CHAPTER 165 Evidence Base
Neurostimulation for Pain
This chapter, a critical examination of the evidentiary basis for the use of neurostimulation therapies to treat chronic noncancer pain, is unprecedented in the history of neurosurgical textbooks; it is bound to be controversial and to contradict other chapters in this section. Although many works of this sort tabulate previous reports about pain therapies, critical deconstruction of the nature and quality of those publications is unusual.1–3 This chapter is based on previous work by the authors and others who examined their work critically to establish whether and how we really know what we think we know about neurosurgical therapies—including those for chronic pain.4–11 Despite the apparently narrow focus of this work, our methods apply to the evaluation of other medical therapies and to any field amenable to empirical observation. The vocabulary of evidence-based medicine is in vogue and may reflect a serious trend.12 Nonetheless, most evidence-based reviews continue to replicate the biases and flaws of previous reports.13 In a similar vein, evidence-based econometric studies, which advocate private or national health system reimbursement coverage for particular neurostimulation therapies, serve economic ends and suffer from considerable input- and assumption-based biases.14–19 The problematic nature of econometric analyses has been examined in detail in the context of another category of medical devices, implantable cardioverter-defibrillators, which, arguably, are easier to evaluate than neurostimulation pain therapies because the efficacy end point (death) is unequivocal.20–25
Background
Patients with chronic pain, which may last indefinitely according to International Association for the Study of Pain definitions, now constitute about half of individuals who receive unemployment or disability benefits in industrialized countries.26,27 Many have work-related injuries, disorders of the lumbosacral spine, or pain that persists after multiple operations. It is now easier for many unemployed workers in the United States to obtain permanent, pain-related disability benefits than to find a new job.28,29 Reasons for this trend involve socioeconomic policies that intersect with and have nourished the growth of pain medicine as a specialty over the past three decades.
History
Mid-20th century experiments that led investigators to stimulate the ventral posterolateral and posteromedial sensory thalamic nuclei and periaqueductal-periventricular gray matter (VPL, VPM, and PAG-PVG) and to the gate theory, which led to stimulation of the dorsal columns of the spinal cord in patients with chronic intractable pain, are reviewed elsewhere.4,8,9,30–36 Reports of analgesic effects after MCS appeared in the 1980s,37 and reports of ONS to treat head pain disorders appeared in the 1990s.38,39 In the 1980s, the U.S. Food and Drug Administration (FDA) reviewed implantable neurostimulation devices and, on the basis of available publications and expert testimony, ruled that spinal cord and peripheral nerve stimulation devices could continue to be marketed in the United States without formal clinical trials of efficacy. Until recently, approvals of newer-generation devices were based on limited safety and usability trials and other provisions of the Code of Federal Regulations.40 In contrast, because DBS leads were substantial-risk investigational devices, new implants in the United States could proceed only in approved clinical trials, or by compassionate use. FDA approval of the Activa DBS system (Medtronic, Inc., Minneapolis, MN) for medically refractory tremor in 1997, Parkinson’s disease in 2002, and dystonia (humanitarian use) in 2003, respectively, made DBS devices available again for the treatment of pain, but as an unlabeled indication for an approved device. At present, MCS is unlabeled in the United States for any indication. A European multicenter trial of MCS for pain commenced in 2005 and was closed by the sponsor in 2007 because of slow enrollment and lack of a clear efficacy signal (Final report on clinical investigation with medical devices: A prospective, randomized, double blind, crossover, multicenter study to evaluate the safety and efficacy of motor cortex stimulation with the cortical stimulation lead model 2976 in patients with neuropathic pain [hereafter referred to as CONCEPT trial], Medtronic Europe, Tolochenaz, Switzerland, March 26, 2008). One multinational feasibility trial of ONS for chronic migraine using leads approved for SCS recently was completed (Clinical report, 3-month data: Occipital nerve stimulation for the treatment of chronic migraine headaches [hereafter referred to as ONSTIM trial], Medtronic, Inc., Minneapolis, MN, June 24, 2008), and other industry-sponsored trials are underway. A preview of our analysis of hundreds of publications, reviews, and (to date) unpublished multicenter clinical trials of neurostimulation modalities to treat chronic noncancer pain reveals that no study or industry-sponsored trial of any neurostimulation modality that employed adequate blinding, randomization, or a valid contemporaneous control group has shown clinically meaningful long-term analgesic efficacy. Other controlled, uncontrolled, blinded, or unblinded nonindustry neurostimulation studies, if carried beyond a few months, or at most, beyond 2 years, also revealed no statistically detectable analgesic effects.
Methods
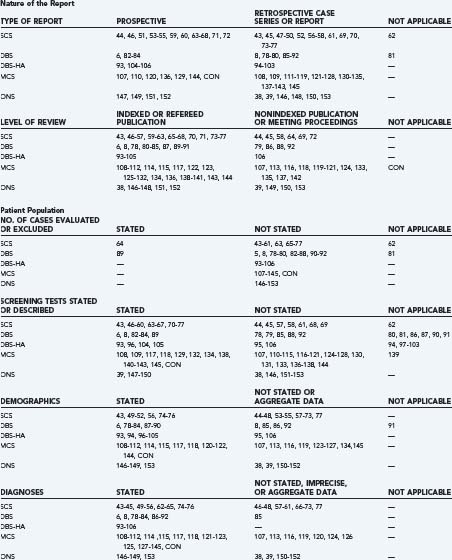
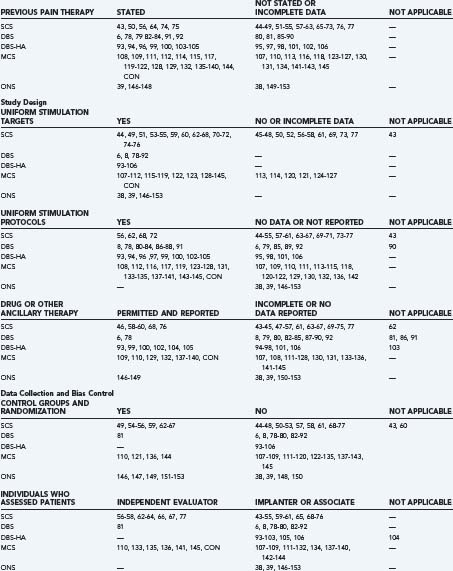
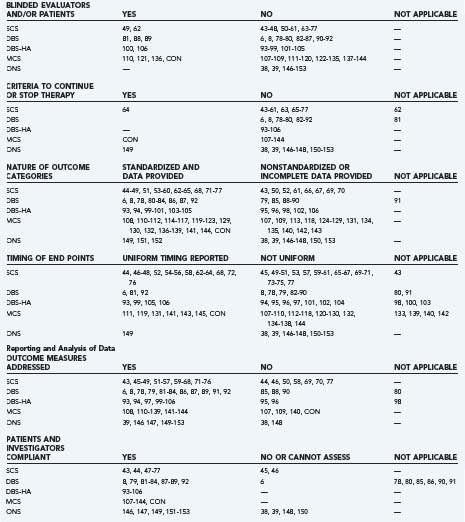
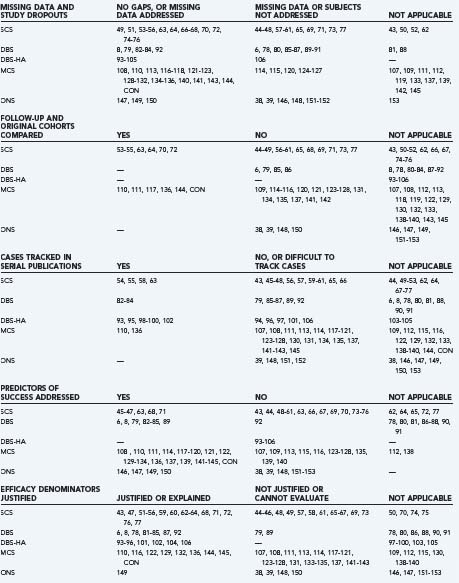
The authors identified publications for analysis using the U.S. National Library of Medicine PubMed database and the following search strategies: “spinal cord stimulation AND pain,” “deep brain stimulation AND pain,” “deep brain stimulation AND cluster,” “motor cortex stimulation AND pain,” “occipital nerve stimulation AND pain,” or “occipital nerve stimulation AND cluster.” Search limits included “language = English” (or an English language abstract), and “human.” We identified additional historical references from the bibliographies of indexed publications and reviews and drew on our recent critical analyses of individual neurostimulation therapies.6,4,8,9 Publications that described clinical efficacy were selected for analysis of the features listed in Table 165-1.41,42 We focused on publications and accompanying editorials or correspondence that contained or analyzed clinical efficacy data and that appeared in print between March 31, 2004, the closure date for a previous analysis, and February 29, 2008. The aggregate results are summarized in Table 165-2.38,39,43–154 The current work excluded publications that described technical or procedural innovations or complications, imaging or anatomic observations, and physiologic or neurophysiologic phenomena apart from analgesic efficacy.
