Chapter 22 Evidence-Based Summary of Guidelines from the American Venous Forum and the Society for Vascular Surgery
Chronic venous disorders are among the world’s oldest afflictions and are responsible for substantial socioeconomic morbidity in the Western world. Varicose veins are the most common clinical manifestation of chronic venous disease, occurring in one fourth to one third of Western adult populations,1,2 while severe chronic venous insufficiency with skin changes and ulceration is present in 2% to 5% of Western populations.3 There have been many technological advances in the management of venous disease over the past decade, and many of these have had substantial benefits for afflicted patients. Unfortunately, many of these treatments are quite costly and are often approved, marketed, and adopted by clinicians without solid evidence supporting their use.
Although the current federal regulatory processes are effective in encouraging the development of new technology and do provide some mechanism for ensuring safety, they do not favor proof of clinically relevant efficacy.4,5 It is therefore becoming increasingly important for the clinician to have the skills to evaluate the clinical evidence and recommend the best treatment for their patients. The American Venous Forum has taken the lead in providing the clinical data clinicians require in caring for patients with venous disease and have published, together with the Society for Vascular Surgery, the most up-to-date practice guidelines for the care of patients with venous disorders.6
Evidence-Based Medicine and Practice Guidelines
Evidence-based medicine is perhaps best defined as “the conscientious, explicit, and judicious use of the current best evidence in making decisions about the care of individual patients.”7 This specifically involves integrating clinical expertise, the patient’s individual values and preferences, and the best available clinical evidence. Older approaches to evaluating clinical evidence relied primarily on a hierarchy of study methodology, a system that provides little guidance to physicians in daily practice. While randomized clinical trials are usually less prone to bias and are less likely to lead to false-positive conclusions, it is unrealistic to expect data from rigorously conducted trials to guide every clinical question that arises. Furthermore, they may not be appropriate for all diseases and interventions.8 Randomized trials are difficult to justify when interventions are clearly harmful or show a large beneficial treatment effect (risk ratio <0.4) in observational studies or when the treatment effect is so small (risk ratios 0.9 to 1.0) that sample size requirements preclude adequately powered randomized trials. Perhaps most importantly, clinicians are less interested in the precise study methodology than they are in reliable estimates of the benefits and harms associated with a therapy.9 For high-quality evidence, the effects of therapy are precise, and further research is unlikely to change our confidence in the estimate of effect. In contrast, the estimated effect provided by poor-quality evidence may be unclear and subject to change as better-quality evidence becomes available.
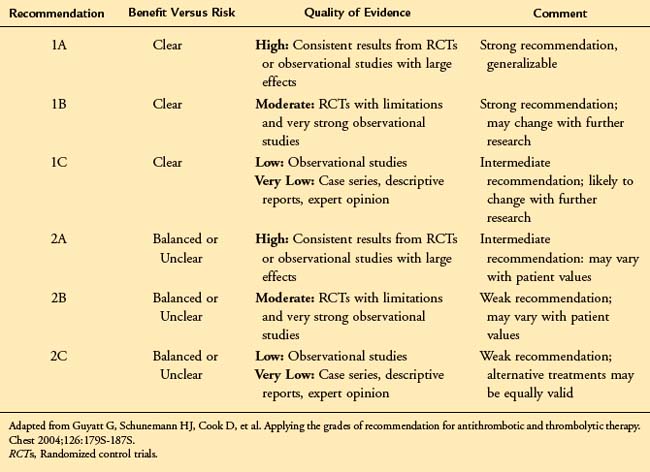
Current approaches to the evaluation of clinical evidence account for these concerns and are based largely on an assessment of the estimate of effect (beneficial or ill) associated with a treatment. The approach developed by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) working group9 has been adopted by the American Venous Forum in developing practice guidelines.9 According to this system, there are two components to any treatment recommendation—the first a designation of the strength of the recommendation (1 or 2) based upon the degree of confidence that the recommendation will do more good than harm, the second an evaluation of the strength of the evidence (A to D) based upon the confidence that the estimate of effect is correct. (Table 22-1).
In accordance with the American College of Chest Physicians (ACCP) guidelines for the antithrombotic treatment of venous thromboembolic disease,10 the American Venous Forum has adopted the language of “recommending” the use of strong Grade 1 guidelines and “suggesting” the use of weaker Grade 2 guidelines. These guidelines should be viewed as a summary of the best available clinical evidence to guide the management of patients with chronic venous disease. However, consistent with the goals of evidence-based medicine, they are subject to the physician’s clinical judgment, resources, and expertise and the patient’s individual values and preferences. They should not be interpreted as a rigid “standard of care.” The key elements of the evidence-based guidelines are outlined in Table 22-2.
