly sensitive to suboptimal CSF drainage. Clinically, increased levels of GFAP protein have been found in the CSF of patients with normal-pressure hydrocephalus and those who developed secondary hydrocephalus due to subarachnoid hemorrhage.156–160 The possibility of using GFAP protein levels as a diagnostic tool for hydrocephalus is currently being explored.158,161
It is likely that gliosis may dramatically change the mechanical properties of the brain so that it becomes more rigid (less compliant) and resistant to increases in CSF pressure and flow.126,127,130,131,162–171 The importance of these properties in hydrocephalus is illustrated by the finding that reduced compliance, measured using the pressure-volume index, is a better predictor of shunt success than measurements of ventricular size.162,172,173 Unfortunately, no studies have directly examined the relationship between glial alterations and intracranial compliance. Compliance can be measured in animals using CSF infusion tests,174–176 and in hydrocephalus, resistance to CSF outflow is increased over a wide range of intracranial pressures.176 Compliance is usually lower in hydrocephalic animals, but it varies depending on intracranial pressure.
Most recently, an interesting and potentially powerful relationship has been suggested between astrocytes and aquaporin channels, which can have major impact on CSF absorption. Aquaporins are cell membrane proteins, and most are permeable only to water.177–180 Aquaporin 4 (AQP4) is found primarily on the endfeet of astrocytes that contact microvessels in the periventricular white matter and the subpial region of the cerebral cortex,181 as well as in ependymal cells lining the lateral ventricles. These locations place astrocyte aquaporin channels in a good position to transport CSF from the brain to the vascular system, and several investigators have suggested that they may play a major role in CSF absorption.182 Aquaporin channels require connections to a basal lamina that specifically contains collagen XVIII molecules; if these connections are not present, hydrocephalus ensues.65,183,184 One structural response of endothelial cells to chronic hypoperfusion is an abnormal basal lamina,185 so it is not surprising that aquaporin changes have been reported in experimental hydrocephalus.56,186–188 More directly, expression of AQP4 (but not AQP1 or AQP9) increases in the cerebral cortex and hippocampus of rats with mechanically induced hydrocephalus, suggesting that this water channel plays a compensatory role in CSF absorption during ventriculomegaly.186 Fibrosis in the form of reactive astrocytosis could therefore have an important impact on CSF absorption, which might not recover with ventricular shunting alone.
Minocycline has recently shown promise as a specific inhibitor of microglial cells,189–192 one of the main elements of glial scar formation in hydrocephalus.89,152,193,194 Although the mechanism of action is still unknown, minocycline has multiple benefits in brain injury,191,195–227 and its promise as a neuroprotective agent is illustrated by the recent initiation of clinical trials in Parkinson’s disease,228,229 Huntington’s disease,230–233 amyotrophic lateral sclerosis,234 multiple sclerosis,235,236 and schizophrenia.237,238 Based on previous studies showing positive effects in white matter,195,203,215,239–242 Miller and McAllister243 initiated tests of minocycline’s ability to inhibit gliosis in the H-Tx rat model of congenital hydrocephalus. Preliminary results are encouraging, in that both numbers of glia (astrocytes and microglia) and cortical mantle thickness were significantly reduced when minocycline was administered after hydrocephalus had progressed considerably (Fig. 189-2).
Intracranial Pulsatility
Thirty years ago, Di Rocco and coworkers244 showed that ventricular dilation can be caused by abnormal intracranial pulsatility in the absence of a physical obstruction to CSF flow. Abnormal intracranial pulsations are also clearly related to clinical hydrocephalus; intraventricular pulsatility, detected as CSF pulsations in the aqueduct on cine MRI, are often dramatically elevated in hydrocephalus245–248 and can be a useful diagnostic criterion in normal-pressure hydrocephalus. Although authors have posited theories on the cause of these elevated pulsations,249–251 no study has provided a clear link between abnormal pulsations and the underlying pathophysiology of hydrocephalus.
Furthermore, the dissipation of cerebral arterial pulsatility, resulting in minimal (homeostatic) capillary and venous pulse pressure, is believed to be critical for normal cerebrovascular function.251–254 Although dissipation is easily accomplished in other organs, the closed cranium requires a more complex system for this function; CSF and venous blood serve this purpose. The major arteries entering the cranium are located within the CSF-filled subarachnoid spaces, allowing the efficient coupling and transfer of pulsation. Pulsations can be transferred out of the cranium either directly via CSF flow through the foramen magnum into the compliant spinal subarachnoid space or indirectly via coupling to the sagittal sinus within the convexity. An important component of the pathophysiology of hydrocephalus is a change in intracranial compliance, which may lead to a redistribution of the pulsation dissipation mechanism.250,251 One potential pathway is directly into parenchymal capillaries. Thus, hydrocephalus may be caused by a breakdown in this pulse dissipation mechanism (or a failed windkessel),251,253,255–258 which normally protects the capillary bed from excessive pulsatile shear forces.
Increased capillary pulsatility may have several pathophysiologic consequences. Structural responses may lead to the loss of parenchymal microvessels, and in fact, decreased capillary density has been shown in experimental hydrocephalus.4,259–264 In addition, the integrity of the blood-brain barrier is compromised in hydrocephalus.265–267 Both these effects might explain the marked loss of cerebral perfusion that has been well documented in clinical268–282 and experimental hydrocephalus.34,67,276,278,283–289 Importantly, it was recently shown that excessive pulsatile stress can impair hemodynamics through changes in endothelial cell homeostasis290–295 mediated by nitric oxide.296 A marked increase in nitric oxide synthase immunoreactivity has also been reported in a kaolin hydrocephalus rat model during the first few weeks of ventriculomegaly.286 These examples all provide a compelling case for the importance of pulsation dissipation in maintaining normal capillary function.
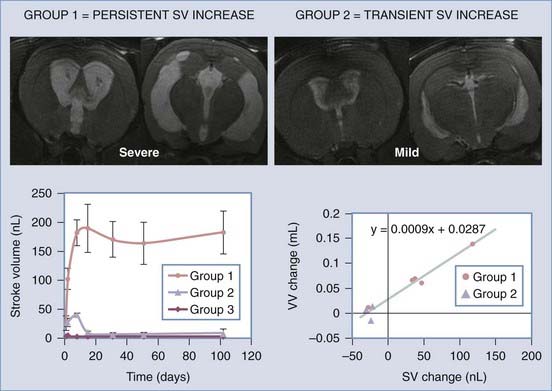
Zou and colleagues297 and Wagshul and associates,298 using two different types of analyses, recently showed that adult dogs normally exhibit a pulse dissipation mechanism termed a notch (because when intracranial pressure is graphed against frequency, it appears as a trough) and that this notch changes as pressure is raised. Further studies are needed to determine whether this change in intracranial hydrodynamics is causative or a secondary response to ventriculomegaly. One series of investigations that addressed this issue used the novel model of communicating hydrocephalus in adult rats described earlier.78 Using high-field-flow MRI in this model, Wagshul and coworkers299 demonstrated that aqueductal pulsatility (stroke volume) increased within 24 hours of basal cistern obstruction, and the severity of ventriculomegaly was associated with the maintenance of high stroke volume. In contrast, only mild ventriculomegaly developed in animals that exhibited an early but transient increase in aqueductal pulsatility. These experiments clearly indicate that pulsatility plays a role in the pathophysiology of experimental hydrocephalus (Fig. 189-3).