TABLE 165-1 Factors Used to Evaluate Reports of Neurostimulation for Chronic Pain*
| LEVELS OF EVIDENCE | CRITERIA |
|---|---|
* Adapted and modified from Canadian Task Force on the Periodic Health Examination. The periodic health examination: 2. 1987 update. Can Med Assoc J. 1988;138:618-626; and Weintraub M. How to evaluate reports of clinical trials. Pharm Therapeut. 1990;14:1463-1476.
Results
Success Criteria and Reported Efficacy Rates
A historically accepted success criterion in neurostimulation studies for pain is that greater than or equal to 50% of individual patients should report greater than or equal to 50% pain relief (PPR; or ≥50% reduction in the visual analog scale [VAS] of pain intensity) at follow-up—commonly after 6 to 24 months. Until the 1990s, most trials and studies had used minor variations of the 50 : 50 standard.6,47 By definition, and to prevent a few cases from skewing the results, average or aggregate pain ratings formerly were avoided in efficacy rate calculations. After historical series failed to achieve the 50 : 50 efficacy criterion, studies of SCS between the 1990s and the present were structured to determine whether statistically significant aggregate or average pain relief scores differed after treatment over relatively short periods (<2 years) compared with the patient’s baseline. According to our previous analyses and data summarized in Table 165-2, assessments commonly were performed by the implanters or their associates or did not reach the 50 : 50 efficacy threshold, or both. Studies of novel programming techniques or lead designs also found limited efficacy or lacked sufficient data to draw long-term conclusions. Most of the studies that we reviewed had open-label designs, and all compared the results with the patient’s pretreatment baseline. None reported greater than or equal to 50% long-term relief in greater than or equal to 50% of subjects unless efficacy was analyzed without regard for the duration of follow-up or unless subjects who were lost or disqualified from follow-up were excluded from the calculations.
Long-term, prospective, randomized, crossover comparisons of SCS and reoperation in subjects with persistent pain after previous back surgery, performed over about a 10-year interval, were not designed to measure the degree of pain relief afforded by SCS. Despite the preference of subjects for SCS compared with reoperation, the effect size of the analgesia afforded by SCS or of repeat low back surgery remained undefined.64,66 A problem with such study designs, in addition to the assignment of one cohort to repeat low back surgery, a treatment that had previously not relieved their pain, is that subjects with a demonstrable anatomic cause for their pain were as likely to be assigned to SCS as to another low back operation. Likewise, subjects with no demonstrable spinal abnormalities had a 50% likelihood of being assigned to undergo another spine operation despite the absence of surgically amenable pathology. Neither treatment served as an adequate control measure for the other, leaving the study uncontrolled, unblinded, and unlikely to answer fundamental questions about the efficacy of SCS.67 One randomized controlled trial, among nearly 600 articles on SCS for RSD or CRPS, found that SCS plus physical therapy (PT) significantly reduced pain after 6 months and 2 years compared with PT alone, but not after 5 years.53–5563 The early results did not achieve the traditional 50 : 50 success criterion, and the long-term results revealed no statistical difference between the SCS plus PT group and the PT alone group.
In all DBS clinical series reported until recently, fewer patients reported pain relief after 6 to 24 months of therapy than during the early postimplantation period, and no study achieved the 50 : 50 success level unless short-term results carried the same analytical weight as follow-up that lasted months to years.6,5,8 Two industry-sponsored open-label DBS trials for pain enrolled 246 patients from 1989 to 1995. The coated-wire DBS lead used in the first trial became obsolete and was withdrawn from the market (N = 196); a trial of the current model DBS lead was closed because of slow enrollment and unexpectedly low efficacy (N = 50).6 Nandi and associates83–85 originally reported that seven patients with internalized DBS systems (among eight patients with implanted electrodes) experienced 32% to 46% pain relief after 3 to 30 months. Subsequent reports from the same group described better results than the first cohorts. However, it eventually became impossible for reviewers to follow patients through the entire series of publications.86–88 In another recent publication, investigators, one of whom had decades of experience in the field, described the results of a small 10-year case series that divided patients retrospectively according to diagnosis, and in which more than half of patients experienced no clinically meaningful pain relief at any time after DBS lead implantation.90
Articles and reviews by investigators in Milan (8 of 14 reviewed in Table 165-2), nearly all covering the same overlapping patient cohort at different stages of follow-up, and all describing dramatic efficacy, have dominated the discussion of hypothalamic DBS to treat cluster headache and other intractable trigeminal autonomic cephalgias. The experience at other centers has been less dramatic, and a proposed U.S. trial to examine hypotheses generated during a physician-sponsored pilot study may eventually achieve funding-agency approval.106 However, a randomized, controlled, and blinded French multicenter trial recently failed to demonstrate a statistically significant difference in the prespecified primary efficacy variable (the weekly frequency cluster headache attacks) between the stimulation “on” and “off” periods.107
The industry-sponsored European multinational study of MCS to treat facial (trigeminal) deafferentation pain and central poststroke pain enrolled and implanted only 24 subjects in 2 years, out of a planned cohort of 104 enrollments calculated to yield 82 evaluable subjects for the primary efficacy end point, before being closed by the sponsor because of slow enrollment. Although one can draw only limited conclusions because the prospectively planned N number was not achieved, 7 of 24 subjects (29%) withdrew or were discontinued from the study after lead implantation, but before randomization (to sham or active MCS) because of lack of efficacy during either the trial stimulation or early postinternalization study phases. Among the 11 randomized subjects who completed blinded one-way crossover MCS (on to off, or off to on; 4 weeks duration, each) and who completed all scheduled follow-up visits, none expressed a preference for the MCS “on” condition regardless of the on-off sequence to which they were randomized. No other large-scale blinded and controlled trial of MCS has been undertaken to date. Results of the MCS trial, albeit incomplete, fail to validate the more positive findings of the other controlled or uncontrolled single-center studies that we reviewed in Table 165-2.