![]() TABLE 22–2 Key Treatment Recommendations for Chronic Venous Disease
TABLE 22–2 Key Treatment Recommendations for Chronic Venous Disease
| Guideline | Grade of Recommendation |
|---|---|
| Diagnosis of Chronic Venous Disease | |
| We recommend initial clinical evaluation including a thorough history, focusing on the underlying etiology (congenital, primary, or secondary), symptoms, and risk factors for venous disease. | 1A |
| We recommend duplex ultrasonography, including an evaluation of reflux in the upright position, as the initial diagnostic test in patients with CVD. | 1A |
| We recommend threshold values for reflux of >500 ms for reflux in the saphenous, deep femoral, tibial, and perforating veins and >1 s in the femoral and popliteal veins. | 1B |
| We recommend adjunctive studies including plethysmography, CT/MR venography, and IVUS in selected patients in whom the pathophysiology is incompletely defined by ultrasound or in whom a surgical or endovenous intervention is planned. | 1B |
| Management of C1-C3 Chronic Venous Disease | |
| We suggest the use of venoactive drugs such as horse chestnut seed extract for amelioration of the symptoms of pain and swelling. | 2B |
| We suggest the use of 20–30 mm Hg compression stockings for patients with symptomatic varicose veins who are not candidates for superficial venous intervention. | 2C |
| We recommend stripping or ablation of the saphenous vein in preference to compression stockings in patients who are suitable candidates. | 1B |
| We recommend endothermal venous ablation in preference to high ligation and stripping or foam sclerotherapy for the management of saphenous vein incompetence. | 1B |
| We recommend sclerotherapy for the treatment of reticular veins, telangiectasias, and recurrent varicose veins. | 1B |
| We recommend phlebectomy over sclerotherapy for the treatment of tributary varicosities once axial reflux has been addressed. | 1B |
| We recommend against the treatment of incompetent perforating veins in patients with C2 CVD. | 1B |
| Management of C4-C6 Chronic Venous Disease | |
| We recommend compression therapy for the treatment of venous leg ulcers. | 1A |
| We suggest pentoxifylline as an adjunct for the healing of venous leg ulcers. | 2B |
| We recommend sharp debridement of venous ulcers associated with significant slough and nonviable tissue. | 1C |
| Depending on the specific agent, specialized wound dressings can be only weakly suggested. | 2 B/C |
| We recommend the treatment of saphenous incompetence to reduce the recurrence of venous leg ulcers. | 1A |
| We suggest the treatment of pathologic perforating veins—defined as incompetent perforating veins ≥3.5 mm in diameter with outward flow ≥500 ms in duration and located beneath a healed or open venous ulcer. | 2B |
| The benefit of deep venous valvular reconstruction is poorly established and is suggested only in centers with substantial experience and after failure of treatments supported by more substantial data. | 2C |
CT/MR, Computed tomography/magnetic resonance; CVD, chronic venous disease; IVUS, intravascular ultrasound.