Cerebrospinal Fluid Absorption—Lymphatic, Arachnoid, Microvascular
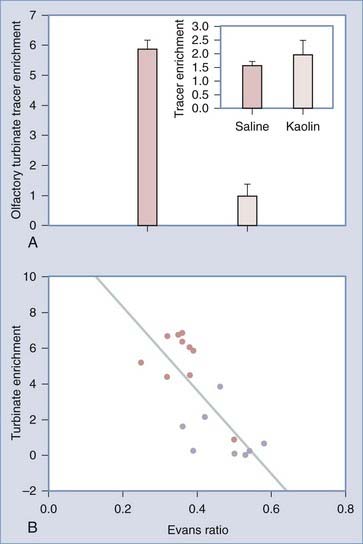
The traditional view of CSF absorption exclusively via arachnoid granulations into the superior sagittal sinus or the veins adjacent to the spinal roots has been challenged by a series of experiments.300–310 Initially, the results of these experiments were challenged because they were performed on animals or human cadavers and consisted of latex Microfil infusions to reveal potential CSF pathways from the subarachnoid space. However, the latest in vivo studies clearly demonstrate that cranial lymphatics, most notably the nasal pathways surrounding the olfactory nerves, are capable of transporting large volumes of CSF. Most important, these pathways are impaired in adult rats with communicating hydrocephalus (Fig. 189-4).
Bateman GA. The role of altered impedance in the pathophysiology of normal pressure hydrocephalus, Alzheimer’s disease and syringomyelia. Med Hypotheses. 2004;63:980.
Bergsneider M, Egnor MR, Johnston M, et al. What we don’t (but should) know about hydrocephalus. J Neurosurg. 2006;104:157.
Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317.
Del Bigio MR. Future directions for therapy of childhood hydrocephalus: a view from the laboratory. Pediatr Neurosurg. 2001;34:172.
Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am. 2001;12:639.
Del Bigio MR, McAllister JPII. Pathophysiology of Hydrocephalus. In: Choux M, DiRocco R, Hockley AD, Walker ML, editors. Pediatric Neurosurgery. 4 ed. Philadelphia: Churchill Livingstone; 1999:217-236.
Eskandari R, McAllister JPII, Miller JM, et al. Effects of hydrocephalus and ventriculoperitoneal shunt therapy on afferent and efferent connections in the feline sensorimotor cortex. J Neurosurg. 2004;101:196.
Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27:145.
Harris NG, McAllister JPII, Jones HC. The effect of untreated and shunt-treated hydrocephalus on cortical pyramidal neurone morphology in the H-Tx rat. Eur J Pediatr Surg. 1993;3(suppl 1):31.
Jones HC, Harris NG, Rocca JR, Andersohn RW. Progressive tissue injury in infantile hydrocephalus and prevention/reversal with shunt treatment. Neurol Res. 2000;22:89.
Klinge PM, Samii A, Muhlendyck A, et al. Cerebral hypoperfusion and delayed hippocampal response after induction of adult kaolin hydrocephalus. Stroke. 2003;34:193.
Li J, McAllister JPII, Shen Y, et al. Communicating hydrocephalus in adult rats with kaolin obstruction of the basal cisterns or the cortical subarachnoid space. Exp Neurol. 2008;211:351.
Mangano FT, McAllister JP, Jones HC, et al. The microglial response to progressive hydrocephalus in a model of inherited aqueductal stenosis. Neurol Res. 1998;20:697.
McAllister JPII, Chovan P. Neonatal hydrocephalus. Mechanisms and consequences. Neurosurg Clin N Am. 1998;9:73.
Miller JM, Kumar R, McAllister JP, Krause GS. Gene expression analysis of the development of congenital hydrocephalus in the H-Tx rat. Brain Res. 2006;1075:36.
Miller JM, McAllister JP. Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5.
Miller JM, McAllister JP II. Minocycline reduces gliosis and increases cortical thickness in experimental hydrocephalus. Paper presented at Goteburg, Sweden, 2006.
Sweger EJ, Casper KB, Scearce-Levie K, et al. Development of hydrocephalus in mice expressing the G1-coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci. 2007;27:2309.
Tullberg M, Blennow K, Mansson JE, et al. Ventricular cerebrospinal fluid neurofilament protein levels decrease in parallel with white matter pathology after shunt surgery in normal pressure hydrocephalus. Eur J Neurol. 2007;14:248.
Vio K, Rodriguez S, Yulis C, et al. The subcommissural organ of the rat secretes Reissner’s fiber glycoproteins and CSF-soluble proteins reaching the internal and external CSF compartments. Cerebrospinal Fluid Res. 2008;5:3.
Wagshul ME, Chen JJ, Egnor MR, et al. Amplitude and Phase of cerebrospinal fluid pulsations: experimental studies and review of the literature. J Neurosurg. 2006;104:810.
Wagshul ME, Kelly EJ, Yu HJ, et al. Resonant and notch behavior in intracranial pressure dynamics. J Neurosurg Pediatr. 2009;3:354.
Wagshul ME, McAllister JPII, Rashid S, et al. Ventricular dilation and elevated aqueductal pulsations in a new experimental model of communicating hydrocephalus. Exp Neurol. 2009;218:33.
Williams MA, McAllister JP, Walker ML, et al. Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. J Neurosurg Pediatr. 2007;107:345.
Zou R, Park EH, Kelly EM, et al. Intracranial pressure waves: characterization of a pulsation absorber with notch filter properties using systems analysis: laboratory investigation. J Neurosurg Pediatr. 2008;2:83.
1 Bergsneider M, Egnor MR, Johnston M, et al. What we don’t (but should) know about hydrocephalus. J Neurosurg. 2006;104:157.
2 Williams MA, McAllister JP, Walker ML, et al. Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. J Neurosurg Pediatr. 2007;107:345.
3 Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am. 2001;12:639.
4 McAllister JP, Chovan PII. Neonatal hydrocephalus. Mechanisms and consequences. Neurosurg Clin N Am. 1998;9:73.
5 Del Bigio MR, McAllister JPII. Pathophysiology of hydrocephalus. In: Choux M, DiRocco R, Hockley AD, Walker ML, editors. Pediatric Neurosurgery. 4 ed. Philadelphia: Churchill Livingstone; 1999:217-236.
6 Weller RO, Kida S, Harding BN. Aetiology and pathology of hydrocephalus. In: Schurr PH, Polkey CE, editors. Hydrocephalus. New York: Oxford University Press; 1993:48-99.
7 Weller RO, Mitchell J, Griffin RL, Gardner MJ. The effects of hydrocephalus upon the developing brain. Histological and quantitative studies of the ependyma and subependyma in hydrocephalic rats. J Neurol Sci. 1978;36:383.
8 Weller RO, Shulman K. Infantile hydrocephalus: clinical, histological, and ultrastructural study of brain damage. J Neurosurg. 1972;36:255.
9 Rekate HL, Trimurti D, Nadkarni MC, Wallace D. The importance of the cortical subarachnoid space in understanding hydrocephalus. J Neurosurg Pediatr. 2008;2:1.
10 Hochwald GM. Animal models of hydrocephalus: recent developments. Proc Soc Exp Biol Med. 1985;178:1.
11 Johanson C, Del Bigio MR, Kinsman S, et al. New models for analysing hydrocephalus and disorders of CSF volume transmission. Br J Neurosurg. 2001;15:281.
12 Kohn DF, Chinookoswong N, Chou SM. A new model of congenital hydrocephalus in the rat. Acta Neuropathol. 1981;54:211.
13 Jones HC. Molecular genetics of congenital hydrocephalus. Exp Neurol. 2004;190:79.
14 Miller JM, Kumar R, McAllister JP, Krause GS. Gene expression analysis of the development of congenital hydrocephalus in the H-Tx rat. Brain Res. 2006;1075:36.
15 Jones HC, Depelteau JS, Carter BJ, et al. Genome-wide linkage analysis of inherited hydrocephalus in the H-Tx rat. Mamm Genome. 2001;12:22.
16 Jones HC, Yehia B, Chen GF, Carter BJ. Genetic analysis of inherited hydrocephalus in a rat model. Exp Neurol. 2004;190:79.
17 Cai X, McGraw G, Pattisapu JV, et al. Hydrocephalus in the H-Tx rat: a monogenic disease? Exp Neurol. 2000;163:131.
18 Jones HC, Totten CF, Mayorga DA, et al. Genetic loci for ventricular dilatation in the LEW/Jms rat with fetal-onset hydrocephalus are influenced by gender and genetic background. Cerebrospinal Fluid Res. 2005;2:2.