Success rates in two early case series of ONS for head pain disorders were 64.5% and 100% during a mean follow-up of 18 months.38,39 In one report of 25 patients, 9 (36%) still had moderately or severely disabling headaches after 9 to 36 months.39 However, results of the randomized, controlled, industry-sponsored ONS clinical feasibility trial, which met prospectively defined enrollment, follow-up, and analysis goals (N = 110 enrolled subjects), showed no clear efficacy signal.150 The difference in the primary efficacy end point “number of headache days per month” was not statistically significant among the adjustable stimulation group (active therapy) and three different control groups: a group with preset stimulation (1 minute of stimulation per day, automatically programmed), a group with medical management (no device implant), or an ancillary group of eight subjects who were implanted and received adjustable stimulation, but who had failed to respond to an occipital nerve block during the preimplantation screening phase. The positive results reported from other case series and studies analyzed in Table 165-2 do not comport with the findings of this monitored and audited industry-sponsored trial.
The Neurostimulation Literature
Nature of the Reports
Evidence-related features of the recent neurostimulation clinical literature are listed in detail in Table 165-2 and organized according to published criteria for the evaluation of medical evidence.41,42 The prospective studies of SCS in RSD and of SCS compared with reoperation for low back pain each employed control groups and randomization. However, as noted previously, patients and evaluators were not blinded.53–55,63–67 Industry-sponsored studies of MCS and ONS were controlled, randomized, and blinded and revealed no clear signals of efficacy. This leaves unexplained the one prospective and partially blinded single-institution study of MCS that reported positive results.145 The remainder of neurostimulation publications consisted of retrospective case series, case reports, or meeting abstracts (see Table 165-2).
Diagnoses and Patient Selection
Diagnoses, demographic data, the number of eligible patients who were evaluated or excluded, the pretreatment duration of pain, and previous pain therapies were reported unevenly or not at all in several publications (see Table 165-2). The assignment of diagnoses or pain categories, including nociceptive, deafferentation, central, mixed pain, and headache disorders, was inconsistent among centers, and sometimes among multiple reports from the same institution. Patients treated successfully with ONS for occipital neuralgia in one series were followed by a second series that had the newly minted diagnosis of chronic or transformed migraine headache using criteria that continued to evolve after commencement of the ONS trials.38,149,155
Reports and reviews continued to describe selection criteria believed to maximize the likelihood of success for neurostimulation therapies, including pain in contiguous regions that can be covered by stimulation-induced paresthesias, favorable responses to transcutaneous electrical nerve stimulation (TENS), diagnoses previously reported to respond favorably, and rejection of patients with presumably unfavorable psychological or personality profiles, drug addiction, or pending litigation. In other reports, the results of psychological or pharmacologic screening either were not reported or had little influence on whether patients were accepted for therapy (see Table 165-2). The recently completed ONS feasibility trial revealed no predictive value for occipital nerve blocks. Psychological selection criteria for other neurostimulation therapies also had little predictive value.5,156,157
Stimulation Targets, Test Simulation, and Ancillary Treatments
The neural target for SCS was the dorsal columns of the spinal cord at cervical or thoracic levels to treat upper or lower extremity pain, respectively, and using percutaneous or surgically implanted epidural leads. DBS targets for classic indications included the VPL-VPM thalamic nuclei or the PAG-PVG region, or both, depending on the surgeon’s judgment, the patient’s symptoms, or the results of preoperative screening tests. Hypothalamic target coordinates for DBS to treat cluster headache are very close to the classic PVG target.158 MCS lead implantation techniques evolved during the past 20 years to include neuroimaging guidance, electrophysiologic localization, single-stage surgery, and epidural, instead of subdural, lead placement.5,9 ONS involved percutaneous fluoroscopically guided insertion of SCS leads into the subcutaneous tissue at the C1 level. A transverse trajectory placed the electrical contact surfaces across the course of the greater and lesser occipital nerves. Technical reports and small case series described procedures for ONS lead placement under direct vision; however, those techniques have not yet been studied in formal clinical trials. For all neurostimulation therapies, the use or duration of a trial period before system internalization, the methods used to select active electrode contacts, the stimulation parameters that were employed, and the daily schedule of treatment sessions varied among centers and among individual patients. Many publications contained no information about how analgesic medications, psychoactive drugs, or other concomitant therapies were managed, and all information regarding decreased pain medication intake relied on patient self-reports that were not verified by pill counts, drug testing of body fluids, or other quantitative methods (see Table 165-2).
Follow-up, Outcome Measures, Data Collection, Reporting, and Analysis
Other than the industry-sponsored trials, relatively few studies employed independent evaluators (see Table 165-2). In most studies, the implanters or their associates, who were aware of the patients’ history and previous assessments and who were responsible for their ongoing care, performed follow-up evaluations. Again, with the exception of industry studies, the duration of follow-up often was unclear, not reported, or varied from months to years in the same series. And multiple reports of the same patient cohort frequently did not track individual cases through the series of publications. Because follow-up periods were not uniform, because subjects frequently did not comply with long-term follow-up, and because life table analyses were not performed, different durations of therapy carried equal weight in efficacy calculations. The studies that had well-defined follow-up periods are listed in Table 165-2 as “uniform timing reported” and included all of the recent and historical industry-sponsored trials.
Reviews, Meta-analyses, and Practice Guidelines
Few reviews, editorials, or journal correspondence critically analyzed efficacy claims. As a rule, review articles recapped previous claims without analyzing the validity of the underlying methods and data, or the plausibility of the claims themselves. This held true for articles, chapters, meta-analyses, and quasi-official publications of evidence-based practice guidelines for neurostimulation therapies.13,159–166 Most reviewers accepted the hypothesis that neurostimulation provided pain relief as described in the original reports, and some put forth mechanisms to explain stimulation-produced analgesia in the absence of evidence that analgesia actually occurs.167
Discussion
The paradox of pain, its simultaneous reality and subjectivity, makes assessment of pain therapies susceptible to observer- or patient-related influences. Unintentional cues, learned responses, or knowledge that a treating physician or the physician’s representative is conducting the assessment all affect how patients rate analgesic treatments. The latter are particularly important when patients depend on physician approval to receive tangible and intangible benefits—discussed later under “Expectations and Reporting Bias.” Reliable methods to control for such influences are used in psychological and behavioral studies and should color our assessment of reports about the efficacy of neurostimulation for pain.167,168
Because every clinical trial to date that employed contemporaneous control groups, blinding, randomization, or prospectively defined methods of analysis has failed to reveal analgesic effects comparable to those reported in case series and uncontrolled trials, several common notions have, for all practical purposes, been falsified. These include the prognostic value of diagnostic categories (nociceptive, deafferentation, or central pain); the utility of preimplantation screening using psychological, personality, or pharmacologic tests; and the value of nerve blocks or temporary stimulation trials. Other obsolete notions include the concepts of stimulation tolerance and that efficacy decays over time because chronic noncancer pain syndromes worsen. Finally, reports and reviews of neurostimulation therapies demonstrate a bias toward positive interpretation of negative data, a phenomenon associated with novel therapies, even though neurostimulation has been used to treat pain for more than 40 years.169
Diagnoses and Prognostic Factors
Patient selection is uniformly reported to influence the success of neurostimulation therapies. One fundamental selection criterion is the diagnosis or etiology of a patient’s pain. Table 165-2 indicates that individual diagnoses and broader diagnostic categories sometimes have been arbitrary or malleable. A few recent trials, which also showed no analgesic or salutary physiologic effects, attempted to narrowly specify diagnoses in the study protocols (e.g., SCS for angina pectoris, MCS for central poststroke pain) and to require documentation of the diagnosis in each subject’s medical record (e.g., imaging studies, electrophysiologic tests). Both factors, diagnostic consistency and assurance that each subject’s diagnosis has a sound anatomic and physiologic basis, are crucial to the successful investigation of any therapy for chronic pain.
