Chapter 94 Ex vivo and in situ hypothermic hepatic resection
Overview
The limits of liver surgery are constantly evolving, propelled by advances in surgical technique and imaging. Extended right and left hepatectomies, previously considered to be pushing the boundaries of resection, have become relatively standard procedures for experienced liver surgeons. Strategies such as preoperative portal vein embolization (PVE) (see Chapter 93A, Chapter 93B ) to allow growth of the planned liver remnant allows even more aggressive resections to be performed with a morbidity similar to that of less extensive resections. Advances in surgical technique have been paralleled by advances in hepatic imaging, giving the liver surgeon greater ability to assess tumor position in relation to the intrahepatic vascular and biliary structures. Over the same period, liver transplantation has thrived and proliferated, and more recently the development of living donor liver transplantation (LDLT) has led to a variety of techniques that also can be applied in nontransplant liver surgery. Standard components of LDLT include resection and reconstruction of portal venous, hepatic arterial, biliary, and hepatic venous structures (see Chapters 90D and 98B). These same techniques are now more frequently being considered for the resection of hepatic tumors by surgeons experienced with liver resection and transplantation techniques.
The principal difficulty of vascular reconstruction during hepatic resection is the resulting period of hepatic ischemia. The liver can tolerate short periods of ischemia surprisingly well. During standard liver resections, the use of hepatic inflow occlusion is often used during the parenchymal transection to control excessive blood loss. The use of a combination of inflow occlusion with maintenance of low central venous pressure (CVP) allows relatively bloodless transection of the liver. Intermittent hepatic inflow occlusion for 15 minutes with 5 minutes of reperfusion at the end of each 15-minute interval can reduce the degree of liver injury (Man et al, 1997) and may be particularly useful in more complex resections.
Ischemic preconditioning seems to protect the liver from subsequent ischemic injury (Clavien et al, 2003) and is performed by applying a Pringle maneuver for 10 minutes, then reperfusing the liver for at least 10 minutes before reapplying inflow occlusion. The mechanisms by which ischemic preconditioning protects the liver from subsequent ischemia have not been fully elucidated, but even longer periods of ischemia are relatively well tolerated. The normal liver can tolerate 60 minutes or more of continuous inflow occlusion and warm ischemia (Huguet et al, 1994; Azoulay et al, 2005), but patients with cirrhosis or altered liver function from biliary obstruction or prolonged chemotherapy may tolerate significantly less ischemic insult before sustaining irreversible injury (Hannoun et al, 1996); the same may be true of older patients (Selzner et al, 2009). Even with normal liver function, the risk of irreversible liver damage following prolonged periods of continuous ischemia is significant.
Beyond inflow occlusion, tumors that involve the retrohepatic inferior vena cava (IVC) or that involve the hepatic veins at their junction with the vena cava may require total vascular isolation (see Chapter 91A). Total vascular isolation may increase the degree of ischemic injury to the liver more than inflow occlusion alone, as there is some evidence that backward diffusion of hepatic venous blood into the liver attenuates ischemic injury (Smyrniotis et al, 2003). In most cases, however, the procedure can be planned such that most of the hepatic parenchymal division is performed without total vascular exclusion, and caval clamping is reserved for the relatively short time that is required to deal with the IVC or hepatic veins.
History of Hypothermic Perfusion and Ex Vivo Techniques
Fortner and colleagues (1974) first described the use of hypothermic perfusion during liver resection to protect the liver from ischemic injury in a series of 29 patients. Technical improvements in liver surgery over the next 2 decades, along with the growing understanding of the liver’s ability to tolerate normothermic ischemia, made the use of hypothermic perfusion unnecessary in most cases. During the same period, liver transplantation had been applied to technically unresectable primary and secondary liver malignancy with dismal results (see Chapter 97A). Although the procedure was technically feasible, transplantation for large, unresectable primary liver tumors, and especially for metastatic lesions, resulted in the rapid recurrence of malignancy, either in the new liver or elsewhere shortly after transplantation.
In response to patients with unresectable tumors who were considered inappropriate for liver transplantation, Pichlmayr and associates (1988) developed hypothermic perfusion with ex vivo liver resection. During ex vivo liver resection, the liver is removed completely from the body and perfused with cold preservation solution on the back table. The liver resection is performed on the back table in a completely bloodless field such that reconstruction of hepatic venous outflow is performed under ideal conditions; however, the morbidity and mortality rates from this procedure are relatively high; as a result, in situ and ante situm hypothermic perfusion techniques have been explored.
In situ hypothermic perfusion is performed using standard liver resection mobilization techniques, but the liver is placed in total vascular isolation (see Chapter 91A) and cold perfused via the portal vein. In the ante situm procedure, the liver is cold-perfused via the portal vein, with the hilar structures left otherwise intact; but the suprahepatic IVC is divided, and the liver is rotated forward, allowing improved access to the area of the liver and IVC centered around the hepatic vein confluence. To a great extent, the three techniques—in situ hypothermic liver perfusion, ante situm hypothermic liver perfusion, and ex vivo liver resection—overlap, and all three mirror aspects of LDLT. The procedure and role for each technique are described below.
In Situ Hypothermic Perfusion
In situ hypothermic perfusion is the least technically demanding of the three techniques. In situ perfusion has been recommended (Hannoun et al, 1996) for liver resections that require total vascular isolation for periods exceeding 1 hour. More recently Azoulay and colleagues (2005) demonstrated that hypothermic perfusion of the liver is associated with better tolerance to ischemia in the setting of total vascular isolation of any duration.