Evidence-Based Management of Venous Disease
Evaluation and Follow-up of the Patient with Chronic Venous Disease
Evaluation of the patient with venous disease should include a thorough history, focusing on the underlying etiology (congenital, primary, or secondary), symptoms, and risk factors for venous disease (Grade 1A). This should include an assessment of the degree of disability and effect on the patient’s quality of life. Physical examination should focus on specific features of venous disease and exclusion of other etiologies of the patient’s signs and symptoms. In clinical practice, every patient should be characterized using both the basic CEAP classification and the Venous Clinical Severity Score (VCSS).11,12
For research investigations, the use of patient-important outcomes is strongly recommended, while the use of technical or surrogate outcome measures should be restricted to early feasibility studies. Several disease-specific quality of life measures are available for this purpose.13–15
Treatment of Mild (C2-C3) Chronic Venous Disease
Compression Therapy
Despite the absence of methodologically sound data, compression stockings are often considered first-line therapy for mild to moderate chronic venous disorders. The majority of comparative studies of compression stockings have evaluated surrogate hemodynamic parameters rather than patient-important outcomes, and it does appear that stockings improve a variety of hemodynamic measurements.16 However, the clinical benefits are less clear. Comparisons to placebo are very limited but do suggest some improvement in symptoms with the use of compression stockings.16 Others have reported improvement in up to one third of patients with compression stockings.17 Definitive data regarding the optimal degree of compression are lacking. However, symptomatic improvement is clearly less than after surgical treatment of varicose veins,17 and the authors of one systematic review concluded that the benefits of compression as a first-line treatment are limited.16 Although 20 to 30 mm Hg compression stockings are suggested for patients with symptomatic varicose veins (Grade 2C), the American Venous Forum recommends against their use as the primary treatment in patients who are candidates for superficial venous intervention (Grade 1B). Compression stockings should be considered only after a thorough history and measurement of the ankle-brachial index to exclude arterial disease and should be fitted by appropriately trained personnel.16
Pharmacologic Therapy
Phlebotonic agents have been used to address many of the symptoms of chronic venous disorders, including leg pain, swelling, and pruritus. These include a heterogeneous group of plant extracts (rutosides, hidrosmine, diosmine, and others) as well as synthetic drugs with similar properties. A systematic review of 44 randomized, placebo-controlled trials evaluating oral phlebotonics suggested efficacy for some signs such as edema, although the global evidence for their efficacy was insufficient to recommend routine use.18 While other preparations are available abroad, horse chestnut seed extract is the most studied preparation available in the United States. A systematic review of 17 randomized, controlled trials of horse chestnut seed extract suggests significant benefits with respect to leg pain, edema, and pruritus.19 Two of these trials demonstrated similar improvements with horse chestnut seed extract and compression. Although the data are somewhat heterogeneous and the consequences of long-term use are poorly documented, there is at least a suggestion that the venoactive drugs may have some benefit in patients with C2-C3 disease (Grade 2B).
Surgical Management
The surgical management of varicose veins has evolved over the past century, with high ligation and stripping of the great saphenous vein being the standard approaches in recent decades. High ligation alone fails to control reflux in a high proportion of patients. In comparison to high ligation alone, high ligation and stripping reduced the need for reoperation by two thirds after 5 years of follow-up.20 Surgical treatment of varicose veins has been shown to be more efficacious and cost-effective than management with compression stockings. The REACTIV trial17 randomized 246 patients to conservative management (lifestyle advice and compression hosiery) versus surgery (saphenous ligation, stripping, and phlebectomy). There was significantly greater improvement in symptoms and quality of life in the surgical group and surgery was cost-effective with an incremental cost-effectiveness of £1936 per quality-adjusted life year (QALY) over a 10-year period. Notably 31% of patients did have some improvement in symptoms with compression hosiery alone, although 51.6% of patients assigned to conservative management crossed over to surgical treatment by the third year of follow-up. These data are supported by a Markov model demonstrating a variety of interventions for varicose veins, including surgical stripping, ultrasound-guided foam sclerotherapy and endovenous thermal ablation, to be more cost effective than conservative management.21 Based upon this reasonably compelling evidence, surgical management of saphenous vein incompetence is recommended over compression stockings in suitable patients (Grade 1B).
An array of endovenous techniques, including thermal and chemical ablation, has largely replaced high ligation and stripping for the management of saphenous reflux. A recent meta-analysis that included all available evidence (randomized trials, observational studies, cases series) and used saphenous obliteration as a weak surrogate outcome concluded that ultrasound-guided foam sclerotherapy was equivalent to and the endovenous techniques superior to saphenous vein stripping.22 With respect to the endovenous techniques, initial closure rates (92.9% versus 88.8%) and intermediate-term durability (94.5% versus 79.9% at 5 years) were better for laser ablation than for the first-generation radiofrequency (RF) device (ClosurePLUS [VNUS Medical Technologies, San Jose, CA]). Among larger studies, the EVOLVes trial randomized 85 patients to high ligation and stripping or endovenous RF ablation of the great saphenous vein. Early follow up demonstrated a more rapid return to usual activities and work among patients undergoing RF ablation. This is supported by smaller trials demonstrating less pain,23,24 edema,25 and bruising23–25 after endovenous ablation. However, Rasmussen26 randomized 121 patients to high ligation and stripping of the saphenous veins or endovenous laser ablation (EVLA) and found the procedures to be equally efficacious at 6 months. Despite slightly greater pain over the first 7 days after surgical treatment (high ligation and stripping), there was no difference in analgesic requirements or return to normal activities or work. Based on the evidence that the early efficacy and safety of the endovenous techniques are at least equivalent to high ligation and stripping, with potential advantages with respect to early postoperative pain and pending long-term studies evaluating clinically relevant outcome measures (e.g., recurrence, quality of life), the endovenous techniques can be recommended over saphenous vein stripping (Grade 1B).
