Evaluation of the Patient with Pulmonary Disease
Evaluation on a Macroscopic Level
Wheezes are high-pitched, continuous sounds generated by airflow through narrowed airways. Causes of such narrowing include airway smooth muscle constriction, edema, secretions, intraluminal obstruction, and collapse because of poorly supported walls. These individual pathophysiologic features are discussed in Chapters 4 through 7. For reasons that are also described later, the diameter of intrathoracic airways is less during expiration than inspiration, and wheezing generally is more pronounced or exclusively heard in expiration. However, because sufficient airflow is necessary to generate a wheeze, wheezing may no longer be heard if airway narrowing is severe. In conditions such as asthma and chronic obstructive pulmonary disease, wheezes originate in multiple narrowed airways and are generally polyphonic, meaning they are a combination of different musical pitches that start and stop at different times during the expiratory cycle. In contrast, wheezing sounds tend to be monophonic when they result from focal narrowing of the trachea or large bronchi. When the site of narrowing is the extrathoracic airway (e.g., in the larynx or the extrathoracic portion of the trachea), the term stridor is used to describe the inspiratory wheezing-like sound that results from such narrowing. Physiologic factors that relate the site of narrowing and the phase of the respiratory cycle most affected are described later in this chapter and shown in Figures 3-20 and 3-21.
Table 3-1 summarizes some of the pulmonary findings commonly seen in selected disorders affecting the respiratory system. Many of these are mentioned again in subsequent chapters when the specific disorders are discussed in more detail.
Table 3-1
TYPICAL CHEST EXAMINATION FINDINGS IN SELECTED CLINICAL CONDITIONS
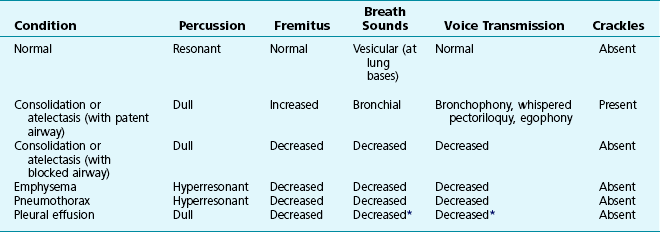
*May be altered by collapse of underlying lung, which will increase transmission of sound.
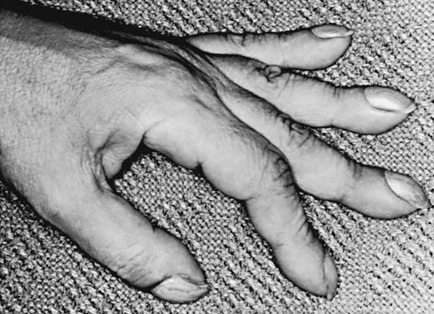
Clubbing is a change in the normal configuration of the nails and the distal phalanx of the fingers or toes (Fig. 3-1). Several features may be seen: (1) loss of the normal angle between the nail and the skin, (2) increased curvature of the nail, (3) increased sponginess of the tissue below the proximal part of the nail, and (4) flaring or widening of the terminal phalanx. Although several nonpulmonary disorders can result in clubbing (e.g., congenital heart disease with right-to-left shunting, endocarditis, chronic liver disease, inflammatory bowel disease), the most common causes clearly are pulmonary. Occasionally, clubbing is familial and of no clinical significance. Carcinoma of the lung (or mesothelioma of the pleura) is the single leading etiologic factor. Other pulmonary causes include chronic intrathoracic infection with suppuration (e.g., bronchiectasis, lung abscess, empyema) and some types of interstitial lung disease. Uncomplicated chronic obstructive lung disease is not associated with clubbing, so the presence of clubbing in this setting should suggest coexisting malignancy or suppurative disease.
Chest Radiography
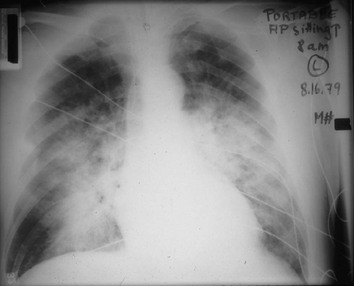
Chest radiographs usually are taken in two standard views—posteroanterior (PA) and lateral (Fig. 3-2). For a PA film, the x-ray beam goes from the back to the front of the patient, and the patient’s anterior chest is adjacent to the film. The lateral view is taken with the patient’s side against the film, and the beam is directed through the patient to the film. If a film cannot be taken with the patient standing and the chest adjacent to the film, as in the case of a bedridden patient, then an anteroposterior view is taken. For this view, which is generally obtained using a portable chest radiograph machine in the patient’s hospital room, the film is placed behind the patient (generally between the patient’s back and the bed), and the beam is directed through the patient from front to back. Lateral decubitus views, either right or left, are obtained with the patient in a side-lying position, with the beam directed horizontally. Decubitus views are particularly useful for detecting free-flowing fluid within the pleural space and therefore are often used when a pleural effusion is suspected.