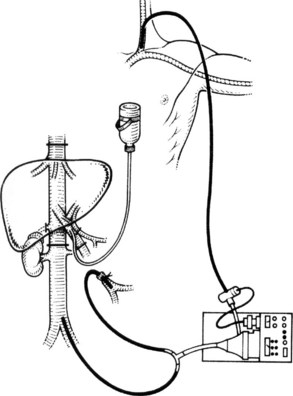
To perform in situ cold perfusion as originally described, the liver is mobilized as for total vascular isolation (see Chapter 91A) with control of the suprahepatic and infrahepatic IVC and the portal structures. The main portal vein is dissected out for insertion of a perfusion catheter. Although the portal vein can be dissected from the right of the bile duct, for in situ perfusion, it is usually easier to dissect it from the left of the bile duct after dissecting out the hepatic artery; the hepatic artery eventually requires control in any event. A sufficient length of portal vein (3 to 4 cm) is exposed to place a perfusion catheter and the portal venous cannula of venovenous bypass, if this is to be used. Most patients tolerate total vascular isolation without venovenous bypass; however, the standard use of bypass reduces the time pressures involved in these cases and reduces the gut edema associated with prolonged portal clamping. Prior to clamping, the patient is bolused with at least 5000 U of heparin intravenously. The infrahepatic cava is clamped, and the patient is placed on the caval portion of venovenous bypass.
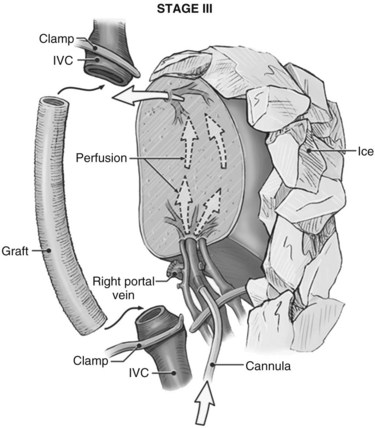
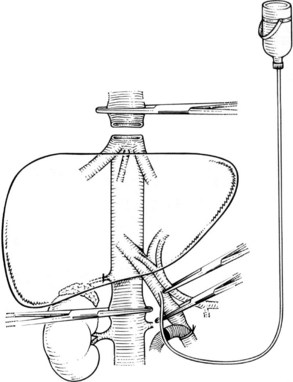
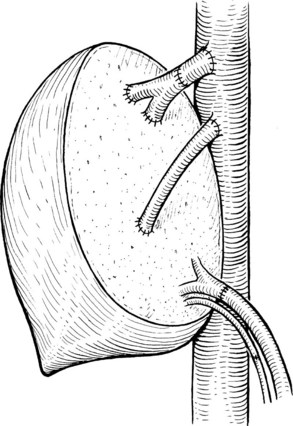
It is generally advisable, although not absolutely necessary, to ligate and divide the right adrenal vein before clamping the infrahepatic IVC, because it otherwise has a tendency to become avulsed during some particularly inconvenient portion of the procedure. A portal clamp is placed relatively superiorly on the portal vein with bypass instituted below. The portal cannula can be inserted down toward the superior mesenteric vein after complete division of the portal vein or by dividing just the anterior wall of the portal vein and sliding the cannula down the back wall. Full venovenous bypass is started. The liver side of the portal vein is cannulated with the perfusion solution tubing, and the hepatic artery is clamped. The suprahepatic cava is clamped, and a transverse venotomy is created in the infrahepatic IVC just above the clamp. Cold perfusion of the liver is begun with preservation solution, and the effluent is suctioned from the venotomy in the infrahepatic cava (Fig. 94.1).
Preservation solution is either histidine-tryptophan-ketoglutarate (HTK) (Gubernatis et al, 1990) or University of Wisconsin (UW) solution (Kalayoglu et al, 1988). The liver resection proceeds in a bloodless field with excellent visualization of intrahepatic structures. The liver can be cooled continuously throughout the parenchymal transection by slow infusion of cooling solution (see below), or it can be cooled intermittently every 30 minutes by bolus infusion; at completion of the liver resection, the liver should be flushed of cold preservation solution, which can be done by flushing the portal vein with cold 5% albumin before restoring flow to the liver, or by allowing the initial 300 to 500 mL of venous effluent from the reperfused liver to be vented out the infrahepatic cava venotomy after reperfusion but before removing the suprahepatic cava clamp. Next, the portal bypass cannula is removed, and the portal vein is repaired or reanastomosed if divided. If the liver has been flushed with 5% albumin, the infrahepatic venotomy is closed, and the suprahepatic cava clamp is removed to assess hepatic venous bleeding, which should be controlled if present. Portal and hepatic arterial inflow is reestablished.
We rarely use in situ perfusion as just described. The advent of LDLT (see Chapters 90D and 98B) and the more frequent use of the anterior approach to liver resection (Liu et al, 2000) have improved the ability to divide the liver parenchyma and dissect along the hepatic veins without the need for extensive periods of inflow occlusion. In patients in whom a single hepatic vein or the vena cava requires reconstruction, most of the parenchymal transection can be performed without inflow occlusion under low CVP conditions. As the parenchymal transection is near completion, total vascular isolation is applied to divide and reconstruct the vascular structures only. This practice results in substantially shorter periods of hepatic ischemia and allows a different method of applying in situ cold perfusion that is simpler and generally does not require bypass.