Appropriately conducted trials comparing the different endovenous modalities are beginning to appear. In a randomized comparison of the first-generation ClosurePLUS device and the 810-nm laser, RF ablation was associated with less perioperative bruising and discomfort, but greater 1-year anatomic failure, than laser ablation.27 Closure rates appear to have been improved with the second generation ClosureFAST (VNUS Medical Technologies) device. Two randomized trials and one cohort study have compared ClosureFAST RF ablation to 980-nm laser ablation. In a cohort study,28 RF ablation was associated with significantly less pain (median pain scores 13 mm versus 23.3 mm, p = .014) and more rapid return to work (median 5 versus 9 days, p = .022) in comparison to EVLA. Among 87 limbs randomized to RF or EVLA, venous occlusion was achieved in all patients at 1 month.29 However, RF ablation was associated with significantly less pain, fewer complications, and improved quality of life at 1 and 2 weeks but not at 4 weeks. A second randomized trial demonstrated significantly less pain and analgesic use at 3 days with RF in comparison to EVLA, although this did not translate into more rapid recovery or improved quality of life at 6 weeks.30
Several new fiber designs, including radially firing fibers and those with wavelengths (1320 and 1470 nm) targeting water rather than hemoglobin, are now available.31,32 Reduced hemoglobin absorption may theoretically be associated with less vein wall perforation and fewer complications such as pain and bruising.32 Although early data with these fibers suggest excellent efficacy with reduced postprocedural pain,32 robust data comparing these fiber designs to either older designs or RF ablation are lacking, and these fibers can be only weakly suggested over those with hemoglobin-specific wavelengths (Grade 2C).
Sclerotherapy has previously been considered an alternative to surgery for varicose veins. Several randomized trials have directly compared liquid sclerotherapy to surgery, with the results suggesting that although sclerotherapy is effective in early follow-up, the 5-year results substantially favor surgery.33 Despite these findings, foam sclerotherapy has been demonstrated to be more efficacious than liquid and has emerged as an alternative to foam elsewhere in the world. Three-month GSV occlusion rates were significantly higher among veins randomized to treatment with polidocanol foam (54%) in comparison to veins treated with liquid polidocanol (17%).34 Even higher occlusion rates have been reported in single-center studies using different protocols. A meta-analysis of 69 studies including over 9000 patients reported venous occlusion in a mean of 87% of patients (range 60% to 98.2%).35 Among randomized trials evaluating venous occlusion as an outcome, foam sclerotherapy was more effective than liquid sclerotherapy (relative risk [RR] 1.39; 95% confidence interval [CI] 0.91 to 2.11) but less effective than surgery (RR 0.86, 95% CI 0.67 to 1.10). Serious complications of foam sclerotherapy included DVT in 0.02% to 0.7%, ulceration in 0% to 4%, arterial events in 0% to 2.1%, and rare case reports of stroke and myocardial infarction. Other adverse events included matting or pigmentation in 17.8%, thrombophlebitis in 4.7%, visual disturbances in 1.4%, headache in 4.2%, and cough, chest tightness, and vasovagal events in 0% to 2.8% of patients. It is clear that this is a high maintenance procedure, with occlusion rates declining to 35% at 5 years and reintervention rates ranging from 6.7% to 16.5% per year.36 Although initial costs of ultrasound-guided foam sclerotherapy are lower than other treatment strategies, the need for retreatment is likely associated with reduced quality of life and increased costs. Despite somewhat higher costs, Gohel et al. concluded that other treatment strategies might in fact be associated with better value.21 Based upon currently available evidence, endovenous thermal ablation of the incompetent saphenous vein is recommended over foam sclerotherapy (Grade 1B).
Once saphenous reflux has been eliminated, the most widely used options for the management of tributary varicosities include sclerotherapy and ambulatory phlebectomy. A systematic review of the sclerotherapy literature found the data to be of marginal quality but to generally support the current role of sclerotherapy in treating reticular veins, telangiectasias, and recurrent varicose veins37 (Grade 1B). There are little robust data to guide sclerosant choice, type of compression, or length of compression. Data directly comparing ambulatory phlebectomy to sclerotherapy for varicose veins are also sparse. One randomized trial demonstrated significantly higher 2-year recurrence rates for liquid sclerotherapy (37.5%) in comparison to ambulatory phlebectomy (2.1%).38 A further small randomized trial suggested that phlebectomy performed at the time of ablation is associated with early improvements in quality of life in comparison to sequential phlebectomy.39 Based upon the results of these trials, which did have reasonable methodology, phlebectomy is recommended over sclerotherapy for the treatment of tributary varicosities once saphenous reflux has been addressed (Grade 1B).