19 Jones HC, Carter BJ, Morel L. Characteristics of hydrocephalus expression in the LEW/Jms rat strain with inherited disease. Childs Nerv Syst. 2003;19:11.
20 Sasaki S, Goto H, Nagano H, et al. Congenital hydrocephalus revealed in the inbred rat, LEW/Jms. Neurosurgery. 1983;13:548.
21 Yamada H, Oi SZ, Tamaki N, et al. Prenatal aqueductal stenosis as a cause of congenital hydrocephalus in the inbred rat LEW/Jms. Childs Nerv Syst. 1991;7:218.
22 Borit A, Sidman RL. New mutant mouse with communicating hydrocephalus and secondary aqueductal stenosis. Acta Neuropathol. 1972;21:316.
23 das Neves L, Duchala CS, Tolentino-Silva F, et al. Disruption of the murine nuclear factor I-A gene (Nfia) results in perinatal lethality, hydrocephalus, and agenesis of the corpus callosum. Proc Natl Acad Sci U S A. 1999;96:11946.
24 Stoddart JHJ, Ladd D, Bronson RT, et al. Transgenic mice with a mutated collagen promoter display normal response during bleomycin-induced fibrosis and possess neurological abnormalities. J Cell Biochem. 2000;77:135.
25 Jones HC. Cerebrospinal fluid pressure and resistance to absorption during development in normal and hydrocephalic mutant mice. Exp Neurol. 1985;90:162.
26 Galbreath E, Kim SJ, Park K, et al. Overexpression of TGF-beta 1 in the central nervous system of transgenic mice results in hydrocephalus. J Neuropathol Exp Neurol. 1995;54:339.
27 Dahme M, Bartsch U, Martini R, et al. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346.
28 Bruni JE, Del Bigio MR, Cardoso ER, Persaud TV. Neuropathology of congenital hydrocephalus in the SUMS/NP mouse. Acta Neurochir. 1988;92:118.
29 Kume T, Deng KY, Winfrey V, Gould DB, et al. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell. 1998;93:985.
30 Moinuddin SM, Tada T. Study of cerebrospinal fluid flow dynamics in TGF-beta 1 induced chronic hydrocephalic mice. Neurol Res. 2000;22:215.
31 Robinson ML, Allen CE, Davy BE, et al. Genetic mapping of an insertional hydrocephalus-inducing mutation allelic to hy3. Mamm Genome. 2002;13:625.
32 Jones HC, Dack S, Ellis C. Morphological aspects of the development of hydrocephalus in a mouse mutant (SUMS/NP). Acta Neuropathol. 1987;72:268.
33 Matsumoto S, Yamasaki S, Shirataki K, Fujiwara K. Comparative study of various models of experimental hydrocephalus. Childs Brain. 1975;1:236.
34 Wozniak M, McLone DG, Raimondi AJ. Micro- and macrovascular changes as the direct cause of parenchymal destruction in congenital murine hydrocephalus. J Neurosurg. 1975;43:535.
35 Kuwamura K, McLone DG, Raimondi AJ. The central (spinal) canal in congenital murine hydrocephalus: morphological and physiological aspects. Childs Brain. 1978;4:216.
36 McLone DG, Bondareff W, Raimondi AJ. Hydrocephalus-3, a murine mutant. II. Changes in the brain extracellular space. Surg Neurol. 1973;1:233.
37 McLone DG, Bondareff W, Raimondi AJ. Brain edema in the hydrocephalic hy-3 mouse: submicroscopic morphology. J Neuropathol Exp Neurol. 1971;30:627.
38 McLone DG, Bondareff W. Developmental morphology of the subarachnoid space and contiguous structures in the mouse. Am J Anat. 1975;142:273.
39 Raimondi AJ, Clark SJ, McLone DG. Pathogenesis of aqueductal occlusion in congenital murine hydrocephalus. J Neurosurg. 1976;45:66.
40 Raimondi AJ, Bailey OT, McLone DG, et al. The pathophysiology and morphology of murine hydrocephalus in Hy-3 and Ch mutants. Surg Neurol. 1973;1:50.
41 Tada T, Kanaji M, Kobayashi S. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta 1. J Neuroimmunol. 1994;50:153.
42 Wyss-Coray T, Feng L, Masliah E, et al. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol. 1995;147:53.
43 Aliev G, Miller JP, Leifer DW, et al. Ultrastructural analysis of a murine model of congenital hydrocephalus produced by overexpression of transforming growth factor-beta1 in the central nervous system. J Submicrosc Cytol Pathol. 2006;38:85.
44 Crews L, Wyss-Coray T, Masliah E. Insights into the pathogenesis of hydrocephalus from transgenic and experimental animal models. Brain Pathol. 2004;14:312.
45 Hayashi N, Leifer DW, Cohen AR. Chronologic changes of cerebral ventricular size in a transgenic model of hydrocephalus. Pediatr Neurosurg. 2000;33:182.
46 Nakazato F, Tada T, Sekiguchi Y, et al. Disturbed spatial learning of rats after intraventricular administration of transforming growth factor-beta 1. Neurol Med Chir (Tokyo). 2002;42:151.
47 Batiz LF, Paez P, Jimenez AJ, et al. Heterogeneous expression of hydrocephalic phenotype in the hyh mice carrying a point mutation in [alpha]-SNAP. Neurobiol Dis. 2006;23:152.
48 Wagner C, Batiz LF, Rodriguez S, et al. Cellular mechanisms involved in the stenosis and obliteration of the cerebral aqueduct of hyh mutant mice developing congenital hydrocephalus. J Neuropathol Exp Neurol. 2003;62:1019.
49 Jimenez AJ, Tome M, Paez P, et al. A programmed ependymal denudation precedes congenital hydrocephalus in the hyh mutant mouse. J Neuropathol Exp Neurol. 2001;60:1105.
50 Perez-Figares JM, Jimenez AJ, Perez-Martin M, et al. Spontaneous congenital hydrocephalus in the mutant mouse hyh. Changes in the ventricular system and the subcommissural organ. J Neuropathol Exp Neurol. 1998;57:188.
51 Vio K, Rodriguez S, Yulis C, et al. The subcommissural organ of the rat secretes Reissner’s fiber glycoproteins and CSF-soluble proteins reaching the internal and external CSF compartments. Cerebrospinal Fluid Res. 2008;5:3.
52 Johanson CE, Szmydynger-Chodobska J, Chodobski A, et al. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2-induced hydrocephalus. Am J Physiol. 1999;277:R263.
53 Fransen E, D’Hooge R, Van Camp G, et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum Mol Genet. 1998;7:999.
54 Kamiguchi H, Hlavin ML, Lemmon V. Role of L1 in neural development: what the knockouts tell us. Mol Cell Neurosci. 1998;12:48.
55 Rolf B, Kutsche M, Bartsch U. Severe hydrocephalus in L1-deficient mice. Brain Res. 2001;891:247.
56 Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006;26:1527.
57 Fan Y, Kong H, Shi X, et al. Hypersensitivity of aquaporin 4-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine and astrocytic modulation. Neurobiol Aging. 2008;29:1226.
58 Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. 2007;22:778.
59 Verkman AS, Binder DK, Bloch O, et al. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta—Biomembranes. 2006;1758:1085.
60 Bryan JH. The immotile cilia syndrome. Mice versus man. Virchows Arch A Pathol Anat Histopathol. 1983;399:265.
61 Topczewska JM, Topczewski J, Solnica-Krezel L, Hogan BL. Sequence and expression of zebrafish foxc1a and foxc1b, encoding conserved forkhead/winged helix transcription factors. Mech Dev. 2001;100:343.
62 Hong HK, Lass JH, Chakravarti A. Pleiotropic skeletal and ocular phenotypes of the mouse mutation congenital hydrocephalus (ch/Mf1) arise from a winged helix/forkhead transcriptionfactor gene. Hum Mol Genet. 1999;8:625.