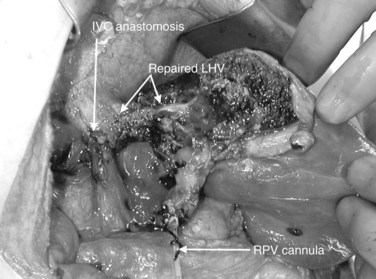
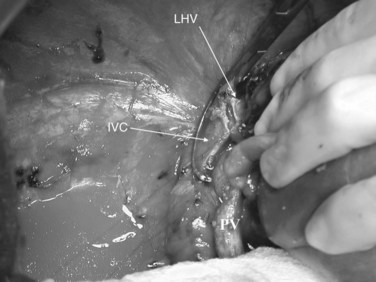
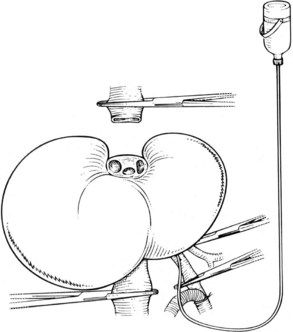
In this technique, the liver is mobilized as for total vascular isolation. The portal vein is dissected to the right and left branches, and perfusion tubing is placed into the portal vein branch on the side of the liver to be removed (Fig. 94.2). The branch is divided, maintaining portal flow to the side of the liver to be left in but allowing access for cold perfusion. Alternatively, the anterior wall of the main portal vein can be used as a site of cannula insertion (Fig. 94.3). As much of the hepatic parenchyma as possible is divided under low CVP, maintaining hepatic perfusion until it becomes necessary to divide the hepatic veins or IVC that requires reconstruction.
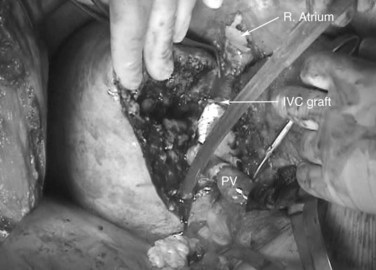
At this point, the patient is volume loaded, and clamps are placed sequentially on the infrahepatic cava, the portal vein, hepatic artery, and the suprahepatic IVC. If only the hepatic vein requires reconstruction, caval flow can be maintained by controlling the offending hepatic vein by placing a clamp tangentially down onto the IVC, across the hepatic vein orifice, but only partially occluding the IVC (Fig. 94.4). The anterior wall of the IVC or hepatic vein is incised, and cold perfusion of the liver is instituted through the portal cannula. Only the side of the liver remaining in place is perfused. The vessels are transected, and the specimen is removed. The vessels, hepatic veins, or IVC can be reconstructed in a bloodless field without time pressures (Fig. 94.5). Before completing the anastomoses, the liver can be flushed with cold 5% albumin. Alternatively, the liver can be flushed with 300 to 400 mL of portal vein blood with the caval clamps still in place, whereupon the portal vein clamp is reapplied, the caval venotomy is closed, and all clamps are removed. With the shorter ischemic periods involved, we have not had to use venovenous bypass with this technique (Dubay et al, 2009; Hemming et al, 2002, 2004, 2008); even longer periods of total vascular isolation do not necessarily require venovenous bypass.
Ante Situm Procedure
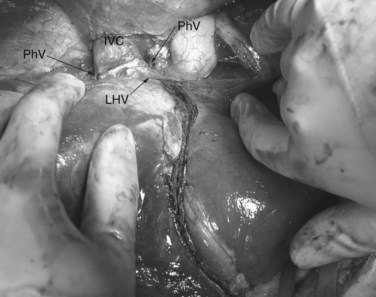
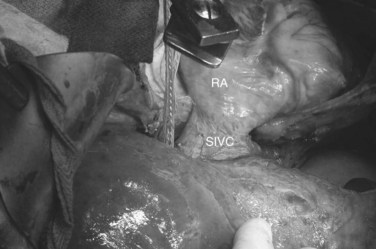
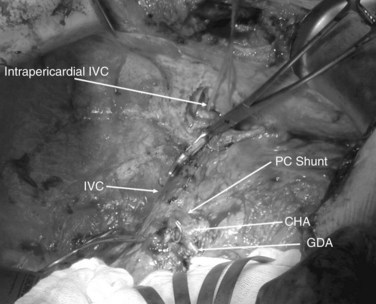
The ante situm technique of liver resection can be used when resection of the IVC and hepatic veins is expected to be difficult, and when improved access to the hepatic veins and IVC is required. We have used this technique when combined cava and hepatic vein reconstruction is required (Azoulay et al, 2006). The ante situm technique uses the same technique as in situ cold perfusion with several caveats. The suprahepatic IVC requires more extensive dissection to achieve enough length to place a clamp on, divide, and subsequently reanastomose. Greater exposure of the suprahepatic cava can be obtained by dividing the phrenic veins and gently dissecting the IVC away from the diaphragm (Fig. 94.6). We frequently also open the pericardium directly anterior to the IVC and loop the intrapericardial vena cava; alternatively, a sternotomy provides superb exposure of the entire area (Fig. 94.7).
Control of the intrapericardial cava allows placement of the clamp on the vena cava or caudal right atrium within the pericardium as a primary option or as a secondary option in case technical difficulties arise with placement of the original suprahepatic cava clamp. We perform as much of the liver transection as possible without inflow occlusion before cold perfusing the liver. Venovenous bypass is generally recommended for this procedure; however, many patients tolerate caval clamping without difficulty. Institution of cold perfusion is as originally described for in situ perfusion; however, the perfusate is vented via the suprahepatic IVC as the suprahepatic cava is divided. Dividing the suprahepatic IVC allows the liver to be rotated forward and upward toward the abdominal wall, enabling greater access to the area immediately around the IVC–hepatic vein junction (the hepatocaval confluence) (Figs. 94.8 and 94.9).
