Exogenous Hormones and their Effects on the Endometrium
Estrogens
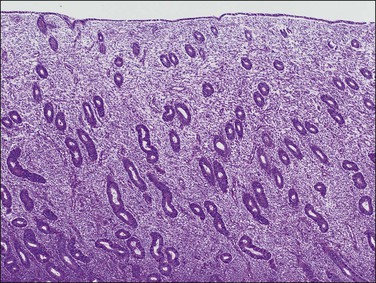
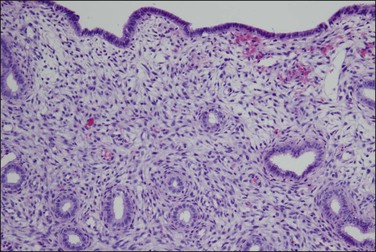
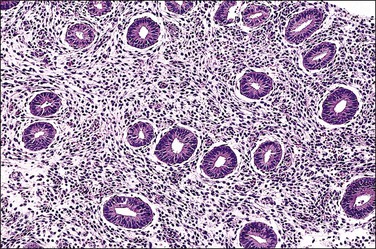
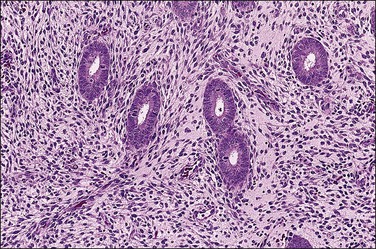
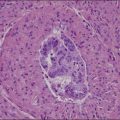
The basic effect of estrogens on the endometrium is to induce proliferation of the endometrial glands and stroma, including vascular endothelium. The degree of proliferation can vary in proportion to the estrogenic stimulus. Very low levels of estrogen or a very weak estrogen will lead to an inactive or atrophic endometrium. Higher levels lead to proliferation, which when excessive can produce a hyperplastic endometrium (Figure 15.1). The degree of proliferative activity can usually be assessed by the mitotic activity in both the glandular epithelium and the stroma. Women who are many years postmenopausal demonstrate profound endometrial atrophy, secondary to lack of estrogen, but even atrophic endometrium remains estrogen responsive to quite advanced age (Figure 15.2). A number of estrogenic drugs on the market are listed in Table 15.1.1
Table 15.1 Representative Estrogenic Agents Available in the United States Adapted from Schimmer and Parker (2012).1
| Generic Name | U.S. Brand Name |
| Estradiol | Estrace |
| Estradiol valerate | Delestrogen, Gynogen, Valergen |
| Estradiol (transdermal) | Estraderm, Climara, Fempatch |
| Diethylstilbestrol | |
| Conjugated equine estrogens | Premarin |
| Synthetic conjugated estrogens | Cenestin, Enjuvia |
| Mestranol | |
| Ethinyl estradiol | Estinyl |
| Estropipate | Ogen, Ortho-Est |
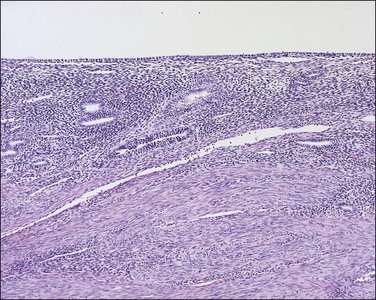
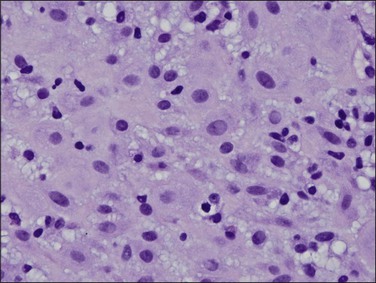
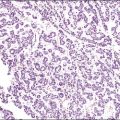
Figure 15.2 Atrophic endometrium. The few glands present have minimal cytoplasm and small nuclei. The endometrial stroma is dense.
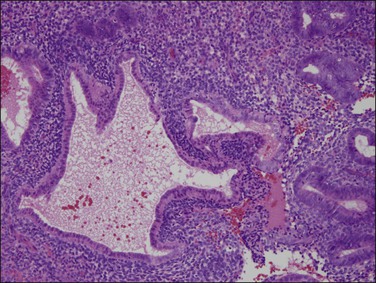
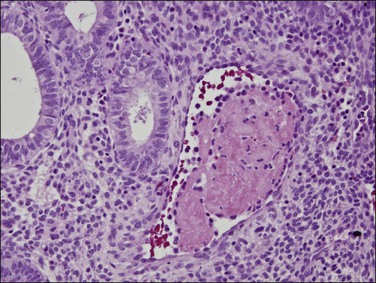
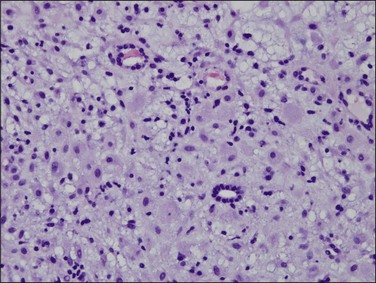
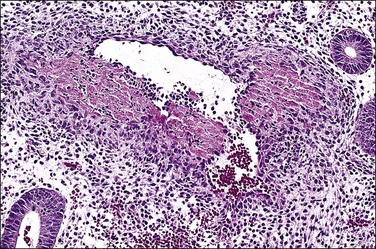
Persistent exposure to a significant estrogenic stimulus, such as occurs in anovulation, leads to a pattern of continued, unrelieved proliferation. The endometrium cannot support such continued growth. Whether the estrogen source is endogenous or exogenous, the histologic consequence is the same: a combination of proliferative endometrium with episodic coexisting stromal breakdown, or shedding, which accounts for the clinical presentation of irregular bleeding (Figure 15.3). This pattern is also called ‘anovulatory bleeding’ or ‘anovulatory shedding.’ Because the endometrial vessels become abnormally large, bleeding can also be quite severe. Some pathologists mistakenly refer to the shedding endometrium as ‘menstrual,’ but it is important to distinguish this appearance from the appearance of menstrual endometrium occurring at the end of a normal cycle. In normal endometrium, the endometrial glands transform soon after ovulation to a secretory pattern and by the time menstrual bleeding begins will still show some residual changes, most commonly secretory exhaustion (see Chapter 14). This change is absent in anovulatory-type bleeding or where the drug administered is progestin poor. Another common difference is the presence of large fibrin thrombi in the vessels of anovulatory shedding endometrium (Figure 15.4). They are absent from menstrual endometrium because of the marked fibrinolytic activity typical of normal menstruation.
With prolonged estrogen exposure, the proliferating glands tend to lose their uniformity of size, shape, and distribution, leading to so-called disordered proliferative endometrium. Cystic dilatation of glands and ‘tubal’ metaplasia are commonly present. With continued, longer term, unopposed estrogen exposure, a high proportion of patients will ultimately develop non-atypical endometrial hyperplasia. A small number will also go on to develop endometrial intraepithelial neoplasia (EIN) (also called ‘atypical hyperplasia’) or even adenocarcinoma (see Chapters 17 and 18).