The incidence of deep venous thrombosis (DVT) detected by routine ultrasound imaging after saphenous vein surgery has been reported to be 5.3%, with the vast majority of these cases having been asymptomatic and isolated to the calf veins.40 The incidence of DVT was not influenced by a single preoperative dose of low-molecular-weight heparin. Others have shown rates of symptomatic venous thromboembolism to be as low as 0.18% after varicose vein surgery, with perioperative prophylaxis having no effect on VTE rates in low-risk patients.41 In a study of 460 limbs treated with EVLA, rates of true DVT, pulmonary embolism, and saphenofemoral thrombus extension were 0.7%, 0.2%, and 7.2%, respectively.42 These rates were not influenced by institution of a risk-based thromboprophylaxis policy. These data tend to support the general ACCP guidelines recommending no specific thromboprophylaxis other than early ambulation in patients without thromboembolic risk factors (Grade 2B) and consideration of prophylaxis with unfractionated heparin, low-molecular-weight heparin, or fondaparinux in patients with additional thromboembolic risk factors (Grade 1C).
Class 4 to 6 Chronic Venous Disease
Compression
Although imperfect, treatment of venous ulcers with compression dressings represents the “gold standard” to which all other therapy must be compared. Proposed mechanisms include a reduction in ambulatory venous pressure, improvements in skin and subcutaneous tissue microcirculation, and augmented diffusion of nutrients and oxygen secondary to edema reduction. There are several options for active and maintenance treatment including the “Unna” boot, the multilayer compression bandage, compression stockings, and devices such as the CircAid (CircAid Medical Products, Inc., San Diego, CA). A systematic review of 23 randomized and controlled clinical trials concluded that compression clearly increases healing rates in comparison to no compression and that high compression is more efficacious than low compression.43 However, it is not clear that there are substantial differences between the high compression alternatives. Compression is the most widely validated treatment for venous ulcers and can be considered a Grade 1A recommendation for ulcer healing.
Compression is, however, limited by substantial rates of recurrence. Although there are no randomized trials comparing ulcer recurrence with and without compression, there is strong observational evidence of higher recurrence rates without compression. The available data suggest recurrence rates of 32% to 64% without compression in comparison to 19% to 34% among compliant patients.44 Based on strong observational evidence, the use of compression stockings for the prevention of ulcer recurrence is a Grade 1B recommendation.
Adjuncts to Compression
At least one observational study has suggested that sharp debridement of chronic ulcers with significant slough and nonviable tissue accelerates wound healing, and this is a Grade 1C recommendation. Although there does appear to be a correlation between bacterial density and delayed wound healing, the data regarding the use of systemic or topical antibiotics are poor and provide few solid conclusions. Based on the lack of any clear benefit and an association with bacterial resistance, current evidence argues against the use of systemic antibiotics in the absence of clinically significant infection45 (Grade 1B). Among topical preparations, only cadexomer iodine has been associated with significantly improved rates of complete wound healing (RR 6.72; 95% CI 1.56 to 28.95)45 and can be considered a Grade 2B recommendation. One randomized trial has demonstrated a larger reduction in wound area with a sustained-release silver foam dressing in comparison to a hydrocellular dressing.46 However, area reduction is a surrogate measure of unclear clinical significance, and such dressings can be only weakly recommended (Grade 2C) based on the current evidence.
There are few pharmacologic agents available for the treatment of venous ulcers and none are as efficacious as high-level compression. Pentoxifylline is the only agent available in the United States and is postulated to act through the suppression of tumor necrosis factor alpha and leukocyte adhesion molecules.47,48 Among five trials comparing pentoxifylline to placebo with background compression, patients in the treatment group were 30% more likely to completely heal their ulcer than were those receiving placebo.47 Unfortunately, none of these trials were of sufficient duration to address their role in preventing ulcer recurrence. Randomized trials have similarly shown micronized purified flavanoid fraction, which is unavailable in the United States, to increase the odds of ulcer healing by 32% in comparison to compression and local wound care alone.48 Despite the efficacy of theses pharmacologic adjuncts, they are probably indicated in only selected patients (with large or long-standing ulcers) and are therefore a Grade 2B recommendation.