Only small unblinded series supported selection based on responses to analgesic or sedative drugs or to local anesthetic injections. The investigators’ conclusions most likely resulted from patient suggestibility or from misattribution of predictive value to random fluctuations within small data sets. Other selection criteria have included the subjects’ past medical history and demographic and psychosocial factors. These were investigated in studies of SCS and were reviewed in the DBS literature, with disparate or inconclusive findings.155,156 The most important limitation of previous analyses is that the influence of putative prognostic factors (e.g., the results of psychological or personality tests) could not be measured after those same factors were employed as inclusion or exclusion criteria in the same studies.
Trial Stimulation and Blinded Programming
Temporary stimulation with externalized leads allows patients to experience stimulation-induced effects, allows physicians to adjust parameters to minimize unpleasant side effects, and can help to avoid further investment in a therapy that may not match a patient’s analgesic needs or expectations. In actual clinical practice, trial stimulation or intensive postimplantation programming can influence patients to respond in an affirmative manner, especially if they are instructed to identify the settings that provide the best pain relief from among the choices presented to them. This phenomenon may explain in part why the proportion of patients who experienced long-term relief after an initial trial for the same diagnoses in different series has varied from near 0% to near 100%. One report described attempts to salvage postimplantation MCS analgesic efficacy by hospitalization of patients for intensive device reprogramming when they ceased to report analgesic efficacy after initial success.115 The entire series was not published, and it is plausible to speculate that at least some of the patients provided affirmative responses simply to escape from the hospital. Patient and evaluator blinding and the random or repeated presentation of different stimulation settings by a neutral individual and at prespecified or uniform intervals can reduce the occurrence and impact of unintentional coercion, cues, or suggestions.
Expectations and Reporting Bias
Neurostimulation pain therapies are administered in a context of optimism and positive expectations.12 Physicians and patients expect that previously reported findings should translate into positive results. In contrast, the phenomenon of therapeutic confusion occurs when investigators in a clinical trial believe that the primary purpose for intervention is to help patients, rather than to determine whether the therapy in question is safe and effective. The difference between the two attitudes is not merely semantic. Paradoxically, it can be too late, historically, to undertake a properly controlled trial if patients or physicians believe they already know that a therapy is effective, especially if they have access to the same therapy outside of a clinical trial. The combination of therapeutic confusion on the part of physicians and the accessibility of MCS therapy to European patients outside of the industry-sponsored multicenter trial undoubtedly contributed to the inability of the study to meet its recruitment goals. On the one hand, some investigators believed it was unethical to withhold MCS therapy from subjects who were eligible, but who would not consent to participate in the trial. Implantation of those patients off-study using noninvestigational (but off-label) devices created a selection bias. Conversely, some patients’ eagerness to undergo MCS implantation and therapy in the absence of rigorous evidence of efficacy amplified the selection bias; they knew the therapy was effective and consequently withdrew themselves from the pool of potential subjects.
Government or insurance regulations and social policies also influence the physician-patient relationship because many chronic pain patients are dependent on physician approval to obtain valuable benefits. Tangible benefits include disability and medical insurance coverage, absence from or modification of work duties, renewal of narcotic drug prescriptions, and referrals for physical therapy or other ancillary treatments. One intangible benefit is validation of the patient’s illness status. Loss of approval brings the opposite into play, making patients vulnerable to economic loss if physicians withdraw authorization for such benefits. Consequently, patients learn to maximize the likelihood that benefits will continue. In contrast, patients who seek physician approval to continue insurance or unemployment payments, narcotic prescriptions, and other benefits after failing to report pain relief from neurostimulation (or other) therapies risk being labeled as manipulative or nonorganic. The cycle of expectations and responses is a feature of human behavior that is observed in a variety of situations.168 It can cause caregivers and patients to reinforce each other’s beliefs and should not to be confused with or dismissed as a placebo effect—which can only occur in a clinical trial setting in which sham therapy is administered deliberately. The phenomenon of mutually reinforcing expectations and responses also accounts for the finding that patients express subjective global satisfaction even when their pain scores do not change appreciably during neurostimulation clinical trials or outcome surveys. Such ordinary behavioral and cognitive phenomena were among the reasons why control groups and blinding were introduced in clinical trials 260 and 60 years ago, respectively,170,171 and contributed to the design of controlled trials in the 1970s that successfully debunked other ineffective neurostimulation therapies.172–175
Selective or multiple reporting of the same patient cohort has influenced the reported success rate of neurostimulation therapies and has contributed to the difficulty one encounters in tracking individual cases through serial publications (see Table 165-2). The loss of patients to follow-up limited the interpretation of long-term efficacy for other interventional pain modalities,7 and the assignment of patient outcomes by individuals who have a stake in seeing the therapy succeed creates a conflict of interest that also influences reported success rates.
Recommendations and Conclusion
Clinical Trial Design
We have proposed that new data would be required to investigate the efficacy of neurostimulation for pain because mining or meta-analyses of the available data could not repair deficits in the existing data and knowledge bases, nor provide accurate answers. New clinical investigations would take into account the methods for evaluation of medical evidence summarized in Table 165-1 and should include as many features as feasible from Table 165-3. Even formal compliance with level I criteria, namely, randomization of subjects and the presence of a control group, may yield data subject to significant limitations. Unintentional study design loopholes can include lack of blinding, ineffective blinding, and the selection of qualitatively different treatments for the active treatment compared with control arms (implant compared with no implant, or substantially different treatments). We recently discussed these issues and related ones in detail in a publication available online as full text.5
TABLE 165-3 Optimal Clinical Trial Design Features to Investigate the Efficacy of Neurostimulation
| OPTIMAL DESIGN FEATURES | RATIONALE, DETAILS, AND EXECUTION |
|---|---|
| Investigator equipoise | Agree that hypotheses under study remain to be verified or refuted |
| Team of at least three individuals | Blinded implanter and evaluator, blinded or neutral programmer |
| Multiarm or multiphase project | Three-arm study example: active vs. sham vs. deliberately ineffective |
| Well-defined diagnostic criteria for eligibility | Unequivocal diagnosis supported by imaging and electrophysiologic data |
| Inclusive selection, limited exclusions | Candidates with the correct diagnosis and no exclusions are eligible |
| Personality tests and psychological or pharmacologic screening may be performed and the results recorded | Investigators, evaluators, and patients are blinded to results, which do not affect subject eligibility |
| Prospectively defined efficacy denominator | Either intention to treat, or last value carried forward for implanted-internalized subjects |
| Optimization period is brief and blinded | Optimization or trial period is shorter than the blinded study period, and subject and evaluator are unaware of stimulation parameters |
| Days to weeks interval between trial and randomization | Permits washout of stimulation effects; helps to maintain blinding |
| Long-duration blinded randomized study period | As long as feasible, consistent with subject retention for chronic therapy |
| Drug and ancillary therapy tracked and reported | Significant decrease in opioid and other drug intake verified by testing |
| Prospective data collection, analysis, and success criteria | 50% pain relief or other standard vs. control group (not difference from baseline) at 1- to 2-year end point |
In conclusion, extraordinarily durable biases on the part of investigators, a positive reporting bias in the clinical literature, and the nature of chronic pain have created a broad gulf between the expectations of patients and physicians and less optimistic findings of structured analyses of the clinical literature. One readily passes off expressions of marginally rational ideas about health and well-being among the lay public, a commonly observed phenomenon in social and cognitive psychological studies (e.g., positive attitudes prevent recurrence of breast cancer), as the product of uninformed minds.176 In the case of physicians and other professional elites, highly educated individuals who should know better, the neurophysiological basis for the persistence of demonstrably false ideas is just now being elucidated.177–180
Acknowledgement
The authors are grateful to Denys Fontaine, M.D., Ph.D., Department of Neurosurgery, Centre Hospitalier Universitaire de Nice, UNSA, Nice, France, for supplying an advance draft of reference 9 and for supplying prepublication data from the French multicenter study of DBS for cluster headache, references 106 and 157.