Progestins
The effect of progestins on the endometrium depends on ‘priming’ by estrogen, which induces progesterone receptors in the endometrial cells. One important feature of progestins is that they act to downregulate estrogen and progesterone receptors; in other words, they reduce the sensitivity of the endometrium to both of these hormones. Prolonged exposure to progestins can thus lead to a histologic picture that is paradoxically similar to the atrophy seen in a postmenopausal or hormone-suppressed patient. Some commonly used progestins are shown in Table 15.2.1
Table 15.2 Representative Progestational Agents Available in the United States
Adapted from Schimmer and Parker (2012).1
| Generic Name | Brand Name |
| Progesterone | Prometrium, Crinone |
| Hydroxyprogesterone | Hylutin, Makena |
| Medroxyprogesterone | Provera, Prodrox, Cycrin, Amen, Curretab |
| Megestrol acetate | Megace |
| Norgestrel | Neogest |
| Drospirenone | |
| Levonorgestrel | Norplant, Plan B |
| Norethindrone | Aygestin |
| Norethynodrel | |
| Desogestrel | Cerazette |
| Norgestimate |
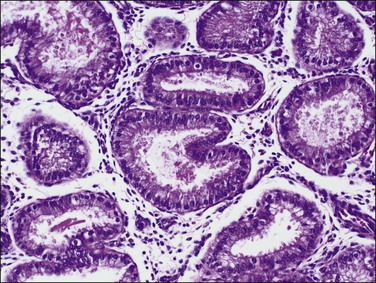
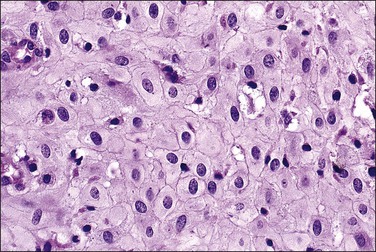
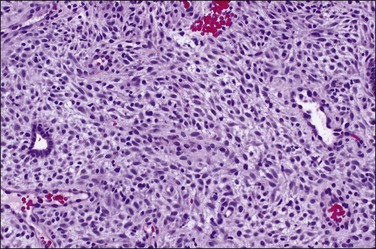
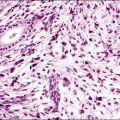
In the short term, however, progestins induce secretory differentiation in endometrial glands and decidual-type change in the stroma, i.e., the classic changes of a normal secretory endometrium. The glands develop large glycogen vacuoles that are then secreted into the increasingly complex gland lumina (Figures 15.5 and 15.6). At the same time, the stromal cells enlarge strikingly, acquire an abundant cytoplasm, and appear relatively cohesive (Figure 15.7). Even when pathology is present, the stroma may demonstrate pseudodecidual changes, but progesterone responsiveness is often diminished in disease states such as endometritis, and in polyps.
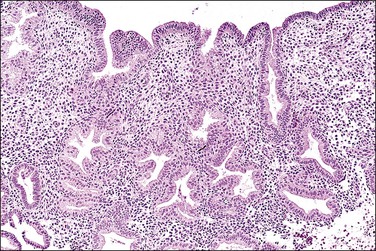
Figure 15.5 Secretory endometrium, demonstrating exogenous progesterone effect. The appearance is indistinguishable from a normal cycle. The woman, who was older, had received progesterone for 20 days.
With continued exposure or repeated cycles, the receptor downregulation causes the glands to lose their sensitivity to estrogens and progestins, leading to progressively less secretory change (‘secretory exhaustion’), such that they ultimately appear atrophic. At this stage, which only occurs after prolonged exposure or multiple cycles, the appearance is that of decidualized stroma with widely dispersed atrophic glands, often referred to as a ‘pill’ endometrium (Figure 15.8). More slowly, the decidualized stroma also begins to be suppressed by the receptor downregulation, and over months to years will become attenuated and atrophic, ultimately losing most of its decidual features. At this point, the appearance resembles endometrial atrophy due to menopause or hormone suppression.
Exposure to high-dose progestins (often given for dysfunctional uterine bleeding and usually with a good initial response) can, paradoxically, cause secondary necrosis of the superficial endometrium, often in the form of wedge-shaped infarcts, and renewed bleeding. The hysteroscopic appearances are often alarming. Both the continued bleeding and the hysteroscopic findings lead the clinician to perform a dilatation and curettage to exclude hyperplasia or malignancy and yielding abundant, macroscopically suspicious tissue fragments. Occasionally, patients will spontaneously pass large intact sheets of decidualized endometrium (‘decidual casts’).
Oral Contraceptives
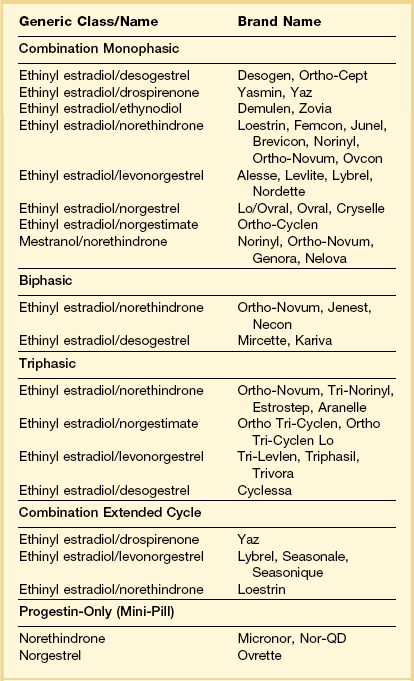
Oral contraceptives (OCs) are one of the most widely used medications in the developed world. They are used as treatment for several common medical problems in addition to contraception, including dysfunctional uterine bleeding and endometriosis. A variety of formulations and administration schedules have been developed (Table 15.3).1 Sequential OCs contain only estrogen during the first half of the cycle, with progestin added during the second half. Because of the increased risk of endometrial cancer with some sequential formulations,2 the most commonly used types today are combined estrogen–progestin and progestin-only formulations administered over 21 consecutive days of the cycle, followed by 7 days of placebo tablets yielding a withdrawal week. Combined pills can be monophasic, in which there is a fixed dose of estrogen and a progestin in combination for the 21 days of each cycle, or they can be biphasic or triphasic, depending on whether the progestin dose is altered once or twice during the cycle. In contrast to some sequential OCs and estrogen-only hormone replacement regimens, which increase the risk of endometrial carcinoma, combined OCs are protective, with the degree of protection increasing with the length of therapy. Patients with 10 years of OC use have about a 75% reduction in endometrial carcinoma.3,4 OC use is also associated with a 30–50% decrease in the risk of ovarian carcinoma; this lowered risk persists for at least 20 years after cessation of their use and is also seen in BRCA1 and BRCA2 mutation carriers.5 Recently, a variety of extended cycle (91 day) and even continuous cycle (365 day) OCs have been introduced that appear to have similar effectiveness to traditional OCs.6 The histologic effects of these newer formulations have not been well studied.
Table 15.3 Composition of Representative OCs Available in the United States
Adapted from Schimmer and Parker (2012).1
Combined OCs
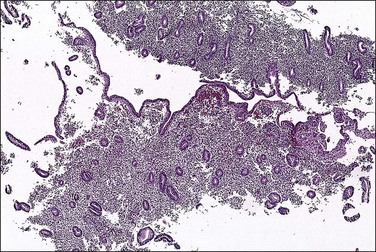
The histologic appearance of the endometrium in a patient on combined OCs is extremely variable, but is dominated by the progestin effects. It depends on various factors, some poorly understood, including the type of pill, the duration of therapy, precise dose of the individual pill, levels of compliance with the regimen, and endogenous hormone synthesis and metabolism.7–9 Certain generalizations are possible, however. Within the first several cycles of a combined OC, there is a mixture of proliferative and secretory features seen in the endometrium (‘asynchronous’ or ‘discordant’ endometrium). The glands tend to remain relatively straight and narrow, resembling proliferative endometrial glands but with cuboidal rather than columnar epithelium, and with very minimal mitotic activity. Subnuclear vacuoles may be seen, especially during the first 2 weeks of the cycle. Decidual change can be seen early but is usually more evident after several cycles have been completed (Figures 15.9 and 15.10). Despite the stromal changes, spiral arterioles do not develop normally.10 With prolonged therapy over many cycles the glands develop secretory exhaustion and become increasingly small, inactive, and ultimately atrophic. As this occurs, the stromal decidual changes become increasingly well developed, until the endometrium is uniformly decidualized, with only rare, atrophic-appearing glands (Figures 15.11 and 15.12). This is the classic appearance of the so-called ‘pill’ endometrium. Prominent ectatic thin-walled veins are also seen, which often contain thromboses in patients having breakthrough bleeding (Figure 15.13). The classic pill endometrium is not seen in all patients, and there is a spectrum from well-developed stromal changes to complete atrophy, with only a very thin endometrium showing little or no identifiable decidual change. The atrophic changes are seen more frequently with some of the lower dose regimens.8
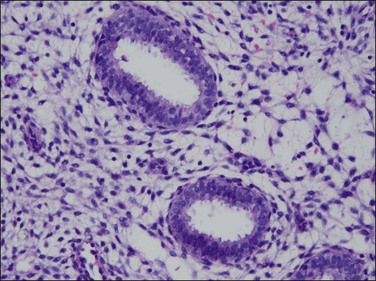
Figure 15.10 Combined OC, secretory exhaustion. The glands are small and show only rudimentary evidence of secretory activity in the form of apical snouts.