The ante situm approach gives better access to the cava–hepatic vein junction than does simple in situ cold perfusion. The exposure is not as good as with a complete ex vivo approach. The advantage to the ante situm approach is that biliary and hepatic arterial anastomoses are not required, reducing the ischemic time to the liver and reducing the potential for complications resulting from these additional anastomoses. Currently, we use the ante situm approach when a combination of cava resection and hepatic vein reconstruction is required, and we expect to implant only a single hepatic vein orifice, whether from a single vein or one or more veins plastied together, into the cava (Figs. 94.10 through 94.12. In the few cases where the reconstruction is expected to be significantly more complex, we use a complete ex vivo approach.
Ex Vivo Liver Resection
Patient Selection and Preoperative Workup
One of the benefits of planning the ex vivo approach—or for that matter, planning any of the hypothermic perfusion techniques—is that frequently at operation, what initially was thought to be unresectable by any method short of complete removal of the liver is found to be resectable with a less complex procedure. General assessment of the patient is similar to that for liver transplantation, with particular assessment of cardiac risk factors. In patients older than 50 years of age or those with any cardiac abnormalities, a functional stress test, such as dobutamine stress echocardiogram, is performed. Any significant cardiac abnormality is a contraindication. Even mild renal dysfunction has been shown to increase the risk of standard extended hepatectomy (Melendez et al, 2001), and a creatinine level greater than 1.3 mg/dL should be considered a contraindication. Ex vivo liver resection should be attempted only in otherwise healthy, well-selected patients.
Preoperative imaging is crucial to stage the tumor and to assess its position in relation to hepatic vascular anatomy. Our current standard is triphasic spiral computed tomography (CT) with three-dimensional (3D) reconstructions, to assess the liver anatomy and tumor position, and CT of the chest and pelvis (see Chapter 16). In some instances, magnetic resonance imaging (MRI) is required, including MR angiography and MR venography, particularly when all three hepatic veins are involved, and some degree of hepatic venous obstruction is present that prevents adequate flow of contrast material into the hepatic veins during CT (see Chapter 17). With the combination of CT and MRI and the present availability of 3D reconstruction, invasive angiography is usually unnecessary. Contrast ultrasound provides a dynamic view of the relationship of the tumor to the hepatic veins and cava, and it is particularly useful for gauging extent of invasion of vascular structures by the tumor.
Remnant liver volume assessment can be performed as for extended hepatectomy. In a standard liver resection, assuming normal liver function, 25% of total liver volume (TLV) after resection is considered adequate for resection without the need for preoperative PVE (see Chapter 93A). One caveat is that patients who have undergone or are undergoing prolonged and aggressive preoperative chemotherapy may require more liver volume because of chemotherapy-induced steatohepatitis. With cold preservation and reperfusion, additional ischemic injury occurs over and above what happens during standard liver resection. We have arbitrarily chosen a projected liver remnant of 40% of TLV as a cutoff for consideration for preoperative PVE in patients who require extended hepatectomy who may need complex vascular reconstruction and cold-perfusion techniques (Hemming et al, 2003, 2008). A positron-emission tomography (PET) scan should also be considered for all patients to assess for the possibility of otherwise undetected extrahepatic disease (see Chapter 15).
Accurate imaging of the intrahepatic architecture is paramount to assess the possibilities for reconstruction. Three-dimensional imaging techniques occasionally may reveal unusual anatomy that makes vascular reconstruction unnecessary, such as the presence of a large inferior hepatic vein that makes reconstruction of the main right hepatic vein unnecessary. Alternatively, anatomy may be discovered that requires additional reconstruction, such as a large segment VI vein that drains into the middle hepatic vein (Lang et al, 2004).
Anesthesia
Anesthetic management for ex vivo liver resections is similar to that for liver transplantation (see Chapter 97B). Maintaining patient temperature is a key component of anesthesia management and includes the use of forced-air warming blankets and warming of all fluids. In addition to standard monitoring for liver resection, a Swan-Ganz catheter is inserted for hemodynamic monitoring and assessment of blood temperature in the pulmonary artery. Access for venovenous bypass, which originally was achieved by cutdowns, has been simplified by percutaneous insertion techniques.
Surgical Procedure
Pichlmayr and Hauss (1994) described the procedure of ex vivo liver resection in the second edition of this textbook; they appropriately described the procedure in terms similar to liver transplantation. The following description of technique is taken largely from Pichlmayr’s original description in this textbook with some minor modifications by the current authors. The procedure comprises three phases: 1) assessment of resectability and removal of the liver, 2) liver resection and vascular reconstruction on the back table, and 3) reimplantation of the liver autograft. As with the preceding discussion, many of the recommendations found below are applicable to any of the three hypothermic perfusion techniques described in this chapter.
Assessment of Resectability and Removal of the Liver
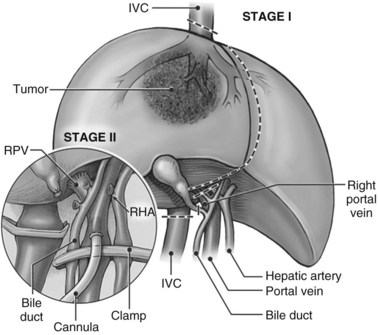
1 The hilar structures are prepared. The arterial anatomy is dissected, and an appropriate site is chosen for planned division. This usually is at the level of the common hepatic artery–gastroduodenal artery junction. If aberrant arterial anatomy is evident, an alternative site may be required, depending on the particular anatomy and the portion of the liver that remains after the resection. In most cases, to preserve small arterial communications to the biliary plate and tree, the artery is not dissected high into the hilum. If the tumor involves the hilar structures, the artery must be dissected far enough to determine that there is a usable, tumor-free portion of the respective hepatic artery branch to reconstruct. The portal vein is identified, and the common bile duct is encircled without skeletonization. A cholecystectomy is performed, and the bile duct is reflected off the portal vein, taking care to preserve its blood supply, and the neural and lymphatic tissue around the portal vein is cleared.
