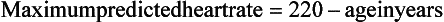Chapter 44
Exercise and the Heart
1. What is the difference between physical activity and exercise?
2. What is the difference between isometric and isotonic exercise?
3. What is the training effect?
4. What are the acute cardiovascular changes that occur with exercise?
5. What are the chronic cardiovascular changes that occur with exercise?
The increase in cardiac output associated with isotonic exercise creates a volume load that results in left ventricular dilation with minimal increase in wall thickness. The vasoconstriction and increased afterload associated with isometric exercise produces a pressure load that results in left ventricular hypertrophy without dilation.
6. How is exercise intensity defined?
7. How much exercise is necessary to maintain cardiovascular fitness?
8. What is the effect of exercise on cardiac risk factors?
Exercise has beneficial effects on hypertension, diabetes, hyperlipidemia, and obesity. In addition, exercise has favorable effects on endothelial function, thrombosis, inflammation, and autonomic tone (Table 44-1).
TABLE 44-1
BENEFICIAL EFFECTS OF ENDURANCE EXERCISE ON ATHEROSCLEROTIC RISK FACTORS
| FACTOR | EFFECT OF EXERCISE |
| Hypertension | Modest ↓ in both SBP (approximately 4 mm Hg) and DBP (approximately 3 mm Hg) |
| Diabetes | ↑ Insulin sensitivity, ↓ hepatic glucose production, preferential use of glucose over fatty acids by exercising muscle |
| Hyperlipidemia | Significant ↓ in TG, modest ↑ in HDL, minimal change in LDL |
| Obesity | Modest weight loss (2-3 kg), ↓ in body fat necessary to maintain weight loss |
| Thrombosis | ↓ Fibrinogen, ↓ platelet activation |
| Endothelial function | Improved vasodilation, possibly through ↑ NO synthesis |
| Autonomic tone | ↑ Vagal tone, ↓ sympathetic tone |
| Inflammation | ↓ Inflammatory markers (CRP, TNF-α, IL-6) |
9. Do endurance training and resistance training have similar benefits?
10. What is the effect of exercise on mortality?
11. Is it ever too late to obtain the benefits of exercise?
12. Is it safe for patients with known coronary artery disease (CAD) to exercise?
13. How long after MI can a patient begin an exercise program?
Patients who are clinically stable after MI can begin an exercise program as part of inpatient CR within 1 to 2 days of their infarction. Initial activity may be limited to range-of-motion exercises, but is rapidly increased to assisted walking. Activity is then gradually increased so that most patients can independently perform activities of daily living at the time of hospital discharge. Current AHA/American College of Cardiology (ACC) guidelines suggest that after MI all stable patients should be referred to formal outpatient CR programs. This is especially true for patients with multiple cardiac risk factors and for moderate- or high-risk patients (e.g., patients with residual CAD, patients with depressed left ventricular [LV] systolic function) for whom a supervised exercise program is appropriate. Stable patients can usually enroll in these programs 2 to 3 weeks after MI.
14. Is exercise safe for patients with heart failure?
15. Does exercise benefit patients who are limited by leg claudication?
16. What is an exercise prescription?
17. How is an exercise prescription developed after MI?
18. Should patients with heart disease perform resistance training exercise?
Yes. Resistance training is now a standard part of a comprehensive exercise regimen for patients with cardiovascular disease, and appears to be particularly beneficial in the elderly, patients with stable heart failure, and those with diabetes. The improvement in muscular strength and endurance that accompanies resistance training aids in the performance of activities of daily living and may facilitate return to the workplace. Resistance training is prescribed at an intensity of 10 to 15 repetitions per set, using a load that is based on the maximum load (ML) that the patient can lift a single time (upper body: 30% to 40% ML; lower body: 50 to 60% ML). Patients should perform 1 to 3 sets of 8 to 10 different upper- and lower-body exercises at a frequency of 2 to 3 times per week. Common modalities for resistance training include free weights, weight machines, wall pulleys, elastic bands, and calisthenics.
19. What is aerobic interval training?
20. What are the cardiovascular risks of exercise?
21. Should patients be screened before enrolling in an exercise program?
22. What are the contraindications to participation in an exercise program?
Bibliography, Suggested Readings, and Websites
1. Maron, B.J., Zipes, D.P. 36th Bethesda Conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005;45:2–64.
2. American Association of Cardiovascular and Pulmonary Rehabilitation. The AACVPR website. Available at http://www.aacvpr.org. Accessed March 13, 2013
3. American College of Sports Medicine. The ACSM website. Available at http://www.acsm.org. Accessed March 13, 2013
4. American Diabetes Association. Physical activity/exercise and diabetes. Diabetes Care. 2006;29:1433–1438.
5. American Heart Association. The AHA website (search Exercise). Available at http://www.heart.org. Accessed March 13, 2013
6. Awtry, E.A., Balady, G.J. Exercise and physical activity. In Topol E.J., ed.: Textbook of cardiovascular medicine, ed 3, Philadelphia: Lippincott Williams and Wilkins, 2007.
7. Balady, G.J., Ades, P.A. Exercise and Sports Cardiology. In: Bonow R.O., Mann D.L., Zipes D.P., Libby P., eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. ed 9. Philadelphia: Saunders; 2012:1784–1792.
8. Balady, G.J., Ades, P.A., Bittner, V.A., et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960.
9. Balady, G.J., Williams, M.A., Ades, P.A., et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update. A statement from the American Heart Association. Circulation. 2007;115:2675–2682.
10. Downing, J., Balady, G.J. The role of exercise training in heart failure. J Am Coll Cardiol. 2011;58:561–569.
11. Hamburg, N.M., Balady, G.J. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123:87–97.
12. Hammill, B.G., Curtis, L.H., Schulman, K.A., et al. 2010 Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70.
13. Haskell, W.L., Lee, I.-M., Pate, R.P., et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093.
14. Maron, B.J., Thompson, P.D., Ackerman, M.J., et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update. A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;115:1643–1655.
15. Murphy, T.P., Cutlip, D.E., Regensteiner, J.G., et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study. Circulation. 2012;125:130–139.
16. O’Connor, C.M., Whellan, D.J., Lee, K.L., et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450.
17. Thompson, P.D. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112:2354–2363.
18. Thompson, P.D., Franklin, B.A., Balady, G.J., et al. Exercise and acute cardiovascular events: placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115:2358–2368.
19. Thompson W.R., ed. American College of Sports Medicine Guidelines for Exercise Testing and Prescription, ed 8, Philadelphia: Lippincott, Williams and Wilkins, 2010.
20. Williams, M.A., Haskell, W.L., Ades, P.A., et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update. A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584.
21. Wisløff, U., Støylen, A., Loennechen, J.P., et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094.