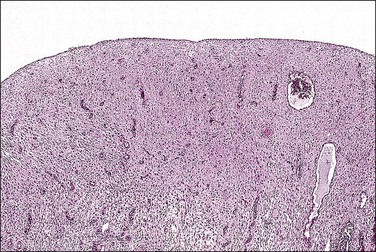
Figure 15.11 Combined OC (Loestrin), with classic pill endometrium. The stroma is massively decidualized and contains widely scattered, atrophic glands.
Formulations that include short estrogen-only periods have been used in an attempt to lower the overall dose of hormones. Mircette, for example, has 21 days of combination treatment followed by a 2 day, hormone-free interval and 5 days of unopposed estrogen. Technically, this is a sequential OC, but to date it has not had the safety issues associated with the older, higher dose sequential therapies. This formulation has a distinctly different histologic pattern than the combined OCs. Biopsies taken during the estrogen-only portion of the cycle show proliferative endometrium while biopsies taken during the combined portion of the cycle show secretory endometrium.11
Pure Progestin OCs (‘Mini-Pill’)
In contrast to the combined OCs, the pure progestin OC (the mini-pill, 300 μg norethisterone) is taken daily without interruption during the cycle. The mechanism of contraception differs from combined OCs in that ovulation is not consistently suppressed. Instead, contraception is due to the production of relatively thick cervical mucus that is impermeable to sperm, and to the atrophic endometrium that will not support implantation. The endometrial changes seen with the mini-pill are not distinctly different from those seen with combined OCs, but marked atrophy is more common and biopsy frequently yields only scanty material. In addition, proliferative endometrium is not infrequently seen, and this correlates with abnormal bleeding.12
Long-Term, Progestin-Only OCs
Several systems have been developed for long-term, progestin-only contraception. Medroxyprogesterone acetate microcrystals in an aqueous solution (Depo-Provera) are used as an intramuscular injection. The slow dissolution of the crystals maintains effective progestin levels for several months, allowing for an injection schedule of every 3 months. Levels of progestin are typically higher than with the mini-pill. Early after injection, some women develop exaggerated hypersecretory changes that resemble gestational endometrium, including the presence of Arias-Stella reaction. By 3–6 months these changes have resolved and stromal decidual changes develop, resembling those seen with combined OCs as described earlier. Long-term treatment can result in atrophy or pure decidua-like change (Figure 15.14), just as with combined OCs.8,13
The Norplant system consists of several Silastic tubes containing levonorgestrel. The tubes are slightly permeable to steroids, and the progestin is slowly released for a period of 3–5 years. As in other progestin-only regimens, ovulation occurs in about one-third of cycles, and contraception is by production of thick cervical mucus and an endometrium inimical to implantation. There are relatively few studies on the histologic findings with Norplant,14,15 but the findings seem to be similar to other progestin-only regimens, with atrophy being the most common finding. Proliferative endometrium can also be seen, and correlates with irregular bleeding patterns.16
Devices that supply progestins directly to the endometrium are also used. The most common of these uses a slow-release formulation of levonorgestrel in combination with an intrauterine device (Mirena). Although physically located within the endometrium, absorption is systemic and they can be used for treatment of extrauterine disease such as endometriosis as well as abnormal uterine bleeding.17–19 Histologically, the endometrium shows extensive decidualization.20
Hormone Replacement Therapy
The number of patients receiving long-term (>5 years) HRT dropped precipitously after large studies showed an increase in cardiovascular risk rather than the predicted protective effects.21 In the United States, the number of prescriptions for HRT in the national Medicaid program fell by 57% between 2002 and 2004.22 Nonetheless, HRT continues to be commonly used as short-term therapy for symptoms related to menopause.
Unopposed Estrogen HRT
Historically, estrogen alone was given as hormone replacement for postmenopausal women, and the beneficial effects of this therapy in preventing osteoporosis and cardiovascular disease have been well documented. Then a series of case–control studies subsequently demonstrated a markedly increased risk of endometrial adenocarcinoma in women using long-term estrogens unopposed by progestins. Patients receiving unopposed estrogens for 5 years or more have an approximately sixfold increase in endometrial carcinoma. With the addition of progestins, the risk of endometrial carcinoma fell to near control levels.23–25 The risk of endometrial carcinoma is dose related, but low-dose estrogens (0.3 mg conjugated estrogens or equivalent) continue to be associated with a risk of carcinoma if not combined with a progestin.26 Even estrogens applied as vaginal creams have sufficient systemic absorption to increase the risk.27 Although current practice is treatment with combined therapy, occasional patients are still treated with estrogen alone. It should be noted that, although addition of a progestin reduces the risk of endometrial cancer from HRT, combined HRT is associated with a significant increase in the risk of breast cancer.28
The endometrium from a woman being treated with unopposed estrogens will most commonly appear proliferative, and may in fact be indistinguishable from a normal proliferative endometrium in a premenopausal patient. If the estrogen dose is low, there may also be a lesser degree of proliferation that is described as weakly proliferative.29 If the patient is bleeding, the endometrium will often have proliferative glands with associated stromal breakdown deep below the uterine surface lining (Figure 15.3), a feature that specifically suggests unopposed estrogen exposure. Unfortunately, conventional microscopy cannot distinguish these changes due to exogenous estrogens from the effects of endogenous estrogen, as in anovulatory bleeding, or from the effects of other agents that may act as estrogenic agents in the endometrium. These include tamoxifen, various drugs and pharmaceutical agents such as digitalis or phenothiazines, and herbal preparations such as ginseng.