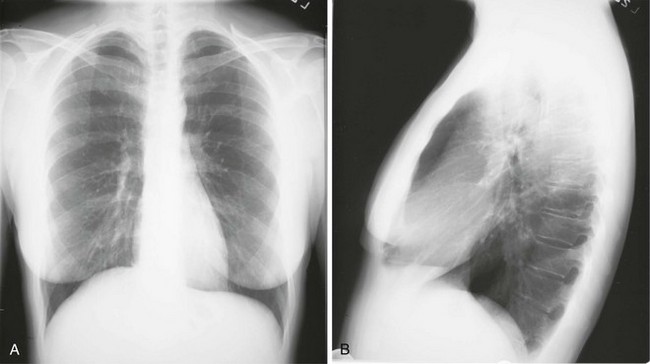
Figure 3-2 Normal chest radiograph. A, Posteroanterior view. B, Lateral view. Compare with Figure 3-3 for position of each lobe.
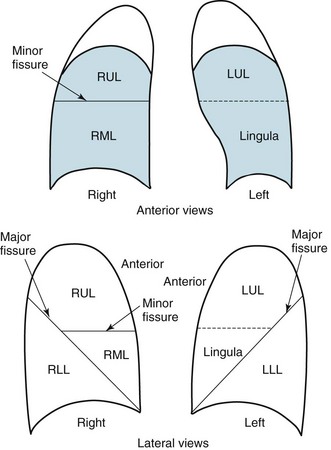
Knowledge of radiographic anatomy is fundamental for interpretation of consolidation or collapse (atelectasis) and for localization of other abnormalities on the chest film. Lobar anatomy and the locations of fissures separating the lobes are shown in Figure 3-3. Localization of an abnormality often requires information from both the PA and lateral views, both of which should be taken and interpreted when an abnormality is being evaluated. As can be seen in Figure 3-3, the major fissure separating the upper (and middle) lobes from the lower lobe runs obliquely through the chest. Thus it is easy to be misled about location on the basis of the PA film alone; a lower lobe lesion may appear in the upper part of the chest, whereas an upper lobe lesion may appear much lower in position.
When a lobe becomes filled with fluid or inflammatory exudate, as in pneumonia, it contains water rather than air density and therefore is easily delineated on the chest radiograph. With pure consolidation the lobe does not lose volume, so it occupies its usual position and retains its usual size. An example of lobar consolidation on PA and lateral radiographs is shown in Figure 3-4.
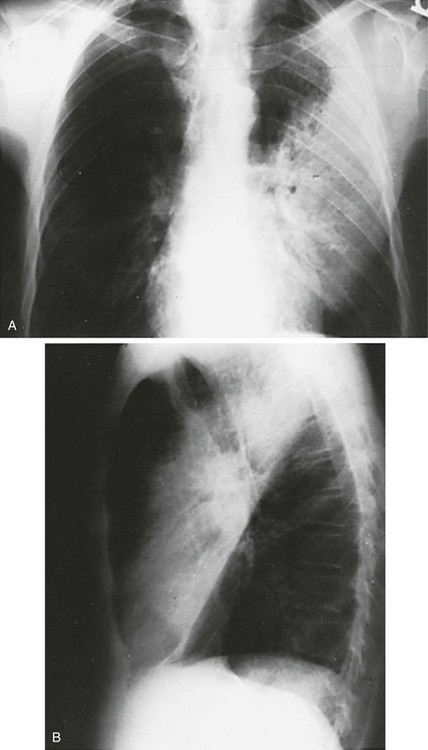
Figure 3-4 Posteroanterior (A) and lateral (B) chest radiographs of patient with left upper lobe consolidation due to pneumonia. Anatomic boundary is best appreciated on lateral view, where it is easily seen that normally positioned major fissure defines lower border of consolidation (compare with Figure 3-3). Part of left upper lobe is spared. (Courtesy Dr. T. Scott Johnson.)
In contrast, when a lobe has airless alveoli and collapses, it not only becomes more dense but also has features of volume loss characteristic for each individual lobe. Such features of volume loss include change in position of a fissure or the indirect signs of displacement of the hilum, diaphragm, trachea, or mediastinum in the direction of the volume loss (Fig. 3-5). A common mechanism of atelectasis is occlusion of the airway leading to the collapsed region of lung, caused, for example, by a tumor, aspirated foreign body, or mucous plug. All the aforementioned examples reflect either pure consolidation or pure collapse. In practice, however, a combination of these processes often occurs, leading to consolidation accompanied by partial volume loss.