Aamodt S, Wang S. Can you trust your brain? In: Welcome to Your Brain: Why You Lose Your Car Keys but Never Forget How to Drive and Other Puzzles of Everyday Life. New York: Bloomsbury; 2008:2-8.
Berger M, Honig P, Spatz I. Medicare and cost effectiveness analysis. N Engl J Med. 2006;354:208.
Boswell MV, Trescot AM, Datta S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
Burchiel KJ. Neurostimulation for chronic noncancer pain. J Neurosurg. 2006;105:174.
Canadian Task Force on the Periodic Health Examination. The periodic health examination: 2. 1987 update. Can Med Assoc J. 1988;138:618-626.
Coffey RJ, Lozano AM. Neurostimulation for chronic noncancer pain: an evaluation of the clinical evidence and recommendations for future trial designs. J Neurosurg. 2006;105:175-189.
Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Medicine. 2001;2:183-192.
Doleys DM. Psychologic evaluation for patients undergoing neuroaugmentative procedures. Neurosurg Clin N Am. 2003;14:409-417.
Doleys DM. Psychological factors in spinal cord stimulation therapy: brief review and discussion. Neurosurg Focus. 2006;21(6):E1.
Duncan GH, Bushnell MC, Marchand S. Deep brain stimulation: a review of basic research and clinical studies. Pain. 1991;45:49-59.
Gallagher RM. Intrathecal drug delivery for chronic back pain: better science for clinical innovation. Pain Medicine. 2004;5:1-3.
Goldman L. Cost-effectiveness in a flat world—can ICDs help the United States get rhythm? N Engl J Med. 2005;353:14.
Goldman L. Medicare and cost effectiveness analysis. N Engl J Med. 2006;354:208-209.
Goodman SJ, Young RF. Dorsal column stimulation in the treatment of multiple sclerosis. Surg Forum. 1978;29:507-509.
Haines S. How do you know? Contemp Neurosurg. 1997;44:1-15.
Hamani C, Schwalb JM, Rezai AR, et al. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain. 2006;125:188-196.
Hardy SG. Analgesia elicited by prefrontal stimulation. Brain Res. 1985;339:281-284.
Headache Classification Committee of the International Headache Society. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004;24;:1-160.
Lind J. A treatise of the scurvy. In three parts. Containing an inquiry into the nature, causes and cure of that disease. Together with a critical and chronological view of what has been published on the subject. London: A. Millar; 1753.
Lowenstein R. Help wanted. NY Times Magazine. September 5, 2004; 52-69.
McCabe DP, Castel AD. Seeing is believing: the effect of brain images on judgments of scientific reasoning. Cognition. 2008;107:343-352.
Medical Research Council. Streptomycin treatment for pulmonary tuberculosis. Br Med J. 1948;2:769.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed. Seattle: IASP Press; 1994.
Mortimer JT, Shealy CN, Wheeler C. Experimental nondestructive electrical stimulation of the brain and spinal cord. J Neurosurg. 1970;32:553-559.
Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353:14.
Penn RD, Myklebust BM, Gottlieb GL, et al. Chronic cerebellar stimulation for cerebral palsy. Prospective and double-blind studies. J Neurosurg. 1980;53:160-165.
Penn RD. Chronic cerebellar stimulation for cerebral palsy: a review. Neurosurgery. 1981;10:116-121.
Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444-445.
Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter–defibrillators. N Engl J Med. 2005;353:1471-1480.
Schulman K. Medicare and cost effectiveness analysis. N Engl J Med. 2006;354:207-208.
Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489-491.
Singer M. The vitality of mythical numbers. The Public Interest. Spring 1971;23:3-9.
Spanos N. Multiple Identities and False Memories. A Sociocognitive Perspective. Washington, DC: American Psychological Association; 1996.
Stewart DE, Cheung AM, Duff S, et al. Attributions of cause and recurrence in long-term breast cancer survivors. Psychooncology. 2001;10:179-183.
Tall AR. C-Reactive protein reassessed. New Engl J Med. 2004;350:1450-1452.
Tasker RR, Vilela OF. Deep brain stimulation for neuropathic pain. Stereotact Funct Neurosurg. 1995;65:122-124.
Tierney J: Can anyone unseat FDR? NY Times. Jan 23, 2005.
Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995;37:1088-1096.
Turner JA, Loeser JD, Deyo RA, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2001;108:137-147.
Warms CA, Turner JA, Marshall HM, et al. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. 2002;18:154-163.
Weintraub M. How to evaluate reports of clinical trials. Pharm Ther. 1990;14:1463-1476.
Weisberg DS, Keil FC, Goodstein J, et al. The seductive allure of neuroscience explanations. J Cogn Neurosci. 2008;20:470-477.
Young RF, Goodman SJ. Dorsal spinal cord stimulation in the treatment of multiple sclerosis. Neurosurgery. 1979;5:225-230.
1 Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995;37:1088-1096.
2 Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-147.
3 Warms CA, Turner JA, Marshall HM, Cardenas DD. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. 2002;18:154-163.
4 Burchiel KJ. Neurostimulation for chronic noncancer pain. J Neurosurg. 2006;105:174.
5 Coffey RJ, Lozano AM. Neurostimulation for chronic noncancer pain: an evaluation of the clinical evidence and recommendations for future trial designs. J Neurosurg. 2006;105:175-189.
6 Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001;2:183-192.
7 Gallagher RM. Intrathecal drug delivery for chronic back pain: better science for clinical innovation. Pain Med. 2004;5:1-3.
8 Hamani C, Schwalb JM, Rezai AR, et al. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain. 2006;125:188-196.
9 Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: a critical review of the literature. J Neurosurg. 2009;110:251-256.
10 Raslan AM, McCartney S, Burchiel KJ. Management of severe pain: cerebral neuromodulatory and neuroablative approaches. Acta Neurochir Suppl. 2007;97:17-26.
11 Tasker RR, Vilela OF. Deep brain stimulation for neuropathic pain. Stereotact Funct Neurosurg. 1995;65:122-124.
12 Haines S. How do you know? Contemp Neurosurg. 1997;44:1-15.
13 Birknes JK, Sharan A, Rezai A. Treatment of chronic pain with neurostimulation. In: Pollak BE, editor. Guiding Neurosurgery by Evidence. Prog Neurol Surg, Vol 19. Basel: Karger; 2006:197-207.
14 Beersen N, Redekop WK, de Bruijn JH, et al. Quality based social insurance coverage and payment of the application of a high cost medical therapy: the case of spinal cord stimulation for chronic non-oncologic pain in The Netherlands. Health Policy. 2005;71:107-115.
15 North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007;61:361-368.
16 Mekhail NA, Aeschbach A, Stanton-Hicks M. Cost benefit analysis of neurostimulation for chronic pain. Clin J Pain. 2004;20:462-468.
17 Taylor RS, Taylor RJ, Van Buyten J-P, et al. The cost effectiveness of spinal cord stimulation in the treatment of pain: a systematic review of the literature. J Pain Symptom Manage. 2004;27:370-378.
18 Taylor RJ, Taylor RS. Spinal cord stimulation for failed back surgery syndrome: a decision-analytic model and cost-effectiveness analysis. Int J Technol Assess Health Care. 2005;21:351-358.
19 Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101.
20 Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471-1480.