A number of wound dressings are available as adjuncts to compression therapy. These include nonocclusive and semiocclusive/occlusive dressings, growth factors, and human skin equivalents. A recent meta-analysis suggests that although most of these dressings afford little advantage over compression alone, five (zinc oxide paste–impregnated bandage, Tegasorb, perilesional injection of granulocyte-macrophage colony-stimulating factor, porcine small-intestinal submucosa, and cultured human skin equivalent) did show statistically significant benefits.49 However, given the modest benefits and variation in wound characteristics, drainage capabilities, ease of appli-cation, and cost, even those dressings that may be efficacious are at best a Grade 2A or 2B recommendation.
Surgery
The ESCHAR trial50 has provided that most definitive evidence regarding the role of superficial venous surgery in the management of venous ulcers. Five hundred patients with healed (C5) or open (C6) ulcers were randomized to compression, using a multilayer bandage for those with open ulcers, or surgery, which included great or small saphenous vein stripping and phlebectomy. Although there was no difference in 24-week healing rates, which were 65% in both arms, patients randomized to surgery had significantly lower 12-month recurrence rates (12% versus 28%). These results were durable through 4 years of follow-up, at which point ulcer recurrence rates were significantly lower among patients randomized to compression plus surgery (31% versus 56%).51 After 3 years, patients randomized to surgery had 15 weeks more ulcer-free time than those receiving compression alone. Although superficial venous surgery is of questionable value in ulcer healing, it can be considered a Grade 1A recommendation for the prevention of ulcer recurrence.
Most of the data supporting the use of endovenous ablation in the management of venous ulceration are indirect, extrapolated from the results in patients with mild to moderate venous disease. However, one small randomized trial has demonstrated that in comparison to compression alone, laser ablation of the great and/or small saphenous veins was associated with higher 12-month healing rates (24% versus 81.5%) and lower recurrence rates.52 The reason for the discrepancy in wound healing between the ESCHAR trail and this study are unclear, although the 12-month ulcer healing rate with compression alone was remarkably low and should be questioned. However, based on weak indirect data and pending further direct evidence, endovenous ablation for the treatment of saphenous reflux associated with ulceration should be considered equivalent to stripping for the prevention of recurrence (Grade 1C).
Unfortunately, the data supporting the use of surgery for deep venous and perforator incompetence are substantially weaker. A variety of minimally invasive techniques, including subfascial endoscopic perforator surgery, thermal ablation, and sclerotherapy are available for the treatment of perforating veins. One systematic review of subfascial endoscopic perforator surgery (SEPS),53 with or without saphenous extirpation, reported a recurrence rate of 13% after a mean follow-up of 29 months. This is close to the 12-month recurrence rate of 12% reported in the ESCHAR trial with superficial venous surgery alone. In a small randomized trial that specifically excluded patients with ulcers, the addition of SEPS to high ligation, stripping, and phlebectomy decreased the number of perforators present at 12 months but was not associated with significant differences in the clinically important measures of pain, mobility, cosmetic appearance, or quality of life.54 One other trial randomized 200 ulcerated legs to compression alone or compression with SEPS and, when indicated, surgery of the superficial system.55 Unfortunately, this trial used ulcer-free period, a composite of ulcer healing and recurrence, as the primary endpoint. As demonstrated by the ESCHAR trial,50 surgical intervention may have different effects on the components of this composite measure. In comparing conservative therapy with SEPS, no significant differences were noted in the primary endpoint, ulcer-free period (53% versus 72%), or in the secondary endpoints of ulcer healing (73% versus 83%) and recurrence (23% versus 22%). Based on the currently available data, perforator interruption cannot be recommended in the treatment of C2 disease (Grade 2B). However, because the data remain flawed and could change if better evidence becomes available, the potential value of interruption of pathologic perforators (>3.5 mm, reflux ≥0.5 second, located near the ulcer bed) in C5-C6 disease cannot be excluded and can be weakly suggested in appropriate patients (Grade 2B).