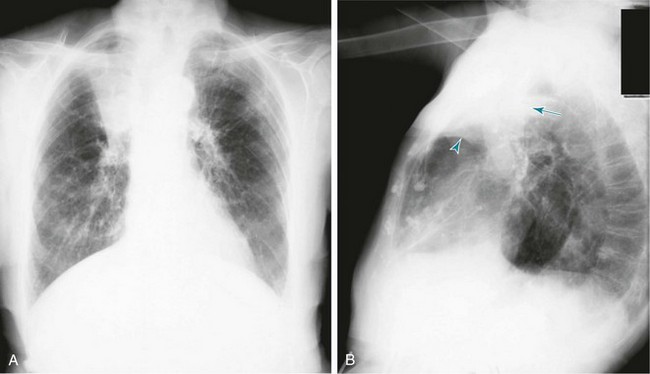
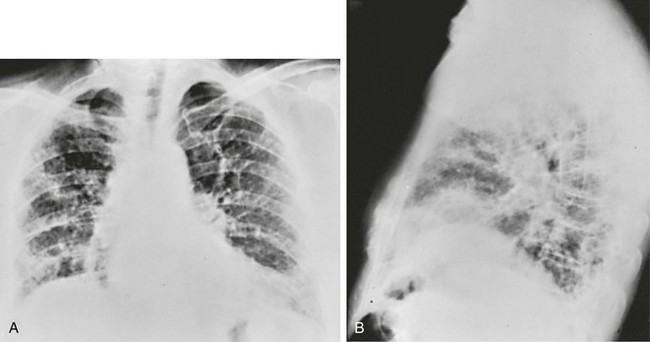
An interstitial pattern generally is described as reticular or reticulonodular, consisting of an interlacing network of linear and small nodular densities. In contrast, an alveolar pattern appears fluffier, and the outlines of air-filled bronchi coursing through the alveolar densities are often seen. This latter finding is called an air bronchogram and is due to air in the bronchi being surrounded and outlined by alveoli that are filled with fluid. This finding does not occur with a purely interstitial pattern. Examples of chest radiographs that show diffuse abnormality as a result of interstitial disease and alveolar filling are shown in Figures 3-6 and 3-7, respectively.
Computed Tomography
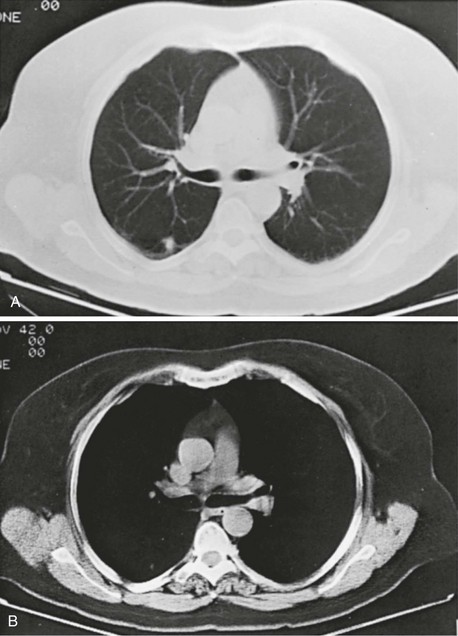
Compared with the plain chest radiograph, CT of the chest provides greater anatomic detail but is more expensive and exposes patients to a significantly higher dose of radiation. With this technique, a narrow beam of x-rays is passed through the patient and sensed by a rotating detector on the other side of the patient. The beam is partially absorbed within the patient, depending on the density of the intervening tissues. Computerized analysis of the information received by the detector allows a series of cross-sectional images to be constructed (Fig. 3-8). Use of different “windows” allows different displays of the collected data, depending on the densities of the structures of interest. With the technique of helical (spiral) CT scanning, the entire chest is scanned continuously (typically during a single breathhold and using multiple detectors) as the patient’s body is moved through the CT apparatus (the gantry).
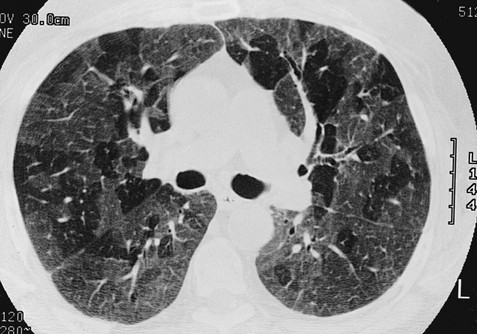
Chest CT is used extensively in evaluating pulmonary nodules and the mediastinum. It is also quite valuable in characterizing chest wall and pleural disease. As the technology has advanced, CT has become progressively more useful in the diagnostic evaluation of various diseases affecting the pulmonary parenchyma and the airways. With high-resolution CT, the thickness of individual cross-sectional images is reduced to 1 to 2 mm instead of the traditional 5 to 10 mm. As a result, exceptionally fine detail can be seen, allowing earlier recognition of subtle disease and better characterization of specific disease patterns (Fig. 3-9).
Magnetic Resonance Imaging
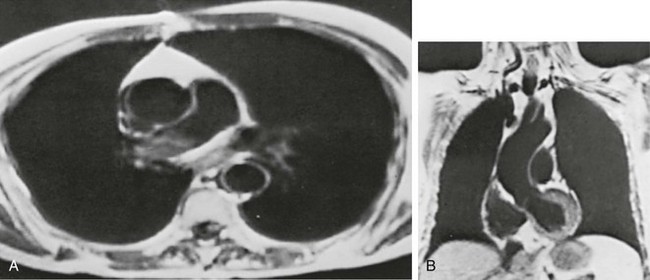
MRI has several important features in the evaluation of intrathoracic disease. First, flowing blood produces a “signal void” and appears black, so blood vessels can be readily distinguished from nonvascular structures without the need to use intravenous contrast agents. Second, images can be constructed in any plane so that the information obtained can be displayed as sagittal, coronal, or transverse (cross-sectional) views. Third, differences can be seen between normal and diseased tissues that are adjacent to each other, even when they are of the same density and therefore cannot be distinguished by routine radiography or CT. Some of these features are illustrated in Figure 3-10.