2 The infrahepatic IVC is dissected out down to the level of the renal veins and encircled. Planned vascular clamp placement is immediately above the renal veins, although division of the vena cava may be substantially more cephalad. The right adrenal vein should be ligated and divided, and small caudate veins should be divided if accessible. The large size of the tumor may make access to the caudate veins difficult, in which case they can be dealt with on the back table.
3 The liver is freed from surrounding attachments. If the tumor is infiltrating the diaphragm, the portion of the diaphragm involved is resected en bloc with the tumor.
4 The suprahepatic IVC is prepared. The phrenic veins are divided bilaterally, allowing the diaphragm to be dissected away from the IVC, providing an increased length of intraabdominal IVC for subsequent clamping and reconstruction. We currently also open the pericardium directly anterior to the IVC and loop the intrapericardial IVC, or we use a midline sternotomy for the same reason. Control of the intrapericardial IVC allows placement of the clamp on the vena cava within the pericardium as a primary option for tumors that are very large, or as a secondary option in case technical difficulties arise with placement of the original suprahepatic cava clamp.
5 Clamp placement and subsequent transection lines are assessed, along with the need for vascular conduits for reconstruction, and alternatives for reconstruction are contemplated before removing the liver. Hepatic venous branches may be reconstructed using hepatic vein or portal vein segments from the side of the liver resected or from autologous vein grafts, such as superficial femoral vein, proximal left renal vein, gonadal vein, or saphenous vein panel grafts. Cryopreserved femoral vein grafts can be used for reconstruction of long segments of hepatic veins or IVC, although long-term patency is currently unclear. The IVC alternatively can be replaced with a 20-mm ringed polytetrafluoroethylene (Gore-Tex) tube graft. Planning for these eventualities should occur before placement of clamps and removal of the liver.
6 Percutaneous access of the internal jugular vein and femoral vein is achieved, the IVC is clamped, and the cava portion of the bypass is started. The portal vein is clamped just below the bifurcation, and the portal cannula is inserted down toward the superior mesenteric vein and secured. The portal vein is completely transected approximately 1.5 to 2 cm below the bifurcation, and the portal flow is added to the bypass circuit, while the liver continues to be perfused by arterial blood.
7 The liver is removed, and the bile duct is transected sharply approximately 1 to 1.5 cm below the bifurcation. To permit a duct-to-duct anastomosis on reimplantation, we do not ligate either end. The gastroduodenal artery is ligated and divided, the common hepatic artery is clamped, and the hepatic artery is transected at the takeoff of the gastroduodenal artery such that a branch patch is formed on either side of the arterial transection if possible. The suprahepatic IVC is clamped either below the diaphragm or within the pericardium, and the suprahepatic vena cava is transected, far enough away from tumor to provide an adequate oncologic margin but close enough to leave room to perform the suprahepatic cava anastomosis at the time of reimplantation. If the vena cava has been freed of its posterior attachments before clamp placement, the infrahepatic IVC is divided as cephalad as possible, and the liver is removed and placed in an ice bath. Cold perfusion through the portal vein and hepatic artery is initiated on the back table. As with any hypothermic perfusion technique, cold perfusion can be with either HTK or UW solution. If the retrohepatic IVC has not been freed up previously because of the large tumor size and difficulty with access, the liver can be cold perfused via the portal vein after the suprahepatic IVC is divided. The liver is rotated forward, as in the ante situm approach, and the retrohepatic IVC is freed of attachments. The infrahepatic IVC is divided, and the liver is removed and placed in the ice bath.
8 Bleeding is controlled, and the bypass circuit is assessed. Flows of 3 to 6 L/min would be expected on total bypass (see Fig. 94.1). The abdomen is loosely packed and covered, and attention is turned to the back table.
An alternative method can be performed in patients with complex hepatic vein involvement without IVC involvement with tumor. If access allows, the liver can be mobilized completely off of the retrohepatic IVC, until it is suspended only by the hepatic veins. The hilar structures are divided as for standard ex vivo resection, but the patient is not placed on bypass. A vascular clamp is placed partially across the IVC, occluding the hepatic vein orifices but allowing cava flow to remain uninterrupted. The hepatic veins are transected, and the liver is removed and cold flushed on the back table. A temporary portacaval shunt is constructed (Fig. 94.13) to decompressing the enteric circulation; this option avoids bypass, but it can be used only when there is no IVC involvement, and when the IVC can be preserved in situ. It is worth assessing whether the tumor can be dissected away from the IVC in most patients, because preoperative imaging can be inaccurate in determining true IVC involvement (Okada et al, 2003).
Ex Situ Liver Resection
Hilar structures are dissected out and divided. Great care must be taken not to divide segmental arteries that supply portions of the liver that are to remain, as their caliber is too small to reconstruct with confidence. Main segmental divisions of the portal vein usually can be repaired and reconstructed. Parenchymal transection can be performed using a variety of techniques, including ultrasonic and water-jet dissection or even sharp division with a knife. Small bile ducts and vessels are ligated or oversewn, and large hepatic veins are cut sharply and are assessed later for reconstruction (Figs. 94.14 to 94.17). The surgical tumor margin should not be compromised in order to minimize the subsequent complexity of vascular reconstruction.
