Evaluation of patients at high risk for weaning failure with doppler echocardiography
(CONSULTANT LEVEL EXAMINATION)
Overview
Weaning failure is a major complication of mechanical ventilation with associated morbidity and mortality. It is usually defined as an unsuccessful spontaneous breathing trial (SBT) or need for tracheal reintubation within 48 hours following extubation.1 According to study populations and diagnostic criteria, the incidence of weaning failure ranges from 25% to 42% in large cohorts.1 Although the mechanisms are varied and potentially complex, failure to wean a critically ill patient from the ventilator frequently has a cardiac origin. Indeed, the transition from positive-pressure ventilation to spontaneous breathing abruptly alters cardiac loading conditions and has been compared with an exercise test that increases cardiac and breathing workload and metabolic expenditure.2 Accordingly, patients at high risk for weaning failure have long been identified as those with cardiovascular disease or chronic obstructive pulmonary disease (COPD).3,4
Echocardiographic examination
Since transthoracic echocardiography (TTE) is usually informative in this specific clinical setting, transesophageal echocardiography (TEE) is rarely required and performed only as an adjunct to TTE in a patient reconnected to the ventilator. The apical four-chamber view is mainly used to obtain the requested information because it allows evaluation of global and regional ventricular systolic function, left ventricular (LV) diastolic properties and filling pressure, valvular function, intraventricular pressure gradient, and systolic pulmonary artery pressure (Figure 34-1).
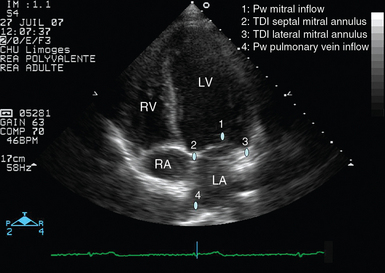
Figure 34-1 Apical four-chamber view disclosing the positioning of pulsed wave Doppler and tissue Doppler imaging samples for assessment of left ventricular filling pressure (blue dots). In addition, this transthoracic echocardiographic view allows evaluation of ventricular function and identification of mitral or tricuspid regurgitations via color Doppler mapping and continuous wave Doppler. LA, Left atrium; LV, left ventricle; Pw, pulsed wave Doppler; RA, right atrium; RV, right ventricle; TDI, tissue Doppler imaging.
Interestingly, TTE may be combined with chest ultrasound to best guide the diagnostic workup of patients with weaning failure at the bedside. After completion of TTE, chest ultrasonography can be performed to obtain valuable morphologic information on the lungs and pleurae, both of which are potentially involved in the weaning failure. For example, chest ultrasonography allows a quantitative diagnosis of pleural effusion and helps in deciding whether to perform thoracentesis in hypoxemic ICU patients.5
Hemodynamic changes induced by spontaneous breathing trials
The transition from positive-pressure ventilation to spontaneous breathing abruptly increases ventricular preload and LV afterload, decreases effective LV compliance,3 and may even induce cardiac ischemia.6 All these factors tend to increase LV filling pressure, which may result in weaning-induced cardiogenic pulmonary edema, especially in patients with left-sided heart disease.7 Nevertheless, in the absence of left heart failure (e.g., COPD patients), the rise in pulmonary artery occlusion pressure (PAOP) remains limited.8
In 117 ICU patients fulfilling the criteria for weaning, we performed TTE immediately before and at the end of a 30-minute SBT. The SBT significantly increased LV stroke volume (and hence cardiac output) and LV filling pressure over baseline values as reflected by a higher mitral Doppler E/A ratio and shortened E-wave deceleration time.9 Similarly, Ait-Ouffela et al10 showed that SBTs increase the mitral E/A ratio and shorten the E-wave deceleration time.