63 Blackshear PJ, Graves JP, Stumpo DJ, et al. Graded phenotypic response to partial and complete deficiency of a brain-specific transcript variant of the winged helix transcription factor RFX4. Development. 2003;130:4539.
64 MacDonald BA, Raab G, Goishi K, et al. Heparin-binding epidermal growth factor overexpression is implicated in the development of hydrocephalus in transgenic mice. Paper presented at the American Association of Neurological Surgeons Annual Meeting; 2005.
65 Utriainen A, Sormunen R, Kettunen M, et al. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet. 2004;13:2089.
66 Sweger EJ, Casper KB, Scearce-Levie K, et al. Development of hydrocephalus in mice expressing the G1-coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci. 2007;27:2309.
67 Cosan TE, Gucuyener D, Dundar E, et al. Cerebral blood flow alterations in progressive communicating hydrocephalus: transcranial Doppler ultrasonography assessment in an experimental model. J Neurosurg. 2001;94:265.
68 Cosan TE, Guner AI, Akcar N, et al. Progressive ventricular enlargement in the absence of high ventricular pressure in an experimental neonatal rat model. Childs Nerv Syst. 2002;18:10.
69 Daniel GB, Edwards DF, Harvey RC, Kabalka GW. Communicating hydrocephalus in dogs with congenital ciliary dysfunction. Dev Neurosci. 1995;17:230.
70 Deo-Narine V, Gomez DG, Vullo T, et al. Direct in vivo observation of transventricular absorption in the hydrocephalic dog using magnetic resonance imaging. Invest Radiol. 1994;29:287.
71 James AEJr, Burns B, Flor WF, et al. Pathophysiology of chronic communicating hydrocephalus in dogs (Canis familiaris). Experimental studies. J Neurol Sci. 1975;24:151.
72 James AEJr, Flor WJ, Novak GR, et al. Evaluation of the central canal of the spinal cord in experimentally induced hydrocephalus. J Neurosurg. 1978;48:970.
73 Schurr PH, McLaurin RL, Ingraham FD. Experimental studies on the circulation of the cerebrospinal fluid and methods of producing communicating hydrocephalus in the dog. J Neurosurg. 1953;10:515.
74 Strecker EP, James AEJr, Konigsmark B, Merz T. Autoradiographic observations in experimental communicating hydrocephalus. Neurology. 1974;24:192.
75 Price DL, James AEJr, Sperber E, Strecker EP. Communicating hydrocephalus. Arch Neurol. 1976;33:15.
76 Del Bigio MR, Bruni JE. Cerebral water content in silicone oil-induced hydrocephalic rabbits. Pediatr Neurosci. 1987;13:72.
77 Diggs J, Price AC, Burt AM, et al. Early changes in experimental hydrocephalus. Invest Radiol. 1986;21:118.
78 Li J, McAllister JPII, Shen Y, et al. Communicating hydrocephalus in adult rats with kaolin obstruction of the basal cisterns or the cortical subarachnoid space. Exp Neurol. 2008;211:351.
79 Davis LE. Communicating hydrocephalus in newborn hamsters and cats following vaccinia virus infection. J Neurosurg. 1981;54:767.
80 Wiesmann M, Koedel U, Bruckmann H, Pfister HW. Experimental bacterial meningitis in rats: demonstration of hydrocephalus and meningeal enhancement by magnetic resonance imaging. Neurol Res. 2002;24:307.
81 Fiori MG, Sharer LR, Lowndes HE. Communicating hydrocephalus in rodents treated with beta,beta’-iminodipropionitrile (IDPN). Acta Neuropathol. 1985;65:209.
82 Lovely TJ, McAllister JPII, Miller DW, et al. Effects of hydrocephalus and surgical decompression on cortical norepinephrine levels in neonatal cats. Neurosurgery. 1989;24:43.
83 Lovely TJ, Miller DW, McAllister JPII. A technique for placing ventriculoperitoneal shunts in a neonatal model of hydrocephalus. J Neurosci Methods. 1989;29:201.
84 Hale PM, McAllister JPII, Katz SD, et al. Improvement of cortical morphology in infantile hydrocephalic animals after ventriculoperitoneal shunt placement. Neurosurgery. 1992;31:1085.
85 McAllister JPII, Cohen MI, O’Mara KA, Johnson MH. Progression of experimental infantile hydrocephalus and effects of ventriculoperitoneal shunts: an analysis correlating magnetic resonance imaging with gross morphology. Neurosurgery. 1991;29:329.
86 Harris NG, McAllister JPII, Jones HC. The effect of untreated and shunt-treated hydrocephalus on cortical pyramidal neurone morphology in the H-Tx rat. Eur J Pediatr Surg. 1993;3(suppl 1):31.
87 Harris NG, Jones HC, McAllister JP II. 1-NMR spectroscopy of cerebral cortex in hydrocephalic H-Tx rats with and without shunt treatment. Society for Research into Hydrocephalus and Spina Bifida. 1995.
88 Eskandari R, McAllister JPII, Miller JM, et al. Effects of hydrocephalus and ventriculoperitoneal shunt therapy on afferent and efferent connections in the feline sensorimotor cortex. J Neurosurg. 2004;101:196.
89 Miller JM, McAllister JP. Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5.
90 Wehby-Grant MC, Olmstead CE, Peacock WJ, et al. Metabolic responses of the neonatal rabbit brain to hydrocephalus and shunting. Pediatr Neurosurg. 1996;24:79.
91 Fukuhara T, Luciano MG, Brant CL, Klauscie J. Effects of ventriculoperitoneal shunt removal on cerebral oxygenation and brain compliance in chronic obstructive hydrocephalus. J Neurosurg. 2001;94:573.
92 Bayston R, Brant C, Dombrowski SM, et al. An experimental in-vivo canine model for adult shunt infection. Cerebrospinal Fluid Res. 2008;5:17.
93 Aoyama Y, Kinoshita Y, Yokota A, Hamada T. Neuronal damage in hydrocephalus and its restoration by shunt insertion in experimental hydrocephalus: a study involving the neurofilament-immunostaining method. J Neurosurg. 2006;104:332.
94 Yamada H, Yokota A, Furuta A, Horie A. Reconstitution of shunted mantle in experimental hydrocephalus. J Neurosurg. 1992;76:856.
95 Oi S, Matsumoto S. Morphological findings of postshunt slit-ventricle in experimental canine hydrocephalus. Aspects of causative factors of isolated ventricles and slit-ventricle syndrome. Childs Nerv Syst. 1986;2:179.
96 Murata T, Handa H, Mori K, Nakano Y. The significance of periventricular lucency on computed tomography: experimental study with canine hydrocephalus. Neuroradiology. 1981;20:221.
97 Dohrmann GJ. The choroid plexus in experimental hydrocephalus. A light and electron microscopic study in normal, hydrocephalic, and shunted hydrocephalic dogs. J Neurosurg. 1971;34:56.
98 Jones HC, Harris NG, Rocca JR, Andersohn RW. Progressive tissue injury in infantile hydrocephalus and prevention/reversal with shunt treatment. Neurol Res. 2000;22:89.
99 Jones HC, Harris NG, Briggs RW, Williams SC. Shunt treatment at two postnatal ages in hydrocephalic H-Tx rats quantified using MR imaging. Exp Neurol. 1995;133:144.
100 Harris NG, Jones HC, Patel S. Ventricle shunting in young H-Tx rats with inherited congenital hydrocephalus: a quantitative histological study of cortical grey matter. Childs Nerv Syst. 1994;10:293.
101 Jones HC, Rivera KM, Harris NG. Learning deficits in congenitally hydrocephalic rats and prevention by early shunt treatment. Childs Nerv Syst. 1995;11:655.
102 Harris NG, McAllister JPII, Conaughty JM, Jones HC. The effect of inherited hydrocephalus and shunt treatment on cortical pyramidal cell dendrites in the infant H-Tx rat. Exp Neurol. 1996;141:269.