Summary
Although many aspects of the treatment of chronic venous disorders have remained the same for almost a century, several recent advances have provided new and beneficial treatment opportunities for patients with chronic venous disorders. Unfortunately, the current regulatory environment does not favor proof of clinically relevant efficacy prior to widespread adoption of new technology. The efficient use of limited health care resources therefore demands that the clinician have some ability to interpret the clinical evidence. Fortunately, methods of evaluating clinical evidence based solely on a hierarchy of study design have been replaced by more clinically relevant systems using an assessment of risk versus benefit, the degree of confidence in the estimate of effect, and the likelihood that recommendations will change with future research. Unfortunately, much of the evidence in venous disease continues to rely on case series and the use of irrelevant and possibly misleading surrogate outcome measures. Optimal care of our patients and the health care system requires that we understand both the strengths and weakness of the evidence surrounding any clinical question and effectively apply it to the care of our patients. Clinical practice guidelines must continually evolve with the development of new technology and generation of new evidence, but the guidelines jointly published by the American Venous Forum are based on the best evidence currently available.6 Consistent with the goals of evidence-based medicine, they should be appropriately influenced by the physician’s expertise and individual patient’s values and preferences.
1 Fowkes FG, Lee AJ, Evans CJ, et al. Lifestyle risk factors for lower limb venous reflux in the general population: Edinburgh Vein Study. Int J Epidemiol. 2001;30:846-852.
2 Bradbury A, Evans CJ, Allan P, et al. The relationship between lower limb symptoms and superficial and deep venous reflux on duplex ultrasonography: The Edinburgh Vein Study. J Vasc Surg. 2000;32:921-931.
3 Krijnen RMA, de Boer EM, Bruynzeel DP. Epidemiology of venous disorders in the general and occupational populations. Epidemiol Rev. 1997;19:294-309.
4 Newburger AE. Cosmetic medical devices and their FDA regulation. Arch Dermatol. 2006;142:225-228.
5 Nygaard I. What does “FDA Approved” mean for medical devices? Obstet Gynecol. 2008;111:4-6.
6 Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5 Suppl):2S-48S.
7 Sackett DL. Evidence-based medicine. Spine. 1998;23:1085-1086.
8 Ioannidis JPA, Haidich AB, Lau J. Any casualties in the clash of randomised and observation evidence? BMJ. 2001;322:879-880.
9 Group GW. Grading quality of evidence and strength of recommendation. BMJ. 2004;328:1490-1494.
10 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
11 Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248-1252.
12 Rutherford RB, Padberg FT, Comerota AJ, et al. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307-1312.
13 Garratt AM, Ruta DA, Abdalla MI, et al. Responsiveness of the SF-36 and a condition-specific measure of health for patients with varicose veins. Qual Life Res. 1996;5:223-234.
14 Lamping DL, Schroter S, Kurz X, et al. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg. 2003;37:410-419.
15 Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539-554.
16 Palfreyman SJ, Michaels JA. A systematic review of compression hosiery for uncomplicated varicose veins. Phlebology. 2009;24(Suppl 1):13-33.
17 Michaels JA, Campbell WB, Brazier JE, et al. Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial). Health Technol Assess. 2006;10:1-196. iii-iv
18 Martinez MJ, Bonfill X, Moreno RM, et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev 2005;(3):CD003229.
19 Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev 2006;(1):CD003230.
20 Dwerryhouse S, Davies B, Harradine K, et al. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg. 1999;29:589-592.
21 Gohel MS, Epstein DM, Davies AH. Cost-effectiveness of traditional and endovenous treatments for varicose veins. Br J Surg. 2010;97:1815-1823.
22 van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: A meta-analysis. J Vasc Surg. 2009;49:230-239.
23 Hinchliffe RJ, Ubhi J, Beech A, et al. A prospective randomised controlled trial of VNUS closure versus surgery for the treatment of recurrent long saphenous varicose veins. Eur J Vasc Endovasc Surg. 2006;31:212-218.
24 Stotter L, Schaaf I, Bockelbrink A. Comparative outcomes of radiofrequency ablation, invagination stripping, and cryostripping in the treatment of great saphenous vein insufficiency. Phlebology. 2006;12:60-64.
25 de Medeiros CA, Luccas GC. Comparison of endovenous treatment with an 810 nm laser versus conventional stripping of the great saphenous vein in patients with primary varicose veins. Dermatol Surg. 2005;31:1685-1694.
26 Rasmussen LH, Bjoern L, Lawaetz M, et al. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results. J Vasc Surg. 2007;46:308-315.
27 Gale SS, Lee JN, Walsh ME, et al. A randomized, controlled trial of endovenous thermal ablation using the 810-nm wavelength laser and the ClosurePLUS radiofrequency ablation methods for superficial venous insufficiency of the great saphenous vein. J Vasc Surg. 2010;52:645-650.
28 Shepherd AC, Gohel MS, Lim CS, et al. Pain following 980-nm endovenous laser ablation and segmental radiofrequency ablation for varicose veins: a prospective observational study. Vasc Endovascular Surg. 2010;44:212-216.
