Chapter 16 Exfoliants, Moisturizers and More: AHAs, BHAs, and PHAs
ALPHA HYDROXY ACIDS (AHAs)
• Histologic effects
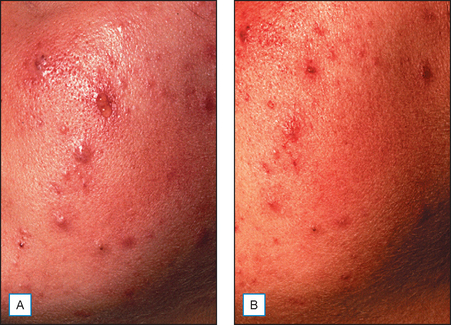
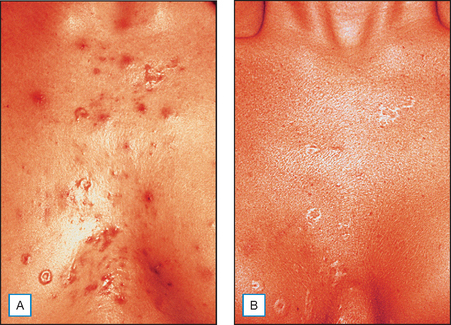
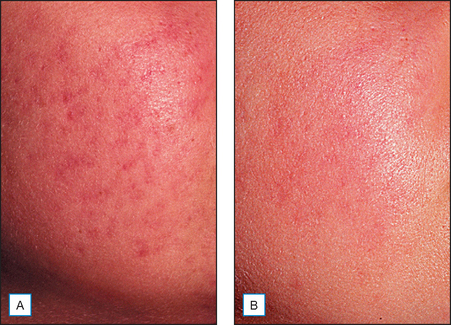
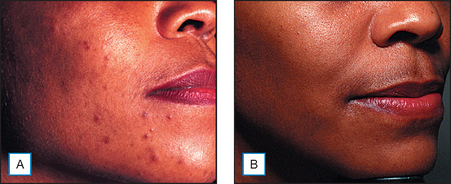
It has been long held that lower concentrations of AHAs, when applied topically, reduce the thickness of the hyperkeratotic stratum corneum by reducing corneocyte cohesion in the lower levels of the stratum corneum. When applied in higher concentrations and low pH values, these same AHAs can cause epidermolysis as they have been found to work at the desmosomal attachment sites of the basilar layer. This effect can then produce varying degrees of exfoliation of the skin and therefore AHAs are useful in the management of various cosmetic and dermatologic conditions such as dry skin, seborrheic dermatitis, callosities, acne (Fig. 16.1), scarring (Fig. 16.2) actinic and seborrheic keratoses, and warts as well as photodamaged skin.
• Mechanism of action
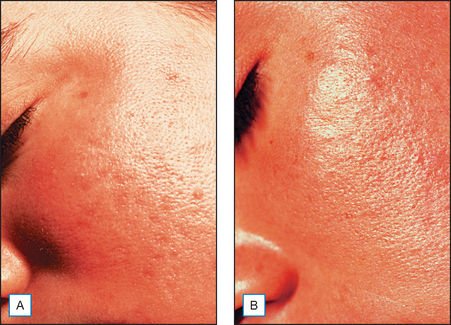
The mechanism of action of AHAs has not been fully determined. It is postulated that AHAs act as a chelating agent and thereby decrease local calcium ion concentrations from cation-dependent cell adhesion molecules. This calcium loss from cadherins of desmosomes, adherens junctions and tight junctions causes a decrease in desmosomal attachments. This makes the usually protected endogenous stratum corneum chymotryptic enzymes on cadherins vulnerable to proteolysis. When calcium is decreased, cellular adhesions are disrupted and exfoliation takes place (Fig. 16.3).