Lung Scanning
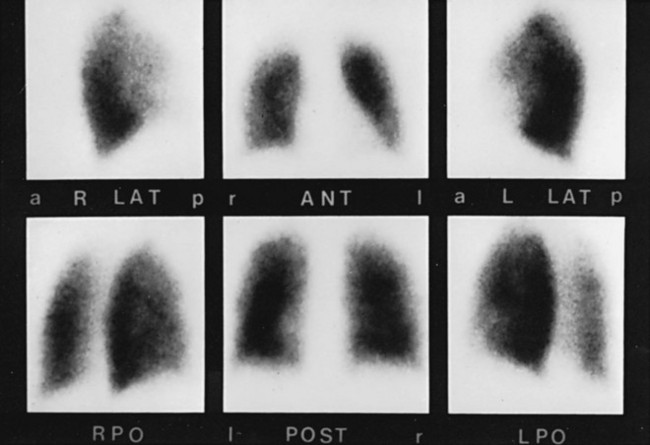
Injected or inhaled radioisotopes readily provide information about pulmonary blood flow and ventilation. Imaging of the γ radiation from these isotopes produces a picture showing the distribution of blood flow and ventilation throughout both lungs (Fig. 3-11). Other isotopes have been used for detecting and evaluating infectious, inflammatory, and neoplastic processes affecting the lungs.
Perfusion and Ventilation Scanning
Perfusion and ventilation scans are chiefly performed for two reasons: detection of pulmonary emboli and assessment of regional lung function. When a pulmonary embolus occludes a pulmonary artery, blood flow ceases to the lung region normally supplied by that vessel, and a corresponding perfusion defect results. Generally, ventilation is preserved, and a ventilation scan does not show a corresponding ventilation defect. In practice, many pieces of information are considered in the interpretation of the scan, including the appearance of the chest radiograph and the size and distribution of the defects on the perfusion scan. These issues are discussed in greater detail in Chapter 13.
Pulmonary Angiography
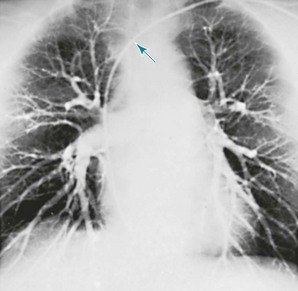
Pulmonary angiography is a radiographic technique in which a catheter is guided from a peripheral vein through the right atrium and ventricle and into the main pulmonary artery or one of its branches. A radiopaque dye is injected, and the pulmonary arterial tree is visualized on a series of rapidly exposed chest films (Fig. 3-12). This test is primarily used for diagnosing pulmonary embolism. A clot in a pulmonary vessel appears either as an abrupt termination (“cutoff”) of the vessel or as a filling defect within its lumen. Previously, pulmonary angiography was often used when the diagnosis of pulmonary embolism was uncertain after lung scanning, or CTA was inconclusive. However, with advances in CT techniques, a pulmonary angiogram is rarely needed.
Bronchoscopy
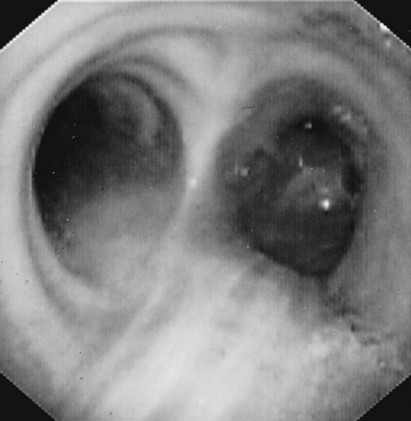
Direct visualization of the airways is possible by bronchoscopy, originally performed with a hollow, rigid metal tube, but now much more commonly with a thin, flexible instrument (Fig. 3-13). The flexible instrument transmits images either via flexible fiberoptic bundles (traditional fiberoptic bronchoscope) or more commonly via a digital chip at the tip of the bronchoscope that displays the images on a monitor screen. Because the bronchoscope is flexible, the bronchoscopist can bend the tip with a control lever and maneuver into airways at least down to the subsegmental level.
The bronchoscopist can obtain an excellent view of the airways (Fig. 3-14) and collect a variety of samples for cytologic, pathologic, and microbiologic examination. Sterile saline can be injected through a small hollow channel in the bronchoscope and suctioned back into a collection chamber. This technique, called bronchial washing, samples cells and, if present, microorganisms from the lower respiratory tract. When the bronchoscope is passed as far as possible and wedged into an airway before saline is injected, the washings are able to sample the contents of the alveolar spaces; this technique is called bronchoalveolar lavage (BAL).
Evaluation on a Microscopic Level
Obtaining Specimens
BAL has become an increasingly popular method for obtaining specimens from the lower respiratory tract. The fluid obtained by BAL has been used quite effectively for detecting P. jiroveci, particularly in patients with AIDS. In some diffuse parenchymal lung diseases (see Chapters 9 and 11), analysis of the cellular and biochemical components of BAL may provide information that is useful diagnostically and for research about basic disease mechanisms.