21 Goldman L. Cost-effectiveness in a flat world—can ICDs help the United States get rhythm? N Engl J Med. 2005;353:14.
22 Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353:14.
23 Schulman K. Medicare and cost effectiveness analysis. N Engl J Med. 2006;354:207-208.
24 Berger M, Honig P, Spatz I. Medicare and cost effectiveness analysis. N Engl J Med. 2006;354:208.
25 Goldman L. Medicare and cost effectiveness analysis. N Engl J Med. 2006;354:208-209.
26 . European Federation of IASP Chapters: About Pain. Last revised September 22, 2004 http://www.efic.org/about_pain.htm
27 Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed. Seattle: IASP Press; 1994.
28 Lowenstein R. Help wanted. NY Times Magazine. September 5, 2004; 52-69.
29 Tierney J. Can anyone unseat FDR? NY Times Jan 23, 2005.
30 Heath RG, Mickle WA. Evaluation of seven years’ experience with depth electrode studies in human patients. In: Ramey ER, O’Doherty DS, editors. Electrical Studies on the Unanesthetized Brain. New York: Hoeber; 1960:214-247.
31 Sheer DE, editor. Electrical Stimulation of the Brain. An Interdisciplinary Survey of Neurobehavioral Integrative Systems. Austin: University of Texas, 1961.
32 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
33 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489-491.
34 Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444-445.
35 Mortimer JT, Shealy CN, Wheeler C. Experimental nondestructive electrical stimulation of the brain and spinal cord. J Neurosurg. 1970;32:553-559.
36 Mazars GJ, Merienne L, Ciolocca C. Traitement de certain types de douleurs pars de stimulateurs thalamiques implantables. Neurochirurgie. 1974;20:117-124.
37 Hardy SG. Analgesia elicited by prefrontal stimulation. Brain Res. 1985;339:281-284.
38 Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217-221.
39 Popeney CA, Alo KM. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43:369-375.
40 Federal Register: Volume 42 FR 42526, August 23, 1977 (21CFR807: Revised April 1, 2004) Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=807&showFR=1 (Accessed March 17, 2005)
41 Canadian Task Force on the Periodic Health Examination. the periodic health examination: 2. 1987 update. Can Med Assoc J. 1988;138:618-626.
42 Weintraub M. How to evaluate reports of clinical trials. Pharm Therapeut. 1990;14:1463-1476.
43 Ahmed SU. Complex regional pain syndrome type I after myocardial infarction treated with spinal cord stimulation. Reg Anesth Pain Med. 2003;28:245-247.
44 Barolat G. A prospective multicenter study to assess the efficacy of spinal cord stimulation utilizing a multi-channel radio-frequency system for the treatment of intractable low back and lower extremity pain. Initial considerations and methodology. Neuromodulation. 1999;2:179-183.
45 Bennett DS, Alo KM, Oakley J, Feler CA. Spinal cord stimulation for complex regional pain syndrome I [RSD]: a retrospective multicenter experience from 1995 to 1998 of 101 patients. Neuromodulation. 1999;2:202-210.
46 Burchiel KJ, Anderson VC, Brown FD, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21:2786-2794.
47 Burchiel KJ, Anderson VC, Wilson BJ, et al. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995;36:1101-1110.
48 Calvillo O, Racz G, Didie J, Smith K. Neuroaugmentation in the treatment of complex regional pain syndrome of the upper extremity. Acta Orthop Belg. 1998;64:57-63.
49 Daousi C, Benbow SJ, MacFarlane IA. Electrical spinal cord stimulation in the long-term treatment of chronic painful diabetic neuropathy. Diabetes Med. 2005;22:393-398.
50 Ebel H, Balogh A, Volz M, Klug N. Augmentative treatment of chronic deafferentation pain syndromes after peripheral nerve lesions. Minim Invasive Neurosurg. 2000;43:44-50.
51 Harke H, Gretenkort P, Ladleif HU, Rahman S. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective clinical study. Eur J Pain. 2005;9:363-373.
52 Hord ED, Cohen SP, Ahmed SU, et al. The predictive value of sympathetic block for the success of spinal cord stimulation. Neurosurgery. 2003;53:626-632.
53 Kemler MA, Barendse GA, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618-624.
54 Kemler MA, de Vet HC, Barendse GA, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292-298.
55 Kemler MA, de Vet HC, Barendse GA, et al. Spinal cord stimulation for chronic reflex sympathetic dystrophy—five-year final follow-up. N Engl J Med. 2006;354:2394-2396.
56 Kumar K, Toth C, Nath RK. Spinal cord stimulation for chronic pain in peripheral neuropathy. Surg Neurol. 1996;46:363-369.
57 Kumar K, Nath RK, Toth C. Spinal cord stimulation is effective in the management of reflex sympathetic dystrophy. Neurosurgery. 1997;40:503-509.
58 Kumar A, Felderhof C, Eljamel MS. Spinal cord stimulation for the treatment of refractory unilateral limb pain syndromes. Stereotact Funct Neurosurg. 2003;81:70-74.
59 Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179-188.
60 Turner JA, Deyo RA, Loeser JD. Spinal cord stimulation: stimulating questions. Pain. 2007;132:10-11.
61 Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58:481-496.
62 Marchand S, Bushnell MC, Molina-Negro P, et al. The effects of dorsal column stimulation on measures of clinical and experimental pain in man. Pain. 1991;45:249-257.
63 Marius A, Kemler MA, De Vet HCW, et al. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13-18.
64 North RB, Kidd DH, Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir Suppl. 1995;64:106-108.
65 North RB, Kidd DH, Olin J, et al. Spinal cord stimulation for axial low back pain: a prospective, controlled trial comparing dual with single percutaneous electrodes. Spine. 2005;30:1412-1418.
66 North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98-106.
67 Cooper P. Discussion of: Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:106-107.
68 Ohnmeiss DD, Rashbaum RF, Bogdanffy GM. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine. 1996;21:1344-1350.
69 Qigley DG, Arnold J, Eldridge PR, et al. Long-term outcome of spinal cord stimulation and hardware complications. Stereotact Funct Neurosurg. 2003;81:50-56.
70 Rainov NG, Heidecke V, Burkert W. Short test-period spinal cord stimulation for failed back surgery syndrome. Minim Invasive Neurosurg. 1996;39:41-44.
71 Sindou MP, Mertens P, Bendavid U, et al. Predictive value of somatosensory evoked potentials for long-lasting pain relief after spinal cord stimulation: practical use for patient selection. Neurosurgery. 2003;52:1374-1383.
72 Slavin KV, Burchiel KJ, Anderson VC, Cooke B. Efficacy of transverse tripolar stimulation for relief of chronic low back pain. Stereotact Funct Neurosurg. 1999;73:126-130.
73 Sundaraj SR, Johnstone C, Noore F, et al. Spinal cord stimulation: a seven-year audit. J Clin Neurosci. 2005;12:264-270.
74 Vallejo R, Kramer J, Benyamin R. Neuromodulation of the cervical spinal cord in the treatment of chronic intractable neck and upper extremity pain: a case series and review of the literature. Pain Physician. 2007;10:305-311.
75 Hagen JE, Bennett DS. Request for additional pertinent information regarding 4 extremity stimulation coverage from c2 spinal cord stimulation lead placement. Pain Physician. 2007;10:515-516.
76 Verdolin MH, Stedje-Larsen ET, Hickey AH. Ten consecutive cases of complex regional pain syndrome of less than 12 months duration in active duty United States military personnel treated with spinal cord stimulation. Anesth Analg. 2007;104:1557-1560.
77 Villavicencio AT, Leveque J-C, Rubin L, et al. Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery. 2000;46:399-405.