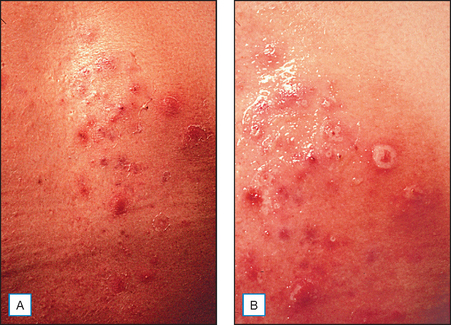
Another proposed mechanism for AHA induced exfoliation is an increase in apoptosis. In one study, lactic acid (LA) was shown to cause a concentration-dependent increase in apoptotic cells. In this same study, vascular endothelial growth factor (VEGF) was increased at least 2.5-fold over vehicle control with either a 1.5 or 3% concentration of LA. Angiogenin secretion was decreased by LA in a concentration-dependent manner. It was concluded that topical AHAs modulate secretion of cytokines by keratinocytes and that this regulation may account in part for their effects in skin disorders as well as photoaging. Another study in 2003 confirms that glycolic acid (GA) directly accelerates collagen synthesis by fibroblasts and modulates matrix degradation and collagen synthesis through keratinocyte-released cytokines (Fig. 16.4). The primary mediator for this matrix degradation is interleukin 1α (IL-1α).
• Role in moisturization
Unlike salicylic acid, AHAs have the ability not only to cause exfoliation but also moisturization. This dual nature of AHAs has been noted but not completely understood. It is postulated that the AHAs induced increased mucopolysaccharide content, in particular dermal glycosaminoglycans (GAGs), in the skin, which may account for the increased moisturization. It was shown in one study that 20% GA treatment of forearm skin as compared to vehicle demonstrated an increase in hyaluronic acid content in the epidermis and dermis. An increase in collagen mRNA gene expression was found in the AHA-treated sites only.
There are relatively few data on the effects of AHA on stratum corneum lipids. In their unpublished data, Motta and Berardesca indicate that an increase in production of ceramides occurs with AHA use. This would provide one explanation for the moisturization and barrier fortification that these products deliver (Fig. 16.5).
• Their role in skin barrier function
In 2004, Song et al found that skin barrier function is damaged after a GA peel and also after aluminum oxide microdermabrasion but recovers within 1–4 days after treatment. Therefore, these authors felt that repeat peeling at 2-week intervals would allow sufficient time for the skin to recover.
• Effects on pigmentation
Interestingly, in that same year an in vitro study showed that GA and LA in doses of 300 or 500 μg/mL suppressed melanin formation by directly inhibiting tyrosinase activity. Adjusting the pH up to 5.6 did not affect tyrosinase activity and this effect was then deemed independent of the acidic nature of these AHAs. The authors postulate that GA and/or LA might work on pigmented lesions by accelerating epidermal turnover and by directly inhibiting melanin formation in melanocytes. There have been conflicting clinical studies showing no benefit to a positive benefit in patients with hyperpigmented skin conditions. It is our belief that the AHAs may actually work clinically by enhancing penetration of other bleaching agents such as hydroquinones or retinoids and/or by directly inducing skin turnover which improves the appearance of hyperpigmentation due to hyperkeratinization (Fig. 16.6).
• Cosmeceutical effects
In 1996, topical 8% GA and LA creams were compared in a double-blind, vehicle-controlled, randomized clinical trial of the effects on photodamaged skin of 74 women aged 40–70 years over 22 weeks. The study showed that AHA creams were well tolerated and found to be modestly beneficial in ameliorating the clinical signs of photodamage. After 10 weeks of treatment, both GA and LA significantly reduced mottled hyperpigmentation of the forearms compared with the vehicle. This effect was maximized by 14 weeks of treatment. At the end of this study, only LA showed a continued significant advantage over the vehicle. AHA induced significant reductions in skin sallowness as observed at 10 weeks, maximized at 14 weeks and maintained throughout the 22 weeks of the study over vehicle. There was a 16% greater diminution of tactile roughness with AHAs as compared to vehicle alone. Only the subjects using AHAs noted improvement from baseline in number of fine wrinkles, firmness, age spots or evenness of color.