103 Harris NG, Plant HD, Briggs RW, Jones HC. Metabolite changes in the cerebral cortex of treated and untreated infant hydrocephalic rats studied using in vitro 31P-NMR spectroscopy. J Neurochem. 1996;67:2030.
104 Boillat CA, Jones HC, Kaiser GL, Harris NG. Ultrastructural changes in the deep cortical pyramidal cells of infant rats with inherited hydrocephalus and the effect of shunt treatment. Exp Neurol. 1997;147:377.
105 Tenti G, Drake JM, Sivaloganathan S. Brain biomechanics: mathematical modeling of hydrocephalus. Neurol Res. 2000;22:19.
106 Sivaloganathan S, Stastna M, Tenti G, Drake JM. A viscoelastic model of the brain parenchyma with pulsatile ventricular pressure. Appl Math Comput. 2005;165:687.
107 Sivaloganathan S, Stastna M, Tenti G, Drake JM. Biomechanics of the brain: A theoretical and numerical study of Biot’s equations of consolidation theory with deformation-dependent permeability. Int J Non-Linear Mechanics. 2005. In Press
108 Drake JM, Mostachfi O, Tenti G, Sivaloganathan S. Realistic simple mathematical model of brain biomechanics for computer simulation of hydrocephalus and other brain abnormalities. Can J Neurol Sci. 1996;23:S5.
109 Davis GB, Kohandel M, Sivaloganathan S, Tenti G. The constitutive properties of the brain paraenchyma Part 2. Fractional derivative approach. Med Eng Phys. 2006;28:455.
110 Miller K, Chinzei K. Mechanical properties of brain tissue in tension. J Biomech. 2002;35:483.
111 Pang D, Altschuler E. Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery. 1994;35:643.
112 Pena A, Bolton MD, Whitehouse H, Pickard JD. Effects of brain ventricular shape on periventricular biomechanics: a finite-element analysis. Neurosurgery. 1999;45:107.
113 Penar PL, Lakin WD, Yu J. Normal pressure hydrocephalus: an analysis of aetiology and response to shunting based on mathematical modeling. Neurol Res. 1995;17:83.
114 Shapiro K, Fried A, Marmarou A. Biomechanical and hydrodynamic characterization of the hydrocephalic infant. J Neurosurg. 1985;63:69.
115 Shapiro K, Takei F, Fried A, Kohn I. Experimental feline hydrocephalus. The role of biomechanical changes in ventricular enlargement in cats. J Neurosurg. 1985;63:82.
116 Dutta-Roy T, Wittek A, Miller K. Biomechanical modelling of normal pressure hydrocephalus. J Biomech. 2008;41:2263.
117 Linninger AA, Sweetman B, Penn R. Normal and hydrocephalic brain dynamics: the role of reduced cerebrospinal fluid reabsorption in ventricular enlargement. Ann Biomed Eng. 2009;37:1434.
118 Linninger AA, Xenos M, Sweetman B, et al. A mathematical model of blood, cerebrospinal fluid and brain dynamics. J Math Biol. 2009;59:729.
119 Penn R, Sweetman B, Linninger AA. Blood, cerebrospinal fluid and brain dynamics in communicating hydrocephalus. Clin Neurol Neurosurg. 2008;110:S3.
120 Zhu DC, Xenos M, Linninger AA, Penn RD. Dynamics of lateral ventricle and cerebrospinal fluid in normal and hydrocephalic brains. J Magn Reson Imaging. 2006;24:756.
121 Linninger AA, Tsakiris C, Zhu DC, et al. Pulsatile cerebrospinal fluid dynamics in the human brain. IEEE Trans Biomed Eng. 2005;52:557.
122 Chiang WW, Takoudis CG, Lee SH, et al. Relationship between ventricular morphology and aqueductal cerebrospinal fluid flow in healthy and communicating hydrocephalus. Invest Radiol. 2009;44:192.
123 Drapaca CS, Tenti G, Rohlf K, Sivaloganathan S. A quasi-linear viscoelastic constitutive equation for the brain: application to hydrocephalus. J Elasticity. 2006;85:65.
124 Kiefer M, Eymann R, Steudel WI. The dynamic infusion test in rats. Childs Nerv Syst. 2000;16:451.
125 Meier U, Kiefer M. The ICP-dependency of resistance to cerebrospinal fluid outflow: a new mathematical method for CSF-parameter calculation in a model with H-Tx rats. Acta Neurochir Suppl. 2003;86:539.
126 Tans JT, Poortvliet DCJ. Relationship between compliance and resistance to outflow of CSF in adult hydrocephalus. J Neurosurg. 1989;71:59.
127 Shulman K. Pressure/volume relationships in intracranial disease. Z Kinderchir. 1987;25:289.
128 Shapiro K, Fried A. Pressure-volume relationships in shunt-dependent childhood hydrocephalus. The zone of pressure instability in children with acute deterioration. J Neurosurg. 1986;64:390.
129 Sahuquillo J, Rubio E, Codina A, et al. Reappraisal of the intracranial pressure and cerebrospinal fluid dynamics in patients with the so-called “normal pressure hydrocephalus” syndrome. Acta Neurochir. 1991;112:50.
130 Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg. 1975;43:523.
131 Czosnyka M, Czosnyka ZH, Whitfield PC, et al. Age dependence of cerebrospinal pressure-volume compensation in patients with hydrocephalus. J Neurosurg. 2001;94:482.
132 Bateman GA. Vascular compliance in normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2000;21:1574.
133 Bateman GA. The role of altered impedance in the pathophysiology of normal pressure hydrocephalus, Alzheimer’s disease and syringomyelia. Med Hypotheses. 2004;63:980.
134 Gefen A, Gefen N, Zhu Q, et al. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003;20:1163.
135 Gefen A, Margulies SS. Are in vivo and in situ brain tissues mechanically similar? J Biomech. 2004;37:1339.
136 Muthupillai R, Lomas DJ, Rossman PJ, et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854.
137 Yin M, Rouviere O, Glaser KJ, Ehman RL. Diffraction-biased shear wave fields generated with longitudinal magnetic resonance elastography drivers. Magn Reson Imaging. 2008;26:770.
138 Del Bigio MR, Zhang YW. Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol. 1998;154:157.
139 Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317.
140 Del Bigio MR. Future directions for therapy of childhood hydrocephalus: a view from the laboratory. Pediatr Neurosurg. 2001;34:172.
141 Deren KE, Forsyth J, Abdullah O, et al. Low levels of amyloid-beta and its transporters in neonatal rats with and without hydrocephalus. Cerebrospinal Fluid Res. 2009;6:4.
142 Khan OH, Enno TL, Del Bigio MR. Brain damage in neonatal rats following kaolin induction of hydrocephalus. Exp Neurol. 2006;200:311.
143 Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317.
144 Del Bigio MR, da Silva MC, Drake JM, Tuor UI. Acute and chronic cerebral white matter damage in neonatal hydrocephalus. Can J Neurol Sci. 1994;21:299.
145 Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85:573.
146 Del Bigio MR, Bruni JE. Periventricular pathology in hydrocephalic rabbits before and after shunting. Acta Neuropathol. 1988;77:186.
147 Del Bigio MR, Bruni JE, Fewer HD. Human neonatal hydrocephalus. An electron microscopic study of the periventricular tissue. J Neurosurg. 1985;63:56.
148 Bruni JE, Del Bigio MR, Clattenburg RE. Ependyma: normal and pathological. A review of the literature. Brain Res Rev. 1985;356:1.
149 Glees P, Hasan M. Ultrastructure of human cerebral macroglia and microglia: maturing and hydrocephalic frontal cortex. Neurosurg Rev. 1990;13:231.
150 Hasan M, Glees P. The fine structure of human cerebral perivascular pericytes and juxtavascular phagocytes: their possible role in hydrocephalic edema resolution. J Hirnforsch. 1990;31:237.