Finally, fluid in the pleural space is frequently sampled in the evaluation of a patient with a pleural effusion. A small needle is inserted through the chest wall and into the pleural space, and fluid is withdrawn. The fluid can be examined for malignant cells and microorganisms. Chemical analysis of the fluid (see Chapter 15) often provides additional useful diagnostic information. A biopsy specimen of the parietal pleural surface (the tissue layer lining the pleural space) also may be obtained either blindly, with a special needle passed through the chest wall, or under direct visualization using a thoracoscope. The tissue can be used for microscopic examination and microbiologic studies.
Processing Specimens
Organisms other than the common bacterial pathogens and mycobacteria often require other specialized staining and culture techniques. Fungi may be diagnosed by special stains, such as methenamine silver or periodic acid–Schiff stains, applied to tissue specimens. Fungi also can be cultured on special media favorable to their growth. P. jiroveci, a pathogen now classified as a unique category of fungi (see Chapter 25) and most common in patients with impaired defense mechanisms, is stained in tissue and tracheobronchial secretions by methenamine silver, toluidine blue, or Giemsa stain. An immunofluorescent stain using monoclonal antibodies against Pneumocystis is particularly sensitive for detecting the organism in sputum and BAL fluid. The organism identified in 1976 as Legionella pneumophila, the causative agent of Legionnaires’ disease, can be diagnosed by silver impregnation or immunofluorescence staining. The organism also can be grown (with difficulty) on some special media.
Assessment on a Functional Level
Pulmonary Function Tests
Three main categories of information can be obtained with routine pulmonary function testing:
1. Lung volumes, which provide a measurement of the size of the various compartments within the lung
2. Flow rates, which measure maximal flow within the airways
3. Diffusing capacity, which indicates how readily gas transfer occurs from the alveolus to pulmonary capillary blood
Before examining how these tests indicate what type of functional lung disease a patient has, we briefly describe the tests themselves and how they are performed.
Lung Volumes
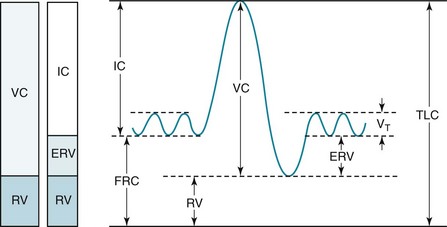
Although the lung can be subdivided into compartments in different ways, four volumes are particularly important (Fig. 3-15):
1. Total lung capacity (TLC): total volume of gas within the lungs after a maximal inspiration
2. Residual volume (RV): volume of gas remaining within the lungs after a maximal expiration
3. Vital capacity (VC): volume of gas expired when going from TLC to RV
4. Functional residual capacity (FRC): volume of gas within the lungs at the resting state—that is, at the end of expiration during the normal tidal breathing pattern
1. Dilution tests: A known volume of an inert gas (usually helium) at a known concentration is inhaled into the lungs. This gas is diluted by the volume of gas already present within the lungs, and the concentration of expired gas (relative to inspired) therefore reflects the initial volume of gas in the lungs.
2. Body plethysmography: The patient, sitting inside an airtight box, performs a maneuver that causes expansion and compression of gas within the thorax. By measuring volume and pressure changes and by applying Boyle’s law, the volume of gas in the thorax can be calculated.
Flow Rates
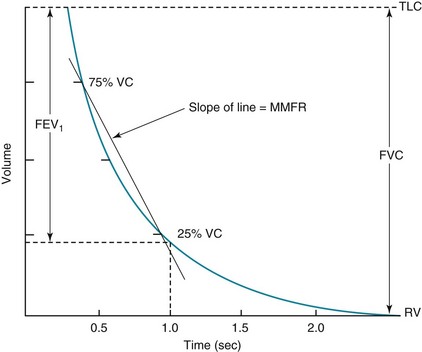
Measurement of flow rates on routine pulmonary function testing involves assessing airflow during maximal forced expiration—that is, with the patient blowing out as hard and as fast as possible from TLC down to RV. The volume expired during the first second of such a forced expiratory maneuver is called the forced expiratory volume in 1 second (FEV1) (Fig. 3-16). When pulmonary function tests are interpreted, FEV1 is routinely compared with VC, or with VC specifically measured during the forced expiratory maneuver, called the forced vital capacity (FVC). In interpreting flow rates, the ratio between these two measurements (FEV1/VC or FEV1/FVC) is the most important number used to determine the presence of obstruction to airflow. Another parameter often calculated from the forced expiratory maneuver is the maximal midexpiratory flow rate (MMFR), which is the rate of airflow during the middle one half of the expiration (between 25% and 75% of the volume expired during the FVC). MMFR is frequently called the forced expiratory flow (FEF) between 25% and 75% of vital capacity (FEF25%–75%). The MMFR or FEF25%–75% is a relatively sensitive index of airflow obstruction and may be abnormal when the FEV1/FVC ratio is still preserved.