29 Almeida JI, Kaufman J, Gockeritz O, et al. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study). J Vasc Interv Radiol. 2009;20:752-759.
30 Shepherd AC, Gohel MS, Brown LC, et al. Randomized clinical trial of VNUS ClosureFAST radiofrequency ablation versus laser for varicose veins. Br J Surg. 2010;97:810-818.
31 Pannier F, Rabe E, Rits J, et al. Endovenous laser ablation of great saphenous veins using a 1470 nm diode laser and the radial fibre: follow-up after six months. Phlebology. 2011;26:35-39.
32 Proebstle TM, Moehler T, Gul D, et al. Endovenous treatment of the great saphenous vein using a 1,320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31:1678-1683. discussion 1683-1684
33 Rigby KA, Palfreyman SJ, Beverley C, et al. Surgery versus sclerotherapy for the treatment of varicose veins. Cochrane Database Syst Rev 2004(4):CD004980.
34 Rabe E, Otto J, Schliephake D, et al. Efficacy and safety of great saphenous vein sclerotherapy using standardised polidocanol foam (ESAF): a randomised controlled multicentre clinical trial. Eur J Vasc Endovasc Surg. 2008;35:238-245.
35 Jia X, Mowatt G, Burr JM, et al. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;94:925-936.
36 Chapman-Smith P, Browne A. Prospective five-year study of ultrasound-guided foam sclerotherapy in the treatment of great saphenous vein reflux. Phlebology. 2009;24:183-188.
37 Tisi PV, Beverley C, Rees A. Injection sclerotherapy for varicose veins. Cochrane Database Syst Rev 2006(4):CD001732.
38 de Roos KP, Nieman FH, Neumann HA. Ambulatory phlebectomy versus compression sclerotherapy: results of a randomized controlled trial. Dermatol Surg. 2003;29:221-226.
39 Carradice D, Mekako AI, Hatfield J, et al. Randomized clinical trial of concomitant or sequential phlebectomy after endovenous laser therapy for varicose veins. Br J Surg. 2009;96:369-375.
40 van Rij AM, Chai J, Hill GB, et al. Incidence of deep vein thrombosis after varicose vein surgery. Br J Surg. 2004;91:1582-1585.
41 Enoch S, Woon E, Blair SD. Thromboprophylaxis can be omitted in selected patients undergoing varicose vein surgery and hernia repair. Br J Surg. 2003;90:818-820.
42 Knipp BS, Blackburn SA, Bloom JR, et al. Endovenous laser ablation: venous outcomes and thrombotic complications are independent of the presence of deep venous insufficiency. J Vasc Surg. 2008;48:1538-1545.
43 Cullum N, Nelson EA, Fletcher AW, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev 2001;Issue 2:Art No.:CD000265. doi:10.1002/14651858.CD000265.
44 Nelson EA, Bell-Syer SE, Cullum NA. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2000(4):CD002303.
45 O’Meara S, Al-Kurdi D, Ovington LG. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2008(1):CD003557.
46 Jorgensen B, Price P, Andersen KE, et al. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J. 2005;2:64-73.
47 Jull AB, Waters J, Arroll B. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 2002(1):CD001733.
48 Meissner MH, Eklof B, Smith PC, et al. Secondary chronic venous disorders. J Vasc Surg. 2007;46(Suppl S):68S-83S.
49 O’Donnell TFJr, Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. J Vasc Surg. 2006;44:1118-1125.
50 Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;5(363):1854-1859.
51 Gohel MS, Barwell JR, Taylor M, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. 2007;335:83.
52 Viarengo LM, Poterio-Filho J, Poterio GM, et al. Endovenous laser treatment for varicose veins in patients with active ulcers: measurement of intravenous and perivenous temperatures during the procedure. Dermatol Surg. 2007;33:1234-1242.
53 Tenbrook JAJr, Iafrati MD, O’Donnell TFJr, et al. Systematic review of outcomes after surgical management of venous disease incorporating subfascial endoscopic perforator surgery. J Vasc Surg. 2004;39:583-589.
54 Kianifard B, Holdstock J, Allen C, et al. Randomized clinical trial of the effect of adding subfascial endoscopic perforator surgery to standard great saphenous vein stripping. Br J Surg. 2007;94:1075-1080.
55 van Gent WB, Hop WC, van Praag MC, et al. Conservative versus surgical treatment of venous leg ulcers: a prospective, randomized, multicenter trial. J Vasc Surg. 2006;44:563-571.