Identification of patients at high risk for pulmonary edema during weaning
We previously showed that ICU patients who failed ventilator weaning had a significantly lower LV ejection fraction (median [25th to 75th percentiles]: 36% [27% to 55%] vs. 51% [43% to 55%], P = .04) and higher LV filling pressure (E/E′ ratio: 7.0 [5.0 to 9.2] vs. 5.6 [5.2 to 6.3]; P = .04) before the SBT than did patients who were successfully extubated.9 Papanikolaou et al11 reported that a lateral E/E′ ratio higher than 7.8 at baseline (pressure-support ventilation) predicted SBT failure in 50 ventilated ICU patients with a sensitivity and specificity of 79% and 100%, respectively.
Identification of pulmonary edema during weaning
Cardiogenic pulmonary edema induced by weaning from the ventilator is suspected in high-risk patients when alternative causes of weaning failure have been confidently ruled out.7 It is a leading cause of weaning failure. In a TTE study performed in 117 ventilated ICU patients, we showed that weaning failure was of cardiac origin in 20 of 23 patients (87%) with an unsuccessful SBT or extubation.9 In patients in whom weaning-induced pulmonary edema develops, echocardiography depicts the presence of increased LV filling pressure and helps identify the potential underlying cause to guide therapy.
Identification of elevated left ventricular filling pressure
Using right-heart catheterization, Lemaire et al3 have long reported that patients in whom weaning-induced pulmonary edema developed exhibited a marked increase in PAOP from a mean value of 8 mm Hg to 25 mm Hg after disconnection from the ventilator.
TTE diagnosis of pulmonary edema induced by weaning relies on depiction of elevated LV filling pressure during spontaneous breathing by means of mitral inflow and pulmonary vein Doppler velocity profiles (Figure 34-2). Combined assessment of early diastolic mitral annulus displacement with tissue Doppler imaging provides valuable information on LV relaxation and may be used to more precisely assess filling pressure (Chapter 32). In ventilated ICU patients, a mitral E/A ratio higher than 2, a systolic fraction of pulmonary vein flow lower than 40%, and an E/E′ ratio higher than 9.5 best predict a PAOP higher than 18 mm Hg.12 In contrast, we showed in 88 ventilated ICU patients that a mitral E/A ratio of 1.4 or lower, a pulmonary vein S/D ratio higher than 0.65, a systolic fraction of pulmonary vein flow higher than 44%, and a lateral E/E′ ratio of 8 or greater best predicted a PAOP of 18 mm Hg or higher.13
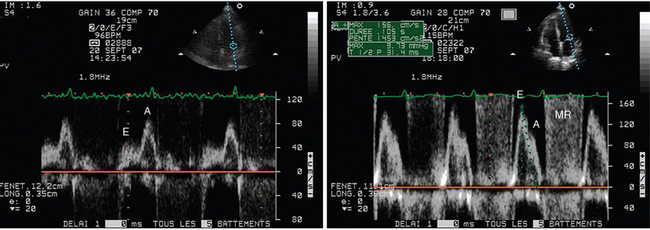
Figure 34-2 Mitral pulsed wave Doppler velocity profiles recorded from an apical four-chamber view in a patient who failed ventilator weaning. Under pressure-support ventilation, the patient exhibited abnormal relaxation with an inverted E/A ratio and a prolonged E-wave deceleration time at baseline (left panel). After extubation, the patient’s clinical status deteriorated secondary to pulmonary edema induced by weaning. The mitral Doppler pattern markedly changed, with a predominant E wave and shortened E-wave deceleration time, which were consistent with elevated left ventricular filling pressure (right panel). Note the development of associated mitral regurgitation (MR) and global acceleration of anterograde mitral Doppler velocity. All these factors contributed to the development of weaning-induced pulmonary edema.