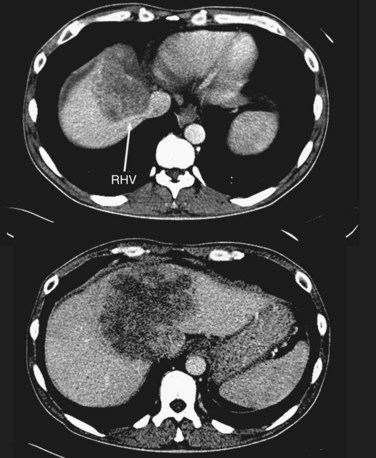
FIGURE 94.14 A single colorectal metastasis (arrow) involving all three hepatic veins. RHV, right hepatic vein.
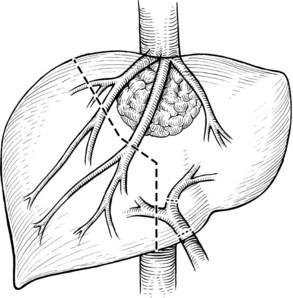
FIGURE 94.15 The planned hepatic transection line for the tumor shown in Figure 94.14. Multiple hepatic vein branches require reconstruction.
Hepatic Vein and Inferior Vena Cava Reconstruction
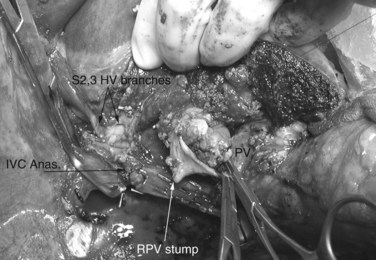
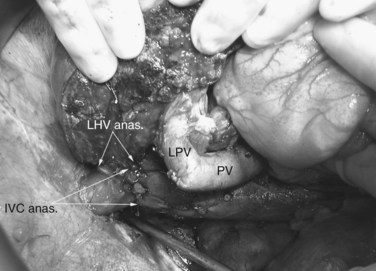
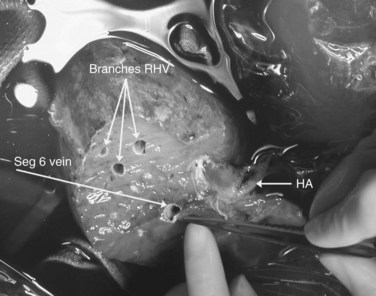
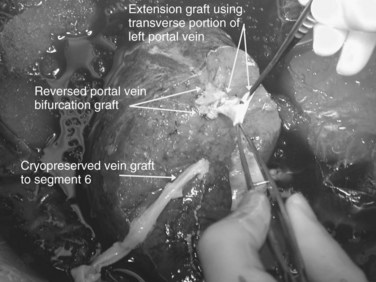
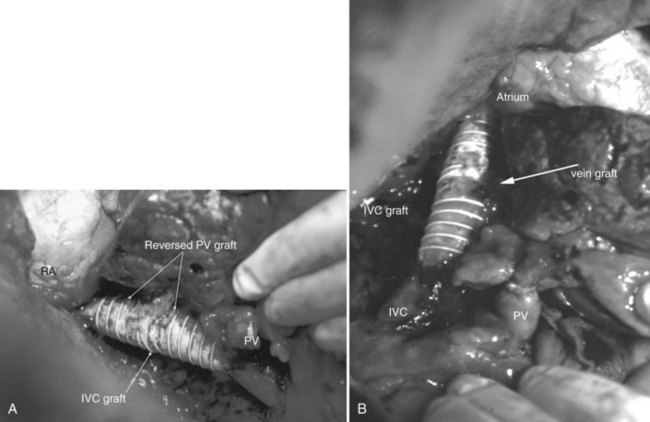
Multiple orifices of hepatic veins can be plastied together or implanted into venous conduits (Figs. 94.18 to 94.20). The farther away from the IVC that the hepatic vein is transected, the thinner the wall of the hepatic vein, and the more difficult it is to reimplant the vein directly into the IVC. We have harvested the portal vein bifurcation, reversed it, and used it to reconstruct multiple hepatic vein branches into a single outflow vessel on several occasions (Figs. 94.21 to 94.23; Hemming & Cattral, 1999). Grafts of saphenous, superficial femoral, internal jugular, and cryopreserved vein all have been used to reconstruct hepatic veins (Dong et al, 2004; Kaneoka et al, 2000; Kishi et al, 2004; Kubota et al, 1997, 1998). In addition, segments of uninvolved hepatic vein from the side of the liver resected can be salvaged and used for grafts or patches.
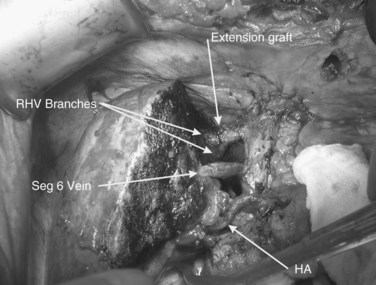
FIGURE 94.20 The reimplanted liver as in Figure 94.19. HA, hepatic artery; RHV, right hepatic vein; Seg 6, segment VI vein.