Patterns of Pulmonary Function Impairment
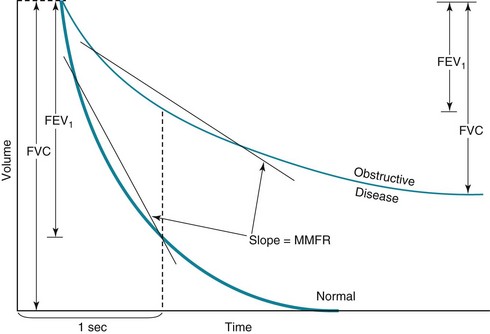
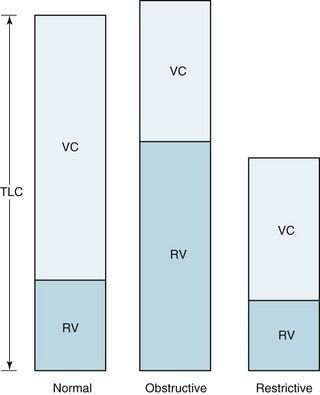
An obstructive pattern, as seen in patients with asthma, chronic bronchitis, and emphysema, consists of a decrease in rates of expiratory airflow and usually manifests as a decrease in MMFR and FEV1/FVC ratio (Fig. 3-17). There is generally a high RV and an increased RV/TLC ratio, indicating air trapping due to closure of airways during forced expiration (Fig. 3-18). Hyperinflation, reflected by an increased TLC, is often found, particularly in patients with emphysema. Diffusing capacity tends to be decreased in patients who have loss of alveolar-capillary bed (as seen in emphysema) but not in those without loss of available surface area for gas exchange (as in chronic bronchitis and asthma).
The hallmark of restrictive disease is a reduction in lung volumes, whereas expiratory airflow is normal (see Fig. 3-18). Therefore, TLC, RV, VC, and FRC all tend to be reduced, whereas MMFR and FEV1/FVC are preserved. In some patients with significant loss of volume resulting from restrictive disease, MMFR is decreased because less volume is available to generate a high flow rate. Interpreting a low MMFR in the face of significant restrictive disease is difficult unless MMFR is clearly decreased out of proportion to the decrease in lung volumes.
A simplified guide to the interpretation of pulmonary function tests is presented along with several sample problems in Appendix B.
Other Tests
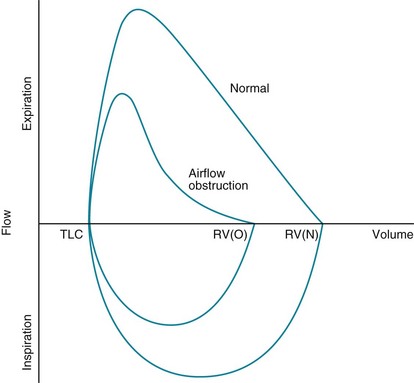
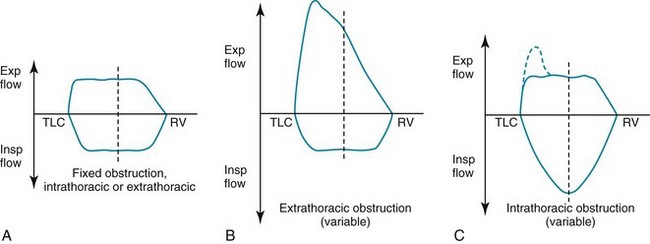
In patients with evidence of airflow obstruction, flow rates at a given volume are decreased, often giving the curve a “scooped out” or coved appearance. The flow data obtained from maximal expiratory flow-volume loops can be interpreted quantitatively (comparing observed flow rates at specified volumes with predicted values) or qualitatively (visually analyzing the shape and concavity of the expiratory portion of the curve). When routine spirometric parameters reflecting airflow obstruction (MMFR, FEV1/FVC) are abnormal, the flow-volume loop generally is abnormal. However, in patients with early airflow obstruction, perhaps localized to small airways, the contour of the terminal part of the expiratory curve may be abnormal even when the FEV1/FVC ratio is normal. Examples of flow-volume loops in a normal patient and in a patient with obstructive lung disease are shown in Figure 3-19.
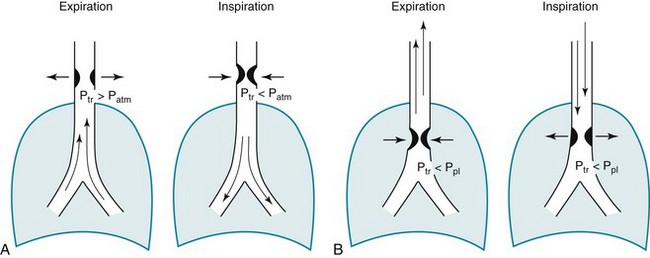
Another important application of flow-volume loops is for diagnosing and localizing upper airway obstruction. By analyzing the contour of the inspiratory and expiratory portions of the curve, the obstruction can be categorized as fixed or variable, as well as intrathoracic or extrathoracic. In a fixed lesion, changes in pleural pressure do not affect the degree of obstruction, and a limitation in peak airflow (a plateau) is seen on both the inspiratory and expiratory portions of the curve. In a variable lesion, the amount of obstruction is determined by the location of the lesion and the effect of alterations in pleural and airway pressure with inspiration and expiration (Fig. 3-20). A variable intrathoracic lesion is characterized by expiratory limitation of airflow and a plateau on the expiratory portion of the flow-volume curve, whereas a variable extrathoracic lesion demonstrates inspiratory limitation of airflow and a plateau on the inspiratory portion of the flow-volume curve (Fig. 3-21).