Lamia et al14 recently performed a TTE study in 39 ICU patients with a right-heart catheter after two consecutive SBT failures. PAOP greater than 18 mm Hg (n = 17) was consistently associated with weaning failure. A combined mitral E/A ratio higher than 0.95 and an E/E′ ratio higher than 8.5 at the end of the SBT predicted a PAOP of 18 mm Hg or greater with a sensitivity of 82% and a specificity of 91%.14
Identification of etiology
Myocardial ischemia
The increased cardiac workload and increased load of breathing, especially in patients with COPD,7 induced by SBT may result in myocardial ischemia, which in turn may participate in weaning failure.6 The development of regional wall motion abnormalities (RWMAs) secondary to myocardial ischemia occurs earlier than electrocardiographic changes in the ischemic cascade (Figure 34-3). Accordingly, a new-onset RWMA as evidenced by TTE in a patient who fails ventilator weaning is consistent with myocardial ischemia secondary to unmatched myocardial oxygen demand. Depending on its location and extension, myocardial ischemia may result in a significant increase in LV filling pressure, LV systolic failure, or acute mitral regurgitation (MR) of various mechanisms.15
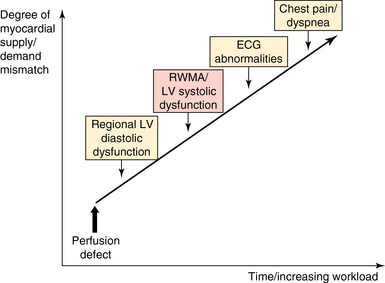
Figure 34-3 Schematic representation of the ischemic cascade of the heart. Regional wall motion abnormality (RWMA) as seen on echocardiography occurs before electrocardiographic (ECG) abnormalities and symptoms. Myocardial ischemia may result in chest pain, as well as in rest dyspnea, secondary to pulmonary venous congestion, especially with exercise.
Transient or exacerbated mitral regurgitation
MR is a frequent occurring valvulopathy in which the regurgitant volume may be altered acutely by changes in LV loading conditions. Specifically, any abrupt increase in LV afterload may worsen MR, especially when central and “functional” (i.e., related to dilatation of the mitral annulus). It may be particularly pronounced in patients with associated LV diastolic dysfunction and chronically elevated filling pressure. Although transient MR is not present in spontaneously breathing patients who arrive at the emergency department with acute pulmonary edema,16 it may be observed during an SBT. In the presence of marked systolic hypertension, arterial vasodilators may be used to offset the deleterious effects of SBT-induced sympathic stimulation on LV afterload.
Ischemic MR has been observed in patients who were admitted with acute pulmonary edema but no evidence of acute ischemia or arrhythmia.17 In these patients, semisupine bicycle exercise increased MR volume and pulmonary artery pressure as assessed by TTE and was associated with exercise-limiting dyspnea. Accordingly, an SBT may potentially induce transient, yet relevant MR (Figure 34-4) secondary to tethering of the mitral leaflets, which tents the leaflets toward the LV apex.17 When eccentric, MR may result in asymmetric or unilateral pulmonary edema since the regurgitant flow increases pulmonary vein pressure unilaterally.18 In this case, papillary muscle dysfunction associated with concomitant RWMA is frequently observed, and TEE has greater diagnostic accuracy than TTE does. Regardless of its mechanism, ischemic MR induced or worsened by an SBT may lead to performance of percutaneous coronary angioplasty to facilitate weaning from the ventilator. Ischemic MR induced or worsened by an SBT may lead to an evaluation of the performance of a coronary revascularization procedure in patients who cannot be weaned from the ventilator after the optimization of medical therapy and repeated SBT failures.
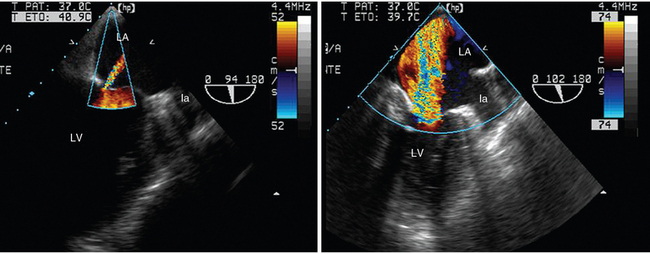
Figure 34-4 Transesophageal echocardiographic diagnosis of acute massive mitral regurgitation in a patient reconnected to the ventilator because of a failed spontaneous breathing trial. Under pressure-support ventilation, the transesophageal two-chamber view disclosed trivial central mitral regurgitation (left panel). Disconnection from the ventilator was rapidly interrupted because of the abrupt onset of weaning-induced pulmonary edema. In a similar echocardiographic view, a large regurgitant jet consistent with massive mitral insufficiency was found to be the origin of the acute respiratory failure (right panel). A concomitant new-onset regional wall motion abnormality strongly suggested myocardial ischemia. la, Left appendage; LA, left atrium; LV, left ventricle.