78 Bittar RG, Otero S, Carter H, Aziz TZ. Deep brain stimulation for phantom limb pain. J Clin Neurosci. 2005;12:399-404.
79 Katayama Y, Yamamoto T, Kobayashi K, et al. Motor cortex stimulation for post-stroke pain: comparison of spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77:183-186.
80 Kringelbach ML, Jenkinson N, Green AL, et al. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport. 2007;18:223-228.
81 Marchand S, Kupers RC, Bushnell MC, Duncan GH. Analgesic and placebo effects of thalamic stimulation. Pain. 2003;105:481-488.
82 Nandi D, Smith H, Owen S, et al. Peri-ventricular grey stimulation versus motor cortex stimulation for post stroke neuropathic pain. J Clin Neurosci. 2002;9:557-561.
83 Nandi D, Liu X, Joint C, et al. Thalamic field potentials during deep brain stimulation of periventricular gray in chronic pain. Pain. 2002;97:47-51.
84 Nandi D, Aziz T, Carter H, Stein J. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation—a series of eight cases. Pain. 2003;101:97-107.
85 Nandi D, Aziz TZ. Deep brain stimulation in the management of neuropathic pain and multiple sclerosis tremor. J Clin Neurophysiol. 2004;21:31-39.
86 Owen SL, Green AL, Nandi DD, et al. Deep brain stimulation for neuropathic pain. Acta Neurochir Suppl. 2007;97:111-116.
87 Owen SL, Green AL, Stein JF, Aziz TZ. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain. 2006;120:202-206.
88 Phillips NI, Bhakta BB. Affect of deep brain stimulation on limb paresis after stroke. Lancet. 2000;356:222-223.
89 Rasche D, Rinaldi P, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21:E8.
90 Romanelli P, Heit G. Patient-controlled deep brain stimulation can overcome analgesic tolerance. Stereotact Funct Neurosurg. 2004;82:77-79. 2004
91 Spooner J, Yu H, Kao C, et al. Neuromodulation of the cingulum for neuropathic pain after spinal cord injury. Case report. J Neurosurg. 2007;107:169-172.
92 Yamamoto T, Katayama Y, Obuchi T, et al. Thalamic sensory relay nucleus stimulation for the treatment of peripheral deafferentation pain. Stereotact Funct Neurosurg. 2006;84:180-183.
93 Bartsch T, Pinsker MO, Rasche D, et al. Hypothalamic deep brain stimulation for cluster headache: experience from a new multicase series. Cephalalgia. 2008;28:285-295.
94 Broggi G, Franzini A, Leone M, Bussone G. Update on neurosurgical treatment of chronic trigeminal autonomic cephalalgias and atypical facial pain with deep brain stimulation of posterior hypothalamus: results and comments. Neurol Sci. 2007;28(suppl 2):S138-S145.
95 Bussone G, Franzini A, Proietti Cecchini A, et al. Deep brain stimulation in craniofacial pain: seven years’ experience. Neurol Sci. 2007;28(Suppl 2):S146-S149.
96 Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52:1095-1101.
97 Green AL, Owen SLF, Davies P, et al. Deep brain stimulation for neuropathic cephalalgia. Cephalalgia. 2006;26:561-567.
98 Leone M, Franzini A, Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. 2001;345:1428-1429.
99 Leone M, Franzini A, Broggi G, et al. Long-term follow-up of bilateral hypothalamic stimulation for intractable cluster headache. Brain. 2004;127:2259-2264.
100 Leone M, Franzini A, D’Andrea G, et al. Deep brain stimulation to relieve drug-resistant SUNCT. Ann Neurol. 2005;57:924-927.
101 Leone M. Deep brain stimulation in headache. Lancet Neurol. 2006;5:873-877.
102 Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic stimulation for intractable cluster headache: long-term experience. Neurology. 2006;67:150-152.
103 Owen SLF, Green AL, Davies P, et al. Connectivity of an effective hypothalamic surgical target for cluster headache. J Clin Neurosci. 2007;4:955-960.
104 Schoenen J, Di Clemente L, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128:940-947.
105 Starr PA, Barbaro NM, Raskin NH, Ostrem JL. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106:999-1005.
106 Fontaine D, Lazorthes Y, Mertens P, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11:23-31.
107 Brown JA, Nguyen JP, Preul MC, et al. Epidural motor cortex stimulation for neuropathic facial pain: interim analysis of results from treatment of 13 patients in a multicenter prospective trial (abstract). J Neurosurg. 1999;90:405A-406A.
108 Brown J, Pilitsis J. Motor cortex stimulation for central and neuropathic facial pain: a prospective study of 10 patients and observations of enhanced sensory and motor function during stimulation. Neurosurgery. 2005;56:290-297.
109 Canavero S, Bonicalzi V, Castellano G, et al. Painful supernumerary phantom arm following motor cortex stimulation for central poststroke pain. J Neurosurg. 1999;91:121-123.
110 Carroll D, Joint C, Maartens N, et al. Clinical note. Motor cortex stimulation for chronic neuropathic pain: a preliminary study of 10 cases. Pain. 2000;84:431-437.
111 Ebel H, Rust D, Tronnier V, et al. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien). 1996;138:1300-1306.
112 Franzini A, Ferroli P, Servello D, Broggi G. Reversal of thalamic hand syndrome by long-term motor cortex stimulation. J Neurosurg. 2000;93:873-875.
113 Garcia-Larrea L, Peyron R, Mertens P, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neurosurg. 1997;68:141-148.
114 Garcia-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83:259-273.
115 Henderson J, Boongird A, Rosenow J, et al. Recovery of pain control by intensive reprogramming after loss of benefit from motor cortex stimulation for neuropathic pain. Stereotact Funct Neurosurg. 2004;82:207-213.
116 Herregodts P, Stadnik T, De Ridder F, D’Haens J. Cortical stimulation for central neuropathic pain: 3-D surface MRI for easy determination of the motor cortex. Acta Neurochir Suppl (Wien). 1995;64:132-135.
117 Katayama Y, Fukaya C, Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. J Neurosurg. 1998;89:585-591.
118 Katayama Y, Tsubokawa T, Yamamoto T. Chronic motor cortex stimulation for central deafferentation pain: experience with bulbar pain secondary to Wallenberg syndrome. Stereotact Funct Neurosurg. 1994;62:295-299.
119 Katayama Y, Yamamoto T, Kobayashi K, et al. Motor cortex stimulation for phantom limb pain: comprehensive therapy with spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77:159-162.
120 Mertens P, Nuti C, Sindou M, et al. Precentral cortex stimulation for the treatment of central neuropathic pain: results of a prospective study in a 20-patient series. Stereotact Funct Neurosurg. 1999;73:122-125.
121 Meyerson BA, Lindblom U, Linderoth B, et al. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien). 1993;58:150-153.
122 Migita K, Uozumi T, Arita K, Monden S. Transcranial magnetic coil stimulation of motor cortex in patients with central pain. Neurosurgery. 1995;36:1037-1039.
123 Montes C, Mertens P, Convers P, et al. Cognitive effects of precentral cortical stimulation for pain control: an ERP study. Neurophysiol Clin. 2002;32:313-325.
124 Nguyen JP, Keravel Y, Feve A, et al. Treatment of deafferentation pain by chronic stimulation of the motor cortex: report of a series of 20 cases. Acta Neurochir Suppl (Wien). 1997;68:54-60.
125 Nguyen JP, Lefaucheur JP, Decq P, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245-251.
126 Nguyen JP, Lefaucheur JP, Le Guerinel C, et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res. 2000;31:263-265.