Arterial Blood Gases
Arterial PO2 normally is between 80 and 100 mm Hg, but the expected value depends significantly on the patient’s age and the simultaneous level of PCO2 (reflecting alveolar ventilation, an important determinant of alveolar and, secondarily, arterial PO2). From the arterial blood gases, the alveolar-arterial oxygen gradient (AaDO2) can be calculated, as discussed in Chapter 1. Normally the difference between alveolar and arterial PO2 is less than 10 to 15 mm Hg in a healthy young person, and this difference increases with patient age. The oxygen content of the blood does not begin to fall significantly until the arterial PO2 drops below approximately 60 mm Hg (see Chapter 1). Therefore, an abnormally low PO2 generally does not affect O2 transport to the tissues until it drops below this level and the saturation falls.
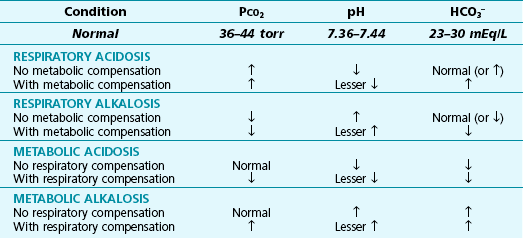
In practice, the clinician considers three fundamental questions in defining all acid-base disturbances: (1) Is there an acidosis or alkalosis? (2) Is the primary disorder of respiratory or metabolic origin? (3) Is there evidence for respiratory or metabolic compensation? Table 3-2 summarizes the findings in the major types of acid-base disturbances. Unfortunately, matters are not always so simple in clinical practice, and it is quite common to see complex mixtures of acid-base disturbances in patients who have several diseases and are receiving a variety of medications.
A simplified guide to the interpretation of arterial blood gases is presented along with several sample problems in Appendix C.
Loudon, R, Murphy, RLH, Jr. Lung sounds. Am Rev Respir Dis. 1984;130:663–673.
Maitre, B, Similowski, T, Derenne, J-P. Physical examination of the adult patient with respiratory diseases: inspection and palpation. Eur Respir J. 1995;8:1584–1593.
Marrie, TJ, Brown, N. Clubbing of the digits. Am J Med. 2007;120:940–941.
Martin, L, Khalil, H. How much reduced hemoglobin is necessary to generate central cyanosis? Chest. 1990;97:182–185.
Murphy, RL. In defense of the stethoscope. Respir Care. 2008;53:355–369.
Myers, KA, Farquhar, DRE. Does this patient have clubbing? JAMA. 2001;286:341–347.
Pasterkamp, H, Kraman, SS, Wodicka, GR. Respiratory sounds. Advances beyond the stethoscope. Am J Respir Crit Care Med. 1997;156:974–987.
Vyshedskiy, A, Alhashem, RM, Paciej, R, et al. Mechanism of inspiratory and expiratory crackles. Chest. 2009;135:156–164.
Chiles, C. A radiographic approach to diffuse lung disease. Radiol Clin North Am. 1991;29:919–929.
Fraser, RS, Colman, N, Müller, NL, et al, eds. Fraser and Paré’s diagnosis of diseases of the chest, ed 4, vol I. Philadelphia: WB Saunders, 1999.
Fraser, R, Colman, N, Müller, N, et al. Synopsis of diseases of the chest, ed 3. Philadelphia: Elsevier; 2005.
Goodman, LR. Felson’s principles of chest roentgenology: a programmed text, ed 3. Philadelphia: WB Saunders; 2007.
McAdams, HP, Samei, E, Dobbins, J, 3rd., et al. Recent advances in chest radiography. Radiology. 2006;241:663–683.
Raoof, S, Feigin, D, Sung, A, et al. Interpretation of plain chest roentgenogram. Chest. 2012;141:545–558.
Ferretti, GR, Bricault, I, Coulomb, M. Virtual tools for imaging of the thorax. Eur Respir J. 2001;18:381–392.
Kumamaru, KK, Hoppel, BE, Mather, RT, et al. CT angiography: current technology and clinical use. Radiol Clin North Am. 2010;48:213–235. vii
Naidich, DP, Webb, WR, Müller, NL, et al. Computed tomography and magnetic resonance of the thorax, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2007.
Webb, WR, Müller, NL, Naidich, DP. High-resolution CT of the lung, ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001.
Hatabu, H, Stock, KW, Sher, S, et al. Magnetic resonance imaging of the thorax. Past, present, and future. Radiol Clin North Am. 2000;38:593–620.
Müller, NL. Computed tomography and magnetic resonance imaging: past, present and future. Eur Respir J. 2002;19:3s–12s.