Long-term estrogen exposure can lead to a full spectrum of appearances, ranging from disordered proliferative changes to non-atypical endometrial hyperplasia, EIN, or even adenocarcinoma.7 Disordered proliferative endometrium shows a basic pattern of proliferative endometrium, with the addition of irregularly dilated and focally branched glands. This condition is most commonly seen in untreated women during the perimenopause and is not felt to be premalignant (see Chapter 17). Tubal metaplasia is common. The hallmark of non-atypical endometrial hyperplasia is the development of endometrial compartment-wide irregular gland shape and distribution, which in many areas has glandular crowding to more than a 1 : 1 gland to stroma ratio. EIN may emerge from non-atypical endometrial hyperplasia as a localized lesion with discrete cytology.30 Adenocarcinomas are of the endometrioid type, exhibit severe glandular crowding, usually with a cribriform or papillary architecture, and at least mild nuclear atypia (see Chapter 18). Tumors developing from unopposed estrogen exposure, whether endogenous or exogenous, are usually low grade with high long-term survival rates. Patients who develop adenocarcinomas usually have a minimum of 2–3 years of unopposed estrogen use. The risk increases over time, with the highest risk in patients who have taken estrogens for 10 or more years.9
Cyclic Estrogen–Progesterone HRT
Because unopposed estrogens elevate the risk of endometrial adenocarcinoma, HRT today nearly always includes a progestin (if the patient has a uterus). The most commonly used agents are conjugated equine estrogen (Premarin) in combination with medroxyprogesterone acetate (Provera). Cyclic or sequential HRT uses daily estrogen for the first 21–25 days of the month with daily progestin added for the last 10–13 days. Consistent reduction of mitotic rates in glandular epithelium is found after generally only 9 or more days of progesterone administration in each cycle.31,32 This regimen mimics to some degree the normal progression of these hormones during a menstrual cycle and typically results in a withdrawal bleed at the end of each cycle. Longer cycle lengths (i.e., 12 instead of 4 weeks) have shown a decrease in the protective effect of the progestin and are not generally recommended.33
The pathologic findings in the endometrium are somewhat but not entirely predictable based on our understanding of normal endometrial cycles. Not surprisingly, biopsies taken from the estrogen-only portion of the cycle typically have a proliferative appearance, and may be histologically identical to a normal proliferative endometrium (Figures 15.15–15.17). Biopsies taken after the initiation of the progestin are more variable. Most show some degree of secretory change, beginning about 3 days after the beginning of the progestin therapy, but the changes lack the well-ordered daily progression seen in normal secretory endometrium and cannot be ‘dated’ in the same fashion (see Chapter 14).34–36 Frequently, the glandular component develops no further than an early secretory appearance, with variably developed glycogen vacuoles persisting even late into the artificial cycle. The stroma shows a variable response to the progestin, and may develop a spotty decidual response by day 10 after progestin initiation. This is often described in pathology reports as gland–stromal asynchrony, and can be due to various other factors, including intrauterine devices (IUDs), OCs, underlying mass lesions such as leiomyomas or polyps, chronic endometritis, and other types of hormone such as mifepristone, clomiphene, and gonadotropins. Although this sequence of incomplete cycling is the most common picture with cyclic HRT, other patterns are not infrequent. Some patients will have normal menstrual changes (Figure 15.18) impossible to distinguish from women who have never taken HRT. Up to one-fifth of patients will have an inactive endometrium. This group of patients generally does not experience monthly withdrawal bleeds.
Combined Estrogen–Progesterone HRT
Continuous combined estrogen–progestin HRT protects against the carcinogenic effects of unopposed estrogen. These regimens generally have a relatively predictable and uniform effect on the endometrium. As described previously, the continuous long-term use of progesterone leads to downregulation of both estrogen and progesterone receptors, diminishing the responsiveness of the endometrium. This leads to the most common appearance, namely an atrophic or inactive endometrium.8,37 There may be weak, poorly developed decidual change in the stromal cells, and the glandular component may show a few glycogen vacuoles suggesting progestin effect in the glands, but these findings are variable. Least common, there is a well-developed stromal decidual change, similar to that seen with OCs (Figure 15.19). Metaplastic changes such as tubal metaplasia, eosinophilic metaplasia, squamous metaplasia, and papillary syncytial metaplasia are more common with combined continuous HRT.7
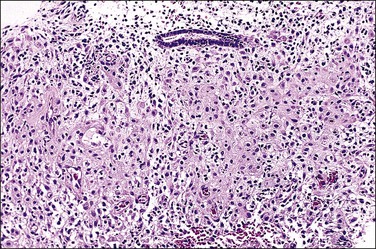
Figure 15.19 Combined HRT (Prempro), extensive stromal decidual change. The stroma shows a well-developed decidual change, with inactive glands. This pattern can mimic “pill” endometrium.
A clearly proliferative pattern in the endometrium would suggest an inadequate dose of the progestational agent, especially if there is ongoing breakdown (i.e., an anovulatory-like pattern) (Figures 15.20 and 15.21).
Other Hormonal Agents
Tamoxifen
Tamoxifen has been widely used in the treatment and now the prevention of breast cancer, where it functions as an antiestrogen.38 Toremifene is a closely related agent that is available in several countries; it will be considered together with tamoxifen as they have very similar actions.39 In the endometrium, tamoxifen is a strong competitive inhibitor of circulating estrogens, but also a weak estrogen agonist. In the presence of high estrogen levels, as found in premenopausal women, it competes for receptors, thereby reducing overall estrogen stimulation. In the presence of very low ambient estrogen levels, i.e., in the postmenopausal patient, the agonist activity of tamoxifen can result in mild activation of the endometrial estrogen receptor.40 While most postmenopausal women will continue to have atrophic or (regular or cystic) inactive endometrium (Figure 15.22), a few will develop the pathologic changes associated with chronic unopposed estrogen stimulation.41 As might be expected, women receiving tamoxifen who are symptomatic have far greater frequencies of endometrial pathologies than women who are asymptomatic (93% vs 25%).42–44
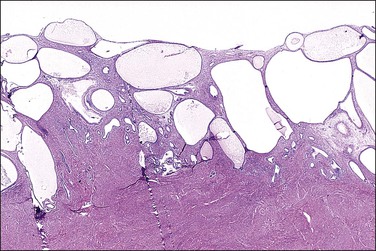
Figure 15.22 Tamoxifen, cystic atrophy. Large cystic glands are present in the endometrium.
(Courtesy of Dr. H. Krigman, University of Minnesota.)
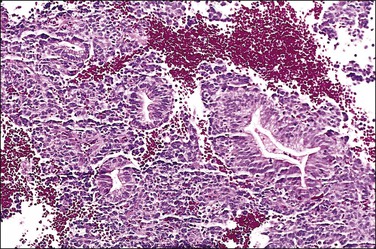
Multiple reports have described unusual-appearing endometrial polyps in tamoxifen-treated patients, with cystically dilated glands sometimes containing luminal secretions, a variety of metaplasias, massive fibrosis and, more rarely, decidualized stroma (Figures 15.23 and 15.24).44,45 Some of these changes may be dose related.46 Many of the polyps are 20 mm or more in size, which is larger than seen with any other form of HRT. No studies have yet to indicate why this drug alone has such a peculiar effect on the endometrial stroma. Malignancies are occasionally found in polyps associated with tamoxifen therapy and at rates much higher than in healthy nonusers (3% vs 0.5%).46
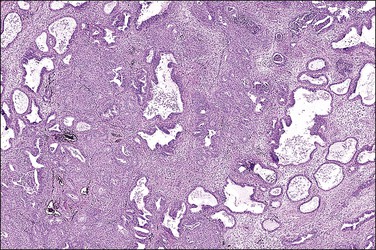
Figure 15.23 Tamoxifen, polyp. This polyp also showed extensive squamous metaplasia, a not uncommon finding.
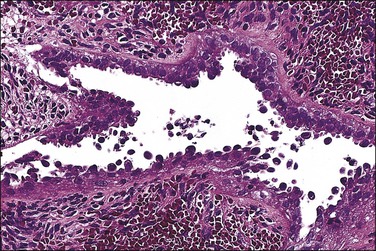
Figure 15.24 Tamoxifen, serous-like epithelial atypia in polyp.
(Courtesy of Dr. H. Krigman, University of Minnesota.)
Endometrial carcinomas arise at approximately the same rate as with unopposed estrogen and at far greater rates than women not receiving HRT. The risk is related to the duration of therapy, and is increased approximately fourfold in patients exposed to tamoxifen for more than 5 years.47 Some initial anecdotal reports and small series suggested that tamoxifen-induced carcinomas might be more frequently high grade,48 but larger subsequent series including results from the NSABP B-14 study have shown a similar proportion of high-grade tumors to those arising sporadically.49,50 Most cancers are low-grade, low-stage endometrioid adenocarcinomas. Uterine sarcomas also occur rarely in association with tamoxifen therapy,51 but most of these examples are carcinosarcomas that the current body of opinion places into the category of ‘metaplastic carcinomas’ (see Chapter 18).