SALICYLIC ACID
• Toxicity
Hairless Skh:hr1 mice were irradiated with UVB light for 14 weeks and were then subjected (with or without treatment) every 2 weeks to 30% salicylic acid in polyethylene glycol (PEG) for a total of 18 weeks. In this study, it was interesting to note that the total number of skin tumors was greatly reduced in the treated versus the control mice. Skin tumor development was also slower in the treated versus the control mice. Fractions of T and B lymphocytes and natural killer cells from spleens of both groups of mice were comparable, and interferon-gamma production did not differ. It was then suggested by the authors that chemical peeling with salicylic acid in PEG may help to prevent as well as to reduce the number of UVB-induced skin tumors.
POLYHYDROXY ACIDS (PHAs)
• Definition
PHAs have been found to be compatible with clinically sensitive skin, including rosacea and atopic dermatitis, and can be used after cosmetic procedures. PHAs such as gluconolactone or lactobionic acid may be used in combination with other products, ingredients, or procedures such as laser and microdermabrasion to provide additional benefits to therapy or to enhance the therapeutic effect.
Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure and safety. American Journal of Clinical Dermatology. 2003;4:473–492.
Alpha-hydroxy acids: unapproved uses or indications. SKINmed. 2004;3:141–144.
Berardesca E, Distante F, Vignoli GP, Oresajo C, Green B. Alpha hydroxyacids modulate stratum corneum barrier function. British Journal of Dermatology. 1997;137:934–938.
Bernstein EF, Lee J, Brown DP, Yu R, Van Scott EJ. Glycolic acid increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatologic Surgery. 2001;27:429–433.
Bernstein EF, Brown DB, Schwartz MD, Kaidbey K, Ksenzenko SM. The polyhydroxy acid gluconolactone protects against UV radiation in an in vitro model of cutaneous photoaging. Dermatologic Surgery. 2004;30(2 Pt 1):189-195. discussion 196
Brody HJ. Chemical peeling and resurfacing, 2nd edn., St Louis: Mosby; 1997:1.
Corcuff P, Fiat F, Gracia AM, Leveque JL 1996 Hydroxyl acid induced desquamation of the human stratum corneum: a comparative ultrastructural study. 19th International Federation Society of Cosmetic Chemists Congress, Sydney, Australia (3):85–94
Cosmetic Ingredient Review Expert Panel. Safety assessment of salicylic acid. International Journal of Toxicology. 2003;22(Suppl 3):1–108.
Ditre CM, Griffin TD, Murphy GF, et al. Effects of alpha hydroxy acids on photoaged skin: a pilot clinical, histologic, and ultrastructural study. Journal of the American Academy of Dermatology. 1996;34:187–195.
Ditre CM, Nini KT, Vagley RT. Introduction: Practical use of glycolic acid as a chemical peeling agent. Journal of Geriatric Dermatology. 1996;4(Suppl B):2B-7B.
Dorland’s Illustrated Medical Dictionary, 25th edn. Philadelphia: WB Saunders. 1974:556.
Edison BL, Green BA, Wildnauer RH, Sigler ML. A polyhydroxy acid skin care regimen provides antiaging effects comparable to an alpha-hydroxyacid regimen. Cutis. 2004;73(Suppl 2):14–17.
Green B. After 30 years … the future of hydroxyacids. Journal of Cosmetic Dermatology. 2005;4:44–45.
Green BA, Edison BL, Wildnauer RH, Hwu R. Cosmetic uses of benzilic acid—a lipophilic alpha-hydroxyacid (AHA). Barcelona, Spain: European Academy of Dermatology and Venereology Poster Exhibit, 2003 October 15–18.
Grimes PE, Green BA, Wildnauer RH, Edison BL. The use of polyhydroxy acids (PHAs) in photoaged skin. Cutis. 2004;73(Suppl 2):3–13.
Hermann M. Salicylic acid: an old dog, new tricks, and staphylococcal disease. Journal of Clinical Investigation. 2003;112:149–151.
Kim TH, Choi EH, Kang YC, Lee SH, Ahn SK. The effects of topical alpha-hydroxyacids on the normal skin barrier of hairless mice. British Journal of Dermatology. 2001;144:267–273.
Kligman AM. The compatibility of combinations of GA and tretinoin in acne and in photoaged facial skin. Journal of Geriatric Dermatology. 1995;3(Suppl A):25A-28A.