151 Glees P, Voth D. Clinical and ultrastructural observations of maturing human frontal cortex. Part I (Biopsy material of hydrocephalic infants). Neurosurg Rev. 1988;11:273.
152 Mangano FT, McAllister JP, Jones HC, et al. The microglial response to progressive hydrocephalus in a model of inherited aqueductal stenosis. Neurol Res. 1998;20:697.
153 Ulfig N, Bohl J, Neudorfer F, Rezaie P. Brain macrophages and microglia in human fetal hydrocephalus. Brain Dev. 2004;26:307.
154 Yoshida Y, Koya G, Tamayama K, et al. Development of GFAP-positive cells and reactive changes associated with cystic lesions in HTX rat brain. Neurol Med Chir. 1990;30:445.
155 Yoshida Y, Koya G, Tamayama K, et al. Histopathology of cystic cavities in the cerebral white matter of HTX rats with inherited hydrocephalus. Neurol Med Chir. 1990;30:229.
156 Albrechtsen M, Sorensen PS, Gjerris F, Bock E. High cerebrospinal fluid concentration of glial fibrillary acidic protein (GFAP) in patients with normal pressure hydrocephalus. J Neurol Sci. 1985;70:269.
157 Krueger RCJr, Wu H, Zandian M, et al. Neural progenitors populate the cerebrospinal fluid of preterm patients with hydrocephalus. J Pediatr. 2006;148:337.
158 Petzold A, Keir G, Kerr M, et al. Early identification of secondary brain damage in subarachnoid hemorrhage: a role for glial fibrillary acidic protein. J Neurotrauma. 2006;23:1179.
159 Tullberg M, Rosengren L, Blomsterwall E, et al. CSF neurofilament and glial fibrillary acidic protein in normal pressure hydrocephalus. Neurology. 1998;50:1122.
160 Tullberg M, Blennow K, Mansson JE, et al. Ventricular cerebrospinal fluid neurofilament protein levels decrease in parallel with white matter pathology after shunt surgery in normal pressure hydrocephalus. Eur J Neurol. 2007;14:248.
161 Beems T, Simons KS, Van Geel WJ, et al. Serum- and CSF-concentrations of brain specific proteins in hydrocephalus. Acta Neurochir (Wien). 2003;145:37.
162 Boon AJ, Tans JT, Delwel EJ, et al. Dutch normal-pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J Neurosurg. 1997;87:687.
163 Czosnyka M, Maksymowicz W, Batorski L, et al. Comparison between classic-differential and automatic shunt functioning on the basis of infusion tests. Acta Neurochir. 1990;106:1.
164 Czosnyka M. Pulsatility index. J Neurosurg. 2001;94:685.
165 Czosnyka M, Whitehouse H, Smielewski P, et al. Testing of cerebrospinal compensatory reserve in shunted and non-shunted patients: a guide to interpretation based on an observational study. J Neurol Neurosurg Psychiatry. 1996;60:549.
166 Ekstedt J. CSF hydrodynamic studies in man. 1. Method of constant pressure CSF infusion. J Neurol Neurosurg Psychiatry. 1977;40:105.
167 Katzman R, Hussey F. A simple constant-infusion manometric test for measurement of CSF absorption. I. Rationale and method. Neurology. 1970;20:534.
168 Maksymowicz W, Czosnyka M, Koszewski W, et al. The role of cerebrospinal compensatory parameters in the estimation of functioning of implanted shunt system in patients with communicating hydrocephalus (preliminary report). Acta Neurochir. 1989;101:112.
169 Marmarou A. Progress in the analysis of intracranial pressure dynamics. In: Miller JD, editor. Intracranial Pressure. VI ed. Berlin: Springer-Verlag; 1986:781-788.
170 Marmarou A, Shulman K, Rosende RM. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg. 1978;48:332.
171 Tisell M, Edsbagge M, Stephensen H, et al. Elastance correlates with outcome after endoscopic third ventriculostomy in adults with hydrocephalus caused by primary aqueductal stenosis. Neurosurgery. 2002;50:70.
172 Tans JT, Boon AJW, Dutch NPH Study Group. How to select patients with normal pressure hydrocephalus for shunting. Acta Neurochir Suppl. 2002;81:3.
173 Tans JT, Poortvliet DCJ. Reduction of ventricular size after shunting for normal pressure hydrocephalus related to CSF dynamics before shunting. J Neurol Neurosurg Psychiatry. 1988;51:521.
174 Jones HC, Deane R, Bucknall RM. Developmental changes in cerebrospinal fluid pressure and resistance to absorption in rats. Brain Res. 1987;430:23.
175 Jones HC, Bucknall RM. Changes in cerebrospinal fluid pressure and outflow from the lateral ventricles during development of congenital hydrocephalus in the H-Tx rat. Exp Neurol. 1987;98:573.
176 Meier U, Kiefer M, Bartels P. The ICP-dependency of resistance to cerebrospinal fluid outflow: a new mathematical method for CSF-parameter calculation in a model with H-TX rats. J Clin Neurosci. 2002;9:58.
177 Agre P, King LS, Yasui M, et al. Aquaporin water channels—from atomic structure to clinical medicine. J Physiol. 2002;542:3.
178 Badaut J, Regli L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience. 2004;129:971.
179 Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367.
180 Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988;263:15634.
181 Badaut J, Hirt L, Granziera C, et al. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:477.
182 Stephensen H. Understanding CSF dynamics and hydrocephalus: do water channels carry the solution? Hydrocephalus 2006. 2006. Ref Type: Conference Proceeding.
183 Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129:999.
184 Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991.
185 de la Torre JC, Stefano GB. Evidence that Alzheimer’s disease is a microvascular disorder: the role of constiutive nitric oxide. Brain Res Rev. 2000;34:119.
186 Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23:2929.
187 McAllister JP, Miller JM. Aquaporin 4 and hydrocephalus. J Neurosurg. 2006;105:457.
188 Shen XQ, Miyajima M, Ogino I, Arai H. Expression of the water-channel protein aquaporin 4 in the H-Tx rat: possible compensatory role in spontaneously arrested hydrocephalus. J Neurosurg. 2006;105:459.
189 Suk K. Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett. 2004;366:167.
190 Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527.
191 Lin S, Zhang Y, Dodel R, et al. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition of p38 MAPK in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61.
192 Yrjanheikki J, Tikka T, Keinanen R, et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496.
193 Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312.
194 Streit WJ, Conde JR, Fendrick SE, et al. Role of microglia in the central nervous system’s immune response. Neurol Res. 2005;27:685.
195 Lechpammer M, Manning SM, Samonte F, et al. Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leucomalacia. Neuropathol Appl Neurobiol. 2008;34:379.
196 Wu J, Yang S, Xi G, et al. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res. 2009;31:183.
197 Crack PJ, Gould J, Bye N, et al. The genomic profile of the cerebral cortex after closed head injury in mice: effects of minocycline. J Neural Transm. 2009;116:1.
198 Noble W, Garwood C, Stephenson J, et al. Minocycline reduces the development of abnormal tau species in models of Alzheimer’s disease. FASEB J. 2009;23:739.
199 Marchand F, Tsantoulas C, Singh D, et al. Effects of etanercept and minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13:673.
200 Cai ZY, Yan Y, Sun SQ, et al. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci Bull. 2008;24:305.
201 Pinzon A, Marcillo A, Quintana A, et al. A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res. 2008;1243:146.
202 Hoang TX, Akhavan M, Wu J, Havton LA. Minocycline protects motor but not autonomic neurons after cauda equina injury. Exp Brain Res. 2008;189:71.
203 Carty ML, Wixey JA, Colditz PB, Buller KM. Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci. 2008;26:477.
204 Luccarini I, Ballerini C, Biagioli T, et al. Combined treatment with atorvastatin and minocycline suppresses severity of EAE. Exp Neurol. 2008;211:214.
205 Saganova K, Orendacova J, Cizkova D, Vanicky I. Limited minocycline neuroprotection after balloon-compression spinal cord injury in the rat. Neurosci Lett. 2008;433:246.