Other Selective Estrogen Receptor Modulators
There are at least two types of estrogen receptors that are differentially active between reproductive and somatic target organs. This has led to the development of selective estrogen receptor modulators (SERMs), designed for relatively tissue-specific effects.39 Clinically beneficial compounds would minimize the stimulatory effect in endometrial tissue while retaining the beneficial effects in bone and the cardiovascular system. The first of these to come into wide use was tamoxifen, but in recent years a number of additional SERMs have become available.
Raloxifene, an antiestrogen designed initially for breast cancer treatment, has selective beneficial effects on bone and cardiovascular systems, while lacking any evident estrogenic activity within the endometrium itself. Endometrial biopsies from postmenopausal women taking raloxifene show atrophy or inactive endometrium,52 and treated patients show no increase in endometrial thickness as measured by ultrasound.53 Long-term follow-up studies now confirm that patients receiving raloxifene have neither increased endometrial hyperplasia nor carcinoma when compared with control groups,54–56 although there has been an increase in endometrial polyps.
A number of other SERMS are entering clinical use or have shown promise in clinical trials, including lasofoxifene, arzoxifene, bazedoxifene, ospemifene and fulvestrant. None of these have had detailed, long-term evaluations of their endometrial effects, but at least one (lasofoxifene) has been shown to be associated with increased endometrial thickness and polyps.55 It appears that each of these agents has a unique combination of activities and their effects on the endometrium will need to be documented individually rather than as a single class of drugs.
Aromatase Inhibitors
Aromatase inhibitors are a class of endocrine agents that block the peripheral conversion of steroids into estradiol by inhibiting the aromatase enzyme. Letrozole, anastrozole, and exemestane are the three mostly thoroughly studied members of this drug class. These drugs are used primarily to treat estrogen receptor-positive breast cancers in postmenopausal women (in this situation they are highly effective)57,58 and, more recently, endometriosis59 and ovulation induction for in vitro fertilization.60,61 Because they reduce circulating estrogen levels to near zero, the primary effect on the endometrium is atrophy, and the rate of endometrial carcinoma is lower than in tamoxifen-treated patients.62 As measured by transvaginal ultrasound, aromatase inhibitors do not increase endometrial thickness. Some preliminary evidence suggests that aromatase inhibitors could even help treat pre-existing endometrial hyperplasias.63 Although aromatase inhibitors effectively shut down endometrial proliferation, endometrial polyps continue to develop, an observation that contradicts current dogma that endometrial polyps are estrogen induced.
Phytoestrogens and Other Dietary Agents
Dietary and herbal remedies are widely used worldwide for relief of menopausal symptoms. Commonly used herbs include black cohosh (Cimicifuga racemosa), chaste tree berry (Vitex agnus-castus), dong quai (Angelica sinensis), ginseng (Panax ginseng and other Panax species), evening primrose oil (Oenothera biennis), motherwort (Leonurus cardiaca), red clover (Trifolium pratense), and licorice (Glycyrrhiza glabra). In many cases the active ingredients of these herbal preparations have not been clearly identified.64
Phytoestrogens, new forms of SERM still being evaluated, are plant estrogens that occur naturally as constituents of many plants, most notably beans and a variety of grains and seeds. Soybeans and flaxseed are commonly cited in the lay literature as sources of phytoestrogens,65,66 which have a weak estrogen-like effect in the body. One tantalizing observation is that Asian women, who are known to have a much lower incidence of endometrial carcinoma than Western women, consume much higher amounts of phytoestrogen-rich foods, such as soy and tofu. Human studies suggest that a diet high in phytoestrogens may protect against endometrial adenocarcinoma and provide benefits in bone density.67 Nationwide population-based studies, while not specifically evaluating phytoestrogens, suggest that a diet with a low fruit and vegetable intake is associated with higher rates of endometrial cancer.68
The most common types of phytoestrogen in plants and foods are the isoflavones. The richest sources in nature are the leaves of the subterranean red clover with levels of up to 5 g/100 g dry weight, and soybeans with levels up to 300 mg/100 g dry weight. The most commonly studied phytoestrogens within this group are genistein and daidzein.
The initial data available on the effects on the endometrium itself suggest that phytoestrogens likely function as weak estrogen agonists. Long-term exposure to high doses may increase the risk of endometrial hyperplasia in postmenopausal women, but most evidence suggests that short-term exposure to lower doses has no discernible histologic effect on endometrium.69,70 In premenopausal women, the endometrium appears to cycle normally (Figure 15.25).
Wild yam preparations contain diosgenin, which can be converted to progesterone in a laboratory but not in the human body. Whether dietary supplementation with wild yams has any histologic effects on the endometrium is unknown.66
Clomiphene/Ovulation Induction Therapy
Clomiphene citrate (Clomid) is an agent with strong affinity for the estrogen receptor. It binds essentially irreversibly to the receptor, reducing turnover. Its clinical usefulness stems from its effects on the hypothalamus, which interprets this activity as a low level of estrogen, causing increased gonadotropin-releasing hormone (GnRH) stimulation of the pituitary, and hence increased follicle-stimulating hormone (FSH) production. This increases ovarian estrogen production, which then feeds back to the pituitary and causes a luteinizing hormone (LH) surge, followed by ovulation.8 This drug, used to induce ovulation, is usually given on cycle days 5–10, with ovulation occurring 5–10 days after its administration. The increased stimulation of the dominant follicle results in higher serum estrogen and progesterone levels during the resulting secretory phase.
The effects of clomiphene on the endometrium are relatively subtle.71,72 Some, but not all, studies suggest that, in a clomiphene-induced secretory phase, the glandular development is delayed relative to the time of ovulation (Figure 15.26). Although delayed, the early secretory changes are unusually well developed, with large, uniform subnuclear glycogen vacuoles that persist longer than in a normal cycle. Later in the cycle, decidual changes in the stroma are less prominent, and a coiled glandular architecture persists. These findings probably represent the antiestrogenic effects of clomiphene in combination with the high circulating levels of estrogen and progesterone.73
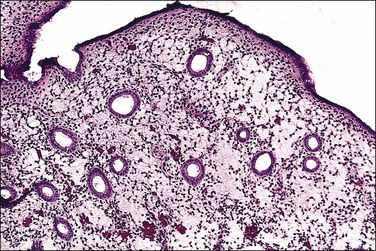
Figure 15.26 Markedly edematous stroma (clomiphene). (Courtesy of Dr. Karen Ireland, Liberty Lake, WA.)
In more recent years, ovulation induction has involved more complex regimens, generally involving administration of GnRH agonists and antagonists, FSH, human chorionic gonadotropin, and aromatase inhibitors. There are few treatment-specific studies on endometrial morphology, and it is difficult to extrapolate results between regimens. At least some studies suggest a relative delay in secretory phase maturation, similar to that seen with clomiphene. Other studies show in-phase or even advanced date endometrium.74,75 The alterations in endometrial function may be responsible for the reduced rate of successful implantation seen in some patients.
Progesterone Receptor Modulators
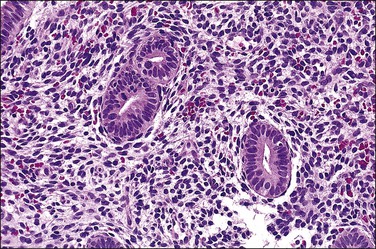
This class of agents modulates the activity of the progesterone receptor. Although developed originally as progesterone receptor antagonists, their actions in vivo appear to be more complex, varying by agent and modified by the hormonal environment of the individual patient. The most widely known agent in the United States is mifepristone, which is primarily used as an agent of very early abortion (‘morning after pill’), but these agents are also effective in the treatment of a variety of common conditions such as endometriosis and leiomyomata.76 Other drugs in this category include asoprisnil, and Proellex (CDB-4124). Several studies have shown unique histologic changes in the endometrium in patients receiving progesterone receptor modulators (PRMs), and a National Institutes of Health-sponsored workshop has suggested the term ‘progesterone receptor modular associated endometrial changes’ (PAEC) as a name for this constellation of findings.77–79 Common findings include the development of multiple cysts, and glandular epithelium with nonphysiologic combinations of estrogen and progesterone effects: mitoses with apoptosis, or secretory vacuoles with proliferative activity (Figures 15.27 and 15.28). Stromal predecidual change is rare, and they differ from unopposed estrogens in lacking fibrin thrombi. PAEC changes are not seen in every treated patient, some of whom have proliferative or atrophic endometria that lack any distinctive features that would suggest exposure to a PRM. Long-term clinical outcomes are generally unknown or poorly understood, as the majority are newly introduced agents. However, as PRMs become more widely used, it will be important for pathologists to be aware of these changes so they are not confused with polyps or unopposed estrogen effects.