Finally, transient SBT-induced MR may be attributed to systolic anterior motion (SAM) of the mitral valve.19
Dynamic left ventricular outflow tract obstruction
Patients with a small LV cavity (e.g., LV hypertrophy) are at risk for the development of dynamic obstruction of the LV outflow tract associated with SAM secondary to SBT-driven adrenergic stimulation.19 A dynamic LV outflow tract increases LV filling pressure and reduces LV stroke volume, whereas SAM induces acute and usually eccentric MR. Echocardiography is best suited to depict these intricate mechanisms that jointly lead to weaning-induced pulmonary edema (Figure 34-5). In these patients, right-heart catheterization may erroneously suggest severe LV systolic dysfunction in the presence of elevated PAOP and low cardiac output and lead to the deleterious administration of diuretics, inotropes, or both. Therapy may include interruption of any inotropic support and blood volume expansion, correction of any associated arrhythmia, use of antihypertensive drugs when necessary, and introduction of β-blockers, especially in those with hypertrophic cardiomyopathy and severe LV diastolic dysfunction.20
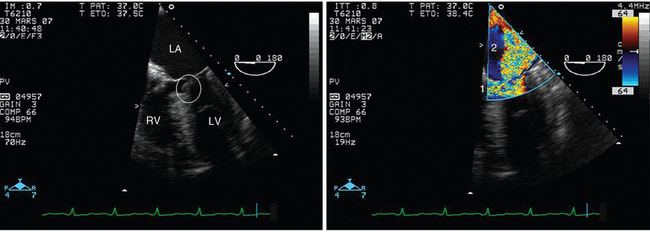
Figure 34-5 Dynamic left ventricular outflow tract obstruction in a hypertrophied left ventricle depicted by transesophageal echocardiography in the four-chamber view. In this patient, who underwent ventilation for severe cardiogenic pulmonary edema, systolic anterior motion of the mitral valve (left panel, circle) was associated with massive mitral regurgitation (right panel, 2). Note that the outflow tract obstruction induces blood flow turbulence (right panel, 1) and may significantly reduce cardiac output. LA, Left atrium; LV, left ventricle; RV, right ventricle.
Monitoring of acute therapy with echocardiography
TTE should be performed before an SBT in high-risk patients to identify any underlying cardiomyopathy and evaluate baseline LV filling pressure. This allows the weaning strategy to be guided and therapy to be adjusted for optimization of LV loading conditions before weaning from the ventilator. When an SBT fails, TTE is valuable in detecting the mechanism of the weaning failure and tailoring therapy (e.g., diuretics, vasodilators). After attaining more favorable LV loading conditions, TTE may confirm the decrease in LV filling pressure before resuming the weaning process and allows close monitoring of hemodynamic tolerance with further SBTs (Figure 34-6).
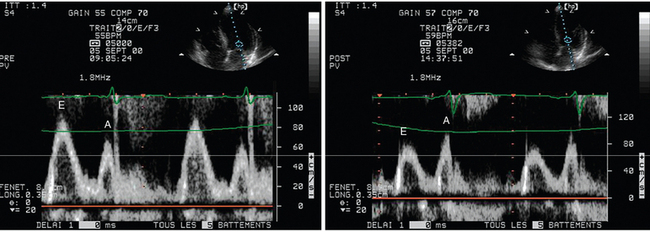
Figure 34-6 Acute effects of left ventricular loading conditions on the mitral Doppler velocity profile recorded from the apical four-chamber view. In this spontaneously breathing hemodialysis patient with shortness of breath at baseline, transthoracic echocardiography recorded a “normalized” mitral Doppler pattern consistent with elevated filling pressure (left panel). The patient’s symptoms disappeared after large-volume ultrafiltration, which induced a drop in left ventricular filling pressure, as reflected by an inverse E/A ratio (right panel).