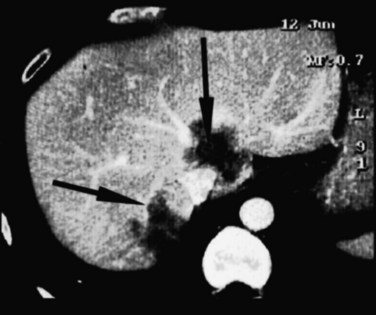
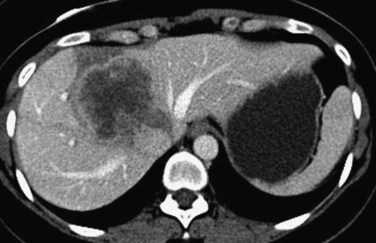
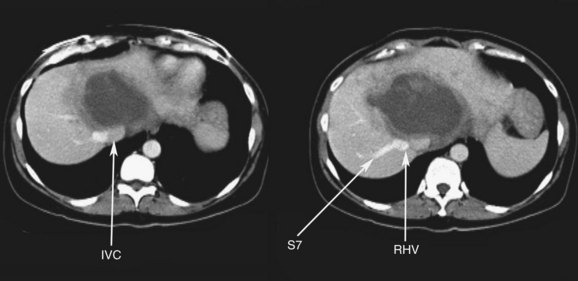
FIGURE 94.21 Computed tomographic scan showing colorectal metastases involving all three hepatic veins. Arrows indicate tumor.
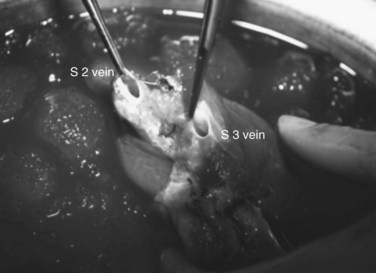
FIGURE 94.22 Remnant liver after ex vivo resection of tumor seen in Figure 94.21. S2 and S3 veins are segments II and III hepatic vein branches.
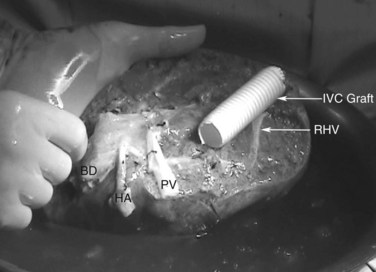
An important issue is how to reconstruct the vena cava. If possible, it is best to reimplant the hepatic veins into native vena cava. Frequently, a large portion of the vena cava that was removed initially with the liver can be salvaged and moved superiorly, with the hepatic veins reimplanted into this newly situated portion of IVC. A ringed 20-mm Gore-Tex tube graft can be used to make a composite cava graft with the Gore-Tex graft placed inferiorly. Alternatively, a section of the IVC remaining in the patient immediately above the renal veins can be harvested and used as the segment of IVC into which the hepatic veins are reimplanted. A ringed 20-mm Gore-Tex tube graft is used to replace the segment of IVC immediately above the renal veins. In some circumstances, it is necessary to reimplant the hepatic veins directly into a relatively stiff artificial graft that is replacing the IVC (Figs. 94.24 and 94.25). In this situation, it is important to make a larger opening in the Gore-Tex than one might expect and to triangulate the anastomosis to prevent anastomotic stricturing (Lodge et al, 2000).
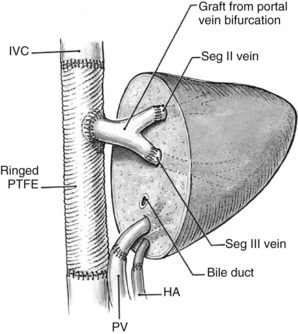
FIGURE 94.25 Diagrammatic representation of Figure 94.24. HA, hepatic artery; IVC, inferior vena cava; PTFE, polytetrafluoroethylene (Gore-Tex); PV, portal vein; Seg, segment.
At completion of the back-table reconstruction, an autograft results that is similar to that of a reduced-size or split-liver allograft (Figs. 94.26 through 94.28). Total cold ischemic time is usually 2 to 4 hours, which is well within acceptable limits, when comparing cold ischemic times for split-liver or reduced-size liver transplantation.
Postoperative Course
An early sign that the autograft is functioning is the return of lactate levels to baseline in the first 12 to 24 hours after surgery. Maintenance of coagulation parameters, in particular prothrombin time or international normalized ratio (INR), suggests recovery of liver function. It is frequently necessary, however, to give fresh frozen plasma for the first few days to maintain an INR target less than 2 (Martin et al, 2003). Hypophosphatemia can occur between postoperative days 1 and 3 as the liver regenerates, and it may be so profound as to require constant intravenous replacement. Without much evidence as to its effectiveness, we have used low-dose intravenous heparin (500 U/h) perioperatively, and we attempt to maintain the hematocrit between 30% and 35%. Patients who have artificial venous caval grafts are started on low-dose aspirin before discharge. This is maintained for life, although no data confirm the need for long-term anticoagulation.