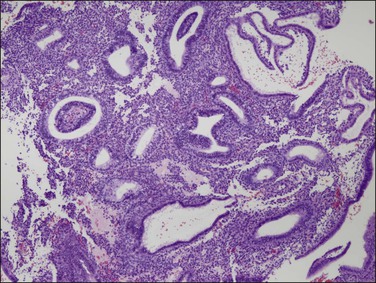
Figure 15.27 Effects of progesterone blockade on the premenopausal endometrium. PAEC caused by CDB-4124 includes epithelial–stromal dyssynchrony, evident as scattered cysts. (Courtesy Repros Therapeutics, Woodlands, TX)
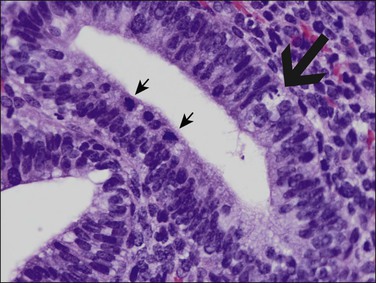
Figure 15.28 Closer view of CDB-4124-treated endometrium shows nonphysiologic combinations of mitoses (small arrows), apoptotic change (large arrow), and secretory vacuoles within glands.
(Courtesy Repros Therapeutics, Woodlands, TX)
GnRH Agonists and Antagonists
Currently, GnRH agonists and antagonists are used to reduce the size of uterine leiomyomas and endometrial stromal sarcomas.80 They are also used in the diagnosis and treatment of endometriosis, and in the suppression of ovulation during oocyte harvest for in vitro fertilization. Histologically, it is not surprising that the endometrium from patients treated chronically with GnRH shows profound atrophy.81
Gonadotropins
Gonadotropins are used primarily to treat infertility. Human menopausal gonadotropins, LH and FSH, are extracted from the urine of postmenopausal women. Human chorionic gonadotropin is closely related to LH, and can be used to simulate the mid-cycle LH surge in ovulation induction. The effects of these agents on the endometrium are not clear. Various conflicting findings report delayed, normal, or advanced histologic maturation relative to the clinical dates, as well as hypersecretory changes similar to Arias-Stella reactions.8,9,82
Danazol
Danazol is a weak androgen that is structurally related to testosterone and is used to treat endometriosis and endometrial hyperplasia. The endometrial changes resemble those seen with progestational agents. As with progestins, long-term therapy usually leads to atrophy83,84 or a reduction in the severity of hyperplasia.85
Treatment of Endometrial Lesions with Progestins
Hormonal therapy, primarily high-dose progestin therapy, has long been used to treat lesions diagnosed as endometrial hyperplasia, EIN, and even well-differentiated adenocarcinomas in women who are poor surgical candidates or who wish to preserve fertility. Relatively high doses of progestins are used, typically 20–40 mg/day oral medroxyprogesterone acetate (Provera), 80 mg/day megestrol acetate (Megace), or intramuscular depo-medroxyprogesterone acetate (Depo-Provera). Levonorgestrel-releasing IUDs are also effective. Repeat biopsy is usually obtained (typically at around 3 months) to evaluate response to therapy. The histologic response to this therapy requires progesterone receptors within lesional tissue, a condition met in almost all non-atypical endometrial hyperplasias and EIN, and well-differentiated adenocarcinomas.7
Endometrium treated with high-dose progestins shows typical secretory changes in the first weeks of therapy. By 3 months, the endometrium in patients who respond completely shows diffuse and profound decidualization, with widely scattered atrophic-appearing glands similar to the decidua seen in pregnancy. A variety of metaplastic epithelial changes are commonly seen with progestin therapy, including squamous, mucinous, secretory, or eosinophilic.86
Progestin Therapy of Persistent Estrogen States (Hyperplasia)
Short-term therapy with oral progestins leads to the formation of secretory vacuoles within the maintained architectural structure of the pre-existing lesion (Figure 15.29). Stromal decidualization and secretory exhaustion follows, but initially with the cysts and irregular glands of the underlying lesion (Figure 15.30). Eventually, and especially if a withdrawal shedding is allowed to occur, the hyperplastic architecture is lost (Figure 15.31). Incomplete responses are common, and may be caused by patient noncompliance, inadequate dose or duration, or lack of endometrial responsiveness. Adequacy of treatment can be assessed by repeat endometrial sampling, in conjunction with clinical symptoms.
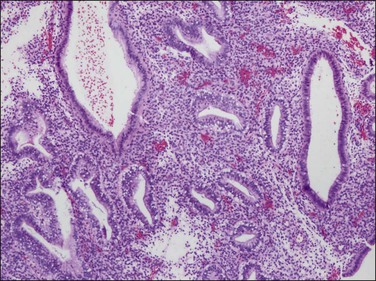
Figure 15.29 Short-term (days) oral progestin treatment of an anovulatory (unopposed estrogen type) endometrium retains the architecture of cysts and disordered glands, but with development of secretory vacuoles. Mitotic activity is not yet suppressed.
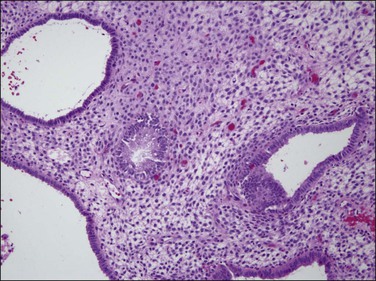
Figure 15.30 Anovulatory (unopposed estrogen type) endometrium treated for 1 week with oral progestins still retains the architecture of cysts and disordered glands, but now the secretory vacuoles are gone and the stroma is developing decidual change.
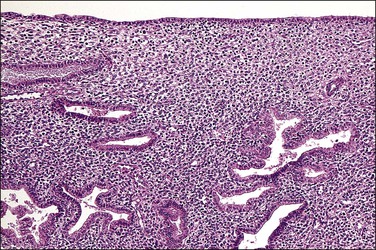
Figure 15.31 Changes in an endometrium treated with progesterone for simple hyperplasia. This woman had initially used high-dose Premarin over a long period, with shedding or involution of the hyperplastic glands. After a short period of receiving Depo-Provera, some of the endometrial glands show secretory changes. The stroma has become decidualized.
If the underlying production of persistent estrogens is not remedied, the endometrium will revert to an anovulatory, or hyperplastic, appearance upon cessation of progestin therapy. Direct intrauterine delivery of progestins by placement of a hormonally impregnated IUD can provide long-term (years) endometrial treatment with the additional advantage of reducing the level of systemic exposure (Figure 15.32).
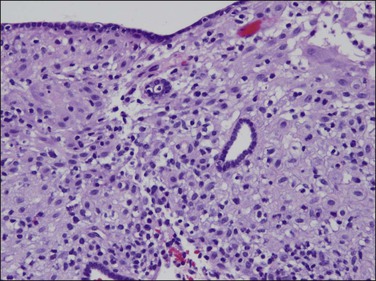
Figure 15.32 Stroma decidual changes, similar to pill endometrium, in a patient treated with a levonorgestrel IUD (Mirena). Long-term treatment has, in some areas, resulted in atrophic glands in a decidualized stroma.