Lévèque JL, Saint-Léger D. Salicylic acid and derivatives. In: Leyden JJ, Rawlings AV, editors. Skin moisturization. New York: Marcel Dekker; 2002:353-364.
Lévèque JL, Corcuff P, Montastier C, et al. Mechanism of action of a lipophilic salicylic acid derivative on normal skin. Skin Research Technology. 1995;1:115–122.
Leyden JJ, Lavker RM, Grove G, Kaidbey K. Alpha hydroxy acids are more than moisturizers. Issues and perspectives of AHAs. Cosmetic Dermatology. 1994;October(Suppl):33A-37A.
Okana Y, Abe Y, Masaki H, et al. Biological effects of glycolic acid on dermal matrix metabolism mediated by dermal fibroblasts and epidermal keratinocytes. Experimental Dermatology. 2003;12(Suppl 2):57–63.
Piérard GE, Rougier A. Nudging acne by topical beta-lipohydroxy acid (LHA), a new comedolytic agent. European Journal of Dermatology. 2002;12:XLVII-XLVIII.
Piérard GE, Nikkels-Tassoudji N, Arrese JE, et al. Dermo-epidermal stimulation elicited by a lipohydroxyacid: a comparison with salicylic acid and all-trans retinoic acid. Dermatology. 1997;194:398–401.
Piérard GE, Kligman AM, Stoudemayer T, Lévèque JL. Comparative effects of retinoic acid, glycolic acid, and a lipophilic derivative of salicylic acid on photodamaged skin. Dermatology. 1999;199:50–53.
Rendl M, Mayer C, Weninger W, Tschachler E. Topically applied lactic acid increases spontaneous secretion of vascular endothelial growth factor by human reconstructed epidermis. British Journal of Dermatology. 2001;145:3–9.
Saint-Léger D, Lévèque JL, Verschoore M. The use of hydroxyl acids on the skin: characteristics of C8-lipohydroxy acid. Journal of Cosmetic Dermatology. 2007;6:59–65.
Song JY, Kang HA, Kim MY, Park YM, Kim HO. Damage and recovery of skin barrier function after glycolic acid chemical peeling and crystal microdermabrasion. Dermatologic Surgery. 2004;30:390–394.
Usuki A, Ohashi A, Sato H, et al. The inhibitory effect of GA and LA on melanin synthesis in melanoma cells. Experimental Dermatology. 2003;12(Suppl 2):43–50.
Van Scott EF, Yu RJ. Hyperkeratinization, corneocyte cohesion and alpha hydroxy acids. Journal of the American Academy of Dermatology. 1984;11(Pt 1):867–879.
Van Scott EF, Yu RJ. Alpha hydroxy acids: procedures for use in clinical practice. Cutis. 1989;43:222–228.
Van Scott EJ, Yu RJ. Alpha hydroxy acids: therapeutic potentials. Canadian Journal of Dermatology. 1989;1:108–112.
Van Scott EJ, Yu RJ. Actions of alpha hydroxy acids on skin compartments. Journal of Geriatric Dermatology. 1995;3(Suppl A):19A-24A.
Van Scott EJ, Ditre CM, Yu RJ. Alpha-hydroxyacids in the treatment of signs of photoaging. Clinics in Dermatology. 1996;14:217–226.
Veda S, Mitsugi K, Ichige K, et al. New formulation of chemical peeling agent: 30% SA in polyethylene glycol. Journal of Dermatological Science. 2002;28:211–218.
Wang X. A theory for the mechanism of action of the AHAs applied to the skin. Medical Hypotheses. 1999;53:380–382.
Yu RJ, Van Scott EJ. Alpha-hydroxy acids: science and therapeutic use. Issues and perspectives of AHAs. Cosmetic Dermatology. 1994;October(Suppl):1–6.
Yu RJ, Van Scott EJ. Bioavailability of alpha-hydroxy acids in topical formulations. Cosmetic Dermatology. 1996;9:54–62.
Yu RJ, Van Scott EJ. Salicylic acid: not a beta-hydroxy acid. Cosmetic Dermatology. 1997;10:27. [letter to the editor]