Current Role of Ex Vivo Liver Resection
The role of such an extensive procedure in advanced malignancies is open for discussion. Although 2 decades have passed since Pichlmayr and colleagues (1988) described the first ex vivo liver resection, relatively few surgeons have attempted the procedure. The largest reported series from Pichlmayr’s own group consists of only 22 patients (Oldhafer et al, 2000). There are several reasons behind the lack of adoption of this technique, not least the fact that the two other hypothermic perfusion techniques described in this chapter, in situ and ante situm hepatic hypothermic perfusion, can be applied to the great majority of difficult vascular reconstructions with the important exception of tumors involving both hilar structures and the hepatic vein and/or the cava. The ex vivo technique requires a surgeon familiar with advanced techniques in liver resection and liver transplantation, which restricts the procedure to relatively few individuals. Many patients who may be candidates for ex vivo resection are deemed unresectable by competent hepatic surgeons and are not referred on. Perhaps the most compelling reason for the lack of adoption of this technique is the relatively high risk-to-benefit ratio that the procedure offers. Most of the literature on ex vivo liver resections has been case reports that describe aspects of technique, and long-term follow-up is not available. Even in well-selected patients, perioperative mortality is between 10% and 30%. By contrast, the perioperative mortality for patients undergoing in situ or ante situm hepatic hypothermic perfusion with vascular reconstruction is around 10% or less (Azoulay et al, 2005; Dubay et al, 2009; Hemming et al, 2008). Long-term survival after ex vivo liver resection is also poor: at best, the 5-year survival for ex vivo resections performed for malignancy is 15% to 30%. In Oldhafer’s series (2000), the six patients who underwent ex vivo resection for colorectal metastases had a median survival of 21 months, although it might be expected that the general improvement in survival following resection for colorectal metastases seen over the last decade would apply to ex vivo liver resections as well.
Notwithstanding the considerations above, there is no doubt that the occasional patient is cured by this aggressive procedure. One patient with resection for cholangiocarcinoma in Oldhafer’s series was alive and disease free at 3.5 years after resection, whereas one of our own patients who underwent ex vivo resection for HCC was alive and disease free more than 5 years after the operation (Hemming et al, 2000). It is worth noting that many patients may benefit by being considered for ex vivo liver resection, simply because a surgeon prepared to perform ex vivo resection realizes that the resection can be done using a less aggressive technique, such as in situ cold perfusion or standard vascular reconstruction.
Azoulay DA, et al. In situ perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg. 2005;241(2):277-285.
Azoulay DA, et al. Combined liver resection and reconstruction of the supra-renal vena cava. Ann Surg. 2006;244(1):80-88.
Clavien PA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843-852.
Dong G, et al. Cadaver iliac vein outflow reconstruction in living donor right lobe liver transplantation. J Am Coll Surg. 2004;199:504-507.
Dubay D, et al. In situ hypothermic liver preservation during radical liver resection with major vascular reconstruction. Br J Surg. 2009;96:1429-1436.
Fortner JG, et al. Major hepatic resection using vascular isolation and hypothermic perfusion. Ann Surg. 1974;180:644-652.
Gubernatis G, et al. HTK-solution (Bretschneider) for human liver transplantation: first clinical experiences. Langenbecks Arch Chir. 1990;375:66-70.
Hannoun L, et al. Major extended hepatic resections in diseased livers using hypothermic protection: preliminary results from the first 12 patients treated with this new technique. J Am Coll Surg. 1996;183:597-605.
Hemming AW, Cattral MS. Ex vivo liver resection with replacement of the inferior vena cava and hepatic vein replacement by transposition of the portal vein. J Am Coll Surg. 1999;189:523-526.
Hemming AW, et al. Ex vivo liver resection. Can J Surg. 2000;43:222-224.
Hemming AW, et al. Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg. 2002;235:850-858.
Hemming AW, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686-693.
Hemming AW, et al. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239:712-721.
Hemming AW, et al. Role for extending hepatic resection using an aggressive approach to liver surgery. J Am Coll Surg. 2008;206(5):870-875.
Huguet C, et al. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454-458.
Kalayoglu M, et al. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617-619.
Kaneoka Y, et al. Hepatic vein reconstruction by external iliac vein graft using vascular clips. World J Surg. 2000;24:377-382.
Kishi Y, et al. Sharing the middle hepatic vein between donor and recipient: left liver graft procurement preserving a large segment VIII branch in donor. Liver Transpl. 2004;10:1208-1212.
Kubota K, et al. Reconstruction of the inferior vena cava using a hepatic venous patch obtained from resected liver. Hepatogastroenterology. 1997;44:378-379.
Kubota K, et al. Reconstruction of the hepatic and portal veins using a patch graft from the right ovarian vein. Am J Surg. 1998;176:295-297.
Lang H, et al. Extended left hepatectomy: modified operation planning based on three-dimensional visualization of liver anatomy. Langenbecks Arch Surg. 2004;389:306-310.
Liu CL, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25-31.
Lodge JP, et al. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231:471-479.
Man K, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704-713.
Martin RC2nd, et al. The use of fresh frozen plasma after major hepatic resection for colorectal metastasis: is there a standard for transfusion? J Am Coll Surg. 2003;196:402-409.
Melendez J, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47-53.
Okada Y, et al. Diagnosis and treatment of inferior vena cava invasion by hepatic cancer. World J Surg. 2003;27:689-694.
Oldhafer KJ, et al. Long-term experience after ex situ liver surgery. Surgery. 2000;127:520-527.
Pichlmayr R, Hauss J. Autotransplantation of the liver and resection in the hypothermic perfused liver. In: Blumgart, LH, editor. Surgery of the Liver and Biliary Tract. 2nd ed. New York: Churchill Livingstone; 1994:1857-1862.
Pichlmayr R, et al. [Ex situ operation on the liver: a new possibility in liver surgery]. Langenbecks Arch Chir. 1988;373:122-126.
Selzner M, et al. Recipient age affects long-term outcome and hepatitis C recurrence in old donor livers following transplantation. Liver Transpl. 2009;15(10):1288-1295.
Smyrniotis V, et al. Effects of hepatovenous back flow on ischemic-reperfusion injuries in liver resections with the Pringle maneuver. J Am Coll Surg. 2003;197:949-954.