Progestin Therapy of Neoplastic Lesions: EIN and Carcinoma
The histologic response of neoplastic (EIN and endometrioid type adenocarcinoma) endometrial tissues to progestin therapy is discussed in Chapters 17 and 18, respectively. Generally, the therapeutic goal is to stabilize or ablate lesional tissues, and when successful the histologic appearance is dominated by hormonally altered background endometrium, which may revert to normal after cessation of therapy. Presently there are no clinically useful parameters to predict which lesions will, or will not, respond to therapy. A decision to treat medically is thus made on a clinical basis once a specific qualifying diagnosis is rendered.
Progestin-altered glands of persistent premalignant EIN lesions and adenocarcinoma may become widely separated by an expanded decidualized stroma, and acquire a bland nuclear cytology. This can make persistent disease difficult to recognize in biopsies taken while the patient is still receiving hormonal therapy. Close follow-up is always warranted even when there has been an apparently complete histologic response.87
References
1. Schimmer, BP, Parker, KL. Contraception and Pharmacotherapy of Obstetrical and Gynecological Disorders. In Brunton L, Chabner B, Knollmann B, eds.: Goodman and Gilman’s the pharmacological basis of therapeutics, 12th ed, New York: McGraw-Hill, 2011.
2. Weiss, NS, Sayvetz, TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980; 302:551–554.
3. Hannaford, PC, Selvaraj, S, Elliott, AM, et al. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception study. BMJ. 2007; 335:651.
4. Bernstein, L. The risk of breast, endometrial and ovarian cancer in users of hormonal preparations. Basic Clin Pharmacol Toxicol. 2006; 98:288–296.
5. Iodice, S, Barile, M, Rotmensz, N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010; 46:2275–2284.
6. Rodolakis, A, Thomakos, N, Akrivos, N, et al. Clinicopathologic insight of simultaneously detected primary endometrial and ovarian carcinomas. Arch Gynecol Obstet. 2012; 285(3):817–821.
7. Deligdisch, L. Hormonal pathology of the endometrium. Mod Pathol. 2000; 13:285–294.
8. Ireland, K, Zaino, RJ. Iatrogenic patterns: what hath the physician wrought? In: Zaino RJ, ed. Interpretation of endometrial biopsies and curettings. New York: Lippincott-Raven; 1996:143–173.
9. Mazur, MT, Kurman, RJ. Effects of hormones. Diagnosis of endometrial biopsies and curettings: a practical approach. New York: Springer-Verlag; 1995.
10. Wynants, P, Ide, P. Endometrial morphology during a normophasic and a triphasic regimen: a comparison. Contraception. 1986; 33:149–157.
11. Archer, DF. Endometrial histology during use of a low-dose estrogen-desogestrel oral contraceptive with a reduced hormone-free interval. Contraception. 1999; 60:151–154.
12. Kim-Bjorklund, T, Landgren, BM, Johannisson, E. Morphometric studies of the endometrium, the fallopian tube and the corpus luteum during contraception with the 300 micrograms norethisterone (NET) minipill. Contraception. 1991; 43:459–474.
13. Rivera, R, Yacobson, I, Grimes, D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999; 181:1263–1269.
14. Hickey, M, Simbar, M, Young, L, et al. A longitudinal study of changes in endometrial microvascular density in Norplant implant users. Contraception. 1999; 59:123–129.
15. Oliveira-Ribeiro, M, Petta, CA, De Angelo Andrade, LA, et al. Correlation between endometrial histology, microvascular density and calibre, matrix metalloproteinase-3 and bleeding pattern in women using a levonorgestrel-releasing intrauterine system. Hum Reprod. 2004; 19:1778–1784.
16. Rhoton-Vlasak, A, Chegini, N, Hardt, N, Williams, RS. Histological characteristics and altered expression of interleukins (IL) IL-13 and IL-15 in endometria of levonorgestrel users with different uterine bleeding patterns. Fertil Steril. 2005; 83:659–665.
17. Abou-Setta, AM, Al-Inany, HG, Farquhar, CM. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006.
18. Busfield, RA, Farquhar, CM, Sowter, MC, et al. A randomised trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006; 113:257–263.
19. Roberts, TE, Tsourapas, A, Middleton, LJ, et al. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ. 2011; 342:d2202.
20. Jones, RL, Critchley, HO. Morphological and functional changes in human endometrium following intrauterine levonorgestrel delivery. Hum Reprod. 2000; 15(Suppl 3):162–172.
21. Anderson, GL, Limacher, M, Assaf, AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004; 291:1701–1712.
22. Udell, JA, Fischer, MA, Brookhart, MA, et al. Effect of the Women’s Health Initiative on osteoporosis therapy and expenditure in Medicaid. J Bone Miner Res. 2006; 21:765–771.
23. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996; 275:370–375.
24. Weiderpass, E, Adami, HO, Baron, JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999; 91:1131–1137.
25. Beral, V, Bull, D, Reeves, G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005; 365:1543–1551.
26. Cushing, KL, Weiss, NS, Voigt, LF, et al. Risk of endometrial cancer in relation to use of low-dose, unopposed estrogens. Obstet Gynecol. 1998; 91:35–39.
27. Labrie, F, Cusan, L, Gomez, JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause. 2009; 16:30–36.
28. Calcagno, A, Grassi, T, Mariuzzi, L, et al. Expression patterns of Aurora A and B kinases, Ki-67 and the estrogen and progesterone receptors determined using an endometriosis tissue microarray model. Hum Reprod. 2011; 26(10):2731–2741.
29. Ettinger, B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas. 2007; 57:81–84.
30. Mutter, GL, Zaino, RJ, Baak, JP, et al. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol. 2007; 26:103–114.
31. Moyer, DL, Felix, JC. The effects of progesterone and progestins on endometrial proliferation. Contraception. 1998; 57:399–403.
32. Moyer, DL, de Lignieres, B, Driguez, P, Pez, JP. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril. 1993; 59:992–997.
33. Cerin, A, Heldaas, K, Moeller, B. Adverse endometrial effects of long-cycle estrogen and progestogen replacement therapy. N Engl J Med. 1996; 334:668–669.
34. Carranza-Lira, S, Martinez-Chequer, JC, Santa Rita, MT, et al. Endometrial changes according to hormone replacement therapy schedule. Menopause. 1998; 5:86–89.
35. Habiba, MA, Bell, SC, Al-Azzawi, F. Endometrial responses to hormone replacement therapy: histological features compared with those of late luteal phase endometrium. Hum Reprod. 1998; 13:1674–1682.
36. Vandermooren, MJ, Hanselaar, AGJM, Borm, GF, Rolland, R. Changes in the withdrawal bleeding pattern and endometrial histology during 17 beta-estradiol—dydrogesterone therapy in postmenopausal women: a 2 year prospective study. Maturitas. 1994; 20:175–180.
37. Piegsa, K, Calder, A, Davis, JA, et al. Endometrial status in post-menopausal women on long-term continuous combined hormone replacement therapy (Kliofem). A comparative study of endometrial biopsy, outpatient hysteroscopy and transvaginal ultrasound. Eur J Obstet Gynecol Reprod Biol. 1997; 72:175–180.
38. Jordan, VC, Brodie, AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007; 72:7–25.
39. Ye, J, Hameed, O, Findeis-Hosey, J, et al. Diagnostic utility of PAX8, TTF-1 and napsin A for discriminating metastatic carcinoma from primary adenocarcinoma of the lung. Biotech Histochem. 2012; 87(1):30–34.
40. Cohen, I, Beyth, Y, Altaras, MM, et al. Estrogen and progesterone receptor expression in postmenopausal tamoxifen-exposed endometrial pathologies. Gynecol Oncol. 1997; 67:8–15.
41. Suh-Burgmann, EJ, Goodman, A. Surveillance for endometrial cancer in women receiving tamoxifen. Ann Intern Med. 1999; 131:127–135.