206 Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211.
207 Frenzel T, Lee CZ, Kim H, et al. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis. 2008;25:157.
208 Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem. 2008;105:1582.
209 Nagel S, Su Y, Horstmann S, et al. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008;1188:198.
210 Fan R, Xu F, Previti ML, et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057.
211 Szymanska A, Biernaskie J, Laidley D, et al. Minocycline and intracerebral hemorrhage: influence of injury severity and delay to treatment. Exp Neurol. 2006;197:189.
212 Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience. 2006;141:27.
213 Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776.
214 Fox C, Dingman A, Derugin N, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138.
215 Lechpammer M, Samonte F, Talos D, et al. Minocycline decreases activated microglia in hypoxic/ischemic cerebral white matter of immature rat [abstract]. Washington, DC: Society for Neuroscience Program No. 222.9; 2005.
216 Baptiste DC, Powell KJ, Jollimore CAB, et al. Effects of minocycline and tetracycline on retinal ganglion cell survival after axotomy. Neuroscience. 2005;134:575.
217 Teng YD, Choi H, Onario RC, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101:3071.
218 Ryu JK, Franciosi S, Sattayaprasert P, Kim MJ. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia. 2004;48:85.
219 Wells JEA, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain Res. 2003;126:1628.
220 Dommergues M-A, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 2003;121:619.
221 Arvin KL, Han BH, Du Y, et al. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54.
222 Tikka TM, Vartiainen NE, Goldsteins G, et al. Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain. 2002;125:722.
223 Zhu S, Stavrovskaya IG, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74.
224 Du Y, Ma Z, Lin S, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669.
225 He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001;909:187.
226 Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393.
227 Chen M, Ona VO, Li M, et al. Minocycline inhibits caspase 1 and caspase 3 expression and delays mortality in a transgenic mouse model of Huntington’s disease. Nat Med. 2000;6:797.
228 Ravina BM, Fagan SC, Hart RG, et al. Neuroprotective agents for clinical trials in Parkinson’s disease: a systematic assessment. Neurology. 2003;60:1234.
229 LeWitt PA. Clinical Trials of neuroprotection for Parkinson’s disease. Neurology. 2004;63:S23.
230 Bonelli RM, Hodl AK, Hofmann P, Kapfhammer HP. Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol. 2005;6:337.
231 Huntington Study Group. Minocycline safety and tolerability in Huntington disease. Neurology. 2004;63:547.
232 Thomas M, Ashizawa T, Jankovic J. Minocyclines in Huntington’s disease: a pilot study. Mov Disord. 2004;19:692.
233 Smith DL, Woodman B, Mahal A, et al. Minocycline and doxycycline are not beneficial in a model of Huntington’s disease. Ann Neurol. 2003;54:186.
234 Gordon PH, Moore DH, Gelinas DF, Miller RG. Placebo-controlled phase I/II studies of minocycline in amyotrophic lateral sclerosis. Neurology. 2004;62:E22.
235 Fong JS, Rae-Grant A, Huang D. Neurodegeneration and neuroprotective agents in multiple sclerosis. Recent Patents CNS Drug Discov. 2008;3:153.
236 Zhang Y, Metz LM, Yong VW, et al. Pilot study of minocycline in relapsing-remitting multiple sclerosis. Can J Neurol Sci. 2008;35:185.
237 Miyaoka T. Clinical potential of minocycline for schizophrenia. CNS Neurol Disord Drug Targets. 2008;7:376.
238 Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2008.
239 Cai Z, Lin S, Fan LW, et al. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425.
240 Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005;158:58.
241 Cai Z, Lin S, Fan L, et al. Minocycline protects developing oligodendrocytes from hypoxia—ischemia induced injury in the neonatal rat brain [abstract]. Washington, DC: Society for Neuroscience Program No. 222.10; 2005.
242 Li W, Setzu A, Zhao C, Frankovic R. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte prgenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2004;158:58.
243 Miller JM, McAllister JP II. Minocycline reduces gliosis and increases cortical thickness in experimental hydrocephalus. Paper presented at Goteburg, Sweden, 2006.
244 Di Rocco C, Di Trapani G, Pettorossi VE, Caldarelli M. On the pathology of experimental hydrocephalus induced by artificial increase in endoventricular CSF pulse pressure. Childs Brain. 1979;5:81.
245 Bradley WGJr, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198:523.
246 Dixon GR, Friedman JA, Luetmer PH, et al. Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculoperitoneal shunting for idiopathic normal-pressure hydrocephalus. Mayo Clin Proc. 2002;77:509.
247 Luetmer PH, Huston J, Friedman JA, et al. Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2002;50:534.
248 Baledent O, Gondry-Jouet C, Meyer ME, et al. Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Invest Radiol. 2004;39:45.
249 Bradley WG. Cerebrospinal fluid dynamics and shunt responsiveness in patients with normal-pressure hydrocephalus. Mayo Clin Proc. 2002;77:507.
250 Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27:145.
251 Egnor MR, Zheng L, Rosiello A, et al. A model of pulsations in communicating hydrocephalus. Pediatr Neurosurg. 2002;36:281.
252 Egnor MR, Rosiello A, Zheng L. A model of intracranial pulsations. Pediatr Neurosurg. 2001;35:284.
253 Egnor MR, Wagshul M, Zheng L, Rosiello A. Resonance and the synchrony of arterial and CSF pulsations. Pediatr Neurosurg. 2003;38:273-276.
254 Egnor MR, Wagshul ME, Madsen JR, et al. The cerebral windkessel and its relevance to hydrocephalus: the notch filter model of cerebral blood flow. Cerebrospinal Fluid Res. 2006;3(suppl 1):S48.
255 Wagshul ME, Chen JJ, Egnor MR, et al. Amplitude and phase of cerebrospinal fluid pulsations: experimental studies and review of the literature. J Neurosurg. 2006;104:810.
256 Madsen JR, Egnor MR, Zou R. Cerebrospinal fluid pulsatility and hydrocephalus: The fourth circulation. In: Clinical Neurosurgery. Lippincott Williams & Wilkins; 2006:48-52.
257 Wagshul ME, Egnor MR, McCormack EJ, et al. The cranium as an oscillator: analysis of phase relationships in intracranial blood and CSF pulsations using flow sensitive MRI. Cerebrospinal Fluid Res. 2006;3(suppl 1):S12.
258 McCormack EJ, Egnor MR, Wagshul ME. Improved cerebrospinal fluid flow measurements using phase contrast balanced steady-state free precession. Magn Reson Imaging. 2007;25:172.
259 Ayzman I, Weaver M, Luciano MG, McAllister JP II. Effects of infantile hydrocephalus and surgical decompression on the vascularization of cerebral cortex. Paper presented at Lende Neurosurgical Meeting, 1996.
260 Luciano MG, Skarupa DJ, Booth AM, et al. Cerebrovascular adaptation in chronic hydrocephalus. J Cereb Blood Flow Metab. 2001;21:285.
261 Del Bigio MR, Bruni JE. Changes in periventricular vasculature of rabbit brain following induction of hydrocephalus and after shunting. J Neurosurg. 1988;69:115.
262 Jones HC, Bucknall RM, Harris NG. The cerebral cortex in congenital hydrocephalus in the H-Tx rat: a quantitative light microscopy study. Acta Neuropathol. 1991;82:217.
263 Oka N, Nakada J, Endo S, Takaku A. Angioarchitecture in experimental hydrocephalus. Pediatr Neurosci. 1985;12:294.
264 Okuyama T, Hashi K, Sasaki S, et al. Changes in cerebral microvasculature in congenital hydrocephalus of the inbred rat LEW/Jms: light and electron microscopic examination. Surg Neurol. 1987;27:338.
265 Castejon OJ. Transmission electron microscope study of human hydrocephalic cerebral cortex. J Submicrosc Cytol Pathol. 1994;26:29.