Naidich, DP, Webb, WR, Müller, NL, et al. Computed tomography and magnetic resonance of the thorax. Philadelphia: Lippincott Williams & Wilkins; 2007.
Wielputz, M, Kauczor, HU. MRI of the lung: state of the art. Diagn Interv Radiol. 2012;18:344–353.
Gould, MK, Maclean, CC, Kuschner, WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions. A meta-analysis. JAMA. 2001;285:914–924.
Kumar, A, Dutta, R, Kannan, U, et al. Evaluation of mediastinal lymph nodes using F-FDG PET-CT scan and its histopathologic correlation. Ann Thorac Med. 2011;6:11–16.
Vansteenkiste, JF, Stroobants, SG. The role of positron emission tomography with 18F-fluoro-2-deoxy-D-glucose in respiratory oncology. Eur Respir J. 2001;17:802–820.
Sadigh, G, Kelly, AM, Cronin, P. Challenges, controversies, and hot topics in pulmonary embolism imaging. AJR Am J Roentgenol. 2011;196:497–515.
Stein, PD, Fowler, SE, Goodman, LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327.
Beckh, S, Bölcskei, PL, Lessnau, K-D. Real-time chest ultrasonography. A comprehensive review for the pulmonologist. Chest. 2002;122:1759–1773.
Koenig, SJ, Narasimhan, M, Mayo, PH. Thoracic ultrasonography for the pulmonary specialist. Chest. 2011;140:1332–1341.
Becker, HD. Bronchoscopy: the past, the present, and the future. Clin Chest Med. 2010;31:1–18.
Bolliger, CT, Sutedja, TG, Strausz, J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258–1271.
Dooms, C, Seijo, L, Gasparini, S, et al. Diagnostic bronchoscopy: state of the art. Eur Respir Rev. 2010;19:229–236.
Haas, AR, Vachani, A, Sterman, DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med. 2010;182:589–597.
Meyer, KC, Raghu, G, Baughman, RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014.
Sheski, FD, Mathur, PN. Endobronchial ultrasound. Chest. 2008;133:264–270.
Wahidi, MM, Herth, FJF, Ernst, A. State of the art. Interventional pulmonology. Chest. 2007;131:261–274.
Obtaining and Processing Specimens
Bartlett, JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis. 2011;52:S296–S304.
Dorman, SE. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin Infect Dis. 2010;50:S173–S177.
Epstein, RL. Constituents of sputum: a simple method. Ann Intern Med. 1972;77:259–265.
Leslie, KO, Helmers, RA, Lanza, LA, et al. Processing and evaluation of lung biopsy specimens. Eur Respir Mon. 2000;5:55–62.
Mahony, JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. 2008;21:716–747.
Mayaud, C, Cadranel, J. A persistent challenge: the diagnosis of respiratory disease in the non-AIDS immunocompromised host. Thorax. 2000;55:511–517.
Peterson, LR. Molecular laboratory tests for the diagnosis of respiratory tract infection due to Staphylococcus aureus. Clin Infect Dis. 2011;52:S361–S366.
Reynolds, HY. Use of bronchoalveolar lavage in humans: past necessity and future imperative. Lung. 2000;178:271–293.
Talbot, HK, Falsey, AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 2010;50:747–751.
Assessment on a Functional Level
American Thoracic Society and American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277.
Arena, R, Sietsema, KE. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011;123:668–680.
ATS statement. guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111.
Chupp, GL. Pulmonary function testing. (ed). Clin Chest Med. 2001;22:599–859.
Evans, SE, Scanlon, PD. Current practice in pulmonary function testing. Mayo Clin Proc. 2003;78:758–763.
Flesch, JD, Dine, CJ. Lung volumes. Measurement, clinical use, and coding. Chest. 2012;142:506–510.
Hughes, JMB, Pride, NB. Examination of the carbon monoxide diffusing capacity (DLCO) in relation to its KCO and VA components. Am J Respir Crit Care Med. 2012;186:132–139.
Hughes JMB, Pride NB, eds. Lung function tests: physiological principles and clinical applications. London: WB Saunders, 2000.
MacIntyre, N, Crapo, R, Viegi, G, et al. ATS/ERS Task Force Standardisation of the single breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735.
Miller, MR, Crapo, R, Hankinson, J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161.
Narins, RG, Emmett, M. Simple and mixed acid-base disorders: a practical approach. Medicine. 1980;59:161–187.
Palange, P, Ward, SA, Carlsen, K-H, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29:185–209.
Pellegrino, R, Viegi, G, Brusasco, V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968.
Raffin, TA. Indications for arterial blood gas analysis. Ann Intern Med. 1986;105:390–398.
Wasserman, K. Diagnosing cardiovascular and lung pathophysiology from exercise gas-exchange. Chest. 1997;112:1091–1101.
Wasserman, K, Hansen, JE, Sue, DY, et al. Principles of exercise testing and interpretation: including pathophysiology and clinical applications, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2012.
Williams, AJ. ABC of oxygen: assessing and interpreting arterial blood gases and acid-base balance. BMJ. 1998;317:1213–1216.