42. Seoud, M, Shamseddine, A, Khalil, A, et al. Tamoxifen and endometrial pathologies: a prospective study. Gynecol Oncol. 1999; 75:15–19.
43. Deligdisch, L, Kalir, T, Cohen, CJ, et al. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000; 78:181–186.
44. Kennedy, MM, Baigrie, CF, Manek, S. Tamoxifen and the endometrium: review of 102 cases and comparison with HRT-related and non-HRT-related endometrial pathology. Int J Gynecol Pathol. 1999; 18:130–137.
45. Schlesinger, C, Kamoi, S, Ascher, SM, et al. Endometrial polyps: a comparison study of patients receiving tamoxifen with two control groups. Int J Gynecol Pathol. 1998; 17:302–311.
46. Cohen, I, Perel, E, Tepper, R, et al. Dose-dependent effect of tamoxifen therapy on endometrial pathologies in postmenopausal breast cancer patients. Breast Cancer Res Treat. 1999; 53:255–262.
47. Bernstein, L, Deapen, D, Cerhan, JR, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999; 91:1654–1662.
48. Bland, AE, Calingaert, B, Secord, AA, et al. Relationship between tamoxifen use and high risk endometrial cancer histologic types. Gynecol Oncol. 2009; 112:150–154.
49. Silva, EG, Tornos, CS, Follenmitchell, M. Malignant neoplasms of the uterine corpus in patients treated for breast carcinoma—the effects of tamoxifen. Int J Gynecol Pathol. 1994; 13:248–258.
50. Treilleux, T, Mignotte, H, Clement-Chassagne, C, et al. Tamoxifen and malignant epithelial-nonepithelial tumours of the endometrium: report of six cases and review of the literature. Eur J Surg Oncol. 1999; 25:477–482.
51. Arenas, M, Rovirosa, A, Hernandez, V, et al. Uterine sarcomas in breast cancer patients treated with tamoxifen. Int J Gynecol Cancer. 2006; 16:861–865.
52. Boss, SM, Huster, WJ, Neild, JA, et al. Effects of raloxifene hydrochloride on the endometrium of postmenopausal women. Am J Obstet Gynecol. 1997; 177:1458–1464.
53. Davies, GC, Huster, WJ, Shen, W, et al. Endometrial response to raloxifene compared with placebo, cyclical hormone replacement therapy, and unopposed estrogen in postmenopausal women. Menopause. 1999; 6:188–195.
54. DeMichele, A, Troxel, AB, Berlin, JA, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008; 26:4151–4159.
55. Pinkerton, JV, Goldstein, SR. Endometrial safety: a key hurdle for selective estrogen receptor modulators in development. Menopause. 2010; 17:642–653.
56. Grady, D, Herrington, D, Bittner, V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002; 288:49–57.
57. Lonning, PE. Adjuvant endocrine treatment of early breast cancer. Hematol Oncol Clin North Am. 2007; 21:223–238.
58. Smith, IE, Dowsett, M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003; 348:2431–2442.
59. Ferrero, S, Venturini, PL, Ragni, N, et al. Pharmacological treatment of endometriosis: experience with aromatase inhibitors. Drugs. 2009; 69:943–952.
60. Wagman, I, Levin, I, Kapustiansky, R, et al. Clomiphene citrate vs. letrozole for cryopreserved-thawed embryo transfer: a randomized, controlled trial. J Reprod Med. 2010; 55:134–138.
61. Pritts, EA. Letrozole for ovulation induction and controlled ovarian hyperstimulation. Curr Opin Obstet Gynecol. 2010; 22:289–294.
62. Eisen, A, Trudeau, M, Shelley, W, et al. Aromatase inhibitors in adjuvant therapy for hormone receptor positive breast cancer: a systematic review. Cancer Treat Rev. 2008; 34:157–174.
63. Li, HZ, Chen, XN, Qiao, J. Letrozole as primary therapy for endometrial hyperplasia in young women. Int J Gynaecol Obstet. 2008; 100:10–12.
64. Borrelli, F, Ernst, E. Alternative and complementary therapies for the menopause. Maturitas. 2010; 66:333–343.
65. Nicolae, A, Goyenaga, P, McCluggage, WG, et al. Endometrial intestinal metaplasia: a report of two cases, including one associated with cervical intestinal and pyloric metaplasia. Int J Gynecol Pathol. 2011; 30:492–496.
66. Suh, S, Kim, KW. Diabetes and cancer: is diabetes causally related to cancer? Diabetes Metab J. 2011; 35:193–198.
67. Tanaka, T, Bai, T, Utsunomiya, H, et al. STAT3 enhances intracellular Fas-mediated apoptotic signals in HHUA human endometrial epithelial cells. Mol Med Report. 2011; 4:307–312.
68. Wrenn, DC, Saigal, K, Lucci, JA, 3rd., et al. A Phase I Study using low-dose fractionated whole abdominal radiotherapy as a chemopotentiator to full-dose cisplatin for optimally debulked stage III/IV carcinoma of the endometrium. Gynecol Oncol. 2011; 122:59–62.
69. Tempfer, CB, Froese, G, Heinze, G, et al. Side effects of phytoestrogens: a meta-analysis of randomized trials. Am J Med. 122, 2009. [939–46.e9].
70. Bandera, EV, Williams, MG, Sima, C, et al. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control. 2009; 20:1117–1127.
71. Sereepapong, W, Suwajanakorn, S, Triratanachat, S, et al. Effects of clomiphene citrate on the endometrium of regularly cycling women. Fertil Steril. 2000; 73:287–291.
72. Marotti, JD, Glatz, K, Parkash, V, Hecht, JL. International Internet-based assessment of observer variability for diagnostically challenging endometrial biopsies. Arch Pathol Lab Med. 2011; 135:464–470.
73. Benda, JA. Clomiphene’s effect on endometrium in infertility. Int J Gynecol Pathol. 1992; 11:273–282.
74. Tropea, A, Miceli, F, Minici, F, et al. Endometrial evaluation in superovulation programs: relationship with successful outcome. Ann N Y Acad Sci. 2004; 1034:211–218.
75. Simon, C, Oberye, J, Bellver, J, et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005; 20:3318–3327.
76. Spitz, IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009; 21:318–324.
77. Ioffe, OB, Zaino, RJ, Mutter, GL. Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol. 2009; 22:450–459.
78. Mutter, GL, Bergeron, C, Deligdisch, L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008; 21:591–598.
79. Fiscella, J, Bonfiglio, T, Winters, P, et al. Distinguishing features of endometrial pathology after exposure to the progesterone receptor modulator mifepristone. Hum Pathol. 2011; 42:947–953.
80. Lethaby, A, Vollenhoven, B, Sowter, M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001.
81. Sowter, MC, Lethaby, A, Singla, AA. Pre-operative endometrial thinning agents before endometrial destruction for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002.
82. Heller, DS. Hormonal effects on the endometrium—dysfunctional uterine bleeding, iatrogenic hormonal effects, and luteal phase defects. New York: Igaku-Shoin Medical Publishing; 1994.
83. Matias-Guiu, X, Oliva, E. Pathology of the endometrium. Introduction. Semin Diagn Pathol. 2010; 27:197–198.
84. Cheng, W, Wang, YJ, Zhang, X, Gao, XM. [The effect on angiogenesis of endometrium after transcervical resection of polyp]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010; 41:854–857.
85. Gomez-Lopez, N, Vadillo-Perez, L, Nessim, S, et al. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011; 204:364.e9–364.e16.
86. Miranda, MC, Mazur, MT. Endometrial squamous metaplasia: an unusual response to progestin therapy of hyperplasia. Arch Pathol Lab Med. 1995; 119:458–460.
87. Wheeler, DT, Bristow, RE, Kurman, RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007; 31:988–998.