266 Nakagawa Y, Cervós-Navarro J, Artigas J, Cervos-Navarro J. Tracer study on a paracellular route in experimental hydrocephalus. Acta Neuropathol. 1985;65:247.
267 Sada Y, Moriki T, Kuwahara S, et al. Immunohistochemical study on blood-brain barrier in congenitally hydrocephalic HTX rat brain. Zentralbl Pathol. 1994;140:289.
268 Owler BK, Pena A, Momjian S, et al. Changes in cerebral blood flow during cerebrospinal fluid pressure manipulation in patients with normal pressure hydrocephalus: a methodological study. J Cereb Blood Flow Metab. 2004;24:487.
269 Brooks DJ, Beaney RP, Powell M, et al. Studies on cerebral oxygen metabolism, blood flow, and blood volume, in patients with hydrocephalus before and after surgical decompression, using positron emission tomography. Brain. 1986;109:613.
270 Chang CC, Kuwana N, Ito S, et al. Cerebral haemodynamics in patients with hydrocephalus after subarachnoid haemorrhage due to ruptured aneurysm. Eur J Nucl Med Mol Imaging. 2003;30:123.
271 Graff-Radford NR, Rezai K, Godersky JC, et al. Regional cerebral blood flow in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1987;50:1589.
272 Greitz TVB, Grepe AOL, Kalmér MSF, Lopez J. Pre- and postoperative evaluation of cerebral blood flow in low-pressure hydrocephalus. J Neurosurg. 1969;31:644.
273 Klinge P, Berding G, Brinker T, et al. PET-studies in idiopathic chronic hydrocephalus before and after shunt-treatment: the role of risk factors for cerebrovascular disease (CVD) on cerebral hemodynamics. Acta Neurochir Suppl. 2002;81:43-45.
274 Klinge PM, Brooks DJ, Samii A, et al. Correlates of local cerebral blood flow (CBF) in normal pressure hydrocephalus patients before and after shunting–a retrospective analysis of [(15)O]H(2)O PET-CBF studies in 65 patients. Clin Neurol Neurosurg. 2008;110:369.
275 Larsson A, Bergh AC, Bilting M, et al. Regional cerebral blood flow in normal pressure hydrocephalus: diagnostic and prognostic aspects. Eur J Nucl Med. 1994;21:118.
276 Lee EJ, Hung YC, Chang CH, et al. Cerebral blood flow velocity and vasomotor reactivity before and after shunting surgery in patients with normal pressure hydrocephalus. Acta Neurochir. 1998;140:599.
277 Mori K, Maeda M, Asegawa S, Iwata J. Quantitative local cerebral blood flow change after cerebrospinal fluid removal in patients with normal pressure hydrocephalus measured by a double injection method with N-isopropyl-p-[(123)I] iodoamphetamine. Acta Neurochir (Wien). 2002;144:255.
278 Nakada J, Oka N, Nagahori T, et al. Changes in the cerebral vascular bed in experimental hydrocephalus: an angio-architectural and histological study. Acta Neurochir. 1992;114:43.
279 Shirane R, Sato S, Sato K, et al. Cerebral blood flow and oxygen metabolism in infants with hydrocephalus. Childs Nerv Syst. 1992;8:118.
280 Tanaka A, Kimura M, Nakayama Y, et al. Cerebral blood flow and autoregulation in normal pressure hydrocephalus. Neurosurgery. 1997;40:1161.
281 Vorstrup S, Christensen J, Gjerris F, et al. Cerebral blood flow in patients with normal-pressure hydrocephalus before and after shunting. J Neurosurg. 1987;66:379.
282 Waldemar G, Schmidt JF, Delecluse F, et al. High resolution SPECT with [99mTc]-d,l-HMPAO in normal pressure hydrocephalus before and after shunt operation. J Neurol Neurosurg Psychiatry. 1993;56:655.
283 da Silva MC, Michowicz S, Drake JM, et al. Reduced local cerebral blood flow in periventricular white matter in experimental neonatal hydrocephalus:restoration with CSF shunting. J Cereb Blood Flow Metab. 1995;15:1057.
284 Dombrowski SM, Schenk S, Leichliter A, et al. Chronic hydrocephalus-induced changes in cerebral blood flow: mediation through cardiac effects. J Cereb Blood Flow Metab. 2006;26:1298.
285 Jones HC, Richards HK, Bucknall RM, Pickard JD. Local cerebral blood flow in rats with congenital hydrocephalus. J Cereb Blood Flow Metab. 1993;13:531.
286 Klinge PM, Samii A, Muhlendyck A, et al. Cerebral hypoperfusion and delayed hippocampal response after induction of adult kaolin hydrocephalus. Stroke. 2003;34:193.
287 Ransohoff J, DiMattio J, Hochwald GM, Epstein F. Cerebral fluid dynamics and brain regional blood flow in experimental hydrocephalus. Childs Brain. 1975;1:183.
288 Richards HK, Bucknall RM, Jones HC, Pickard JD. Uncoupling of LCBF and LCGU in two different models of hydrocephalus: a review. Childs Nerv Syst. 1995;11:288.
289 Vasthare US, McAllister JPII, Hale PM, et al. Cerebrovascular and evoked potential effects of neonatal hydrocephalus. Soc Neurosci Abstr. 17, 1991.
290 de la Torre JC, Aliev G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: spatial memory and immunocytochemical changes. J Cereb Blood Flow Metab. 2005;25:663.
291 Loscalzo J. Endothelial injury, vasoconstriction, and its prevention. Tex Heart Inst J. 1995;22:180.
292 Luscher TF, Rubanyi GM, Masaki T, et al. Introduction: endothelium as regulator of tone and growth. Circulation. 1993;87:1.
293 Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:1145.
294 Rubanyi GM, Freay AD, Kauser K, et al. Mechanoreception by the endothelium: mediators and mechanism of pressure- and flow-induced vascular responses. Blood Vessels. 1990;27:246.
295 Ziegler T, Bouzourene K, Harrison VJ, et al. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686.
296 Bilfinger TV, Stefano GB. Human aortocoronary grafts and nitric oxide release: relationships to pulsatile pressure. Ann Thorac Surg. 2000;69:480.
297 Zou R, Park EH, Kelly EM, et al. Intracranial pressure waves: characterization of a pulsation absorber with notch filter properties using systems analysis: laboratory investigation. J Neurosurg Pediatr. 2008;2:83.
298 Wagshul ME, Kelly EJ, Yu HJ, et al. Resonant and notch behavior in intracranial pressure dynamics. J Neurosurg Pediatr. 2009;3:354.
299 Wagshul ME, McAllister JPII, Rashid S, et al. Ventricular dilation and elevated aqueductal pulsations in a new experimental model of communicating hydrocephalus. Exp Neurol. 2009;218:33.
300 Boulton M, Flessner M, Armstrong D, et al. Lymphatic drainage of the CNS: effects of lymphatic diversion/ligation on CSF protein transport to plasma. Am J Physiol. 1997;272:R1613.
301 Boulton M, Armstrong D, Flessner M, et al. Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol. 1998;275:R889.
302 Boulton M, Flessner M, Armstrong D, et al. Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol. 1998;274:R88.
303 Boulton M, Flessner M, Armstrong D, et al. Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am J Physiol. 1999;276:R818.
304 Johnston M, Papaiconomou C. Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci. 2002;17:227.
305 Mollanji R, Bozanovic-Sosic R, Zakharov A, et al. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1593.
306 Papaiconomou C, Bozanovic-Sosic R, Zakharov A, Johnston M. Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol Regul Integr Comp Physiol. 2002;283:R869.
307 Zakharov A, Papaiconomou C, Djenic J, et al. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol Appl Neurobiol. 2003;29:563.
308 Johnston M, Zakharov A, Papaiconomou C, et al. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2.
309 Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6.
310 Nagra G, Koh L, Zakharov A, et al. Quantification of cerebrospinal fluid transport across the cribriform plate into lymphatics in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1383.