127 Nguyen JP, Lefaucheur JP, Le Guerinel C, et al. Treatment of central and neuropathic facial pain by chronic stimulation of the motor cortex: value of neuronavigation guidance systems for the localization of the motor cortex. Neurochirurgie. 2000;46:483-491.
128 Nguyen JP, Pollin B, Feve A, et al. Improvement of action tremor by chronic cortical stimulation. Mov Disord. 1998;13:84-88.
129 Nuti C, Peyron R, Garcia-Larrea L, et al. Motor cortex stimulation for neuropathic pain: four year outcome and predictors of efficacy. Pain. 2005;118:43-52.
130 Peyron R, Garcia-Larrea L, Deiber MP, et al. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain. 1995;62:275-286.
131 Rainov NG, Fels C, Heidecke V, Burkert W. Epidural electrical stimulation of the motor cortex in patients with facial neuralgia. Clin Neurol Neurosurg. 1997;99:205-209.
132 Rasche D, Ruppolt M, Stippich C, et al. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10-year experience. Pain. 2006;121:43-52.
133 Rodriguez RF, Contreras N. Bilateral motor cortex stimulation for the relief of central dysesthetic pain and intentional tremor secondary to spinal cord surgery: a case report. Neuromodulation. 2002;5:189-195.
134 Saitoh Y, Hirano S-I, Kato A, et al. Motor cortex stimulation for deafferentation pain. Neurosurg Focus. 11, 2001. Article 1
135 Saitoh Y, Shibata M, Hirano S, et al. Motor cortex stimulation for central and peripheral deafferentation pain. Report of 8 cases. J Neurosurg. 2000;92:150-155.
136 Smith H, Joint C, Schlugman D, Nandi D, et al. Motor cortex stimulation for neuropathic pain. Neurosurg Focus. 11, 2001. Article 2
137 Sol JC, Casaux J, Roux FE, et al. Chronic motor cortex stimulation for phantom limb pain: correlations between pain relief and functional imaging studies. Stereotact Funct Neurosurg. 2001;77:172-176.
138 Son BC, Kim MC, Moon DE, Kang JK. Motor cortex stimulation in a patient with intractable complex regional pain syndrome type II with hemibody involvement. Case report. J Neurosurg. 2003;98:175-179.
139 Son B, Lee S, Choi E, et al. Motor cortex stimulation for central pain following a traumatic brain injury. Pain. 2006;123:210-216.
140 Tani N, Saitoh Y, Hirata M, et al. Bilateral cortical stimulation for deafferentation pain after spinal cord injury. J Neurosurg. 2004;101:687-689.
141 Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl. 1991;52:137-139.
142 Tsubokawa T, Katayama Y, Yamamoto T, et al. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol. 1991;14:131-134.
143 Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78:393-401.
144 Velasco F, Arguelles C, Carrillo-Ruiz J, et al. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomized double-blind trial. J Neurosurg. 2008;108:698-706.
145 Yamamoto T, Katayama Y, Hirayama T, Tsubokawa T. Pharmacological classification of central post-stroke pain: comparison with the results of chronic motor cortex stimulation therapy. Pain. 1997;72:5-12.
146 Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369:1099-3106.
147 Magis D, Allena M, Bolla M, et al. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6:314-321.
148 Matharu MS, Bartsch T, Ward N, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220-230.
149 Saper J, Goadsby PJ, Silberstein S, et al. Occipital nerve stimulation (ONS) for treatment of intractable migraine headache: 3-month results from the ONSTIM feasibility study. Cephalalgia. 2010 (in press).
150 Picaza JA, Hunter SE, Cannon BW. Pain suppression by peripheral nerve stimulation. Chronic effects of implanted devices. Appl Neurophysiol. 1977-1978;40:223-234.
151 Schwedt TJ, Dodick DW, Hentz J, et al. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
152 Vogel HB. Occipital nerve stimulation for chronic headache. Cephalalgia. 2007;27:1288.
153 Schwedt TJ, Dodick DW, Trentman TL, Zimmerman RS. Occipital nerve stimulation for chronic cluster headache and hemicrania continua: pain relief and persistence of autonomic features. Cephalalgia. 2006;26:1025-1027.
154 Headache Classification Committee of the International Headache Society. International classification of headache disorders, 2nd ed. Cephalalgia. 2004;24:1-160.
155 Doleys DM. Psychologic evaluation for patients undergoing neuroaugmentative procedures. Neurosurg Clin N Am. 2003;14:409-417.
156 Doleys DM. Psychological factors in spinal cord stimulation therapy: brief review and discussion. Neurosurg Focus. 2006;21:E1.
157 Fontaine D, Lanteri-Minet M, Ouchchane L, et al. Anatomical location of effective deep brain stimulation electrodes in chronic cluster headache. Brain. 2010;13:1214-1223.
158 Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine. 2005;30:152-160.
159 Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(suppl 4):S13-S19.
160 Taylor RS, Niv D, Raj PP. Exploration of the evidence. Pain Pract. 2006;6:10-21.
161 Bittar RG, Kar-Purkayastha I, Owen SL, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515-519.
162 North R, Shipley J. Prager J, for the American Academy of Pain Medicine: Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8(suppl 4):S200-S275.
163 Boswell MV, Trescot AM, Datta S, for the American Society of Interventional Pain Physicians. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
164 Cruccu G, Aziz TZ, Garcia-Larrea L, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952-970.
165 Leone M, May A, Franzini A, et al. Deep brain stimulation for intractable chronic cluster headache: proposals for patient selection. Cephalalgia. 2004;24:934-937.
166 Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006;31(suppl 4):S6-S12.
167 Duncan GH, Bushnell MC, Marchand S. Deep brain stimulation: a review of basic research and clinical studies. Pain. 1991;45:49-59.
168 Spanos N. Multiple Identities and False Memories. A Sociocognitive Perspective. Washington, DC: American Psychological Association; 1996.
169 Tall AR. C-Reactive protein reassessed. N Engl J Med. 2004;350:1450-1452.
170 Lind J. A treatise of the scurvy. In three parts. Containing an inquiry into the nature, causes and cure of that disease. Together with a critical and chronological view of what has been published on the subject. London: A. Millar; 1753.
171 Medical Research Council. Streptomycin treatment for pulmonary tuberculosis. BMJ. 1948;2:769.
172 Goodman SJ, Young RF. Dorsal column stimulation in the treatment of multiple sclerosis. Surg Forum. 1978;29:507-509.
173 Young RF, Goodman SJ. Dorsal spinal cord stimulation in the treatment of multiple sclerosis. Neurosurgery. 1979;5:225-230.
174 Penn RD, Myklebust BM, Gottlieb GL, et al. Chronic cerebellar stimulation for cerebral palsy. Prospective and double-blind studies. J Neurosurg. 1980;53:160-165.
175 Penn RD. Chronic cerebellar stimulation for cerebral palsy: a review. Neurosurgery. 1981;10:116-121.
176 Stewart DE, Cheung AM, Duff S, et al. Attributions of cause and recurrence in long-term breast cancer survivors. Psychooncology. 2001;10:179-183.
177 Aamodt S, Wang S. Can you trust your brain? in, Welcome to Your Brain: Why You Lose Your Car Keys but Never Forget How to Drive and Other Puzzles of Everyday Life. New York: Bloomsbury; 2008. 2-8
178 McCabe DP, Castel AD. Seeing is believing: the effect of brain images on judgments of scientific reasoning. Cognition. 2008;107:343-352.
179 Singer M. The vitality of mythical numbers. Public Interest. 1971;23:3-9.
180 Weisberg DS, Keil FC, Goodstein J, et al. The seductive allure of neuroscience explanations. J Cogn Neurosci. 2008;20:470-477.