Pearls and highlights
• Cardiogenic pulmonary edema is frequently responsible for failure to wean patients from the ventilator. Patients at high risk should be screened with TTE so that they can best be managed before the planned extubation.
• In case of weaning failure, TTE allows rapid confirmation of the diagnosis of weaning-induced pulmonary edema by depicting increased LV filling pressure and disclosing a potentially associated cardiopathy.
• Echocardiography helps the intensivist in tailoring therapy to facilitate the weaning process.
References
1. Boles, JM, Bion, J, Connors, A, et al, Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–1056.
2. Pinsky, MR, Breathing as exercise: the cardiovascular response to weaning from mechanical ventilation. Intensive Care Med. 2000;26(9):1164–1166.
3. Lemaire, F, Teboul, JL, Cinotti, L, et al. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988; 69(2):171–179.
4. Richard, C, Teboul, JL, Archambaud, F, et al. Left ventricular function during weaning of patients with chronic obstructive pulmonary disease. Intensive Care Med. 1994; 20(3):181–186.
5. Vignon, P, Chastagner, C, Berkane, V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005; 33(8):1757–1763.
6. Hurford, WE, Favorito, F. Association of myocardial ischemia with failure to wean from mechanical ventilation. Crit Care Med. 1995; 23(9):1475–1480.
7. Teboul, JL, Monnet, X, Richard, C, Weaning failure of cardiac origin: recent advances. Crit Care. 2010;14(2):211.
8. Teboul, JL, Abrouk, F, Lemaire, F. Right ventricular function in COPD patients during weaning from mechanical ventilation. Intensive Care Med. 1988; 14(suppl 2):483–485.
9. Caille, V, Amiel, JB, Charron, C, et al, Echocardiography: a help in the weaning process. Crit Care. 2010;14(3):R120.
10. Ait-Oufella, H, Tharaux, PL, Baudel, JL, et al, Variation of natriuretic peptides and mitral flow indexes during successful ventilatory weaning: a preliminary study. Intensive Care Med. 2007;33(7):1183–1186.
11. Papanikolaou, J, Makris, D, Saranteas, T, et al, New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med. 2011;37(12):1976–1985.
12. Vignon, P, Colreavy, F, Slama, M, Pulmonary edema: which role for echocardiography in the diagnostic work-upDe Backer D, Cholley BP, Slama M, et al, eds.. Hemodynamic monitoring using echocardiography in the critically ill. Springer: Berlin, 2011; 177–194.
13. Vignon, P, Ait Hssain, A, Françoise, B, et al, Noninvasive assessment of pulmonary artery occlusion pressure in ventilated patients: a transesophageal study. Crit Care. 2008;12(1):R18.
14. Lamia, B, Maizel, J, Ochagavia, A, et al. Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med. 2009; 37(5):1696–1701.
15. Marwick, TH, Lancelloti, P, Pierard, L, Ischemic mitral regurgitation: mechanisms and diagnosis. Heart. 2009;95(20):1711–1718.
16. Gandhi, SK, Powers, JC, Nomeir, AM, et al. The pathogenesis of acute pulmonary oedema associated with hypertension. N Engl J Med. 2001; 344(1):17–22.
17. Piérard, LA, Lancellotti, P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med. 2004; 351(16):1627–1634.
18. Lesieur, O, Lorillard, R, Ha Thi, H, et al, Unilateral pulmonary oedema complicating mitral regurgitation: diagnosis and demonstration by transesophageal echocardiography. Intensive Care Med. 2000;26(4):466–470.
19. Adamopoulos, C, Tsagourias, M, Arvaniti, K, et al. Weaning failure from mechanical ventilation due to hypertrophic obstructive cardiomyopathy. Intensive Care Med. 2005; 31(5):734–737.
20. Chockalingam, A, Dorairajan, S, Bhalla, M, et al, Unexplained hypotension: the spectrum of dynamic left ventricular outflow tract obstruction in critical care settings. Crit Care Med. 2009;37(2):729–734.